Abstract
A 65-year-old man experienced cough and shortness of breath 3 days after receiving the first dose of the Pfizer-BioNTech coronavirus disease 2019 (COVID-19) vaccine. Chest X-ray revealed bilateral infiltrates, and the desaturation deteriorated rapidly. The symptoms and radiographic abnormalities rapidly improved after the initiation of corticosteroid therapy. Intradermal testing of the Pfizer-BioNTech COVID-19 vaccine showed a delayed positive reaction. Based on these findings, the patient was diagnosed with COVID-19 vaccine-induced pneumonitis. The timing of the onset of pneumonitis after vaccination and the results of intradermal testing suggest that Type IV hypersensitivity against COVID-19 vaccine may have been responsible for this clinical condition.
Keywords: COVID-19, mRNA vaccine, vaccine-induced pneumonitis, intradermal testing
Introduction
The global pandemic caused by severe acute respiratory syndrome coronavirus infection (SARS-CoV-2) and the resulting coronavirus disease 2019 (COVID-19) has tormented people all over the world (1). Vaccination against and treatment for COVID-19 are ways to potentially combat this situation. COVID-19 vaccines, such as viral vector vaccines (2) and mRNA vaccines (3), have been developed and administered to many people. They have a high efficacy and immunogenicity. Although most vaccine-associated adverse events are mild, severe adverse reactions, such as anaphylaxis, myocarditis, and thrombotic events, rarely but occasionally occur (4-6). However, there have been no reports of COVID-19 vaccine-induced pneumonitis.
We herein deliver a preliminary report of a case of COVID-19 vaccine-induced pneumonitis.
Case Report
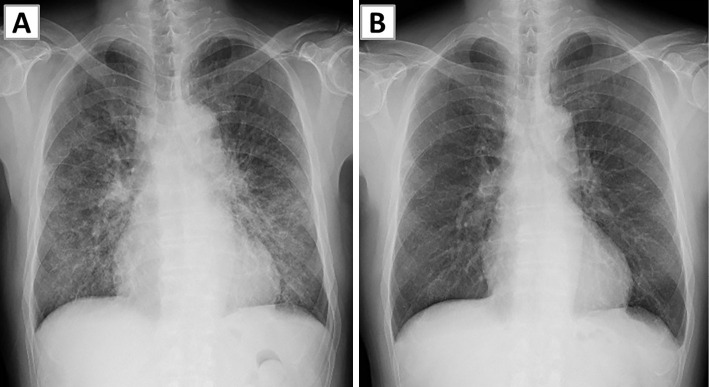
A 65-year-old man was admitted to our department with bilateral lung infiltrates and diffuse ground-glass opacity (GGO) on chest X-ray (Fig. 1A). Five days earlier, he had received the first dose (0.03 mg) of the Pfizer-BioNTech COVID-19 vaccine. He developed a low-grade fever two days after the vaccination, and cough and shortness of breath appeared the next day. No local side effect of vaccination appeared, and he was not using any antipyretics. He had a medical history of myocardial infarction, hyperlipidemia, and hypertension, which were well-controlled with aspirin, carvedilol, rosuvastatin, and enalapril maleate. His medication had not been altered for several years, and he had not used any supplements. He had undergone percutaneous coronary intervention nine years earlier. He was an ex-smoker with a smoking history of 7.5 pack-years. He had received the seasonal influenza vaccine every year and used isotonic PEG solution (NiflecⓇ) as a premedication for colonoscopy 10 years earlier. He had no history of allergic reactions to foods, drugs, or vaccines.
Figure 1.
Chest X-ray findings. (A) Chest X-ray on admission showed infiltration and ground-glass opacities in both lungs. (B) Chest X-ray obtained four days after the initiation of systemic steroid treatment showed complete resolution of infiltration in both lungs.
