Highlights
-
•
30% of the Bangladeshi population were found to be seropositive for severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) immunoglobulin G antibodies.
-
•
The highest seroprevalence rate (64%) was found in slum areas in Bangladesh.
-
•
Thirty-eight percent and 29% of participants from urban and rural areas were SARS-CoV-2 seropositive.
-
•
The highest seroprevalence rate for coronavirus disease 2019 was observed in August 2020.
Keywords: Seroprevalence, Antibodies, Bangladesh, COVID-19
Abstract
Design
A cross-sectional study was conducted amongst household members in 32 districts of Bangladesh to build knowledge about disease epidemiology and seroepidemiology of coronavirus disease 2019 (COVID-19).
Objective
Antibody responses to severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) were assessed in people between April and October 2020.
Results
The national seroprevalence rates of immunoglobulin G (IgG) and IgM were estimated to be 30.4% and 39.7%, respectively. In Dhaka, the seroprevalence of IgG was 35.4% in non-slum areas and 63.5% in slum areas. In areas outside of Dhaka, the seroprevalence of IgG was 37.5% in urban areas and 28.7% in rural areas. Between April and October 2020, the highest seroprevalence rate (57% for IgG and 64% for IgM) was observed in August. IgM antibody was more prevalent in younger participants, while older participants had more frequent IgG seropositivity. Follow-up specimens from patients with COVID-19 and their household members suggested that both IgG and IgM seropositivity increased significantly at day 14 and day 28 compared with day 1 after enrolment. Conclusions: SARS-CoV-2 had spread extensively in Bangladesh by October 2020. This highlights the importance of monitoring seroprevalence data, particularly with the emergence of new SARS-CoV-2 variants over time.
Introduction
The first case of coronavirus disease 2019 (COVID-19) was identified on 31 December 2019 in Wuhan, China (Guo et al.; 2020, World Health Organization, 2020). The Government of Bangladesh reported the first case of COVID-19 in Bangladesh on 8 March 2020 (GARDAWORLD, 2020). As of 29 June 2021, 896,770 confirmed cases have been identified in Bangladesh, including 14,276 deaths (Management Information System Directorate General of Health Services, 2021). Bangladesh is estimated to be at high risk for COVID-19 due to its population density, poor sanitary practices, and limited infrastructure and infection control measures. A second wave of COVID-19 occurred in Bangladesh between April and May 2021, with 90% of cases due to the beta variant of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). By August 2021, all cases of COVID-19 in Bangladesh were due to the delta variant, and the death rate was higher than that in the first wave in 2020 (Rahman et al., 2021). To better ascertain the burden of COVID-19 in Bangladesh, the Institute of Epidemiology, Disease Control and Research (IEDCR) directed a national level investigation to evaluate the prevalence of COVID-19 in Bangladesh, in collaboration with the International Centre for Diarrhoeal Disease Research, Bangladesh (ICDDR,B), with support from the US Agency for International Development (USAID) and the Bill and Melinda Gates Foundation.
Much epidemiological information about this newly emerging disease remains unknown, including estimates of the proportion of COVID-19 cases in the community, particularly for lower-income regions and countries such as Bangladesh, making it difficult for government policy makers to design optimal containment and mitigation strategies. Prior research has indicated that there may be a considerable number of asymptomatic cases of COVID-19 (Anderson et al., 2020). Other areas requiring further exploration include the incidence rate, prevalence rate, secondary infection rate, incubation period, serial interval and reproductive number of COVID-19 in various settings. Although there have been attempts to gather some of these data in previous studies worldwide, most estimates have been based on small-scale data or on information collected from relatively narrow geographic regions (Anderson et al., 2020). It is also important to determine and characterize the immune responses to SARS-CoV-2 infection to understand how well the response protects people against future SARS-CoV-2 infection and how long this protection lasts (Sutton et al., 2020). In this context, serological investigation has the potential to provide information about the true number of SARS-CoV-2 infections, allowing for robust estimates of the infection fatality rates (Fontanet et al., 2020), and to guide public health decision-making. Therefore, a nationwide seroprevalence study of SARS-CoV-2 was conducted in Bangladesh, with follow-up data on cases of COVID-19 and their household members, in order to enhance knowledge about the seroepidemiology of SARS-CoV-2 in Bangladesh.
