Abstract
Objectives
To determine the status of immune responses after primary and booster immunization for SARS-CoV-2 variants and evaluate the differences in disease resistance based upon titers of neutralizing antibodies (NAbs) against the variants.
Methods
Participants aged 18–59 years received 2 doses of inactivated COVID-19 vaccine, 14 days apart, and a booster dose after 12 months. Blood samples were collected before vaccination (baseline), 1 and 6 months after primary immunization, and at multiple instances within 21 days of the booster dose. NAbs against the spike protein of Wuhan-Hu-1 and 3 variants were measured using pseudovirus neutralization assays.
Results
Of 400 enrolled participants, 387 completed visits scheduled within 6 months of the second dose and 346 participants received the booster dose in the follow-up research. After 1 month of primary immunization, geometric mean titers (GMTs) of NAbs peaked for Wuhan-Hu-1, whereas GMTs of other variants were <30. After 6 months of primary immunization, GMTs of NAbs against all strains were <30. After 3 days of booster immunization, GMTs were unaltered, seroconversion rates reached approximately 50% after 7 days, and GMTs of NAbs against all strains peaked at 14 days.
Conclusion
Two-dose of inactivated COVID-19 vaccine induced the formation of NAbs and memory-associated immune responses, and high titers of NAbs against the variants obtained after booster immunization may further improve the effectiveness of the vaccine.
Keywords: SARS-CoV-2 variants, Primary and booster immunization, Pseudovirus neutralization assay, Inactivated COVID-19 vaccine
Introduction
The pathogen causing COVID-19 has overwhelmed the human immune system and led to severe morbidity and mortality on a global scale. However, scientists all over the world have taken quick, unprecedented, and coordinated action to develop vaccines and antiviral agents for ending this pandemic. To date, billions of people have been vaccinated with various types of COVID-19 vaccines, including inactivated viruses, viral mRNA, and adenovirus vectors. Incidentally, the available evidence demonstrates that all of these vaccines have a commendable expectancy in preventing COVID-19-associated hospitalization and death. The estimated vaccine effectiveness is 60%–80% in preventing hospitalization and severe disease outcome (Moghadas et al., 2021) and >80% in preventing death from COVID-19 (Roghani, 2021). However, with respect to preventing infection, the effectiveness of the vaccines varies at different time points after immunization (Dagan et al., 2021; Rossman et al., 2021). This is possibly related to the continuous decline of neutralizing antibodies (NAbs) over time, thereby weakening the effectiveness of the vaccine. In this study, a set of pseudovirus neutralizing antibody assays were established to understand the status of the immune responses at different time points and evaluate the effects of cross immunization against SARS-CoV-2 variants after primary and booster immunization.
Methods
Study design, participants, and sample collection
This study was performed between July 2020 and October 2021 in Beijing, China. A total of 400 participants, aged between 18-59 years, were recruited. The main exclusion criteria included a history of severe acute respiratory syndrome coronavirus (SARS-CoV), SARS-CoV-2, or Middle East respiratory syndrome infection; high-risk epidemiology history within 14 days before enrolment (including a history of travel or residence in communities with case reports or contact with a SARS-CoV-2-infected individual), axillary temperature >37.0°C, and history of allergy to any of the vaccine components. A complete list of exclusion criteria is included in the protocol.
Every participant was familiarized with the aim of the study and asked to sign an informed consent agreement. Subsequently, they received 2 doses of 3 µg of inactivated COVID-19 vaccine (CoronaVac, Sinovac Life Sciences, Beijing, China), 14 days apart according to the product manual. Blood samples were collected from the participants on day 0 (day of first dose vaccine) as well as 1 month and 6 months after the second dose of primary immunization. Of the 400 participants, 387 completed the visits scheduled within 6 months of the second dose.
According to the product manual, the booster immunization can be carried out 6 months after the primary immunization. After 12 months of receiving the second vaccine dose, 346 of 400 participants took part in the follow-up research, and they were administered a single dose of booster immunization. Thereafter, they were divided into 5 groups. The participants were included in 1 of the 5 groups, according to their wishes. Blood samples were collected separately from 46, 41, 40, 100, and 119 individuals on days 3, 7, 10, 14, and 21 to detect antibodies against the COVID-19 pathogen. Information related to the number of participants and schedule of sample collection is presented in Figure 1 .
