Abstract
Background
Non-exclusive voluntary licensing that is access-oriented has been suggested as an option to increase access to medicines to address the COVID-19 pandemic. To date, there has been little research on the effect of licensing, mainly focused on economic and supply chain considerations, and not on the benefits in terms of health outcomes. We aimed to study the economic and health effect of voluntary licensing for medicines for HIV and hepatitis C virus (HCV) in low-income and middle-income countries (LMICs).
Methods
A robust modelling framework was created to examine the difference between scenarios, with (factual) and without (counterfactual) a Medicines Patent Pool (MPP) licence for two medicines, dolutegravir and daclatasvir. Data were obtained from MPP licensees, as well as a large number of external sources. The primary outcomes were the cost savings and health impact between scenarios with and without MPP licences across all LMICs. Through its licences, MPP had access to the volumes and prices of licensed generic products sold in all covered countries on a quarterly basis. These data informed the volumes, prices, and uptake for the past factual scenarios and were the basis for modelling the future factual scenarios. These scenarios were then compared with a set of counterfactual scenarios in the absence of the studied licences.
Findings
Cumulatively, between 2017 and 2032, the model's central assumptions predicted an additional uptake of 15·494 (range 14·406–15·494) million patient-years of dolutegravir-based HIV treatments, 151 839 (34 575–312 973) deaths averted, and US$3·074 (1·837–5·617) billion saved through the MPP licence compared with the counterfactual scenario. For daclatasvir-based HCV treatments, the cumulative effect from 2015 to 2026 was predicted to be an additional uptake of 428 244 (127 584–636 270) patients treated with daclatasvir, 4070 (225–6323) deaths averted, and $107·593 (30·377–121·284) million saved with the licence compared with the counterfactual scenario.
Interpretation
The chain of effects linking upstream licensing to downstream outcomes can be modelled. Accordingly, credible quantitative estimates of economic and health effects arising from access-oriented voluntary licensing were obtained based on assumptions that early generic competition leads to price reductions that influence procurement decisions and enable the faster and broader uptake of recommended medicines, with beneficial economic and health effects.
Funding
Unitaid.
Introduction
The non-exclusive voluntary licensing of intellectual property rights in an access-oriented manner has been put forward as one option to enable affordable access to quality medicines to address the ongoing COVID-19 pandemic.1, 2 WHO has also included licensing as a strategy to foster broader access to health products in their roadmap for access to medicines, vaccines, and other health products.3 Additionally, the Lancet Commission on essential medicines policies has listed patent-related interventions such as encouragement of voluntary licensing and patent pools4 as part of its recommended policies to reduce the prices of essential medicines.
Several authors have attempted to describe the effect of voluntary licensing and access to generic drugs for key medicines in low-income and middle-income countries (LMICs). Studies looking at the effect of voluntary licences have focused on reduced procurement prices and cost savings, as well as the increased treatment uptake resulting from access to more affordable versions of the medicines.5, 6, 7 These findings have aligned with observations of generic drug prices being lower than originator prices, even when tiered pricing or other voluntary price discount mechanisms were in place.8, 9, 10 However, little has been said on the public health effect of enabling more people access to such treatments.
In this Article we assess the economic and health effects of access-oriented voluntary licensing, with case studies based on the experience in HIV and hepatitis C virus (HCV) of the Medicines Patent Pool (MPP; a UN-backed public health organisation established to improve access to affordable, quality-assured medicines in LMICs through access-oriented voluntary licensing).11, 12, 13 The methods presented revisit previous impact assessment assumptions done by the MPP, exploring licensing contributions to affordability, scale-up rates, and uptake volumes, as well as the associated effect on health and cost savings more broadly. The methods developed and the results obtained are presented for two case studies: the MPP licences for the HIV and HCV medicines dolutegravir and daclatasvir.14, 15 Since 2018, dolutegravir has been recommended by WHO as part of the preferred first-line regimen for the treatment of HIV, a chronic disease requiring lifelong care for which there is a large market in LMICs (because of substantial donor funding and national treatment programmes that are well established). Daclatasvir is used as part of one of multiple WHO-recommended regimens for HCV that can be treated fully (generally in 8–24 weeks) but for which markets are small because of little donor funding, diagnosis, and treatment scale-up in most LMICs.
Research in context.
Evidence before this study
We searched PubMed (and manually inspected results) using “voluntary licenses”, “access to medicines”, “generic drugs”, “intellectual property licensing”, and “Medicines Patent Pool” as keywords, all in English, from the creation of the Medicines Patent Pool (MPP) in 2010 to the end of 2020. In addition, we consulted the Geneva Graduate Institute Global Health Centre's Knowledge Portal on innovation and access to medicines, in particular the research synthesis on affordability, compulsory licensing, patent pools, tiered pricing, and voluntary licensing.
Several peer-reviewed studies have attempted to describe the effect of voluntary licensing of intellectual property rights in an access-oriented and non-exclusive manner, in particular patents, to enable affordable access to quality medicines through enhanced generic competition in low-income and middle-income countries (LMICs). Studies looking at the effect of voluntary licences have focused on reduced procurement prices and cost savings, as well as the increased treatment uptake resulting from access to more affordable versions of the medicines.
For example, Beck and colleagues (2019) presented projections for global HIV antiretroviral therapy costs for which expenses were contrasted between using exclusively originator or generic antiretrovirals. The total projected drug costs were higher in the scenario using originator products only. These findings have aligned with the observations of generic drug prices being lower than originator prices, even when tiered pricing (ie, differential pricing or market segmentation often based on country income level) or other voluntary price discount mechanisms (ie, reduced prices offered by manufacturers through negotiations with governments, procurement agencies, and other buyers) were in place. Consumer welfare implications and the affordability of tiered prices have also been discussed and contrasted with generic prices offered from originator-led bilateral licences. Little has been reported, however, on the health effect of enabling more people access to such treatments.
