Abstract
Introduction
Even if now we have available the weapon of vaccination against SARS-CoV-2, the patients with cancer remains a very frail population in which frequently the immunologic response to vaccination may be impaired. In this setting, the SARS-CoV-2 infection screening retains a great value. However, there are still limited data on the feasibility and efficacy of combined screening procedures to assess the prevalence of SARS-CoV-2 infection (including asymptomatic cases) in cancer outpatients undergoing antineoplastic therapy.
Patients and results
From May 1, 2020, to June 15, 2020, during the first wave of SARS-CoV-2 pandemic, 860 consecutive patients, undergoing active anticancer therapy, were evaluated and tested for SARS-CoV-2 with a combined screening procedure, including a self-report questionnaire, a molecular nasopharyngeal swab (NPS) and a rapid serological immunoassay (for anti-SARS-CoV-2 IgG/IgM antibodies). The primary endpoint of the study was to estimate the prevalence of SARS-CoV-2 infection (including asymptomatic cases) in consecutive and unselected cancer outpatients by a combined screening modality. A total of 2955 SARS-CoV-2 NPS and 860 serological tests, in 475 patients with hematologic cancers and in 386 with solid tumors, were performed. A total of 112 (13%) patients self-reported symptoms potentially COVID-19 related. In 1/860 cases (< 1%) SARS-CoV-2 NPS was positive and in 14 cases (1.62%) the specific serological test was positive (overall prevalence of SARS-CoV-2 infection 1.62%). Of the 112 cases who declared symptoms potentially COVID-19-related, only 2.7% (3/112) were found SARS-CoV-2 positive.
Conclusions
This is the largest study reporting the feasibility of a combined screening procedure (including triage, NPS and serologic test) to evaluate the prevalence of SARS-CoV-2 infection in cancer patients receiving active therapy, during the first epidemic wave and under the restrictive lockdown measures, in one of the active areas of the SARS-CoV-2 circulation. Lacking specific recommendations for the detection of asymptomatic SARS-CoV-2 cases, a combined diagnostic screening might be more effective to detect the exact prevalence of SARS-CoV-2 in neoplastic patient population. The prevalence can obviously change according to the territorial context, the entity of the restrictive measures adopted and the phase of the epidemic curve. However, its exact and real-time knowledge could be important to balance risks/benefits of oncologic treatments, avoiding (if the prevalence is low) the reduction of dose intensity or the selection of less intensive (but also less effective) anti-cancer therapies.
1. Introduction
SARS-CoV-2 infection and related disease (COVID-19) has been an ongoing global health emergency since early 2020 [1–3]. The epidemic in Europe reached its first peak in March 2020 and, unfortunately, the European Community has been involved in further, and even more intense, epidemic waves with over 51 million confirmed cases of infection as of May 2021 and over 1 million related deaths (over 125.000 related deaths only in Italy).
Epidemiological, clinical, and therapeutic knowledge on this disease is still partial and in rapid update and there are still few epidemiological data on its real prevalence (including symptomatic and asymptomatic cases), but it has become increasingly evident that the role of asymptomatic carriers is very important for the infection spreading and for maintenance of a human viral reservoir [4–7]. Furthermore, the worldwide emergence of SARS-CoV-2 variants is a further cause of concern and need to be monitored closely [8]. Even if we have now available the vaccination option against SARS-CoV-2, the patients with cancer remains a very frail population in which the efficacy of vaccination may be unsatisfactory with a higher risk for a severe COVID-19. In this scenario the management of cancer patients underling antineoplastic therapy remains very challenging [9–15]. Furthermore, in patients with solid and hematological tumors, a clinical anamnestic screening by specific triage alone may not be appropriate to intercept SARS-CoV-2 infection cases since some of the symptoms of malignancy may be similar as those of SARS-CoV-2 infection [16, 17]. For this reason, it is important, in cancer populations, especially in the outpatient setting, to define the most effective and targeted monitoring strategies in order to detect, as accurately as possible, the prevalence of SARS-CoV-2 infection in a definite temporal context and geographical area.
Herein, we report the feasibility and efficacy of a combined triple screening strategy (including triage, nasopharyngeal swabs and serological test) to detect the SARS-CoV-2 infection prevalence, in a large cohort of cancer patients undergoing active antineoplastic therapy between May and June 2020.
2. Patients and methods
This is a prospective cohort study including 860 consecutive outpatients with solid cancer or hematological malignancies treated at the University Hospital of Udine-ASUFC, Italy, during the first wave of COVID-19 pandemic.
The primary goal was to assess the prevalence of SARS-CoV-2 infection (symptomatic or asymptomatic cases) using a combined triple screening strategy (including triage, nasopharyngeal swabs and serological test), in patients with active cancer requiring antineoplastic therapy, between May 01 and June 15, 2020. The choice of this period of epidemiological analysis was performed taking into account that it was a temporal phase of active circulation of SARS-CoV-2 in Italy and that, given the maximum incidence of the infection recorded in March 2020, there was an appropriate time frame for the development of an antibody response in potentially exposed cases that were tested.
