SUMMARY
The lung is the primary site of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-induced immunopathology whereby the virus enters the host cells by binding to angiotensin-converting enzyme 2 (ACE2). Sophisticated regeneration and repair programs exist in the lungs to replenish injured cell populations. However, known resident stem/progenitor cells have been demonstrated to express ACE2, raising a substantial concern regarding the long-term consequences of impaired lung regeneration after SARS-CoV-2 infection. Moreover, clinical treatments may also affect lung repair from antiviral drug candidates to mechanical ventilation. In this review, we highlight how SARS-CoV-2 disrupts a program that governs lung homeostasis. We also summarize the current efforts of targeted therapy and supportive treatments for COVID-19 patients. In addition, we discuss the pros and cons of cell therapy with mesenchymal stem cells or resident lung epithelial stem/progenitor cells in preventing post-acute sequelae of COVID-19. We propose that, in addition to symptomatic treatments being developed and applied in the clinic, targeting lung regeneration is also essential to restore lung homeostasis in COVID-19 patients.
KEYWORDS: cell therapy, lung injury, resident epithelial stem/progenitor cells, mesenchymal stem cells, post-acute sequelae of COVID-19
INTRODUCTION
The mammalian lungs are continuously challenged by environmental particles, pollutants, and pathogens. Epithelia that cover the conducting airways and alveoli space play multiple roles in maintaining lung hemostasis and building immunity against inhaling content. Classically, lung epithelial cells with tight junctions are considered to form a “great wall” that prevents infiltration of inhaled substances and removes them through mucociliary clearance (1). Increasing evidence has shown that the lung epithelium orchestrates the pulmonary innate immune response because it expresses pathogen-associated molecular patterns (PAMPs) (2). In host defense against infection, lung epithelial cells are also found to produce antimicrobial peptides and proteins, antiviral interferons, and chemokines that drive the recruitment of inflammatory cells (3). As the lungs’ respiratory units, alveoli are tiny sacs lined by flat and thin alveolar type 1 (AT1) epithelial cells that mediate oxygen/carbon dioxide exchange. In contrast, AT2 epithelial cells secrete surfactants (SPA, SPB, SPC, and SPD) to reduce alveolar surface tension. To break through the defense, inhaled pathogens, including the rising acute respiratory syndrome coronavirus 2 (SARS-CoV-2), usually invade lung epithelial cells or induce cell apoptosis or death.
Since the first case of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection was reported in 2019, the number of infections has exceeded 248 million, with over 5 million deaths worldwide by November 5, 2021 (https://covid19.who.int/). Nasal epithelial cells are the primary target of SARS-CoV-2 because of the expression of viral entry factors (4–6). Therefore, the nasal mucosa is considered the primary infection site, from which SARS-CoV-2 can be further transmitted to the respiratory tract (7). This builds a basis for nasal swabs for the detection of SARS-CoV-2. Evidence suggests that viral load peaks in the upper airways and elicits a robust interferon response prior to symptom onset (8). However, the lungs may be the primary site of immunopathology, especially in patients with severe COVID-19. Sophisticated programs have evolved to maintain lung homeostasis following lung injury. This review addresses the mechanisms by which the lung epithelium is repaired after lung injury and how the repair program is disrupted during SARS-CoV-2 infection. We also discuss current therapeutic efforts for coronavirus disease 2019 (COVID-19) patients and highlight how targeted lung regeneration strategies with stem cell therapy are important to maximally restore lung homeostasis in COVID-19 patients.
EPITHELIAL CELLS IN THE LUNG
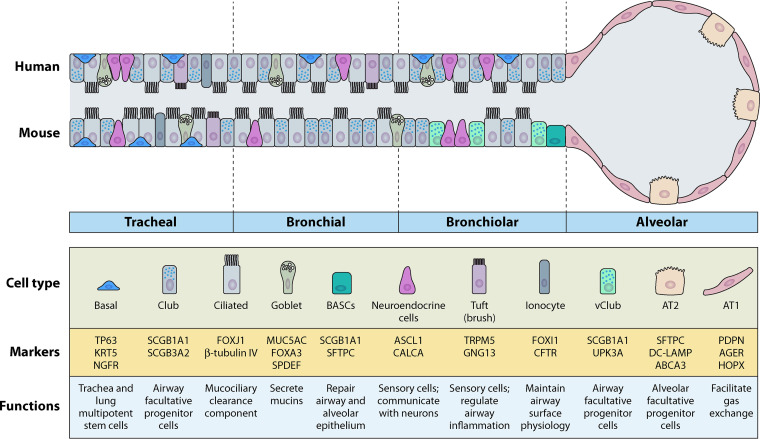
At present, approximately a dozen main epithelial cell types have been identified in the lungs. The composition of the lung epithelium is region-specific. The trachea is covered with a pseudostratified columnar epithelium, and the main cell types are club secretory cells, goblet cells, ciliated cells, and basal cells (Fig. 1) (9). Moreover, there are also submucosal glands as well as a few neuroendocrine clusters. A more simplified columnar epithelium defines intrapulmonary conducting airways, including bronchi and bronchioles. Ciliated and club cells are the most abundant cell types in this region. There are two main epithelial cell types in the alveoli: type-1 (AT1) cells, which cover 95% of the alveolar surface area and are responsible for gas exchange, and type-2 (AT2) cells, which generate large amounts of surfactant protein to reduce the surface tension of the alveoli and prevent lung collapse upon every breath (10, 11).
FIG 1.
General epithelial populations in mammalian lungs. Pseudostratified epithelium is observed in the trachea of both human and mice. Basal cells are situated on basal membrane and serve as stem cells. Basal cells are restricted to the tracheal region in mice but distributed from proximal to distal axis in human lungs. Club cells and ciliated cells represent two of the most predominant cell types in conducting airways. Bronchioalveolar stem cells (BASCs) are found in distal conducting airways of mice but not human. Alveolar epithelia in human is similar to that in mice, including AT1 and AT2 cells. Markers and functions of each epithelial cell type are also indicated.
The current understanding of the mechanisms underlying human lung epithelial maintenance is largely based on the extensive knowledge that has been collected in counterpart mouse models in past decades. This is because the cellular composition is very similar between humans and mice, such as the trachea and alveoli. However, it is worth noting that there are some differences between the intrapulmonary conducting airways of humans and mice. For example, basal cells are distributed throughout the pseudostratified epithelium from the trachea to the bronchioles in human lungs; however, in mice, basal cells are restricted to the trachea and proximal extrapulmonary airways (12). In the distal conducting airways of mice, there are also more neuroendocrine cells than their human counterparts (13). In addition, the distal conducting airways of mice terminate at the BAL junction, which contains bronchioalveolar stem cells (BASCs). The distal airways of humans have no evidence of BASCs and are connected to the alveoli through the respiratory bronchioles, which are not found in mice. These differences must be considered in translational lung medicine.
LUNG EPITHELIAL REGENERATION BY ENDOGENOUS STEM/PROGENITOR CELLS
As the inner surface area of an adult lung is approximately 80 to 100 square meters, the regenerative mechanism in the lung has evolved into a region-specific manner. Unlike other solid organs, multiple regional lung stem/progenitor cells (LSPCs) are positioned in the lung epithelium from the proximal to the distal axis to maintain epithelial homeostasis and/or to perform a repair after lung injury. These stem/progenitor cells include basal cells, club cells, variant club cells, pulmonary neuroendocrine cells, BASCs, and AT2 cells. Mouse BASCs can give rise to both airway and alveolar lineages in mice (14, 15). We focused on the three most studied epithelial stem/progenitor cells in the lungs.
Tracheal Basal Cells
Basal cells, marked by transcription factor transformation-related protein 63 (Trp63 or p63) and cytokeratin 5 (Krt5), are capable of self-renewal, are multipotent, and generate multiple types of epithelial cells when necessary, such as club cells, secretory cells, goblet cells, and ciliated cells (16, 17). Basal cells are thought to be a homogeneous population. However, using mouse lineage tracing of Krt5, Watson et al. found that basal cells are heterogeneous and comprise at least two subsets. One acts more as a self-renewing stem cell, with the other cells expressing low Krt8 levels, focusing on intracavitary differentiation (18). Through single-cell transcriptomic analysis and mouse lineage tracing technology, basal cells were shown to produce tuft cells known as solitary chemosensory cells and may play a role in lung dysplastic remodeling after injury (19, 20).
Adult basal airway cells normally maintain a quiescent state. Knockout of yes-associated protein 1 (Yap) results in the loss of mouse basal cells in the airways (21). Intracellular reactive oxygen species increase to activate nuclear factor erythroid 2-related factor 2 (Nrf2) to stimulate human airway basal cell self-renewal (22). FGFR1 signaling maintains the slow turnover rate of mouse basal cells through downstream SPRY2 inhibitory regulation of receptor tyrosine kinases (23). In contrast, FGFR2 signaling favors mouse airway basal cell self-renewal, and its ligands, FGF7 and FGF10, can promote basal cell growth (24). In addition, basal cell proliferation is also facilitated by epidermal growth factor and Wnt/β-catenin signaling pathways, while restrained by SMAD, bone morphogenetic protein (BMP), and TGFβ signaling pathways in mice (25–29). Conditional deletion of Lef-1, a downstream transcription factor of Wnt/β-catenin, stalls mouse basal cell proliferation (30). β-catenin has also been shown to direct the differentiation of basal cells into club cells or ciliated cells (31, 32). Notch signaling mediated by NOTCH1 and NOTCH3 is essential for human basal cell differentiation into ciliated cells, but sustained activation in human basal cells favors secretory cell differentiation (33, 34). Interleukin 6 (IL-6)/signal transducer and activator of transcription 3 (STAT3) enhance ciliated cell differentiation from basal cells in both humans and mice (35).
Airway Club Cells
Club cells are marked by secretoglobin 1a1 (scgb1a1 or CCSP) and are considered to be epithelial progenitor cells in bronchial and bronchiolar airways; they exhibit limited self-renewal capacity but are capable of giving rise to ciliated cells and goblet cells under both normal homeostasis and injury conditions (36–39). Club cells are heterogeneous and distinct in the proximal versus distal conducting airways and show differential responses to bleomycin-induced lung injury (38). Club cells metabolize lipophilic compounds through cytochrome P450, making them susceptible to injury by lipophilic compounds (40). When club cells are damaged, the variant club (vClub) cells, which are located in the neuroendocrine bodies and bronchioalveolar duct junction (BADJ), rapidly proliferate and repopulate the lost club cells and ciliated cells (41). Club cells can de-differentiate into basal cells during influenza virus infection (36, 42, 43).
The tumor suppressor p53 loss maintains the self-renewal and proliferative capacity of club cells by limiting ciliated cell differentiation (44). BMP signaling is required for mouse airway club cell regeneration but not for ciliated cell differentiation (45). Notch jagged ligand blockade induces rapid loss of club cells and drives ciliated cell differentiation but reduces goblet cell metaplasia in a preclinical asthma model (46, 47). Tyrosine kinase (RYK) is a WNT coreceptor, and goblet cell hyperplasia and mucus overproduction were observed after lung injury in mouse airways when Ryk was genetically deleted in club cells (48). We adopted a loss-of-function strategy to find that autophagy and glucose metabolism sustain the self-renewal, proliferation, and regeneration of mouse club cells at a steady state or during lung inflammation (49). Organoid cultures demonstrated that autophagy inhibits ciliated and goblet cell differentiation (49).
Alveolar AT2 Cells
The epithelial repair program in the alveoli is injury-specific. Bleomycin-induced lung fibrosis is usually associated with apoptosis of alveolar type 2 (AT2) and AT1 cells, replenished mainly by the surviving AT2 cells, AT2 cell subset, BASCs, and distal airway club cells (10). Rare integrin β4-positive lineage-negative epithelial stem/progenitor (LNEP) cells have been identified within the normal distal lung (50). A subset of these cells, which express high H2-K1 levels, are club-like, differentiate into alveolar epithelial cells, and rescue lung function after bleomycin injury (51). Lineage-tracing studies have demonstrated that AT1 cells, thought to be terminally differentiated, proliferate and dedifferentiate into AT2 cells following partial pneumonectomy (52). Rare Trp63 and Krt5 dual positive basal cells in the bronchioles were shown to respond to H1N1 infection (53). p63+/Krt5− cells among LNEP cells can produce Krt5+ basal-like pots in the alveoli after influenza infection (54). This clearly shows the cell plasticity in distal lung regeneration after injury. It is worth noting that all stem-like progenitor cells generate AT2 cells under different injury conditions. This is not surprising given that AT2 cells represent an important facultative progenitor cell for maintaining alveolar-capillary barrier function.
The regulation of AT2 cell self-renewal, proliferation, and differentiation by distinct niche cells and signals has been well documented (55, 56). Delta-like 1 homolog (Dlk1) interacts with Notch to regulate AT1 differentiation in mouse AT2 cells (57). In addition to its role in basal cell regulation, BMP is active in mouse AT2 cells and favors mouse AT1 differentiation at the expense of proliferation after pneumonectomy (58). During lipopolysaccharide (LPS)-induced lung injury, TGFβ is upregulated in the lung and serves as a halting signal for AEC2 proliferation; inactivation of TGFβ allows AT1 differentiation (59). Hippo-yes-associated protein 1 (YAP1) is increased in AT2 cells after LPS-induced mouse lung injury, and interfering with YAP1 expression impedes AT2 proliferation (60). Lung microvascular endothelial cells secrete lipid sphingosine-1-phosphate (S1P) to promote AT2 to AT1 differentiation through S1PR2/YAP signaling after bacterial lung injury (61). In addition, we observed that endothelial thrombospondin-1/CD36 signaling promotes AT2 cell proliferation (62). Thrombospondin-1 could also be a Wnt signaling target in the regulation of human AT1 differentiation (63). Activation of Wnt/β-catenin signaling promotes AT2 cell survival and differentiation into AT1 cells during BLM-induced mouse lung injury (64, 65). FGFR2 is also essential for AT2 survival and prevents lung fibrosis after bleomycin (66). Autophagy is upregulated in surviving AT2 cells and is required for AT2 proliferation during regeneration after lung injury (67).
Inflammatory cytokines produced by locally injured lung tissues directly or indirectly affect the proliferation and differentiation of airway and alveolar epithelial cells. After pneumonectomy, activated platelets secrete stromal-cell-derived factor-1 through its receptor on pulmonary endothelial cells to trigger AT2 cell proliferation and differentiation through the membrane-type matrix metalloproteinase (MMP14) (68). Macrophages and C motif chemokine receptor 2 (CCR2)+ monocytes are recruited to the lung after pneumonectomy and differentiate into M2-like macrophages in the presence of IL-13, which is produced by group-2-innate lymphoid cells and promotes lung regeneration (69). Trefoil factor 2/Wnt signaling in macrophages is essential for stimulating epithelial proliferation and promoting epithelial barrier restoration following lung injury (70). The expression of IL-1β and tumor necrosis factor-alpha (TNF-α) is promoted in the AT2 niche following influenza-induced injury, enhancing the proliferation of surviving AT2 cells and sustaining their differentiation potential (71).
IMMUNE RESPONSE TO SARS-CoV-2 IN THE LUNG
The homotrimers of the spike glycoprotein (S protein) constitute the spikes on the surface of SARS-CoV-2 and are responsible for binding to the host receptor angiotensin-converting enzyme 2 (ACE2) (72). In addition, transmembrane serine protease (TMPRSS) 11A, TMPRSS11D, and TMPRSS2 are proteases on the cell surface that cleave the SARS-CoV-2 S protein (73). TMPRSS2 is now known as the main host protease that activates S proteins and mediates virus entry into cells (74). FURIN and cathepsin L are intracellular proteases that cleave the coronavirus S protein through the endolysosome pathway to allow SARS-CoV to enter the cell (75).
Animal models have been established to facilitate the understanding of SARS-CoV-2 induced immunopathology and accelerate the testing of vaccines and therapeutics. Mice, nonhuman primates, hamsters, ferrets, and others have been adopted in COVID-19 research for different purposes (76–80). The comparison of these animal models for SARS-CoV-2 infection has been studied and reviewed (81–84).
As part of innate immunity, lung epithelial cells are the first line to sense invading SARS-CoV-2 through ACE2 and TMPRSS2. SARS-CoV-2 cell entry and infectivity can be facilitated by TMPRSS2, the cysteine protease cathepsins B and L, and furin, or neuropilin-1 (85, 86). Cytokines, chemokines, and growth factors are then produced by the infected epithelium and resident immune cells in the acute immune response. This leads to the recruitment and activation of various inflammatory cells, including neutrophils, monocytes, and natural killer cells, to build a powerful innate immune defense system (87). Many immune cells in the lungs express CD147, which may mediate infection by SARS-CoV-2, but do not express ACE2 or TMPRSS2 (88). In infected cells, single-stranded RNA from the virus can be recognized by pattern recognition receptors (PRRs), including Toll-like receptor 7 (TLR7) and TLR8, whereas double-stranded RNA during replication is sensed by TLR3, retinoic acid-inducible gene I (RIG-I), and melanoma differentiation-associated protein 5 (MDA5) (89). Antiviral expression of type I and III interferons (IFNs) is then triggered by downstream activation of the transcription factor interferon regulatory factor-3 (IRF-3)/IRF-7 via TIR-domain-containing adapter-inducing interferon-β (TRIF). In addition, nuclear factor kappa light-chain-enhancer of activated B cells (NF-κB) is activated via myeloid differentiation factor 88 (MyD88) and translocated to the nucleus to stimulate the production of pro-inflammatory cytokines/chemokines (e.g., TNF-α, CCL-2, and CXCL-8) (90).
Neutrophils are the first immune cells recruited to the lungs upon infection; they ingest microbes and kill them with reactive oxygen species, antimicrobial proteins, and degradative enzymes. Neutrophils also act as antigen-presenting cells. Neutrophils amplify inflammation by releasing chemokines to attract monocytes and dendritic cells. Monocyte-derived macrophages and alveolar macrophages present antigens, phagocytose microbes, or apoptotic cells. They can be polarized into the pro-inflammatory M1 phenotype in the presence of LPS, IFN-γ, or an anti-inflammatory phenotype in IL-4 and IL-13 (91). M1 macrophages secrete TNF-α, IL-1β, and IL-6, causing tissue injury, while M2 macrophages produce IL-10 and TGF-β, associated with tissue repair and fibrosis (92). Degranulated mast cells mediate a potent inflammatory response by releasing many inflammatory factors, including histamine, proteases, leukotrienes, cytokines, and chemokines (93). In addition to promoting the killing of inhaled microbes, vasoactive contents of mast cell granules may also induce lung tissue damage by affecting the vascular tone, smooth muscle, and mucous glands. Like neutrophils, eosinophils also contain granules but are filled with different components contributing to immune defense and tissue damage (94, 95). Other innate immune cells include basophils, innate lymphoid cells, and dendritic cells (87, 96, 97). Dendritic cells serve as messengers between innate and adaptive immune systems. Dendritic cells recognize, ingest microbes, and present antigens. Compared with adults, children with mild COVID-19 exhibit reduced circulating monocytes, dendritic cells, and natural killer cells during acute SARS-CoV-2 infection (98).
In adaptive immune response, viral antigen presentation triggers both T cell-mediated cellular immunity and B cell-mediated humoral immunity (99). T cells, macrophages, and natural killer cells secrete IFN-γ to exert antiviral effects. Successful host defense prevents the invasion of microbes-induced lung tissue damage. Complete removal of invading microbes, apoptosis of inflammatory cells, and switching from a pro-inflammatory to an anti-inflammatory state leads to the resolution of acute inflammation. However, chronic inflammation occurs when any of these inflammation resolution processes fail. Lung tissue damage is often seen in chronic inflammation, prolonging disease progression and making the tissue susceptible to challenges from other respiratory microbes.
