Abstract
The retention of the capsule used during small bowel capsule endoscopy (SBCE) is a serious complication that can occur in patients with known or suspected small bowel stenosis, and a prior evaluation of the patency of the gastrointestinal (GI) tract is therefore essential. Patency capsule (PC) is a non-diagnostic capsule the same size as the diagnostic SBCE. To date, there are no clear guidelines regarding the contraindications for undergoing a PC evaluation prior to SBCE. Each small bowel disorder has specific occasions to inhibit the progress of PC and SBCE, even though they do not have any stenotic symptoms or abnormalities on imaging. In this review, we summarize the indications and limitations of PC prior to SBCE, especially the contraindications, and discuss clinical scenarios in which even PC should be avoided, and therefore such areas of stenosis should be evaluated by alternative modalities. We thus propose this new algorithm to evaluate the patency of the GI tract for patients with suspected and known small bowel stenosis in order that they may undergo SBCE safely.
Keywords: algorithm, capsule endoscopy, Crohn's disease, patency capsule, retention
Introduction
Small bowel capsule endoscopy (SBCE) is a valuable tool for diagnosing small bowel diseases; however, it is contraindicated in patients with swallowing disorders, known severe gastrointestinal (GI) stricture, and those with implanted electro medical devices (1-11). Reducing the risk of capsule retention is the main safety consideration in SBCE (12). The manufacturing company that introduced the PillCam SB has recently developed an innovative system (Patency capsule; PC, manufactured by Medtronic, Minneapolis, USA) to confirm the patency of the digestive tract in a non-invasive way. The Agile™ patency capsule (APC) is a dummy capsule with a radio-frequency identification (RFID) tag, identical in size to the PillCam SB3, which can be used to evaluate the patency of the GI tract prior to SBCE. The cellophane-walled capsule cylinder, filled with lactose admixed with barium, is protected by hollow plugs that allow the influx of intestinal fluid and the dissolution of lactose. If the APC remains within the intestine, then the timer plug erodes after 30 hours, thus allowing the penetration of body fluids into the capsule and the subsequent dissolution of the capsule. In addition to barium, which allows for radiological detection, the APC contains an inner RFID tag that enables detection by a hand-held radio-frequency scanner. It has been previously utilized for patients with suspected small bowel stenosis (13,14). Although the RFID tag was useful for identifying the APC in the GI tract without radiation exposure, it had the potential risk of small bowel obstruction due to the impaction of the tag into the stenotic lesion (15). The APC significantly improved the overall safety of SBCE, but further research is required to optimize its use in routine clinical practice. The PillCam™ patency capsule (PPC) is another dummy capsule without an RFID tag that is identical in size to the PillCam SB3. It was first introduced into clinical practice in Japan in July 2012, and multiple single center studies have reported its effectiveness (16-18). Since the PPC does not have an RFID tag, confirmation of patency was performed by confirming that it was excreted from the body in intact form or by plain X-ray or computerized tomography (CT) confirmation of its location. The inability to identify the PC on an abdominal radiograph was defined as complete PC passage. In addition, other types of SBCE, such as the EndoCapsule (19), OMOM (20) and MiroCam (21), which have functions similar to that of the PillCam, have also been introduced. Although they do not have a PC system, the APC and PPC may be applied.
The decision to use a PC prior to CE is usually left to the clinician, and the frequency of its use varies. However, PC cannot always be safely performed and their use can be as challenging as SBCE within 33 hours from ingestion. Furthermore, a PC can also be retained in cases with severe small bowel stenoses. Seike et al. reported a case where a PC was retained, despite a prior CT scan failing to show positive findings (22). Kopylov et al. reported the high frequency of abdominal symptoms during PC examination (23). Therefore, the contraindications for the use of PC should also be discussed.
Since its introduction into clinical practice, prospective studies have assessed the clinical usefulness and safety of the PC in patients with clinical and/or radiological evidence of intestinal strictures; however, the optimal strategy for confirming the patency of the GI tract has not been fully established. If the PC is not applied for patients with a potential risk of SBCE retention, such as long-term users of non-steroidal anti-inflammatory drugs (NSAIDs) and established Crohn's disease (CD) patients, the retention rate would increase with direct SBCE examinations. On the other hand, if every patient undergoes the PC prior to SBCE, combined PC and SBCE would thus be a relatively complicated examination. This review describes the appropriate use of the PC for evaluating small bowel disorders, including contraindications, and proposes a novel strategy to minimize the risks of capsule retention in SBCE.
Causes of Small Bowel Stenosis
Small bowel stenosis can be asymptomatic in the absence of severe stenosis. It is caused by several mechanisms including mechanical obstruction, non-mechanical obstruction, and vascular ischemia (24).
Stenosis can often be identified by CT scans or abdominal ultrasonography and it is characterized by thickening of the small bowel wall and dilatation of the lumen ahead of the stenosis. Stenosis can also occur without any direct damage to the small bowel, as seen in cases of stenosis secondary to abdominal adhesions. Other causes of stenosis include CD, malignant tumors, ischemic enteritis, intussusception, inflammatory adhesions, and NSAID-induced diaphragm disease (25). The symptoms caused by small bowel stenosis can vary depending on its length and severity, and these factors also influence the subsequent management of stenosed lesions. In cases of NSAID-induced diaphragm disease (26), where the narrow segment has a short length, the patient may not develop obstructive symptoms and stenotic lesions may be missed on cross-sectional imaging (27). It should also be noted that differentiating between neoplastic and inflammatory lesions can be difficult, because these lesions have similar clinical features and cross-sectional findings on CT or magnetic resonance imaging. In such cases, direct observation by endoscopy is useful as it allows for a more detailed evaluation. Therefore, SBCE is a useful modality for the investigation of unknown small bowel stenosis.