The study protocol was approved by the Ethics Committee of Friuli Venezia Giulia Region-IT (N° CERU FVG-2020-Os-187) and was conducted in accordance with the Declaration of Helsinki.
The criteria for inclusion in the study were: 1) diagnosis of malignancy under active anticancer therapy; 2) access to the onco-hematologic outpatient department, from May 01, 2020, to June 15, 2020, with a completion of a self-reported triage questionnaire; 3) performance of at least one molecular naso-pharyngeal swab (NPS) and one rapid serologic test for SARS-CoV-2 during the study period; 4) age > 18 years; 5) signature of written informed consent.
The collected and analyzed information’s included: specific patient biographical data, type of cancer (by site), stage of neoplasm (advanced vs. early), type of therapy (conventional chemotherapy, immunotherapy, target therapy), line of therapy (first line, salvage, palliative), potentially COVID-19 related symptoms (fever, sore throat, cough or dyspnea, ageusia, anosmia, headache, pharyngitis, diarrhea, nausea, vomiting), the number of SARS-CoV-2 molecular NPS performed in the study period, the percentage of positive SARS-CoV-2 NPS, the number of rapid serological tests performed and percentage of positive.
2.1 Prevention and social distancing measures adopted during the study period
The epidemiological analysis was performed during a first lockdown period (first epidemic wave) in which schools in Italy were closed to in-person activity, the work activities were reduced, and remote work was encouraged. Circulation was permitted with a surgical mask; access to store and bar activities was restricted. All patients with active cancer were recommended to reduce as much as possible extra-family contacts. Access to the day hospital department was allowed only with surgical mask, and after completion of a triage questionnaire. All healthcare workers carried out their activities with personal protective equipment (PPE) and were also tested for SARS-CoV-2 with an RT-PCR NPS every two weeks, by active surveillance.
2.2 Questions included in the Day Hospital Pre-Access Triage procedure
The triage procedure consisted of a self-report questionnaire including the following key questions: (1) “Have you had fever ≥37°C in the last 14 days?” (2) “Have you had any of the following symptoms in the last 14 days: sore throat, cough or breathing difficulty, loss of taste and smell?” (3) “Have you been in close contact with confirmed SARS-CoV-2 infected persons in the last 14 days?” (4) “Have you been asked to self-quarantine and/or have you (or one of your family members) been tested positive for the SARS-CoV-2 virus?”. The triage questionnaire was considered positive if the patient reported at least one positive answer. Besides, patients received concurrent measurement of body temperature (BT) and were asked to declare the results of any previous SARS-CoV-2 test. All positively triaged patients underwent the same day molecular NPS control and returned home until the response of the swab test was available (generally available within 12–24 h).
2.3 Molecular nasopharyngeal swab (NPS) and Serological Test
The RT-PCR NPS was performed by dedicated and trained nursing staff, at least once in all 860 patients and before each access to the day hospital department, regardless of the triage result; it was additionally performed in all cases of self-reported potentially COVID-19 related symptoms. In addition to the molecular NPS, all patients were tested with one rapid qualitative serological test, in order to intercept, as extensively as possible, patients with a previous contact with the SARS-CoV-2 virus. To assess the presence of anti-SARS-CoV-2 antibodies we used a qualitative commercially available point-of-care lateral flow chromatographic immunoassay (Cellex qSARS-CoV-2 IgG/IgM cassette Rapid Test, Cellex, Inc., NC, USA) that can simultaneously detect IgM and IgG antibodies against SARS-CoV-2 in human blood, with a reported overall sensitivity of 98,4% and specificity of 96,4%.
2.4 Statistical analysis
Basic descriptive statistics were used to analyze and report patients’ characteristics. Quantitative variables are described as the mean±standard deviation or median and range, whereas qualitative variables were described as number and percentages. Differences in categorical variables were analyzed using the Fisher exact test. The chi-square test was used to analyze proportions when appropriate. All tests were performed two-sided at a significance level of 0.05. The small number of events precludes multivariate analysis. Statistical and graphical analyses were performed using MedCalc (version 19.2.1).
3. Results
3.1 Characteristics of the tested patients and of symptomatic cases
Eight hundred and sixty patients with malignancy were tested, of whom 474/860 (55%) had hematologic malignancies and 386/860 (45%) had solid tumors (Table 1). The most frequent neoplasms were lymphomas in 198/860 (23%) cases, breast cancer in 103/860 (12%) cases, multiple myeloma in 103/860 (12%) cases, acute leukemia in 83/860 (9.5%) cases, and lung cancer in 81/860 (9%) cases. The median age of patients was 64 years (range 17–91). As reported in Table 1, 41% (356/860) of patients had newly diagnosed cancer while 59% (504/860) had a relapsed or advanced/refractory neoplastic disease. All patients were receiving an active anticancer therapy and specifically: 327/860 (38%) chemotherapy alone, 373/860 (43%) immunotherapy (in 185 cases alone and in 188 cases in combination), 122/860 (14%) target therapy±immunotherapy, and 49/860 (6%) other supportive/palliative treatments. None of these patients had received anti SARS-CoV-2 vaccination.