A previous attempt by Juneja and colleagues (2017) to assess the effect of MPP licences did not estimate any health impact and calculated cost savings without adjusting for the effect of higher drug prices on procurement decisions (ie, the demand for the specific drugs) in the absence of licensing (when prices are assumed to be higher). In a step towards characterising the health effect of expanding treatment programmes, Simmons and colleagues (2019) explored the effect of licensing on the uptake of hepatitis C virus (HCV) medicines. Their approach looked at year and country variations of the implementation of licenses, comparing HCV treatment uptake in the presence and absence of voluntary licences, with a panel of 35 intervention and control LMICs between 2004 and 2016. Findings pointed to voluntary licences leading to increases in the annual numbers of people being given those medicines. Notably, the study did not report on the resulting health effect of treating more people.
Added value of this study
The study presented here provides a robust modelling framework that includes a realistic assessment of counterfactual scenarios, to not only produce estimates of cost savings but also to inform on the health effects arising from the earlier access to and accelerated uptake of optimal treatments that are quality-assured and affordable in LMICs. It shows, using case studies for the HIV and HCV medicines dolutegravir and daclatasvir, that access-oriented voluntary licences for recommended treatments have both economic and health benefits for people in LMICs, saving both money and lives. For some products of particular importance (including those investigated in this study), the effect of access-oriented voluntary licensing can be substantial, with deaths averted in the tens of thousands or more, and costs saved in the hundreds of millions or even billions of US dollars.
Implications of all the available evidence
The effect of voluntary licensing interventions that are access-oriented on health outcomes and cost savings are dependent on the size of the licensed territories (and the inclusion of specific countries with a high disease burden, especially when they would have accessed drugs only at a much higher price in the absence of generic competition); the breadth and speed of licensing, generic product development, registration, and the resulting uptake of licensed medicines; the extent of price reductions resulting from access to more affordable products; the health benefits of transitioning to optimal treatments (and positioning of products in normative guidance); and the time between licensing and patent expiry. Conversely, the role of access-oriented voluntary licensing in increasing the manufacturing capacity in response to a public health demand for prioritised products supports drug introduction plans by country and international donor programmes. These considerations are relevant to the debates around the access to potential patented COVID-19 medicines under development, for which the timely availability of affordable treatments in many countries and possibly in high volumes might become a priority. More generally, the study can help to inform policy decisions to incentivise the use of access-oriented voluntary licensing and other access strategies for medicines of global public health importance. With regards to the implications for the research agenda, the study provides the basis for more nuanced economic and health impact modelling of other voluntary licensing agreements that are access-oriented and non-exclusive, including other existing ones from MPP. In addition, the model sets the basis for the prospective impact estimation of hypothetical licences for medicines of global public health interest.
Methods
The model
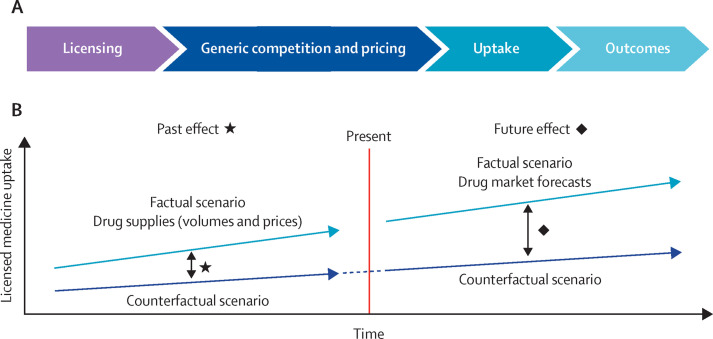
The model calculates the chain of effects linking licensing to health and economic outcomes (figure 1 ), both with and without the licence, retrospectively (from 2012) and in the future (until 2032), so that outcomes can be compared as differences between factual and counterfactual scenarios. The model, which allows for the testing of sensitivities and the estimation of ranges (ie, scenarios of central, low, and high health and economic impact, factoring in key health benefits and generic competition), is constructed on a series of evidence-based assumptions on how each factor affects the subsequent one. The difference between factual and counterfactual scenarios is the change that is estimated to have been caused by the contribution of the licence (ie, the effect arising from negotiating and managing the licence and supporting in-country uptake). The model was built using Microsoft Excel version 2109.
Figure 1.
Approach used to estimate effect of access-oriented licensing
(A) Chain of effects linking licensing to outcomes. Parallel factual and counterfactual scenarios are modelled following this chain of effect. (B) Modelling past and future outcomes of access-oriented licensing in a factual scenario in comparison to a counterfactual scenario allows for estimating the effect on uptake, cost savings, and health benefits.
Through its licences, MPP has access to the volumes and prices of licensed generic products sold in all countries that have procured or been supplied with the licenced medicines (appendix pp 11–12) on a quarterly basis. These data inform the volumes, prices, and uptake for the past factual scenarios and are the basis for modelling the future factual scenarios. These scenarios are then compared with a set of counterfactual scenarios in the absence of the studied licences.
