Abstract
Brazil has the highest SARS-CoV-2 case-fatality rate in pregnant women in the Americas. In this study, clinical and virological findings of five mildly symptomatic pregnant women and their infected fetuses/newborns treated at a referral hospital for COVID19-pregnant women in Midwestern Brazil are reported. Mother and fetal samples were tested by RT-qPCR, ECLIA and Illumina MiSeq sequencing. From the five cases, one resulted in spontaneous abortion, one was stillborn, two were preterm births and one full-term birth. Maternal and fetal placenta, newborn and stillborn secretions were SARS-CoV-2+; one neonate developed ground-glass opacities in his lungs. One neonate's umbilical cord was IgG+ and all were IgM negative upon hospital discharge. Genomes recovered from two placentas belong to the B.1.1.28 and B.1.1.33 lineages and present nonsynonymous mutations associated with virus fitness and infectivity; other not frequently reported mutations (B.1.1.33: NSP3 V2090G, M A2S and ORF3ab S253P and Y264N; B.1.1.28: NSP3 E995D, NSP12 R240K, NSP14H1897Y and in ORF7b V21F) were found in proteins involved in viral replication, viral induction of apoptosis, viral interference on interferon and on NF-Κβ pathways. Phylogeny indicates the south of Brazil as the possible origin of these lineages circulating in Mato Grosso State. These findings contribute to describe SARS-CoV-2 infection and outcomes in pregnant women and their fetuses, at any stage of gestation and even in mild symptomatic cases.
Keywords: Coronavirus, Neonatal infection, Vertical transmission, Clinical virology, Molecular virology
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is an enveloped 29,740 bp ssRNA + Sarbecovirus encoding 16 nonstructural proteins (NSP), four structural and six accessory proteins from Betacoronavirus genus, Orthocoronavirinae subfamily, Coronaviridae family. This agent most likely crossed host range barriers from bats and reached an unknown vertebrate reservoir and humans in late 2019 in China, emerging as a public health emergency of international concern [[1], [2], [3], [4], [5]]. Yet, SARS-CoV-2 origins remain to be elucidated.
Despite the exponential growth of SARS-CoV-2 infection in pregnant women, data regarding 2019 coronavirus disease (COVID19) effects on mother and fetus are still scarce, mostly based on IgM detection in the first hour of life, when all hospital control measures are taken to prevent peri- and postpartum transmissions. Therefore, SARS-CoV-2 vertical transmission has been considered unusual [[6], [7], [8], [9], [10]]. Overall, literature reviews indicate fetal demise occurs in approximately 0.7% of pregnant women, and a mean of 3.7% newborns from mothers testing positive for SARS-CoV-2 during pregnancy [11].
The number of SARS-CoV-2-infected pregnant women grew in parallel to the increasing number of COVID19 cases during the first wave of infections in Brazil, resulting in the hospitalization of 521 COVID19 pregnant women between February and May, 2020 [12]. Between January 2020 and May 2021, Brazil reported 5931 SARS-CoV-2 infections in pregnant women, 428 deaths and, a 7.22% case-fatality rate, the highest lethality in America [13].
The state of Mato Grosso (MT), in the middle west of Brazil, registered more than 512,036 laboratory-confirmed infections, 13,163 deaths and an overall 377,8 mortality rate per 100 thousand inhabitants until August, 2021; 546 of these confirmed cases in pregnant women, with 32.19% admitted to an intensive care unit (ICU), 15.49% intubated, 39 deaths, representing an 8.88% death rate [14,15].
In Brazil, the virus was detected in the amniotic fluid and placenta of two out of five dead fetuses from SARS-CoV-2-positive pregnant women [16]. Spike protein was detected in the umbilical cord, lung and liver from an aborted fetus of a SARS-CoV-2 pregnant woman that developed severe placental thromboembolism [17]. Despite the availability of obstetric data obtained from pregnant women in Brazil, few maternal–fetal data can be retrieved from literature until the present.
Viruses that have recently crossed host-range barriers are more prone to mutations to adapt to the tissues of the new host. Emerging SARS-CoV-2 variants carrying several amino acid changes, mainly in Spike protein, leading to increased viral load, transmissibility, and immune evasion phenotype have been reported worldwide since late 2020 [[18], [19], [20], [21]].
The significant numbers of COVID-19 infection in pregnant women [15] and, the probable registers of vertical transmission, prompted us to describe clinical, virological and genomic aspects of SARS-CoV-2 detected in maternal–fetal samples during the first wave of infection in the middle west of Brazil.
1. Material and methods
1.1. Sampling and ethical aspects
Patients were sampled by the epidemiological surveillance nucleus during their clinical treatment at the Julio Muller University Hospital (HUJM), local reference for severe COVID19 patients, from June to July 2020.
Childbirth occurred in surgery rooms equipped with exhaust fan and HEPA filter. Cesarean-section newborns had intact membranes; all babies received medical care at least two meters apart from their mothers, without any physical contact. Biological samples were collected in the delivery room or in the first hour of life at the neonatal intensive care unit (NICU), under strict infection control procedures. Additional laboratory results and clinical data were recovered from the Hospital's archives.
This study was approved by HUJM ethics committee (CAAE: 44693621.0.0000.5541).
1.2. Laboratorial and imaging exams
Electrochemiluminescence (ECLIA; Abbott Laboratories, Chicago, Illinois, Brazil) was used for anti-SARS-CoV-2 IgM and IgG detection. Standard reference values for total antibodies were the following: non-reagent: <1.0 UA/mL, reagent: ≥1,0 UA/mL; and for IgM or IgG detection: non-reagent: <0.9 UA/mL, undetermined: 0.9 to 1.0 UA/mL, reagent: ≥1.1 UA/mL. COVID-19 IgG/IgM ECO immunochromatographic test was used for two mothers (Eco Diagnóstica, Nova Lima-MG, Brazil).
D-dimer degradation determined by quantitative fluorescent immunoassay (Celer Biotecnologia SA, Belo Horizonte-MG, Brazil) had reference values of <0,5 mg/L (normal) and >0.5 mg/L (increased). C Reactive Protein (CRP) quantified by dry chemistry (Vitros Chemistry System 250, Raritan–NJ, USA) presented reference values considered normal when <10 mg/L and increased when ≥10 mg/L.
Chest computed tomography (Chest CT) scans with the patient in supine position, following standard guideline procedures (Philips Diamond Select Brilliance CT of 16 channels; Philips Healthcare, Amsterdam, Netherlands), were analyzed according to the Ministry of Health criteria for SARS-CoV-2 infection.
