Summary
Background
In view of emerging variants of concern (VOCs), we aimed to evaluate the impact of various allocation strategies of COVID-19 vaccines and antiviral such that the pandemic exit strategy could be tailored to risks and preferences of jurisdictions in the East Asia and Pacific region (EAP) to improve its efficiency and effectiveness.
Methods
Vaccine efficacies were estimated from the titre distributions of 50% plaque reduction neutralization test (PRNT50), assuming that PRNT50 titres of primary vaccination decreased by 2-10 folds due to antibody waning and emergence of VOCs, and an additional dose of vaccine would increase PRNT50 titres by 3- or 9-fold. We then used an existing SARS-CoV-2 transmission model to assess the outcomes of vaccine allocation strategies with and without the use of antivirals for symptomatic patients in Japan, Hong Kong, and Vietnam.
Findings
Increasing primary vaccination coverage was the most important contributing factor in reducing the total and peak number of COVID-19 hospitalisations, especially when population vaccine coverage or vaccine uptake among older adults was low. Providing antivirals to 50% of symptomatic infections only further reduced total and peak hospitalisations by 10-13%. The effectiveness of an additional dose of vaccine was highly dependent on the immune escape potential of VOCs and antibody waning, but less dependent on the boosting efficacy of the additional dose.
Interpretation
Increasing primary vaccination coverage should be prioritised in the design of allocation strategies of COVID-19 vaccines and antivirals against emerging VOCs, such as Omicron, in the EAP region. Heterologous vaccination with any available vaccine as the additional dose could be considered when planning pandemic exit strategies tailored to the circumstances of EAP jurisdictions.
Funding
Health and Medical Research Fund, General Research Fund, AIR@InnoHK
Keywords: SARS-CoV-2, COVID-19, Vaccination, Antiviral, Booster vaccination, Immune escape, Antibody waning, Allocation strategy, Pandemic exit strategy, Variant, Variant of concern, VOC, Delta variant, Omicron variant
Research in context.
Evidence before this study
In view of emerging variants of concern (VOCs), we searched PubMed and preprint archives for articles published up to 13 December 2021, that contained information about the control measures against COVID-19 outbreak using the terms “coronavirus”, “COVID-19”, “SARS-CoV-2”, “variant”, “VOC”, “vaccin*”, “vaccine”, “antiviral”, “treatment” and “allocation”. Previous studies have focused on the optimal allocation of vaccines to reduce disease burden of COVID-19 pandemic. However, to our knowledge, there is no study to investigate the impacts of waning vaccine effectiveness on COVID-19 vaccine and antiviral allocation strategies in the East Asia and Pacific (EAP) region against emerging VOCs.
Added value of this study
We used an existing SARS-CoV-2 transmission model to assess the outcomes of vaccine allocation strategies with and without the use of antivirals for symptomatic patients in Japan, Hong Kong, and Vietnam. We found that increasing primary vaccination coverage was the most important contributing factor in reducing the total and peak number of COVID-19 hospitalisations, especially when population vaccine coverage or vaccine uptake among older adults was low. The effectiveness of an additional dose of vaccine was highly dependent on the immune escape potential of VOCs and antibody waning, but less dependent on the boosting efficacy of the additional dose.
Implications of all the available evidence
Our study provided support to the prioritization of increasing primary vaccination coverage among all the allocation strategies of vaccine and antivirals considered. Administering antivirals would only have limited impact in reducing total and peak hospitalisations. Heterologous vaccination with any available vaccines as the additional dose, as long as it is safe, could be considered as potential options when planning the pandemic exit strategies tailored to the preference of EAP jurisdictions, especially for low- and middle-income countries and in anticipation of further emergence of new VOCs.
Alt-text: Unlabelled box
Introduction
By implementing public health measures rapidly and effectively, most countries in the East Asia and Pacific region (EAP) kept COVID-19 cases and deaths much lower than in the US, Europe, and South America in 2020. However, the emergence of variants of concern (VOCs), especially the Delta and Omicron variants which are more transmissible and have greater immune escape potential, changed the game the put stress on the EAP region in 2021. Many low- and middle-income countries in EAP were left behind to develop or purchase COVID-19 vaccines and antivirals. By mid-August 2021, more than half of the populations in the United States, United Kingdom, and more than a dozen of high-income and upper-middle-income countries have been fully vaccinated according to the initially designed immunisation schedule (mostly with two doses); while conversely, only 1.2% of population in low-income countries have received at least one dose.1 Molnupiravir and Paxlovid, the antivirals that were reported to reduce the risk of COVID-19 hospitalisation and death by 30-50% and 89% respectively in the phase 2/3 trial,2, 3, 4 cost US$700 per course which means they might not be available in most low- and middle-income countries in the short term.
Moreover, Delta and Omicron have caused large outbreaks in highly vaccinated populations5 and it has led to calls for an additional dose of COVID-19 vaccine, e.g. a third dose for most vaccines and a second dose for some adeno-vectored vaccines.6, 7, 8, 9 On one hand, “breakthrough” infections and reduction of in vitro serum neutralizing antibodies over time have been observed,8,10 suggesting a potential waning in vaccine efficacy against infection11; on the other hand, the severity of disease in vaccinated individuals is nevertheless much reduced compared to unvaccinated individuals, indicating that the vaccines are still highly effective in reducing hospitalisations and deaths.11, 12, 13
While equity is important in health decision making, EAP jurisdictions would have various expectation of vaccines and antivirals due to differences in the society and economy. The first group of jurisdictions, which has pursued a Zero-COVID or elimination strategy to stringently control COVID-19 within their borders, includes mainland China, Hong Kong, Macau, and Taiwan. A vaccine-driven exit strategy is the most likely path for these jurisdictions: if they were to minimise infections of VOCs in a highly susceptible population in the absence of public health measures, higher protection from vaccines would be required, which might only be attained by fully vaccinating all the eligible individuals with both primary and additional doses of the most efficacious vaccines.14 The second group of jurisdictions, which is in transition to a Living-with-COVID strategy, includes Australia, Japan, Singapore, South Korea, and most of the Southeast Asian countries. If their goals were to minimise COVID-19 hospitalisations to avoid overwhelming the health system, additional doses of vaccines might not be as necessary if the primary vaccination coverages are high and sufficient supplies of antivirals are secured. Furthermore, vaccine effectiveness also depends on the type of vaccines available, age distribution and underlying health condition of vaccinees and the population, emergence of new VOCs and waning of protection over time.15
Therefore, it is important to evaluate the impact of various allocation strategies of vaccines and antiviral such that the pandemic exit strategy could be tailored to jurisdictions’ risks and preferences to improve its efficiency and effectiveness. Here, we used an existing SARS-CoV-2 transmission model to assess the outcomes of primary and additional vaccine allocation strategies with and without the presence of antivirals. We defined primary vaccination as completion of the initially designed immunisation schedule, which consisted of two doses for most vaccines and one dose for some adeno-vectored vaccines. Vaccines that were given beyond primary vaccination were defined as additional vaccination. We assumed primary vaccination, i.e., vaccinating the never-vaccinated as much as possible, should be always prioritized. Given that the vaccines that many EAP countries receive from the COVAX initiative compose of different types, we analysed hypothetical jurisdictions which mainly used 1) inactivated virus vaccines, 2) mRNA vaccines, 3) adeno-vectored vaccines and 4) a mix of inactivated/adeno-vectored virus and mRNA vaccines, with and without antivirals.