Indications and Contraindications for the Use of the Patency Capsule
The PC is used to reduce the risk of SBCE retention, defined as the presence of the capsule in the GI tract for at least 2 weeks after ingestion with retention confirmed on abdominal radiography or at the time of endoscopic or surgical retrieval of the capsule (28-30). We identified 264 articles from October 2003 to July 2020 by searching the PubMed database for the following terms: “small bowel capsule endoscopy” and “retention.” We found 15 etiologies that cause SBCE retention (Table 1) (7-11,13,28-32). In a pooled analysis of 22,840 SBCE procedures, the overall retention rate was as low as 1.4% [confudence interval (CI): 1.2-1.6] (32). Out of 104 reported cases of capsule retention, 88 were asymptomatic (85%) and 16 had signs of partial or total intestinal obstruction. Of the retained capsules, 58.7% were removed surgically, 12.5% endoscopically, 15.8% passed spontaneously or after medical treatment, and the details of the others were not reported in detail. In one meta-analysis of SBCE retention, causes of retention included CD (35.3%), neoplastic lesions (22.1%), NSAID-induced enteropathy (18.4%), postsurgical stenosis (7.4%), ulceration (3.7%), intestinal adhesions (2.9%), tuberculosis or radiation enteritis (2.2% each), ischemia-induced stenosis, Meckel's diverticulum or pouch (1.5% each), and a peptic ulcer scar with stricture or cryptogenic multifocal ulcerous stenosing enteritis (0.7% each) (32). In such cases, physicians should consider the need for a PC test prior to SBCE. Although several useful modalities exist to evaluate small bowel stenosis, including CT enterography, MR enterography (MRE), and balloon-assisted endoscopy (33-36), SBCE allows for the visualization of the mucosa of the entire small bowel at one time and can localize the lesion for further examination (37). Therefore, SBCE is especially recommended for whole small bowel observation even if the patient has suspected small bowel stenosis and potential risk of capsule retention. MRE can help identify high-risk patients prior to APC and provide additional clinical information (7,38). Rondonotti et al. (39) suggested that APC should be performed for patients at high risk for CE retention despite negative cross-sectional imaging. The European Society for Gastrointestinal Endoscopy advised against the use of cross-sectional imaging or APC prior to SBCE in patients with suspected CD in the presence of obstructive symptoms, while imaging techniques and APC are recommended prior to SBCE in established CD (40).
Table 1.
Gastrointestinal Disorders with a Potential Risk for Capsule Retention.
| Established Crohn’s disease |
| Suspected Crohn’s disease with any abdominal symptom |
| Neoplasm (cancer, malignant lymphoma, NET *, metastatic cancer) |
| Non-steroidal anti-inflammatory drug-induced enteropathy |
| Radiation enteritis |
| Adhesions |
| Tuberculosis |
| Postoperative anastomosis |
| Ischemic enteritis |
| Duplication cyst |
| Meckel’s diverticulum |
| Eosinophilic gastroenteritis |
| Peptic ulcer scar |
| CMUSE ** |
| Delayed transit |
* Neuroendocrine tumor
** Cryptogenic multifocal ulcerous stenosing enteritis
It should be noted that PC has the same dynamic features as SBCE during the first 33 hours after ingestion and has associated risks that should be mentioned. Abdominal pain due to PC retention and intestinal perforation within 24 hours of PC ingestion, even in the absence of known small bowel obstruction or imaging findings of small bowel stenosis, have been previously reported (41,42). Blanco-Velasco et al. (43) reported the case of a patient with 2 episodes of bowel obstruction secondary to CD. The patient developed obstructive symptoms a few hours after PC ingestion, and a plain abdominal radiograph revealed intestinal dilatation within the GI tract. The patient subsequently expelled the PC and his symptoms thereafter improved spontaneously. Rasmussen et al. (44) reported symptomatic PC retention in 2 patients with suspected CD. A multicenter study of 1,615 patients that underwent a PC test reported symptomatic retention in 20 patients (1.2%), only one of whom required surgery. The rest of the cases resolved spontaneously or after corticosteroid therapy (23). Although the PC dissolves upon contact with intestinal fluids and passes spontaneously in the majority of cases, slow dissolution or even failure of complete dissolution have been reported (23). Disintegration or painful passage of the PC may be associated with a clinically relevant small bowel stricture and a high probability of surgery (45).
Although certain contraindications for the use of the PC have been proposed, further research is required. Rozendorn et al. (46) found that a stricture length ≥10 cm and more than two prestenotic dilations on MRE were associated with PC retention, and the use of a PC should be avoided in these cases (Fig. 1a-c). Similarly, Lee et al. (47) reported that stricture length ≥20 mm on MRE was associated with the disintegration of the PC or capsule retention.
Figure 1a.
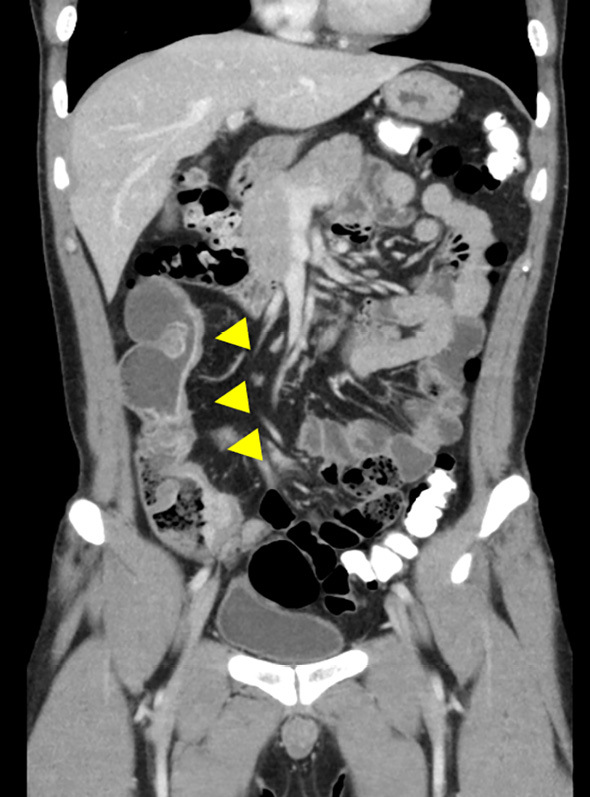
The patency capsule was not excreted from the small bowel in time and the patient developed abdominal pain during patency capsule examination. A CT scan showed several stenoses (arrow heads) and a dilated lumen in the ileum.
Figure 1b.
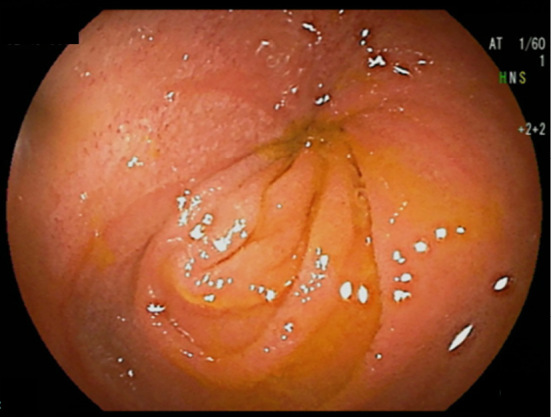
Subsequent double-balloon endoscopy revealed severe stenosis in the ileum, which led to an incomplete patency capsule examination.