1.3. SARS-CoV-2 real time RT-PCR (RT-qPCR) testing of biological samples
Nucleic acid extracted from nasopharyngeal swabs (NPS), bronchoalveolar lavage (BAL), newborn gastric aspiration (GA) (0.2 mL), placenta sections (∼50 mg) using MagMAX™ Viral/Pathogen Nucleic Acid Isolation Kit (Applied Biosystems) was immediately tested with TaqPath COVID-19 CE-IVD RT-qPCR kit (Applied Biosystems). Positive control (50 copies/uL), bacteriophage MS2 Phage extraction control and no template control (DEPC water) were included in all reactions. Mean cycle threshold (CT) values were obtained from CT values of each amplified region of the virus (ORF1ab, Spike and Nucleocapsid genes) with CT < 37 as cut off value.
1.4. Illumina MiSeq sequencing and bioinformatics
Viral RNA extracted with QIAamp Viral RNA Mini Kit (QIAGEN, Hilden, Germany) was reverse transcribed (Superscript IV first-strand synthesis kit; Invitrogen, San Diego–CA, USA), amplified [19,20], purified (Agencourt AMPure XP beads, Beckman Coulter, Pasadena–CA, USA), quantified (Qubit dsDNA HS Assay kit, Invitrogen, San Diego, CA, USA) and subjected to short-read DNA libraries (Nextera XT DNA Sample Preparation kit; Illumina, San Diego, CA, United States) synthesis. After quality-testing on Agilent 2100 Bioanalyzer (Agilent, Santa Clara, CA, United States), samples were subjected to paired-end sequencing (Illumina MiSeq; San Diego, CA, United States).
Adapters and low-quality readings (>Q30) were filtered from sequences, which were mapped back to SARS-CoV-2 hCoV-19/Wuhan/WIV04/2019 reference strain, aligned to obtain the consensus (CLC Genomics Workbench 20.0) and classified using Pangolin (pangolin.cog-uk.io; v.3.1.14) and Nextclade (https://clades.nextstrain.org/; v.1.5.4). Mutation profile identified on NEXTCLADE was compared with the most frequent lineage mutations using outbreak.info Lineage Reports [22].
Spike tridimensional images were obtained from CoVsurver (A∗STAR Bioinformatics Institute (BII), Singapore). Genomic sequences were deposited at GISAID (Global Initiative on Sharing Avian Influenza Data) database EpiCoV (https://www.gisaid.org/) (access numbers EPI_ISL_1550878; EPI_ISL_1550388) and at GenBank (NCBI) (access numbers MW962307, MW962308).
Alignment of Brazilian SARS-CoV-2 complete genomes coding sequences obtained from GenBank (NCBI) and from GISAID database (Supplementary Tables 1 and 2), representing B.1.1.28 and B.1.1.33 lineages circulating in Brazil between 2020 and 2021, was performed on a multiple alignment sequence program (MAFFT v.7.490) [23]. Alignment was edited on AlliView v.1.27 [24]. The maximum clade credibility tree with best fit General Time Reversible (GTR) nucleotide substitution model, effective sample size of 890.6, was constructed with Bayesian Evolutionary Analysis Sampling Tree (BEAST2 v.2.6.6; http://beast2.org), with strict molecular clock (5.621E-4), coalescent Bayesian skyline inference (−115.09). The tree was processed on TreeAnnotator (BEAST package v.2.6.6) with 10% Burnin and edited on FigTree (v1.4.4, http://tree.bio.ed.ac.uk/software/figtree) to include time scale and posterior values.
2. Results
2.1. Case studies
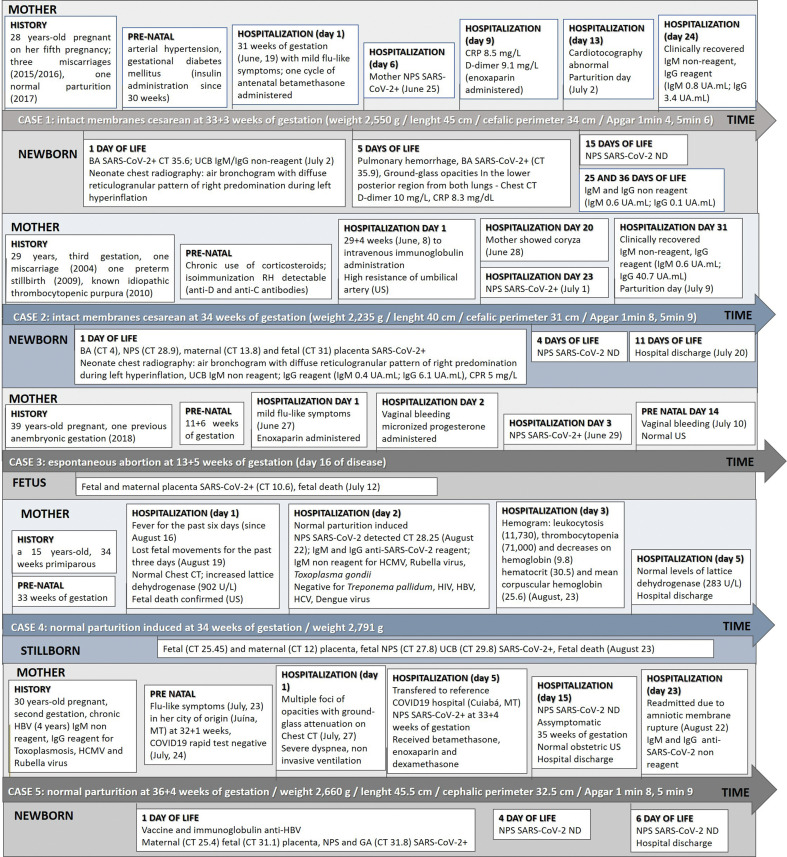
During June and August 2020, 16 mother/baby binomials from SARS-CoV-2 pregnant women, confirmed infected through NPS RT-qPCR, were investigated. The virus was detected in the maternal–fetal interface of five cases. The evolution of the disease during pregnancy and after delivery of infected women (upper boxes) and their fetuses/newborns (lower boxes) is shown on Fig. 1 .
Fig. 1.
Clinical and laboratorial evolution of five COVID19+ mothers (upper boxes) and their neonates/fetus (lower boxes) attended at a reference University Hospital from June to August, 2020. NPS: nasopharyngeal swab; Chest TC: chest computerized tomography; w: weeks; g: grams; cm: centimeters; US: ultrasonography; BA: Bronchoalveolar aspirate; GA: gastric aspirate; UCB: umbilical cord blood; ND: not detectable; HCMV: Human Cytomegalovirus; HBV: B hepatitis virus; HCV: C hepatitis virus; HIV: human immunodeficiency virus; US: ultrasonography CT value is indicated when available.
