To the Editor: The highly transmissible omicron (B.1.1.529) variant of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is of mounting concern globally. The omicron variant carries a large number of spike mutations, including at least 15 mutations in the receptor-binding domain, which is a major target of neutralizing antibodies.1 To assess the potential susceptibility of this variant to the mRNA-1273 vaccine, neutralization of the omicron variant by serum samples obtained from vaccinated recipients was compared with neutralization of the prototypical D614G variant and the beta (B.1.351) and delta (B.1.617.2) variants. In a pilot study, neutralization of the omicron variant after the primary two-dose regimen of the mRNA-1273 vaccine was lower than that of the D614G and beta variants but increased substantially after a booster dose of the mRNA-1273 vaccine (Figs. S1 through S3 in the Supplementary Appendix, available with the full text of this letter at NEJM.org).
To confirm and extend these initial findings, we evaluated omicron neutralization by serum samples obtained from participants who had received the primary two-dose regimen of the mRNA-1273 vaccine (100 μg in each dose) in the Coronavirus Efficacy (COVE) phase 2 and phase 3 trials of that vaccine2,3 and who had been randomly selected to receive one booster dose of the mRNA-1273 vaccine (at a dose of either 50 or 100 μg), the bivalent mRNA-1273.211 vaccine (a 1:1 mix of mRNA-1273 vaccine and beta variant messenger RNAs [mRNAs], for a total dose of either 50 or 100 μg), or the bivalent mRNA-1273.213 vaccine (a 1:1 mix of beta and delta variant mRNAs, for a total dose of 100 μg) (Table S1). The characteristics of the participants, including age and sex, were generally balanced among the groups.
The neutralizing activity of these serum samples was also assessed against the prototypical D614G variant, which was dominant in the pandemic globally during the time period when the COVE trial showed that the mRNA-1273 vaccine had 93% efficacy in preventing symptomatic coronavirus disease 2019.3 The neutralization titers against the D614G variant that were measured in the pseudovirus assay used in our study were a correlate of vaccine efficacy in the COVE trial.4
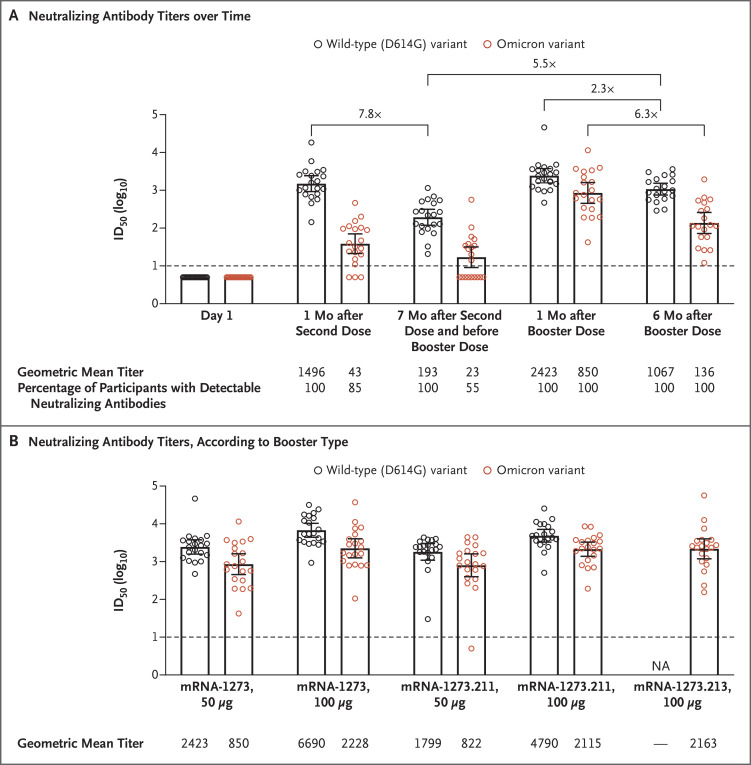
We found that the primary two-dose regimen of the mRNA-1273 vaccine elicited detectable neutralizing antibodies against the omicron variant in 85% of the participants 1 month after the second dose. The 50% inhibitory dilution (ID50) geometric mean titer was 35.0 times lower than that against the D614G variant (Figure 1A). Similar results were observed in live-virus focus-reduction and pseudovirus neutralization assays performed independently (Figs. S1, S4, and S5).