Step 1: licensing
The core impact pathway for licensing is to enable more competition for a particular product in a particular market and for a particular year. Product availability in the counterfactual scenario is the first major assumption in the model because there is inherent uncertainty with regards to what would have happened in the absence of a given licence (eg, what would a patent holder have decided to do if not licensing a product to MPP). The model's approach to the counterfactual licensing scenario is largely based on patent holders' stated access policies before MPP licences were announced. The model takes into consideration the probable timing of the entry of a generic drug into the market in different countries, bearing in mind alternative licence arrangements and the patent landscape in each country. The model accounts for the possibility of earlier in-country uptake, with MPP reflecting accelerated timelines for in-licensing, as well as the proactive management of sublicences leading to faster development and regulatory approvals, and work with governments and other stakeholders to accelerate uptake. The appendix (pp 3–4) provides additional details, and factual and counterfactual licensing assumptions for dolutegravir and daclatasvir (appendix pp 11–13).
Step 2: generic competition and pricing
Translating licensing assumptions into impact requires making assumptions about how licensing affects generic competition and how competition affects pricing (appendix p 4). There is empirical evidence that a greater number of generic products serving the product market in a specific country decreases prices (up to a specific saturation point that depends on the market size).16 The model estimates a price for every product-country-year (every product in every country for each year) with and without MPP licences on the basis of factual price data and estimations of future price evolution. The difference between the average country-level number of suppliers with and without MPP is translated into a mark-up on the basis of estimates from the literature that are added to the generic price with MPP to give a counterfactual price without MPP (appendix p 4).16 The licence's effect on price is assumed to end shortly after patents expire, allowing full generic competition. However, the health and economic effect continues after this point because uptake with and without a licence converges more slowly than prices, and health benefits can occur over multiple years after treatment.
Step 3: uptake
The model used the weighted scoring of regimen prices and clinical guidance (from WHO, EASL, and Indian national guidance across multiple years; appendix p 3) to predict which regimens are preferred by each country in every year and for every subgroup of the treatment population. The results presented are based on equal relative weights for the prices and guidelines, as defined by sensitivity testing and the inspection of global and country-specific uptake and effect outputs (appendix pp 4–5). The model splits the potential treatment population into subgroups. For HIV, the model distinguishes between first-line and second-line antiretroviral therapy, as per WHO recommendations (appendix p 6), and for HCV the model splits the potential treatment population by genotypes 1–6 and disease stages (non-cirrhotic, cirrhotic [compensated and decompensated], or hepatocellular carcinoma; appendix p 6). These population splits allow the model to predict intermediate levels of scale-up, such as using dolutegravir for second-line treatments only or using daclatasvir for a specific genotype or disease stage only.
Uptake curves are modelled for both counterfactual and factual scenarios individually, for all countries. For HIV, the model assumes that people living with HIV on antiretroviral therapy can only change regimen gradually for clinical reasons, and are unlikely to move back to a previous suboptimal regimen after having made the switch to a better option. When a country changes its preferred HIV treatment, the population of people living with HIV on antiretroviral therapy in the relevant subgroup will transition over several years, following a trajectory described by an s-shaped logistic function (appendix p 8). For HCV, the model contains no barriers to changing regimen from one year to the next, since it is considered that subsequent treatments are used to treat different people and the continuity of treatment, which lasts 8–24 weeks, is therefore irrelevant. Modelled volumes are adjusted to mirror the actual sales, collected from MPP licensees, for past uptake and to align with the best available market share forecasts for future uptake, obtained from the Polaris Observatory (appendix p 9).17, 18 Modelled uptake trajectories are transformed into the numbers of people treated in each country-year through matching with available epidemiological information, obtained from UNAIDS.18
Step 4: outcomes
The model applies evidence-informed assumptions to translate uptake into outcomes, including health outcomes (mortality, morbidity, and adverse effects linked to disease progression or the medicines used; appendix pp 9–10) and economic outcomes (drugs costs and health system costs associated with untreated disease progression; appendix pp 3, 9–10). The effect is derived from the comparison of factual and counterfactual country-level outcomes (ie, the difference between scenarios; appendix pp 9–10).
The effect of the MPP licence for dolutegravir is modelled with the following assumptions: (1) alternatives to regimens based on generic dolutegravir licensed by MPP that are considered to be counterfactual treatment options include the use of first-line regimens based on generic efavirenz, first-line and second-line regimens based on originator and bilaterally licensed generic dolutegravir, and second-line regimens based on lopinavir and ritonavir, among other options; (2) cost savings are obtained from comparing the costs of products based on dolutegravir that are MPP licensed for adults across all lines of treatment (as per WHO recommendations) with a weighted average cost of the regimens that would have been used in the counterfactual scenario (ie, in the absence of the MPP license); and (3) the health impact considers the scale of uptake of dolutegravir and its benefits compared with alternative treatments (ie, efavirenz as a first-line alternative).19
The effect of the MPP licence for daclatasvir is modelled on the following assumptions: (1) alternatives to regimens based on generic daclatasvir licensed by MPP considered as counterfactual treatment options include the use of more expensive originator and bilaterally licensed generic sofosbuvir and daclatasvir; sofosbuvir and ledipasvir; sofosbuvir and velpatasvir; sofosbuvir and ribavirin; sofosbuvir, daclatasvir, and ribavirin; sofosbuvir, ledipasvir, and ribavirin; and glecaprevir and pibrentasvir (as either fixed-dose combinations or individual components); (2) cost savings are obtained from comparing the costs of products based on daclatasvir that are MPP licensed with those of other treatments used in the counterfactual scenario, and additional expenses from scaling up treatment are subtracted from these savings; and (3) the health impact triggered by access to more affordable (ie, cheaper) recommended treatment options is obtained from diagnosed HCV cases being given treatment earlier and treatment programmes being expanded (ie, more people being treated and cured).