Mother 1 was hospitalized with clinical suspicion of COVID19, showing coryza, headache, hyposmia and ageusia when tested SARS-CoV-2+. Fetal distress led to a preterm cesarean section at 33+3 weeks of gestation, 13 days after symptoms had begun. The mother presented hyposmia on the day the C-section was performed. The premature female baby required early clamping of the umbilical cord for neonatal cardiopulmonary resuscitation and was then transferred to the isolation NICU in a transport incubator. She remained in mechanical ventilation until the 11th day of life, with noninvasive ventilation for two additional days. After clinical diagnosis of SARS-CoV-2 infection on the delivery day, the chest CT on the fifth day of life showed a viral pattern of infection in the lungs of neonate. Clinical evolution leaded to NICU discharge when the baby had two consecutive negative RT-qPCRs, after 18 days of life, breathing environmental air and breastfeeding.
The second pregnant woman had been hospitalized for 20 days due to detectable isoimmunization against fetal Rh factor. She was sharing the room with another pregnant woman that tested positive after four days of hospitalization, when she developed coryza. The mother's NPS resulted positive on the following day. Ever since hospitalization, ultrasonography scans indicated high resistance of the umbilical artery leading to intact membrane C-section on the 34th week, 10 days after SARS-CoV-2 diagnosis. The umbilical cord was clamped after 1 min; male newborn presented mild respiratory distress in the first hour of life and was transferred to the isolation room in the NICU. SARS-CoV-2 genetic material was detected on fetal and maternal sides of placenta and in the newborn secretions; IgG was detected in umbilical cord blood (UCB) and in the mother's serum on delivery day. Respiratory distress was stabilized within 6 h of life; no clinical signs of COVID19 were detected in the neonate. Newborn coombs direct test was positive; intravenous immunoglobulin and phototherapy improved neonatal condition, leading to hospital discharge on July 17 (8 days of life), when was breastfeeding and both mother and newborn were asymptomatic to SARS-CoV-2.
The third pregnant woman sought medical attention with mild flu-like symptoms characterized by a sore throat, myalgia, anosmia, headache, no fever, after having traveled during the weekend to another state. She received a prescribed anticoagulant. On the following day, she developed a vaginal bleeding, containing with micronized progesterone. This patient tested positive to SARS-CoV-2 on the following day, at 12+1 weeks of gestation. Fully recovered from respiratory symptoms 14 days later, at 13+5 weeks of gestation, another vaginal bleeding occurred, presenting normal morphological US. Two days later, 16 days after seeking medical care for the first time, she had an intense abdominal pain and expelled the fetus and placenta completely. SARS-CoV-2 was detected in placenta samples.
The fourth pregnant woman reported sudden onset of fever lasting for six days and loss of fetal movements during three days previous to hospital admission. Chest CT on admission showed absence of any pattern related to viral infection, despite the mother's infection was confirmed in laboratory exams on the next day. Fetal death was confirmed through US; labor for a normal delivery was medically induced 24 h after admission. SARS-CoV-2 was detected in stillborn NPS, UCB, fetal and maternal sides of placenta.
The fifth pregnant woman was admitted in her hometown with COVID19 suspicion, needing noninvasive ventilation due to dyspnea. Flu-like symptoms had lasted for five days and she tested negative for SARS-CoV-2 on antigen rapid test. However, chest CT revealed multiple focuses of opacities with ground-glass attenuation in the lungs. The patient was transferred to the state capital five days after hospitalization, presenting with severe dyspnea ten days after symptoms onset and at 33+4 weeks of gestation. She tested positive in the RT-qPCR on this day. Betamethasone was administered to accelerate pulmonary maturation on the fetus and the patient was treated with enoxaparin and dexamethasone. Her condition improved and she was medically discharged after 19 days of hospitalization. On August 22, 36+4 weeks of gestation, 30 days after clinical onset, patient was readmitted due to amniotic membrane rupture. After normal delivery, the newborn was taken to an isolation room in a transport incubator. SARS-CoV-2 was detected by RT-qPCR on maternal and fetal placenta, neonate NPS and GA from first hour of life. The baby was breastfed by mother, who wore protective mask. After two consecutive NPS negative results, on the fourth day of life, the newborn was discharged from hospital.
3.2. Phylogenetic analysis and mutation profile of SARS-CoV-2 sequences
SARS-CoV-2 sequences were retrieved from cases #3 (hCoV-19/Brazil/MT21774/2020) and #4 (hCoV-19/Brazil/MT21770/2020). Library construction and viral isolation did not progress from the other three cases.
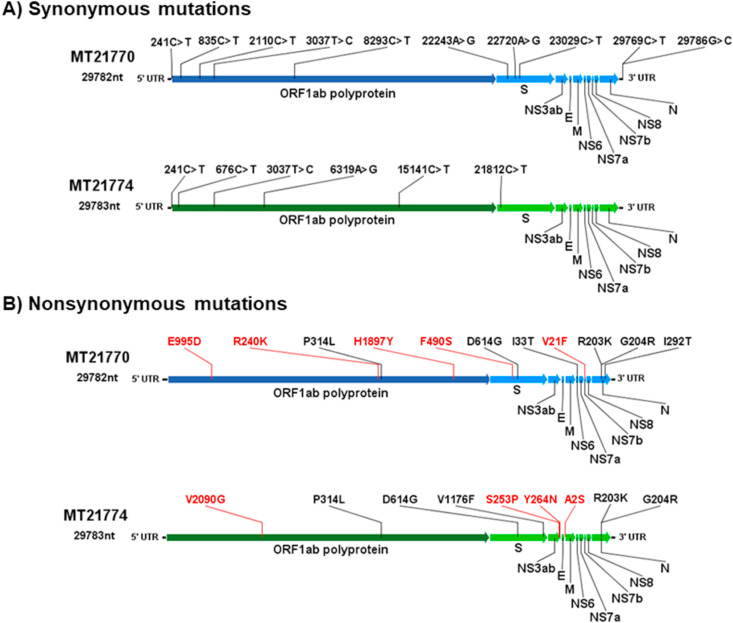
These two genome sequences belong to lineages B.1.1.28 clade 1 and B.1.1.33 clade 2, respectively and share 99.84% and 99.76% amino acid (aa) identity, respectively, with reference sequence hCoV-19/Wuhan/WIV04/2019 and 99.9% aa similarity with other two SARS-CoV-2 sequences obtained from placenta in the same time period (USA/Connecticut-Yale-050 MT886396 from lineage B.1.1 and human/BRA/Rio-P1/2020 MT939654.1 from lineage B.1.1.33). Sequences from this study also share four nonsynonymous mutations, P314L (Nsp12), D614G (Spike), R203K and G204R (Nucleocapsid), with these placenta sequences, in addition to two synonymous mutations (214C > T, 3037C > T).