Methods
Predicting the reduction in vaccine efficacy due to emerging VOCs
Currently, three types of vaccines are most widely used in the world, including the adenovirus vector vaccines (e.g. AZD1222 from AstraZeneca), mRNA vaccines (e.g. BNT162b2 from BioNTech) and inactivated virus vaccines (e.g. CoronaVac from Sinovac).15,16 Data from a cohort study of 49 BNT162b2 vaccinees and 49 CoronaVac vaccinees in Hong Kong showed that both vaccines elicited significant immune response against the original virus.17 At one month after the second dose of vaccine, the logarithmic of 50% plaque reduction neutralization test (PRNT50) titres among vaccinees follow a normal distribution, with the mean titre against the original virus of 251.60 (± 1SD: 146.66 – 431.63) among BNT162b2 vaccinees and 69.45 (± 1SD: 28.07 – 171.77) among CoronaVac vaccinees respectively (Figure S1).
Although comparable data about the AZD1222 vaccinees were not available in Hong Kong, it has been reported that the mean titres of pseudo-virus and live virus neutralization tests among AZD1222 vaccinees were 135-172 and 166-261 respectively,18 and the efficacies of AZD1222 vaccines against the original virus lied between CoronaVac and BNT162b2 vaccines.19 Therefore, without loss of generalization, we assumed that the mean PRNT50 titre against the original virus of AZD1222 vaccinees were 133.82 (± 1SD: 78.59 –227.85), which was the average of CoronaVac and BNT162b2 vaccinees in the log scale.
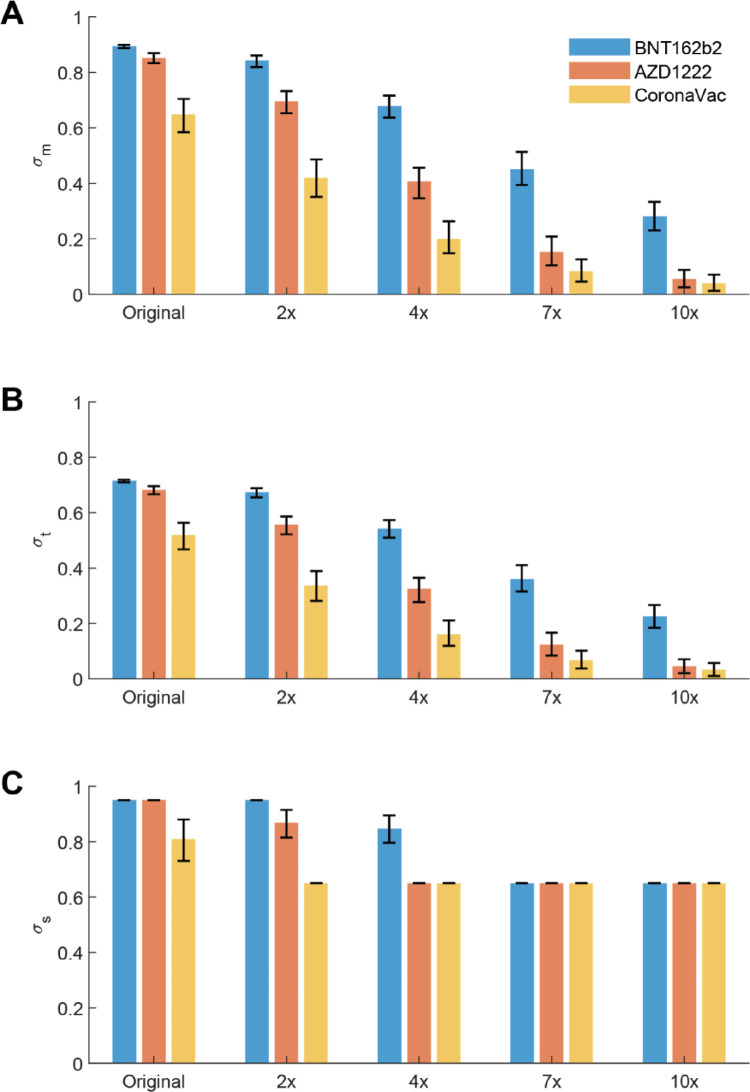
We assumed that vaccine efficacies were reduced mainly due to antibody waning and emergence of VOCs.13,20, 21, 22, 23 Comprehensive analyses of neutralization using isolates of distinct SARS-CoV-2 variants revealed a range of reduction in the neutralization titres among vaccinees who were previously not infected, with an average of 0.5-3.4, 1.6-6.9, 2.3-13.2 and 5.5-41.4 folds of decrease in neutralization titre for the Alpha (B.1.1.7), Delta (B.1.617.2), Beta (B.1.351) and Omicron (B.1.1.529) variants, varied by type of tests, population, and the time since the second dose of mRNA vaccines.10,24, 25, 26 As such, we estimated the vaccine efficacy against infections (i.e., σm) of emerging VOCs by reducing the mean PRNT50 titre level against the original virus strain by a factor of 2, 4, 7, or 10 for hypothetical variants of concern (see Appendix for details).