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
The early licensing of dolutegravir from ViiV Healthcare to MPP in 2014, less than a year after the US Food and Drug Administration approval of the originator product, and sublicensing to a large number of generic manufacturers, have enabled the uptake of generic versions that are quality-assured and affordable (including the once-per-day oral fixed-dose combination of tenofovir disoproxil fumarate [300 mg], lamivudine [300 mg], and dolutegravir [50 mg]) with unprecedented speed, all which can be found in the MPP.14, 20 As of June, 2020, 11 generic manufacturers had quality-assured dolutegravir-based products on the market, and supplies had been delivered to 106 countries (appendix pp 13–14) after rapid and extensive price reductions (initiated by the early negotiated pricing for generic tenofovir disoproxil fumarate, lamivudine, and dolutegravir at US$75 per person per year announced in 2017).21
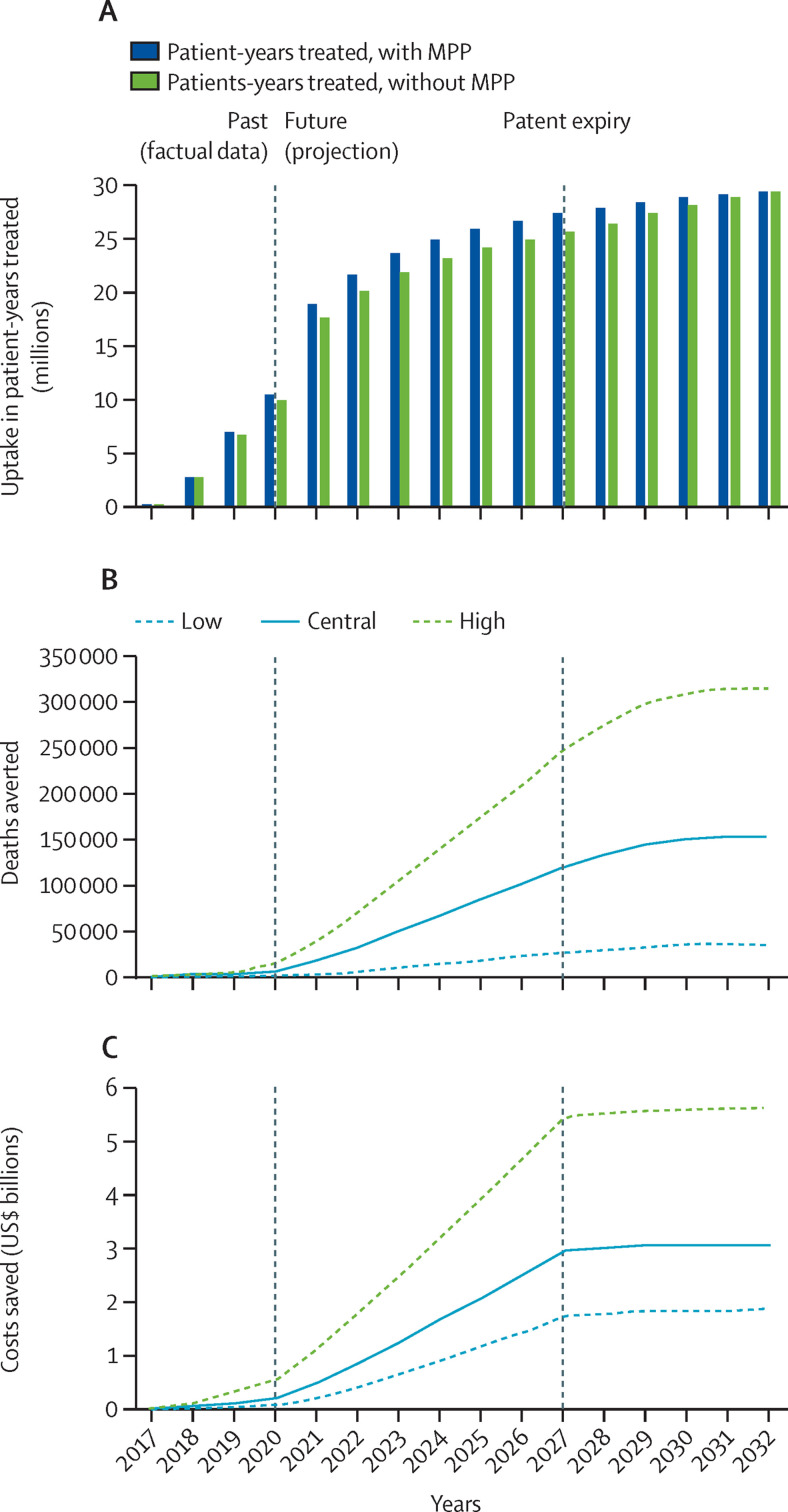
Cumulatively, between 2017 and 2032 (ie, until five years following effective patent expiry in 2026/2027), the model predicted 332·6 million patient-years treated with dolutegravir through an additional uptake of 15·494 (range 14·406–15·494) million patient-years of dolutegravir-based products compared with the counterfactual scenarios. The model also predicted 151 839 (34 575–312 973) deaths averted cumulatively, and $3·074 (1·837–5·617) billion saved cumulatively by the MPP licence for dolutegravir, which can be found on MedsPaL. Furthermore, 1·086 (0·247–2·239) million disability-adjusted life-years, 1·394 (0·288–2·789) million virological failures, and 4555 (908–9436) mother-to-child transmissions were also predicted to be averted during this period (figure 2 and appendix pp 15–18, including data disaggregated by World Bank country income category and geographical region).
Figure 2.