Table 1. Patients’characteristics.
| Solid Cancers | N°(%) | Hematologic Cancers | N°(%) |
|---|---|---|---|
| Patients | 386 | Patients | 474 |
| Sex (Male/Female) | 166/220 | Sex (Male/Female) | 265/209 |
| Median Age (range) | 64 (31–85) | Median Age (range) | 63 (17–91) |
|
Type of Disease • Breast cancer • Lung cancer • Colorectal cancer • Pancreatic cancer • Gastric cancer • Skin cancer and Melanoma • Ovarian cancer • Head and neck cancer • Prostatic cancer • Renal and Urothelial cancer • Gynecological cancer (ovarian excluded) • Biliary tract cancer • Endocrine cancer • Other° |
103 81 54 34 24 22 16 8 9 15 10 3 2 5 |
Type of Disease • Lymphoma • Non-Hodgkin • Hodgkin • Cronic Lymphocytic leukemia • Acute Leukemia • Myelodysplastic syndrome • Multiple Myeloma • Myeloproliferative neoplasms • Other** |
198 152 46 59 83 6 103 14 11 |
|
Status of Disease • First Diagnosis • Relapsed/Refractory disease |
98 288 |
Status of Disease • First Diagnosis • Relapsed/Refractory disease |
258 216 |
|
Ongoing Therapy • Chemotherapy alone • Chemotherapy ±Immunotherapy±TT • Immunotherapy alone • Target Therapy (TT) alone • Immunotherapy + TT |
186 73 84 35 8 |
Ongoing Therapy • Chemotherapy alone • Chemotherapy ±Immunotherapy±TT • Immunotherapy alone • Target Therapy (TT) alone • Immunotherapy + TT • Other^^ |
141 104 101 76 3 49 |
° 1 esophageal cancer, 1 peritoneal cancer, 1 testicular cancer, 1 tymoma, 1 glioblastoma
** 4 amyloidosis; 4 myelodysplastic syndrome+emoglobinuria; 3 hystiocytosis
^^ mainly steroids ± radiotherapy.
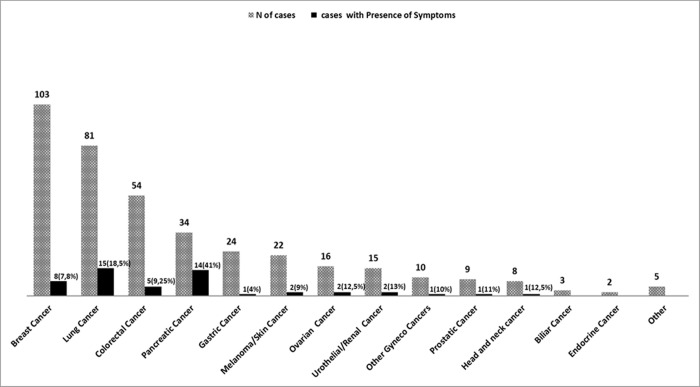
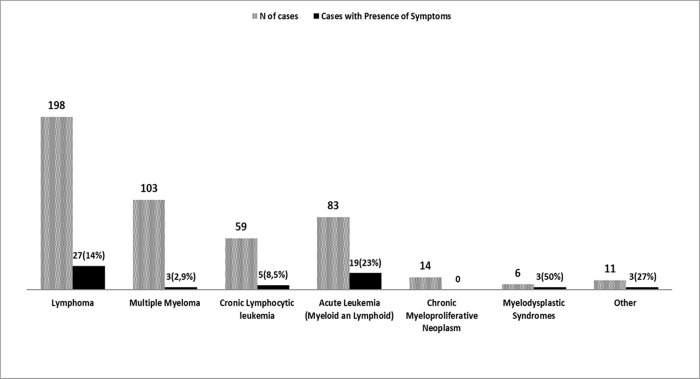
Of the 860 tested patients, 13% (112/860) declared one or more symptoms potentially COVID-19-related at the specific triage procedure performed at each day hospital access during the study period, without significant differences between patients with solid tumors and those with hematologic malignancies (13.5% vs. 13%). The most frequent declared symptom was fever > 37°C, that was reported in 7% (27/386) of patients with solid tumors and in 9,3% (44/474) of cases with hematological neoplasms. Among solid tumors, the presence of clinical symptoms (particularly the presence of fever > 37°C) was more relevant in patients with pancreatic (symptoms in 41% of cases) and lung cancers (symptoms in 18.5% of cases), as shown in Fig 1. In patients with hematological neoplasms, the presence of clinical symptoms (mainly fever > 37°C) was more frequent in patients with acute leukemia (23%), myelodysplastic syndromes (50%) and lymphomas (14%)-Fig 2.