Assumption about efficacy of vaccines and antivirals
The analyses above estimated vaccine efficacy against SARS-CoV-2 infection. However, it is thought that the immune response after vaccination, especially cellular immunity, may provide greater protection from severe disease than from mild or asymptomatic infection.27 Khoury et al estimated that the neutralization titre level for 50% protection from infection was comparable to the level required for about 85% protection from severe disease.28 Moreover, T cell responses or reactivation of memory B cell responses may also play an important role in protection from severe diseases.27,29, 30, 31 Therefore, we assumed that the vaccine efficacy in reducing symptomatic diseases and admission to hospital (i.e., σs) was 25% higher than σm, i.e., σs = 1.25 × σm and fell within the range between 65% and 95% for emerging VOCs, given that there were limited data about the association between T cell response level and σs. Our assumption about σs was more conservative compared with the observed data on the existing VOCs for most vaccines.21,27 Similarly, we assumed that the vaccine efficacy in reducing infectiousness if infected (i.e., σt) was 20% lower than σm, i.e., σt = 0.8 × σm, for variants of concern, following similar assumptions used by Scientific Advisory Group for Emergencies of the UK.32 For antivirals, we assumed the efficacies were εm = 0 in reducing susceptibility to infection, εt = 0 in reducing infectivity if infected and εs = 0.5 in reducing symptomatic diseases and hospitalisations, since Molnupiravir and Paxlovid are currently only recommended to patients within 5 days of symptom onset.2
Modelling allocation strategies of COVID-19 vaccines and antivirals
We adapted an existing age-structured susceptible-infectious-removed model of SARS-CoV-2 transmission dynamics that can be parameterized with country- or region-specific age demographics and contact patterns to simulate the effect of vaccination (i.e., including primary vaccination and booster vaccination) on transmission.14,33
In three hypothetical populations, we parameterized the model with population demographics and age-specific primary vaccine uptake as of 1 August 2021 from Japan (medium population coverage of 39.7% and high coverage of 87.6% among older adults aged 65 or above), Hong Kong (medium population coverage of 44.5% and low coverage of 19% among older adults aged 70 or above) and Vietnam (low population coverage of 6.0% and minimal coverage among older adults aged 65 or above), to assess the effectiveness of allocation strategies (Figure S2). The three jurisdictions were selected as exemplars of countries in the EAP region based on the types of vaccines available, age-specific vaccination coverage, and timing of booster vaccination programmes (see Appendix for details).
Then we assumed: 1) 100% of vaccinees received BNT162b2 vaccines in Japan, 2) 60% and 40% of vaccinees received BNT162b2 and CoronaVac vaccines in Hong Kong, and 3) 40%, 30% and 30% of vaccinees received BNT162b2, AZD1222, CoronaVac vaccines in Vietnam. Since giving additional vaccines to vaccinees who have completed primary vaccination would have lower marginal effectiveness compared with vaccinating individuals who were never vaccinated (inferred from the titre distribution after the third dose34), therefore, throughout the analysis, we hypothesised that primary vaccination should always be prioritised and considered the following vaccine allocation strategies (Table 1):
Table 1.
Allocation strategies of COVID-19 vaccines and antivirals.
| Strategy | Vaccine and antiviral allocation |
|---|---|
| Strategy 0 |
|
| Strategy 1: PV |
|
| Strategy 2: PV+AR |
|
| Strategy 3: PV+AV+AR |
|
| Strategy 4: PV+ selective AV+AR |
|
| Strategy 5: PV+ universal AV+AR |
|
Strategy 1: Vaccinate the never-vaccinated individuals only as much as possible (PV)
Considering the potential vaccine hesitancy and refusal, we assumed that the maximum primary vaccination coverage would achieve 70%, 80% or 90% in three scenarios.
Strategy 2: Vaccinate the never-vaccinated individuals only as much as possible and provide antivirals to 50% of the symptomatic patients (PV+AR)
We assumed that the maximum primary vaccination coverage would achieve 70%, 80% or 90% in three scenarios. We assumed 40% of vaccinees (i.e., completed the primary vaccination) and 60% of unvaccinated individuals would develop symptoms if they were infected (Table S5), and antivirals would be provided to 50% of the symptomatic patients.
Strategy 3: Vaccinate the never-vaccinated individuals as much as possible, give an additional dose to only AZD1222 or CoronaVac vaccinees and provide antivirals to 50% of the symptomatic patients (PV+AV+AR)
We assumed that the maximum vaccination coverage would achieve 70%, 80% or 90% and that all AZD1222/CoronaVac vaccinees were willing to take the additional dose regardless of the type of vaccine that would be given. We assumed that the PRNT50 titres of AZD1222/CoronaVac vaccinees would increase by 3 or 9 folds after the additional dose, which was consistent with the findings in the recent studies about the immunogenicity of a third dose of AZD1222/CoronaVac vaccine (i.e. 2-4 folds increase in antibody titres and T cell response among AZD1222 vaccinees,7 and 3-4 folds increase of antibody titres in healthy adults aged 18 to 59 and ≥7 folds among the older adults aged 60 or above among CoronaVac vaccinees8,35).
Strategy 4: Vaccinate the never-vaccinated individuals as much as possible, give an additional dose to all vaccinated individuals with PRNT50 titre lower than a certain threshold, and provide antivirals to 50% of the symptomatic patients (PV+ selective AV+AR)
Similarly, we assumed that the maximum vaccination coverage would achieve 70%, 80% or 90% and that all vaccinees were willing to take the additional dose if given. We assumed that the PRNT50 titre of BNT162b2 vaccinees would at least increase by 3 or 9 folds after the additional dose.34 The additional dose would only be given to vaccinees with PRNT50 titres lower than the prespecified thresholds. We considered three scenarios in which the additional dose would be given to vaccinees with the original PRNT50 titre lower than 25.9, 39.9 and 74.1, which corresponded to the estimated thresholds of 50%, 60% and 70% protection against infection of the original virus.