Figure 1c.
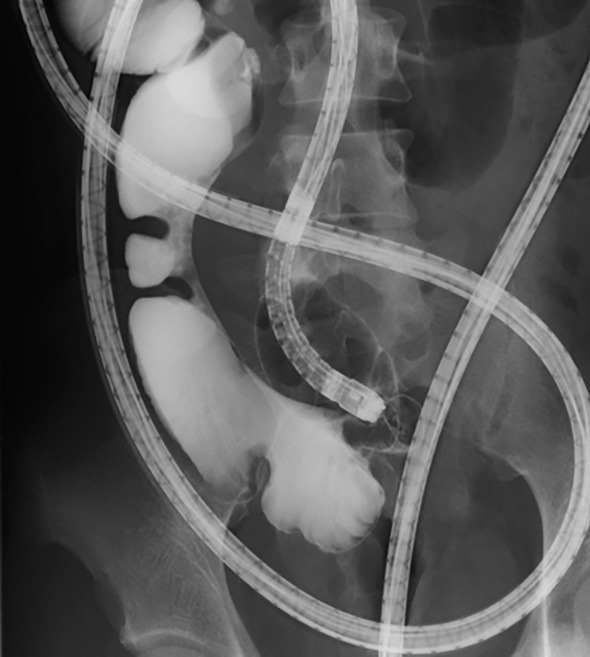
Gastrografin enterography showed severe stenoses on the mesenteric side.
PPC combined with ultrasonography before SBCE has also been reported to be a reliable indicator of functional patency to predict and minimize the risk of impaction in suspected or even known cases with small bowel stricture (48,49).
Indication for PC in Small Bowel Disorders
In addition to the disorders listed in Table 1, obtaining additional data regarding each small bowel disorder is required in order to reduce the risk of SBCE retention since pre-procedure CT scans do not predict the risk of capsule retention (6,50-52).
1. Obscure gastrointestinal bleeding
The most common causes of obscure gastrointestinal bleeding (OGIB) are vascular lesions (22.8% of all cases), ulcerations and erosions (16.7%), tumors and polyps (13.0%), diverticulosis (6.2%), and others (41.3%) (53). Since vascular lesions rarely occlude the entire GI lumen, they are relatively unlikely to cause SBCE retention. Chronic kidney disease has been identified as an independent predictor for the presence of a vascular lesion in overt GI bleeding patients, while a history of NSAID use is an independent risk factor for erosive lesions (54). In their multivariate analysis, Shahidi et al. also identified a significant association of an increasing number of esophagogastroduodenoscopies, increasing transfusion requirements, and connective tissue disease with identification of positive findings for OGIB by SBCE (55). Ohmiya et al. showed that vascular lesions were associated with underlying diseases, namely, cardiovascular disease, liver cirrhosis, and chronic kidney disease, whereas the causes of OGIB in patients aged under 50 years without any underlying disease were mainly bleeding from ulceration in CD and Meckel's diverticulum; these pose a potential risk of SBCE retention (56,57). Furthermore, in a systematic review of patients with overt OGIB, the diagnostic and therapeutic yields of SBCE have been reported to decrease as endoscopy was delayed and SBCE was recommended to be performed within two days from bleeding (58). Since PC takes time to evaluate the patency of the digestive tract, cross-sectional imaging, which can provide a rapid evaluation of GI patency, can be preferable in patients who develop overt OGIB and require patency evaluation prior to SBCE.
In summary, to avoid the risk of SBCE retention in patients with OGIB, PC is recommended for younger patients without a history of underlying disease as well as patients in the high-risk group, as shown in Table 1.
2. Crohn's disease
A previous meta-analysis demonstrated that SBCE is a more sensitive method for the diagnosis of small bowel CD, with an incremental diagnostic yield 30% greater than other imaging modalities (59). Mensink et al. (60) stated that double-balloon endoscopy (DBE) is a valuable diagnostic modality for the evaluation of small bowel lesions in CD patients and that adjusting the therapy in the majority of these patients based on DBE findings leads to significant and sustained clinical remission. Oshitani et al. (61) suggested that special attention should be paid to mesenteric longitudinal ulcers during insertion and that the overtube balloon of the DBE should not be inflated if a clear intestinal view is not possible, as this increases the risk of perforation. At present, the endoscopic evaluation of CD is considered to be invasive, but nevertheless, it is important to guide subsequent management, and is recommended by a number of studies as part of the treat-to-target strategy (62,63). The European Society of Gastrointestinal Endoscopy recommends PC testing when SBCE is indicated in patients with established CD but does not recommend routine small-bowel imaging or PC examination prior to SBCE in patients with suspected CD (40). In patients with suspected CD, the risk of small-bowel capsule retention is low and comparable to that of SBCE for GI bleeding. Esaki et al. reported that suspected CD, with or without symptoms, is an indication for PC prior to SBCE based on evidence from a Japanese multicenter trial (64). Alternatively, patients with established CD, especially those with known intestinal stenosis, are predicted to have a higher risk of small-bowel capsule retention. In these patients, clinicians should try to exclude small-bowel strictures by taking a thorough clinical history and obtaining radiographic imaging before SBCE. However, it should be noted that radiographic studies cannot definitively predict the risk of small-bowel capsule retention (65).
In another review, the authors suggested that direct SBCE should not be used in patients with known or suspected stricture, as capsule retention has been described in up to 13% (ICCE consensus) of patients who underwent a capsule study for CD (66). Instead, the authors recommended that patients with known small bowel CD undergo small bowel imaging or a patency study prior to SBCE. In a retrospective multicenter study of 406 patients with established CD, Nemeth et al. (67) reported that SBCE retention rates without PC, after patency confirmation with PC, and after patency not confirmed with PC were 2.3% (3/132), 2.1% (4/193), and 11.1% (2/18), respectively. The authors then proposed two different patency utilization strategies: the selective strategy (only in patients with obstructive symptoms, history of intestinal obstruction or surgery, or per the treating physician's request, n=180) and the nonselective strategy (all patients with CD, n=162), but the SBCE retention rates were 1.3% (2/180) and 1.6% (2/162), respectively, which did not differ significantly. They suggested that the PC test should be used selectively in patients who have obstructive symptoms or prior abdominal surgery and thus were at high risk of capsule retention.