Additional nucleotide and aa changes leading to synonymous and nonsynonymous mutations, respectively, compared with reference sequence hCoV-19/Wuhan/WIV04/2019 are presented in Fig. 2 . The characteristic nonsynonymous mutations of both lineages present in these sequences are identified in their respective genome positions in black. Changes not reported in databases as characteristic of these lineages are marked in red.
Fig. 2.
Representation of synonymous (panel A) and nonsynonymous (panel B) mutations found in each coding sequence, MT21770/2020 (lineage B.1.1.33) and MT21774/2020 (lineage B.1.1.28). Mutations marked in black are the characteristic lineage mutations reported to nexclade and outbreak.info, and those marked in red are the mutations found in these sequences not reported elsewhere related to these lineages.
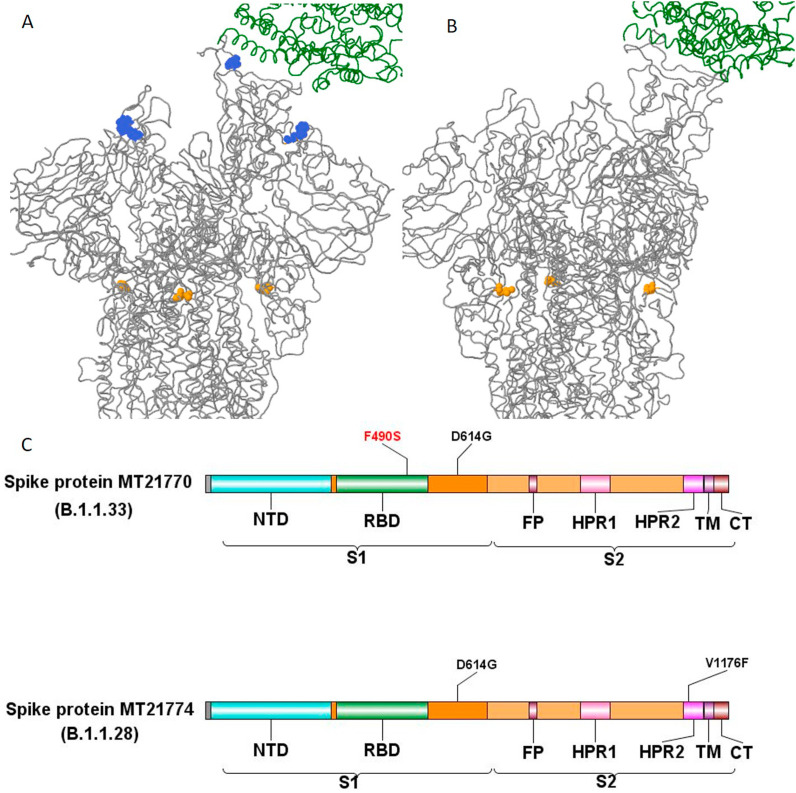
Spike protein has a S1 subunit (14–685 residues) comprised by the N-terminal extracellular domain (NTD; 13–305) and the receptor binding domain (RBD; 319–541); S2 subunit (686–1273 residues) contains the fusion peptide (FP; 788–806), heptapeptide repeat 1 (HPR1; 912–984), heptapeptide repeat 2 (HPR2; 1163–1212), transmembrane (TM; 1213–1236) and C-terminal domains (CTD; 1237–1273). In this study, the detected Spike aa changes were located in the S1 subunit (D614G) of both sequences, and in the RDB of MT21770/2020 (F490S) (Fig. 3 A and B). An additional aa change was also present in S2 subunit HRP2 region (V1176F) of MT21774/2020 (Fig. 3C).
Fig. 3.
Tridimensional structure of MT21770/2020 (A) and MT21774/2020 (B) Spike protein (grey) in complex with ACE2 receptor (green) showing the receptor binding domain (RBD) mutation F490S (blue) and the S1 mutation D614G (yellow). (C) Schematic representation of Spike protein pointing out the location of aa changes found in both sequences obtained in this study.
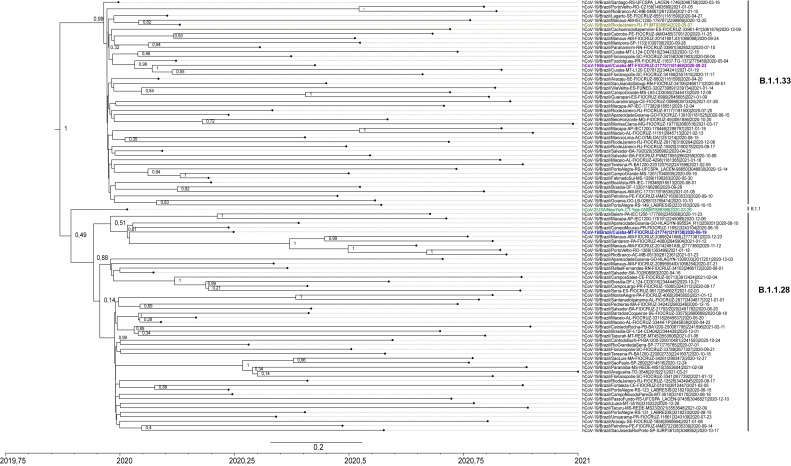
Phylogenetic tree showed two bigger clades (posterior value = 1) representing linages B.1.1.33 and B.1.1.28, placing the two sequences obtained in this study, MT21770/2020 and MT21774/2020, inside each lineage. Lineage B.1.1.33 showed an ancestral sequence from the state of Rio Grande do Sul (posterior value = 0.99). MT21770/2020 formed a small subclade with other sequences from states in the south of Brazil distant from the other sequence of this lineage obtained from placenta in Rio de Janeiro in May, 2020. MT21770/2020 sequence may have originated from sequences obtained in Paraná (posterior value = 0.96), having descendants in Cuiabá, capital of Mato Grosso, and Florianopolis in the state of Santa Catarina (posterior value = 1).