Figure 1.
Design of study and schedule of sample collection.
A total of 400 participants aged 18–59 years were enrolled for the study in Beijing, China. All of them received 2 doses of the inactivated COVID-19 vaccine, 14 days apart. Blood samples were collected from all participants on day 0 (just before vaccination; baseline) and from 387 individuals who came for the scheduled visits after 1 and 6 months of the second dose. Among them, 346 individuals participated in the follow-up research, where they received 1 dose of booster immunization; blood samples were collected from them at multiple instances within 21 days of the booster dose.
Abbreviations: COVID-19 = coronavirus disease 2019.
The study protocol was approved by the Ethics Committee of Beijing CDC (2020-28), and the entire study was performed in accordance with the requirements of Good Clinical Practice of China and the International Conference on Harmonisation.
Pseudovirus preparation and titration
The vesicular stomatitis virus (VSV)-based SARS-CoV-2 pseudoviruses consisting of the Wuhan-Hu-1 (GenBank: MN908947), B.1.1.7, B.1.351, or B.1.617.2 spike proteins were prepared by the National Institutes for Food and Drug Control (NIFDC). They were generated by transfection with a plasmid expressing 1 of the spike proteins of SARS-CoV-2 or the SARS-CoV-2 variant strain and concurrent infection with G*ΔG-VSV (Kerafast) in which the G gene is replaced with the firefly luciferase (Fluc) reporter gene. The cell supernatant containing the pseudotyped virus was harvested after 24 and 48 hours, filtered (0.45-μm pore size, Millipore, SLHP033RB), aliquoted (1 mL/tube), and stored at -80°C for further use. Before the pseudovirus titration, a single aliquot of the pseudotyped virus was taken out from -80°C to avoid repeated freezing and thawing. Subsequently, the titration was carried out by serial dilution to infect the target cells Huh-7 in 96-well plates. The 50% tissue culture infectious dose (TCID50) of the pseudotyped virus was calculated according to the Reed-Muench method. The abovementioned procedures were performed according to previously published protocols (Li et al., 2020; Nie et al., 2020a; Nie et al., 2020b).
Pseudovirus neutralization assay (pVNT)
The titers of NAbs were quantified using the pseudovirus neutralization assay for Wuhan-Hu-1 and SARS-CoV-2 variants. First, 100 μL of serial threefold diluted human serum (starting at 1:10) was incubated with 50 μL of pseudovirus (1300 TCID50/mL) for 1 hour at 37°C in the 96-well plates. Thereafter, Huh-7 cells were added (2 × 104 cells/100 μL per well), and the plates were incubated at 37°C in a humidified atmosphere with 5% CO2. Duplicated wells were analyzed for each sample. The cell control (CC), which contained only Huh-7 cells, and the virus control (VC), which contained the virus and the Huh-7 cells, were set up for each plate. After incubation for 24 hours, chemiluminescence signals were detected by Molecular Devices SpectraMax® iD5 using the luciferase substrate (PerkinElmer). The Reed-Muench method was used to calculate the half-maximal inhibition dilution (ID50), and ID50 values ≥30.0 were considered positive.
Statistical analyses
The sample size for this study was based on practical considerations rather than statistical power calculations. Statistical analyses were conducted with GraphPad Prism 8.0.1. NAbs were presented as geometric mean titers (GMTs) with 95% confidence intervals (CIs). The calculations were performed with log10 values of the original data and subsequent application of anti-log transformation. Wilcoxon matched-pairs signed-rank test was used to compare differences among groups. Two-sided p-values < 0.05 were considered statistically significant.