Taken together, the above results suggest that PC should be performed for established CD and considered in suspected CD, especially when there is known small bowel stenosis or abdominal symptoms. Another important consideration in CD patients is to ensure that the interval between the PC examination and SBCE is minimal in order to minimize the number of false-positive patency results. This is because the disease activity and the severity of the small bowel stenosis are likely to change within a short period of time.
3. Suspected small bowel tumor
A previous study reported that the most common tumor of the small bowel is malignant lymphoma (21.5%), followed by gastrointestinal stromal tumor (18.8%), Peutz-Jeghers syndrome (15.3%), familial adenomatous polyposis (9.7%), and carcinoma (9.7%) (68). Despite their prevalence, the diagnosis of small bowel tumors is often delayed (69,70) due to the fact that they are usually asymptomatic in the early stage (71). The aforementioned study did not report the predictive factors for small bowel tumors before SBCE. Small bowel tumors are usually diagnosed incidentally after a patient presents with iron deficiency anemia or OGIB, and those with a history of severe anemia or previous blood transfusion may be required to undergo a CT scan prior to SBCE to rule out serious pathology. Rondonotti et al. (72) reported that among 5,129 patients undergoing SBCE, 124 (2.4%) had small-bowel tumors (112 primary, 12 metastatic) and capsule retention occurred in 9.8%. The metastatic tumors consisted of 5 adenocarcinomas, 3 malignant lymphomas, 1 carcinoid, 1 gastrointestinal stromal tumor, 1 angiosarcoma, and 1 metastasis of colon cancer. Notably, 95% of the patients underwent surgical management, which also allowed for the excision of the retained capsule.
In summary, in the case of elderly patients with OGIB not complicated by underlying disease, namely heart failure or chronic kidney disease, PC may be recommended due to the potential risk of small bowel masses.
4. Intestinal motility disorders
The delayed transit of the SBCE can also lead to retention. However, in the majority of these cases, the SBCE will eventually be excreted, unless there is a large diverticulum in the GI tract or previous small bowel resection resulting in a blind loop. Therefore, no intervention is required to remove the SBCE in cases of intestinal motility disorders. Since the size and weight of the SBCE is similar to that of the PC, delayed transit of both can occur in patients with intestinal motility disorders (73,74). This can lead to incomplete examination via SBCE, and patients should be given prokinetics or bowel preparation liquid prior to or during the evaluation of the GI tract (75).
Alternative Modalities
Several modalities exist to evaluate the patency of the GI tract. In a retrospective study, PC and imaging studies (CT and MRE) had a similar sensitivity (57% vs 71%; p=1.00) and specificity (86% vs 97%; p=0.22) to detect clinically significant small-bowel strictures (76). Klang et al. (77) evaluated the ability of MR diffusion-weighted imaging (DWI) to predict PC retention in CD. They found that the sensitivity and negative predictive value of restricted diffusion for PC retention were 100%, suggesting that DWI may predict the capability of the GI tract to pass the SBCE. The presence or absence of stricture, a fistula, abscess, or inflammatory mass was correctly determined by preoperative CT enterography in 100, 94, 100, and 97 percent of cases, respectively (78). These findings demonstrate the value of cross-sectional imaging in predicting the patency of the GI tract and informing clinical practice. Unlike the PC test, a cross-sectional examination provides real-time images that allow rapid assessment of GI tract patency without having to wait for the PC to be dissolved or excreted. In emergency cases where the rapid evaluation of GI patency is required, such as cases of GI bleeding with potential small bowel stenosis, imaging is preferable to SBCE. However, the fact that cross-sectional imaging modalities are not always available in every hospital is an issue. PC can be more widely used in daily clinical practice due to its advantages of no radiation exposure and simple evaluation methodology.
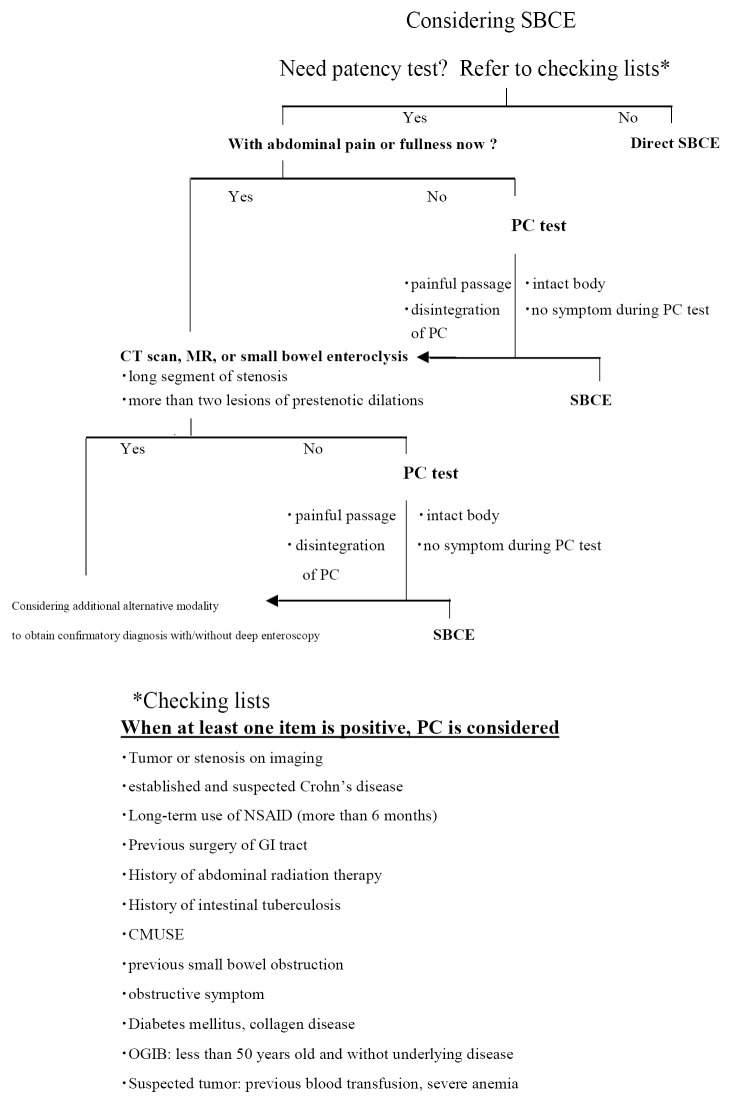
Proposed Algorithm for SBCE in Patients with Small Bowel Stenosis
When considering SBCE for patients with suspected or known small bowel stenosis, our proposed flow chart may help clinicians perform a safer examination (Fig. 2, Table 2). First, the patient interview and medical chart review are important to determine whether the patient requires a patency test. Even when the clinician decides to perform a direct SBCE, the patients should be educated about SBCE retention before giving their informed consent. OGIB in some young patients would be caused by ulcerations due to CD and be complicated with small bowel stenosis. We can imagine small bowel stenosis by a suspected small bowel tumor with decreased blood hemoglobin level. In such situations, the clinician will need to think about patency evaluation prior to SBCE. Clinicians will have to consider the adverse events associated with PC, such as the impaction of the PC at the stenotic site or intestinal perforation within 33 hours. The SBCE retention rate is reported to be approximately 1% (74,79). If a hospital's retention rate is higher than 1%, the indications and contraindications of PC may need to be reconsidered. It is also important for the patient to be interviewed about stenotic symptoms in more detail before PC. If there are stenotic symptoms, cross-sectional imaging would be used instead of PC to determine the underlying long-segmental stenosis or number of stenotic sites. We sometimes cannot correctly evaluate whether abdominal symptoms in CD patients are caused by GI stenosis. Cross-sectional imaging would be initially recommended to avoid any painful passage of the PC. If the images do not show a dilated lumen or wall thickness, a PC test will be subsequently performed. In fact, there is a possibility for either active lesions or stricture to exist in the small bowel even in asymptomatic CD patients with normal c-reactive protein levels. Additionally, false negative cases may be observed with short stricture by cross sectional imaging, or even MRE (80). This short stricture has a potential to be the responsible lesion of SBCE retention.