An exaggerated innate immune response, delayed adaptive immunity, or altered coordination between innate and adaptive immunity may induce pathogenic inflammation in the lungs of patients with COVID-19 (100). The disease severity of COVID-19 is determined by the balance between an effective antiviral response and a dysregulated immune response. Aberrant activation of macrophages and neutrophils has been observed in patients with severe COVID-19 and contributes to the development of acute respiratory distress syndrome (ARDS) (101–103). The T-cell count was low, especially in severe COVID-19 patients (104). As evidence accumulates, it is becoming clear that longer hospitalization and higher incidence of critical disease are related to reduced and delayed neutralizing antibody response or production of IFN-λ and type I IFN, along with the rampant production of pro-inflammatory cytokines, such as TNF, IL-6, and IL-8 (105–109). Furthermore, downregulation of specialized pro-resolving lipid mediators also contributes to increased disease severity and dysregulated phagocyte function in COVID-19 (110). Recent studies have suggested that eosinopenia confers a higher risk of severe progression (111). The nasopharyngeal microbiome has been shown to regulate local and systemic immune responses and impact disease outcomes in acute SARS-CoV-2 infection (112, 113). Post-acute sequelae of COVID-19 (PASC) are associated with the severity of acute SARS-CoV-2 infection, pre-existing medical conditions, immune exhaustion, or abnormal immunometabolism and mitochondrial dysfunction (114, 115).
SARS-CoV-2 INDUCED LUNG EPITHELIAL INJURY
When lung epithelial cells, the initial source of inflammatory cytokines, are infected by SARS-CoV-2, they interact with the recruited immune cells, contributing to inflammatory lung damage and respiratory failure (116). This mechanism has been suggested by single-cell RNA sequencing analyses of lung samples from COVID-19 patients (117). Prolonged and uncontrolled cytokine storm may lead to epithelial barrier damage; lung fibrosis progresses once lung epithelial regeneration is suppressed under such conditions.
SARS-CoV-2 injures a variety of lung epithelial cells because of the expression of ACE2 or TMPRSS2 (Table 1). In the airway epithelium, ACE2 is expressed on basal cells, intermediate cells, club cells, mucous cells, and ciliated cells (118). A single-cell RNA sequence analysis suggested that the proportion of ACE2 in mucous cells was higher than in other epithelial cells (119). Both AT2 and AT1 cells can be infected with SARS-CoV-2, and AT2 cells appear to be the first to become infected (120). However, it has been suggested that only a small portion of AT2 cells express ACE2 (121). In contrast, TMPRSS2 is expressed at low levels in the basal cells of the undifferentiated airway epithelium and is more clearly expressed in differentiated airway epithelium. TMPRSS11A, TMPRSS11D, TMPRSS2, FURIN, and cathepsin L are expressed in the small airway epithelium (118). TMPRSS2, FURIN, and ACE2 co-express in club cells, ciliated cells, AT1, and AT2 cells (122, 123).
TABLE 1.
Lung epithelial cells that SARS-CoV-2 targetsa
| Cell type | Host | Tool | ACE2 | TMPRSS2 | FURIN | Reference |
|---|---|---|---|---|---|---|
| Basal cells | Human | RNA-seq, scRNA-seq and microarray | √ | Zhang et al. (118) | ||
| Intermediate cells | Human | RNA-seq, scRNA-seq and microarray | √ | Zhang et al. (118) | ||
| Mucous cells | Human | RNA-seq, scRNA-seq and microarray | √ | Zhang et al. (118) | ||
| Club cells | Human and nonhuman primates (M. mulatta and M. fascicularis) | RNA-seq, scRNA-seq and microarray, whole-mount immunostaining, qRT-PCR, immunohistochemistry, scRNA-ISH, Western blotting, snRNA-seq | √ | √ | √ | Zhang et al. (118), Hou et al. (120), Ziegler et al. (122), Lukassen et al. (123) |
| Ciliated cells | Human and nonhuman primates (M. mulatta and M. fascicularis) | RNA-seq, scRNA-seq and microarray, whole-mount immunostaining, qRT-PCR, immunohistochemistry, scRNA-ISH, Western blotting, snRNA-seq | √ | √ | √ | Zhang et al. (118), Hou et al. (120), Ziegler et al. (122), Lukassen et al. (123) |
| AT1 cells | Human and nonhuman primates (M. mulatta and M. fascicularis) | scRNA-seq, whole-mount immunostaining, qRT-PCR, immunohistochemistry, scRNA-ISH, Western blotting, snRNA-seq | √ | √ | √ | Hou et al. (120), Ziegler et al. (122), Lukassen et al. (123) |
| AT2 cells | Human and nonhuman primates (M. mulatta and M. fascicularis) | scRNA-seq, whole-mount immunostaining, qRT-PCR, immunohistochemistry, scRNA-ISH, Western blotting, snRNA-seq, multiplex RT-PCR, immunofluorescent staining | √ | √ | √ | Hou et al. (120), Ziegler et al. (122), Lukassen et al. (123), Zou et al. (121), Mossel et al. (246) |
RNA-seq, RNA sequencing; scRNA-seq, single-cell RNA sequencing; snRNA-seq, single-nuclei RNA sequencing; qRT-PCR, quantitative real-time PCR.
After SARS-CoV-2 infection, the lung initiates the regeneration and repair mechanism, and obvious proliferation of LSPCs appears in the trachea, large and small airways, and alveoli (124). Basal cell proliferation is evident in both the trachea and the large airways. AT2 cell proliferation increases in the alveoli, and AT2 differentiation is initiated by the 38th day after the onset of initial symptoms (125). Analysis of serum cellular markers in 115 COVID-19 patients indicated that damage to AT1 cells and endothelial cells was no longer evident 2 weeks after virus clearance (126). However, the damage to the AT2 cells and lung structures persisted. However, the time scale for complete lung regeneration remains unknown. The lung histopathological characteristics of COVID-19 patients include diffuse alveolar damage (DAD), reactive alveolar hyperplasia, and fibroblast hyperplasia (127). Significant exudation of proteins and fibrin has also been observed (128). SARS-CoV-2 infection induces fibronectin production in alveolar epithelial cells, and its deposition may lead to pulmonary fibrosis in COVID-19 patients (129). Based on a recent cohort study, pulmonary impairment was reported in COVID-19 survivors 6 months after acute infection (130).
CURRENT PREVENTION AND TREATMENTS FOR COVID-19
SARS-CoV-2 infection usually causes fever, cough, dyspnea, pneumonia, and other clinical symptoms, while some mild patients have no obvious symptoms. Elderly patients or patients with underlying medical conditions have a higher chance of developing severe conditions, including myocardial damage, acute respiratory distress syndrome, septicemia, septic shock, secondary infection, and acute heart injury, with a high mortality rate.
Vaccines
Safe and effective SARS-CoV-2 vaccines are game-changing tools in the fight against the COVID-19 pandemic. To date, more than a dozen COVID-19 vaccines have been approved, including mRNA vaccines (BNT16b2 and mRNA-1273), viral vector vaccines (AZD1222, Sputnik V, Sputnik V Light, Ad5-nCoV, Ad26.COV2.S), inactivated and live-attenuated vaccines (CoronaVac, BBIBP-CorV, Wuhan Sinopharm inactivated vaccine, Covaxin, QazVac, KoviVac, COVIran Barekat), and protein-based vaccines (EpiVacCorona, ZF2001, Abdala). A narrative review of these vaccines has been conducted in terms of their safety and effectiveness in preventing severe COVID-19, hospitalization, and death against all variants of concern (131). Over 7 billion vaccine doses had been administered by November 5, 2021, according to the World Health Organization (WHO). As long-lasting B-cell and T-cell immunity to SARS-CoV-2 is established by vaccination globally, we do not have far to go to bring the pandemic under control. To achieve this goal, there is still much to do regarding booster doses, dosing intervals, and duration of vaccine immunity against variants of concern (132).
Targeted Therapies
Approximately 5% to 10% of COVID-19 patients have severe or even life-threatening illnesses that require urgent intervention. Early antiviral treatment for these COVID-19 patients is essential, especially in the presence of poor prognostic predictors (133). The antiviral drug remdesivir, previously tested against Ebola virus infection, was approved by the Food and Drug Administration in October 2020 to treat hospitalized COVID-19 patients. Its activity against SARS-CoV-2 infection has been demonstrated in Vero E6 cells (134). In an animal model of SARS-CoV-2 infection, remdesivir treatment was shown to lower viral titers and prevent lung tissue damage (135). Pooled observational clinical studies have indicated that remdesivir may shorten the median recovery time of adult patients with severe COVID-19 (136). Similar to remdesivir, the anti-malarial drugs chloroquine (CQ) or hydroxychloroquine (HCQ) have been assessed in several clinical trials regarding their efficacy, effectiveness, and safety managing COVID-19. CQ is a proton-base that can concentrate in acidic organelles and have glycosylation interactions with the ACE-2 receptor, thus preventing viruses from binding and fusing into the cells (133). However, the results of clinical trials were conflicting due to the improper design of the studies and the small cohort of patients in most of these studies (137). Other anti-SARS-CoV-2 drug candidates, including umifenovir, lopinavir/ritonavir, darunavir, favipiravir, and nitazoxanide, have also been recently reviewed (138).
SARS-CoV-2 elicited a humoral immune response by generating abundant immunoglobulin M (IgM) antibodies approximately 2 weeks after infection, followed by the production of IgA- and IgG-protective neutralizing antibodies that remain in the blood of recovered COVID-19 patients for months (139). Therefore, convalescent plasma transfusion (CPT) has naturally raised the potential to tackle the COVID-19 pandemic. CPT therapy has been previously evaluated in patients with SARS and Ebola (140, 141). Based on the limited clinical trials available, CPT therapy appears to be safe in treating COVID-19 patients. It effectively eliminates SARS-CoV-2 RNA, increasing neutralizing antibody titers, improving oxygen and lymphocyte counts, downregulating inflammatory markers, alleviating clinical symptoms, and reducing mortality (142). Early transfusion of high-titer convalescent plasma may prevent severe progression in older adults (143). It is difficult to use CPT therapy as the main intervention method for COVID-19 because of its obvious limitations. For example, convalescent plasma is a mixture of antibodies created in response to the virus infection. The titer of neutralizing antibodies in donated plasma varies from donor to donor, posing a huge obstacle to the standardization of CPT therapy. The constituents of non-antibody fractions in plasma and their long-term deleterious effects on recipients are not fully understood. To overcome these shortcomings, monoclonal neutralizing antibodies should be isolated from convalescent plasma or developed experimentally to block SARS-CoV-2 pathogenicity (144). Most of the research conducted screened neutralizing antibodies that bind to the SARS-CoV-2 receptor-binding domain (144, 145). Casirivimab and imdevimab have recently been recommended for emergent use to treat COVID-19 patients with mild to moderate symptoms. Although monoclonal antibodies are very effective in neutralizing the virus, large-scale production of monoclonal antibodies is usually expensive and is time-consuming. Secondary antibody responses are usually induced in recipients to target these monoclonal antibodies to limit their therapeutic effects. Impaired and exhausted NK cells are associated with the severity of COVID-19 (146, 147). NK-based therapies could be developed to promote early viral control.
Immunomodulatory Therapies
Impaired innate and adaptive immunity and unwanted inflammatory cytokine storms drive disease progression after SARS-CoV-2 infection. A decrease in the counts of T and B lymphocytes and natural killer cells was more obvious in severe patients than in mild cases (148, 149). Pro-inflammatory cytokines (e.g., IL-2, IL-6, IL-8, and TNF) are significantly increased in the plasma of patients with severe symptoms (150). In addition, IL-6 targeting monoclonal antibodies, such as tocilizumab and sarilumab, can reduce acute inflammatory responses in COVID-19 patients (151, 152). Ciclesonide, an inhaled glucocorticoid, reduces local inflammation through its anti-inflammatory properties and inhibits viral replication by targeting the SARS-CoV-2 NSP15 endonuclease (153, 154). A retrospective cohort study by Yamasaki et al. indicated that the incidence of severe pneumonia was lower in COVID-19 patients treated with ciclesonide than in the control group (155). Dexamethasone and cortisone inhibit systemic inflammatory responses (156). Although steroids do not significantly reduce mortality, they effectively stabilize hemodynamics and shortening mechanical ventilation in patients (157). Although many drugs have been proposed to enhance antiviral immunity and eliminate the inflammatory cytokine storm (149), only a few drugs are recommended for certain COVID-19 patients to relieve severe symptoms. JAK signaling inhibitors such as baricitinib and tofacitinib act on multiple critical pathways to suppress cytokine production, modulate interferons and IL-6, and interfere with viral infection (149). Baricitinib treatment has been shown to resolve inflammation in severe COVID-19 patients or SARS-CoV-2-infected rhesus macaques (158, 159). Adult COVID-19 patients who received oral tofacitinib exhibited a lower risk of death or respiratory failure through day 28 than placebo (160).
Symptomatic and Supportive Therapies
Symptomatic therapy remains the leading solution to relieve the clinical symptoms associated with SARS-CoV-2 infection. For example, certain medicines can reduce fever and pain, suppress cough, and stop nausea, vomiting, and dyspnea when necessary. Fluid supplementation and nutritional support are critical for meeting the needs of the body’s metabolism. Psychological assistance, usually ignored, is also necessary to tackle the anxiety normally observed in COVID-19 patients (161).
Microbial coinfection occurs in some cases of COVID-19, including respiratory viruses, fungi, and bacteria (162). Symbiotic ecosystems in the lung are restored or maintained by the proper administration of antibacterial drugs for therapeutic or prophylactic use. Some COVID-19 patients may require intubation and mechanical ventilation to relieve hypoxia symptoms (163). Trioxy therapy, known as trioxy autohemotherapy or immunotrioxy transfusion therapy, is a raised and tested COVID-19 treatment because of its potential capacity to stimulate the innate immune system (164).
Possible Effects of Existing Treatments on Lung Regeneration
Whether or not such mixed interventions may result in long-term side effects is missing. Many lung epithelial cells, including stem/progenitor cells, are targeted and injured by SARS-CoV-2. The plasma AT2 injury marker was observed to maintain a high level even after SARS-CoV-2 was cleared (126). Alveolar epithelial cell injury has been observed in mechanically ventilated COVID-19 patients (165). It remains unknown whether such injury is due to an inflammatory storm and/or clinical treatments. In vitro experiments suggested that dexamethasone or transdehydroandrosterone significantly decreased lung epithelial injuries associated with mechanical ventilation, but this was not tested in vivo, especially during viral infections (166). An ambidirectional cohort study showed that three-quarters of surviving COVID-19 patients retained at least one common symptom 6 months after discharge (130). The length of these significant sequelae remains unclear. As we learned from SARS in 2003, a significant proportion of surviving patients still had respiratory and cardiac problems 12 years after recovery (167). The lung is the most severely damaged organ in these patients; thus, treatments for COVID-19 must choose medical options that do not impair lung regeneration. Lung injury could be caused by hyperbaric oxygen during ventilation therapy due to the toxicity of the drugs administered or from a virus-associated inflammatory storm (168). Therefore, the ideal therapy for COVID-19 does not sacrifice lung regeneration.
STEM CELL THERAPY AND COVID-19
Mesenchymal Stem Cell-Based Therapy
Mesenchymal stem cells (MSCs) can be obtained from various tissues, including the stroma of the bone marrow, adipose tissue, placenta, umbilical cord tissue, or amniotic fluid. MSCs are multipotent and can differentiate into multiple types of cells within the body, including osteoblasts, chondroblasts, adipocytes, cardiomyocytes, hepatocytes, renal tubular epithelial cells, and pancreatic islet beta cells (169). In this regard, MSC therapy has led to a robust clinical agenda for treating various diseases associated with tissue damage (170). MSCs exert their functions through paracrine factors (soluble mediators), including anti-inflammatory cytokines, anti-apoptotic and antimicrobial peptides, angiogenic growth factors, and extracellular vesicles (171, 172). Exosomes and microvesicles carry transcription factors, cytokines, growth factors, miRNAs, and mRNAs to affect target cells upon binding or internalization (173). MSC-derived extracellular vesicles (MSC-EVs) exhibit exceptional immunosuppressive capacities by regulating the migration, proliferation, activation, and polarization of various immune cells and promoting a tolerogenic immune response (174).
The SARS-CoV-2 infection causes injury to multiple organs, including the lungs, heart, brain, intestine, kidney, liver, other blood vessels, pancreas, bile ducts, and conjunctiva (175). Therefore, MSC-based therapy has become an attractive and promising strategy for the treatment of COVID-19 and was immediately raised at the beginning of the pandemic (176). ARDS is associated with high mortality and morbidity in many pulmonary diseases, including COVID-19. MSC-based therapy provides a potential alternative strategy for treating ARDS by targeting the pathological events associated with ARDS, as reviewed by Qin and Zhao (177). The compassionate use of MSC-based therapy for COVID-19 patients in the absence of pre-clinical data was encouraged by its application in ARDS induced by lipopolysaccharide (LPS), Escherichia coli, and influenza virus. In preclinical animal studies, administration of MSCs derived from bone marrow, adipose, menstrual blood, or umbilical cord was demonstrated to reduce lung inflammation, mitigate lung damage, and improve lung function during LPS-induced ARDS in animals (178–182). Similar benefits were observed in MSC therapy for ARDS induced by Escherichia coli in animals (183–188).
The therapeutic effects of MSCs were obvious in H9N2 and H5N1, but not H1N1 influenza virus-induced acute lung injury in mice (189–191). In H5N1-induced acute lung injury in mice, the therapeutic effect of umbilical cord MSCs was better than that of bone marrow MSCs, attributed to higher levels of angiopoietin-1 and HGF (191). In clinical trials, the transplantation of MSCs derived from bone marrow or adipose tissue was tolerated in patients with ARDS without adverse effects (192, 193). Moreover, a phase 2a safety trial of bone marrow MSC therapy for ARDS showed that patients’ plasma angiopoietin 2 (a mediator of lung epithelial injury) decreased and oxygenation improved (194). Using MSCs to treat ARDS caused by H7N9 infection, Chen et al. found that the transplantation of allogeneic menstrual blood-derived MSCs significantly decreased the mortality of the experimental group, and no harmful effects were observed in four patients within the 5-year follow-up period (195). As for the cell-free strategy, MSC-derived extracellular vesicles or exosomes have shown good therapeutic effects in acute lung injury induced by Escherichia coli, LPS, or influenza virus (196–200).
By November 9, 2021, 365 clinical trials were registered on ClinicalTrials.gov, and 53 studies were completed. Among these, three trials reported death, but two trials reported that death was unrelated to MSC administration (Table 2) (201, 202). The side effects associated with MSC therapy include transient facial flushing, fever, transient hypoxia, liver dysfunction, heart failure, and allergic rashes (203, 204). Other published clinical trials reported no side effects or deaths, suggesting that MSCs are well tolerated and relatively safe for COVID-19 treatment (Table 2). COVID-19 patients in most of these trials included both male and female adults in severe or critical conditions. The sources of MSCs included umbilical cords, menstrual blood, bone marrow, or adipose tissue, while some studies did not indicate their sources. MSC exosomes were used in one study. Most trials delivered MSCs to COVID-19 recipients intravenously at 1 to 2 million cells per kilogram of body weight. Approximately half of the MSC therapy trials had no placebo control. After the administration, typical symptoms of SARS-CoV-2 infection, including fever, weakness, fatigue, and shortness of breath, were shown to be improved, and inflammatory indicators showed reductions, oxygenation indices improved, lymphocyte counts increased, and ground-glass opacities and pneumonia infiltration were reduced on chest radiographs (Table 2).
TABLE 2.