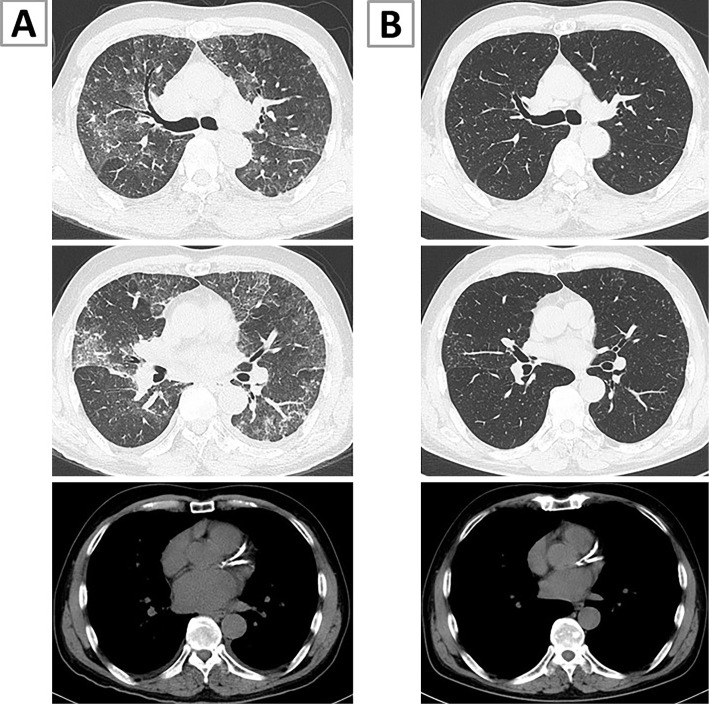
At the first visit, his arterial blood oxygen saturation was 85% on room air, and his respiratory rate was increased to 24 breaths/minute. The other vital signs and physical features were normal. Computed tomography (CT) revealed patchy GGO with a crazy-paving pattern and interlobular thickening (Fig. 2A). Minimal bilateral pleural effusion and left atrium enlargement were observed, but no lymph-node swelling was noted.
Figure 2.
Computed tomography (CT) findings of the lung. (A) CT on admission showed patchy ground-glass opacities with a crazy-paving pattern, interlobular thickening, minimal bilateral pleural effusion, and slight left atrium enlargement. (B) CT performed seven days after the initiation of systemic steroid treatment showed almost complete resolution of these findings.
The laboratory test results are shown in Table. A complete blood count revealed leukocytosis and neutrophilia. Other than elevated lactate dehydrogenase and C-reactive protein levels, there were no signs of mycosis, acute heart failure, vasculitis, or connective tissue disease.
Table.
Laboratory Data on Admission.
| <Hematology> | <Serology> | |||||||
| WBC | 16,200 | /μL | CRP | 5.49 | mg/dL | |||
| Neutrophils | 78.2 | % | PCT | 0.03 | pg/mL | |||
| lymphocytes | 9.7 | % | KL-6 | 214 | U/mL | |||
| Monocytes | 5.5 | % | SP-D | 73.1 | ng/mL | |||
| Eosinophils | 5.9 | % | BNP | 46.8 | pg/mL | |||
| Basophils | 0.7 | % | CEA | 3.8 | ng/mL | |||
| Hb | 14.5 | g/dL | β-D-glucan | <5.0 | pg/mL | |||
| Plt | 169×103 | /μL | ANA | <40 | fold | |||
| <Biochemistry> | Anti-ARS Ab | <5.0 | index | |||||
| T-Bil | 0.8 | mg/dL | MPO-ANCA | <1.0 | U/mL | |||
| AST | 22 | IU/L | PR3-ANCA | <1.0 | U/mL | |||
| ALT | 21 | IU/L | ACE | 7.1 | IU/L | |||
| LDH | 281 | IU/L | IgE | 40.4 | U/mL | |||
| CPK | 62 | IU/L | <Virology> | |||||
| TP | 5.9 | g/dL | COVID-19 PCR | (-) | ||||
| Alb | 3.3 | g/dL | COVID-19 Ag | (-) | ||||
| BUN | 14 | mg/dL | Influenza Ag | (-) | ||||
| Cr | 0.89 | mg/dL | <Blood Gas analysis (room air)> | |||||
| Na | 141 | mEq/L | pH | 7.448 | ||||
| K | 3.9 | mEq/L | PaCO2 | 37.4 | Torr | |||
| BS | 101 | mg/dL | PaO2 | 44.5 | Torr | |||
| HCO3- | 25.3 | mmol/L | ||||||
TP: total protein, Alb: albumin, BS: blood sugar, CRP: C-reactive protein, PCT: procalcitonin, KL-6: Krebs von den Lungen-6, SP-D: surfactant protein-D, BNP: brain natriuretic peptide, CEA: carcinoembryonic antigen, ANA: anti-nuclear-antibody, ARS: aminoacyl-tRNA synthetase, MPO-ANCA: myeloperoxidase antineutrophil cytoplasmic antibody, PR3-ANCA: proteinase-3 anti-neutrophil cytoplasmic antibody, ACE: angiotensin-converting enzyme
