Abstract
The surgical treatment of long bone defects in septic environments remains a challenge for any orthopedic surgeon. The two-stage reconstruction technique described by Masquelet AC is a better alternative in our regions where expertise in microsurgical techniques is rare. We report our first experience with this technique through the reconstruction of the humeral diaphyseal bone defect. We presented a 12-year-old boy diagnosed with chronic osteomyelitis of the left humerus with sequestrum, a pathologic fracture with overly joint involvement. The first stage consisted of a sequestrectomy removing the entire humerus shaft (25 cm) with conservation of the humerus paddle followed by the implantation of cement spacer into the bone defect and stabilization with 2 Kirschner wires (22/10th) and a thoraco-brachial cast. Eleven months later, we performed a cancellous autograft associated with a free non-vascularised fibula graft (12 cm). The bone corticalisation was obtained after 11 months. At the 43-month follow-up, despite joint stiffness and unequal length of brachial segments, the patient and his parents were satisfied.
Keywords: Children, chronic osteomyelitis, free non-vascularised fibula graft, induced membrane technique
INTRODUCTION
Uncommon in developed countries, chronic osteomyelitis (COM) is a common cause of morbidity in paediatric surgical settings.[1] The total surgical debridement of infected long bones often leads to large segmental bone defects (>5 cm) which are a real therapeutic challenge in most sub-Saharan countries where the technical facilities for microvascular surgery are deficient. Different surgical strategies have been reported in the literature to address this, especially the Masquelet-induced membrane technique (IMT).[2] IMT has the advantage of being indicated in septic, tumour, congenital or traumatic environments,[3,4] with a success rate varying between 88% and 100%.[2,4] This two-stage surgery based on the induction of a foreign-body granulation membrane allows reconstruction of large bone defects up to 25 cm also.[2,4,5] The first stage involves a total debridement of devitalised soft tissue and non-viable bone following by bone stabilisation to maintain length and alignment. Then, the insertion of an antibiotic-impregnated cement spacer to fill the segmental bone defect. The second stage performing 6–8 weeks later consists into removing the spacer, the posterior induced membrane left in place. The periosteal cavity is filled up by morselised cancellous bone autograft harvested from numerous locations including iliac crests, femur, proximal tibia and calcaneus.[5,6,7] Despite the numerous publications in the literature regarding IMT, reconstruction of humeral diaphyseal osteomyelitis defect is poorly described. We report our first experience with IMT through the total reconstruction of the humerus in an adolescent in an unfavourable environment, emphasising the technical difficulties.
CASE REPORT
The adolescent S. C., 12-year-old male, was presented to our department with a febrile swelling of the left shoulder evolving for more than a month, previously treated by self-medication and traditional therapy, after a notion of lung infection not well documented. It is due to stiffness of the ipsilateral shoulder that the parents consulted. Clinical examination revealed a fever at 38°9 C, painful swelling of the arm and a limitation of shoulder and arm joint mobility. The review of the other systems devices was unremarkable, and a left humerus COM was suspected.
The laboratory investigations revealed a hyperleucocytosis at 14630 cells/mm3 with 79% neutrophils. The haemoglobin level was 8.4 g/dL. The C-reactive protein was 108 mg/L, and erythrocyte sedimentation rate was 68 mm at the 1st h. The blood culture was negative. Haemoglobin electrophoresis and retroviral serology were normal. Culture of the metaphyseal puncture fluid isolated Staphylococcus aureus. Arm X-ray showed a pandiaphysitis with bone sequestrum and proximal humeral epiphyseal detachment [Figure 1]. The patient was therefore classified type B4 according to the Beit Cure classification.[2] Parenteral antibiotic therapy based on Amoxicillin + clavulanic acid® (100 mg/kg/24 h) and Gentamicin® (5 mg/kg/24 h) was initiated. Gentamicin® was administered for only 5 days due to its nephrotoxicity.
Figure 1.
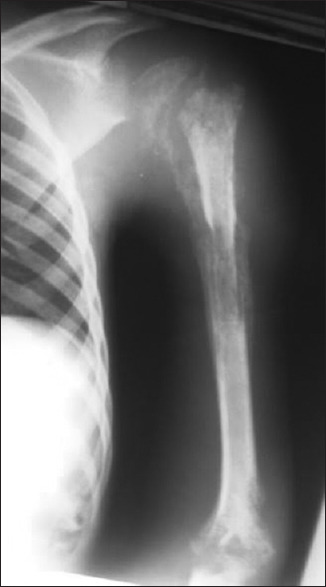
Anteroposterior radiograph of the arm showing pandiaphysis with bone sequestration and pathologic epiphyseal detachment
Surgery was performed 9 days later due to financial constraints. Peroperatively, the sequestrectomy removed the entire humeral diaphysis (25 cm) with preservation of the humerus paddle. The insertion of an antibiotic-impregnated cement spacer and an internal fixation with two Kirschner wires (22/10th) for bone stabilisation following by immobilisation by a thoraco-brachial cast were performed [Figure 2a]. Postoperatively, antibiotics were continued for 6 weeks based on the culture report. Post-operative follow-up was irregular.