Methods
Study sites, design and household selection
This first national-level cross-sectional study in Bangladesh incorporated data from April 2020 to October 2020. To assess the seroprevalence of SARS-CoV-2 infection in Dhaka, a total of 25 wards were selected at random out of the 129 wards, one mahalla (the smallest geographical unit of urban area) was selected at random from each ward, and 120 households were selected at random from each mahalla. To evaluate the seroprevalence in slum areas, an additional eight slums from Dhaka were included in the study. Each participant was asked if they had any of the four probable COVID-19 symptoms [i.e. fever (body temperature >38°C), cough, sore throat and breathing difficulties within the last 7 days], and were also asked for other sociodemographic information (e.g. shared bathroom, household size, number of living rooms, and if they had been tested for COVID-19 before the survey) on the day of the interview or within 7 days preceding sample collection (Nazneen et al., 2021).
In addition, 32 districts, including Dhaka, were selected at random out of the 64 districts of Bangladesh. One village (the smallest geographical unit of a rural area) and one mahalla (the smallest geographical unit of an urban area) were selected at random from each selected district for data collection.
A nasopharyngeal swab and 3–4 mL of blood were collected from each participant in Dhaka and outside Dhaka. The SARS-CoV-2-positive participants from Dhaka, as determined from nasopharyngeal swab samples collected on enrolment day (day 1), were followed-up and serological responses in their household members were measured on days 1, 14 and 28 after enrolment.
Processing of specimens
Blood specimens were collected from study participants at the time of the interview. In addition, blood specimens were collected on days 14 and 28 after enrolment from household members of individuals in Dhaka who tested positive for SARS-CoV-2 on reverse transcriptase polymerase chain reaction (RT-PCR). Serum was separated from whole blood after centrifugation of the tubes at 700x g for 15 min and kept frozen (-80 °C) until the time of laboratory analysis. Enzyme-linked immunosorbent assay (ELISA) was used to determine SARS-CoV-2-specific immunoglobulin G (IgG) and IgM antibodies against COVID-19 in the collected serum samples, as below.
SARS-CoV-2-specific enzyme-linked immunosorbent assay
For analysis of antibodies to SARS-CoV-2 in this study, COVID-19-specific antibody measurements were performed using an in-house ELISA assay for IgG and IgM isotypes against the receptor binding domain (RBD) of the spike protein of SARS-CoV-2 (Iyer et al., 2020; Shirin et al., 2020). RBD-specific antibody concentrations (ng/mL) were quantified using isotype-specific anti-RBD monoclonal antibodies. Briefly, 96-well Nunc MaxiSorp plates (Thermo Fisher Scientific, Waltham, MA, USA) were coated with 100 µL of SARS CoV-2 RBD antigen (1 μg/mL in carbonate buffer) and incubated for 1 h at room temperature (RT). Plates were blocked for 30 min at RT with 300 µL of 5% non-fat milk in phosphate-buffered saline (PBS). Heat-inactivated serum samples [serially diluted samples 1:100, 1:400, 1:1600 and 1:6400 in 5% milk-1x PBS 0.05% Tween (PBST)] were added to the plate (100 μL/well) and incubated for 1 h at 37°C. A specific monoclonal antibody to RBD of known concentration (Mab CR3022) was added to the plate; two-fold serial dilutions were performed starting at 25 ng/mL for both the IgG and IgM monoclonal antibodies. Individual serum samples were tested and quantified to determine the concentration of specimens based on the monoclonal antibody. At the end of incubation, plates were washed five times with PBST. Goat anti-human IgG and IgM horseradish peroxidase-conjugated secondary antibodies (Jackson ImmunoResearch, West Grove, PA, USA) diluted at 1:5000 in 5% milk in PBST were added to plates (100 μL/well) and incubated at RT for 30 min followed by five washes with PBST and one wash with 1X PBS. Bound secondary antibodies were detected using ortho phenylenediamine (Sigma, St Louis, MO, USA; 200 μL/well) in 0.1 M sodium citrate buffer (pH 4.5) and 30% H2O2. Plates were incubated at RT for 20 min in the dark. Optical density (OD) was measured at 450 nm and 570 nm in the Eon (Biotek, Winooski, VT, USA) ELISA Reader; OD values were adjusted by subtracting the OD at 570 nm from the OD at 450 nm.