An electrocardiogram was normal, and an ultrasonic echocardiogram showed a normal wall motion with a left ventricular ejection fraction of 65% and slight left atrium enlargement (43 mm in diameter). The serum brain natriuretic peptide level was 58.2 pg/mL. These findings were not very different from those observed half a year before. Two days after hospitalization, bronchoalveolar lavage (BAL) was performed from the right B3 and B5 with 150 mL saline each. The recovery rate of BAL fluid was not sufficient (17.2%) probably because of the peripheral air way obstruction. A transbronchial lung biopsy was not performed because the patient was taking an antiplatelet drug. The total cell count in BAL fluid was 0.20×105/mL. The cell differential count of BAL fluid was 14% lymphocytes, 7% eosinophils, 1% macrophages, and 78% neutrophils. No infectious organisms or malignant cells were observed. The CD4+/CD8+ ratio was 0.62.
The patient's symptoms, chest X-ray findings, and respiratory status deteriorated, and he required high-flow nasal oxygen therapy after a bronchoscopy examination. Intravenous methylprednisolone was administered at 1,000 mg daily for 3 days immediately after the bronchoscopy examination, followed by prednisolone at 60 mg (1.0 mg/kg/day) daily without any diuretic. The patient's symptoms and radiographic and laboratory abnormalities rapidly improved (Fig. 1B, 2B), and systemic corticosteroids were quickly tapered off within 15 days.
We performed drug sensitivity testing more than two weeks after the withdrawal of corticosteroids. A drug lymphocyte stimulation test performed using peripheral lymphocytes showed a negative reaction to the COVID-19 vaccine, with a stimulation index of 113% (normal <180%). Similarly, patch testing showed a negative reaction. Intradermal testing (IDT) was performed on the volar aspect of the forearm with 0.02 mL of COVID-19 vaccine or normal 0.9% saline (negative control). At 48 h, the IDT showed a positive reaction with a rash and a wheal larger than 30 mm in diameter (Fig. 3), although there was a negative reaction at 20 min. No change in his vital sign, symptoms, or blood test results was caused by the IDT.
Figure 3.
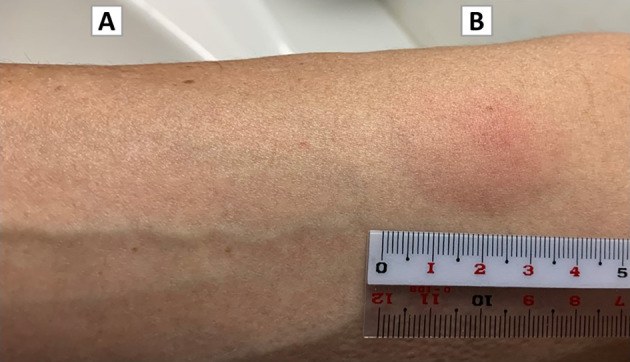
Intradermal testing results after 48 hours. (A) A negative response to normal 0.9% saline. (B) A positive response to the COVID-19 vaccine at a dose of 0.02 mL. A positive reaction was seen, with a rash and a wheal larger than 30 mm in diameter.
We ultimately diagnosed him with COVID-19 vaccine-induced pneumonitis based on the delayed IDT reaction and clinical course. There was no other trigger except for vaccine administration. Although cardiogenic pulmonary edema induced by acute lung failure should be considered as a major differential diagnosis of these chest CT findings, the results of the ultrasonic echocardiogram and serological testing as well as the clinical course did not suggest severe cardiac dysfunction. To avoid possible recurrence of pneumonitis, the patient's appointment to receive the second dose of the COVID-19 vaccine was cancelled.