Figure 2.
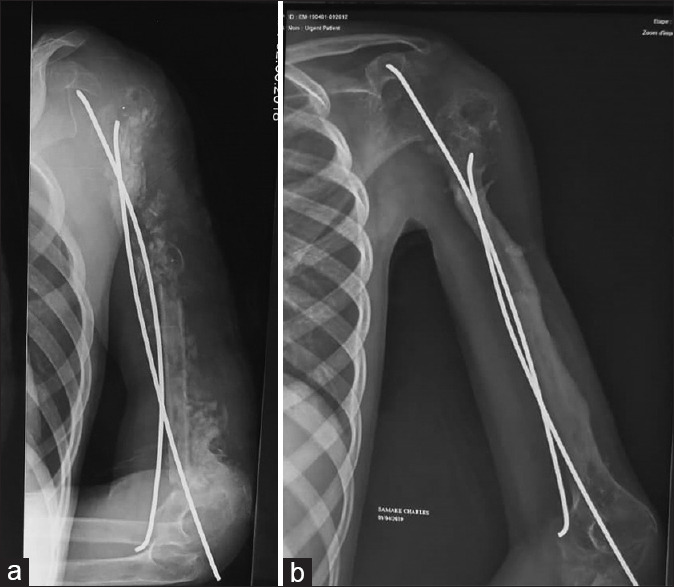
Anteroposterior radiograph of the left arm showing (a) the cancellous graft with the free nonvascularised fibula graft and (b) corticalisation at 10 month
Eleven months after the first procedure, the spacer was removed and an anterior bi-iliac cancellous graft combined with a free non-vascularised fibula graft (12 cm) of the ipsilateral leg were performed, bone stabilised by an internal fixation. The patient was placed in a thoraco-brachial cast for immobilisation. Ten months after the second stage, corticalisation was judged satisfactory and nails removed [Figure 2b].
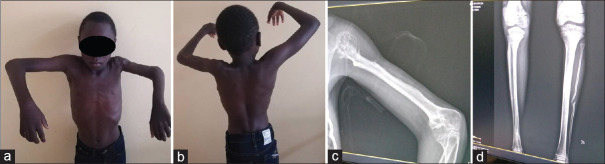
At M43 follow-up, the patient presented an arm length inequality (4 cm), a limited shoulder, and an elbow fixed at 90° with a limitation of prosupination [Figure 3]. Non-union of the fibular donor site was noted. Despite those functional orthopaedic sequelae, parents were satisfied.
Figure 3.
Appearance at the latest follow-up. (A) Clinical aspect of the patient on a front (a) and back (b) view. Radiographic aspect of the arm (c) and leg (d)
DISCUSSION
Reconstruction of large bone defects in septic environments is challenging, and the main objectives are to sterilise the infectious site and to reconstruct the bone defect. In this case, after debridement, a large bone defect of 25 cm was developed. Debate exists on the best approach to address bone loss with critical size. The most reported techniques are the Papineau technique, the vascularised fibular transplant, the Ilizarov intercalary bone transport and the IMT. Among the two surgical procedures (IMT and free vascularised fibula graft) recommended by Masquelet et al.[3] for the reconstruction of bone defect larger than 5 cm at the upper limb, we opted for IMT because our technical platform did not allow the transfer of free vascularised fibula graft. Our first experience with IMT has its pitfalls. The delay in the surgical management after diagnosis was due to economic constraints. The spacer was expensive (145$ US) and parents did not have a health insurance. The technical error in the placement of the cement spacer was related to our lack of experience. The non-union of the fibular donor site was probably related to the long size of the graft. The bone union was achieved in our patient even if he developed some complications. Follow-up was difficult in this unfavourable environment. We are aware that corticalisation time must be interpreted with caution.
The time needed between the two stages remains debated. For our patient, the interval was long (>5 months) which is similar with that of Gindraux et al.[1] who found an average time of 5.6 months between the two stages. Some authors recommended performing the second stage early after the first one, particularly because of a higher rate of growth factors reporting several weeks after the first stage.[8] Others recommended modulating this delay according to the site of the defect like the femur and humerus where the consolidation of the membrane will take too long because of one main vascular axis.[8] We agree with Gindraux et al.[1] and Giannoudis and Harwood[9] that the long delay (>8 weeks) between the two operative stages did not have significant impact on the osteo-inductive and osteogenic properties of the induced membrane.