Validation and cut-off calculation for seropositivity
Before testing current study specimens, ELISA was performed using serum specimens collected from RT-PCR-positive cases of COVID-19 (n=38), and pre-pandemic healthy controls collected from Dhaka (n=73), Khulna and Rajshahi (n=203) to determine the cut-offs for the different isotypes of antibodies and validate the assay. In addition, sera obtained from patients with influenza (n=59) and patients from the Japanese encephalitis surveillance platform (n=20) were tested (Shirin et al., 2020), and the responses were compared with commercially available ELISA kits (Euroimmun, Gross Sarau, Germany for IgG; Wantai, Beijing, China for IgM). For IgG, 100% sensitivity, 98% specificity and 97% positive predictive value were observed. For IgM, 89% sensitivity, 95% specificity and 94% positive predictive value were observed. The upper limit of the range of concentrations of SARS-CoV-2 IgG and IgM antibodies from random available pre-pandemic serum samples (n=200) collected from different parts of Bangladesh (before 2019) was used to determine the cut-off for seropositivity. Based on this, 500 ng/mL (0.5 µg/mL) was determined as the cut-off value for both IgG and IgM antibodies (Tables S1 and S2, see online supplementary material).
Data analyses
For each stage of sampling, the selection probability of sampling units was estimated using the existing sampling frame, and then all the selection probabilities obtained from each stage were multiplied to estimate the selection probability for each selected individual. Next, individual-level sampling weight was estimated by taking the inverse of the selection probabilities. Finally, the weights were normalized and used to estimate the parameters. The seroprevalence of SARS-CoV-2 antibodies with 95% confidence interval (CI) was estimated, adjusting for design weight and clustering effects. For the concentration, geometric mean (GM) with 95% CI is presented. A line plot is presented over the study period for seroprevalence estimated by 15-day interval. Pearson's Chi-squared test was used to measure the difference in seroprevalence between different study groups, and a difference of two GMs was considered significantly different at 5% level of significance if 95% CI were non-overlapping. Antibody responses (IgG and IgM) on different days in household members of the RT-PCR-positive participants were analysed using the Mann–Whitney U-test. P<0.05 was considered to indicate significance. All analyses were performed using GraphPad Prism 6.0 and STATA Version 15. Clusters and individuals within each cluster were selected using probability sampling. For each stage of sampling, the selection probability of sampling units was estimated using the existing sampling frames, and then all selection probabilities obtained at different stages were multiplied to determine the selection probability of each selected individual. Individual-level sampling weight was estimated by taking the inverse of the selection probabilities and normalizing them. As appropriate weights were used, the estimates are generalizable to the rest of the country.
Ethical consideration
The Research Review Committee and the Ethical Review Committee of ICDDR,B approved the study protocol. The institutional review board of IEDCR also approved the study. Informed written consent was obtained from all participants before enrolment and data collection.
Results
Study participants
Demographic information was collected from all participants (Table 1). Blood specimens were collected from 2582 participants at national level, comprising Dhaka (n=701, non-slum areas) as well as rural (n=819) and urban areas (n=1062) outside Dhaka. In addition, blood specimens were collected from selected people (n=126) living in slum areas in Dhaka. Gender distribution was comparable (51% male and 49% female participants). As this survey was based on households, participants from all age groups were enrolled. Among the study population, 20% of participants were aged <20 years. The largest proportion of participants (41%) were aged 20–39 years. Based on recorded symptoms, 14% of people had fever, 11% had cough, 3% had sore throat and 2% had shortness of breath on the day of enrolment and within the past 7 days. The majority of participants (83%) did not use shared bathrooms and 47% of enrolled households had four members or more. Almost all participants (99%) had not been tested for COVID-19 before inclusion in this study (Table 1).
Table 1.