Uptake and effect of the MPP licence for dolutegravir
(A) Dolutegravir uptake in patient-years treated in low-income and middle-income countries with or without the MPP license, in the central scenario by year. Low, central, and high health and economic impact scenarios for cumulative deaths averted (B) and cost savings (C) from the implementation of the license were obtained by considering a set of ranges for key health and generic competition variables (appendix p 12). MPP=Medicines Patent Pool.
Daclatasvir was licensed from Bristol-Myers Squibb to MPP in 2015.15 As of June, 2020, three generic manufacturers had daclatasvir-based products that were quality-assured on the market, and supplies had been delivered to 30 countries (appendix p 19), with prices decreasing substantially over time.22 Although the daclatasvir patent expiry should have been between 2027 and 2030, the patent holder decided to let patents lapse after market withdrawal in 2020; this has effectively truncated the period of impact.
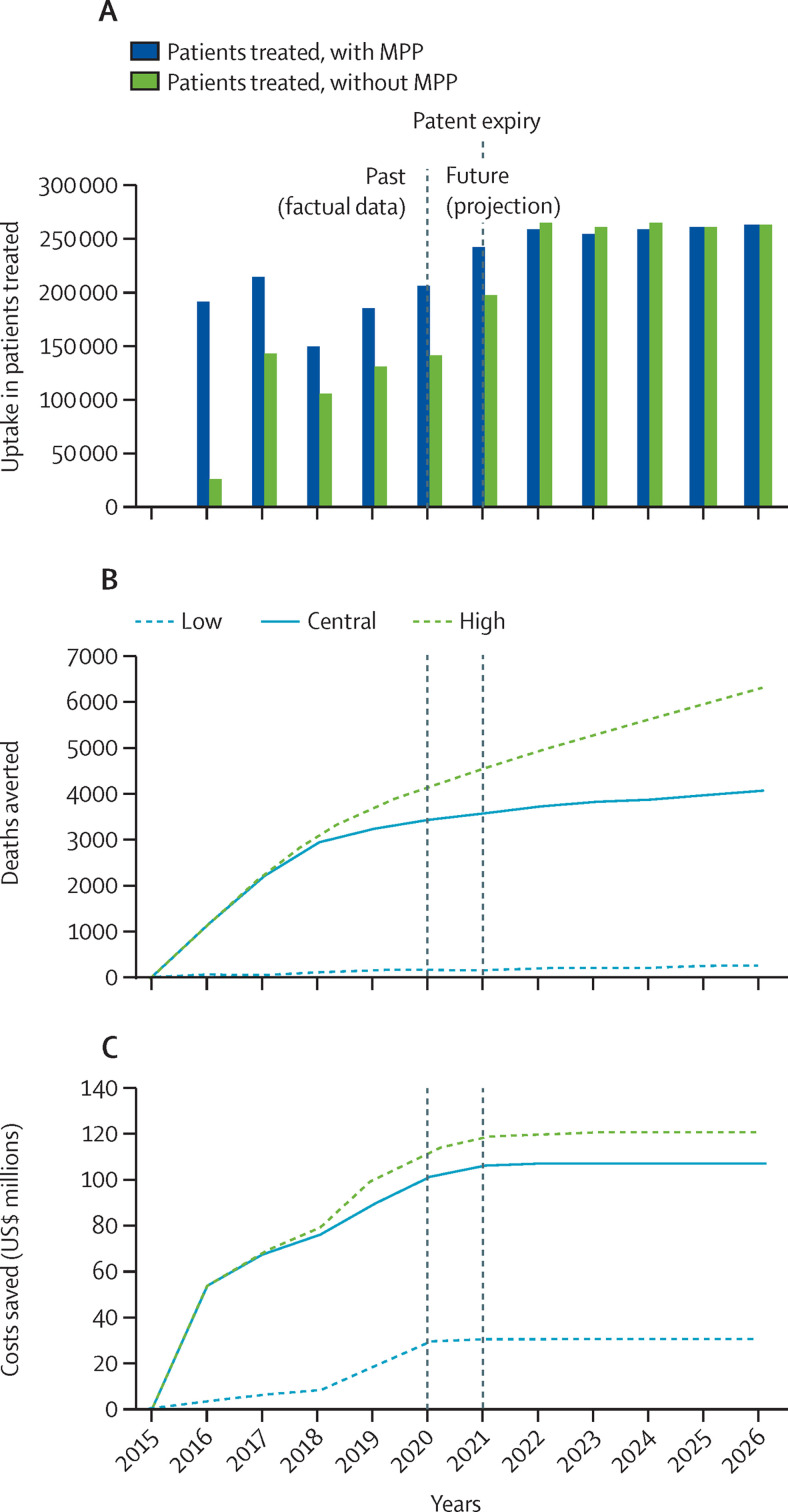
Cumulatively, between 2015 and 2026 (ie, until 5 years after the effective patent expiry in 2020 and 2021), the model predicted 2·477 million patients treated with once-per-day oral daclatasvir (60 mg) licensed by MPP through an additional uptake of 428 244 (range 127 584–636 270) patients treated with daclatasvir-based products compared with the counterfactual scenario, including 14 003 (1610–43 766) people with HCV who would otherwise not have been treated at all. The model also predicted 4070 (225–6323) deaths averted, and $107·593 (30·377–121·284) million saved because of the MPP licence for daclatasvir. In addition to reduced mortality, intermediate health impact areas were also modelled and included 1765 (321–6033) cases of compensated cirrhosis, 165 (32–695) cases of decompensated cirrhosis, and 136 (26–468) cases of hepatocellular carcinoma averted, with $20·233 (2·044–34·316) million in additional health system costs associated with the progression of HCV disease avoided (figure 3 and appendix pp 19–21, including data disaggregated by World Bank country income category and geographical region).