Phylogeny placed the placenta sequence belonging to B.1.1 lineage from USA (March 2020) as an older sequence (posterior value = 0.49) in separated clades from lineages B.1.1.28 and B.1.1.33 sequences obtained in Brazil along the entire pandemic.
MT21774/2020 fits in a bigger subclade of B.1.1.28 sequences with posterior value = 0.88, closer to sequences obtained from the states of Paraná and Goiás, and seems to branch more recent sequences from patients from several states in the north of Brazil: Amazonas, Pará, Rondônia, Acre and, from Goiás in the middle west (Fig. 4 ).
Fig. 4.
Maximum clade credibility tree with node posterior values of SARS-CoV-2 sequences obtained from placenta in Middle west of Brazil, with lineages B.1.1.28 and B.1.1.33 sequences reported in Brazilian States between 2020 and 2021. Sequences obtained in this study (21770 from B.1.1.33 and 21774 from B.1.1.28 lineages) are marked in purple and in blue, respectively. Sequences obtained from placenta in Rio de Janeiro (B.1.1.33) and New York (B.1.1) are marked in yellow and green, respectively.
3. Discussion
Initial descriptions of SARS-CoV-2 adverse outcomes during pregnancy did not mention SARS-CoV-2 vertical transmission [25]. Despite the reports of Spike protein immunostaining in placenta and fetal tissues [16,17], few documented cases and genomic data from SARS-CoV-2 maternal–fetal samples are currently available in databases. In this study clinical, laboratorial, and virological data of five mild antenatally diagnosed COVID19 cases in pregnant women from a reference hospital in the middle west of Brazil are reported; two were hospitalized to treat other medical conditions, three were already clinically recovered when their gestational outcome occurred, four had preterm labor, two had intact membrane C-sections, two had normal delivery of a stillborn and an asymptomatic newborn at 36 weeks of gestation and one had a spontaneous abortion 14 days after the mother's infection was confirmed, when she was already asymptomatic. Maternal and fetal placenta, GA, BA or NPS collected from stillborn baby and neonates in their first hour of life under hospital control measures, suggest the presence of the virus in the intrauterine environment (Fig. 1). Data show evidence of SARS-CoV-2 genome presence in the maternal–fetal interface, and genomic characteristics of two sequences recovered from placenta.
Narang et al. [26] showed that the most frequent gestational age at delivery of viable pregnancies in infected women was ≥36 weeks (96/148 pregnancies) and the neonates' outcome was 99.31% live births and 0.68% stillbirths. One other report shows five stillborn cases due to the intense placental inflammatory reaction caused by the SARS-CoV-2 infection, discarding other possible reasons that may cause fetal death [16]. Another study shows a case of fetal death after 14 days of mother's infection with coagulation disorders and showing that SARS-CoV-2 may cause thromboembolic lesions in the placental tissue leading to poor vascular perfusion [17] and thus may cause syncytiotrophoblast necrosis as shown in other studies [27].
Syncytiotrophoblasts and cytotrophoblasts of the chorionic villi express acetylcholine (ACE2) receptor 2 and low levels of transmembrane serine protease 2 (TMPRSS2). Focal infection of these surface cells has been found in the placenta from SARS-CoV-2 positive mothers with several non-characteristic histopathological findings [28,29].
Maternal viremia may allow the infection of the outer layer of the chorionic villi's multinucleated cells, consistent with the higher viral load found in maternal placenta rather than in fetal placenta of COVID19 mothers. Findings of intervillous macrophage invasion (intervillositis), lymphohistiocytic villitis and fibrin deposition in intervillous spaces of placenta are consistent with the histological findings in the lung of SARS-CoV-2 patients. However, these histopathological changes have not been consistently reported in placentas from SARS-CoV-2 positive mothers [28,30]. Other frequently reported histological changes in these patients are maternal and fetal placenta malperfusion and syncytiotrophoblast necrosis [28]. These cases show that the presence of the virus in the maternal–fetal interface may compromise pregnancy maintenance leading to fetal death, miscarriage, or preterm labor.
Congenitally transmitted viruses, such as Human Cytomegalovirus, Human Immunodeficiency virus and Zika virus infect syncytiotrophoblasts without causing characteristic histological abnormalities. Syncytiotrophoblasts connect maternal and fetal circulation in the placenta and are involved in IgG translocation from mother to fetus circulation during pregnancy. In a similar fashion, it has been proposed that SARS-CoV-2 may infect the fetus by antibody-dependent transcytosis, mediated by the neonatal Fc receptor (FcRn), leading to the presence of viral particles in the amniotic fluid, which could be inhaled or ingested by the fetus [28,31].
SARS-CoV-2 vertical infection was first reported after IgM detection in two neonates with negative NPS on the first day of life, born from COVID19 symptomatic mothers [10]. Retrospective data revealed additional 3/33 neonates with positive NPS at two days of life born from COVID-19 pregnant women in Wuhan, China. An asymptomatic IgM and IgG reagent neonate, NPS negative at 2 h of life was born from a COVID19 mother 25 days after she presented nasal congestion, respiratory difficulties and fever [6]. Since IgM is not translocated through placenta, these cases were indicative of recent fetal exposure to the virus.
Since there is not enough information on vertical transmission, there are some proposals to classify the infection in pregnant women, fetuses and neonates or defining the transmissions as intrauterine, intrapartum, or superficial exposure to SARS-CoV-2 [31,32]. As established in the classification system of Shah et al. [32], all cases in this study are considered as confirmed maternal infections during pregnancy because SARS-CoV-2 was detected in each case.
For case 1, the newborn had a clinical evolution consistent of infection. The BA tested positive for SARS-CoV-2 in the first and fifth day of life. Additionally, the chest CT images confirmed a characteristic viral-pattern infection and need for mechanical ventilation. This was the first neonate detected positive after being born from intact membrane C-section, with all hospital control measures to prevent horizontal transmission. However, the baby maintained negative serology (IgM/IgG) on 36th day of life, emphasizing that these tests alone underestimate the diagnosis of intrauterine infection, reinforcing the importance of RT-qPCR and other laboratorial tests to aid diagnosis and monitor clinical evolution.
The live born neonate in case 2 may be classified as confirmed congenital infection since SARS-CoV-2 was detected in placental tissue and in NPS sampled after intact membrane C-section [32]. This newborn was also reagent to anti-SARS-CoV-2 IgG in the umbilical cord blood collected during delivery, indicating he probably received these antibodies from his recovered mother by passive transfer.