Demographic characteristics of the study population.
| Characteristic | National (n=2582) Frequency (%) | Dhaka |
Outside Dhaka |
||
|---|---|---|---|---|---|
| Non-slum (n=701) Frequency (%) | Slum (n=126) Frequency (%) | Urban (n=1062) Frequency (%) | Rural (n=819) Frequency (%) | ||
| Sex | |||||
| Male | 51.4 | 56.5 | 54.0 | 49.1 | 50.2 |
| Female | 48.6 | 43.5 | 46.0 | 50.9 | 49.8 |
| Age (years) | |||||
| <10 | 2.9 | 2.0 | 4.8 | 2.9 | 3.7 |
| 10–14 | 7.0 | 6.6 | 7.9 | 6.9 | 7.7 |
| 15–19 | 10.1 | 8.4 | 11.1 | 11.6 | 9.5 |
| 20–39 | 41.4 | 46.9 | 51.6 | 40.2 | 38.1 |
| 40–59 | 28.2 | 27.7 | 19.8 | 29.1 | 27.5 |
| ≥60 | 10.4 | 8.4 | 4.8 | 9.3 | 13.6 |
| Symptoms (day of visit and preceding 7 days) | |||||
| Fever | 14.0 | 18.0 | 11.1 | 13.9 | 10.7 |
| Cough | 10.8 | 13.3 | 7.1 | 11.1 | 8.5 |
| Sore throat | 3.4 | 4.4 | 1.6 | 3.8 | 2.2 |
| Shortness of breathing | 1.9 | 3.0 | 2.4 | 2.0 | 1.0 |
| Shared bathroom | |||||
| No | 82.7 | 76.6 | 23.0 | 84.1 | 86.1 |
| Yes | 17.3 | 23.4 | 77.0 | 15.9 | 13.9 |
| Household size | |||||
| ≤4 members | 53.5 | 54.4 | 59.5 | 53.6 | 52.6 |
| ≥4 members | 46.5 | 45.6 | 40.5 | 46.4 | 47.4 |
| Number of living rooms | |||||
| 1 | 15.1 | 23.8 | 61.9 | 11.9 | 11.7 |
| 2 | 31.2 | 29.2 | 29.4 | 32.8 | 30.9 |
| ≥3 | 53.7 | 46.9 | 8.7 | 55.4 | 57.4 |
| Tested for COVID-19 before the survey | |||||
| No | 99.4 | 99.0 | 98.4 | 99.3 | 99.8 |
| Yes | 0.6 | 1.0 | 1.6 | 0.7 | 0.2 |
COVID-19, coronavirus disease 2019.
Seroprevalence of SARS-CoV-2-specific antibodies in Bangladesh
At national level, 30.4% of the population had IgG responses against the SARS-CoV-2 RBD antigen and 39.7% had IgM antibodies in Bangladesh between April and October 2020 (Table 2). Overall, 51.8% of individuals tested seropositive against SARS-CoV-2 for IgG and/or IgM antibody in Bangladesh during the abovementioned period. During the study period, significantly higher concentrations of IgM antibodies (GM 355 ng/mL) were observed in comparison with IgG (GM 176 ng/mL).
Table 2.
Seroprevalence of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2)-specific immunoglobulin G (IgG) and IgM antibodies in Bangladesh, including Dhaka and outside Dhaka.
| Nationala | Dhaka |
Outside Dhaka |
|||||
|---|---|---|---|---|---|---|---|
| Non-sluma | Slumb | P-valuec | Urbana | Rurala | P-valued | ||
| Seropositivity, % (95% CI) | |||||||
| IgG | 30.4 (24–37) | 35.4 (24–47) | 63.5e (55–72) | <0.05e | 37.5 (29–46) | 28.7 (21–37) | >0.05 |
| IgM | 39.7 (33–46) | 26.5 (20–33) | 38.1 (29–47) | >0.05 | 45.1 (38–52) | 38.7 (31–47) | >0.05 |
| Geometric mean concentration (ng/mL, 95% CI) | |||||||
| IgG | 176 (164–189) | 250 (214–294) | 425 (281–642) | >0.05 | 246 (220–275) | 162 (142–184) | <0.05e |
| IgM | 355 (340–369) | 226 (205–248) | 307 (248–380) | <0.05e | 351 (328–376) | 360 (335–386) | >0.05 |
CI, confidence interval.
Weighted estimates.
Unweighted estimates.
Statistical analyses were performed between slum and non-slum areas of Dhaka.
Statistical analyses were performed between urban and rural populations outside of Dhaka.
P<0.05 was considered to indicate significance.