Discussion
Drug-induced infiltrative lung disease caused by many kinds of drugs shows various patterns of lung involvement on imaging (7,8). Occasionally, intravesical BCG therapy or vaccination can produce acute pneumonia (9,10). The diagnostic criteria for drug-induced infiltrative lung disease established by Camus et al. do not include drug sensitivity testing (7). Drug sensitivity testing, including drug-induced lymphocyte stimulation test (DLST), a patch test, and IDT, has widely been performed in many cases. While a median DLST positive rate of 66.9% was reported for patients with drug-induced pneumonia (11), Suzuki et al. reported that the sensitivity of DLST was only 14.9%, and the false negative result of DLST was 85.1% for anti-tuberculosis drug (12). IDT has been reported to be more sensitive to T-cell mediated adverse drug reactions than patch testing (13). It was also reported that the false negative rate of IDT was lower than that of patch testing (14). In our case, only IDT showed a positive reaction, possibly due to the relatively high sensitivity of IDT compared to other testing methods.
The pneumonitis in the present case was considered to have been caused by the Pfizer-BioNTech COVID-19 vaccine that had been administered three days before the onset of symptoms. The Pfizer-BioNTech COVID-19 vaccine BNT162b2, a lipid nanoparticle-formulated nucleoside-modified RNA vaccine, was shown to be 95% effective in preventing COVID-19 (15) and treating a wide range of COVID-19-related outcomes (16). Furthermore, it reportedly has a low incidence of serious adverse events. The majority of the COVID-19 vaccine's safety profile is characterized by mild- to-moderate fatigue, headache, a fever, chills, muscle pain, and joint pain. These events are more common after the second dose than with the first (3). Recently, anaphylaxis was reported after the first dose of the Pfizer-BioNTech COVID-19 vaccine (17). The mRNA in the COVID-19 vaccines have been chemically modified and packaged in lipid nanoparticles (LNPs), which decreases its reactivity and protects the nucleic acid from degradation (18). The LNPs with mRNA in the COVID-19 vaccines are presumably responsible for the anaphylaxis (19). The components of the LNPs in the Pfizer-BioNTech and Moderna COVID-19 vaccines are polyethylene glycol (PEG), ionizable lipid, neutral lipid, cholesterol, and mRNA (19). PEG is widely used in medical, cosmetic, and household products and has been reported to be an allergen that causes systemic allergic reactions (20,21). IgE-mediated Type I hypersensitivity to PEG can reportedly be evaluated by skin testing using the skin prick and IDT techniques (22) or the basophil activation test (BAT) (23). While the BAT using a flow cytometry assay is useful in research, it is unsuitable for clinical use. IDT shows both immediate and delayed reactions. Delayed drug hypersensitivities are predominantly the result of T-cell mediated Type IV hypersensitivity. There have been reports of IDT with delayed reactions, such as maculopapular exanthema, acute generalized exanthematous pustulosis, and drug reaction with eosinophilia and systemic symptoms (24). Recently, SARS-CoV-2-exposed individuals showed delayed-type hypersensitivity reactions (25).
The concentration of PEG-2000 in the Pfizer-BioNTech vaccine has been show to elicit a delayed intradermal response in some vaccinated individuals who were not allergic to PEG (4). However, it was also considered that PEG induces a non-IgE-mediated reaction (26). The investigation of PEG is complicated, and PEG, LNPs of COVID-19 vaccine, or any protein-associated CD4+ T cell or CD8+ T cell activation induced by mRNA of COVID-19 vaccine may cause adverse reactions (19,27). The timing of the onset of pneumonitis after vaccination and the results of IDT suggest that Type IV hypersensitivity against COVID-19 vaccine may be responsible for this clinical condition.
In our case, the patient has a history of repeated seasonal influenza vaccine injection without any obvious allergic reaction. However, he also had a history of NiflecⓇ single administration as a premedication for colonoscopy. These medical histories suggest that the PEG contained in the COVID-19 vaccine might indeed be the causative ingredient of the allergic reaction observed in this case. While no local adverse reaction occurred after the COVID-19 vaccine intramuscular injection, IDT of COVID-19 vaccine showed a positive result. The discrepancy in the local reaction between these two administrations of the COVID-19 vaccine might be due to differences in the site of vaccine injection.