Summary of clinical trials of MSC therapy for COVID-19 patientsa
| Reference | Country of study | No. of COVID-19 patients |
Illness severity (no. of patients) | MSC source (no. of patients) | Administration route | Control group included? | Investigation time window | Main findings |
|---|---|---|---|---|---|---|---|---|
| Sengupta et al. (247) | United States | 27 (10 female, 17 male, age 29 to 84) | Critical (24) | BM-MSC exosomes (ExoFlo™) | Intravenously, 15 ml of ExoFlo added to 100 mL of saline | No | 14 days after injection | Clinical status and oxygenation improved, neutrophil count decreased, lymphopenia improved, acute phase reactants declined. |
| Lanzoni et al. (248) | United States | 12 (7 female, 5 male, age 59 ± 16 yrs) | Mild-to-moderate (3), Moderate-to-severe (9) | hUC-MSC | Intravenously, twice (on day 0 and 3) of 100 ± 20 × 106 cells | 12 COVID-19 patients | 0 and 6 days after the injection | GM-CSF, IFN-γ, IL-5, and IL-6 were significantly reduced. The recovery and survival time of patients improved. No serious adverse events related to UC-MSC infusion were observed. |
| Shi et al. (249) | China | 65 (28 female, 37 male, age, median 61) | Severe (65) | hUC-MSCs | Intravenously, 4 × 107 cells every time (day 0, 3, and 6) | 35 COVID-19 patients | baseline to day 28 | The volume of whole lung lesions and the 6-min walk test distance increased in the MSCs treatment group. |
| Guo et al. (201) | China | 31 (6 female, 25 male, IQR, 61 to 71 yrs) | Severe (23), Critical (8) | UC-MSCs | Intravenously, 1 × 106 cells/kg wt, once (11), twice (9), 3 times (11) | No | Not specified | Lymphocyte count increased, procalcitonin, IL-6, D-dimer and CRP decreased, PaO2/FiO2 elevated. In 14 days after the first infusion, 30 patients’ SARS-CoV-2 PCR results became negative. Four died. |
| Xu et al. (250) | China | 26 (9 female, 17 male, age 58 ± 12 yrs) | Severe (16), Critical (10) | Menstrual blood-derived MSCs | Intravenously, three times (3 × 107 cells each time) every other day | 18 COVID-19 patients for control group (concomitant medications) | 7, 14, and 30 days after injection | The time required for treatment has been shortened and the survival rate of patients has been improved. The oxygen saturation and PaO2 were significantly improved. |
| Chen et al. (203) | China | 25 (5 female, 20 male, IQR, 59 to 71 yrs) | Severe (25) | Not described | Intravenously, 1 × 106 cells/kg wt. One time (7), twice (7), three times (11) | No | 2 to 3 days after the first injection | WBC counts, CRP, procalcitonin, and IL-6 not changed. The serum levels of cardiac troponin T, creatine kinase-MB and lactate elevated. Three cases experienced treatment related side effects including liver dysfunction, heart failure, and allergic rash. |
| Shu et al. (251) | China | 12 (8 male and 4 female, IQR, 50 to 70 yrs) | Severe (12) | hUC-MSCs | Intravenously, 2 × 106 cells/kg wt | 29 COVID-19 patients | 14 days after injection | Symptoms of weakness and fatigue, shortness of breath improved. CRP and IL-6 reduced, the oxygenation index recovered faster, the recovery of lymphocyte count accelerated. Chest CT showed lung inflammation decreased. |
| Meng et al. (204) | China | 9 (2 female, 7 male, IQR, 37 to 52 yrs) | Moderate (5), Severe (4) | hUC-MSCs | Intravenously, 3 times (3 × 107cells each time) | 9 COVID-19 patients | 14 days after the first injection | Inflammatory cytokines reduced, PaO2/FiO2 improved. Transient facial flushing and fever shortly after MSC therapy. |
| Leng et al. (252) | China | 7 (4 male and 3 female, IQR, 51 to 65 yrs) | Critical (1), severe (4), common (2) | Not described | Intravenously, 1 × 106 cells/kg wt | 3 COVID-19 patients | 10 days after injection | Pulmonary function significantly improved, 2 common and 1 severe patients recovered and discharged in 10 days after treatment. |
| Tang et al. (253) | China | 2 (male 71 yrs, female 37 yrs) | Severe (2) | MB-MSCs | Intravenously, 1 × 106 cells/kg wt, 3 times | No | 12 days after the first injection | Lymphocyte count increased, the inflammation indicators decreased, and the absorption of the exudate lesions in the lungs. |
| Liang et al. (254) | China | 1 (female, 65 yrs) | Critical (1) | hUC-MSCs | Intravenously, 3 times (5 × 107 cells each time) with a 3-day interval | No | 17 days after the first MSC injection | Remission of inflammatory symptoms, the patient discharged. |
| Peng et al. (255) | China | 1 (female, 66 yrs) | Severe (1) | hUC-MSCs | Intravenously, 1 × 106 cells/kg wt, 3 times | No | 4 days after the first injection | Bilateral infiltration absorbed, dyspnea and dry cough significantly improved. |
| Zhang et al. (256) | China | 1 (male, 54 yrs) | Severe (1) | hWJCs | Intravenously, 1 × 106 cells/kg wt | No | 6 days after injection | Fever, shortness of breath disappeared, lymphocyte count increased, IL-6, TNFα, and CRP decreased, ground-glass opacity and pneumonia infiltration reduced. |
| Zhu et al. (257) | China | 1 (male, 48 yrs) | Critical (1) | hUC-MSC | Intravenously, 1 × 106 cells/kg wt | No | 14 days after injection | The non-invasive ventilator was removed 6 days later. On the 13th day, the coronavirus nucleic acid test was negative. |
| Dilogo et al. (258) | Indonesia | 20 (15 male, 5 female, <40 yrs, 4; 40-60 yrs, 8; >60 yrs, 8) | Critical | UC-MSCs | Intravenously, 1 × 106 cells/kg wt | 20 COVID-19 patients | 15 days after injection | The survival rate of patients has increased. The level of IL-6 decreased. |
| Sánchez-Guijo et al. (202) | Spain | 13 (12 male and 1 female, IQR, 55 to 66 yrs) | Severe (13) | AT-MSC | Intravenously, 1 × 106 cells/kg wt, once (2), twice (10), 3 times (1) | No | 16 days after the first injection | CRP, IL-6, ferritin, LDH and D-dimer decreased, lymphocyte count increased. Two patients died unrelated to MSC therapy, one from massive gastrointestinal bleeding and another one secondary fungal pneumonia. |
| Haberle et al. (259) | Germany | 5 (2 female, 3 male, IQR, 32 to 50 yrs) | Severe (5) | Allogeneic hMSC | Intravenously, 1 × 106 cells/kg wt. twice (3), three times (2) | 18 COVID-19 patients | Day 1, day 5 and discharge day | Ferritin and D-dimer increased significantly. Leukocytes and neutrophils decreased, while lymphocytes increased. The lung function improved overall and the mortality rate decreased. |
| Hashemian et al. (260) | Iran | 11 (3 female, 8 male, age 54 ± 10 yrs) | Critical (11) | UC-MSCs (6) PL-MSCs (5) |
Intravenously, three times (2 × 108 cells each time) | No | 1 to 2 days after the injection | Six patients survived. The oxygen saturation is significantly improved. The opacity of the ground glass in the lungs obviously disappeared. IL-8, TNF-α, C-reactive protein, IL-6, and IFN-v° decreased. During the first PL-MSC infusion, two patients experienced shaking, which was relieved by supportive treatment. |
| Saleh et al. (261) | Iran | 5 (2 female, 3 male, age 45 to 54) | Severe (5) | hWJCs | Intravenously, 3 times 3 days apart (1.5 × 108 cells each time) | No | 0, 3, 6, 14, and 28 days after the first injection | Absolute lymphocyte count, percentage of lymphocytes, CD4+ T cells and CD8+ T cells increased. Ferritin decreased significantly. 14 days after treatment, stromal cell-derived factor 1 and IL-10 increased, while TGF-β, IFN-γ, VEGF, IL-6 and TNFα decreased. |
| Iglesias et al. (262) | México | 5 (1 female, 4 male, IQR, 38 to 64 yrs) | Severe (5) | Allogeneic human umbilical cord MSCs | Intravenously, 1 × 106 cells/kg wt | No | 21 days after injection | On the 13th and 15th days, two patients died. From 1st to 7th day, PaO2/FiO2 gradually improved. Plasma thrombin-antithrombin complex and D-dimer will increase transiently. |
| Senegaglia et al. (263) | Brazil | 1 (male, 51 yrs old) | Severe | UC-MSCs | Intravenously, 3 times (on day 1, 3, and 5), 5 × 105 cells each time | No | On the day of cell infusion, and D14, D60, and D120 after the first infusion | Creatinine, ferritin, D-dimer, and C-reactive protein decreased. On the 14th day, the relative viral quantification was undetectable. lymphocytes and T cells increased. Ground-glass opacity reduced. |
| Zengin et al. (264) | Turkey | 1 (male, 72 yrs) | Critical (1) | UC-MSCs | Intravenously (0.7 × 106 cells/kg) and intratracheally (0.3 × 106 cells/kg), second dose at 5 days later | No | 7 days after the first injection | Acidosis, electrolyte imbalance, and hypoxemia improved, CRP decreased, chest X-ray showed regression in the ground-glass imaged infiltration and the low-density. |
hUC-MSCs, human umbilical cord derived mesenchymal stem cells; BM-MSCs, bone marrow derived mesenchymal stem cells; MB-MSCs, menstrual blood derived mesenchymal stem cells; hWJCs, human umbilical cord Wharton's jelly derived mesenchymal stem cells; AT-MSCs, adipose tissue derived mesenchymal stromal cells; CRP, C-reactive protein; WBC, white blood cells; IL-6, interleukin-6; LDH, lactic acid dehydrogenase.
Multiple potential mechanisms are underlying MSC-based therapy for COVID-19. MSCs have the potential to differentiate into AT2 cells. MSCs derived from bone marrow, decidua, or amniotic fluid were induced by Wnt/β-catenin to express AT2 cell markers and/or secret surfactants in vitro (205–209). MSC-derived secretomes contain soluble factors (chemokines, growth factors, etc.) and extracellular vesicles that may control cytokine storm, reduce apoptosis of alveolar epithelial cells and endothelial cells, and promote angiogenesis and alveolar epithelial regeneration in COVID-19 patients (210–212). MSC or MSC exosomes also reduced fibroblast activation and ECM deposition (213–216). In addition, MSC-EVs were shown to fuse with lung epithelial cells, thereby inhibiting influenza virus replication in a swine model of acute lung injury (200). It remains unknown whether MSC-EVs play a similar role in COVID-19.
Although the sympathetic application of MSCs appears to be promising in COVID-19 treatments, there are still several issues that need to be addressed: (i) the rational strategy of using MSCs against SARS-CoV-2 infection is based on the proven beneficial effects of MSC therapy in treating ARDS, but they lack relevant pre-clinical studies; (ii) the number of recruited COVID-19 patients was small, and most of the completed clinical trials had no control group, making the efficacy of MSC therapy plausible; (iii) MSCs accumulate in the lung after intravenous transplantation, creating a favorable opportunity to reclaim the pulmonary microenvironment. However, severe to critical COVID-19 patients are usually in various degrees of hypercoagulable states and are at high risk for thromboembolism and thrombotic multi-organ failure. Patients’ intravascular coagulation needs to be evaluated when standardizing a safe and effective dosage of MSCs; (iv) many companies are commercializing MSC-based cell therapies. However, the translation of such cell-based therapy is impaired by many steps that introduce batch heterogeneity to MSCs; (v) different sources of MSCs have been used in clinical trials, but no comparison has been conducted; and (vi) studies have demonstrated that priming steps are critical for improving the therapeutic effects of MSCs (217). Altogether, much work needs to be done considering the consistency and uniformity of MSC quality in future cell therapy for COVID-19.
Rational LSPC Therapy for COVID-19
The restoration of lung immunological homeostasis is only part of the entirety of lung recovery in COVID-19 patients. The epithelium injured by SARS-CoV-2 in both the airways and alveolar spaces must be repopulated to limit lung vulnerability to inhaled microbes and substances and prevent the progression of lung fibrosis in these patients. Increasing evidence strongly suggests that the restoration of the lung structure is far less efficient than expected, possibly contributing to sequelae observed in recovered COVID-19 patients (126, 130). Therefore, LSPC-based therapy is also needed to improve the quality of life of recovered COVID-19 patients, in addition to MSC therapies.
Screening for small molecules that can promote the regenerative potential of LSPCs would be an effective strategy. This strategy is beneficial not only for COVID-19 patients but also for others with regenerative lung defects. In the past decade, tremendous efforts have been made to identify the signaling pathways that regulate the functions of LSPCs in animal models. Emerging and recently sophisticated in vitro three-dimensional organoid technology provides a powerful platform for large-scale drug screening in regenerative medicine (218, 219). The most likely candidates are the existing drug candidates for COVID-19 that have been demonstrated to tackle the SARS-CoV-2 life cycle (219). In addition to small molecules, we could screen active ingredients of MSC-derived exosomes and microvesicles favorable for lung epithelial repair.
Unlike MSCs, transplantation of tissue stem/progenitor cells is not often observed in clinical trials. One concern is the potential of implanted LSPCs for neoplasms in recipients if mutations are introduced into the genome during the myriad of isolation and expansion steps. LSPCs are possible cells of origin in lung cancer (11). In addition, tens of millions of LSPCs are needed for transplantation, but establishing a well-controlled method for in vitro expansion has been challenging for autologous cell transplantation. Furthermore, multiple LSPCs that can repair alveolar epithelia are targeted by SARS-CoV-2. Transplanting a mixture of LSPCs is impractical in clinical practice. It is relatively more feasible to test individual types of LSPC in clinical trials following reliable pre-clinical studies. In 2007, Serrano-Mollar found that intratracheal transplantation of AT2 cells reduced the bleomycin-induced severity of pulmonary fibrosis in rats (220). The beneficial effects of AT2 cell therapy could be associated with the restoration of AT2 cell abundance and lung surfactant protein levels, which is critical for proper respiratory function (221). Based on this, this group underwent allogeneic AT2 cell transplantation through fiberoptic bronchoscopy in 16 patients with moderate and progressive lung fibrosis. They observed that AT2 cell therapy was well tolerated in these patients (222). More recently, autologous human SOX9-positive basal cells were isolated by bronchoscopic brushing, expanded in vitro, and transplanted into two bronchiectasis patients (223). High-resolution computed tomography and spirometry tests indicated an improvement in pulmonary function. However, the safety and efficacy of this strategy require additional verification in a much larger cohort.
MSC/LSPC Therapy for COVID-19 Patients
COVID-19 patients with prolonged immunological alterations without available treatment options may consider MSC-based therapies. Efficient and robust epithelial regeneration contributes to the resolution of lung inflammation and limits fibrotic lung progression. LSPC-based therapy can be used to treat COVID-19 patients with persistent lung epithelial damage. Cell damage results in the release of cell-specific proteins into the circulation. For example, the receptor for advanced glycation products (RAGE) is a well-characterized marker to evaluate AT1 cell injury; we and others showed that RAGE is elevated in the plasma of COVID-19 patients and associated with ARDS outcome in these patients (126, 224). In addition, we found that plasma SPD also has prognostic and pathogenic values in evaluating AT2 cell injury in COVID-19 patients (126). In this study, we observed that plasma SPD remained at a high level 2 weeks after discharge. When lung structural damage and immunological alterations occur in patients after SARS-CoV-2 infection, MSC-based therapy alone may not be sufficient to restore lung homeostasis. MSC/LSPC double cell therapy may be a solution when no effective treatments are available to prevent lung fibrosis development and critical condition progression. PASC patients with persistent lung injury will most likely require MSC/LSPC-based stem cell therapies. This MSC/LSPC double stem cell therapy strategy targets two important pathological elements of COVID-19 simultaneously: immunological imbalance and structural distortion of the lung tissue.
Contribution of Other Stem/Progenitor Cells to Lung Repair
The successful in vitro differentiation of induced pluripotent stem cells (iPSCs) and embryonic stem cells (ESCs) into lung epithelial cells has ushered in a new era of regenerative medicine (225). This suggests that iPSCs and ESCs have great potential as sources of MSCs or LSPCs for stem cell therapy. When Mou et al. transplanted lung progenitor cells derived from human iPSCs into immunodeficient mice, they found that these cells formed the respiratory epithelium (226). Finn et al. successfully differentiated human pluripotent stem cells into basal cells (227). Pooja et al. proposed a method to differentiate iPSCs into pulmonary neuroendocrine cells (228). Yamamoto et al. described a strategy for obtaining alveolar epithelial cells and alveolar organoids derived from human iPSCs (229). In addition, many studies have shown that embryonic stem cells can generate respiratory epithelial cells (226, 230–233).
Bone marrow-derived endothelial progenitor cells (EPCs) are generally considered to be involved in angiogenesis and regulation of vascular homeostasis (234, 235). However, lung injury animal models have shown that EPC treatment is beneficial to lung repair after injury and the recovery of lung function. For example, exosomes derived from EPCs improve LPS-induced acute lung injury in mice (236). Ju et al. found that EPC transplantation improved ventilator-induced lung injury (237). Studies have also suggested that pulmonary capillary endothelial cells can stimulate epithelial progenitor cells, including bronchoalveolar stem cells and alveolar epithelial cells, to proliferate, promoting alveolar regeneration by producing paracrine growth factors (238). EPC therapy is also considered a potential treatment for cell-based pulmonary hypertension (239, 240). These cell-based therapy strategies may inspire the treatment of COVID-19.
Host Response to Stem Cell Therapy
The efficacy of stem cell therapy is greatly influenced by host factors. As seen in many cell therapies, one of the main challenges in stem cell therapy is the rapid and massive loss of exogenously delivered cells in the recipient. This is presumably caused by the potential immunogenicity of stem cells and their differentiated products, which can be cleared by the host innate and/or adaptive immune systems (241). In addition, it may also be due to the altered hostile tissue environments, such as in COVID-19, inflammatory, fibrotic, and oxidative niche, which is not conducive for the implanted LSPCs or MSCs to engraft and survive (242, 243). Although MSCs exhibit potent immunoregulatory abilities, the contribution of surviving MSCs (only a small portion of the implanted MSCs) to the therapeutic benefits for COVID-19 patients is questionable. In clinical trials without appropriate controls, the efficacy of MSC-based cell therapy must be evaluated cautiously. Excessive reactive oxygen species are known to induce cell senescence, which may abrogate the regenerative capacity of LSPC (67, 244). High levels of reactive oxygen species cause DNA damage and promote MSC aging via multiple mechanisms, including DNA methylation and histone acetylation (245). COVID-19 patients who need stem cell therapy are usually older with oxidative imbalance; therefore, much work remains to prepare the host tissue for stem cell therapy. There are many ways to improve the efficacy of stem cell therapy. Genetic modification of stem cells may minimize their immunogenicity to avoid rejection (241). The immune responsiveness of the host can be modified by the use of immunosuppressive drugs prior to implantation. These must be verified by preclinical animal studies before clinical trials of stem cell therapy for COVID-19 patients can be initiated.
CONCLUDING REMARKS
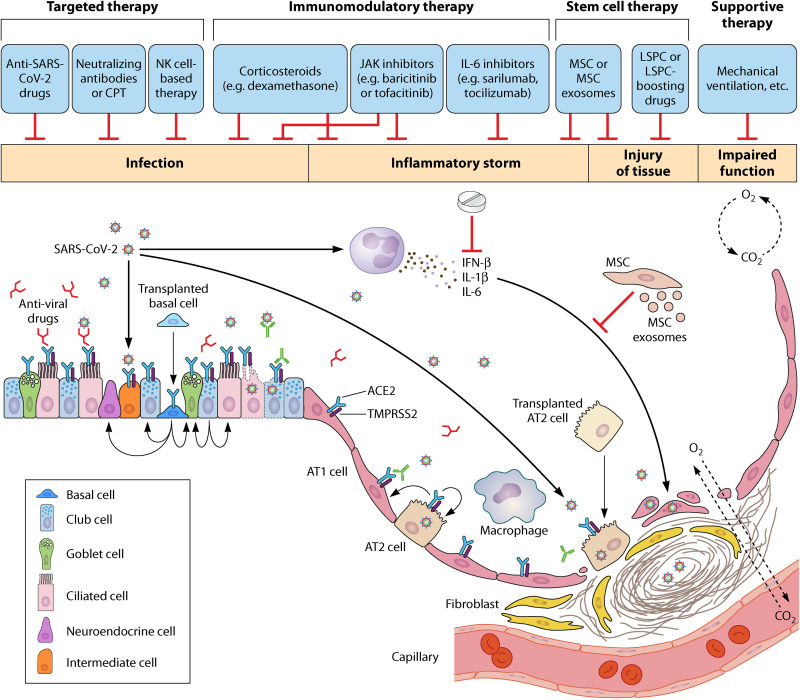
To eliminate the virus and inflammation associated with infection and restore immune homeostasis and repair the structure of the lung, it is beneficial not only to regain lung function in COVID-19 patients but also to better control the proven sequela in these patients after recovery. However, any single intervention may not be sufficient to ensure full recovery of lung homeostasis. In addition to targeted therapy being developed and symptomatic treatments being applied, future therapeutic efforts targeting lung tissue regeneration are also important to restore lung homeostasis and function in COVID-19 patients (Fig. 2). The ideal COVID-19 treatment formula could include: (i) antiviral targeted drugs or neutralizing antibodies to eliminate the virulence of SARS-CoV-2; (ii) immune-targeted therapies including oral corticosteroids or MSC administration to enhance the host’s immunity and limit lung inflammation; (iii) stem cell therapy, either MSCs, MSC exosomes, or LSPCs, if available and appropriate, to enhance the recovery of lung homeostasis. Drugs that boost stem cell therapy should be developed, and (iv) supportive care (for example, mechanical ventilation) is also necessary when required. Continuous optimization of clinical treatment strategies will certainly reduce the severity and mortality of COVID-19 patients and improve their prognosis after discharge.