The stillborn and the spontaneous abortion cases may be classified as confirmed congenital infection because SARS-CoV-2 was detected in placental tissue [32]. The miscarriage case occurred in the first trimester of pregnancy. The virus was replicating in significant levels in placenta when abortion occurred, despite the fact that the mother was already clinically recovered from respiratory disease. In the stillborn case, the mother sought medical care showing respiratory disease that had lasted for six days and loss of fetal movements for three days. SARS-CoV-2 was detected in the stillborn's respiratory secretion, umbilical cord blood, and in the maternal and fetal placenta. The viral genome was directly sequenced from RNA isolated from the placenta of these two cases (3 and 4).
The mother from case five had already recovered from COVID19 when labor started, however, the live born neonate may be classified as a congenital infection since the virus was detected in NPS at birth and in placental tissue, even though no clinical manifestations were present in the newborn.
Two of the three newborns in this study did not present COVID19-related symptoms. However, the newborn in case one, presented respiratory distress which needed mechanical ventilation for eleven days. Newborns may not present symptoms at the time of birth, however, they may develop clinical signs a few days later such as neurological manifestations [8] or respiratory distress with ground glass opacities in the lungs [9].
The data obtained from these five cases reinforce the necessity to broaden SARS-CoV-2 investigation in every newborn of mothers with confirmed diagnosis at any stage of pregnancy, regardless of the presence or absence of clinical symptoms. They also reinforce the necessity to investigate the placenta during prenatal care, since histological alterations could lead to poor pregnancy outcomes such as mentioned above.
There is scarce genomic data on SARS-CoV-2 recovered from placenta in databases. Two sequences recovered from placenta samples in this study represent the most frequent lineages circulating in Brazil at the time these cases were detected.
According to outbreak.info [22], lineage B.1.1.33 has being detected so far in at least 27 countries all over the world and was first detected in Brazil on March 1, 2020, presenting a cumulative prevalence of 3%. It was first detected in the state of Mato Grosso on March 30, 2020 with cumulative prevalence of 6%. The MCC phylogenetic analysis showed that the B.1.1.33 sequences used in this study had an ancestral from the state of Rio Grande do Sul, having the first case of this lineage detected on March 12, 2020 [19,22]. Lineage B.1.1.28 has been detected in 46 countries and has a cumulative prevalence of 4% in Brazil, including Mato Grosso, identified for the first time in this state in April, 2020 [22].
Both sequences were allocated into clades along with other sequences of these lineages circulating in the south of Brazil. Sequence MT21774 was placed in proximity to sequences of lineage B.1.1.28 obtained in patients from states in the north of Brazil in subsequent months. The other two SARS-CoV-2 sequences obtained from placenta retrieved from GenBank had a high aa identity with our sequences, despite the fact that they were not grouped in the phylogeny. All four sequences have four nonsynonymous aa changes located on Spike, NSP12 and nucleocapsid proteins in common [28], which have already been reported in literature as being involved in virus fitness and increased transmissibility.
The aa change D614G in Spike S1 subunit locates outside the RBD binding site and, was found in both sequences of this study. It is well known that this aa change has been distributed in most of the first SARS-CoV-2 sequences obtained worldwide during 2020, and has been implicated in fitness advantage linked to increased viral titers and SARS-CoV-2 transmissibility [33].
F490S was identified in MT21770 sequence. It is a Spike S1 subunit mutation located in a critical neutralizing antibody recognition epitope on RBD (Fig. 2; Fig. 3). It is found in G, GR and GV groups of B1 lineages. It was first reported in England in April, 2020 (hCoV-19/England/BRIS-12321F/2020), and later on in other countries. Mutations at the RBD region of Spike protein are highly associated with virus fitness, evolution and immune evasion since this region is a major target for SARS-CoV-2 neutralizing antibodies [[34], [35], [36], [37]]. The V1176F aa change (MT21774 sequence) locate in the short S2 subunit, region involved in viral envelope fusion with cell membrane after ACE2-receptor binding, TMRPSS2 priming and endocytosis [[36], [37], [38]].
Mutations R203K/G204R in the Nucleocapsid gene were found in both sequences. These mutations increase the transmission and virulence of SAR-CoV-2. Experimental studies have shown increased viral fitness and replication in hamster cell lines and human airway tissue in vitro [39]. Nucleocapsid is the only coronavirus structural protein known to interact with the replicative complex (NSP12-NSP7-NSP8) and its interaction with NSP3 increases infectivity of coronaviruses. A critical polymorphic region of this protein is the serine–arginine region located in amino acids 183–206, exactly where the R203K and G204R aa changes locate [35,37].
Mutation P314L in NSP12 (RdRp) has also been linked to increased viral fitness. The presence of both aa changes, D614G in S protein and P314L in NSP12 has been associated with the fast SARS-CoV-2 dissemination worldwide in 2020 [35,37,38].
MT21770 sequence presents other nonsynonymous mutations besides the most frequent mutations of the B.1.1.33 lineage. These other aa changes are located in ORF1ab, specifically in NSP3 (E995D), NSP12 (R240K) and NSP14 (H1897Y) and in ORF7b (V21F), however, no other information about their relevance has been reported in literature. Mutations in ORF7b have not been frequently reported, but some reports in India and USA were associated to loss of transmembrane anchoring protein function [40].
MT21774 also has one NSP3 (V2090G), one Membrane protein (A2S) and two ORF3ab (S253P and Y264N) aa changes in addition to the characteristic mutations reported worldwide for its lineage. NSP3 or PLpro is a protease involved in coronavirus polyprotein processing, interference with the IFN pathway signaling and, in NF-Ƙβ activation. Membrane protein is a structural signaling protein required for virus budding. ORF3ab is a transmembrane ion channel accessory protein linked to Spike protein, involved in NF-Ƙβ expression and caspase-dependent activation of host cell apoptosis. Mutations in this protein may alter its pro-apoptotic activity [41].
We found few synonymous mutations in these sequences, if compared with the number of nonsynonymous mutations. Synonymous changes are not considered totally neutral. If associated with selective pressures, these nucleotide changes may become permanent over time in a certain population within the species [42].
SARS-CoV-2 lineages have presented an increasing number of nt and aa changes since mid-2020, resulting from massive dispersion of the virus and intense host adaptation events leading to infection of different tissue cells and, new outbreak waves in Brazil.
The virus seems to be constantly altering its codon preferences in order to increase the efficiency of viral protein expression, function, host immune response evasion and virus replication, improving virus fitness and ultimately leading to adaptation and persistence in the relatively new host.
Literature supports that SARS-CoV-2 infects the outer layer and other placenta cells in the maternal–fetus interface. This study reports clinical and virological data corroborating these studies [15,16].