Figure 3.
Uptake and effect of the MPP licence for daclatasvir
(A) Daclatasvir uptake in patients treated in low-income and middle-income countries with and without the MPP license, in the central scenario by year. Low, central, and high health and economic impact scenarios for cumulative deaths averted (B) and cost savings (C) from the implementation of the license were obtained by considering a set of ranges for key health and generic competition variables (appendix p 13). MPP=Medicines Patent Pool.
Discussion
Access-oriented voluntary licensing has emerged as an effective strategy to make quality-assured versions of priority patented essential medicines available at lower prices (and thereby improving affordability) in LMICs, and it has been recognised accordingly in industry benchmark studies such as the Access to Medicine Index studies.23 Although there have previously been efforts at measuring the effect of some voluntary licences, the focus has been limited to economic and uptake aspects.
In 2017, Juneja and colleagues6 presented the impact assessment model used so far by MPP. This model offered a straightforward method for estimating what would have been the costs of procuring equivalent volumes of specific products in the absence of MPP licences. It provided a simple, consistent way of estimating the impact across a range of different products and diseases, providing insight into how much it would have cost countries to achieve the same health outcomes without MPP licences. Using this approach, the authors had estimated that the cost savings from MPP licences for HIV medicines between 2010 and 2028 would be US$2·3 billion saved, equivalent to 24 million patient-years of first-line HIV treatment as per the costs of these treatments in 2016. The study6 did not report on health outcomes and assumed that uptake of these specific medicines without MPP licences would have been identical, regardless of the price of those medicines (appendix p 2).
In a step towards characterising the health impact of expanding treatment programmes, Simmons and colleagues7 explored the effect of licensing on the uptake of HCV medicines. Their approach looked at year and country variations of licensing implementation. The authors compared HCV treatment uptake in the presence and absence of voluntary licences, with a panel of 35 intervention and control LMICs between 2004 and 2016. Findings pointed to voluntary licences leading to increases in the annual numbers of people being given those medicines of 54 (range 26–82) per 1000 people diagnosed. A lag time after the licensing agreements was observed, with a trend towards an increased effect over time; this finding was based on a few years of observation only, using data available until 2016, with no longer term visibility. That study did not report on the resulting health impact of treating more people.7
Although market competition from access to multiple sources of generic HIV and HCV medicines has largely driven prices down in LMICs, it is important to recognise the benefits not only of access to cheaper drugs, but also of earlier access to medicines with a sometimes higher efficacy, better side-effect profile, improved adherence, reduced likelihood for treatment resistance, failure, and mortality over time, or a combination of these factors.24
This study provides, for the first time, a robust modelling framework that includes a realistic assessment of counterfactual scenarios, to not only produce estimates of cost savings, but also to inform on some of the health effects arising from earlier access to and accelerated uptake of quality-assured, affordable optimal treatments in LMICs.
Our model showed that the chain of effects linking upstream licensing to downstream outcomes can be modelled to give credible quantitative estimates of the economic and health impacts arising from access-oriented voluntary licensing, based on assumptions that generic competition leads to price reductions influencing procurement decisions, in turn enabling faster and broader access to medicines, with beneficial economic and health outcomes. The effect of access-oriented voluntary licensing interventions on health outcomes and cost savings was dependent on the timing and breadth of the in-country generic uptake, the extent of the competition (and corresponding price reductions), the scope of the licensed territories, the remaining time to patent expiry, and the clinical benefits of the licensed treatments (and their positioning in normative guidance).
In developing the assumptions for the model, a conservative approach was taken in several instances to avoid an overestimation of impact. For example, in selecting between alternative studies to estimate the effect of the number of generic companies on prices, the study selected16 was the most conservative among those reviewed, leading to lower estimated cost savings from MPP licences (appendix p 4). Further, the country-level number of competing generic manufacturers with MPP differs from the total number of MPP licensees and the model generally takes a conservative approach to this assumption. For example, although MPP had 17 licensees for dolutegravir and 11 companies producing quality-assured products, the country-level number of competing generics considered by the model was four (appendix pp 4, 12). This assumption was because not all licensees have developed the product, not all have registered the product in a given country, and not all are willing or ready to supply a given country at any given time. The licensing assumptions in the counterfactual scenarios also recognise probable access programmes that originator companies would have rolled out. For example, for countries that would have accessed generic dolutegravir in the absence of the MPP license, only small cost savings are considered (given the moderately stronger competition with the MPP-licensed drug, appendix pp 4, 12–13), and no health effect is modelled (since it is considered that these countries would have switched to dolutegravir-based regimens anyway in the absence of the MPP license).
The capability and adaptability of the model were bound by practical constraints and limitations. The results relied on assumptions at several points, such as the effect of licensing on generic competition, generic competition on price, price on uptake, and uptake on outcomes. Assumptions were based on studies providing reasonable estimates for the effects being explored. Other factors associated with the speed and breadth of uptake (such as the inclusion of a medicine in WHO and other clinical guidelines, the manufacturing capacity to match the anticipated demand, international donor funding and support, and government-led and other programmes supporting scale-up) were treated exogenously. The appendix includes details on how key variables affected the scale and composition of the estimated effect and sensitivity analyses for some of the factors (appendix pp 5, 9). In addition, the uncertainty in some input variables of key importance was also recognised by using multiple (low, central, and high) health and economic impact scenarios that led to the estimated ranges in the modelled outcomes.