A systematic search of PubMed and the Pneumotox website for published reports on drug-induced respiratory disease associated with the COVID-19 vaccine yielded only a few results. To our knowledge, ours is the first case report of COVID-19 vaccine-associated pneumonitis. Previous reports on seasonal influenza vaccine-induced pneumonitis described the appearance of multiple nodules or consolidations and GGOs in both lungs on CT (10,28). Our case showed patchy GGO with a crazy-paving pattern and interlobular thickening in both lungs on CT, findings that were similar to the drug-induce pulmonary infiltrates with eosinophilia (PIE) pattern reported by Camus et al. (7). PIE is caused by a variety of chemically unrelated drugs, including aspirin and other nonsteroidal anti-inflammatory drugs, angiotensin-converting enzyme inhibitors, antidepressants, interferons, minocycline, phenytoin, and radiographic contrast material, among others (29). PIE is diagnosed by the presence of eosinophilia in peripheral blood, BAL fluid, or lung tissue. When eosinophilia is absent, it is diagnosed based on the histology. In the present case, mild eosinophilia in the BAL fluid (7%) was observed. Non-IgE-mediated hypersensitivity reportedly causes clinical symptoms, such as airway obstruction with dyspnea (26). This might account for the poor recovery rate of the BAL fluid in this case. However, treatment with systemic corticosteroids rapidly improved the symptoms and radiographic abnormalities. The pneumonitis might have been due to an allergic reaction induced by the mRNA or other ingredients in the COVID-19 vaccine. The further accumulation of cases and investigations into COVID-19 vaccine-induced pneumonitis are needed to elucidate the detailed mechanism underlying this clinical condition.
Conclusion
The findings from the present case suggest the possible risk of the COVID-19 vaccine causing a severe adverse reaction of pneumonitis. The mechanism may involve an allergic reaction of Type IV hypersensitivity.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
We thank Ph Reiko Kobori of the Department of Pharmacy at Tatebayashi Kosei General Hospital for the arrangement of testing with the Pfizer-BioNTech COVID-19 vaccine.
References
- 1. Helmy YA, Fawzy M, Elaswad A, Sobieh A, Kenney SP, Shehata AA. The COVID-19 pandemic: a comprehensive review of taxonomy, genetics, epidemiology, diagnosis, treatment, and control. J Clin Med 9: 1225, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhu FC, Li TH, Guan XH, et al. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: a dose-escalation, open-label, non-randomised, first-in-human trial. Lancet 395: 1845-1854, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jackson LA, Anderson EJ, Rouphael NG, et al. An mRNA vaccine against SARS-CoV-2-preliminary report. N Engl J Med 383: 1920-1931, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Turner PJ, Ansotegui IJ, Cambell DE, et al. ; WAO Anaphylaxis Comittee. COVID-19 vaccine-associated anaphylaxis: a statement of the World Allergy Organization Anaphylaxis Committee. World Allergy Organ J 14: 100517, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Moucu SA, Roguin A, Hellou E, et al. Myocarditis following COVID-19 mRNA vaccination. Vaccine 39: 3790-3793, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kantarcioglu B, Iqbal O, Walenga JM, et al. An update on the pathogenesis of COVID-19 and the reportedly rare thrombotic events following vaccination. Clin Appl Thromb Hemost 27: 10760296211021498, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Camus P, Fanton A, Bonniaud P, Camus C, Foucher P. Interstitial lung disease induced by drug and radiation. Respiration 71: 301-326, 2004. [DOI] [PubMed] [Google Scholar]
- 8. Rossi SE, Erasmus JJ, McAdams HP, Sporn TA, Goodman PC. Pulmonary drug toxicity: radiologic and pathologic manifestations. Radiographics 20: 1245-1259, 2000. [DOI] [PubMed] [Google Scholar]