In conclusion, results herein reported show evidence of virus presence in the maternal–fetal interface even in mildly infected and already recovered pregnant women. These findings indicate the necessity for clinical follow up and laboratory investigation of SARS-CoV-2 in pregnant women and in neonates born from infected mothers, independently of their symptoms and pregnancy stage at the time of infection.
Financial support
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. JARP receives a CAPES MASTER scholarship [the Brazilian Government; code 001]. RDS receives a CNPq research grant [PQ 309750/2020-2].
Declaration of competing interest
The authors have nothing to disclose.
Acknowledgments
Ana Carla Santos Oliveira, Alessandro Gonçalves da Silva, Fernanda Maria Pigatto Correia Vilela, Lorena de Amorim Grando, Natalia Galbiatti Silveira Campesatto, Rene Diaz Rodriguez, Sandra Aparecida Moreira Gomes Monteiro, Taiane Caporossi Martins for their assistance on patient management at the HUJM COVID19 neonatal unit. Alex Pauvolid Correa, Paola Cristina Resende and Marilda Siqueira from Instituto Oswaldo Cruz Laboratory of Respiratory Viruses and Measles for sequencing and virus isolation attempts. Rodrigo Profeta from UFMG for his assistance on phylogenetic analysis and Juliana Helena Chavez Pavoni from UFR for critically reviewing the manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.micinf.2022.104949.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
. SARS-CoV-2 sequences from lineage B.1.1.33 included in the phylogenetic analysis. Acknowledgment table to GISAID database submissions
. SARS-CoV-2 sequences from lineage B.1.1.28 included in the phylogenetic analysis. Acknowledgment table to GISAID database submissions.
References
- 1.ICTV - Coronaviridae Study Group of the International Committee on Taxonomy of Viruses. https://talk.ictvonline.org/information/members-606089945/w/members/471/coronaviridae-study-group. (Accessed July 22 2021).
- 2.WHO – World Health Organization. COVID-19 as a Public Health Emergency of International Concern (PHEIC) under the IHR. Addendum to Fact Sheet 15 on National Implementation Measures for the International Health Regulations 2005 (IHR). https://extranet.who.int/sph/sites/default/files/document-library/document/FS15A_IHR_COVID19_EN_MAY_2020.pdf. (Accessed July 22 2021).
- 3.Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., et al. Genomic characterization and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holmes E.C., Goldstein S.A., Rasmussen A.L., Robertson D.L., Crits-Christoph A., Wertheim J.O., et al. The origins of SARS-CoV-2: a critical review. Cell. 2021;184:1–9. doi: 10.1016/j.cell.2021.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., et al. The China novel coronavirus investigating and research team. A novel coronavirus from patients with pneumonia in China. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dong L., Tiang J., He S., Zhu C., Wang J., Liu C., et al. Possible vertical transmission of SARS-CoV-2 from an infected mother to her newborn. JAMA. 2020;323:1846–1848. doi: 10.1001/jama.2020.4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu Y., Chen H., Tang K., Guo Y. Clinical manifestations and outcome of SARS-CoV-2 infection during pregnancy. J Infect. 2020;S0163-4453(20):30102–30109. doi: 10.1016/j.jinf.2020.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vivanti A.J., Vauloup-Fellous C., Prevot S., Zupan V., Suffee C., Do Cao J., et al. Transplacental transmission of SARS-CoV-2 infection. Nat Commun. 2020;11:3572. doi: 10.1038/s41467-020-17436-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xiong Y., Zhang Q., Zhao L., Shao J., Zhu W. Clinical and imaging features of COVID-19 in a neonate. Chest J. 2020;158(1):e5–e7. doi: 10.1016/j.chest.2020.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zeng L., Xia S., Yuan W., Yan K., Xiao F., Shao J., et al. Neonatal early-onset infection with SARS-CoV-2 in 33 neonates born to mothers with COVID-19 in Wuhan, China. JAMA Pediatr. 2020;174(7):722–725. doi: 10.1001/jamapediatrics.2020.0878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amaral W.N., Moraes C.L., Rodrigues A.P.S., Noll M., Arruda J.T., Mendonça C.R. Maternal coronavirus infections and neonates born to mothers with SARS-CoV-2: a systematic review. Healthcare. 2020;8(4):511. doi: 10.3390/healthcare8040511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.MS – Brazilian Ministry of Health, Health Surveillance Secretary. Special Epidemiological Bulletim coronavirus disease 19 40 COVID19. https://www.gov.br/saude/pt-br/assuntos/media/pdf/2020/dezembro/11/boletim_epidemiologico_covid_40-1.pdf (Accessed September 9 2021).
- 13.PAHO/WHO . 18 May 2021. Epidemiological update: coronavirus disease (COVID-19)https://www.paho.org/ [Google Scholar]
- 14.MS - Brazilian Ministry of Health. 2021. COVID19 Panel. https://covid.saude.gov.br/(Accessed August 25 2021).
- 15.OOBr COVID-19. Observatório Obstétrico de COVID19. https://observatorioobstetrico.shinyapps.io/covid_gesta_puerp_br/(Accessed August 28 2021).
- 16.Richtmann R., Torloni M.R., Oyamada Otani A.R., Levi J.E., Crema Tobara M., de Almeida Silva C., et al. Fetal deaths in pregnancies with SARS-CoV-2 infection in Brazil: a case series. Case Rep Womens. Health. 2020;27 doi: 10.1016/j.crwh.2020.e00243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marinho P.S., da Cunha A.J.L.A., Chimelli L., Avvad-Portari E., Andreiuolo F.M., Oliveira-Szejnfeld P.S., et al. Case Report: SARS-CoV-2 Mother-to-child transmission and fetal death associated with severe placental thromboembolism. Front Med. 2021;8:677001. doi: 10.3389/fmed.2021.677001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rambaut A., Holmes E.C., O'Toole A., Hill V., McCrone J.T., Ruis C., et al. Dynamic nomenclature proposal for SARS-CoV-2 lineages to assist genomic epidemiology. Nat Microbiol. 2020;5:1403–1407. doi: 10.1038/s41564-020-0770-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Resende P.C., Delatorre E., Gräf E., Mir D., Motta F.C., Appolinario L.R., et al. Evolutionary dynamics and dissemination pattern of the SARS-CoV-2 lineage B.1.1.33 during the early pandemic phase in Brazil. Front Microbiol. 2021;11:615280. doi: 10.3389/fmicb.2020.615280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Resende P.C., Naveca F.G., Lins R.D., Dezordi F.Z., Ferraz M.V.F., Moreira E.G., et al. MedRxiv; 2021. The ongoing evolution of variants of concern and interest of SARS-CoV-2 in Brazil revealed by convergent indels in the amino (N)-terminal domain of the Spike protein. 03.19.21253946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Voloch A.C., Francisco R.S., Jr., Almeida L.G.P., Cardoso C.C., Brustolini O.J., Gerber A.L., et al. Genomic characterization of a novel SARS-CoV-2 lineage from Rio de Janeiro, Brazil. J Virol. 2021;95(10):e00119–e00121. doi: 10.1128/JVI.00119-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mullen J, Tsueng G, Latif A, Alkuzweny M, Cano M. et al. outbreak.info. https://outbreak.info/(Accessed September 10 2021).