Strategy 5: Vaccinate the never-vaccinated individuals as much as possible, give an additional dose to all vaccinated individuals and provide antivirals to 50% of the symptomatic patients (PV+ universal AV+AR)
We assumed that the maximum vaccination coverage would achieve 70%, 80% or 90% and that all vaccinees were willing to receive the additional dose. We assumed both additional dose of homologous and heterologous vaccines would elicit similar increases in PRNT50 titres, though only limited data were currently available from selected patient groups.36
Comparative effectiveness of allocation strategies
Similar to our previous analysis,14 we assumed that vaccines were first allocated to never vaccinated individuals in proportion to the age-specific vaccine uptake as of 1 August 2021 until the population vaccination coverage achieved 70%, 80% or 90% or the vaccine uptake of a specific age group achieved 100%. Then the additional doses were allocated to vaccinees following the five allocation strategies above. In the simulation, we assumed antivirals would be directly allocated to 50% symptomatic infections who would seek medical care, and antivirals would only reduce the risk of hospitalisation by 50% (Table 1).
We assumed that the population was completely susceptible before vaccination, i.e., the population immunity was only obtained from vaccination. In view of the emergence of VOCs, we assumed the pre-vaccination effective reproductive (Re) was 6 even with limited public health and social measures in place (e.g. the basic reproductive number of the Delta variant was assumed to be 6, i.e., ∼300% higher than the original virus strain37). We then simulated the epidemics and estimated the total number of hospitalisations and the maximum daily number of hospitalisations.
We estimated the total number of vaccine doses required for every COVID-19 hospitalisation averted in Strategy 1-5 compared with the existing vaccination uptake as of 1 August 2021 (Strategy 0). We also estimated the number of primary and additional vaccine doses required for every COVID-19 hospital admission averted in Strategy 1-5, compared with Strategy 0. Other parameter values were shown in the Appendix.
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Reduction in vaccine efficacy due to antibody waning and VOCs
We estimated that vaccine efficacy in reducing susceptibility (σm) to infection decreased substantially due to VOCs and antibody waning (Figure 1): σm of BNT162b2 vaccinees decreased from 89% to 84%, 68%, 45% and 28% when PRNT50 titres decreased by 2, 4, 7 and 10 folds; σm of AZD1222 vaccinees decreased from 85% to 70%, 41%, 15% and 6%; and σm of CoronaVac vaccinees decreased from 65% to 42%, 20%, 8% and 4% respectively. Similarly, we estimated σt (i.e., vaccine efficacy in reducing infectiousness) decreased from 72% to 67 %, 54%, 36%, 23% for BNT162b2 vaccinees, from 68% to 56%, 33%, 12% and 5% for AZD1222 vaccinees, and from 52% to 33%, 16%, 7% and 3% for CoronaVac vaccinees. For vaccine efficacy in reducing symptomatic disease and hospitalisations, we estimated σs reduced from 95% to 95%, 85%, 65%, 65% for BNT162b2 vaccinees, from 95% to 87%, 65%, 65%, 65% for ADZ1222 vaccinees, and from 81% to 65%, 65%, 65%, 65% for CoronaVac vaccinees. The estimated vaccine efficacies were largely consistent with findings from case-control trials or real world observations about Alpha and Delta variants,15,38 for which PRNT50 titres were estimated to decreased by 1-4 folds and 2-7 folds respectively.10
Figure 1.
Estimated vaccine efficacies of BNT162b2, AZD1222 and CoronaVac vaccines from PRNT50 titre distributions. The mean PRNT50 titres of vaccinees against the hypothetical variants of concern were assumed to decrease by 2, 4, 7, and 10 folds respectively compared with the PRNT50 titres against the original virus strain. The vaccine efficacies were estimated by bootstrapping the PRNT50 titres of 100 vaccinees by 1000 times. The error bars showed the 95% CI of the estimates with the uncertainty from PRNT50 titre distributions. (A) Vaccine efficacy in reducing susceptibility to infection (σm). (B) Vaccine efficacy in reducing infectiousness if infected (σt). (C) Vaccine efficacy in reducing symptomatic disease and hospital admission (σs).
Effectiveness of allocation strategies of vaccines and antivirals
We assessed the effectiveness of allocation strategies of vaccines and antivirals in Japan, Hong Kong, and Vietnam (Figure 2-4). As expected, increasing primary vaccination coverage was the most important contributing factor in reducing the total and peak number of COVID-19 hospitalisations in Strategy 1-5 (Figure 2-4), especially when the population vaccine coverage or the vaccine uptake among older adults was low (Figure 2-3).
Figure 2.
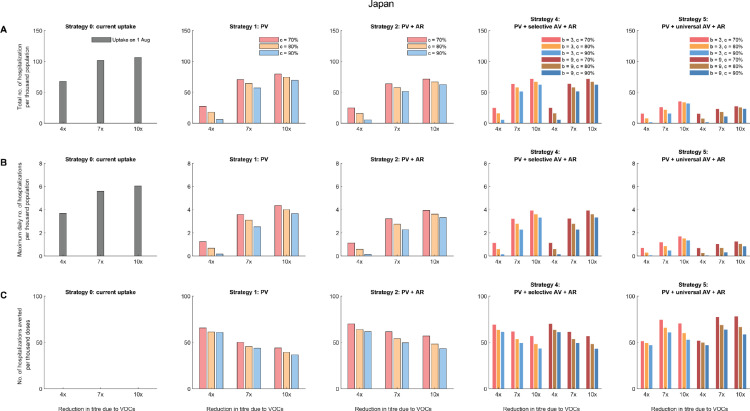
Comparative effectiveness of allocation strategies of vaccines and antivirals in Japan assuming all vaccinees received the BNT162b2 vaccines. As of 1 August 2021, 39.7% of the population had taken at least first dose of vaccine and all of them received the BNT162b2 vaccines. We assumed that the effective reproductive number before the vaccination program (Re) was 6 and that the effects of vaccination (characterized by σm, σt, and σs) were realized instantaneously after the target vaccine uptake was achieved. For Strategy 4, the additional dose would be given to vaccinees with the original PRNT50 titre lower than 74.1, which corresponded to the estimated thresholds of 70% protection against the original virus. The epidemics were seeded with one introduction event immediately after the target vaccine uptake was achieved. We assumed PRNT50 titres reduced by 4, 7 or 10 folds due to VOCs and one dose of booster vaccine increased the PRNT50 titres by 3 or 9 folds (i.e., b = 3 or 9). We assumed the target vaccination coverage (c) was 70%, 80% or 90%. (A) The total number of hospitalisations per thousand population. (B) The maximum daily number of hospitalisations per thousand population. (C) The number of hospitalisations averted per thousand vaccine doses, compared with the existing vaccine uptake as of 1 August 2021 (i.e., about 39.7% coverage).