The model did not capture all possible effect channels, and there are other ways in which voluntary licences that are access-oriented and non-exclusive could have an effect. Facilitating the development of new formulations, such as new fixed-dose combinations (eg, tenofovir disoproxil fumarate, lamivudine, and dolutegravir), can contribute to improving adherence to treatment (and thus health outcomes), or reduce supply chain requirements (leading to cost savings). Licensing might also facilitate the development of adapted paediatric formulations or have a role in the timing of changes to public-health oriented clinical guidelines (eg, from WHO).
The model was also not a comprehensive epidemiological representation of the studied disease areas. It applied the reported variables to estimate approximate health outcomes but did not model transmission dynamics and other feedback loops, subpopulation geographical diversity, and resistance dynamics (although it reported some outcomes related to disease progression, transmission, and resistance). The model also did not calculate any indirect effect on prices for competing products (eg, the availability of MPP-licensed generic products was not assumed to affect the price of other products).
For some products of particular importance, the effect of access-oriented voluntary licensing can be substantial. This effect was observed for the MPP licence for dolutegravir, for which the projected cumulative cost savings are in the same range as the total The Global Fund HIV investments in Nigeria, South Africa, and Zimbabwe combined between 2002 and 2020, and the projected cumulative deaths averted correspond to the total number of AIDS-related deaths in 2019 in Nigeria, Mozambique, Uganda, Kenya, and Democratic Republic of the Congo combined.25
It is important to note that this study focused on the effect of specific MPP licences and did not cover the full effect of licensing the products to generic manufacturers. Rather, we explored the incremental effects of these MPP licences (compared with what would have otherwise happened in the absence of the MPP licence—eg, if patent holders had decided to bilaterally license their products to a smaller number of generic manufacturers or for a smaller geographical area; appendix pp 4, 11–13). Also, the impact calculated for MPP licences does not imply the sole attribution of this effect to the MPP licences; this effect requires multiple contributions from other parties, including patent holders (originators), generic manufacturers, and various other organisations in the global public health landscape, such as those involved in normative guidance, regulatory approvals, funding, procurement, service delivery, advocacy (including for treatment literacy and demand creation), and others.
Voluntary licences have enabled broad access to quality-assured, affordable versions of high-volume, low-cost, oral, tablet drug formulations, and this study has shown that voluntary MPP licences for WHO-recommended treatments that are access-oriented lead to both economic and health benefits for people in LMICs, saving money and lives. These considerations are relevant to the debates around access to the patented medicines under development for COVID-19, for which the timely availability of affordable treatments in a large number of countries and possibly in large volumes is considered a priority.1, 2 This study can help to inform policy decisions on the use of access-oriented voluntary licensing and other access strategies for COVID-19 and other essential medicines, and pipeline candidates of global health importance.3, 4, 26, 27 With this in mind, governments and their research and development funding agencies should explore ways to further incentivise voluntary licensing that is access-oriented and non-exclusive (including through MPP) of medicines that are needed for LMICs, and patent holders should feel encouraged that licensing some of these medicines could lead to a large positive health and economic effect for populations. To enable this effect, procurement agencies, funders, governments, civil society, and communities of people affected by HIV, HCV, and other diseases should support the rapid initial scale-up and sustained uptake of WHO-recommended treatment options benefiting from access-oriented and non-exclusive voluntary licensing agreements.
Data sharing
Information on patents and licences of MPP-licensed and other patented essential medicines is available through MPP's MedsPaL database at https://www.medspal.org, and a quarterly updated overview of country-level registration and supply of MPP-licensed products is available on the MPP website at https://medicinespatentpool.org/progress-achievements/access-to-medicines-tracker (appendix).
Declaration of interests
SM is an employee of the MPP and reports grants from Unitaid. HBM is an employee of MPP and reports grants from Unitaid. EB is an employee of MPP and reports grants from Unitaid. OB-H has acted as a consultant for MPP for this work. CvD has acted as a consultant for MPP for this work. JVL has acted as a consultant for MPP for this work.
Acknowledgments
Acknowledgments
We thank Andrew Phillips and several colleagues and partners at MPP, Unitaid, and the CDA Foundation for their support during this project. We also thank Gilead Sciences for sharing bilateral generic licensees' data on the volumes and prices of treatments for hepatitis C virus (HCV) and allowing their use in informing part of this modelling exercise. We also thank Unitaid for the funding to MPP enabling this work, as well as all stakeholders: patent holders (in particular, Bristol-Myers Squibb and ViiV Healthcare), generic manufacturers, procurement agencies, funders, governments, civil society, and the communities of people affected by HIV, HCV, and other diseases for their essential contributions in enabling the positive effect of MPP licences.
Contributors
OB-H and CvD developed the model, with technical guidance and inputs from SM, HBM, EB, and JVL. SM, JVL, and EB conceptualised the paper, which was assembled by SM, and EB was responsible for the decision to submit for publication. OB-H, CvD, and SM accessed and verified the data. All authors reviewed the results or contributed to the manuscript, or both.