IgG seroprevalence varied with type of study site. It was highest in the slum areas of Dhaka (64%), followed by urban areas outside Dhaka (38%), non-slum areas of Dhaka (35%) and rural areas outside Dhaka (29%). The higher seropositivity rate in individuals living in slum areas may be influenced by the fact that enrolment in these areas started later (July and August 2020) than enrolment in the non-slum areas of Dhaka. In the areas outside Dhaka, the seroprevalence rate was higher in people living in urban areas compared with rural areas (Table 2).
Seroprevalence rates by age and gender
Comparable seroprevalence rates of SARS-CoV-2-specific IgM antibodies were found in all age groups (Table 3). However, IgG seropositivity increased with age. Between 8% and 24% of participants aged <20 years had IgG antibodies, while 32–36% of participants aged >20 years had IgG antibodies. There was no significant difference in the seroprevalence rate between males and females, although females had a higher rate of IgG (33% vs 28%) and IgM seropositivity (48% vs 32%) compared with males (P>0.05).
Table 3.
Weighted distribution of positive severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2 antibodies at national level in Bangladesh among people of different ages and genders.
| IgG, % (95% CI) | IgM, % (95% CI) | |
|---|---|---|
| Age (years) | ||
| <10 | 8.2 (0–19) | 43.1 (20–66) |
| 10–14 | 15.3 (2–29) | 51.7 (38–66) |
| 15–19 | 23.8 (14–34) | 48.8 (35–63) |
| 20–39 | 32.6 (25–40) | 44.7 (36–54) |
| 40–59 | 35.5 (25–46) | 32.4 (24–41) |
| ≥60 | 31.8 (24–40) | 21.3 (9–33) |
| Gender | ||
| Male | 27.8 (21–35) | 32.1 (25–39) |
| Female | 33.4 (26–41) | 48.1 (39–57) |
CI, confidence interval.
Development of SARS-CoV-2 antibodies in Bangladesh
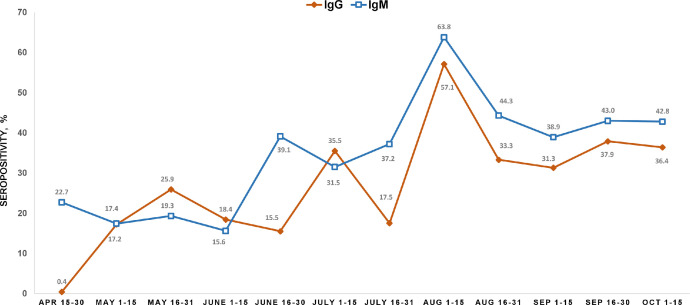
The monthly seropositivity rates (for IgG and IgM antibodies) in Bangladesh between April and October 2020 were analysed (Figure 1). SARS-CoV-2 IgM antibodies were very low in the population in late April 2020. However, approximately 23% of the population were seropositive for SARS-CoV-2 IgG antibody by the end of April 2020. As the first case of COVID-19 in Bangladesh was detected on 8 March 2020, this may explain the IgG antibody responses seen in April 2020. As infections increased and community transmission occurred over the subsequent months in Bangladesh, a continued rise in seropositivity rates was observed, with the highest rates seen in August 2020 for both IgG (57%) and IgM (64%) antibodies. The infection rate decreased between August and October 2020 (Bangladesh, World Health Organization), and decreasing seropositivity was observed over these months. By October 2020, approximately 36% of the population of Bangladesh were seropositive for SARS-CoV-2 IgG antibodies and 43% were seropositive for IgM antibodies.
Figure 1.
Monthly distribution of the seroprevalence (weighted) of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) nationally between April and October 2020. X-axis shows the month and Y-axis shows the rate of seropositivity of SAR-CoV-2 antibodies. The yellow and blue lines show the kinetics of SARS-CoV-2 antibody responses for immunoglobulin G (IgG) and IgM, respectively. The percentage seropositivity for each fortnight has been shown at each time point for each isotype of SARS-CoV-2 antibodies.
Frequency of SARS-CoV-2 antibodies in sequential specimens in individuals exposed in a household
In a subset of participants (n=252) of PCR-positive cases of COVID-19 and their household members in Dhaka, antibody responses were evaluated on days 1, 14 and 28 after enrolment. Seventy-eight percent of these participants were seropositive for SARS-CoV-2 IgG antibodies and 60% of the participants were seropositive for SARS-CoV-2 IgM antibodies by day 28 (Table 4). The GM levels of antibody responses for IgG (GM 852 ng/mL) and IgM (GM 538 ng/mL) also increased significantly by day 28 compared with day 1 (Table 4).