Figure 4.
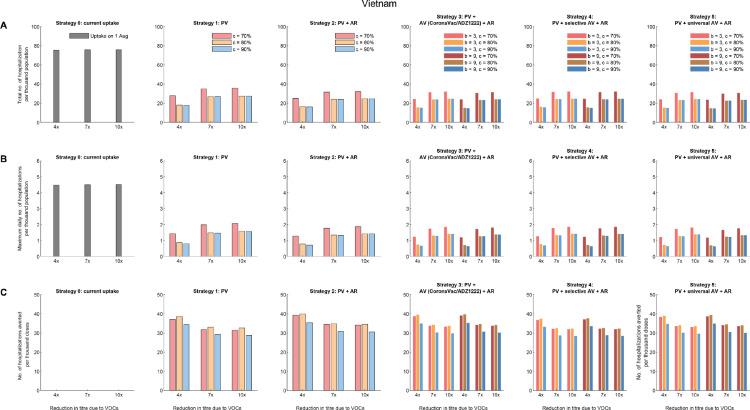
Comparative effectiveness of allocation strategies of vaccines and antivirals in Vietnam assuming 40%, 30% and 30% of vaccinees received BNT162b2, ADZ1222 and CoronaVac vaccines respectively. (A) The total number of hospitalisations per thousand population. (B) The maximum daily number of hospitalisations per thousand population. (C) The number of hospitalisations averted per thousand vaccine doses, compared with the existing vaccine uptake as of 1 August 2021 (i.e., about 6% coverage).
Figure 3.
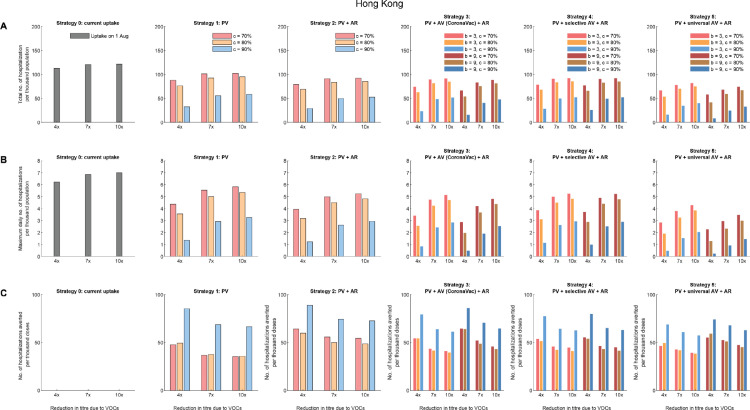
Comparative effectiveness of allocation strategies of vaccines and antivirals in Hong Kong assuming 60% vaccinees received the BNT162b2 vaccines and 40% received CoronaVac vaccines. (A) The total number of hospitalisations per thousand population. (B) The maximum daily number of hospitalisations per thousand population. (C) The number of hospitalisations averted per thousand vaccine doses, compared with the existing vaccine uptake as of 1 August 2021 (i.e., about 44.5% coverage).
In Strategy 1, when PRNT50 titres against VOCs reduced by 4-10 folds (Table 1), increasing primary vaccination coverage from 6% to 70%, 80%, 90% reduced the total hospitalisations by 53-62%, 64-76%, 64-77% in Vietnam, and similar proportions of 54-68%, 65-80%, 65-82% were reduced in peak hospitalisation accordingly. Similarly, in Hong Kong, increasing primary vaccination coverage from 44.5% to 70%, 80%, 90% reduced the total hospitalisations by 15-22%, 22-32%, 52-72% and the peak hospitalisations reduced by 17-30%, 24-43%, 54-78% in Strategy 1.
Compared with Strategy 1, providing antivirals to 50% of symptomatic infections in Strategy 2 only further reduced total and peak hospitalisations by 10-13% and 10-12% in Japan, Hong Kong, and Vietnam across all scenarios. As expected, the number of hospitalisations averted would scale linearly with the antiviral efficacy (εs) and the amount of antiviral prescriptions.
Across Strategy 3-5, the effectiveness of an additional dose of vaccine was highly dependent on the immune escape potential of VOCs or antibody waning. The total and peak hospitalisations increased with the decrease in PRNT50 titres due to VOCs. However, an additional dose of vaccine that generates higher increase in PRNT50 titres would not substantially increase the impact of Strategy 3 and 4: the total and peak hospitalisations, and the number of hospitalisations averted per dose were not significantly different as long as the additional dose increased PRNT50 titres by at least 4 folds. A greater impact was expected in Strategy 5 when the additional dose was given universally to all vaccinees: Strategy 5 had the largest impact in reducing total and peak hospitalisations in Japan where primary vaccine uptake among the elderly was high (Figure 2).
Impacts of different age-specific vaccine uptakes
Across all strategies, the number of hospitalisations averted per thousand doses of vaccines reduced with the increase of primary vaccine uptake from 70% to 90% in Japan (Figure 2 and Figure S3) and Vietnam (Figure 3 and Figure S6-S8). However, in contrast to Japan and Vietnam, the number of hospitalisations averted per thousand doses of vaccines increased significantly with the increase of primary vaccine uptake to 90% in Hong Kong (Figure 3 and Figure S4-S5), which started with a low uptake among the elderly population on 1 August 2021.
Impacts of the increase in PRNT50 titres after the additional dose of vaccine
The impact of an additional dose of vaccine in Strategy 3-5 was small compared with the effects of increasing primary vaccine uptake, especially when the additional dose did not increase PRNT50 titres substantially. Even when the additional dose was given universally to all vaccinees, the impact was dependent on the increase in PRNT50 titres after receiving the additional dose only when a less efficacious vaccine was widely used in the primary vaccination (Figure S3 and Figure S5).