Supplementary Material
References
- 1.WHO WHO Access to COVID-19 Tools (ACT) Accelerator. https://www.who.int/initiatives/act-accelerator
- 2.WHO COVID-19 Technology Access Pool. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/global-research-on-novel-coronavirus-2019-ncov/covid-19-technology-access-pool [DOI] [PubMed]
- 3.WHO Roadmap for access to medicines, vaccines and health product 2019–2023: comprehensive support for access to medicines, vaccines and other health products. 2019. https://apps.who.int/iris/handle/10665/330145
- 4.Wirtz VJ, Hogerzeil HV, Gray AL, et al. Essential medicines for universal health coverage. Lancet. 2017;389:403–476. doi: 10.1016/S0140-6736(16)31599-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beck EJ, Mandalia S, DongmoNguimfack B, et al. Does the political will exist to bring quality-assured and affordable drugs to low- and middle-income countries? Glob Health Action. 2019;12 doi: 10.1080/16549716.2019.1586317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Juneja S, Gupta A, Moon S, Resch S. Projected savings through public health voluntary licences of HIV drugs negotiated by the Medicines Patent Pool (MPP) PLoS One. 2017;12 doi: 10.1371/journal.pone.0177770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simmons B, Cooke GS, Miraldo M. Effect of voluntary licences for hepatitis C medicines on access to treatment: a difference-in-differences analysis. Lancet Glob Health. 2019;7:e1189–e1196. doi: 10.1016/S2214-109X(19)30266-9. [DOI] [PubMed] [Google Scholar]
- 8.Waning B, Diedrichsen E, Moon S. A lifeline to treatment: the role of Indian generic manufacturers in supplying antiretroviral medicines to developing countries. J Int AIDS Soc. 2010;13:35. doi: 10.1186/1758-2652-13-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Waning B, Kyle M, Diedrichsen E, et al. Intervening in global markets to improve access to HIV/AIDS treatment: an analysis of international policies and the dynamics of global antiretroviral medicines markets. Global Health. 2010;6:9. doi: 10.1186/1744-8603-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moon S, Jambert E, Childs M, von Schoen-Angerer T. A win-win solution?: a critical analysis of tiered pricing to improve access to medicines in developing countries. Global Health. 2011;7:39. doi: 10.1186/1744-8603-7-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bermudez J, 't Hoen E. The UNITAID patent pool initiative: bringing patents together for the common good. Open AIDS J. 2010;4:37–40. doi: 10.2174/1874613601004020037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Childs M. Towards a patent pool for HIV medicines: the background. Open AIDS J. 2010;4:33–36. doi: 10.2174/1874613601004020033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoen E, Berger J, Calmy A, Moon S. Driving a decade of change: HIV/AIDS, patents and access to medicines for all. J Int AIDS Soc. 2011;14:15. doi: 10.1186/1758-2652-14-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Medicines Patent Pool Dolutegravir – adult (DTG) April, 2014. https://medicinespatentpool.org/licence-post/dolutegravir-adult-dtg
- 15.Medicines Patent Pool Daclatasvir (DAC) November, 2015. https://medicinespatentpool.org/licence-post/daclatasvir-dac
- 16.Dave C, Hartzema A, Kesselheim AS. Prices of generic drugs associated with numbers of manufacturers. N Engl J Med. 2017;377:2597–2598. doi: 10.1056/NEJMc1711899. [DOI] [PubMed] [Google Scholar]
- 17.Gupta A, Juneja S, Vitoria M, et al. Projected uptake of new antiretroviral (ARV) medicines in adults in low- and middle-income countries: a forecast analysis 2015–2025. PLoS One. 2016;11 doi: 10.1371/journal.pone.0164619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.CDA Foundation Polaris Observatory. 2020. https://cdafound.org/dashboard/polaris/dashboard.html
- 19.Phillips AN, Venter F, Havlir D, et al. Risks and benefits of dolutegravir-based antiretroviral drug regimens in sub-Saharan Africa: a modelling study. Lancet HIV. 2019;6:e116–e127. doi: 10.1016/S2352-3018(18)30317-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Medicines Patent Pool ViiV Healthcare-MPP-Aurobindo. May 4, 2020. https://medicinespatentpool.org/company-post/viiv-healthcare-mpp-aurobindo
- 21.Clinton Health Access Initiative New high-quality antiretroviral therapy to be launched in South Africa, Kenya and over 90 low- and middle-income countries at reduced price. 2017. https://www.clintonhealthaccess.org/new-high-quality-antiretroviral-therapy-launched-south-africa-kenya-90-low-middle-income-countries-reduced-price/
- 22.WHO Accelerating access to hepatitis C diagnostics and treatment. Overcoming barriers in low- and middle-income countries. Jan 27, 2021. https://www.who.int/publications/i/item/9789240019003
- 23.Access to Medicine Foundation 2021 Access to Medicine Index. Jan 26, 2021. https://accesstomedicinefoundation.org/news/now-online-2021-access-to-medicine-index
- 24.Ford N, Wi T, Easterbrook P, Penazzato M, Vitoria M. Global public health efforts to address HIV and related communicable disease syndemics. Curr Opin HIV AIDS. 2020;15:261–265. doi: 10.1097/COH.0000000000000636. [DOI] [PubMed] [Google Scholar]
- 25.The Global Fund Results report 2020. 2020. https://www.theglobalfund.org/media/10103/corporate_2020resultsreport_report_en.pdf
- 26.World Trade Organization Declaration on the TRIPS Agreement and Public Health. Nov 14, 2001. https://www.wto.org/english/thewto_e/minist_e/min01_e/mindecl_trips_e.htm
- 27.Burrone E, Gotham D, Gray A, et al. Patent pooling to increase access to essential medicines. Bull World Health Organ. 2019;97:575–577. doi: 10.2471/BLT.18.229179. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Information on patents and licences of MPP-licensed and other patented essential medicines is available through MPP's MedsPaL database at https://www.medspal.org, and a quarterly updated overview of country-level registration and supply of MPP-licensed products is available on the MPP website at https://medicinespatentpool.org/progress-achievements/access-to-medicines-tracker (appendix).