FIG 2.
SARS-CoV-2 induced lung pathology and treating strategy. SARS-CoV-2 infection causes massive damage to lung epithelium, followed by inflammation storms and possible tissue disruptions. Lung stem/progenitor cells (LSPC) are injured due to the surface expression of ACE2. Monotherapy may not be sufficient to restore lung homeostasis. Combined interventions can be considered including targeted therapy with anti-SARS-CoV-2 infection drugs or monoclonal antibodies, inflammation suppression and host immunity boosts such as corticosteroids, lung structural recovery with LSPC, or MSC therapy. In addition, supportive treatments (for example, mechanical ventilation) are also necessary in the clinical care of COVID-19 patients.
ACKNOWLEDGMENTS
This study was supported by the National Natural Science Foundation of China (81773394, 82070001, 81970001), Natural Science Foundation of Tianjin (20JCQNJC01790, 18ZXDBSY00150), Science and Technology Planning Project of Tianjin Jinnan District (20200118), and Haihe Hospital Fund of China (HHYY-202008). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
We declare that we have no conflicts of interest.
Biographies

Fuxiaonan Zhao obtained her B.S. degree in medical laboratory technology (2019) at Jining Medical University, China. She is in her last year of pursuing a master’s degree in biochemistry and molecular biology in medicine at Tianjin Medical University. She is currently doing research at Tianjin Key Laboratory of Lung Regenerative Medicine, China. She is very interested in stem cell therapy for lung diseases. Her current research focuses on the preclinical cell therapy for idiopathic pulmonary fibrosis by mesenchymal stem cells (MSCs) or lung epithelial stem/progenitor cells (LSPCs).

Qingwen Ma received her B.S. degree in medical laboratory technology from Shanxi Medical University, China. She is an M.Sc. student of biochemistry and molecular biology in medicine at Tianjin Medical University. Since 2020, she has been doing research at the Tianjin Institute of Respiratory Diseases, where her main interest is the investigation of the impact of current clinical treatment options on lung regeneration using three-dimensional organoids and animal models.

Qing Yue obtained a B.S. in medical laboratory technology in Medical School of Dalian University and then continued to pursue her M.Sc. degree in biochemistry and molecular biology in medicine at Haihe Clinical School, Tianjin Medical University. As a second-year graduate student in Dr. Chen’s lab, she is studying molecular mechanisms involved in the regulation of lung epithelial stem/progenitor cells in lung injury and repair.

Huaiyong Chen, Ph.D., is a Principal Investigator of Tianjin Institute of Respiratory Diseases and Director of Tianjin Key Laboratory of Lung Regenerative Medicine, Haihe Hospital, Tianjin University, China. Dr. Chen is also actively involved in graduate training programs at Tianjin Medical University and Zhengzhou University. He is a member of the Basic Research Committee of Chinese Society for Tuberculosis, Chinese Medical Association and advisor of the Committee of Tianjin Clinical Research of Stem Cell Therapy. He obtained his Ph.D. from the Institute of Biophysics, Chinese Academy of Sciences. Thereafter, he was trained as a postdoctoral fellow in the Department of Immunology and then Department of Medicine at Duke University (U.S.A.). Since 2008, Dr. Chen’s research interests have focused on the regulation of lung epithelial stem/progenitor cells in respiratory infections, asthmatic inflammation, and pulmonary fibrosis using organoids, animal models, and human subjects, and stem cell therapy for respiratory diseases.
REFERENCES
- 1.Vareille M, Kieninger E, Edwards MR, Regamey N. 2011. The airway epithelium: soldier in the fight against respiratory viruses. Clin Microbiol Rev 24:210–229. 10.1128/CMR.00014-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hiemstra PS, McCray PB, Jr, Bals R. 2015. The innate immune function of airway epithelial cells in inflammatory lung disease. Eur Respir J 45:1150–1162. 10.1183/09031936.00141514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leiva-Juarez MM, Kolls JK, Evans SE. 2018. Lung epithelial cells: therapeutically inducible effectors of antimicrobial defense. Mucosal Immunol 11:21–34. 10.1038/mi.2017.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sungnak W, Huang N, Becavin C, Berg M, Queen R, Litvinukova M, Talavera-Lopez C, Maatz H, Reichart D, Sampaziotis F, Worlock KB, Yoshida M, Barnes JL, HCA Lung Biological Network . 2020. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med 26:681–687. 10.1038/s41591-020-0868-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahn JH, Kim J, Hong SP, Choi SY, Yang MJ, Ju YS, Kim YT, Kim HM, Rahman MDT, Chung MK, Hong SD, Bae H, Lee CS, Koh GY. 2021. Nasal ciliated cells are primary targets for SARS-CoV-2 replication in the early stage of COVID-19. J Clin Invest 131:e148517. 10.1172/JCI148517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bunyavanich S, Do A, Vicencio A. 2020. Nasal gene expression of angiotensin-converting enzyme 2 in children and adults. JAMA 323:2427–2429. 10.1001/jama.2020.8707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gallo O, Locatello LG, Mazzoni A, Novelli L, Annunziato F. 2021. The central role of the nasal microenvironment in the transmission, modulation, and clinical progression of SARS-CoV-2 infection. Mucosal Immunol 14:305–316. 10.1038/s41385-020-00359-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mick E, Kamm J, Pisco AO, Ratnasiri K, Babik JM, Castaneda G, DeRisi JL, Detweiler AM, Hao SL, Kangelaris KN, Kumar GR, Li LM, Mann SA, Neff N, Prasad PA, Serpa PH, Shah SJ, Spottiswoode N, Tan M, Calfee CS, Christenson SA, Kistler A, Langelier C. 2020. Upper airway gene expression reveals suppressed immune responses to SARS-CoV-2 compared with other respiratory viruses. Nat Commun 11:5854. 10.1038/s41467-020-19587-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rackley CR, Stripp BR. 2012. Building and maintaining the epithelium of the lung. J Clin Invest 122:2724–2730. 10.1172/JCI60519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Basil MC, Katzen J, Engler AE, Guo M, Herriges MJ, Kathiriya JJ, Windmueller R, Ysasi AB, Zacharias WJ, Chapman HA, Kotton DN, Rock JR, Snoeck HW, Vunjak-Novakovic G, Whitsett JA, Morrisey EE. 2020. The cellular and physiological basis for lung repair and regeneration: past, present, and future. Cell Stem Cell 26:482–502. 10.1016/j.stem.2020.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang J, Li X, Chen H. 2020. Organoid models in lung regeneration and cancer. Cancer Lett 475:129–135. 10.1016/j.canlet.2020.01.030. [DOI] [PubMed] [Google Scholar]
- 12.Rock JR, Randell SH, Hogan BL. 2010. Airway basal stem cells: a perspective on their roles in epithelial homeostasis and remodeling. Dis Model Mech 3:545–556. 10.1242/dmm.006031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Volckaert T, De Langhe S. 2014. Lung epithelial stem cells and their niches: Fgf10 takes center stage. Fibrogenesis Tissue Repair 7:8. 10.1186/1755-1536-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim CF, Jackson EL, Woolfenden AE, Lawrence S, Babar I, Vogel S, Crowley D, Bronson RT, Jacks T. 2005. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell 121:823–835. 10.1016/j.cell.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 15.Liu Q, Liu K, Cui G, Huang X, Yao S, Guo W, Qin Z, Li Y, Yang R, Pu W, Zhang L, He L, Zhao H, Yu W, Tang M, Tian X, Cai D, Nie Y, Hu S, Ren T, Qiao Z, Huang H, Zeng YA, Jing N, Peng G, Ji H, Zhou B. 2019. Lung regeneration by multipotent stem cells residing at the bronchioalveolar-duct junction. Nat Genet 51:728–738. 10.1038/s41588-019-0346-6. [DOI] [PubMed] [Google Scholar]
- 16.Hong KU, Reynolds SD, Watkins S, Fuchs E, Stripp BR. 2004. In vivo differentiation potential of tracheal basal cells: evidence for multipotent and unipotent subpopulations. Am J Physiol Lung Cell Mol Physiol 286:L643–9. 10.1152/ajplung.00155.2003. [DOI] [PubMed] [Google Scholar]
- 17.Rock JR, Onaitis MW, Rawlins EL, Lu Y, Clark CP, Xue Y, Randell SH, Hogan BL. 2009. Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc Natl Acad Sci USA 106:12771–12775. 10.1073/pnas.0906850106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Watson JK, Rulands S, Wilkinson AC, Wuidart A, Ousset M, Van Keymeulen A, Gottgens B, Blanpain C, Simons BD, Rawlins EL. 2015. Clonal Dynamics Reveal Two Distinct Populations of Basal Cells in Slow-Turnover Airway Epithelium. Cell Rep 12:90–101. 10.1016/j.celrep.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Montoro DT, Haber AL, Biton M, Vinarsky V, Lin B, Birket SE, Yuan F, Chen S, Leung HM, Villoria J, Rogel N, Burgin G, Tsankov AM, Waghray A, Slyper M, Waldman J, Nguyen L, Dionne D, Rozenblatt-Rosen O, Tata PR, Mou H, Shivaraju M, Bihler H, Mense M, Tearney GJ, Rowe SM, Engelhardt JF, Regev A, Rajagopal J. 2018. A revised airway epithelial hierarchy includes CFTR-expressing ionocytes. Nature 560:319–324. 10.1038/s41586-018-0393-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rane CK, Jackson SR, Pastore CF, Zhao G, Weiner AI, Patel NN, Herbert DR, Cohen NA, Vaughan AE. 2019. Development of solitary chemosensory cells in the distal lung after severe influenza injury. Am J Physiol Lung Cell Mol Physiol 316:L1141–L1149. 10.1152/ajplung.00032.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao R, Fallon TR, Saladi SV, Pardo-Saganta A, Villoria J, Mou H, Vinarsky V, Gonzalez-Celeiro M, Nunna N, Hariri LP, Camargo F, Ellisen LW, Rajagopal J. 2014. Yap tunes airway epithelial size and architecture by regulating the identity, maintenance, and self-renewal of stem cells. Dev Cell 30:151–165. 10.1016/j.devcel.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paul MK, Bisht B, Darmawan DO, Chiou R, Ha VL, Wallace WD, Chon AT, Hegab AE, Grogan T, Elashoff DA, Alva-Ornelas JA, Gomperts BN. 2014. Dynamic changes in intracellular ROS levels regulate airway basal stem cell homeostasis through Nrf2-dependent Notch signaling. Cell Stem Cell 15:199–214. 10.1016/j.stem.2014.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Balasooriya GI, Johnson JA, Basson MA, Rawlins EL. 2016. An FGFR1-SPRY2 signaling axis limits basal cell proliferation in the steady-state airway epithelium. Dev Cell 37:85–97. 10.1016/j.devcel.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Balasooriya GI, Goschorska M, Piddini E, Rawlins EL. 2017. FGFR2 is required for airway basal cell self-renewal and terminal differentiation. Development 144:1600–1606. 10.1242/dev.135681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brechbuhl HM, Li B, Smith RW, Reynolds SD. 2014. Epidermal growth factor receptor activity is necessary for mouse basal cell proliferation. Am J Physiol Lung Cell Mol Physiol 307:L800–10. 10.1152/ajplung.00201.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hsu HS, Liu CC, Lin JH, Hsu TW, Su K, Hung SC. 2014. Repair of naphthalene-induced acute tracheal injury by basal cells depends on beta-catenin. J Thorac Cardiovasc Surg 148:322–332. 10.1016/j.jtcvs.2013.10.039. [DOI] [PubMed] [Google Scholar]
- 27.Mou H, Vinarsky V, Tata PR, Brazauskas K, Choi SH, Crooke AK, Zhang B, Solomon GM, Turner B, Bihler H, Harrington J, Lapey A, Channick C, Keyes C, Freund A, Artandi S, Mense M, Rowe S, Engelhardt JF, Hsu YC, Rajagopal J. 2016. Dual SMAD Signaling Inhibition Enables Long-Term Expansion of Diverse Epithelial Basal Cells. Cell Stem Cell 19:217–231. 10.1016/j.stem.2016.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tadokoro T, Gao X, Hong CC, Hotten D, Hogan BL. 2016. BMP signaling and cellular dynamics during regeneration of airway epithelium from basal progenitors. Development 143:764–773. 10.1242/dev.126656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kiyokawa H, Yamaoka A, Matsuoka C, Tokuhara T, Abe T, Morimoto M. 2021. Airway basal stem cells reutilize the embryonic proliferation regulator, Tgfbeta-Id2 axis, for tissue regeneration. Dev Cell 56:1917–1929 e9. 10.1016/j.devcel.2021.05.016. [DOI] [PubMed] [Google Scholar]
- 30.Jensen-Cody CW, Crooke AK, Rotti PG, Ievlev V, Shahin W, Park SY, Lynch TJ, Engelhardt JF. 2021. Lef-1 controls cell cycle progression in airway basal cells to regulate proliferation and differentiation. Stem Cells 39:1221–1235. 10.1002/stem.3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Giangreco A, Lu L, Vickers C, Teixeira VH, Groot KR, Butler CR, Ilieva EV, George PJ, Nicholson AG, Sage EK, Watt FM, Janes SM. 2012. beta-Catenin determines upper airway progenitor cell fate and preinvasive squamous lung cancer progression by modulating epithelial-mesenchymal transition. J Pathol 226:575–587. 10.1002/path.3962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brechbuhl HM, Ghosh M, Smith MK, Smith RW, Li B, Hicks DA, Cole BB, Reynolds PR, Reynolds SD. 2011. beta-catenin dosage is a critical determinant of tracheal basal cell fate determination. Am J Pathol 179:367–379. 10.1016/j.ajpath.2011.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rock JR, Gao X, Xue Y, Randell SH, Kong YY, Hogan BL. 2011. Notch-dependent differentiation of adult airway basal stem cells. Cell Stem Cell 8:639–648. 10.1016/j.stem.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gomi K, Arbelaez V, Crystal RG, Walters MS. 2015. Activation of NOTCH1 or NOTCH3 signaling skews human airway basal cell differentiation toward a secretory pathway. PLoS One 10:e0116507. 10.1371/journal.pone.0116507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tadokoro T, Wang Y, Barak LS, Bai Y, Randell SH, Hogan BL. 2014. IL-6/STAT3 promotes regeneration of airway ciliated cells from basal stem cells. Proc Natl Acad Sci USA 111:E3641–9. 10.1073/pnas.1409781111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rawlins EL, Okubo T, Xue Y, Brass DM, Auten RL, Hasegawa H, Wang F, Hogan BL. 2009. The role of Scgb1a1+ Clara cells in the long-term maintenance and repair of lung airway, but not alveolar, epithelium. Cell Stem Cell 4:525–534. 10.1016/j.stem.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van Keymeulen A, Blanpain C. 2012. Tracing epithelial stem cells during development, homeostasis, and repair. J Cell Biol 197:575–584. 10.1083/jcb.201201041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen H, Matsumoto K, Brockway BL, Rackley CR, Liang J, Lee JH, Jiang D, Noble PW, Randell SH, Kim CF, Stripp BR. 2012. Airway epithelial progenitors are region specific and show differential responses to bleomycin-induced lung injury. Stem Cells 30:1948–1960. 10.1002/stem.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hong KU, Reynolds SD, Giangreco A, Hurley CM, Stripp BR. 2001. Clara cell secretory protein-expressing cells of the airway neuroepithelial body microenvironment include a label-retaining subset and are critical for epithelial renewal after progenitor cell depletion. Am J Respir Cell Mol Biol 24:671–681. 10.1165/ajrcmb.24.6.4498. [DOI] [PubMed] [Google Scholar]
- 40.Boyd MR. 1977. Evidence for the Clara cell as a site of cytochrome P450-dependent mixed-function oxidase activity in lung. Nature 269:713–715. 10.1038/269713a0. [DOI] [PubMed] [Google Scholar]
- 41.Giangreco A, Reynolds SD, Stripp BR. 2002. Terminal bronchioles harbor a unique airway stem cell population that localizes to the bronchoalveolar duct junction. Am J Pathol 161:173–182. 10.1016/S0002-9440(10)64169-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zheng D, Yin L, Chen J. 2014. Evidence for Scgb1a1(+) cells in the generation of p63(+) cells in the damaged lung parenchyma. Am J Respir Cell Mol Biol 50:595–604. 10.1165/rcmb.2013-0327OC. [DOI] [PubMed] [Google Scholar]
- 43.Tata PR, Mou H, Pardo-Saganta A, Zhao R, Prabhu M, Law BM, Vinarsky V, Cho JL, Breton S, Sahay A, Medoff BD, Rajagopal J. 2013. Dedifferentiation of committed epithelial cells into stem cells in vivo. Nature 503:218–223. 10.1038/nature12777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McConnell AM, Yao C, Yeckes AR, Wang Y, Selvaggio AS, Tang J, Kirsch DG, Stripp BR. 2016. p53 regulates progenitor cell quiescence and differentiation in the airway. Cell Rep 17:2173–2182. 10.1016/j.celrep.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 45.Shafiquzzaman M, Biswas S, Li P, Mishina Y, Li B, Liu H. 2020. The noncanonical BMP signaling pathway plays an important role in club cell regeneration. Stem Cells 38:437–450. 10.1002/stem.3125. [DOI] [PubMed] [Google Scholar]
- 46.Lafkas D, Shelton A, Chiu C, de Leon Boenig G, Chen Y, Stawicki SS, Siltanen C, Reichelt M, Zhou M, Wu X, Eastham-Anderson J, Moore H, Roose-Girma M, Chinn Y, Hang JQ, Warming S, Egen J, Lee WP, Austin C, Wu Y, Payandeh J, Lowe JB, Siebel CW. 2015. Therapeutic antibodies reveal Notch control of transdifferentiation in the adult lung. Nature 528:127–131. 10.1038/nature15715. [DOI] [PubMed] [Google Scholar]
- 47.Carrer M, Crosby JR, Sun G, Zhao C, Damle SS, Kuntz SG, Monia BP, Hart CE, Grossman TR. 2020. Antisense oligonucleotides targeting jagged 1 reduce house dust mite-induced goblet cell metaplasia in the adult murine lung. Am J Respir Cell Mol Biol 63:46–56. 10.1165/rcmb.2019-0257OC. [DOI] [PubMed] [Google Scholar]