Table 4.
Frequency of antibodies in reverse transcription polymerase chain reaction (RT-PCR)-positive participants and their household members from Dhaka at three different time points.
| Seropositivity, n (%, 95% CI) | ||
|---|---|---|
| IgG | IgM | |
| Day 1 (n=252) | 97 (38, 32–44) | 93 (37, 31–43) |
| Day 14 (n=202) | 145 (72, 65–78)a | 113 (56, 49–63)a |
| Day 28 (n=166) | 130 (78, 71–84)a | 100 (60, 52–67)a |
| Geometric mean concentration (95% CI), ng/mL | ||
| Day 1 | 204 (151–274) | 291 (247–345) |
| Day 14 | 592 (460–762)a | 483 (410–568)a |
| Day 28 | 852 (644–1128)a | 538 (446–650)a |
CI, confidence interval; Ig, immunoglobulin.
Significantly higher seropositivity as well as concentrations of both IgG and IgM antibodies found at day 14 and day 28 after enrolment compared with day 1 at enrolment in the severe acute respiratory syndrome coronavirus-2 RT-PCR-positive participants and their household members.
Discussion
There is a critical need for serological surveillance of SARS-CoV-2 to estimate cumulative prevalence, incidence and community distribution in Bangladesh. The preliminary seroprevalence results of the current study provide an important benchmark to assess the state of the COVID-19 epidemic. By assessing the presence of SARS-CoV-2 IgG and IgM antibodies measured in this study, it is estimated that the majority of the population have now been exposed to the virus in Bangladesh.
Transmission of COVID-19 infection can depend on human factors including duration of exposure, proximity to an infected individual and use of personal protective equipment (Park et al., 2020). Despite the high seroprevalence noted nationwide in Bangladesh, a substantial second wave of COVID-19 infection occurred in July 2021. This suggests that antibody responses to the earlier wave were not sufficient to protect the population against the second wave. Most patients were infected by the UK variant (alpha; B.1.1.7) in the 2020 wave, and the South African variant (beta; B.1.351) predominated at the beginning of 2021. Since then, the delta variant (B.1.617.2) and the omicron variant (B.1.1.529) have also been detected in Bangladesh.
Understanding the factors contributing to population immunity is a research priority, including factors enabling transmission in places with a high cumulative incidence, particularly in low to medium income countries. Duration of immunity, the impact of different vaccines on transmission and herd immunity, the role of emerging variants, and vaccine breakthrough are all key areas that need to be understood for control of the COVID-19 pandemic. Herd immunity (Clemente-Suárez et al., 2020) is mathematically related to the propagation and transmission dynamics of the pathogen (Fresnadillo-Martínez et al., 2013; Herrmann and Schwartz, 2020), and the number of individuals in a population susceptible to infection (Fresnadillo-Martínez et al., 2013). Herd immunity is expected to be obtained only when approximately 70% of the population has been infected or vaccinated (Clemente-Suárez et al., 2020). Seroprevalence analyses in large populations and over time may be extremely important tools in understanding community transmission and informing vaccine design and rollout.