Fig 1. Number of cases according to the solid cancer type and percentage of patients who declared one or more symptoms potentially COVID-19-related at the specific triage procedure.
Fig 2. Number of cases according to the hematologic cancer type and percentage of patients who declared one or more symptoms potentially COVID-19-related at the specific triage procedure.
3.2 Molecular NPS and rapid serological tests and results
As shown in Table 2, during the study period (6 weeks), 2955 molecular NPS were performed with a median of 4 NPS/patient (range, 1–9) and oncological patients underwent a higher number of RT-PCR NPS than hematological population (p<0.05). We found only 1 positive NPS/2955 (< 1%) in a patient with solid cancer (gastric carcinoma) symptomatic for fever. Therefore, a positive predictive value (PPV) of 0,89% (95% CI: 0,87–0.91%) was detected.
Table 2. Results of the combined screening procedure.
| TOTAL CASES | Solid Cancers | Hematologic Cancers | |
|---|---|---|---|
| 860 | 386 (45%) | 474 (55%) | |
| Symptoms potentially SARS-COV-2 related 1 | |||
| Present | 112 (13%) | 52 (13,5%) | 60 (13%) |
| Absent | 748 (87%) | 334 (86,5%) | 414 (87%) |
| Nasopharyngeal Swabs (NPS) SARS-COV-2 | |||
| Total number | 2955 | 1692 | 1263 |
| Median NPS/patient | 4 (1–9) | 4 (1–9) | 3 (1–9) |
| Pt Positive | 1 (<1%) | 1 (<1%) | 0 (0%) |
| SARS-COV-2 IgG/IgM Rapid Test | |||
| Total Number | 860 | 386 | 474 |
| Positive | 14 (1,62%) | 6 (1,55%) | 8 (1,7%) |
| IgG and IgM positive | 7 | 3 | 4 |
| Only IgG positive | 1 | 1 | 0 |
| Only IgM positive | 4 | 2 | 4 |
1 Clinical Manifestations that could be attributed to SARS-CoV-2 disease: fever, cough, dyspnea, fatigue, headache, myalgia, vomiting, diarrhea and neurological manifestations (anosmia, ageusia).
During the same period, 860 rapid serologic tests were performed (1 test each patient) and 14/860 (1.62%) were positive without significant differences between hematologic (8/474 positive-1.7%) and solid cancers (6/386 positive-1.55%) (p = 0,88). The overall seroprevalence of anti-SARS-CoV-2 antibodies (IgG and/or IgM) was 1.62%. Of the 14 cases with positive serologic test, IgG and IgM positivity was found in 7/14 (50%), IgG positivity only in 1/14 (7%), and IgM positivity only in 6/14 (43%) (Table 2). Of the 112 cases that declared potentially COVID-19-related symptoms at triage, only 2.7% (3/112) resulted SARS-CoV-2 positive (1 NPS + serologic test positive; 2 with only serologic test positive).
3.3 Characteristics of SARS-CoV-2 positive cases
Features of the 14 SARS-CoV-2 positive cases are reported in S1 Table. Of them, 8 cases had hematologic malignancies (4 non-hodgkin’s lymphomas, 3 multiple myeloma and 1 myelodysplastic syndrome) and 6 cases had solid tumors (2 gastric cancer, 1 colon cancer, 1 lung cancer, 1 pharyngeal cancer and 1 breast cancer). At the triage procedure 3/14 positive cases (21%) declared symptoms (1 fever, 1 cough, 1 fever and diarrhea) while 11/14 of SARS-CoV-2 positive cases (79%) were completely asymptomatic both at the time of testing, during all the study period and in the 2 months before the study starts.
Overall, using a combination of the 2 tests (NPS and serological test), we found an overall prevalence of SARS-CoV-2 infection, in the analyzed population, of 1.62% (14 positive cases /860 cases tested) with a prevalence of symptomatic cases < 1% (3 cases/860) and a prevalence of asymptomatic cases of 1,27% (11 cases/860).
No secondary SARS-CoV-2 infections were detected among healthcare workers who were in close proximity to these patients.
4. Discussion
Since the start of SARS-CoV-2 epidemic, it soon became clear that patients with cancer represent a very vulnerable population with a high risk of severe COVID-19 and a high mortality rate [12, 13, 17–21]. For this reason, both the oncological and hematological societies recommend constant and careful screening to identify and isolate SARS-CoV-2 infected patients, avoiding infection spreading among this very frail population [22–25]. However, in patients with cancer, very limited real-world data are available regarding the efficacy of combined screening procedures to detect the prevalence of SARS-CoV-2 infection [17, 26, 27].