Results
Inactivated COVID‐19 vaccine induces low‐level antibody responses after primary immunization
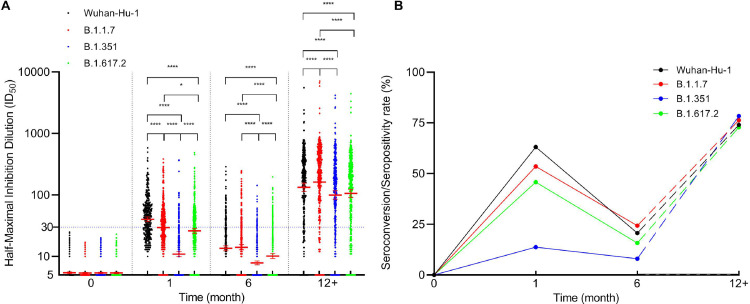
There were no detectable titers of NAbs against the Wuhan-Hu-1 and 3 SARS-CoV-2 variants (baseline) in the sera of the study participants before primary immunization. The seroconversion of the subjects denotes the antibody titer at which their sera convert from negative to positive after 1 month of complete primary vaccination. After 1 month of complete vaccination, the neutralizing antibody titers against Wuhan-Hu-1 increased from baseline to a GMT of 40.2 (95% CI, 36.8–43.8), and the seroconversion rate was 63.0% (244 of 387 participants). However, the GMTs of the other 3 variants, B.1.1.7, B.1.351, and B.1.617.2, were <30, particularly, 29.6 (95% CI, 27.0–32.4), 10.9 (95% CI, 10.0–12.0), and 26.1(95% CI, 23.7–28.7), respectively. There were significant differences among all groups (P <0.05) (Figure 2 A). In addition, their respective seroconversion rates were 53.5% (207 of 387), 13.7% (53 of 387), and 45.7% (177 of 387). Therefore, the GMT and seroconversion rates were the highest for Wuhan-Hu-1 and the lowest for B.1.351 (Figure 2B).
Figure 2.
Status of neutralizing antibody response following primary and booster doses of vaccination.
A, Neutralizing antibody titers against the spike protein of SARS-CoV-2 Wuhan-Hu-1 and 3 variants (B.1.1.7, B.1.351, and B.1.617.2) were measured by pseudovirus neutralization assay. Each data point represents a serum sample. The dotted horizontal line represents the seropositivity threshold. Titers lower than the initial dilution (1:10) are presented as half the limit of detection. The error bars are the GMTs values of neutralizing antibodies with 95% confidence interval (CI). Asterisks indicate statistical significance: * p<0.05, ** p<0.01, *** p<0.001, **** p<0.0001. B, Seroconversion/seropositivity rates of neutralizing antibody after primary and booster immunization. Solid line indicates seroconversion/seropositivity rates of 387 participants after primary vaccination; they were defined as neutralizing antibody levels required for serum conversion from negative to positive concerning the spike protein of SARS-CoV-2 Wuhan-Hu-1 and 3 variants, as the case may be. Dotted line indicates seroconversion rate of 346 participants after booster immunization, and the antibody titer after booster immunization was 4 times higher than that after 6 months of primary immunization. In both figures, the x-axis represents the collection time of blood samples since the primary immunization. Specifically, 0 month indicates blood samples were collected from the participants on the day of the first dose vaccine. One month and 6 month indicates blood sampling at 1 month and 6 months after the second dose of primary immunization. The 12+ months indicates blood sampling after a single dose of booster immunization.
Abbreviations: GMTs = geometric mean titers; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2.
The GMTs of NAbs show a rapidly declining trend after 6 months of primary immunization
After 6 months of the second vaccine dose, we observed a rapid decline in the GMTs of NAbs against all the SARS-CoV-2 pseudovirus strains. The neutralizing antibody GMTs of all 4 pseudovirus strains were <30, namely Wuhan-Hu-1: 13.6 (95% CI, 12.4–14.9), B.1.1.7: 14.1 (95% CI, 12.8–15.6), B.1.351: 7.9 (95% CI, 7.3–8.4), and B.1.617.2: 10.1 (95% CI, 9.3–11.1). Among them, the GMT of B.1.1.7 was the highest, but there were no significant differences between the neutralization antibody GMTs of B.1.1.7 and Wuhan-Hu-1 (P >0 05). On the contrary, the GMT of B.1.351, which was the lowest, significantly differed from that of the other groups (P < 0.0001) (Figure 2A). The antibody-positive rates of Wuhan-Hu-1 and the other 3 variants were Wuhan-Hu-1: 20.7% (80 of 387), B.1.1.7: 24.3% (94 of 387), B.1.351: 8.0% (31 of 387), and B.1.617.2: 15.8% (61 of 387), respectively (Figure 2B).