This study has some important limitations. The ELISA assay was not validated against a plaque reduction neutralization assay, which may provide important information about protection of an individual person. ELISA antibody assays have been found to correlate with neutralizing antibody (NAb) measurements (Holzmann et al., 1996). However, the correlation between the anti-RBD IgG antibody assay and NAb remains unclear as there are contradictory reports on their association (Billon-Denis et al., 2021) in patients with SARS-CoV-2 infection. It has been proposed that other host factors (including age, gender and clinical severity) may also be drivers of the immune response, including antibody and neutralizing antibody responses. It has been suggested that Nab competes with angiotensin-converting enzyme 2 (ACE2) receptor binding of the virus, and so may be a better predictor for virus-neutralizing antibody potency than binding affinity (Ju et al., 2020). Hence, blocking the interaction between RBD and ACE2 may be a useful surrogate for neutralization. However, in the current seroprevalence study, the objective was to analyse population-level seroprevalence in different urban and rural areas in Bangladesh. The authors were not able to analyse cross-reactivity against other coronaviruses due to unavailability of these antigens. The estimates presented here may be affected by waning antibody levels over time, but the authors were not able to track the cumulative infection rates during the study period, which could also impact on the analysis. However, it should be noted that vaccination had not been initiated at the time of the study, and therefore was not likely to influence the seroprevalence estimates. The results show high seroprevalence of SARS-CoV-2 early on in the pandemic (April–October 2020), suggesting protection in the population. However, the authors attempted to analyse data from selected areas in Bangladesh, and the data need to be extrapolated carefully; the seroprevalence levels should not be generalized to Bangladesh as a whole, but rather to the population being studied. In summary, this study found a significant increase in the seroprevalence of SARS-CoV-2 antibodies among the Bangladeshi population during the first wave of COVID-19 between April and October 2020, with 45% positive for IgG antibodies very soon after SARS-CoV-2 was detected in Bangladesh (March 2020). The increase in seropositivity paralleled a substantial rise in the number of PCR-confirmed SARS-CoV-2 infections in Bangladesh (Management Information System Directorate General of Health Services, Bangladesh, 2021). Moreover, throughout the study period, seropositivity of IgM antibodies against SARS-CoV-2 was also observed. As IgM is the primary antibody detected during the acute infection period (Akter et al., 2022), this also indicated the increase in the number of new SARS-CoV-2 infections in the study population. Substantial differences in seroprevalence were observed between slum and non-slum areas of Dhaka, and between urban and rural areas outside Dhaka. Similarly, substantial differences in seroprevalence were found between populations of low and high socio-economic status in Bangladesh (Sattar et al., submitted). There is continuous interest of public health experts to understand and contextualize key drivers of COVID transmission, anticipate future risks, and adopt preventive measures including vaccination to address these problems.
Declaration of Competing Interest
None declared.
Acknowledgments
Acknowledgements
The authors wish to thank USAID for its support towards its research. ICDDR,B is also grateful to the Governments of Bangladesh, Canada, Sweden and the UK for providing core/unrestricted support. In addition, the authors wish to thank Aaron G. Schmidt, Jared Feldman, Blake M. Hauser and Timothy M. Caradonna from Harvard Medical School for providing the SARS-CoV-2 RBD protein and RBD monoclonal antibody. Finally, the authors wish to thank all field and laboratory staff for collection of blood from the community and processing the blood in the laboratory.
Author contributions
AN, FQ, SB, TRB, TS and MR designed, managed and supervised the study. SAS, KCB, ARB, SRT and KH helped to collect the specimens. TRB, MA, AA, FK, SIAR and JF performed the laboratory work and immunological analyses. TRB, MA and NC analysed the data. FQ, TRB, SB, MSF and SEA provided key reagents. FQ, TRB, MA, ASMA, MR, SB, MSF, SBC, ETR, JBH, RCC and RCL reviewed and planned the manuscript. All authors contributed to the interpretation of results and critical review and revision of the manuscript, and have approved the final version.
Funding
This research was supported by USAID (CA#72038819RFA00009) under Alliance for Combating Tuberculosis, Global Emerging Leader Award (K43TW010362), R01 AI130378 of the National Institutes of Health and ICDDR,B.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijregi.2022.01.013.
Appendix. Supplementary materials
References
- Akter A, Ahmed T, Tauheed I, Akhtar M, Rahman SIA, Khaton F, et al. Disease characteristics and serological responses in patients with differing severity of COVID-19 infection: a longitudinal cohort study in Dhaka, Bangladesh. PLoS Negl Trop Dis. 2022;16 doi: 10.1371/journal.pntd.0010102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RM, Heesterbeek H, Klinkenberg D, Hollingsworth TD. How will country-based mitigation measures influence the course of the COVID-19 epidemic? Lancet. 2020;395:931–934. doi: 10.1016/S0140-6736(20)30567-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billon-Denis E, Ferrier-Rembert A, Garnier A, Cheutin L, Vigne C, Tessier E, et al. Differential serological and neutralizing antibody dynamics after an infection by a single SARS-CoV-2 strain. Infection. 2021;49:781–783. doi: 10.1007/s15010-020-01556-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemente-Suárez VJ, Hormeño-Holgado A, Jiménez M, Benitez-Agudelo JC, Navarro-Jiménez E, Perez-Palencia N, et al. Dynamics of population immunity due to the herd effect in the COVID-19 pandemic. Vaccines. 2020;8:236. doi: 10.3390/vaccines8020236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontanet A, Tondeur L, Madec Y, Grant R, Besombes C, Jolly N, et al. Cluster of COVID-19 in Northern France: a retrospective closed cohort study. medRxiv 2020. 04.18.20071134; doi: 10.1101/2020.04.18.20071134. [DOI]
- Fresnadillo-Martínez MJ, García-Sánchez E, García-Merino E, del Rey ÁM, García-Sánchez JE. [Mathematical modelling of the propagation of infectious diseases: where we came from, and where we are going] Rev Esp Quimioter. 2013;26:81–91. [PubMed] [Google Scholar]
- GARDAWORLD. Bangladesh: first cases of COVID-19 confirmed March 8; 2020. Available at: https://www.garda.com/crisis24/news-alerts/320606/bangladesh-first-cases-of-covid-19-confirmed-march-8 (accessed 20 June 2021).