Figure 1. Neutralization of D614G and Omicron SARS-CoV-2 Pseudoviruses in Serum Samples Obtained from Recipients of the mRNA-1273 Primary Vaccine Regimen and Booster.
Panel A shows pseudovirus neutralization assay antibody titers against the wild-type D614G and omicron pseudoviruses measured before the administration of the first dose of the primary two-dose mRNA-1273 vaccine on day 1, 1 month after the second dose (day 57), 7 months after the second dose and before the booster dose, and 1 month and 6 months after the 50-μg mRNA-1273 booster dose. The differences in titers relative to D614G are shown. Panel B shows pseudovirus neutralizing assay titers against D614G and omicron pseudovirus in serum samples obtained from vaccine recipients who initially received the two-dose series of mRNA-1273 vaccine (100 μg in each dose) and who subsequently were randomly selected to receive one booster dose of mRNA-1273 vaccine (either 50 or 100 μg), bivalent mRNA-1273.211 vaccine (either 50 or 100 μg), or bivalent mRNA-1273.213 vaccine (100 μg). Serum samples were obtained from the participants 1 month after they received the booster. The time between vaccination with the second dose of primary vaccine and booster vaccination ranged from 7 to 13 months (Table S1). Twenty participants were selected for each dose of the vaccine and the booster and for each type of booster (mRNA-1273, mRNA-1273.211, or mRNA-1273.213 vaccine). The 50% inhibitory dilution (ID50) neutralizing antibody titers were assayed against pseudoviruses containing the spike protein of the D614G and omicron variants (see the Supplementary Methods section in the Supplementary Appendix). The 𝙸 bars represent 95% confidence intervals, and the circles individual participants. The lower limit of detection (dashed line) of the assay was 10. Values below the lower limit of detection were assigned a value of 5. NA denotes not available for testing.
Seven months after the second dose was administered (before the booster), neutralization of the omicron variant was detected in only 55% of the participants, and the ID50 geometric mean titers were 8.4 times lower than those against the D614G variant (Figure 1A). A booster dose of 50 μg of the mRNA-1273 vaccine, which is currently approved under Emergency Use Authorization for adults who are 18 years of age or older, was associated with ID50 geometric mean titers against the omicron variant that were 20.0 times higher than those assessed 1 month after the second vaccination; these titers were 2.9 times lower than those against the D614G variant.
Neutralization titers against the omicron variant 6 months after the third (booster) dose of vaccine were 6.3 times lower than the peak titers assessed 1 month after the booster injection, but the titers remained detectable in all the participants (Figure 1A). Six months after the booster, neutralization titers against the omicron variant declined faster than those against the D614G variant; however, this decline in titers against the omicron variant was similar to the decline observed in titers against the D614G variant after a second dose of the mRNA-1273 vaccine (by a factor of 7.8 from 1 month to 7 months) (Figure 1A). A similar decline in titers against the D614G variant after a second dose of the mRNA-1273 vaccine has been reported elsewhere.5 The booster dose was associated with improved durability of neutralization of the D614G variant, which was 2.3 times lower 6 months after the booster injection than 1 month after the booster injection.
The 100-μg booster doses of the mRNA-1273, mRNA-1273.211, and mRNA-1273.213 vaccines all generated nearly identical ID50 geometric mean titers against the omicron variant (range, 2115 to 2228); these titers were 2.5 to 2.6 times higher than those assessed after the 50-μg booster dose of the mRNA-1273 vaccine and 1.4 to 1.5 times higher than the peak titers against the D614G variant 1 month after the second dose in the COVE trial (Figure 1B). The strong boosting of neutralization of the omicron variant was similar to the strong boosting of neutralization of the delta and beta variants (Fig. S6).