Memorizing reaction of serum antibody after booster immunization
The GMT of the NAbs against Wuhan-Hu-1 was 133.2 (95% CI, 114.1–155.5), and it had increased significantly after the booster immunization as compared with the respective GMTs after 1 and 6 months of primary immunization. Similar observations were made for the other 3 variants (Figure 3 ). The GMT of NAbs against B.1.1.7 was 161.9 (95% CI, 138.7–189.1). This increase in GMT value is significantly greater than that of Wuhan-Hu-1 (Figure 2A). The GMTs of NAbs against B.1.351 and B.1.617.2 was 99.8 (95% CI, 85.2–116.8) and 106.0 (95% CI, 91.2–123.3), respectively, and these values are 9.2 and 4.1 times greater than their respective GMT values in the peak period of 1 month after primary immunization (Figure 3). Seroconversion was calculated after the booster immunization, and the results indicated that antibody titer was 4 times higher than that after 6 months of primary immunization. The seroconversion rates of Wuhan-Hu-1 and the other 3 variants were Wuhan-Hu-1: 74.0% (256 of 346), B.1.1.7: 76.3% (264 of 346), B.1.351: 78.3% (271 of 346), and B.1.617.2: 72.8% (252 of 346), respectively (Figure 2B).
Figure 3.
The trends of neutralizing antibody titers of different strains at different time points after primary and booster vaccination.
A, Wuhan-Hu-1, B, B.1.1.7 C, B.1.351 D, B.1.617.2. The data of 0, 1, and 6 months refer to the results of 387 participants obtained before primary immunization and at 1 month and 6 months after the second dose of primary immunization, respectively. The data of 12+ month refer to the results of 346 participants obtained at multiple instances within 21 days of receiving the booster dose. Asterisks indicate statistical significance: * p <0.05, ** p <0.01, *** p <0.001, **** p <0.0001.
The GMTs of NAbs at different time points after booster immunization
After 3 days of booster immunization, the GMTs of NAbs showed no change as compared with the respective GMTs observed 6 months after primary immunization; in fact, the values were slightly lower than the GMT levels at 6 months. The GMTs were Wuhan-Hu-1: 8.6 (95% CI, 6.8–10.8), B.1.1.7: 9.4 (95% CI, 7.5–11.9), B.1351: 7.8 (95% CI, 6.3–9.7), and B.1.617.2: 8.6 (95% CI, 7.1–10.3). Interestingly, only 1 individual (1 of 46 participants) had antibody titer values that were 4 times higher than that observed 6 months after primary immunization for 4 pseudovirus strains. After 7 days of booster immunization, the GMT of neutralizing antibody against Wuhan-Hu-1 was 47.7 (95% CI, 35.8–63.5), that is, it had recovered to similar levels as observed 1 month after primary immunization. The GMTs of NAbs against the other 3 variants were >30, namely, B.1.1.7: 71.2 (95% CI, 53.8–94.1; highest), B.1.617.2: 43.4 (95% CI, 32.9–57.2), and B.1.351: 39.1 (95% CI, 28.6–53.5; lowest). The seroconversion rates of the 4 strains were Wuhan-Hu-1: 48.8 % (20 of 41), B.1.1.7: 56.1% (23 of 41), B.1.351: 61.0% (25 of 41), and B.1.617.2: 43.9% (18 of 41). Subsequently, the antibody GMTs against all the strains exhibited a rapidly increasing trend over time. After 10 days of booster immunization, except for a few participants, all others had GMT levels of NAbs >30, and the GMT values peaked on day 14 after booster immunization. These peak GMT levels were Wuhan-Hu-1: 263.9 (95% CI, 223.7–311.3), B.1.1.7: 319.1 (95% CI, 274.1–371.5), B.1.351: 194.9 (95% CI, 160.9–236.1), and B.1.617.2: 202.1 (95% CI, 171.3–238.4), and they were 6.6, 10.8, 17.9, and 7.7 times higher than their respective values in the peak period of 1 month after primary immunization. The seroconversion rates of all the strains were >90%. Thereafter, the neutralizing antibody titers seemed to reach a plateau phase. Apart from the GMT of wild-type, which increased slightly, the GMT levels for all other variants decreased. However, there was no significant difference between the GMTs observed on days 14 and 21 for all the strains (P >0.05) (Figure 4 ).