- 23.Katoh K., Standley D.M. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 2013;30(4):772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Larsson A. AliView: a fast and lightweight alignment viewer and editor for large datasets. Bioinformatics. 2014;30(22):3276–3278. doi: 10.1093/bioinformatics/btu531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karimi-Zarchi M., Neamatzadeh H., Dastgheib S.A., Abbasi H., Mirjalili S.R., Behforouz A. Vertical transmission of coronavirus disease 19 (COVID-19) from infected pregnant mothers to neonates: a review. Fetal Pediatr Pathol. 2020;39(3):246–250. doi: 10.1080/15513815.2020.1747120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Narang K., Enninga E.A.L., Gunaratne M.D., Ibirogba E.R., Trad A.T., Elrefaei A., et al. SARS-CoV-2 infection and COVID-19 during pregnancy: a multidisciplinary review. Mayo Clin Proc. 2020;95(8):1750–1765. doi: 10.1016/j.mayocp.2020.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Menter T., Mertz K.D., Jiang S., Chen H., Monod C., Tzankov A., et al. Placental pathology findings during and after SARS-CoV-2 infection: features of villitis and malperfusion. Pathobiology. 2021;88:69–77. doi: 10.1159/000511324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hosier H., Farhadian S.F., Morotti R.A., Deshmukh U., Lu-Culligan A., Campbell K.H., et al. SARS-CoV-2 infection of the placenta. J Clin Invest. 2020;130(9):4947–4953. doi: 10.1172/JCI139569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hecht J.L., Quade B., Deshpande V., Mino-Kenudson M., Ting D.T., Desai N., et al. SARS-CoV-2 can infect the placenta and is not associated with specific placental histopathology: a series of 19 placentas from COVID-19-positive mothers. Mod Pathol. 2020;33:2092–2103. doi: 10.1038/s41379-020-0639-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shende P., Gaikwad P., Gandhewar M., Ukey P., Bhide A., Patel V., et al. Persistence of SARS-CoV-2 in the first trimester placenta leading to transplacental transmission and fetal demise from an asymptomatic mother. Human Reprod. 2021;36(4):899–906. doi: 10.1093/humrep/deaa367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blumberg D.A., Underwood M.A., Hedriana H.L., Lakshminrusimha S. Vertical transmission of SARS-CoV-2: what is the optimal definition? Am J Perinatol. 2020;37:769–772. doi: 10.1055/s-0040-1712457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shah P.S., Diambomba Y., Acharya G., Morris S.K., Bitnun A. Classification system and case definition for SARS-CoV-2 infection in pregnant women, fetuses and neonates. Acta Obstet Gynecol Scand. 2020;99:565–568. doi: 10.1111/aogs.13870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Korber B., Fischer W.M., Gnanakaran S., Yoon H., Theiler J., Abfalterer W., et al. Tracking changes in SARS-CoV-2 spike: evidence that D614G increases infectivity of the COVID-19 virus. Cell. 2020;182:812–827. doi: 10.1016/j.cell.2020.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weisblum Y., Schmidt F., Zhang F., Silva J., Poston D., Lorenzi J.C.C., et al. BioRxiv; 2020. Escape from neutralizing antibodies by SARS-CoV-2 spike protein variants. 07.21.214759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tang J.W., Tambyah P.A., Hui D.S. Emergence of a new SARS-CoV-2 variant in the UK. J Infect. 2020;28 doi: 10.1016/j.jinf.2020.12.024. S0163-4453(20): 30786-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang Y., Yang C., Xu X.F., Xu W., Liu S.W. Structural and functional properties of SARS-CoV-2 spike protein: potential antivirus drug development for COVID-19. Acta Pharmacol Sin. 2020;41:1141–1149. doi: 10.1038/s41401-020-0485-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garvin M.R., Prates E.T., Pavicic M., Jones P., Amos B.K., Geiger A., et al. Potentially adaptive SARS-CoV-2 mutations discovered with novel spatiotemporal and explainable AI models. Genome Biol. 2020;21:304. doi: 10.1186/s13059-020-02191-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tablizo F.A., Kim K.M., Lapid C.M., Jerrone M., Castro R., Yangzon M.S.L., et al. MedRxiv; 2021. Genome sequencing and analysis of an emergent SARS-CoV-2 variant characterized by multiple spike protein mutations detected from the Central Visayas Region of the Philippines. 03.03.21252812. [DOI] [Google Scholar]
- 39.Wu H., Xing N., Meng K., Fu B., Xue W., Dong P., et al. BioRxiv; 2021. Nucleocapsid mutation R203/G204R increases the infectivity, fitness and virulence of SARS-CoV-2. 05.24.445386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hassan S.S., Choudhury P.P., Roy B. Rare mutations in the accessory proteins ORF6, ORF7b, and ORF10 of the SARS-CoV-2 genomes. Meta Gene. 2021;28:100873. doi: 10.1016/j.mgene.2021.100873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Issa E., Merhi G., Panossian B., Salloum T., Tokajian S. SARS-CoV-2 and ORF3a: non-synonymous mutations, functional domains, and viral pathogenesis. mSystems. 2020;5 doi: 10.1128/mSystems.00266-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vandamme A.M. In: The phylogenetic handbook A practical approach to phylogenetic analysis and hypothesis testing. 2 ed. Lemey P., Salemi M., Vandamme A.M., editors. Cambridge University Press; 2009. Basic concepts of molecular evolution; pp. 3–28. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
. SARS-CoV-2 sequences from lineage B.1.1.33 included in the phylogenetic analysis. Acknowledgment table to GISAID database submissions
. SARS-CoV-2 sequences from lineage B.1.1.28 included in the phylogenetic analysis. Acknowledgment table to GISAID database submissions.