Strategies tailored to the preference of East Asia and Pacific jurisdictions
Strategy 5, which gave the additional dose universally to all vaccinees and provided antivirals to 50% of symptoms, was the most effective in reducing the total and peak hospitalisations, especially when immune escape of VOCs did not result in substantial waning in PRNT50 titres. Facing the threat by Delta and Omicron variants, Strategy 5 might be the only option for EAP jurisdictions aiming to retain Zero-COVID status. For example, in Hong Kong, the maximum daily number of hospitalisations per thousand population was 0.23 if the primary vaccine coverage reached 90%, the PRNT50 titres decreased by 4 folds due to VOCs, antivirals were given to 50% symptomatic patients, and an additional dose that increased PRNT50 titres by 9 folds was given universally to all vaccinees (Figure 3); and this translated to the peak daily number of hospitalisations of 1,725, which was 4.6 times more than the maximum daily number of hospitalisations that Hong Kong would be able to manage (i.e., the maximum daily number of hospitalisations in Hong Kong is estimated to be 375 according to Table S5). As such, even with Strategy 5, some flexible and short-term public health and social measures should be retained during the planning of pandemic exit strategy.
However, for EAP jurisdictions aiming for Living-with-COVID, especially those with high primary vaccination coverage across all age groups with an efficacious vaccine, Strategy 2-4 might be sufficient with no or few additional doses of vaccines. For example, in Japan, the maximum daily number of hospitalisations per thousand population was 0.15 if the primary vaccine coverage reached 90%, the PRNT50 titres decreased by 4 folds due to VOCs, and antivirals were given to 50% of symptomatic patients (Figure 2); and this translated to less than 3 times of the assumed health system capacity (Table S5). Given that Japan had experienced multiple waves of outbreaks and their health system is relatively resilient, additional doses of vaccines could be provided to only selected groups of the population.
Discussion
Our results indicate that increasing primary vaccination coverage was the most important contributing factor to reduce COVID-19 hospitalisations and deaths in the mass vaccination programmes, which is consistent with other modelling studies about EAP countries.39, 40, 41 The effects of increasing primary vaccination coverage were most prominent when the vaccine uptake among older adults was low, such as in the population of Hong Kong, suggesting allocation strategies should prioritise protecting the most vulnerable groups to reduce COVID-19 disease burden through vaccination. Antivirals added minimal impacts in reducing total and peak hospitalisations, although we optimistically assumed 50% of symptomatic patients were provided with antivirals.
Our findings showed that selectively giving additional doses to individuals with lower antibody titres, such as AZD1222/CoronaVac vaccinees only in Strategy 3 or vaccinees with PRNT50 titre lower than a prespecified threshold in Strategy 4, added limited net benefits in terms of reducing the total and peak COVID-19 hospitalisations. Furthermore, when vaccine uptake was low among older adults or a less effective vaccine was widely used, additional doses of vaccines were more effective only when the primary vaccination coverage was higher (such as in Hong Kong in Figure 3 and Figure S5). Our analyses further supported that increasing primary vaccination coverage was essential and should be prioritised.
Interestingly, we found that the effectiveness of the additional dose was negatively associated with the potential immune escapes of VOCs but was less dependent on the boosting efficacy, i.e., the increase in antibody titres generated after the additional dose. Since nearly all COVID-19 vaccines available increased neutralizing antibody titres by at least four folds,34,35,42 heterologous vaccination with any available vaccines, as long as they are safe, could be potential options when planning the allocation strategy.
As of 17 Dec 2021, the newly emerged VOC Omicron has spread rapidly to more than 50 countries and the case numbers rise unprecedentedly fast in countries detected with community transmission.1 Preliminary data show that, though antibodies from a three-shot course of mRNA vaccines produce high levels of neutralising antibodies against Omicron, all the studies report a notable drop in neutralising antibody titres compared with earlier variants such as Delta.25,43 There is an urgent need to understand optimal strategies against Omicron, particularly data about heterologous vaccination and boosters.
Our study has several limitations. First, our assumptions regarding vaccine efficacies against VOCs were based on the statistical association between neutralisation titres and protective efficacy reported in Khoury et al.28. In this study, we have devised a generic method to estimate vaccine efficacy from neutralisation titres which could be applicable before real-world estimates of VEs against VOCs are available (e.g. when new VOCs with increased transmissibility or immune escape potential are first detected).16,23,44 However, the practicability of titre-based vaccine and antiviral allocation strategies would strongly depend on the validity of our method and the availability of appropriate neutralization tests. Second, we did not consider vaccine availability and the level of vaccine hesitancy in different populations. In Hong Kong, the low vaccine uptake among older adults is not because of limited vaccine supply but largely due to worries about vaccine safety among individuals of old age with comorbidities. Whereas for most Southeast Asian countries in the EAP region, increasing primary vaccine uptake to 90% might not be feasible in the short term. Third, our model assumed that the population was completely susceptible to SARS-CoV-2 and that there was no virus circulating before the commencement of vaccination programmes as a case study. Populations that have experienced multiple waves of COVID-19 previously might not need additional doses or antivirals. Recent studies found that natural infections with one dose of vaccines generated higher neutralizing titres and was correlated with highest efficacy against symptomatic infections and hospitalisations.45 Finally, we assumed that limited public health and social measures would be maintained after the primary and additional vaccinations, and a high starting effective reproductive number (Re = 6) of VOCs in the absence of vaccination. Gradual relaxation of public health and social measures would leave more options for the allocation strategies of vaccines and antivirals.
In summary, our results indicate the importance of increasing primary vaccination coverage among all the allocation strategies of vaccine and antivirals considered. Providing antivirals to 50% of symptomatic infections would only reduce total and peak hospitalisations by less than 15%. Heterologous vaccination with any available vaccines as the additional dose, as long as it is safe, could be considered as potential options when planning the pandemic exit strategies tailored to the preference of EAP jurisdictions, especially for low- and middle-income countries and in anticipation of further emergence of new VOCs.
Declaration of interests
The authors declare no competing interests.
Acknowledgments
Contributors
KL, MJ, JTW and GML designed the study. KL, MJ, JTW and GML developed the model and analysed data. KL, MJ, JTW and GML interpreted the results and wrote the manuscript.
Acknowledgement
We thank Shirley Kwok from School of Public Health, The University of Hong Kong for technical support.
Data sharing statement
We collated all data from publicly available data sources. All data included in the analyses are available in the main text or the supplementary materials.