In this prospective study, we analyzed a large cohort of cancer patients treated, at the University Hospital of Udine (Italy), during the first wave of COVID-19 pandemic, in order to assess, using a combined screening procedure, the effective prevalence of SARS-CoV-2 infection (including symptomatic and asymptomatic cases). Of note, in our geographic area (Friuli-Venezia Giulia Region; North-East of Italy), during our study time-lapse, the cumulative incidence of SARS CoV-2 infection was 235 per 100.000 people as of May 1st,2020, and 280 per 100.000 as of June 15th, 2020 [16]. During the six weeks of surveillance, 860 patients with cancer, undergoing active treatment, were strictly monitored with a questionnaire-triage procedure, periodic RT-NPS and a serological test. Our findings suggest that a questionnaire-based triage system, even if accurate and important, has a low positive-predictive value (0,89%; 95% CI: 0,87–0,91%) for the identification of cancer patients with SARS-CoV-2 infection since a differential diagnosis between tumor-related symptoms (fever for leukemia or paraneoplastic syndromes, dyspnea for lung cancer) or treatment-related symptoms (diarrhea and dysgeusia for mucositis, fever for gemcitabine) and COVID-19-related symptoms is always very difficult. In fact, of the 112 patients whose reported potentially COVID-19-related symptoms at triage, only 2.7% (3/112) were actually SARS-CoV-2 positive. This data highlights, as we have recently reported, the opportunity of a triage screening implementation in the onco-hematological setting to avoid unnecessary treatment delays [16, 27, 28]. We also underscore that 11/14 (79%) of our cases, in which a contact with SARS-COV-2 was confirmed, had an asymptomatic course of infection that was documented only by a detection of the specific antibody production. In view of that, a combined screening modality, beyond a single symptom-driven approach, would be very useful to better identify the SARS-CoV-2 prevalence in cancer patients overcoming the possible limitations of a single procedure.
Obviously, the current gold standard confirming SARS-CoV-2 infection remains, as recommended by the Center for Disease Control, the collection of nasopharyngeal swabs followed by SARS-CoV-2 RNA detection using reverse-transcriptase PCR (RT-PCR). However, the results of the molecular NPS test might be affected by stage of infection and/or quality of the sample and the sensitivity and specificity for RT-PCR NPS are around 70% and 95%, mostly evaluated in symptomatic patients [29–31]. Some studies showed a low concentration of viral RNA in samples from asymptomatic patients; therefore, the real sensitivity of NPS in asymptomatic carriers could probably be lower than in the symptomatic cases and also false-negative NPS test results have been reported early in the course of SARS-CoV-2 infection [32–36]. Regarding the serological tests detecting IgG and IgM antibodies against SARS-CoV-2 they might be useful in neoplastic population [17, 18, 37, 38]. They are easy to perform, could intercept previous asymptomatic infections and could help assessing the immune status of the patient. However, serological tests (quantitative or qualitative), if used alone, as a screening procedure, may have various weaknesses. In fact, it is well known that the antibody production may be impaired in immuno-compromised host, resulting in a lower protection against this infection and also a lower response to the vaccination. As a consequence, the possibility of false negative serology test cannot be excluded, resulting in an underestimated SARS-COV-2 prevalence in this setting [39–41]. The declared sensitivity and specificity of the rapid serological tests we used (Cellex qSARS-CoV-2 IgG/IgM Rapid Test) is 93.75% and 96.40%, respectively. Therefore, considering that we performed 860 rapid tests, it has to be considered both the false-positive (especially in only IgM positive cases) and false negative results [38]. Surprisingly, in a SARS-CoV-2 antibody seroprevalence study, performed by Cabezon-Gutierrez et al., high prevalence of IgG/IgM antibodies was detected in a relevant proportion of cancer patients (31,4%, 72/229 patients), mostly asymptomatic [42]. The probability of SARS-CoV-2 seropositivity wasn’t influenced by sex, type of treatment and cancer stage, whereas was significantly higher in cancer patients with pneumonia. However, in the Spanish region where the cancer center is located, a total cumulative incidence of 835 cases per 100,000 inhabitants and a prevalence of IgG in the general population of 20.2% was found, which could possibly explain this high IgG prevalence recorded in the cancer population [42]. Conversely, in a recent French study the reported SARS-CoV-2 seroprevalence under the first epidemic wave, in a large cancer population (1011 cases), was very low (1,7%) [17]. These results have recently been confirmed in an Italian study (performed in the Marche region) in which the SARS-CoV-2 seroprevalence in 949 cancer patients undergoing treatment was 0,7% [43]. Also in our study, according our geographic context, we observed a low prevalence (1,62%) of SARS-CoV-2 infection in a cancer patient population undergoing active therapy. These results suggest that the majority of our cancer patients remained uninfected during the first wave of SARS-CoV-2 pandemic, despite the active circulation of SARS-CoV-2 in our geographic area. Although this is a very good situation, indicating the efficacy of restrictive measures adopted, at the other side of the coin, it means that the neoplastic population in our area, during the study period, was immunologically naive to SARS-CoV-2 and not protected from a subsequent epidemic wave. This scenario suggested us to confirm the restrictive measures even after the peak of the first epidemic wave to maintain the therapeutic area of day hospital virus free.