Figure 4.
Status of neutralizing antibody responses at different time points following booster dose of vaccination.
A, Neutralizing antibody titers against the spike protein of SARS-CoV-2 Wuhan-Hu-1 and 3 variants (B.1.1.7, B.1.351, and B.1.617.2) were measured using pseudovirus neutralization assay. A total of 346 participants received the single dose of booster immunization 12 months after completing the primary vaccination. The day 3 data includes 46 participants, day 7 data includes 41 participants, day 10 data includes 40 participants, day 14 data includes 100 participants, and day 21 data includes 119 participants. Each data point represents a serum sample. The error bars are the GMTs values of neutralizing antibodies with 95% confidence interval (CI). Asterisks indicate statistical significance: * p <0.05, ** p <0.01, *** p <0.001, **** p <0.0001. B, Seroconversion of booster immunization is the antibody titer was 4 times higher than that after 6 months of primary immunization.
Abbreviations: GMTs = geometric mean titers; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2.
Discussion
The present study is a unique large-scale and long-term prospective cohort study on the antibody persistence and secondary immune response of the COVID-19 vaccine. The immunogenicities of 387 participants were evaluated before vaccination, 1 and 6 months after primary immunization, and at different time points after the booster immunization using pseudovirus neutralization assays for SARS-CoV-2. According to previous reports, the peak of neutralizing antibody titers should be seen after 1 month of complete immunization. However, this study demonstrated that after 1 month of primary immunization with inactivated COVID-19 vaccine, there were low levels of antibody response against Wuhan-Hu-1, B.1.1.7, and B.1.617.2 variants, whereas the neutralization capacity for B.1.351 variant was extremely low. Subsequently, 6 months after the second vaccination dose, there was a rapid decline in the GMTs of NAbs, but the antibody-positive rates were still approximately 20% for Wuhan-Hu-1 and B.1.1.7 variants. This is consistent with the antibody persistence results of other COVID-19 vaccines that produced high titers of NAbs after full inoculation (Doria-Rose et al., 2021; Favresse et al., 2021; Pegu et al., 2021). Incidentally, antibody persistence may be associated with immune memory rather than the titer value of NAbs.
After 1 year of complete vaccination, 346 participants received 1 dose of booster immunization. To understand the status of the immune response at different time points after the booster immunization, the participants were divided into 5 groups, and blood samples were collected on days 3, 7, 10, 14, and 21. The neutralizing antibody titers did not change in most participants on day 3 after booster immunization. However, based on the approximately 50% seroconversion rate recorded on day 7 after the booster immunization, it may be suggested that for most of the participants, the antibody titers increased significantly between days 5 and 6 of receiving the booster dose. The seroconversion rate reached 90% at day 10, and the GMT levels peaked on day 14 after booster immunization. This sequence of immune response is consistent with the pattern of memory-associated responses, and it further confirmed that a humoral immune response can be induced in the human body by administering inactivated COVID-19 vaccines similar to other vaccines (Chandrashekar et al., 2020).
The most exciting observation is that all variants show excellent immunogenicity after 10 days of booster immunization. This indicates that a high titer of NAbs is related to the effectiveness of the vaccine against the SARS-CoV-2 variant in vitro; however, this is inconsistent with some studies (Caucci et al., 2021). Although moderate levels of antibody titers have been extrapolated to detect infections, extensive real-world data are necessary to support its relation to the protective effects against the SARS-CoV-2. Hence, it may be suggested that antibody titers were used to indirectly evaluate the effectiveness of the vaccine (McMahan et al., 2021).
This study showed that administration of 2 vaccine doses could not achieve the expected effect against Wuhan-Hu-1 or the variants, and a third dose is necessary (Flaxman et al., 2021). However, the question arises that whether the third dose should be a part of the primary immunization schedule, or should it be included as a booster immunization. Moreover, if the primary immunization requires 3 doses, then the interval between the second and third doses needs to be determined to obtain the best immunogenicity.