- 9. Tobiume M, Shinohara T, Kuno T, et al. BCG-induced pneumonitis with lymphocytic pleurisy in the absence of elevated KL-6. BMC Pulm Med 14: 35, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Numata T, Hida N, Yazaki K, et al. Seasonal influenza vaccine-induced pneumonitis presenting with multiple pulmonary nodules. Intern Med 57: 707-711, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kondo A. Drug-induced pneumonitis. Kekkaku (Bull Jpn Soc Tuberc) 74: 33-41, 1999(in Japanese, Abstract in English). [PubMed] [Google Scholar]
- 12. Suzuki Y, Miwa S, Shirai M, et al. Drug lymphocyte stimulation test in the diagnosis of adverse reactions to antituberculosis drugs. Chest 134: 1027-1032, 2008. [DOI] [PubMed] [Google Scholar]
- 13. Osawa J, Naito S, Aihara M, Kitamura K, Ikezawa Z, Nakajima H. Evaluation of skin test reactions in patients with non-immediate type drug eruptions. J Dermatol 17: 235-239, 1990. [DOI] [PubMed] [Google Scholar]
- 14. Torres MJ, Sánchez-Sabaté E, Alvarez J, et al. Skin test evaluation in nonimmediate allergic reactions to penicillins. Allergy 59: 219-224, 2004. [DOI] [PubMed] [Google Scholar]
- 15. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 383: 2603-2615, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dagan N, Barda N, Kepten E, et al. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med 384: 1412-1423, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shimabukuro T, Nair N. Allergic reaction including anaphylaxis after receipt of the first dose of Pfizer-BioNTech COVID-19 vaccine. JAMA 325: 780-781, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pardi N, Hogan MJ, Poter FW, Weissman D. mRNA vaccines - a new era in vaccinology. Nature Rev Drug Discov 17: 261-279, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Risma KA, Edwards KM, Hummell DS, et al. Potential mechanisms of anaphylaxis to COVID-19 mRNA vaccines. J Allergy Clin Immunol 147: 2075-2082, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wenande E, Garvey LH. Immediate-type hypersensitivity to polyethylene glycols: a review. Clin Exp Allergy 46: 907-922, 2016. [DOI] [PubMed] [Google Scholar]
- 21. Banerji A, Wickner PG, Saff R, et al. mRNA vaccines to prevent COVID-19 disease and reported allergic reactions: current evidence and suggested approach. J Allergy Clin Immunol Pract 9: 1423-1437, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stone CA Jr, Liu Y, Relling MV, et al. Immediate hypersensitivity to polyethylene glycols and polysorbates: more common than we have recognized. J Allergy Clin Immunol Pract 7: 1533-1540, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jover Cerdá V, Rodríguez Pacheco R, Doménech Witek J, Marco de la Calle FM, de la Sen Fernández ML. Immediate hypersensitivity to polyethylene glycols in unrelated products: when standardization in the nomenclature of the components of drugs, cosmetics, and food becomes necessary. Allergy Asthma Clin Immunol 15: 9, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Copaescu A, Gibson A, Li Y, Trubiano JA, Phillips EJ. An updated review of the diagnostic methods in delayed drug hypersensitivity. Front Pharmacol 11: 573573, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Barrios Y, Franco A, Sanchez-Machin I, Poza-Guedes P, Gonzalez-Perez R, Matheu V. A novel application of delayed-type hypersensitivity reaction to measure cellular immune response in SARS-CoV-2 exposed individuals. Clin Immunol 226: 108730, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Klimek L, Novak N, Cabanillas B, Jutel M, Bousquet J, Akdis CA. Allergenic components of the mRNA-1273 vaccine for COVID-19: possible involvement of polyethylene glycol and IgG-mediated complement activation. Allergy 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Versteeg L, Almutairi MM, Hotez PJ, Pollet J. Enlisting the mRNA vaccine platform to combat parasitic infections. Vaccines (Basel) 7: 122, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hibino M, Kondo T. Interstitial Pneumonia associated with the influenza vaccine: a report of two cases. Intern Med 56: 197-201, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Allen JN. Drug-induced eosinophilic lung disease. Clin Chest Med 25: 77-88, 2004. [DOI] [PubMed] [Google Scholar]