To the Editor: There are case reports of lung transplantation for respiratory failure due to coronavirus disease 2019 (Covid-19), but uncertainty surrounds patient selection, clinical outcomes, and the appropriateness of lung transplantation in this context.1-3 We therefore analyzed lung transplantations performed between August 1, 2020, and September 30, 2021, and reported in the United Network for Organ Sharing (UNOS) registry, which collates transplantation data from all patients in participating regions in the United States. The study was approved by the institutional review board at Cedars–Sinai Medical Center; informed consent was waived by the board. Of 3039 lung transplantations, 214 (7.0%) were performed for Covid-19–related respiratory failure, including 140 (4.6%) for acute respiratory distress syndrome and 74 (2.4%) for pulmonary fibrosis. The median number of lung transplantations performed for Covid-19–related respiratory failure per center was 2.5 (range, 1 to 25). Of the 214 lung transplantations, 197 (92.1%) were bilateral lung transplantations (including 2 heart–lung transplantations and 5 lung–kidney transplantations), and 17 (7.9%) were single-lung transplantations (including 1 lung–kidney transplantation).
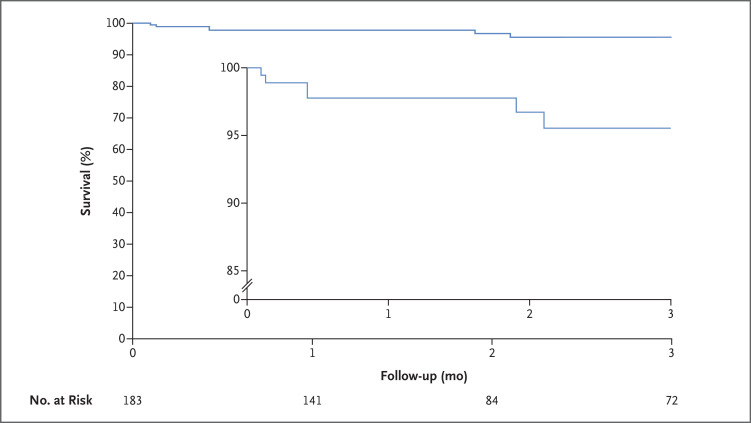
Of the 183 patients with validated data, the median age was 52 years (interquartile range, 43 to 58), 38 (20.8%) were female, and 67 (36.6%) were Hispanic. Preoperatively, 97 (53.0%) received mechanical ventilation, 118 (64.5%) received extracorporeal membrane oxygenation (ECMO), and 9 (4.9%) underwent dialysis; the median lung allocation score (range, 0 to 100, with higher scores indicating a higher priority for a donor lung) was 87.5 (interquartile range, 80.8 to 89.1) (Table S1 in the Supplementary Appendix, available with the full text of this letter at NEJM.org). The median follow-up was 1.9 months (interquartile range, 1.1 to 5.8). There were 9 postoperative deaths during the study period: causes included Covid-19 (1 death), respiratory failure (3), anoxia (1), rejection of the transplant (2), gastrointestinal infection (1), and hyperammonemia (1). The 30-day mortality was 2.2% (4 deaths), and 3-month survival was 95.6% (95% confidence interval, 90.1 to 98.1) (Figure 1). Complications included stroke in 6 of 183 patients (3.3%), new use of ECMO in 8 of 65 patients (12%), and transplant rejection in 11 of 183 patients (6.0%) (Table S2).
Figure 1. Survival through 3 Months after Lung Transplantation among Patients with Respiratory Failure Due to Covid-19.
The inset shows the same data on an enlarged y axis.
Study limitations include incomplete data on the duration of preoperative ventilatory or circulatory support and on preoperative Covid-19 tests and immunization: two negative bronchoalveolar lavages are required for lung-transplant eligibility, but the UNOS did not collect recipient Covid-19 test results. In addition, short-term follow-up precludes firm conclusions about the longer-term usefulness of lung transplantation in this context.
These findings indicate that from August 2020 through September 2021, approximately 7% of lung transplantations nationally were performed in patients with respiratory failure due to Covid-19 (Table S3). Because the 3-month survival among these patients approached that among patients who underwent lung transplantation for reasons other than Covid-19, we believe that lung transplantation may be an acceptable treatment for selected patients with irreversible respiratory failure due to Covid-19.4
Supplementary Appendix
Disclosure Forms
This letter was published on January 26, 2022, at NEJM.org.
Footnotes
Supported by a grant (T32HL116273, to Drs. Roach and Chen) from the National Institutes of Health for research on advanced heart disease.
Disclosure forms provided by the authors are available with the full text of this letter at NEJM.org.
References
- 1.Cypel M, Keshavjee S. When to consider lung transplantation for COVID-19. Lancet Respir Med 2020;8:944-946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holm AM, Mehra MR, Courtwright A, et al. Ethical considerations regarding heart and lung transplantation and mechanical circulatory support during the COVID-19 pandemic: an ISHLT COVID-19 Task Force statement. J Heart Lung Transplant 2020;39:619-626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hawkins RB, Mehaffey JH, Charles EJ, Mannem HC, Roeser M. Lung transplantation for severe post-Coronavirus disease 2019 respiratory failure. Transplantation 2021;105:1381-1387. [DOI] [PubMed] [Google Scholar]
- 4.Valapour M, Lehr CJ, Skeans MA, et al. OPTN/SRTR 2019 annual data report: lung. Am J Transplant 2021;21:441-520. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.