- 48.Kim HT, Yin W, Nakamichi Y, Panza P, Grohmann B, Buettner C, Guenther S, Ruppert C, Kobayashi Y, Guenther A, Stainier DYR. 2019. WNT/RYK signaling restricts goblet cell differentiation during lung development and repair. Proc Natl Acad Sci USA 116:25697–25706. 10.1073/pnas.1911071116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li K, Li M, Li W, Yu H, Sun X, Zhang Q, Li Y, Li X, Li Y, Abel ED, Wu Q, Chen H. 2019. Airway epithelial regeneration requires autophagy and glucose metabolism. Cell Death Dis 10:875. 10.1038/s41419-019-2111-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vaughan AE, Brumwell AN, Xi Y, Gotts JE, Brownfield DG, Treutlein B, Tan K, Tan V, Liu FC, Looney MR, Matthay MA, Rock JR, Chapman HA. 2015. Lineage-negative progenitors mobilize to regenerate lung epithelium after major injury. Nature 517:621–625. 10.1038/nature14112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kathiriya JJ, Brumwell AN, Jackson JR, Tang X, Chapman HA. 2020. Distinct airway epithelial stem cells hide among club cells but mobilize to promote alveolar regeneration. Cell Stem Cell 26:346–358 e4. 10.1016/j.stem.2019.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jain R, Barkauskas CE, Takeda N, Bowie EJ, Aghajanian H, Wang Q, Padmanabhan A, Manderfield LJ, Gupta M, Li D, Li L, Trivedi CM, Hogan BLM, Epstein JA. 2015. Plasticity of Hopx(+) type I alveolar cells to regenerate type II cells in the lung. Nat Commun 6:6727. 10.1038/ncomms7727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kumar PA, Hu Y, Yamamoto Y, Hoe NB, Wei TS, Mu D, Sun Y, Joo LS, Dagher R, Zielonka EM, Wang de Y, Lim B, Chow VT, Crum CP, Xian W, McKeon F. 2011. Distal airway stem cells yield alveoli in vitro and during lung regeneration following H1N1 influenza infection. Cell 147:525–538. 10.1016/j.cell.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xi Y, Kim T, Brumwell AN, Driver IH, Wei Y, Tan V, Jackson JR, Xu J, Lee DK, Gotts JE, Matthay MA, Shannon JM, Chapman HA, Vaughan AE. 2017. Local lung hypoxia determines epithelial fate decisions during alveolar regeneration. Nat Cell Biol 19:904–914. 10.1038/ncb3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Juul NH, Stockman CA, Desai TJ. 2020. Niche cells and signals that regulate lung alveolar stem cells in vivo. Cold Spring Harb Perspect Biol 12:a035717. 10.1101/cshperspect.a035717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang Y, Tang N. 2021. The diversity of adult lung epithelial stem cells and their niche in homeostasis and regeneration. Sci China Life Sci 64:2045–2059. 10.1007/s11427-020-1902-3. [DOI] [PubMed] [Google Scholar]
- 57.Finn J, Sottoriva K, Pajcini KV, Kitajewski JK, Chen C, Zhang W, Malik AB, Liu Y. 2019. Dlk1-mediated temporal regulation of notch signaling is required for differentiation of alveolar type ii to type i cells during repair. Cell Rep 26:2942–2954 e5. 10.1016/j.celrep.2019.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chung MI, Bujnis M, Barkauskas CE, Kobayashi Y, Hogan BLM. 2018. Niche-mediated BMP/SMAD signaling regulates lung alveolar stem cell proliferation and differentiation. Development 145. 10.1242/dev.163014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Riemondy KA, Jansing NL, Jiang P, Redente EF, Gillen AE, Fu R, Miller AJ, Spence JR, Gerber AN, Hesselberth JR, Zemans RL. 2019. Single cell RNA sequencing identifies TGFbeta as a key regenerative cue following LPS-induced lung injury. JCI Insight 4. 10.1172/jci.insight.123637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hu C, Sun J, Du J, Wen D, Lu H, Zhang H, Xue Y, Zhang A, Yang C, Zeng L, Jiang J. 2019. The Hippo-YAP pathway regulates the proliferation of alveolar epithelial progenitors after acute lung injury. Cell Biol Int 43:1174–1183. 10.1002/cbin.11098. [DOI] [PubMed] [Google Scholar]
- 61.Chen Q, Rehman J, Chan M, Fu P, Dudek SM, Natarajan V, Malik AB, Liu Y. 2020. Angiocrine sphingosine-1-phosphate activation of S1PR2-YAP signaling axis in alveolar type ii cells is essential for lung repair. Cell Rep 31:107828. 10.1016/j.celrep.2020.107828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li K, Wu Q, Sun X, Geng Y, Leng D, Li H, Zhang S, Wang Q, Wu J, Xu L, Li X, Li Y, Zhang Q, Kurkciyan A, Liang J, Jiang D, Chen H. 2017. Tsp1 promotes alveolar stem cell proliferation and its down-regulation relates to lung inflammation in intralobar pulmonary sequestration. Oncotarget 8:64867–64877. 10.18632/oncotarget.19952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Abdelwahab EMM, Rapp J, Feller D, Csongei V, Pal S, Bartis D, Thickett DR, Pongracz JE. 2019. Wnt signaling regulates trans-differentiation of stem cell like type 2 alveolar epithelial cells to type 1 epithelial cells. Respir Res 20:204. 10.1186/s12931-019-1176-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Flozak AS, Lam AP, Russell S, Jain M, Peled ON, Sheppard KA, Beri R, Mutlu GM, Budinger GR, Gottardi CJ. 2010. Beta-catenin/T-cell factor signaling is activated during lung injury and promotes the survival and migration of alveolar epithelial cells. J Biol Chem 285:3157–3167. 10.1074/jbc.M109.070326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sun T, Huang Z, Zhang H, Posner C, Jia G, Ramalingam TR, Xu M, Brightbill H, Egen JG, Dey A, Arron JR. 2019. TAZ is required for lung alveolar epithelial cell differentiation after injury. JCI Insight 4. 10.1172/jci.insight.128674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dorry SJ, Ansbro BO, Ornitz DM, Mutlu GM, Guzy RD. 2020. FGFR2 is required for AEC2 homeostasis and survival after bleomycin-induced lung injury. Am J Respir Cell Mol Biol 62:608–621. 10.1165/rcmb.2019-0079OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li X, Wu J, Sun X, Wu Q, Li Y, Li K, Zhang Q, Li Y, Abel ED, Chen H. 2020. Autophagy reprograms alveolar progenitor cell metabolism in response to lung injury. Stem Cell Rep 14:420–432. 10.1016/j.stemcr.2020.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rafii S, Cao Z, Lis R, Siempos II, Chavez D, Shido K, Rabbany SY, Ding BS. 2015. Platelet-derived SDF-1 primes the pulmonary capillary vascular niche to drive lung alveolar regeneration. Nat Cell Biol 17:123–136. 10.1038/ncb3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lechner AJ, Driver IH, Lee J, Conroy CM, Nagle A, Locksley RM, Rock JR. 2017. Recruited monocytes and type 2 immunity promote lung regeneration following pneumonectomy. Cell Stem Cell 21:120–134 e7. 10.1016/j.stem.2017.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hung LY, Sen D, Oniskey TK, Katzen J, Cohen NA, Vaughan AE, Nieves W, Urisman A, Beers MF, Krummel MF, Herbert DR. 2019. Macrophages promote epithelial proliferation following infectious and non-infectious lung injury through a Trefoil factor 2-dependent mechanism. Mucosal Immunol 12:64–76. 10.1038/s41385-018-0096-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Katsura H, Kobayashi Y, Tata PR, Hogan BLM. 2019. IL-1 and TNFalpha contribute to the inflammatory niche to enhance alveolar regeneration. Stem Cell Rep 12:657–666. 10.1016/j.stemcr.2019.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li W, Moore MJ, Vasilieva N, Sui J, Wong SK, Berne MA, Somasundaran M, Sullivan JL, Luzuriaga K, Greenough TC, Choe H, Farzan M. 2003. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 426:450–454. 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hoffmann M, Kleine-Weber H, Schroeder S, Kruger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Muller MA, Drosten C, Pohlmann S. 2020. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181:271–280. 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Iwata-Yoshikawa N, Okamura T, Shimizu Y, Hasegawa H, Takeda M, Nagata N. 2019. TMPRSS2 contributes to virus spread and immunopathology in the airways of murine models after coronavirus infection. J Virol 93:e01815-18. 10.1128/JVI.01815-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bosch BJ, Bartelink W, Rottier PJ. 2008. Cathepsin L functionally cleaves the severe acute respiratory syndrome coronavirus class I fusion protein upstream of rather than adjacent to the fusion peptide. J Virol 82:8887–8890. 10.1128/JVI.00415-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Haga K, Takai-Todaka R, Matsumura Y, Song C, Takano T, Tojo T, Nagami A, Ishida Y, Masaki H, Tsuchiya M, Ebisudani T, Sugimoto S, Sato T, Yasuda H, Fukunaga K, Sawada A, Nemoto N, Murata K, Morimoto T, Katayama K. 2021. Nasal delivery of single-domain antibody improves symptoms of SARS-CoV-2 infection in an animal model. PLoS Pathog 17:e1009542. 10.1371/journal.ppat.1009542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Imai M, Iwatsuki-Horimoto K, Hatta M, Loeber S, Halfmann PJ, Nakajima N, Watanabe T, Ujie M, Takahashi K, Ito M, Yamada S, Fan S, Chiba S, Kuroda M, Guan L, Takada K, Armbrust T, Balogh A, Furusawa Y, Okuda M, Ueki H, Yasuhara A, Sakai-Tagawa Y, Lopes TJS, Kiso M, Yamayoshi S, Kinoshita N, Ohmagari N, Hattori SI, Takeda M, Mitsuya H, Krammer F, Suzuki T, Kawaoka Y. 2020. Syrian hamsters as a small animal model for SARS-CoV-2 infection and countermeasure development. Proc Natl Acad Sci USA 117:16587–16595. 10.1073/pnas.2009799117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rogers TF, Zhao F, Huang D, Beutler N, Burns A, He WT, Limbo O, Smith C, Song G, Woehl J, Yang L, Abbott RK, Callaghan S, Garcia E, Hurtado J, Parren M, Peng L, Ramirez S, Ricketts J, Ricciardi MJ, Rawlings SA, Wu NC, Yuan M, Smith DM, Nemazee D, Teijaro JR, Voss JE, Wilson IA, Andrabi R, Briney B, Landais E, Sok D, Jardine JG, Burton DR. 2020. Isolation of potent SARS-CoV-2 neutralizing antibodies and protection from disease in a small animal model. Science 369:956–963. 10.1126/science.abc7520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shi J, Wen Z, Zhong G, Yang H, Wang C, Huang B, Liu R, He X, Shuai L, Sun Z, Zhao Y, Liu P, Liang L, Cui P, Wang J, Zhang X, Guan Y, Tan W, Wu G, Chen H, Bu Z. 2020. Susceptibility of ferrets, cats, dogs, and other domesticated animals to SARS-coronavirus 2. Science 368:1016–1020. 10.1126/science.abb7015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Blair RV, Vaccari M, Doyle-Meyers LA, Roy CJ, Russell-Lodrigue K, Fahlberg M, Monjure CJ, Beddingfield B, Plante KS, Plante JA, Weaver SC, Qin X, Midkiff CC, Lehmicke G, Golden N, Threeton B, Penney T, Allers C, Barnes MB, Pattison M, Datta PK, Maness NJ, Birnbaum A, Fischer T, Bohm RP, Rappaport J. 2021. Acute respiratory distress in aged, SARS-CoV-2-infected African green monkeys but not rhesus macaques. Am J Pathol 191:274–282. 10.1016/j.ajpath.2020.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lakdawala SS, Menachery VD. 2020. The search for a COVID-19 animal model. Science 368:942–943. 10.1126/science.abc6141. [DOI] [PubMed] [Google Scholar]
- 82.Muñoz-Fontela C, Dowling WE, Funnell SGP, Gsell P-S, Riveros-Balta AX, Albrecht RA, Andersen H, Baric RS, Carroll MW, Cavaleri M, Qin C, Crozier I, Dallmeier K, de Waal L, de Wit E, Delang L, Dohm E, Duprex WP, Falzarano D, Finch CL, Frieman MB, Graham BS, Gralinski LE, Guilfoyle K, Haagmans BL, Hamilton GA, Hartman AL, Herfst S, Kaptein SJF, Klimstra WB, Knezevic I, Krause PR, Kuhn JH, Le Grand R, Lewis MG, Liu W-C, Maisonnasse P, McElroy AK, Munster V, Oreshkova N, Rasmussen AL, Rocha-Pereira J, Rockx B, Rodríguez E, Rogers TF, Salguero FJ, Schotsaert M, Stittelaar KJ, Thibaut HJ, Tseng C-T, et al. 2020. Animal models for COVID-19. Nature 586:509–515. 10.1038/s41586-020-2787-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zeiss CJ, Compton S, Veenhuis RT. 2021. Animal models of COVID-19. I. Comparative virology and disease pathogenesis. ILAR J. 10.1093/ilar/ilab007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Coleman C, Doyle-Meyers LA, Russell-Lodrigue KE, Golden N, Threeton B, Song K, Pierre G, Baribault C, Bohm RP, Maness NJ, Kolls JK, Rappaport J, Mudd JC. 2021. Similarities and differences in the acute-phase response to SARS-CoV-2 in rhesus macaques and African green monkeys. Front Immunol 12:754642. 10.3389/fimmu.2021.754642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shang J, Wan Y, Luo C, Ye G, Geng Q, Auerbach A, Li F. 2020. Cell entry mechanisms of SARS-CoV-2. Proc Natl Acad Sci USA 117:11727–11734. 10.1073/pnas.2003138117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cantuti-Castelvetri L, Ojha R, Pedro LD, Djannatian M, Franz J, Kuivanen S, van der Meer F, Kallio K, Kaya T, Anastasina M, Smura T, Levanov L, Szirovicza L, Tobi A, Kallio-Kokko H, Osterlund P, Joensuu M, Meunier FA, Butcher SJ, Winkler MS, Mollenhauer B, Helenius A, Gokce O, Teesalu T, Hepojoki J, Vapalahti O, Stadelmann C, Balistreri G, Simons M. 2020. Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. Science 370:856–860. 10.1126/science.abd2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Clementi N, Ghosh S, De Santis M, Castelli M, Criscuolo E, Zanoni I, Clementi M, Mancini N. 2021. Viral respiratory pathogens and lung injury. Clin Microbiol Rev 34:e00103-20. 10.1128/CMR.00103-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Radzikowska U, Ding M, Tan G, Zhakparov D, Peng Y, Wawrzyniak P, Wang M, Li S, Morita H, Altunbulakli C, Reiger M, Neumann AU, Lunjani N, Traidl-Hoffmann C, Nadeau KC, O'Mahony L, Akdis C, Sokolowska M. 2020. Distribution of ACE2, CD147, CD26, and other SARS-CoV-2 associated molecules in tissues and immune cells in health and in asthma, COPD, obesity, hypertension, and COVID-19 risk factors. Allergy 75:2829–2845. 10.1111/all.14429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ricci D, Etna MP, Rizzo F, Sandini S, Severa M, Coccia EM. 2021. Innate immune response to SARS-CoV-2 infection: from cells to soluble mediators. Int J Mol Sci 22:7017. 10.3390/ijms22137017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Anaeigoudari A, Mollaei HR, Arababadi MK, Nosratabadi R. 2021. Severe acute respiratory syndrome coronavirus 2: The role of the main components of the innate immune system. Inflammation 44:2151–2169. 10.1007/s10753-021-01519-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cheng P, Li S, Chen H. 2021. Macrophages in lung injury, repair, and fibrosis. Cells 10:436. 10.3390/cells10020436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lee JW, Chun W, Lee HJ, Min JH, Kim SM, Seo JY, Ahn KS, Oh SR. 2021. The role of macrophages in the development of acute and chronic inflammatory lung diseases. Cells 10:897. 10.3390/cells10040897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Robb CT, Regan KH, Dorward DA, Rossi AG. 2016. Key mechanisms governing resolution of lung inflammation. Semin Immunopathol 38:425–448. 10.1007/s00281-016-0560-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Weller PF, Goetzl EJ. 1980. The human eosinophil: roles in host defense and tissue injury. Am J Pathol 100:791–820. [PMC free article] [PubMed] [Google Scholar]
- 95.Cicco S, Cicco G, Racanelli V, Vacca A. 2020. Neutrophil extracellular traps (NETs) and damage-associated molecular patterns (DAMPs): two potential targets for COVID-19 treatment. Mediators Inflamm 2020:7527953. 10.1155/2020/7527953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kumar A, Cao W, Endrias K, Kuchipudi SV, Mittal SK, Sambhara S. 2021. Innate lymphoid cells (ILC) in SARS-CoV-2 infection. Mol Aspects Med 80:101008. 10.1016/j.mam.2021.101008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Murdaca G, Di Gioacchino M, Greco M, Borro M, Paladin F, Petrarca C, Gangemi S. 2021. Basophils and mast cells in COVID-19 pathogenesis. Cells 10:2754. 10.3390/cells10102754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Neeland MR, Bannister S, Clifford V, Dohle K, Mulholland K, Sutton P, Curtis N, Steer AC, Burgner DP, Crawford NW, Tosif S, Saffery R. 2021. Innate cell profiles during the acute and convalescent phase of SARS-CoV-2 infection in children. Nat Commun 12:1084. 10.1038/s41467-021-21414-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Alipoor SD, Mortaz E, Jamaati H, Tabarsi P, Bayram H, Varahram M, Adcock IM. 2021. COVID-19: Molecular and Cellular Response. Front Cell Infect Microbiol 11:563085. 10.3389/fcimb.2021.563085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Liao M, Liu Y, Yuan J, Wen Y, Xu G, Zhao J, Cheng L, Li J, Wang X, Wang F, Liu L, Amit I, Zhang S, Zhang Z. 2020. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat Med 26:842–844. 10.1038/s41591-020-0901-9. [DOI] [PubMed] [Google Scholar]
- 101.Kerget B, Kerget F, Aksakal A, Aşkın S, Sağlam L, Akgün M. 2021. Evaluation of alpha defensin, IL-1 receptor antagonist, and IL-18 levels in COVID-19 patients with macrophage activation syndrome and acute respiratory distress syndrome. J Med Virol 93:2090–2098. 10.1002/jmv.26589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Toor D, Jain A, Kalhan S, Manocha H, Sharma VK, Jain P, Tripathi V, Prakash H. 2020. Tempering macrophage plasticity for controlling SARS-CoV-2 infection for managing COVID-19 disease. Front Pharmacol 11:570698. 10.3389/fphar.2020.570698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Vanderbeke L, Van Mol P, Van Herck Y, De Smet F, Humblet-Baron S, Martinod K, Antoranz A, Arijs I, Boeckx B, Bosisio FM, Casaer M, Dauwe D, De Wever W, Dooms C, Dreesen E, Emmaneel A, Filtjens J, Gouwy M, Gunst J, Hermans G, Jansen S, Lagrou K, Liston A, Lorent N, Meersseman P, Mercier T, Neyts J, Odent J, Panovska D, Penttila PA, Pollet E, Proost P, Qian J, Quintelier K, Raes J, Rex S, Saeys Y, Sprooten J, Tejpar S, Testelmans D, Thevissen K, Van Buyten T, Vandenhaute J, Van Gassen S, Velásquez Pereira LC, Vos R, Weynand B, Wilmer A, Yserbyt J, Garg AD, et al. 2021. Monocyte-driven atypical cytokine storm and aberrant neutrophil activation as key mediators of COVID-19 disease severity. Nat Commun 12:4117. 10.1038/s41467-021-24360-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Iwamura APD, Tavares da Silva MR, Hummelgen AL, Soeiro Pereira PV, Falcai A, Grumach AS, Goudouris E, Neto AC, Prando C. 2021. Immunity and inflammatory biomarkers in COVID-19: A systematic review. Rev Med Virol 31:e2199. 10.1002/rmv.2199. [DOI] [PubMed] [Google Scholar]
- 105.Zheng J, Wang Y, Li K, Meyerholz DK, Allamargot C, Perlman S. 2021. SARS-CoV-2-induced immune activation and death of monocyte-derived human macrophages and dendritic cells. J Infect Dis 223:785–795. 10.1093/infdis/jiaa753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Galani IE, Rovina N, Lampropoulou V, Triantafyllia V, Manioudaki M, Pavlos E, Koukaki E, Fragkou PC, Panou V, Rapti V, Koltsida O, Mentis A, Koulouris N, Tsiodras S, Koutsoukou A, Andreakos E. 2021. Untuned antiviral immunity in COVID-19 revealed by temporal type I/III interferon patterns and flu comparison. Nat Immunol 22:32–40. 10.1038/s41590-020-00840-x. [DOI] [PubMed] [Google Scholar]