- Guo YR, Cao QD, Hong ZS, Tan YY, Chen SD, Jin HJ, et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak – an update on the status. Milit Med Res. 2020;7:11. doi: 10.1186/s40779-020-00240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann HA, Schwartz J-M. Using network science to propose strategies for effectively dealing with pandemics: the COVID-19 example. medRxiv 2020:2020.04.02.20050468.
- Holzmann H, Kundi M, Stiasny K, Clement J, McKenna P, Kunz C, et al. Correlation between ELISA, hemagglutination inhibition, and neutralization tests after vaccination against tick-borne encephalitis. J Med Virol. 1996;48:102–107. doi: 10.1002/(SICI)1096-9071(199601)48:1<102::AID-JMV16>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Iyer AS, Jones FK, Nodoushani A, Kelly M, Becker M, Slater D, et al. Persistence and decay of human antibody responses to the receptor binding domain of SARS-CoV-2 spike protein in COVID-19 patients. Sci Immunol. 2020;5 doi: 10.1126/sciimmunol.abe0367. eabe0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju B, Zhang Q, Ge J, Wang R, Sun J, Ge X, et al. Human neutralizing antibodies elicited by SARS-CoV-2 infection. Nature. 2020;584:115–119. doi: 10.1038/s41586-020-2380-z. [DOI] [PubMed] [Google Scholar]
- Management Information System Directorate General of Health Services. Coronavirus COVID-19 dashboard. 2021. Available at: http://103.247.238.92/webportal/pages/covid19.php.
- Management Information System Directorate General of Health Services, Bangladesh. Coronavirus COVID-19 dashboard. 2021. Available at: http://103.247.238.92/webportal/pages/covid19.php (accessed 17 June 2021).
- Nazneen ASR, Rahman M, Rahman M, Qadri F, Rimi NA, Hossain MK, et al. Prevalence of COVID-19 in Bangladesh, April to October 2020 – a cross-sectional study. IJID Reg. 2021;1:92–99. doi: 10.1016/j.ijregi.2021.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park YJ, Choe YJ, Park O, Park SY, Kim YM, Kim J, et al. Contact tracing during coronavirus disease outbreak, South Korea, 2020. Emerg Infect Dis. 2020;26:2465–2468. doi: 10.3201/eid2610.201315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman M, Shirin T, Rahman S, Rahman MM, Hossain ME, Khan MH, et al. The emergence of SARS-CoV-2 variants in Dhaka city, Bangladesh. Transbound Emerg Dis. 2021;68:3000–3001. doi: 10.1111/tbed.14203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirin T, Bhuiyan TR, Charles RC, Amin S, Bhuiyan I, Kawser Z, et al. Antibody responses after COVID-19 infection in patients who are mildly symptomatic or asymptomatic in Bangladesh. Int J Infect Dis. 2020;101:220–225. doi: 10.1016/j.ijid.2020.09.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton D, Fuchs K, D'Alton M, Goffman D. Universal screening for SARS-CoV-2 in women admitted for delivery. N Engl J Med. 2020;382:2163–2164. doi: 10.1056/NEJMc2009316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . WHO; Geneva: 2020. Coronavirus disease (COVID-19) pandemic.https://www.euro.who.int/en/health-topics/health-emergencies/coronavirus-covid-19/novel-coronavirus-2019-ncov Available at. (accessed 17 June 2021) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.