Figure 2.
Proposed algorithm for SBCE in patients with small bowel stenosis.
Table 2.
Summary of the Recommendations for PC* Use in Each Small Bowel Disorder and the Contraindications.
| PC recommendation | |
| 1 | OGIB** |
| Based on the existing literature | |
| Elderly patients have a potential risk of small bowel tumor (54) | |
| Vascular lesions are frequently complicated with underlying disease and not likely to complicate small bowel stenosis (54, 55) | |
| 50-year-old patients without underlying disease, mainly with bleeding from ulceration in Crohn’s disease and Meckel’s diverticulum (54, 55) | |
| Anemia with hemoglobin level less than 10g/dL is related to capsule retention (73) | |
| PC recommendation | |
| Patients without a history of underlying disease | |
| 2 | CD*** |
| Based on the existing literature | |
| Established CD is an indication for PC (62, 63) | |
| PC for suspected CD is controversial (40, 62-66, 73) | |
| PC only for patients with obstructive symptoms, history of intestinal obstruction or surgery, or per the treating physician’s request (66) | |
| PC recommendation | |
| Established and suspected CD are indications for PC | |
| 3 | Suspected small bowel tumor |
| Based on the existing literature | |
| There is no predictor of small bowel tumor (70, 71) | |
| Elderly patients have potential risk of bleeding from small bowel tumors (54) | |
| PC recommendation | |
| Elderly patients with OGIB | |
| Cross-sectional imaging (optional) for anemia or abdominal symptom | |
| 4 | Intestinal motility disorders |
| Based on the existing literature | |
| PC is not retained but gastrointestinal patency is unlikely to be confirmed (72-74) | |
| PC recommendation | |
| Can be attempted | |
| PC contraindications | |
| Cross-sectional imaging led by medical charts will provide information on PC contraindications (76) | |
| Long-segment stenosis (46, 47) | |
| More than two prestenotic dilations (46) | |
* patency capsule
** obscure gastrointestinal bleeding
*** Crohn’s disease
The PC test provides the useful information prior to SBCE; however, confirmation of GI tract patency is sometimes difficult. One study showed 5 SBCE retentions in 963 cases led by misinterpretation of PC localization (74). Several methods to evaluate the PC localization have been proposed, but no gold standard has yet been established (48,49,81,82). Therefore, future studies should focus on optimizing PC localization to improve the safety of this procedure.
Conclusion
The appropriate use of PC and cross-sectional imaging prior to SBCE is essential in order to minimize the risk of capsule retention in patients with small bowel stenosis. Some of these patients carry a risk of PC retention. Clinicians should therefore exclude any patients with severe stenosis from PC tests by medical interviews and initial examinations, including cross-sectional imaging.
Author's disclosure of potential Conflicts of Interest (COI).
Masanao Nakamura: Honoraria, Janssen Pharma.