Pseudovirus neutralization assays can be conveniently used for evaluating vaccine immunogenicity owing to their safety, and the effectiveness of this method has been established by several studies. Neutralizing antibody titer is the best index to evaluate the immunogenicity of a vaccine (Robbiani et al., 2020), but it has some limitations (Andualem et al., 2020). Even though NAbs are mainly immunoglobulin (Ig) G, isotype IgA and IgM with neutralization abilities may also be present. Interestingly, the dynamic regularity varies not only among the different classes of antibodies but also among the different subclasses of 1 class. In this study, even though the GMT of NAbs against Wuhan-Hu-1 was significantly higher (P <0.0001) than that of B.1.1.7 after 1 month of primary immunization, their immune response levels were similar after 6 months. On the contrary, after 7 days of booster immunization, the GMT of B.1.1.7 antibodies were significantly higher than that of Wuhan-Hu-1 antibodies (P <0.001). It is unclear whether this is related to the class/subclass of antibodies secreted at different time points, and hence, it needs to be explored further. Some studies have reported that IgG subclasses can interfere with the antibody affinity of the SARS-CoV-2 (Luo et al., 2021; Stephens and McElrath, 2020; Suthar et al., 2020). This phenomenon has been observed in other viruses too. A study of antibody responses to primary Rubella infection revealed an initial low-avidity IgM response, followed by low-avidity IgG3 and IgA responses, and finally, IgG1 responses maturing from low to high avidity. Therefore, low-avidity antibodies indicate recent infection, and maturation to high avidity antibodies occurs within 2 months post-exanthem (Susan and Stanley, 2018, Wilson et al., 2006). Future studies can confirm whether the irregular changes in the titers of NAbs in some participants indicate a similar pattern in COVID-19 as in the case of Rubella.
In conclusion, the NAbs induced by 2 doses of inactivated COVID-19 vaccine can be maintained for 6 months, and high titers of NAbs produced after booster immunization can effectively protect against SARS-CoV-2 variants in vitro.
Declaration of Competing Interest
Hui Xie, Xiaojing Wen, Juan Li, Weixin Chen, Meng Chen, Lichi Zhang, Min Lv, Shanshan Zhou, Shuang Bai, Wei Zhao, Jian Wang, and Jiang Wu all declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Funding
This work was supported by the Beijing Municipal Science & Technology Commission (Z211100002521014).
Acknowledgments
We would like to thank all the participants who volunteered for this study. We are also grateful to Prof. Youchun Wang and Prof. Weijin Huang (National Institutes for Food and Drug Control) for helping us with the SARS-CoV-2 pseudovirus preparation and detection.
References
- Andualem H, Kiros M, Getu S, Hailemichael W. Immunoglobulin G2 Antibody as a Potential Target for COVID-19 Vaccine. ImmunoTargets and therapy. 2020;9:143-9. doi: 10.2147/ITT.S274746. [DOI] [PMC free article] [PubMed]
- Caucci S, Corvaro B, Tiano SML, Valenza A, Longo R, Marinelli K, et al. Weak Cross-Lineage Neutralization by Anti SARS-CoV-2 Spike Antibodies after Natural Infection or Vaccination Is Rescued by Repeated Immunological Stimulation. Vaccines. 2021;9 doi: 10.3390/vaccines9101124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrashekar A, Liu J, Martinot AJ, McMahan K, Mercado NB, Peter L, et al. SARS-CoV-2 infection protects against rechallenge in rhesus macaques. Science. 2020;369:812–817. doi: 10.1126/science.abc4776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagan N, Barda N, Kepten E, Miron O, Perchik S, Katz MA, et al. BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Mass Vaccination Setting. The New England journal of medicine. 2021;384:1412–1423. doi: 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doria-Rose N, Suthar MS, Makowski M, O'Connell S, McDermott AB, Flach B, et al. Antibody Persistence through 6 Months after the Second Dose of mRNA-1273 Vaccine for Covid-19. The New England journal of medicine. 