Funding
This research was supported by Health and Medical Research Fund (grant no.: COVID190126, CID-HKU2 and COVID19F05), Health and Medical Research Fund Research Fellowship Scheme (grant no.: 06200097), General Research Fund (grant no.: 17110020), and the AIR@InnoHK administered by Innovation and Technology Commission of the Government of the Hong Kong Special Administrative Region. KL was supported by the Enhanced New Staff Start-up Research Grant from LKS Faculty of Medicine, The University of Hong Kong. MJ received funding from the European Union's SC1-PHE-CORONAVIRUS-2020 - project EpiPose (No 101003688) and the UK's National Institute for Health Research (HPRU-2019-NIHR200908 and HPRU-2019-NIHR200929).
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.lanwpc.2022.100389.
Appendix. Supplementary materials
References
- 1.Ritchie H, Ortiz-Ospina E, Beltekian D, et al. Coronavirus pandemic (COVID-19) Our World in Data. 2021 [Google Scholar]
- 2.Merck. Merck and Ridgeback's Investigational Oral Antiviral Molnupiravir Reduced the Risk of Hospitalization or Death by Approximately 50 Percent Compared to Placebo for Patients with Mild or Moderate COVID-19 in Positive Interim Analysis of Phase 3 Study. 2021. https://www.merck.com/news/merck-and-ridgebacks-investigational-oral-antiviral-molnupiravir-reduced-the-risk-of-hospitalization-or-death-by-approximately-50-percent-compared-to-placebo-for-patients-with-mild-or-moderat/. Accessed 13 December 2021.
- 3.Merck. Merck and Ridgeback Biotherapeutics Provide Update on Results from MOVe-OUT Study of Molnupiravir, an Investigational Oral Antiviral Medicine, in At Risk Adults With Mild-to-Moderate COVID-19. 2021. https://www.merck.com/news/merck-and-ridgeback-biotherapeutics-provide-update-on-results-from-move-out-study-of-molnupiravir-an-investigational-oral-antiviral-medicine-in-at-risk-adults-with-mild-to-moderate-covid-19/. Accessed 13 December 2021.
- 4.Pfizer. Pfizer's novel covid-19 oral antiviral treatment candidate reduced risk of hospitalization or death by 89% in interim analysis of phase 2/3 EPIC-HR study; 2021. https://www.pfizer.com/news/press-release/press-release-detail/pfizers-novel-covid-19-oral-antiviral-treatment-candidate. Accessed 13 December 2021.
- 5.Wadman M. American Association for the Advancement of Science; 2021. Israel's grim warning: Delta can overwhelm shots. [DOI] [PubMed] [Google Scholar]
- 6.Barda N, Dagan N, Cohen C, et al. Effectiveness of a third dose of the BNT162b2 mRNA COVID-19 vaccine for preventing severe outcomes in Israel: an observational study. The Lancet. 2021 doi: 10.1016/S0140-6736(21)02249-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flaxman A, Marchevsky NG, Jenkin D, et al. Reactogenicity and immunogenicity after a late second dose or a third dose of ChAdOx1 nCoV-19 in the UK: a substudy of two randomised controlled trials (COV001 and COV002) The Lancet. 2021;398(10304):981–990. doi: 10.1016/S0140-6736(21)01699-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zeng G, Wu Q, Pan H, et al. Immunogenicity and safety of a third dose of CoronaVac, and immune persistence of a two-dose schedule, in healthy adults: interim results from two single-centre, double-blind, randomised, placebo-controlled phase 2 clinical trials. Lancet Infect Dis. [DOI] [PMC free article] [PubMed]
- 9.Munro APS, Janani L, Cornelius V, et al. Safety and immunogenicity of seven COVID-19 vaccines as a third dose (booster) following two doses of ChAdOx1 nCov-19 or BNT162b2 in the UK (COV-BOOST): a blinded, multicentre, randomised, controlled, phase 2 trial. The Lancet. [DOI] [PMC free article] [PubMed]
- 10.Lucas C, Vogels CBF, Yildirim I, et al. Impact of circulating SARS-CoV-2 variants on mRNA vaccine-induced immunity in uninfected and previously infected individuals. Nature. 2021 doi: 10.1038/s41586-021-04085-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohn BA, Cirillo PM, Murphy CC, Krigbaum NY, Wallace AW. SARS-CoV-2 vaccine protection and deaths among US veterans during 2021. Science. 2021:eabm0620. doi: 10.1126/science.abm0620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lopez Bernal J, Andrews N, Gower C, et al. Effectiveness of Covid-19 Vaccines against the B.1.617.2 (Delta) Variant. N Engl J Med. 2021;385(7):585–594. doi: 10.1056/NEJMoa2108891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Puranik A, Lenehan PJ, Silvert E, et al. Comparison of two highly-effective mRNA vaccines for COVID-19 during periods of Alpha and Delta variant prevalence. medRxiv2021: 2021.08.06.21261707.
- 14.Leung K, Wu JT, Leung GM. Effects of adjusting public health, travel, and social measures during the roll-out of COVID-19 vaccination: a modelling study. Lancet Public Health. 2021 doi: 10.1016/S2468-2667(21)00167-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tregoning JS, Flight KE, Higham SL, Wang Z, Pierce BF. Progress of the COVID-19 vaccine effort: viruses, vaccines and variants versus efficacy, effectiveness and escape. Nat Rev Immunol. 2021 doi: 10.1038/s41577-021-00592-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mallapaty S. China's COVID vaccines have been crucial—now immunity is waning. Nature. 2021;598(7881):398–399. doi: 10.1038/d41586-021-02796-w. [DOI] [PubMed] [Google Scholar]
- 17.Mok CKP, Cohen CA, Cheng SMS, et al. Comparison of the immunogenicity of BNT162b2 and CoronaVac COVID-19 vaccines in Hong Kong. Respirology. 2021 doi: 10.1111/resp.14191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feng S, Phillips DJ, White T, et al. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat Med. 2021;27:2032–2040. doi: 10.1038/s41591-021-01540-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krause PR, Fleming TR, Peto R, et al. Considerations in boosting COVID-19 vaccine immune responses. The Lancet. 2021 Oct;9398(10308):1377–1380. doi: 10.1016/S0140-6736(21)02046-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tang P, Hasan MR, Chemaitelly H, et al. BNT162b2 and mRNA-1273 COVID-19 vaccine effectiveness against the Delta (B.1.617.2) variant in Qatar. Nat Med. 2021;27:2136–2143. doi: 10.1038/s41591-021-01583-4. [DOI] [PubMed] [Google Scholar]
- 21.Tartof SY, Slezak JM, Fischer H, et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: a retrospective cohort study. The Lancet. [DOI] [PMC free article] [PubMed]
- 22.Goel RR, Painter MM, Apostolidis SA, et al. mRNA vaccines induce durable immune memory to SARS-CoV-2 and variants of concern. Science. 0(0):eabm0829. [DOI] [PMC free article] [PubMed]
- 23.Barnard RC, Davies NG, Pearson CAB, Jit M, Edmunds WJ. Modelling the potential consequences of the Omicro SARS-CoV-2 variant in England. 2021. https://cmmid.github.io/topics/covid19/omicron-england.html.