We are aware that this study has some important limitations. The regional incidence of SARS-CoV-2 infection during the study period was not particularly high (around 250–280 cases/100.000 habitants) and there are no available data on concomitant seroprevalence in the general population in our geographic area (North-East of Italy) to compare the prevalence of our cancer population with that of the general population. In addition, our combined screening strategy is quite expensive and could probably be adopted only in a phase of epidemic expansion. Moreover, this study encapsulates and records what happened in mild 2020. However, it remains one of the largest studies evaluating the efficacy of combined screening procedures and prevalence of SARS-CoV-2 infection (including asymptomatic cases) in a cohort of cancer patients receiving anti-cancer treatment, during the first epidemic peak, in one of the areas of the SARS-CoV-2 active circulation. Lacking specific recommendations for the detection of asymptomatic SARS-CoV-2 cases, a coupled screening approach (triage, NPS, serological test) could be useful in improving the detection of SARS-Cov-2 infection prevalence in neoplastic patient populations, including the silent infection cases. Obviously, the prevalence data can be different according to the territorial context, to the entity of the restrictive measures adopted and also to the epidemic curve. Its knowledge is important to balance risks/benefits of oncologic treatments and to avoid, if the prevalence is low, the reduction of dose intensity or the selection of less intensive, but also less effective, anticancer therapies [1, 44].
In the coming months, we should remain cautious and, in this unstable pandemic context, a combined screening procedure could be promptly readopted, to improve the control of virus transmission, in case of additional waves of this highly dangerous infectious disease, particularly in countries or context with a low rate of vaccinated population.
Supporting information
*No symptoms during the study period nor in the 3 months before.
(DOCX)
Data Availability
All relevant data are within the paper. The full database are available from the Division of Hematology, University of Udine (IT) (contact via anna.candoni@asufc.sanita.fvg.it) for researchers who meet the criteria for access to confidential data.
Funding Statement
Funding. This work received no financial support.
References
- 1.Brandes A, Di Nunno V. How toface cancer treatment in the COVID-19 era. Expert Review of Anticancer Therapy. 2020, 6:429–32. doi: 10.1080/14737140.2020.1766355 [DOI] [PubMed] [Google Scholar]
- 2.Klompas M. Coronavirus disease 2019 (COVID-19): Protecting hospitals from the invisible. Ann Intern Med. 2020,11:M20–0751. doi: 10.7326/M20-0751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singh AK, Gillies CL, Singh R, Singh A, Chudasama Y, Coles B, et al. Prevalence of co-morbidities and their association with mortality in patients with COVID-19: A systematic review and meta-analysis. Diabetes Obes Metab. 2020,22(10):1915–1924. doi: 10.1111/dom.14124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oran DP, Topol EJ. Prevalence of Asymptomatic SARS-CoV-2 Infection. A Narrative Review. Ann Intern Med. 2020,173:362–367. doi: 10.7326/M20-3012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gandhi M, Yokoe DS, Havlir DV. Asymptomatic transmission, the Achilles’ heel of current strategies to control COVID-19. N Engl J Med. 2020, 382(22):2158–2160. doi: 10.1056/NEJMe2009758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lipsitch M, Swerdlow DL, Finelli L. Defining the epidemiology of Covid-19 –studies needed. N Engl J Med 2020,382:1194–1196. doi: 10.1056/NEJMp2002125 [DOI] [PubMed] [Google Scholar]
- 7.Gao Z, Xu Y, Sun C, Wang X, Guo Y, Qiu S, et al. A systematic review of asymptomatic infections with COVID-19. J Microbiol Immunol Infect. 2021. Feb;54(1):12–16. doi: 10.1016/j.jmii.2020.05.001 Epub 2020 May 15. ; PMCID: PMC7227597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.European Centre for Disease Prevention and Control (ECDC). Risk Assessment: Risk related to spread of new SARS-CoV-2 variants of concern in the EU/EEA [updated 29 December 2020; cited 14 January 2021]. Available from: https://www.ecdc.europa.eu/en/publications-data/covid-19-risk-assessment-spread-new-sarscov-2-variants-eueea.