References
- 1. Hosoe N, Takabayashi K, Ogata H, Kanai T. Capsule endoscopy for small-intestinal disorders: current status. Dig Endosc 31: 498-507, 2019. [DOI] [PubMed] [Google Scholar]
- 2. Iddan G, Meron G, Glukhovsky A, Swain P. Wireless capsule endoscopy. Nature 405: 417, 2000. [DOI] [PubMed] [Google Scholar]
- 3. Nakamura M, Niwa Y, Ohmiya N, et al. Preliminary comparison of capsule endoscopy and double-balloon enteroscopy in patients with suspected small-bowel bleeding. Endoscopy 38: 59-66, 2006. [DOI] [PubMed] [Google Scholar]
- 4. Yamamoto H, Kita H, Sunada K, et al. Clinical outcomes of double-balloon endoscopy for the diagnosis and treatment of small-intestinal diseases. Clin Gastroenterol Hepatol 2: 1010-1016, 2004. [DOI] [PubMed] [Google Scholar]
- 5. Bandorski D, Kurniawan N, Baltes P, et al. Contraindications for video capsule endoscopy. World J Gastroenterol 22: 9898-9908, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Karagiannis S, Faiss S, Mavrogiannis C. Capsule retention: a feared complication of wireless capsule endoscopy. Scand J Gastroenterol 44: 1158-1165, 2009. [DOI] [PubMed] [Google Scholar]
- 7. Lee HS, Lim YJ, Kim KO, et al. Outcomes and management strategies for capsule retention: a Korean capsule endoscopy nationwide database registry study. Dig Dis Sci 64: 3240-3246, 2019. [DOI] [PubMed] [Google Scholar]
- 8. Makipour K, Modiri AN, Ehrlich A, et al. Double balloon enteroscopy: effective and minimally invasive method for removal of retained video capsules. Dig Endosc 26: 646-649, 2014. [DOI] [PubMed] [Google Scholar]
- 9. Gao Y, Xin L, Wang YX, et al. Double-balloon enteroscopy for retrieving retained small-bowel video capsule endoscopes: a systematic review. Scand J Gastroenterol 55: 105-113, 2020. [DOI] [PubMed] [Google Scholar]
- 10. Van Weyenberg SJ, Van Turenhout ST, Bouma G, et al. Double-balloon endoscopy as the primary method for small-bowel video capsule endoscope retrieval. Gastrointest Endosc 71: 535-541, 2010. [DOI] [PubMed] [Google Scholar]
- 11. Nakamura M, Hirooka Y, Watanabe O, et al. Minimally invasive extraction of a foreign body from the small intestine using double-balloon endoscopy. Nagoya J Med Sci 77: 189-194, 2015. [PMC free article] [PubMed] [Google Scholar]
- 12. Yamamoto H, Ogata H, Matsumoto T, et al. Clinical practice guideline for enteroscopy. Dig Endosc 29: 519-546, 2017. [DOI] [PubMed] [Google Scholar]
- 13. Caunedo-Alvarez A, Romero-Vazquez J, Herrerias-Gutierrez JM. Patency and agile capsules. World J Gastroenterol 14: 5269-5273, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Herrerias JM, Leighton JA, Costamagna G, et al. Agile patency system eliminates risk of capsule retention in patients with known intestinal strictures who undergo capsule endoscopy. Gastrointest Endosc 67: 902-909, 2008. [DOI] [PubMed] [Google Scholar]
- 15. Gay G, Delvaux M, Laurent V, et al. Temporary intestinal occlusion induced by a “patency capsule” in a patient with Crohn's disease. Endoscopy 37: 174-177, 2005. [DOI] [PubMed] [Google Scholar]
- 16. Nakamura M, Hirooka Y, Yamamura T, et al. Clinical usefulness of novel tag-less Agile patency capsule prior to capsule endoscopy for patients with suspected small bowel stenosis. Dig Endosc 27: 61-66, 2015. [DOI] [PubMed] [Google Scholar]
- 17. Nakano M, Oka S, Tanaka S, et al. Clinical usefulness of transabdominal ultrasonography prior to patency capsule for suspected small-bowel strictures. Scand J Gastroenterol 51: 281-287, 2016. [DOI] [PubMed] [Google Scholar]
- 18. Watanabe K, Ohmiya N, Nakamura M, Fujiwara Y. A prospective study evaluating the clinical utility of the tag-less patency capsule with extended time for confirming functional patency. Digestion 102: 180-187, 2021. [DOI] [PubMed] [Google Scholar]
- 19. Hosoe N, Watanabe K, Miyazaki T, et al. Evaluation of performance of the Omni mode for detecting video capsule endoscopy images: a multicenter randomized controlled trial. Endosc Int Open 4: E878-E882, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liao Z, Gao R, Li F, et al. Fields of applications, diagnostic yields and findings of OMOM capsule endoscopy in 2400 Chinese patients. World J Gastroenterol 16: 2669-2676, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dolak W, Kulnigg-Dabsch S, Evstatiev R, Gasche C, Trauner M, Püspök A. A randomized head-to-head study of small-bowel imaging comparing MiroCam and EndoCapsule. Endoscopy 44: 1012-1120, 2012. [DOI] [PubMed] [Google Scholar]
- 22. Seike T, Yamato M, Suda TS, et al. A case of small bowel adenocarcinoma that caused intestinal obstruction after administration of patency capsule. Clin J Gastroenterol 13: 522-526, 2020. [DOI] [PubMed] [Google Scholar]
- 23. Kopylov U, Nemeth A, Cebrian A, et al. Symptomatic retention of the patency capsule: a multicenter real life case series. Endosc Int Open 4: E964-E969, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Goto H. Diagnosis and treatment of small bowel diseases are advanced by capsule endoscopy and double-balloon enteroscopy. Clin J Gastroenterol 3: 219-225, 2010. [DOI] [PubMed] [Google Scholar]
- 25. Ohmiya N, Arakawa D, Nakamura M, et al. Small-bowel obstruction: diagnostic comparison between double-balloon endoscopy and fluoroscopic enteroclysis, and the outcome of enteroscopic treatment. Gastrointest Endosc 69: 84-93, 2009. [DOI] [PubMed] [Google Scholar]
- 26. Flicek KT, Hara AK, De Petris G, Pasha SF, Yadav AD, Johnson CD. Diaphragm disease of the small bowel: a retrospective review of CT findings. Am J Roentgenol 202: 140-145, 2014. [DOI] [PubMed] [Google Scholar]
- 27. Ishihara M, Ohmiya N, Nakamura M, et al. Risk factors of symptomatic NSAID-induced small intestinal injury and diaphragm disease. Aliment Pharmacol Ther 40: 538-547, 2014. [DOI] [PubMed] [Google Scholar]
- 28. Cave D, Legnani P, de Franchis R, Lewis BS. ICCE consensus for capsule retention. Endoscopy 37: 1065-1067, 2005. [DOI] [PubMed] [Google Scholar]