- 24.Laurie MT, Liu J, Sunshine S, et al. SARS-CoV-2 variant exposures elicit antibody responses with differential cross-neutralization of established and emerging strains including Delta and Omicron. J Infect Dis. 2022;635 doi: 10.1093/infdis/jiab635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cele S, Jackson L, Khan K, et al. SARS-CoV-2 Omicron has extensive but incomplete escape of Pfizer BNT162b2 elicited neutralization and requires ACE2 for infection. medRxiv2021: 2021.12.08.21267417.
- 26.Sheward DJ, Kim C, Pankow A, et al. Preliminary Report - Early release, subject to modification - Quantification of the neutralization resistance of the Omicron Variant of Concern. 2021. https://drive.google.com/file/d/1CuxmNYj5cpIuxWXhjjVmuDqntxXwlfXQ/view. Accessed 13 December 2021.
- 27.Cevik M, Grubaugh ND, Iwasaki A, Openshaw P. COVID-19 vaccines: Keeping pace with SARS-CoV-2 variants. Cell. [DOI] [PMC free article] [PubMed]
- 28.Khoury DS, Cromer D, Reynaldi A, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021:1–7. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 29.Sadarangani M, Marchant A, Kollmann TR. Immunological mechanisms of vaccine-induced protection against COVID-19 in humans. Nat Rev Immunol. 2021:1–10. doi: 10.1038/s41577-021-00578-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Geers D, Shamier MC, Bogers S, et al. SARS-CoV-2 variants of concern partially escape humoral but not T cell responses in COVID-19 convalescent donors and vaccine recipients. Sci Immunol. 2021;6(59):eabj1750. doi: 10.1126/sciimmunol.abj1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mateus J, Dan JM, Zhang Z, et al. Low-dose mRNA-1273 COVID-19 vaccine generates durable memory enhanced by cross-reactive T cells. Science; 0(0): eabj9853. [DOI] [PMC free article] [PubMed]
- 32.Scientific Advisory Group for Emergencies. SPI-M-O: Summary of further modelling of easing restrictions – Roadmap Step 4 on 19 July 2021, 7 July 2021. 2021. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1001169/S1301_SPI-M-O_Summary_Roadmap_second_Step_4.2__1_.pdf.
- 33.Wu JT, Leung K, Bushman M, et al. Estimating clinical severity of COVID-19 from the transmission dynamics in Wuhan, China. Nat Med. 2020;26(4):506–510. doi: 10.1038/s41591-020-0822-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Falsey AR, Frenck RW, Walsh EE, et al. SARS-CoV-2 Neutralization with BNT162b2 Vaccine Dose 3. N Engl J Med. 2021 doi: 10.1056/NEJMc2113468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li M, Yang J, Wang L, et al. A booster dose is immunogenic and will be needed for older adults who have completed two doses vaccination with CoronaVac: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. medRxiv2021: 2021.08.03.21261544.
- 36.Bonelli M, Mrak D, Tobudic S, et al. Additional heterologous versus homologous booster vaccination in immunosuppressed patients without SARS-CoV-2 antibody seroconversion after primary mRNA vaccination: a randomised controlled trial. Ann Rheum Dis. 2022 doi: 10.1136/annrheumdis-2021-221558. [DOI] [PubMed] [Google Scholar]
- 37.Dhar MS, Marwal R, Vs R, et al. Genomic characterization and epidemiology of an emerging SARS-CoV-2 variant in Delhi, India. Science. 2021;374(6570):995-999. 10.1126/science.abj9932. Epub 2021; Oct 14. PMID: 34648303; PMCID: PMC7612010. [DOI] [PMC free article] [PubMed]
- 38.Bajema KL. Comparative Effectiveness and Antibody Responses to Moderna and Pfizer-BioNTech COVID-19 Vaccines among Hospitalized Veterans—Five Veterans Affairs Medical Centers, United States, February 1–September 30, 2021. MMWR Morb Mortal Wkly Rep. 2021;70 doi: 10.15585/mmwr.mm7049a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cai J, Yang J, Deng X, et al. Projecting the transition of COVID-19 burden towards the young population while vaccines are rolled out: a modelling study. medRxiv 2021: 2021.10.14.21265032.
- 40.Furuse Y. Simulation of future COVID-19 epidemic by vaccination coverage scenarios in Japan. Journal of Global Health. 2021;11 doi: 10.7189/jogh.11.05025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jayasundara P, Peariasamy KM, Law KB, et al. Sustaining effective COVID-19 control in Malaysia through large-scale vaccination. Epidemics. 2021 doi: 10.1016/j.epidem.2021.100517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zeng G, Wu Q, Pan H, et al. Immunogenicity and safety of a third dose of CoronaVac, and immune persistence of a two-dose schedule, in healthy adults: interim results from two single-centre, double-blind, randomised, placebo-controlled phase 2 clinical trials. Lancet Infect Dis. 2021;8 doi: 10.1016/S1473-3099(21)00681-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wilhelm A, Widera M, Grikscheit K, et al. Reduced Neutralization of SARS-CoV-2 Omicron Variant by Vaccine Sera and Monoclonal Antibodies. medRxiv 2021:2021.12.07.21267432.
- 44.Croda J, Ranzani OT. Booster doses for inactivated COVID-19 vaccines: if, when, and for whom. Lancet Infect Dis. 2021 doi: 10.1016/S1473-3099(21)00696-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reynolds CJ, Pade C, Gibbons JM, et al. Prior SARS-CoV-2 infection rescues B and T cell responses to variants after first vaccine dose. Science. 2021;372(6549):1418–1423. doi: 10.1126/science.abh1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.