- 9.Tian J, Yuan X, Xiao J, Zhong Q, Yang C, Liu B, et al. Clinical characteristics and risk factors associated with COVID-19 disease severity in patients with cancer in Wuhan, China: a multicentre, retrospective, cohort study. Lancet Oncol. 2020,21(7):893–903. doi: 10.1016/S1470-2045(20)30309-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Onder G, Rezza G, Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020,323(18):1775–1776. doi: 10.1001/jama.2020.4683 [DOI] [PubMed] [Google Scholar]
- 11.Liang W, Guan W, Chen R, Wang W, Li J, Xu K, et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020,21(3):335–337. doi: 10.1016/S1470-2045(20)30096-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee LYW, Cazier JB, Angelis V, Arnold R, Bisht V, Campton NA, et al. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet. 2020,395(10241):1919–1926. doi: 10.1016/S0140-6736(20)31173-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Passamonti F, Cattaneo C, Arcaini L, Bruna R, Cavo M, Merli F, et al. Clinical characteristics and risk factors associated with COVID-19 severity in patients with haematological malignancies in Italy: a retrospective, multicentre, cohort stud. Lancet Haematol. 2020,7(10): e737–e745. doi: 10.1016/S2352-3026(20)30251-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cortiula F, Pettke A, Bartoletti M, Puglisi F, Helleday T. Managing COVID-19 in the oncology clinic and avoiding the distraction effect. Ann. Oncol. 2020, 31(5):553–555. doi: 10.1016/j.annonc.2020.03.286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garattini SK, Bin A, Donato R, Mansutti M, Rizzato S, Troiero G, et al. What to do in an oncology department to face the new COVID-19 era challenges? Med Oncol. 2020,37(8):75. doi: 10.1007/s12032-020-01400-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fasola G, Pelizzari G, Zara D, Targato G, Petruzzellis G, Minisini AM, et al. Feasibility and Predictive Performance of a Triage System for Patients with Cancer During the COVID-19 Pandemic. Oncologist. 2021, 26(4): e694–e703. doi: 10.1002/onco.13706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ladoire S, Goussot V, Redersdorff E, Cueff A, Ballot E, Truntzer C, et al. Seroprevalence of SARS-CoV-2 among the staff and patients of a French cancer centre after first lockdown: The canSEROcov study. Eur J Cancer. 2021. May; 148:359–370. doi: 10.1016/j.ejca.2021.02.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saini KS, Tagliamento M, Lambertini M, McNally R, Romano M, Leone M, et al. Mortality in patients with cancer and coronavirus disease 2019: a systematic review and pooled analysis of 52 studies. Eur J Cancer 2020;139: 43–50. doi: 10.1016/j.ejca.2020.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Richards M, Anderson M, Carter P, Ebert BL, Mossialos E. The impact of the COVID-19 pandemic on cancer care. Nat Cancer. 2020,1–3. doi: 10.1038/s43018-020-0074-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuderer NM, Choueiri TK, Shah DP, Shyr Y, Rubinstein SM, Rivera DR, et al. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. 2020,395(10241):1907–1918. doi: 10.1016/S0140-6736(20)31187-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van de Haar J, Hoes LR, Coles CE, Seamon K, Frohling S, Jager D, et al. Caring for patients with cancer in the COVID-19 era. Nat Med. 2020,26:665–671. doi: 10.1038/s41591-020-0874-8 [DOI] [PubMed] [Google Scholar]
- 22.Marron JM, Joffe S, Jagsi R, Spence RA, Hlubocky FJ. Ethics and Resource Scarcity: ASCO Recommendations for the Oncology Community During the COVID-19 Pandemic. J. Clin. Oncol. 2020,38(19):2201–2205. doi: 10.1200/JCO.20.00960 [DOI] [PubMed] [Google Scholar]
- 23.Curigliano G, Banerjee S, Cervantes A, Garassino MC, Garrido P, Girard N, et al. Managing cancer patients during the COVID-19 pandemic: an ESMO multidisciplinary expert consensus. Ann Oncol. 2020, 31(10):1320–1335. doi: 10.1016/j.annonc.2020.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tartarone A, Lerose R. COVID-19 and cancer care: what do international guidelines say? Med Oncol. 2020,37(9):80. doi: 10.1007/s12032-020-01406-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ueda M, Martins R, Hendrie PC, McDonnell T, Crews JR, Wong TL, et al. Managing Cancer Care During the COVID-19 Pandemic: Agility and Collaboration Toward a Common Goal. J Natl Compr Canc Netw. 2020,1–4. doi: 10.6004/jnccn.2020.7560 [DOI] [PubMed] [Google Scholar]