- 29. Argüelles-Arias F, Donat E, Fernández-Urien I, et al. Guideline for wireless capsule endoscopy in children and adolescents: a consensus document by the SEGHNP (Spanish Society for Pediatric Gastroenterology, Hepatology, and Nutrition) and the SEPD (Spanish Society for Digestive Diseases). Rev Esp Enferm Dig 107: 714-731, 2015. [DOI] [PubMed] [Google Scholar]
- 30. Enns RA, Hookey L, Armstrong D, et al. Clinical practice guidelines for the use of video capsule endoscopy. Gastroenterology 152: 497-514, 2017. [DOI] [PubMed] [Google Scholar]
- 31. Rondonotti E, Spada C, Adler S, et al. Small-bowel capsule endoscopy and device-assisted enteroscopy for diagnosis and treatment of small-bowel disorders: European Society of Gastrointestinal Endoscopy (ESGE) technical review. Endoscopy 50: 423-446, 2018. [DOI] [PubMed] [Google Scholar]
- 32. Liao Z, Gao R, Xu C, Li ZS. Indications and detection, completion, and retention rates of small-bowel capsule endoscopy: a systematic review. Gastrointest Endosc 71: 280-286, 2010. [DOI] [PubMed] [Google Scholar]
- 33. Cronin CG, Lohan DG, Browne AM, Alhajeri AN, Roche C, Murphy JM. MR enterography in the evaluation of small bowel dilation. Clin Radiol 64: 1026-1034, 2009. [DOI] [PubMed] [Google Scholar]
- 34. Huprich JE, Fletcher JG. CT enterography: principles, technique and utility in Crohn's disease. Eur J Radiol 69: 393-397, 2009. [DOI] [PubMed] [Google Scholar]
- 35. Takenaka K, Ohtsuka K, Kitazume Y, et al. Magnetic resonance evaluation for small bowel strictures in Crohn's disease: comparison with balloon enteroscopy. J Gastroenterol 52: 879-888, 2017. [DOI] [PubMed] [Google Scholar]
- 36. Hirai F, Andoh A, Ueno F, et al. Efficacy of endoscopic balloon dilation for small bowel strictures in patients with Crohn's disease: a nationwide, multi-centre, open-label, prospective cohort study. J Crohns Colitis 12: 394-401, 2018. [DOI] [PubMed] [Google Scholar]
- 37. Nakamura M, Ohmiya N, Shirai O, et al. Route selection for double-balloon endoscopy, based on capsule transit time, in obscure gastrointestinal bleeding. J Gastroenterol 45: 592-599, 2010. [DOI] [PubMed] [Google Scholar]
- 38. Bandorski D, Kurniawan N, Baltes P, et al. Contraindications for video capsule endoscopy. World J Gastroenterol 22: 9898-9908, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rondonotti E, Soncini M, Girelli CM, Russo A, de Franchis R. Negative small-bowel cross-sectional imaging does not exclude capsule retention in high-risk patients. Eur J Gastroenterol Hepatol 28: 871-875, 2016. [DOI] [PubMed] [Google Scholar]
- 40. Pennazio M, Spada C, Eliakim R, et al. Small-bowel capsule endoscopy and device-assisted enteroscopy for diagnosis and treatment of small-bowel disorders: European Society of Gastrointestinal Endoscopy (ESGE) clinical guideline. Endoscopy 47: 352-376, 2015. [DOI] [PubMed] [Google Scholar]
- 41. Tanabe H, Ando K, Ohdaira H, et al. Successful medical treatment for a Crohn's disease patient with a perforation by a second-generation patency capsule. Endosc Int Open 6: E1436-E1438, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sawai K, Goi T, Takegawa Y, et al. Acute small bowel perforation caused by obstruction of a novel tag-less Agile™ patency capsule. Case Rep Gastroenterol 12: 337-343, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Blanco-Velasco G, Ramos-García J, Zamarripa-Mottú R, Solórzano-Pineda OM, Hernández-Mondragón OV. Symptomatic patency capsule retention in a patient with confirmed Crohn's disease. Rev Gastroenterol Mex 85: 370-372, 2020. [DOI] [PubMed] [Google Scholar]
- 44. Rasmussen B, Nathan T, Jensen MD. Symptomatic patency capsule retention in suspected Crohn's disease. J Crohns Colitis 10: 1445-1447, 2016. [DOI] [PubMed] [Google Scholar]
- 45. Boivin ML, Lochs H, Voderholzer WA. Does passage of a patency capsule indicate small-bowel patency? A prospective clinical trial? Endoscopy 37: 808-815, 2005. [DOI] [PubMed] [Google Scholar]
- 46. Rozendorn N, Klang E, Lahat A, et al. Prediction of patency capsule retention in known Crohn's disease patients by using magnetic resonance imaging. Gastrointest Endosc 83: 182-187, 2016. [DOI] [PubMed] [Google Scholar]
- 47. Lee HS, Lim YJ, Jung JH, et al. Magnetic resonance enterography and capsule endoscopy in patients undergoing patency capsule for the evaluation of small bowel Crohn's disease: a Korean clinical experience. Gastroenterol Res Pract 2020: 8129525, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Shiotani A, Hata J, Manabe N, et al. Clinical relevance of patency capsule combined with abdominal ultrasonography to detect small bowel strictures. Eur J Gastroenterol Hepatol 26: 1434-1438, 2014. [DOI] [PubMed] [Google Scholar]
- 49. Handa O, Shiotani A, Handa Y, et al. Usefulness of radiographic targeting on the evaluation of the location of a patency capsule using abdominal ultrasonography. Eur J Gastroenterol Hepatol. Forthcoming. [DOI] [PubMed] [Google Scholar]
- 50. Rondonotti E, Herrerias JM, Pennazio M, et al. Complications, limitations, and failures of capsule endoscopy: a review of 733 cases. Gastrointest Endosc 62: 712-716, 2005. [DOI] [PubMed] [Google Scholar]
- 51. Sidhu R, Sanders DS, McAlindon ME, et al. Capsule endoscopy for the evaluation of nonsteroidal anti-inflammatory drug induced enteropathy: United Kingdom pilot data. Gastrointest Endosc 64: 1035, 2006. [DOI] [PubMed] [Google Scholar]
- 52. Ho KK, Joyce AM. Complications of capsule endoscopy. Gastrointest Endosc Clin N Am 17: 169-178, 2007. [DOI] [PubMed] [Google Scholar]
- 53. Arakawa D, Ohmiya N, Nakamura M, et al. Outcome after enteroscopy for patients with obscure GI bleeding: diagnostic comparison between double-balloon endoscopy and videocapsule endoscopy. Gastrointest Endosc 69: 866-874, 2009. [DOI] [PubMed] [Google Scholar]
- 54. Sakai E, Endo H, Taniguchi L, et al. Factors predicting the presence of small bowel lesions in patients with obscure gastrointestinal bleeding. Dig Endosc 25: 412-420, 2013. [DOI] [PubMed] [Google Scholar]