- 107.Hadjadj J, Yatim N, Barnabei L, Corneau A, Boussier J, Smith N, Pere H, Charbit B, Bondet V, Chenevier-Gobeaux C, Breillat P, Carlier N, Gauzit R, Morbieu C, Pene F, Marin N, Roche N, Szwebel TA, Merkling SH, Treluyer JM, Veyer D, Mouthon L, Blanc C, Tharaux PL, Rozenberg F, Fischer A, Duffy D, Rieux-Laucat F, Kerneis S, Terrier B. 2020. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science 369:718–724. 10.1126/science.abc6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lin J-W, Tang C, Wei H-C, Du B, Chen C, Wang M, Zhou Y, Yu M-X, Cheng L, Kuivanen S, Ogando NS, Levanov L, Zhao Y, Li C-L, Zhou R, Li Z, Zhang Y, Sun K, Wang C, Chen L, Xiao X, Zheng X, Chen S-S, Zhou Z, Yang R, Zhang D, Xu M, Song J, Wang D, Li Y, Lei SKun, Zeng W, Yang Q, He P, Zhang Y, Zhou L, Cao L, Luo F, Liu H, Wang L, Ye F, Zhang M, Li M, Fan W, Li X, Li K, Ke B, Xu J, Yang H, He S, et al. 2021. Genomic monitoring of SARS-CoV-2 uncovers an Nsp1 deletion variant that modulates type I interferon response. Cell Host Microbe 29:489–502. 10.1016/j.chom.2021.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kawasuji H, Morinaga Y, Tani H, Kimura M, Yamada H, Yoshida Y, Takegoshi Y, Kaneda M, Murai Y, Kimoto K, Ueno A, Miyajima Y, Kawago K, Fukui Y, Sakamaki I, Yamamoto Y. 2021. Delayed neutralizing antibody response in the acute phase correlates with severe progression of COVID-19. Sci Rep 11:16535. 10.1038/s41598-021-96143-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Koenis DS, Beegun I, Jouvene C, Aguirre GA, Souza PR, Gonzalez-Nunez M, Ly L, Pistorius K, Kocher HM, Ricketts W, Thomas G, Perretti M, Alusi G, Pfeffer P, Dalli J. 2021. Disrupted resolution mechanisms favor altered phagocyte responses in COVID-19. Circ Res 129 10.1161/CIRCRESAHA.121.319142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Valverde-Monge M, Canas JA, Barroso B, Betancor D, Ortega-Martin L, Gomez-Lopez A, Rodriguez-Nieto MJ, Mahillo-Fernandez I, Sastre J, Del Pozo V. 2021. Eosinophils and chronic respiratory diseases in hospitalized COVID-19 patients. Front Immunol 12:668074. 10.3389/fimmu.2021.668074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Smith N, Goncalves P, Charbit B, Grzelak L, Beretta M, Planchais C, Bruel T, Rouilly V, Bondet V, Hadjadj J, Yatim N, Pere H, Merkling SH, Ghozlane A, Kerneis S, Rieux-Laucat F, Terrier B, Schwartz O, Mouquet H, Duffy D, Di Santo JP. 2021. Distinct systemic and mucosal immune responses during acute SARS-CoV-2 infection. Nat Immunol 22:1428–1439. 10.1038/s41590-021-01028-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rhoades NS, Pinski AN, Monsibais AN, Jankeel A, Doratt BM, Cinco IR, Ibraim I, Messaoudi I. 2021. Acute SARS-CoV-2 infection is associated with an increased abundance of bacterial pathogens, including Pseudomonas aeruginosa in the nose. Cell Rep 36:109637. 10.1016/j.celrep.2021.109637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Peluso MJ, Deitchman AN, Torres L, Iyer NS, Munter SE, Nixon CC, Donatelli J, Thanh C, Takahashi S, Hakim J, Turcios K, Janson O, Hoh R, Tai V, Hernandez Y, Fehrman EA, Spinelli MA, Gandhi M, Trinh L, Wrin T, Petropoulos CJ, Aweeka FT, Rodriguez-Barraquer I, Kelly JD, Martin JN, Deeks SG, Greenhouse B, Rutishauser RL, Henrich TJ. 2021. Long-term SARS-CoV-2-specific immune and inflammatory responses in individuals recovering from COVID-19 with and without post-acute symptoms. Cell Rep 36:109518. 10.1016/j.celrep.2021.109518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ramakrishnan RK, Kashour T, Hamid Q, Halwani R, Tleyjeh IM. 2021. Unraveling the mystery surrounding post-acute sequelae of COVID-19. Front Immunol 12:686029. 10.3389/fimmu.2021.686029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Thorne LG, Reuschl AK, Zuliani-Alvarez L, Whelan MVX, Turner J, Noursadeghi M, Jolly C, Towers GJ. 2021. SARS-CoV-2 sensing by RIG-I and MDA5 links epithelial infection to macrophage inflammation. EMBO J 40:e107826. 10.15252/embj.2021107826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chua RL, Lukassen S, Trump S, Hennig BP, Wendisch D, Pott F, Debnath O, Thurmann L, Kurth F, Volker MT, Kazmierski J, Timmermann B, Twardziok S, Schneider S, Machleidt F, Muller-Redetzky H, Maier M, Krannich A, Schmidt S, Balzer F, Liebig J, Loske J, Suttorp N, Eils J, Ishaque N, Liebert UG, von Kalle C, Hocke A, Witzenrath M, Goffinet C, Drosten C, Laudi S, Lehmann I, Conrad C, Sander LE, Eils R. 2020. COVID-19 severity correlates with airway epithelium-immune cell interactions identified by single-cell analysis. Nat Biotechnol 38:970–979. 10.1038/s41587-020-0602-4. [DOI] [PubMed] [Google Scholar]
- 118.Zhang H, Rostami MR, Leopold PL, Mezey JG, O'Beirne SL, Strulovici-Barel Y, Crystal RG. 2020. Expression of the SARS-CoV-2 ACE2 receptor in the human airway epithelium. Am J Respir Crit Care Med 202:219–229. 10.1164/rccm.202003-0541OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Duclos GE, Teixeira VH, Autissier P, Gesthalter YB, Reinders-Luinge MA, Terrano R, Dumas YM, Liu G, Mazzilli SA, Brandsma CA, van den Berge M, Janes SM, Timens W, Lenburg ME, Spira A, Campbell JD, Beane J. 2019. Characterizing smoking-induced transcriptional heterogeneity in the human bronchial epithelium at single-cell resolution. Sci Adv 5:eaaw3413. 10.1126/sciadv.aaw3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hou YJ, Okuda K, Edwards CE, Martinez DR, Asakura T, Dinnon KH, 3rd, Kato T, Lee RE, Yount BL, Mascenik TM, Chen G, Olivier KN, Ghio A, Tse LV, Leist SR, Gralinski LE, Schafer A, Dang H, Gilmore R, Nakano S, Sun L, Fulcher ML, Livraghi-Butrico A, Nicely NI, Cameron M, Cameron C, Kelvin DJ, de Silva A, Margolis DM, Markmann A, Bartelt L, Zumwalt R, Martinez FJ, Salvatore SP, Borczuk A, Tata PR, Sontake V, Kimple A, Jaspers I, O'Neal WK, Randell SH, Boucher RC, Baric RS. 2020. SARS-CoV-2 reverse genetics reveals a variable infection gradient in the respiratory tract. Cell 182:429–446 e14. 10.1016/j.cell.2020.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zou X, Chen K, Zou J, Han P, Hao J, Han Z. 2020. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med 14:185–192. 10.1007/s11684-020-0754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ziegler CGK, Allon SJ, Nyquist SK, Mbano IM, Miao VN, Tzouanas CN, Cao Y, Yousif AS, Bals J, Hauser BM, Feldman J, Muus C, Wadsworth MH, 2nd, Kazer SW, Hughes TK, Doran B, Gatter GJ, Vukovic M, Taliaferro F, Mead BE, Guo Z, Wang JP, Gras D, Plaisant M, Ansari M, Angelidis I, Adler H, Sucre JMS, Taylor CJ, Lin B, Waghray A, Mitsialis V, Dwyer DF, Buchheit KM, Boyce JA, Barrett NA, Laidlaw TM, Carroll SL, Colonna L, Tkachev V, Peterson CW, Yu A, Zheng HB, Gideon HP, Winchell CG, Lin PL, Bingle CD, Snapper SB, Kropski JA, Theis FJ, HCA Lung Biological Network, et al. 2020. SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell 181:1016–1035 e19. 10.1016/j.cell.2020.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lukassen S, Chua RL, Trefzer T, Kahn NC, Schneider MA, Muley T, Winter H, Meister M, Veith C, Boots AW, Hennig BP, Kreuter M, Conrad C, Eils R. 2020. SARS-CoV-2 receptor ACE2 and TMPRSS2 are primarily expressed in bronchial transient secretory cells. EMBO J 39:e105114. 10.15252/embj.20105114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Fang Y, Liu H, Huang H, Li H, Saqi A, Qiang L, Que J. 2020. Distinct stem/progenitor cells proliferate to regenerate the trachea, intrapulmonary airways and alveoli in COVID-19 patients. Cell Res 30:705–707. 10.1038/s41422-020-0367-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chen J, Wu H, Yu Y, Tang N. 2020. Pulmonary alveolar regeneration in adult COVID-19 patients. Cell Res 30:708–710. 10.1038/s41422-020-0369-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Shao H, Qin Z, Geng B, Wu J, Zhang L, Zhang Q, Wu Q, Li L, Chen H. 2020. Impaired lung regeneration after SARS-CoV-2 infection. Cell Prolif 53:e12927. 10.1111/cpr.12927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L, Tai Y, Bai C, Gao T, Song J, Xia P, Dong J, Zhao J, Wang FS. 2020. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med 8:420–422. 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tian S, Hu W, Niu L, Liu H, Xu H, Xiao SY. 2020. Pulmonary pathology of early-phase 2019 novel coronavirus (COVID-19) pneumonia in two patients with lung cancer. J Thoracic Oncology 15:700–704. 10.1016/j.jtho.2020.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Xu J, Xu X, Jiang L, Dua K, Hansbro PM, Liu G. 2020. SARS-CoV-2 induces transcriptional signatures in human lung epithelial cells that promote lung fibrosis. Respir Res 21:182. 10.1186/s12931-020-01445-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Huang C, Huang L, Wang Y, Li X, Ren L, Gu X, Kang L, Guo L, Liu M, Zhou X, Luo J, Huang Z, Tu S, Zhao Y, Chen L, Xu D, Li Y, Li C, Peng L, Li Y, Xie W, Cui D, Shang L, Fan G, Xu J, Wang G, Wang Y, Zhong J, Wang C, Wang J, Zhang D, Cao B. 2021. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet 397:220–232. 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Fiolet T, Kherabi Y, MacDonald CJ, Ghosn J, Peiffer-Smadja N. 2021. Comparing COVID-19 vaccines for their characteristics, efficacy and effectiveness against SARS-CoV-2 and variants of concern: A narrative review. Clin Microbio Infection Epub ahead of print. 10.1016/j.cmi.2021.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ramesh S, Govindarajulu M, Parise RS, Neel L, Shankar T, Patel S, Lowery P, Smith F, Dhanasekaran M, Moore T. 2021. Emerging SARS-CoV-2 variants: a review of its mutations, its implications and vaccine efficacy. Vaccines 9:1195. 10.3390/vaccines9101195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Simsek Yavuz S, Unal S. 2020. Antiviral treatment of COVID-19. Turkish J Medical Sciences 50:611–619. 10.3906/sag-2004-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, Shi Z, Hu Z, Zhong W, Xiao G. 2020. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res 30:269–271. 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Williamson BN, Feldmann F, Schwarz B, Meade-White K, Porter DP, Schulz J, van Doremalen N, Leighton I, Yinda CK, Perez-Perez L, Okumura A, Lovaglio J, Hanley PW, Saturday G, Bosio CM, Anzick S, Barbian K, Cihlar T, Martens C, Scott DP, Munster VJ, de Wit E. 2020. Clinical benefit of remdesivir in rhesus macaques infected with SARS-CoV-2. Nature 585:273–276. 10.1038/s41586-020-2423-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Pimentel J, Laurie C, Cockcroft A, Andersson N. 2021. Clinical studies assessing the efficacy, effectiveness and safety of remdesivir in management of COVID-19: a scoping review. Br J Clin Pharmacol 87:2663–2684. 10.1111/bcp.14677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mazhar F, Hadi MA, Kow CS, Marran AMN, Merchant HA, Hasan SS. 2020. Use of hydroxychloroquine and chloroquine in COVID-19: how good is the quality of randomized controlled trials? Int J Infect Dis 101:107–120. 10.1016/j.ijid.2020.09.1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Chopra D, Boparai JK, Bhandari B, Srivastava A, Gupta R. 2020. Pharmacological strategies for COVID-19 - A review of the most promising repurposed antiviral drugs. Infectious Disorders Drug Targets. 10.2174/1871526520666201218151841. [DOI] [PubMed] [Google Scholar]
- 139.Zhang LX, Miao SY, Qin ZH, Wu JP, Chen HY, Sun HB, Xie Y, Du YQ, Shen J. 2020. Preliminary analysis of B- and T-cell responses to SARS-CoV-2. Mol Diagn Ther 24:601–609. 10.1007/s40291-020-00486-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Cheng Y, Wong R, Soo YO, Wong WS, Lee CK, Ng MH, Chan P, Wong KC, Leung CB, Cheng G. 2005. Use of convalescent plasma therapy in SARS patients in Hong Kong. Eur J Clin Microbiol Infect Dis 24:44–46. 10.1007/s10096-004-1271-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.van Griensven J, De Weiggheleire A, Delamou A, Smith PG, Edwards T, Vandekerckhove P, Bah EI, Colebunders R, Herve I, Lazaygues C, Haba N, Lynen L. 2016. The use of Ebola convalescent plasma to treat Ebola virus disease in resource-constrained settings: a perspective from the field. Clin Infect Dis 62:69–74. 10.1093/cid/civ680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Rajendran K, Krishnasamy N, Rangarajan J, Rathinam J, Natarajan M, Ramachandran A. 2020. Convalescent plasma transfusion for the treatment of COVID-19: systematic review. J Med Virol 92:1475–1483. 10.1002/jmv.25961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Libster R, Perez Marc G, Wappner D, Coviello S, Bianchi A, Braem V, Esteban I, Caballero MT, Wood C, Berrueta M, Rondan A, Lescano G, Cruz P, Ritou Y, Fernandez Vina V, Alvarez Paggi D, Esperante S, Ferreti A, Ofman G, Ciganda A, Rodriguez R, Lantos J, Valentini R, Itcovici N, Hintze A, Oyarvide ML, Etchegaray C, Neira A, Name I, Alfonso J, Lopez Castelo R, Caruso G, Rapelius S, Alvez F, Etchenique F, Dimase F, Alvarez D, Aranda SS, Sanchez Yanotti C, De Luca J, Jares Baglivo S, Laudanno S, Nowogrodzki F, Larrea R, Silveyra M, Leberzstein G, Debonis A, Molinos J, Gonzalez M, Perez E, et al. 2021. Early high-titer plasma therapy to prevent severe COVID-19 in older adults. N Engl J Med 384:610–618. 10.1056/NEJMoa2033700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wu Y, Wang F, Shen C, Peng W, Li D, Zhao C, Li Z, Li S, Bi Y, Yang Y, Gong Y, Xiao H, Fan Z, Tan S, Wu G, Tan W, Lu X, Fan C, Wang Q, Liu Y, Zhang C, Qi J, Gao GF, Gao F, Liu L. 2020. A noncompeting pair of human neutralizing antibodies block COVID-19 virus binding to its receptor ACE2. Science 368:1274–1278. 10.1126/science.abc2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Cao Y, Su B, Guo X, Sun W, Deng Y, Bao L, Zhu Q, Zhang X, Zheng Y, Geng C, Chai X, He R, Li X, Lv Q, Zhu H, Deng W, Xu Y, Wang Y, Qiao L, Tan Y, Song L, Wang G, Du X, Gao N, Liu J, Xiao J, Su X-D, Du Z, Feng Y, Qin C, Qin C, Jin R, Xie XS. 2020. Potent neutralizing antibodies against SARS-CoV-2 identified by high-throughput single-cell sequencing of convalescent patients’ B cells. Cell 182:73–84 e16. 10.1016/j.cell.2020.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Witkowski M, Tizian C, Ferreira-Gomes M, Niemeyer D, Jones TC, Heinrich F, Frischbutter S, Angermair S, Hohnstein T, Mattiola I, Nawrath P, Mc Ewen S, Zocche S, Viviano E, Heinz GA, Maurer M, Kolsch U, Chua RL, Aschman T, Meisel C, Radke J, Sawitzki B, Roehmel J, Allers K, Moos V, Schneider T, Hanitsch L, Mall MA, Conrad C, Radbruch H, Duerr CU, Trapani JA, Marcenaro E, Kallinich T, Corman VM, Kurth F, Sander LE, Drosten C, Treskatsch S, Durek P, Kruglov A, Radbruch A, Mashreghi MF, Diefenbach A. 2021. Untimely TGFbeta responses in COVID-19 limit antiviral functions of NK cells. Nature 600:295–301. 10.1038/s41586-021-04142-6. [DOI] [PubMed] [Google Scholar]
- 147.Ghasemzadeh M, Ghasemzadeh A, Hosseini E. 2021. Exhausted NK cells and cytokine storms in COVID-19: whether NK cell therapy could be a therapeutic choice. Human Immunology 8:86–98. 10.1016/j.humimm.2021.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Mansourabadi AH, Sadeghalvad M, Mohammadi-Motlagh HR, Rezaei N. 2020. The immune system as a target for therapy of SARS-CoV-2: A systematic review of the current immunotherapies for COVID-19. Life Sci 258:118185. 10.1016/j.lfs.2020.118185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Yang L, Liu S, Liu J, Zhang Z, Wan X, Huang B, Chen Y, Zhang Y. 2020. COVID-19: immunopathogenesis and Immunotherapeutics. Signal Transduct Target Ther 5:128. 10.1038/s41392-020-00243-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Catanzaro M, Fagiani F, Racchi M, Corsini E, Govoni S, Lanni C. 2020. Immune response in COVID-19: addressing a pharmacological challenge by targeting pathways triggered by SARS-CoV-2. Signal Transduct Target Ther 5:84. 10.1038/s41392-020-0191-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Schijns V, Lavelle EC. 2020. Prevention and treatment of COVID-19 disease by controlled modulation of innate immunity. Eur J Immunol 50:932–938. 10.1002/eji.202048693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wang X, He Z, Zhao X. 2021. Immunoregulatory therapy strategies that target cytokine storms in patients with COVID-19 (Review). Exp Ther Med 21:319. 10.3892/etm.2021.9750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Iwabuchi K, Yoshie K, Kurakami Y, Takahashi K, Kato Y, Morishima T. 2020. Therapeutic potential of ciclesonide inahalation for COVID-19 pneumonia: report of three cases. J Infect Chemother 26:625–632. 10.1016/j.jiac.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kimura H, Kurusu H, Sada M, Kurai D, Murakami K, Kamitani W, Tomita H, Katayama K, Ryo A. 2020. Molecular pharmacology of ciclesonide against SARS-CoV-2. J Allergy Clin Immunol 146:330–331. 10.1016/j.jaci.2020.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Yamasaki Y, Ooka S, Tsuchida T, Nakamura Y, Hagiwara Y, Naitou Y, Ishibashi Y, Ikeda H, Sakurada T, Handa H, Nishine H, Takita M, Morikawa D, Yoshida H, Fujii S, Morisawa K, Takemura H, Fujitani S, Kunishima H. 2020. The peripheral lymphocyte count as a predictor of severe COVID-19 and the effect of treatment with ciclesonide. Virus Res 290:198089. 10.1016/j.virusres.2020.198089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Miyazawa D, Kaneko G. 2021. Clinical trials of inhaled beclomethasone and mometasone for COVID-19 should be conducted. J Med Virol 93:637–638. 10.1002/jmv.26413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Mattos-Silva P, Felix NS, Silva PL, Robba C, Battaglini D, Pelosi P, Rocco PRM, Cruz FF. 2020. Pros and cons of corticosteroid therapy for COVID-19 patients. Respir Physiol Neurobiol 280:103492. 