- 26.Zambelli A, Chiudinelli L, Fotia V, Negrini G, Bosetti T, Callegaro A, et al. Prevalence and Clinical Impact of Sars-CoV-2 Silent Carriers Among Actively Treated Cancer Patients During The COVID-19 Pandemic. Oncologist. 2021,26(4):341–347. doi: 10.1002/onco.13654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Indini A, Cattaneo M, Ghidini M, Rijavec E, Bareggi C, Galassi B, et al. Triage process for the assessment of coronavirus disease 2019-positive patients with cancer: The ONCOVID prospective study. Cancer. 2021,127(7):1091–1101. doi: 10.1002/cncr.33366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Passaro A, Peters S, Mok TSK, Attili I, Mitsudomi T, De Marinis F. Testing for COVID-19 in lung cancer patients. Ann Oncol. 2020,31(7):832–834. doi: 10.1016/j.annonc.2020.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang W, Xu Y, Gao R, Lu R, Han K, Wu G, et al. Detection of SARS-CoV-2 in Different Types of Clinical Specimens. JAMA. 2020,323(18):1843–1844. doi: 10.1001/jama.2020.3786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Woloshin S, Patel N, Kesselheim AS. False Negative Test for SARS-CoV-2 infection—Challenges and Implications. N Engl J Med. 2020,383: e38. doi: 10.1056/NEJMp2015897 [DOI] [PubMed] [Google Scholar]
- 31.Watson J, Whiting PF, Brush JE. Interpreting a covid-19 test result. BMJ. 2020,369:m1808. doi: 10.1136/bmj.m1808 [DOI] [PubMed] [Google Scholar]
- 32.Kucirka LM, Lauer SA, Laeyendecker O, Boon D, Lessler J. Variation in false-negative rate of reverse transcriptase polymerase chain reaction-based SARS-CoV-2 tests by time since exposure. Ann Intern Med. 2020, M20–1495. doi: 10.7326/M20-1495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Loeffelholz MJ, Tang Y-W. Laboratory diagnosis of emerging human coronavirus infections–the state of the art. Emerging Microbes & Infections. 2020,9(1):747–756. doi: 10.1080/22221751.2020.1745095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ternovoi VA, Lutkovsky RY, Ponomareva EP, Gladysheva AV, Chub EV, Tupota NL, et al. Detection of SARS-CoV-2 RNA in nasopharyngeal swabs from COVID-19 patients and asymptomatic cases of infection by real-time and digital PCR. Klin Lab Diagn. 2020,65(12):785–792. doi: 10.18821/0869-2084-2020-65-12-785-792 [DOI] [PubMed] [Google Scholar]
- 35.Wang Y, Kang H, Liu X, Tong Z. Asymptomatic cases with SARS-CoV-2 infection. J Med Virol. 2020,92:1401–1403. doi: 10.1002/jmv.25990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu Y, Yan L-M, Wan L, Xiang T-X, Le A, Liu J-M, et al. Viral dynamics in mild and severe cases of COVID-19. Lancet Infectious Disease. 2020,20(6):656–657. doi: 10.1016/S1473-3099(20)30232-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guo L, Ren L, Yang S, Xiao M, Chang D, Yang F et al. Profiling early humoral response to diagnose novel coronavirus disease (COVID-19). Clin Infect Dis. 2020,71(15):778–85. doi: 10.1093/cid/ciaa310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maple PAC, Sikora K. How Useful is COVID-19 Antibody Testing—A Current Assessment for Oncologists. Clinical Oncology. 2021,33: e73–e81. doi: 10.1016/j.clon.2020.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xiang F, Wang X, He X, Peng Z, Yang B, Zhang J, et al. Antibody detection and dynamic characteristics in patients with Coronavirus Disease 2019. Clin Infect Dis. 2020,71(8):1930–1934. doi: 10.1093/cid/ciaa461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marra A, Generali D, Zagami P, Cervoni V, Gandini S, Venturini S, et al. Seroconversion in patients with cancer and oncology health care workers infected by SARS-CoV-2. Ann Oncol. 2021,32(1):113–119. doi: 10.1016/j.annonc.2020.10.473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Long Q-X, Liu B-Z, Deng H-J, Wu G-C, Deng K, Chen Y-K, et al. Antibody responses to SARSCoV-2 in patients with COVID-19. Nat Med. 2020,26:845–848. doi: 10.1038/s41591-020-0897-1 [DOI] [PubMed] [Google Scholar]
- 42.Cabezon-Gutierrez L, Custodio-Cabello S, Palka-Kotlowska M, Oliveros-Acebes E, Garcia-Navarro MJ, Khosravi-Shahi P. Seroprevalence of SARS-COV-2-specific antibodies in cancer outpatients in Madrid (Spain): a single center, prospective, cohort study and a review of available data. Cancer Treatment Reviews. 2020, 90:102102. doi: 10.1016/j.ctrv.2020.102102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cantini L, Bastianelli L, Lupi A, Pinterpe G, Pecci F, Belletti G, et al. Seroprevalence of SARS-CoV-2-Specific Antibodies in Cancer Patients Undergoing Active Systemic Treatment: A Single-Center Experience from the Marche Region, Italy. J Clin Med. 2021. Apr 4;10(7):1503. doi: 10.3390/jcm10071503 ; PMCID: PMC8038489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hanna TP, Evans GA, Booth CM. Cancer, COVID-19 and the precautionary principle: prioritizing treatment during a global pandemic. Nat. Rev. Clin. Oncol. 2020,17:268–270. doi: 10.1038/s41571-020-0362-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
*No symptoms during the study period nor in the 3 months before.
(DOCX)
Data Availability Statement
All relevant data are within the paper. The full database are available from the Division of Hematology, University of Udine (IT) (contact via anna.candoni@asufc.sanita.fvg.it) for researchers who meet the criteria for access to confidential data.