Together, these results showed that after the primary two-dose series of the mRNA-1273 vaccine, neutralization titers against the omicron variant were 35.0 times lower than those against the D614G variant. These lower titers could lead to an increased risk of severe breakthrough infection. However, a booster dose of mRNA-1273 vaccine was associated with neutralization titers against the omicron variant that were 20.0 times higher than those assessed after the second dose of vaccine, and these titers may substantially reduce the risk of breakthrough infection. The decline in neutralization of the omicron variant 6 months after the booster injection was similar to the decline in neutralization titers against the D614G variant 7 months after the second dose.
The limitations of our study include small sample sets that may not reflect neutralization in diverse populations, differences in the length of time before boosting among the groups, and a lack of post-boost efficacy data. These limitations may be addressed in further studies.
Supplementary Appendix
Disclosure Forms
This trial is ongoing; access to patient-level data and supporting clinical documents may be available to qualified external researchers on request and subject to review once the trial is complete.
This letter was published on January 26, 2022, at NEJM.org.
Footnotes
The study described in this letter was supported by grants from the National Institutes of Health (NIH) (75N93019C00050, to Drs. Montefiori and Shen; U19 AI142790, to Dr. Korber; and UM1 AI148689, to Dr. Lyke), Moderna, and the Intramural Research Program of the Vaccine Research Center, National Institute of Allergy and Infectious Diseases (NIAID), NIH. The Duke and Vaccine Research Center laboratories received funding for sample analysis from Moderna. The Coronavirus Efficacy (COVE) trial (ClinicalTrials.gov number, NCT04470427) was supported by the Office of the Assistant Secretary for Preparedness and Response, Biomedical Advanced Research and Development Authority (BARDA) (contract number, 75A50120C00034) and by the NIAID. The NIAID provides grant funding to the HIV Vaccine Trials Network (HVTN) Leadership and Operations Center (UM1 AI 68614HVTN), the Statistics and Data Management Center (UM1 AI 68635), the HVTN Laboratory Center (UM1 AI 68618), the HIV Prevention Trials Network Leadership and Operations Center (UM1 AI 68619), the AIDS Clinical Trials Group Leadership and Operations Center (UM1 AI 68636), and the Infectious Diseases Clinical Research Consortium leadership group 5 (UM1 AI148684-03). Parts A and B of the phase 2 trial (Dose-Confirmation Study to Evaluate the Safety, Reactogenicity, and Immunogenicity of mRNA-1273 COVID-19 Vaccine in Adults Aged 18 Years and Older; NCT04405076) were supported by federal funds from the Office of the Assistant Secretary for Preparedness and Response, BARDA (contract number, 75A50120C00034) and Moderna. The phase 2–3 trial (A Study to Evaluate the Immunogenicity and Safety of mRNA-1273.211 Vaccine for COVID-19 Variants; NCT04927065) was supported by Moderna.
Disclosure forms provided by the authors are available with the full text of this letter at NEJM.org.
References
- 1.Hastie KM, Li H, Bedinger D, et al. Defining variant-resistant epitopes targeted by SARS-CoV-2 antibodies: a global consortium study. Science 2021;374:472-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chu L, McPhee R, Huang W, et al. A preliminary report of a randomized controlled phase 2 trial of the safety and immunogenicity of mRNA-1273 SARS-CoV-2 vaccine. Vaccine 2021;39:2791-2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.El Sahly HM, Baden LR, Essink B, et al. Efficacy of the mRNA-1273 SARS-CoV-2 vaccine at completion of blinded phase. N Engl J Med 2021;385:1774-1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gilbert PB, Montefiori DC, McDermott AB, et al. Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy clinical trial. Science 2022;375:43-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pegu A, O’Connell SE, Schmidt SD, et al. Durability of mRNA-1273 vaccine-induced antibodies against SARS-CoV-2 variants. Science 2021;373:1372-1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.