2021;384:2259–2261. doi: 10.1056/NEJMc2103916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favresse J, Bayart JL, Mullier F, Elsen M, Eucher C, Van Eeckhoudt S, et al. Antibody titres decline 3-month post-vaccination with BNT162b2. Emerging microbes & infections. 2021;10:1495–1498. doi: 10.1080/22221751.2021.1953403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaxman A, Marchevsky NG, Jenkin D, Aboagye J, Aley PK, Angus B, et al. Reactogenicity and immunogenicity after a late second dose or a third dose of ChAdOx1 nCoV-19 in the UK: a substudy of two randomised controlled trials (COV001 and COV002) Lancet. 2021;398:981–990. doi: 10.1016/S0140-6736(21)01699-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Wu J, Nie J, Zhang L, Hao H, Liu S, et al. The Impact of Mutations in SARS-CoV-2 Spike on Viral Infectivity and Antigenicity. Cell. 2020;182:1284–1294. doi: 10.1016/j.cell.2020.07.012. e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo H, Jia T, Chen J, Zeng S, Qiu Z, Wu S, et al. The Characterization of Disease Severity Associated IgG Subclasses Response in COVID-19 Patients. Frontiers in immunology. 2021;12 doi: 10.3389/fimmu.2021.632814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahan K, Yu J, Mercado NB, Loos C, Tostanoski LH, Chandrashekar A, et al. Correlates of protection against SARS-CoV-2 in rhesus macaques. Nature. 2021;590:630–634. doi: 10.1038/s41586-020-03041-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghadas SM, Vilches TN, Zhang K, Wells CR, Shoukat A, Singer BH, et al. The impact of vaccination on COVID-19 outbreaks in the United States. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2021:ciab079. 10.1093/cid/ciab079. [DOI] [PMC free article] [PubMed]
- Nie J, Li Q, Wu J, Zhao C, Hao H, Liu H, et al. Establishment and validation of a pseudovirus neutralization assay for SARS-CoV-2. Emerging microbes & infections. 2020a;9:680-6. 10.1080/22221751.2020.1743767. [DOI] [PMC free article] [PubMed]
- Nie J, Li Q, Wu J, Zhao C, Hao H, Liu H, et al. Quantification of SARS-CoV-2 neutralizing antibody by a pseudotyped virus-based assay. Nature protocols. 2020;15:3699–3715. doi: 10.1038/s41596-020-0394-5. [DOI] [PubMed] [Google Scholar]
- Pegu A, O'Connell SE, Schmidt SD, O'Dell S, Talana CA, Lai L, et al. Durability of mRNA-1273 vaccine-induced antibodies against SARS-CoV-2 variants. Science. 2021;373:1372–1377. doi: 10.1126/science.abj4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbiani DF, Gaebler C, Muecksch F, Lorenzi JCC, Wang Z, Cho A, et al. Convergent antibody responses to SARS-CoV-2 in convalescent individuals. Nature. 2020;584:437–442. doi: 10.1038/s41586-020-2456-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roghani A. The Influence of COVID-19 Vaccination on Daily Cases, Hospitalization, and Death Rate in Tennessee, United States: Case Study. JMIRx med. 2021;2:e29324. doi: 10.2196/29324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossman H, Shilo S, Meir T, Gorfine M, Shalit U, Segal E. COVID-19 dynamics after a national immunization program in Israel. Nature medicine. 2021;27:1055–1061. doi: 10.1038/s41591-021-01337-2. [DOI] [PubMed] [Google Scholar]
- Stephens DS, McElrath MJ. COVID-19 and the Path to Immunity. Jama. 2020;324:1279–1281. doi: 10.1001/jama.2020.16656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susan ER, Stanley AP. In: Plotkin's Vaccines. Seventh Edition. Plotkin Stanley A., Offit Paul A., Orensteinm Walter A., Edwards Kathryn M., editors. Elsevier Press; Oxford, UK: 2018. 53 - Rubella Vaccines; pp. 970–1000. e18. [DOI] [Google Scholar]
- Suthar MS, Zimmerman MG, Kauffman RC, Mantus G, Linderman SL, Hudson WH, et al. Rapid Generation of Neutralizing Antibody Responses in COVID-19 Patients. Cell reports Medicine. 2020;1 doi: 10.1016/j.xcrm.2020.100040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson KM, Di Camillo C, Doughty L, Dax EM. Humoral immune response to primary rubella virus infection. Clinical and vaccine immunology: CVI. 2006;13:380–386. doi: 10.1128/CVI.13.3.380-386.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]