The arm length inequality could be explained by reaching of the upper extremity of the humerus which is responsible of 80% of the bone growth. The intra-articular malposition of nails and the prolonged immobilization could explain the joint stiffness. The technical errors and complications observed have also been reported by some authors.[2] Some risk factors have been incriminated such as a long pause among the two-stages, a non-stable osteosynthesis, the site (femur) and dimensions of the bone defect.[8,10] Those one could explain our patient sequelae.
The possibility of reconstructing these large bone defects in one step by the modified IMT using a degradable calcium sulfate spacer could flourish in our developing countries where access to care is hampered by economic, cultural and logistical constraints.
CONCLUSION
The IMT is a good option for surgical treatment of post-infectious long bone defects. The adequate strategy for the management of segmental bone loss will depend on the resources available locally and the level of expertise of the treating surgeons. This technique must be mastered by orthopaedic surgeons mostly in less developed countries where chronic haematogenous infections are treated daily due to the delay in presentation.
Consent
Prior written informed consent was obtained from the child’s parents for the publication of this case report and accompanying images.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient has given his consent for his images and other clinical information to be reported in the journal. The patient understands that name and initials will not be published and due efforts will be made to conceal identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors would like to thank M. Ouattara Moussa and Dr Bell Olga for assisting in the language checkup.
REFERENCES
- 1.Gindraux F, Loisel F, Bourgeois M, Oudina K, Melin M, de Billy B, et al. Induced membrane maintains its osteogenic properties even when the second stage of Masquelet's technique is performed later. Europ J Trauma Emerg Surg. 2020;46:301–12. doi: 10.1007/s00068-019-01184-4. [DOI] [PubMed] [Google Scholar]
- 2.Stevenson AJ, Jones HW, Chokotho LC, Beckles VL, Harrison WJ. The Beit CURE classification of childhood chronic haematogenous osteomyelitis--A guide to treatment. J Orthop Surg Res. 2015;10:144. doi: 10.1186/s13018-015-0282-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Masquelet AC, Sales de Gauzy J, Bauer T, Fabre A, Fitoussi F, Hannouche D, et al. Reconstruction of post traumatic diaphyseal bone defects: Preoperative planning, guideline, and future developments. Rev Chir Orthop Traumatol. 2012;98:94–103. [Google Scholar]
- 4.Wang J, Yin Q, Gu S, Wu Y, Rui Y. Induced membrane technique in the treatment of infectious bone defect: A clinical analysis. Orthop Traumatol Surg Res. 2019;105:535–9. doi: 10.1016/j.otsr.2019.01.007. [DOI] [PubMed] [Google Scholar]
- 5.Taylor BC, French BG, Fowler TT, Russell J, Poka A. Induced membrane technique for reconstruction to manage bone loss. J Am Acad Orthop Surg. 2012;20:142–50. doi: 10.5435/JAAOS-20-03-142. [DOI] [PubMed] [Google Scholar]
- 6.Chadayammuri V, Hake M, Mauffrey C. Innovative strategies for the management of long bone infection: A review of the Masquelet technique. Patient Saf Surg. 2015;9:32. doi: 10.1186/s13037-015-0079-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Masquelet AC, Begue T. The concept of induced membrane for reconstruction of long bone defects. Orthop Clin North Am. 2010;41:27–37. doi: 10.1016/j.ocl.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 8.Careri S, Vitiello R, Oliva MS, Ziranu A, Maccauro G, Perisano C. Masquelet technique and osteomyelitis: Innovations and literature review. Eur Rev Med Pharmacol Sci. 2019;23:210–6. doi: 10.26355/eurrev_201904_17495. [DOI] [PubMed] [Google Scholar]
- 9.Giannoudis PV, Harwood PJ, Tosounidis T, Kanakaris NK. Restoration of long bone defects treated with the induced membrane technique: protocol and outcomes. Injury. 2016;47(Suppl 6):S53–61. doi: 10.1016/S0020-1383(16)30840-3. [DOI] [PubMed] [Google Scholar]
- 10.Aurégan JC, Bégué T, Rigoulot G, Glorion C, Pannier S. Success rate and risk factors of failure of the induced membrane technique in children: A systematic review. Injury. 2016;47(Suppl 6):S62–7. doi: 10.1016/S0020-1383(16)30841-5. [DOI] [PubMed] [Google Scholar]