- 55. Shahidi NC, Ou G, Svarta S, et al. Factors associated with positive findings from capsule endoscopy in patients with obscure gastrointestinal bleeding. Clin Gastroenterol Hepatol 10: 1381-1385, 2012. [DOI] [PubMed] [Google Scholar]
- 56. Ohmiya N, Yano T, Yamamoto H, et al. Diagnosis and treatment of obscure GI bleeding at double balloon endoscopy. Gastrointest Endosc 66: S72-S77, 2007. [DOI] [PubMed] [Google Scholar]
- 57. Ohmiya N, Nakamura M, Osaki H, et al. Development of a comorbidity index to identify patients with small bowel bleeding at risk for rebleeding and small bowel vascular diseases. Clin Gastroenterol Hepatol 17: 896-904, 2019. [DOI] [PubMed] [Google Scholar]
- 58. Uchida G, Nakamura M, Yamamura T, et al. Systematic review and meta-analysis of the diagnostic and therapeutic yield of small bowel endoscopy in patients with overt small bowel bleeding. Dig Endosc 33: 66-82, 2021. [DOI] [PubMed] [Google Scholar]
- 59. Leighton JA, Legnani P, Seidman EG. Role of capsule endoscopy in inflammatory bowel disease: where we are and where we are going. Inflamm Bowel Dis 13: 331-337, 2007. [DOI] [PubMed] [Google Scholar]
- 60. Mensink PB, Groenen MJ, van Buuren HR, Kuipers EJ, van der Woude CJ. Double-balloon enteroscopy in Crohn's disease patients suspected of small bowel activity: findings and clinical impact. J Gastroenterol 44: 271-276, 2009. [DOI] [PubMed] [Google Scholar]
- 61. Oshitani N, Yukawa T, Yamagami H, et al. Evaluation of deep small bowel involvement by double-balloon enteroscopy in Crohn's disease. Am J Gastroenterol 101: 1484-1489, 2006. [DOI] [PubMed] [Google Scholar]
- 62. Bouguen G, Levesque BG, Feagan BG, et al. Treat to target: a proposed new paradigm for the management of Crohn's disease. Clin Gastroenterol Hepatol 13: 1042-1050, 2015. [DOI] [PubMed] [Google Scholar]
- 63. Takenaka K, Kitazume Y, Fujii T, Tsuchiya K, Watanabe M, Ohtsuka K. Objective evaluation for treat to target in Crohn's disease. J Gastroenterol 55: 579-587, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Esaki M, Matsumoto T, Watanabe K, et al. Use of capsule endoscopy in patients with Crohn's disease in Japan: a multicenter survey. J Gastroenterol Hepatol 29: 96-101, 2014. [DOI] [PubMed] [Google Scholar]
- 65. Bourreille A, Ignjatovic A, Aabakken L, et al. Role of small-bowel endoscopy in the management of patients with inflammatory bowel disease: an international OMED-ECCO consensus. Endoscopy 41: 618-637, 2009. [DOI] [PubMed] [Google Scholar]
- 66. Kornbluth A, Colombel JF, Leighton JA, Loftus E; ICCE. ICCE consensus for inflammatory bowel disease. Endoscopy 37: 1051-1054, 2005. [DOI] [PubMed] [Google Scholar]
- 67. Nemeth A, Kopylov U, Koulaouzidis A, et al. Use of patency capsule in patients with established Crohn's disease. Endoscopy 48: 373-379, 2016. [DOI] [PubMed] [Google Scholar]
- 68. Mitsui K, Tanaka S, Yamamoto H, et al. Role of double-balloon endoscopy in the diagnosis of small-bowel tumors: the first Japanese multicenter study. Gastrointest Endosc 70: 498-504, 2009. [DOI] [PubMed] [Google Scholar]
- 69. Pennazio M, Rondonotti E, de Franchis R. Capsule endoscopy in neoplastic diseases. World J Gastroenterol 14: 5245-5253, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Dabaja BS, Suki D, Pro B, et al. Adenocarcinoma of the small bowel: presentation, prognostic factor, and outcome of 217 Patients. Cancer 101: 518-526, 2004. [DOI] [PubMed] [Google Scholar]
- 71. Vagholkar K, Mathew T. Adenocarcinoma of the small bowel: a surgical dilemma. Saudi J Gastroenterol 15: 264-267, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Rondonotti E, Pennazio M, Toth E, et al. Small-bowel neoplasms in patients undergoing video capsule endoscopy: a multicenter European study. Endoscopy 40: 488-495, 2008. [DOI] [PubMed] [Google Scholar]
- 73. Sawada T, Nakamura M, Watanabe O, et al. Clinical factors related to false-positive rates of patency capsule examination. Therap Adv Gastroenterol 10: 589-598, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Nakamura M, Watanabe K, Ohmiya N, et al. Tag-less patency capsule for suspected small bowel stenosis: a nationwide multicenter prospective study in Japan. Dig Endosc 33: 151-161, 2021. [DOI] [PubMed] [Google Scholar]
- 75. Kotwal VS, Attar BM, Gupta S, Agarwal R. Should bowel preparation, antifoaming agents, or prokinetics be used before video capsule endoscopy? A systematic review and meta-analysis. Eur J Gastroenterol Hepatol 26: 137-145, 2014. [DOI] [PubMed] [Google Scholar]
- 76. Yadav A, Heigh RI, Hara AK, et al. Performance of the patency capsule compared with nonenteroclysis radiologic examinations in patients with known or suspected intestinal strictures. Gastrointest Endosc 74: 834-839, 2011. [DOI] [PubMed] [Google Scholar]
- 77. Klang E, Kopylov U, Ben-Horin S, et al. Assessment of patency capsule retention using MR diffusion-weighted imaging. Eur Radiol 27: 4979-4985, 2017. [DOI] [PubMed] [Google Scholar]
- 78. Vogel J, da Luz Moreira A, Baker M, et al. CT enterography for Crohn's disease: accurate preoperative diagnostic imaging. Dis Colon Rectum 50: 1761-1769, 2007. [DOI] [PubMed] [Google Scholar]
- 79. Wang YC, Pan J, Liu YW, et al. Adverse events of video capsule endoscopy over the past two decades: a systematic review and proportion meta-analysis. BMC Gastroenterol 20: 364, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Takenaka K, Ohtsuka K, Kitazume Y, et al. Comparison of magnetic resonance and balloon enteroscopic examination of the small intestine in patients with Crohn's disease. Gastroenterology 147: 334-342.e3, 2014. [DOI] [PubMed] [Google Scholar]
- 81. Omori T, Nakamura S, Shiratori K. Localization of the patency capsule by abdominal tomosynthesis. Digestion 91: 318-325, 2015. [DOI] [PubMed] [Google Scholar]
- 82. Omori T, Kambayashi H, Murasugi S, et al. Valuation of intestinal patency with the patency capsule: the twenty-four hour assessment method. Digestion 100: 176-185, 2019. [DOI] [PubMed] [Google Scholar]