10.1016/j.resp.2020.103492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Hoang TN, Pino M, Boddapati AK, Viox EG, Starke CE, Upadhyay AA, Gumber S, Nekorchuk M, Busman-Sahay K, Strongin Z, Harper JL, Tharp GK, Pellegrini KL, Kirejczyk S, Zandi K, Tao S, Horton TR, Beagle EN, Mahar EA, Lee MYH, Cohen J, Jean SM, Wood JS, Connor-Stroud F, Stammen RL, Delmas OM, Wang S, Cooney KA, Sayegh MN, Wang L, Filev PD, Weiskopf D, Silvestri G, Waggoner J, Piantadosi A, Kasturi SP, Al-Shakhshir H, Ribeiro SP, Sekaly RP, Levit RD, Estes JD, Vanderford TH, Schinazi RF, Bosinger SE, Paiardini M. 2021. Baricitinib treatment resolves lower-airway macrophage inflammation and neutrophil recruitment in SARS-CoV-2-infected rhesus macaques. Cell 184:460–475 e21. 10.1016/j.cell.2020.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Bronte V, Ugel S, Tinazzi E, Vella A, De Sanctis F, Cane S, Batani V, Trovato R, Fiore A, Petrova V, Hofer F, Barouni RM, Musiu C, Caligola S, Pinton L, Torroni L, Polati E, Donadello K, Friso S, Pizzolo F, Iezzi M, Facciotti F, Pelicci PG, Righetti D, Bazzoni P, Rampudda M, Comel A, Mosaner W, Lunardi C, Olivieri O. 2020. Baricitinib restrains the immune dysregulation in patients with severe COVID-19. J Clin Invest 130:6409–6416. 10.1172/JCI141772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Guimaraes PO, Quirk D, Furtado RH, Maia LN, Saraiva JF, Antunes MO, Kalil Filho R, Junior VM, Soeiro AM, Tognon AP, Veiga VC, Martins PA, Moia DDF, Sampaio BS, Assis SRL, Soares RVP, Piano LPA, Castilho K, Momesso R, Monfardini F, Guimaraes HP, Ponce de Leon D, Dulcine M, Pinheiro MRT, Gunay LM, Deuring JJ, Rizzo LV, Koncz T, Berwanger O, STOP-COVID Trial Investigators . 2021. Tofacitinib in patients hospitalized with COVID-19 pneumonia. N Engl J Med 385:406–415. 10.1056/NEJMoa2101643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hou WK, Tong H, Liang L, Li TW, Liu H, Ben-Ezra M, Goodwin R, Lee TM. 2021. Probable anxiety and components of psychological resilience amid COVID-19: a population-based study. J Affect Disord 282:594–601. 10.1016/j.jad.2020.12.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lin D, Liu L, Zhang M, Hu Y, Yang Q, Guo J, Guo Y, Dai Y, Xu Y, Cai Y, Chen X, Zhang Z, Huang K. 2020. Co-infections of SARS-CoV-2 with multiple common respiratory pathogens in infected patients. Sci China Life Sci 63:606–609. 10.1007/s11427-020-1668-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Douglas IS, Rosenthal CA, Swanson DD, Hiller T, Oakes J, Bach J, Whelchel C, Pickering J, George T, Kearns M, Hanley M, Mould K, Roark S, Mansoori J, Mehta A, Schmidt EP, Neumeier A. 2021. Safety and outcomes of prolonged usual care prone position mechanical ventilation to treat acute Coronavirus disease 2019 hypoxemic respiratory failure. Crit Care Med 49:490–502. 10.1097/CCM.0000000000004818. [DOI] [PubMed] [Google Scholar]
- 164.Wu J, Tan CS, Yu H, Wang Y, Tian Y, Shao W, Zhang Y, Zhang K, Shao H, Ni G, Shen J, Galoforo AC, Wu Q, Ming D. 2020. Recovery of four COVID-19 patients via ozonated autohemotherapy. Innovation (N Y) 1:100060. 10.1016/j.xinn.2020.100060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Spadaro S, Fogagnolo A, Campo G, Zucchetti O, Verri M, Ottaviani I, Tunstall T, Grasso S, Scaramuzzo V, Murgolo F, Marangoni E, Vieceli Dalla Sega F, Fortini F, Pavasini R, Rizzo P, Ferrari R, Papi A, Volta CA, Contoli M. 2021. Markers of endothelial and epithelial pulmonary injury in mechanically ventilated COVID-19 ICU patients. Crit Care 25:74. 10.1186/s13054-021-03499-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Al-Ruweidi M, Ali FH, Shurbaji S, Popelka A, Yalcin HC. 2021. Dexamethasone and transdehydroandrosterone significantly reduce pulmonary epithelial cell injuries associated with mechanical ventilation. J Appl Physiol (1985) 130:1143–1151. 10.1152/japplphysiol.00574.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Wu Q, Zhou L, Sun X, Yan Z, Hu C, Wu J, Xu L, Li X, Liu H, Yin P, Li K, Zhao J, Li Y, Wang X, Li Y, Zhang Q, Xu G, Chen H. 2017. Altered lipid metabolism in recovered SARS patients twelve years after infection. Sci Rep 7:9110. 10.1038/s41598-017-09536-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Wang J, Li X, Wang A, Zhao F, Wu Q, Li L, Yu H, Wu J, Chen H. 2020. Organoid technology demonstrates effects of potential drugs for COVID-19 on the lung regeneration. Cell Prolif 53:e12928. 10.1111/cpr.12928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Chen Q, Shou P, Zheng C, Jiang M, Cao G, Yang Q, Cao J, Xie N, Velletri T, Zhang X, Xu C, Zhang L, Yang H, Hou J, Wang Y, Shi Y. 2016. Fate decision of mesenchymal stem cells: adipocytes or osteoblasts? Cell Death Differ 23:1128–1139. 10.1038/cdd.2015.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Lukomska B, Stanaszek L, Zuba-Surma E, Legosz P, Sarzynska S, Drela K. 2019. Challenges and controversies in human mesenchymal stem cell therapy. Stem Cells Int 2019:9628536. 10.1155/2019/9628536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Krasnodembskaya A, Song Y, Fang X, Gupta N, Serikov V, Lee JW, Matthay MA. 2010. Antibacterial effect of human mesenchymal stem cells is mediated in part from secretion of the antimicrobial peptide LL-37. Stem Cells 28:2229–2238. 10.1002/stem.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Hu S, Park J, Liu A, Lee J, Zhang X, Hao Q, Lee JW. 2018. Mesenchymal stem cell microvesicles restore protein permeability across primary cultures of injured human lung microvascular endothelial cells. Stem Cells Transl Med 7:615–624. 10.1002/sctm.17-0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Keshtkar S, Azarpira N, Ghahremani MH. 2018. Mesenchymal stem cell-derived extracellular vesicles: novel frontiers in regenerative medicine. Stem Cell Res Ther 9:63. 10.1186/s13287-018-0791-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Harrell CR, Jovicic N, Djonov V, Arsenijevic N, Volarevic V. 2019. Mesenchymal stem cell-derived exosomes and other extracellular vesicles as new remedies in the therapy of inflammatory diseases. Cells 8:1605. 10.3390/cells8121605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Fenrich M, Mrdenovic S, Balog M, Tomic S, Zjalic M, Roncevic A, Mandic D, Debeljak Z, Heffer M. 2020. SARS-CoV-2 dissemination through peripheral nerves explains multiple organ injury. Front Cell Neurosci 14:229. 10.3389/fncel.2020.00229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Atluri S, Manchikanti L, Hirsch JA. 2020. Expanded umbilical cord mesenchymal stem cells (UC-MSCs) as a therapeutic strategy in managing critically ill COVID-19 patients: the case for compassionate use. Pain Physician 23:E71–E83. [PubMed] [Google Scholar]
- 177.Qin H, Zhao A. 2020. Mesenchymal stem cell therapy for acute respiratory distress syndrome: from basic to clinics. Protein Cell 11:707–722. 10.1007/s13238-020-00738-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Huang Z, Liu H, Zhang X, Wen G, Zhu C, Zhao Y, Niu W, Qin Y, Chen H, Bai C, Liu G. 2018. Transcriptomic analysis of lung tissues after hUC-MSCs and FTY720 treatment of lipopolysaccharide-induced acute lung injury in mouse models. Int Immunopharmacol 63:26–34. 10.1016/j.intimp.2018.06.036. [DOI] [PubMed] [Google Scholar]
- 179.Mokhber Dezfouli MR, Jabbari Fakhr M, Sadeghian Chaleshtori S, Dehghan MM, Vajhi A, Mokhtari R. 2018. Intrapulmonary autologous transplant of bone marrow-derived mesenchymal stromal cells improves lipopolysaccharide-induced acute respiratory distress syndrome in rabbit. Crit Care 22:353. 10.1186/s13054-018-2272-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Pedrazza L, Cunha AA, Luft C, Nunes NK, Schimitz F, Gassen RB, Breda RV, Donadio MV, de Souza Wyse AT, Pitrez PMC, Rosa JL, de Oliveira JR. 2017. Mesenchymal stem cells improves survival in LPS-induced acute lung injury acting through inhibition of NETs formation. J Cell Physiol 232:3552–3564. 10.1002/jcp.25816. [DOI] [PubMed] [Google Scholar]
- 181.Silva JD, Lopes-Pacheco M, Paz AHR, Cruz FF, Melo EB, de Oliveira MV, Xisto DG, Capelozzi VL, Morales MM, Pelosi P, Cirne-Lima E, Rocco PRM. 2018. mesenchymal stem cells from bone marrow, adipose tissue, and lung tissue differentially mitigate lung and distal organ damage in experimental acute respiratory distress syndrome. Crit Care Med 46:e132–e140. 10.1097/CCM.0000000000002833. [DOI] [PubMed] [Google Scholar]
- 182.Xiang B, Chen L, Wang X, Zhao Y, Wang Y, Xiang C. 2017. Transplantation of menstrual blood-derived mesenchymal stem cells promotes the repair of lps-induced acute lung injury. Int J Mol Sci 18:689. 10.3390/ijms18040689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Gupta N, Krasnodembskaya A, Kapetanaki M, Mouded M, Tan X, Serikov V, Matthay MA. 2012. Mesenchymal stem cells enhance survival and bacterial clearance in murine Escherichia coli pneumonia. Thorax 67:533–539. 10.1136/thoraxjnl-2011-201176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Kim ES, Chang YS, Choi SJ, Kim JK, Yoo HS, Ahn SY, Sung DK, Kim SY, Park YR, Park WS. 2011. Intratracheal transplantation of human umbilical cord blood-derived mesenchymal stem cells attenuates Escherichia coli-induced acute lung injury in mice. Respir Res 12:108. 10.1186/1465-9921-12-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Alcayaga-Miranda F, Cuenca J, Martin A, Contreras L, Figueroa FE, Khoury M. 2015. Combination therapy of menstrual derived mesenchymal stem cells and antibiotics ameliorates survival in sepsis. Stem Cell Res Ther 6:199. 10.1186/s13287-015-0192-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Devaney J, Horie S, Masterson C, Elliman S, Barry F, O'Brien T, Curley GF, O'Toole D, Laffey JG. 2015. Human mesenchymal stromal cells decrease the severity of acute lung injury induced by E. coli in the rat. Thorax 70:625–635. 10.1136/thoraxjnl-2015-206813. [DOI] [PubMed] [Google Scholar]
- 187.Jackson MV, Morrison TJ, Doherty DF, McAuley DF, Matthay MA, Kissenpfennig A, O'Kane CM, Krasnodembskaya AD. 2016. Mitochondrial transfer via tunneling nanotubes is an important mechanism by which mesenchymal stem cells enhance macrophage phagocytosis in the in vitro and in vivo models of ARDS. Stem Cells 34:2210–2223. 10.1002/stem.2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Sung DK, Chang YS, Sung SI, Yoo HS, Ahn SY, Park WS. 2016. Antibacterial effect of mesenchymal stem cells against Escherichia coli is mediated by secretion of beta- defensin- 2 via toll- like receptor 4 signalling. Cell Microbiol 18:424–436. 10.1111/cmi.12522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Li Y, Xu J, Shi W, Chen C, Shao Y, Zhu L, Lu W, Han X. 2016. Mesenchymal stromal cell treatment prevents H9N2 avian influenza virus-induced acute lung injury in mice. Stem Cell Res Ther 7:159. 10.1186/s13287-016-0395-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Darwish I, Banner D, Mubareka S, Kim H, Besla R, Kelvin DJ, Kain KC, Liles WC. 2013. Mesenchymal stromal (stem) cell therapy fails to improve outcomes in experimental severe influenza. PLoS One 8:e71761. 10.1371/journal.pone.0071761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Loy H, Kuok DIT, Hui KPY, Choi MHL, Yuen W, Nicholls JM, Peiris JSM, Chan MCW. 2019. Therapeutic implications of human umbilical cord mesenchymal stromal cells in attenuating influenza A(H5N1) virus-associated acute lung injury. J Infect Dis 219:186–196. 10.1093/infdis/jiy478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Wilson JG, Liu KD, Zhuo H, Caballero L, McMillan M, Fang X, Cosgrove K, Vojnik R, Calfee CS, Lee JW, Rogers AJ, Levitt J, Wiener-Kronish J, Bajwa EK, Leavitt A, McKenna D, Thompson BT, Matthay MA. 2015. Mesenchymal stem (stromal) cells for treatment of ARDS: a phase 1 clinical trial. Lancet Respir Med 3:24–32. 10.1016/S2213-2600(14)70291-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Zheng G, Huang L, Tong H, Shu Q, Hu Y, Ge M, Deng K, Zhang L, Zou B, Cheng B, Xu J. 2014. Treatment of acute respiratory distress syndrome with allogeneic adipose-derived mesenchymal stem cells: a randomized, placebo-controlled pilot study. Respir Res 15:39. 10.1186/1465-9921-15-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Matthay MA, Calfee CS, Zhuo H, Thompson BT, Wilson JG, Levitt JE, Rogers AJ, Gotts JE, Wiener-Kronish JP, Bajwa EK, Donahoe MP, McVerry BJ, Ortiz LA, Exline M, Christman JW, Abbott J, Delucchi KL, Caballero L, McMillan M, McKenna DH, Liu KD. 2019. Treatment with allogeneic mesenchymal stromal cells for moderate to severe acute respiratory distress syndrome (START study): a randomised phase 2a safety trial. Lancet Respiratory Medicine 7:154–162. 10.1016/S2213-2600(18)30418-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Chen J, Hu C, Chen L, Tang L, Zhu Y, Xu X, Chen L, Gao H, Lu X, Yu L, Dai X, Xiang C, Li L. 2020. Clinical study of mesenchymal stem cell treatment for acute respiratory distress syndrome induced by epidemic influenza A (H7N9) infection: a hint for COVID-19. Engineering (Beijing) 6:1153–1161. 10.1016/j.eng.2020.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Monsel A, Zhu YG, Gennai S, Hao Q, Hu S, Rouby JJ, Rosenzwajg M, Matthay MA, Lee JW. 2015. Therapeutic effects of human mesenchymal stem cell-derived microvesicles in severe pneumonia in mice. Am J Respir Crit Care Med 192:324–336. 10.1164/rccm.201410-1765OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Hao Q, Gudapati V, Monsel A, Park JH, Hu S, Kato H, Lee JH, Zhou L, He H, Lee JW. 2019. Mesenchymal stem cell-derived extracellular vesicles decrease lung injury in mice. J Immunol 203:1961–1972. 10.4049/jimmunol.1801534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Park J, Kim S, Lim H, Liu A, Hu S, Lee J, Zhuo H, Hao Q, Matthay MA, Lee JW. 2019. Therapeutic effects of human mesenchymal stem cell microvesicles in an ex vivo perfused human lung injured with severe E. coli pneumonia. Thorax 74:43–50. 10.1136/thoraxjnl-2018-211576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Jahandideh S, Khatami S, Eslami Far A, Kadivar M. 2018. Anti-inflammatory effects of human embryonic stem cell-derived mesenchymal stem cells secretome preconditioned with diazoxide, trimetazidine and MG-132 on LPS-induced systemic inflammation mouse model. Artif Cells Nanomed Biotechnol 46:1178–1187. 10.1080/21691401.2018.1481862. [DOI] [PubMed] [Google Scholar]
- 200.Khatri M, Richardson LA, Meulia T. 2018. Mesenchymal stem cell-derived extracellular vesicles attenuate influenza virus-induced acute lung injury in a pig model. Stem Cell Res Ther 9:17. 10.1186/s13287-018-0774-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Guo Z, Chen Y, Luo X, He X, Zhang Y, Wang J. 2020. Administration of umbilical cord mesenchymal stem cells in patients with severe COVID-19 pneumonia. Crit Care 24:420. 10.1186/s13054-020-03142-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Sanchez-Guijo F, Garcia-Arranz M, Lopez-Parra M, Monedero P, Mata-Martinez C, Santos A, Sagredo V, Alvarez-Avello JM, Guerrero JE, Perez-Calvo C, Sanchez-Hernandez MV, Del-Pozo JL, Andreu EJ, Fernandez-Santos ME, Soria-Juan B, Hernandez-Blasco LM, Andreu E, Sempere JM, Zapata AG, Moraleda JM, Soria B, Fernandez-Aviles F, Garcia-Olmo D, Prosper F. 2020. Adipose-derived mesenchymal stromal cells for the treatment of patients with severe SARS-CoV-2 pneumonia requiring mechanical ventilation. A proof of concept study. EClinicalMedicine 25:100454. 10.1016/j.eclinm.2020.100454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Chen X, Shan Y, Wen Y, Sun J, Du H. 2020. Mesenchymal stem cell therapy in severe COVID-19: A retrospective study of short-term treatment efficacy and side effects. J Infect 81:647–679. 10.1016/j.jinf.2020.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Meng F, Xu R, Wang S, Xu Z, Zhang C, Li Y, Yang T, Shi L, Fu J, Jiang T, Huang L, Zhao P, Yuan X, Fan X, Zhang JY, Song J, Zhang D, Jiao Y, Liu L, Zhou C, Maeurer M, Zumla A, Shi M, Wang FS. 2020. Human umbilical cord-derived mesenchymal stem cell therapy in patients with COVID-19: a phase 1 clinical trial. Signal Transduct Target Ther 5:172. 10.1038/s41392-020-00286-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Ma N, Gai H, Mei J, Ding FB, Bao CR, Nguyen DM, Zhong H. 2011. Bone marrow mesenchymal stem cells can differentiate into type II alveolar epithelial cells in vitro. Cell Biol Int 35:1261–1266. 10.1042/CBI20110026. [DOI] [PubMed] [Google Scholar]
- 206.Cerrada A, de la Torre P, Grande J, Haller T, Flores AI, Perez-Gil J. 2014. Human decidua-derived mesenchymal stem cells differentiate into functional alveolar type II-like cells that synthesize and secrete pulmonary surfactant complexes. PLoS One 9:e110195. 10.1371/journal.pone.0110195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Li Y, Xu W, Yan J, Xia Y, Gu C, Ma Y, Tao H. 2014. Differentiation of human amniotic fluid-derived mesenchymal stem cells into type II alveolar epithelial cells in vitro. Int J Mol Med 33:1507–1513. 10.3892/ijmm.2014.1705. [DOI] [PubMed] [Google Scholar]
- 208.Liu A-R, Liu L, Chen S, Yang Y, Zhao H-J, Liu L, Guo F-M, Lu X-M, Qiu H-B. 2013. Activation of canonical wnt pathway promotes differentiation of mouse bone marrow-derived MSCs into type II alveolar epithelial cells, confers resistance to oxidative stress, and promotes their migration to injured lung tissue in vitro. J Cell Physiol 228:1270–1283. 10.1002/jcp.24282. [DOI] [PubMed] [Google Scholar]
- 209.Cai SX, Liu AR, Chen S, He HL, Chen QH, Xu JY, Pan C, Yang Y, Guo FM, Huang YZ, Liu L, Qiu HB. 2015. Activation of Wnt/beta-catenin signalling promotes mesenchymal stem cells to repair injured alveolar epithelium induced by lipopolysaccharide in mice. Stem Cell Res Ther 6:65. 10.1186/s13287-015-0060-y. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 210.Song N, Wakimoto H, Rossignoli F, Bhere D, Ciccocioppo R, Chen KS, Khalsa JK, Mastrolia I, Samarelli AV, Dominici M, Shah K. 2021. Mesenchymal stem cell immunomodulation: In pursuit of controlling COVID-19 related cytokine storm. Stem Cells 39:707–722. 10.1002/stem.3354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Chen XX, Tang L, Han ZH, Wang WJ, Meng JG. 2019. Coculture with bone marrowderived mesenchymal stem cells attenuates inflammation and apoptosis in lipopolysaccharidestimulated alveolar epithelial cells via enhanced secretion of keratinocyte growth factor and angiopoietin1 modulating the Tolllike receptor4 signal pathway. Mol Med Rep 19:1891–1902. 10.3892/mmr.2019.9836. [DOI] [PubMed] [Google Scholar]
- 212.Wang HY, Liu C, Wang Y, Zhang LL, Liu XR, Liu HL. 2013. Experimental treatment of pulmonary interstitial fibrosis with human umbilical cord blood mesenchymal stem cells. Chinese J Industrial Hygiene and Occupational Diseases 31:675–680. [PubMed] [Google Scholar]
- 213.Wan X, Chen S, Fang Y, Zuo W, Cui J, Xie S. 2020. Mesenchymal stem cell-derived extracellular vesicles suppress the fibroblast proliferation by downregulating FZD6 expression in fibroblasts via micrRNA-29b-3p in idiopathic pulmonary fibrosis. J Cell Physiol 235:8613–8625. 10.1002/jcp.29706. [DOI] [PubMed] [Google Scholar]
- 214.Mansouri N, Willis GR, Fernandez-Gonzalez A, Reis M, Nassiri S, Mitsialis SA, Kourembanas S. 2019. Mesenchymal stromal cell exosomes prevent and revert experimental pulmonary fibrosis through modulation of monocyte phenotypes. JCI Insight 4. 10.1172/jci.insight.128060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Gad ES, Salama AAA, El-Shafie MF, Arafa HMM, Abdelsalam RM, Khattab M. 2020. The anti-fibrotic and anti-inflammatory potential of bone marrow-derived mesenchymal stem cells and nintedanib in bleomycin-induced lung fibrosis in rats. Inflammation 43:123–134. 10.1007/s10753-019-01101-2. [DOI] [PubMed] [Google Scholar]
- 216.Reddy M, Fonseca L, Gowda S, Chougule B, Hari A, Totey S. 2016. Human adipose-derived mesenchymal stem cells attenuate early stage of bleomycin induced pulmonary fibrosis: comparison with pirfenidone. Int J Stem Cells 9:192–206. 10.15283/ijsc16041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Silva LHA, Antunes MA, Dos Santos CC, Weiss DJ, Cruz FF, Rocco PRM. 2018. Strategies to improve the therapeutic effects of mesenchymal stromal cells in respiratory diseases. Stem Cell Res Ther 9:45. 10.1186/s13287-018-0802-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Li Y, Wu Q, Sun X, Shen J, Chen H. 2020. Organoids as a powerful model for respiratory diseases. Stem Cells Int 2020:5847876. 10.1155/2020/5847876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Han Y, Duan X, Yang L, Nilsson-Payant BE, Wang P, Duan F, Tang X, Yaron TM, Zhang T, Uhl S, Bram Y, Richardson C, Zhu J, Zhao Z, Redmond D, Houghton S, Nguyen DT, Xu D, Wang X, Jessurun J, Borczuk A, Huang Y, Johnson JL, Liu Y, Xiang J, Wang H, Cantley LC, tenOever BR, Ho DD, Pan FC, Evans T, Chen HJ, Schwartz RE, Chen S. 2021. Identification of SARS-CoV-2 inhibitors using lung and colonic organoids. Nature 589:270–275. 10.1038/s41586-020-2901-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Serrano-Mollar A, Nacher M, Gay-Jordi G, Closa D, Xaubet A, Bulbena O. 2007. Intratracheal transplantation of alveolar type II cells reverses bleomycin-induced lung fibrosis. Am J Respir Crit Care Med 176:1261–1268. 10.1164/rccm.200610-1491OC. [DOI] [PubMed] [Google Scholar]
- 221.Guillamat-Prats R, Gay-Jordi G, Xaubet A, Peinado VI, Serrano-Mollar A. 2014. Alveolar type II cell transplantation restores pulmonary surfactant protein levels in lung fibrosis. J Heart Lung Transplant 33:758–765. 10.1016/j.healun.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 222.Serrano-Mollar A, Gay-Jordi G, Guillamat-Prats R, Closa D, Hernandez-Gonzalez F, Marin P, Burgos F, Martorell J, Sanchez M, Arguis P, Soy D, Bayas JM, Ramirez J, Tetley TD, Molins L, de la Bellacasa JP, Rodriguez-Villar C, Rovira I, Fibla JJ, Xaubet A, Pneumocyte Study Group . 2016. Safety and tolerability of alveolar type ii cell transplantation in idiopathic pulmonary fibrosis. Chest 150:533–543. 10.1016/j.chest.2016.03.021. [DOI] [PubMed] [Google Scholar]
- 223.Ma Q, Ma Y, Dai X, Ren T, Fu Y, Liu W, Han Y, Wu Y, Cheng Y, Zhang T, Zuo W. 2018. Regeneration of functional alveoli by adult human SOX9(+) airway basal cell transplantation. Protein Cell 9:267–282. 10.1007/s13238-018-0506-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Kapandji N, Yvin E, Devriese M, de Margerie-Mellon C, Moratelli G, Lemiale V, Jabaudon M, Azoulay E, Constantin JM, Dumas G. 2021. Importance of lung epithelial injury in COVID-19-associated acute respiratory distress syndrome: value of plasma soluble receptor for advanced glycation end-products. Am J Respir Crit Care Med 204:359–362. 10.1164/rccm.202104-1070LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Hawkins F, Kotton DN. 2015. Embryonic and induced pluripotent stem cells for lung regeneration. Ann Am Thorac Soc 12 Suppl 1:S50–3. 10.1513/AnnalsATS.201410-457MG. [DOI] [PubMed] [Google Scholar]
- 226.Mou H, Zhao R, Sherwood R, Ahfeldt T, Lapey A, Wain J, Sicilian L, Izvolsky K, Musunuru K, Cowan C, Rajagopal J. 2012. Generation of multipotent lung and airway progenitors from mouse ESCs and patient-specific cystic fibrosis iPSCs. Cell Stem Cell 10:385–397. 10.1016/j.stem.2012.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Hawkins FJ, Suzuki S, Beermann ML, Barilla C, Wang R, Villacorta-Martin C, Berical A, Jean JC, Le Suer J, Matte T, Simone-Roach C, Tang Y, Schlaeger TM, Crane AM, Matthias N, Huang SXL, Randell SH, Wu J, Spence JR, Carraro G, Stripp BR, Rab A, Sorsher EJ, Horani A, Brody SL, Davis BR, Kotton DN. 2021. Derivation of airway basal stem cells from human pluripotent stem cells. Cell Stem Cell 28:79–95 e8. 10.1016/j.stem.2020.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Hor P, Ichida JK, Borok Z, Ryan AL. 2020. Protocol for differentiation of human iPSCs into pulmonary neuroendocrine cells. STAR Protoc 1:100068. 10.1016/j.xpro.2020.100068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Yamamoto Y, Korogi Y, Hirai T, Gotoh S. 2020. A method of generating alveolar organoids using human pluripotent stem cells. Methods Cell Biol 159:115–141. 10.1016/bs.mcb.2020.02.004. [DOI] [PubMed] [Google Scholar]
- 230.Coraux C, Nawrocki-Raby B, Hinnrasky J, Kileztky C, Gaillard D, Dani C, Puchelle E. 2005. Embryonic stem cells generate airway epithelial tissue. Am J Respir Cell Mol Biol 32:87–92. 10.1165/rcmb.2004-0079RC. [DOI] [PubMed] [Google Scholar]
- 231.Van Vranken BE, Rippon HJ, Samadikuchaksaraei A, Trounson AO, Bishop AE. 2007. The differentiation of distal lung epithelium from embryonic stem cells. Current Protocols in Stem Cell Biology Chapter 1:Unit 1G 1. 10.1002/9780470151808.sc01g01s2. [DOI] [PubMed] [Google Scholar]
- 232.Ali NN, Edgar AJ, Samadikuchaksaraei A, Timson CM, Romanska HM, Polak JM, Bishop AE. 2002. Derivation of type II alveolar epithelial cells from murine embryonic stem cells. Tissue Eng 8:541–550. 10.1089/107632702760240463. [DOI] [PubMed] [Google Scholar]
- 233.Samadikuchaksaraei A, Cohen S, Isaac K, Rippon HJ, Polak JM, Bielby RC, Bishop AE. 2006. Derivation of distal airway epithelium from human embryonic stem cells. Tissue Eng 12:867–875. 10.1089/ten.2006.12.867. [DOI] [PubMed] [Google Scholar]
- 234.Khakoo AY, Finkel T. 2005. Endothelial progenitor cells. Annu Rev Med 56:79–101. 10.1146/annurev.med.56.090203.104149. [DOI] [PubMed] [Google Scholar]
- 235.Attar A, Monabati A, Parsanezhad ME. 2017. Endothelial progenitor cell subsets and preeclampsia: findings and controversies. J Chin Med Assoc 80:615–622. 10.1016/j.jcma.2017.06.013. [DOI] [PubMed] [Google Scholar]
- 236.Zhou Y, Li P, Goodwin AJ, Cook JA, Halushka PV, Chang E, Zingarelli B, Fan H. 2019. Exosomes from endothelial progenitor cells improve outcomes of the lipopolysaccharide-induced acute lung injury. Crit Care 23:44. 10.1186/s13054-019-2339-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Ju YN, Geng YJ, Wang XT, Gong J, Zhu J, Gao W. 2019. endothelial progenitor cells attenuate ventilator-induced lung injury with large-volume ventilation. Cell Transplant 28:1674–1685. 10.1177/0963689719874048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Ding BS, Nolan DJ, Guo P, Babazadeh AO, Cao Z, Rosenwaks Z, Crystal RG, Simons M, Sato TN, Worgall S, Shido K, Rabbany SY, Rafii S. 2011. Endothelial-derived angiocrine signals induce and sustain regenerative lung alveolarization. Cell 147:539–553. 10.1016/j.cell.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Chen H, Strappe P, Chen S, Wang LX. 2014. Endothelial progenitor cells and pulmonary arterial hypertension. Heart Lung Circ 23:595–601. 10.1016/j.hlc.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 240.Little JC, Vaughan MK, Haider N, Smith I, Reiter RJ. 1986. Effects of afternoon injections of O-acetyl-5-methoxytryptophol, melatonin or 5-methoxytryptophol in female Syrian hamsters. J Neural Transm 66:291–301. 10.1007/BF01260921. [DOI] [PubMed] [Google Scholar]
- 241.Bolton EM, Bradley JA. 2015. Avoiding immunological rejection in regenerative medicine. Regen Med 10:287–304. 10.2217/rme.15.11. [DOI] [PubMed] [Google Scholar]
- 242.Acanfora D, Acanfora C, Ciccone MM, Scicchitano P, Bortone AS, Uguccioni M, Casucci G. 2021. The cross-talk between thrombosis and inflammatory storm in acute and long-COVID-19: therapeutic targets and clinical cases. Viruses 13:1904. 10.3390/v13101904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Lee T. 2010. Host tissue response in stem cell therapy. World J Stem Cells 2:61–66. 10.4252/wjsc.v2.i4.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Yao C, Guan X, Carraro G, Parimon T, Liu X, Huang G, Mulay A, Soukiasian HJ, David G, Weigt SS, Belperio JA, Chen P, Jiang D, Noble PW, Stripp BR. 2021. Senescence of alveolar type 2 cells drives progressive pulmonary fibrosis. Am J Respir Crit Care Med 203:707–717. 10.1164/rccm.202004-1274OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Yang SR, Park JR, Kang KS. 2015. Reactive oxygen species in mesenchymal stem cell aging: implication to lung diseases. Oxid Med Cell Longev 2015:486263. 10.1155/2015/486263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Mossel EC, Wang J, Jeffers S, Edeen KE, Wang S, Cosgrove GP, Funk CJ, Manzer R, Miura TA, Pearson LD, Holmes KV, Mason RJ. 2008. SARS-CoV replicates in primary human alveolar type II cell cultures but not in type I-like cells. Virology 372:127–135. 10.1016/j.virol.2007.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Sengupta V, Sengupta S, Lazo A, Woods P, Nolan A, Bremer N. 2020. Exosomes derived from bone marrow mesenchymal stem cells as treatment for severe COVID-19. Stem Cells and Development 29:747–754. 10.1089/scd.2020.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Lanzoni G, Linetsky E, Correa D, Messinger Cayetano S, Alvarez RA, Kouroupis D, Alvarez Gil A, Poggioli R, Ruiz P, Marttos AC, Hirani K, Bell CA, Kusack H, Rafkin L, Baidal D, Pastewski A, Gawri K, Lenero C, Mantero AMA, Metalonis SW, Wang X, Roque L, Masters B, Kenyon NS, Ginzburg E, Xu X, Tan J, Caplan AI, Glassberg MK, Alejandro R, Ricordi C. 2021. Umbilical cord mesenchymal stem cells for COVID-19 acute respiratory distress syndrome: a double-blind, phase 1/2a, randomized controlled trial. Stem Cells Transl Med 10:660–673. 10.1002/sctm.20-0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Shi L, Huang H, Lu X, Yan X, Jiang X, Xu R, Wang S, Zhang C, Yuan X, Xu Z, Huang L, Fu JL, Li Y, Zhang Y, Yao WQ, Liu T, Song J, Sun L, Yang F, Zhang X, Zhang B, Shi M, Meng F, Song Y, Yu Y, Wen J, Li Q, Mao Q, Maeurer M, Zumla A, Yao C, Xie WF, Wang FS. 2021. Effect of human umbilical cord-derived mesenchymal stem cells on lung damage in severe COVID-19 patients: a randomized, double-blind, placebo-controlled phase 2 trial. Signal Transduct Target Ther 6:58. 10.1038/s41392-021-00488-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Xu X, Jiang W, Chen L, Xu Z, Zhang Q, Zhu M, Ye P, Li H, Yu L, Zhou X, Zhou C, Chen X, Zheng X, Xu K, Cai H, Zheng S, Jiang W, Wu X, Li D, Chen L, Luo Q, Wang Y, Qu J, Li Y, Zheng W, Jiang Y, Tang L, Xiang C, Li L. 2021. Evaluation of the safety and efficacy of using human menstrual blood-derived mesenchymal stromal cells in treating severe and critically ill COVID-19 patients: An exploratory clinical trial. Clin Transl Med 11:e297. 10.1002/ctm2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Shu L, Niu C, Li R, Huang T, Wang Y, Huang M, Ji N, Zheng Y, Chen X, Shi L, Wu M, Deng K, Wei J, Wang X, Cao Y, Yan J, Feng G. 2020. Treatment of severe COVID-19 with human umbilical cord mesenchymal stem cells. Stem Cell Res Ther 11:361. 10.1186/s13287-020-01875-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Leng Z, Zhu R, Hou W, Feng Y, Yang Y, Han Q, Shan G, Meng F, Du D, Wang S, Fan J, Wang W, Deng L, Shi H, Li H, Hu Z, Zhang F, Gao J, Liu H, Li X, Zhao Y, Yin K, He X, Gao Z, Wang Y, Yang B, Jin R, Stambler I, Lim LW, Su H, Moskalev A, Cano A, Chakrabarti S, Min KJ, Ellison-Hughes G, Caruso C, Jin K, Zhao RC. 2020. Transplantation of ACE2(-) mesenchymal stem cells improves the outcome of patients with COVID-19 pneumonia. Aging Dis 11:216–228. 10.14336/AD.2020.0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Tang L, Jiang Y, Zhu M, Chen L, Zhou X, Zhou C, Ye P, Chen X, Wang B, Xu Z, Zhang Q, Xu X, Gao H, Wu X, Li D, Jiang W, Qu J, Xiang C, Li L. 2020. Clinical study using mesenchymal stem cells for the treatment of patients with severe COVID-19. Front Med 14:664–673. 10.1007/s11684-020-0810-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Liang B, Chen J, Li T, Wu H, Yang W, Li Y, Li J, Yu C, Nie F, Ma Z, Yang M, Xiao M, Nie P, Gao Y, Qian C, Hu M. 2020. Clinical remission of a critically ill COVID-19 patient treated by human umbilical cord mesenchymal stem cells: A case report. Medicine (Baltimore, MD) 99:e21429. 10.1097/MD.0000000000021429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Peng H, Gong T, Huang X, Sun X, Luo H, Wang W, Luo J, Luo B, Chen Y, Wang X, Long H, Mei H, Li C, Dai Y, Li H. 2020. A synergistic role of convalescent plasma and mesenchymal stem cells in the treatment of severely ill COVID-19 patients: a clinical case report. Stem Cell Res Ther 11:291. 10.1186/s13287-020-01802-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Zhang Y, Ding J, Ren S, Wang W, Yang Y, Li S, Meng M, Wu T, Liu D, Tian S, Tian H, Chen S, Zhou C. 2020. Intravenous infusion of human umbilical cord Wharton's jelly-derived mesenchymal stem cells as a potential treatment for patients with COVID-19 pneumonia. Stem Cell Res Ther 11:207. 10.1186/s13287-020-01725-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Zhu Y, Zhu R, Liu K, Li X, Chen D, Bai D, Luo J, Liu Y, Zhang Y, Li L, Hu J, Xu D, Liu Y, Zhao RC. 2020. Human umbilical cord mesenchymal stem cells for adjuvant treatment of a critically ill COVID-19 patient: a case report. Infect Drug Resist 13:3295–3300. 10.2147/IDR.S272645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Dilogo IH, Aditianingsih D, Sugiarto A, Burhan E, Damayanti T, Sitompul PA, Mariana N, Antarianto RD, Liem IK, Kispa T, Mujadid F, Novialdi N, Luviah E, Kurniawati T, Lubis AMT, Rahmatika D. 2021. Umbilical cord mesenchymal stromal cells as critical COVID-19 adjuvant therapy: a randomized controlled trial. Stem Cells Transl Med 10:1279–1287. 10.1002/sctm.21-0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Haberle H, Magunia H, Lang P, Gloeckner H, Korner A, Koeppen M, Backchoul T, Malek N, Handgretinger R, Rosenberger P, Mirakaj V. 2021. Mesenchymal stem cell therapy for severe COVID-19 ARDS. J Intensive Care Med 36:681–688. 10.1177/0885066621997365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Hashemian SR, Aliannejad R, Zarrabi M, Soleimani M, Vosough M, Hosseini SE, Hossieni H, Keshel SH, Naderpour Z, Hajizadeh-Saffar E, Shajareh E, Jamaati H, Soufi-Zomorrod M, Khavandgar N, Alemi H, Karimi A, Pak N, Rouzbahani NH, Nouri M, Sorouri M, Kashani L, Madani H, Aghdami N, Vasei M, Baharvand H. 2021. Mesenchymal stem cells derived from perinatal tissues for treatment of critically ill COVID-19-induced ARDS patients: a case series. Stem Cell Res Ther 12:91. 10.1186/s13287-021-02165-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Saleh M, Vaezi AA, Aliannejad R, Sohrabpour AA, Kiaei SZF, Shadnoush M, Siavashi V, Aghaghazvini L, Khoundabi B, Abdoli S, Chahardouli B, Seyhoun I, Alijani N, Verdi J. 2021. Cell therapy in patients with COVID-19 using Wharton's jelly mesenchymal stem cells: a phase 1 clinical trial. Stem Cell Res Ther 12:410. 10.1186/s13287-021-02483-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Iglesias M, Butron P, Torre-Villalvazo I, Torre-Anaya EA, Sierra-Madero J, Rodriguez-Andoney JJ, Tovar-Palacio AR, Zentella-Dehesa A, Dominguez-Cherit G, Rodriguez-Reyna TS, Granados-Arriola J, Espisosa-Cruz V, Tellez-Pallares FP, Lozada-Estrada A, Zepeda Carrillo CA, Vazquez-Mezquita AJ, Nario-Chaidez HF. 2021. Mesenchymal stem cells for the compassionate treatment of severe acute respiratory distress syndrome due to COVID 19. Aging Dis 12:360–370. 10.14336/AD.2020.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Senegaglia AC, Rebelatto CLK, Franck CL, Lima JS, Boldrini-Leite LM, Daga DR, Leitao CA, Shigunov P, de Azambuja AP, Bana E, Marsaro DB, Schaidt B, Micosky A, Jamur VR, Schluga Y, Vaz IM, Ribeiro LL, Correa A, Brofman E. 2021. Combined use of tocilizumab and mesenchymal stromal cells in the treatment of severe COVID-19: case report. Cell Transplant 30:9636897211021008. 10.1177/09636897211021008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Zengin R, Beyaz O, Koc ES, Akinci IO, Kocagoz S, Sagcan G, Ovali E, Cuhadaroglu C. 2020. Mesenchymal stem cell treatment in a critically ill COVID-19 patient: a case report. Stem Cell Invest 7:17. 10.21037/sci-2020-024. [DOI] [PMC free article] [PubMed] [Google Scholar]