Abstract
Data on the prognosis of patients treated with oral anticoagulation (OAC) prior to hospital admission for COVID-19 remains controversial and insufficient. Therefore, we endeavored to perform a systematic review and meta-analysis to evaluate the effect of chronic use of OAC prior to the diagnosis of COVID-19 on intensive care unit (ICU) admission and mortality. An electronic search of the Pubmed, Embase, Cochrane library databases was conducted. Meta-analysis and statistical analyses were completed with using the RevMan 5.3 and Stata 12.0. A total of 13 articles representing data from 1,266,231 participants were included in this study. The meta-analysis of unadjusted results showed no decrease in mortality (OR = 1.31, 95% CI: 0.99 to 1.73, P = 0.059) or ICU admission rate (OR = 0.71, 95% CI: 0.29 to 1.77, P = 0.46) in COVID-19 patients with prior OAC therapy at hospital admission compared to patients without prior use of OAC. Moreover, the meta-analysis of adjusted results showed no lower risk of mortality (OR = 1.08, 95% CI: 0.90 to 1.30, P = 0.415) or ICU admission (OR = 1.50, 95% CI: 0.72 to 3.12, P = 0.284) in patients with prior OAC use compared to patients without previous OAC use. In conclusion, the results of this study revealed that the use of OAC prior to hospital admission appeared to be ineffective in reducing the risk of intensive care need and mortality in COVID-19 patients. Randomized controlled trials are needed to evaluate and optimize the use of OAC in COVID-19 infection.
Keywords: COVID-19, Oral anticoagulation, Mortality, meta-analysis
Dear Editor,
The coronavirus disease 2019 (COVID-19) pandemic is characterized by high morbidity and mortality, particularly in patients with concomitant cardiovascular diseases [1,2]. Increasing evidence suggested that alterations in coagulation patterns were associated with more severe outcomes of COVID-19 [3]. Studies have revealed that thrombosis is one of the potential pathophysiologies and complications of COVID-19 infection [4,5]. Some studies showed that anticoagulation with low-molecular-weight heparin (LMWH) could lead to a better prognosis in patients with COVID-19 [6,7]. Anticoagulants, antiplatelet, and antithrombotic strategies have been widely implemented in treating COVID-19 infection due to the frequent occurrence of arterial or venal thrombosis. Nevertheless, data on the prognosis of patients treated with oral anticoagulation (OAC) prior to hospital admission for COVID-19 remains controversial and insufficient. To the best of our knowledge, there have been no previous meta-analysis evaluating the effect of previous OAC use on clinical outcomes in COVID-19. Therefore, we endeavored to perform a systematic review and meta-analysis to evaluate the effect of chronic use of OAC prior to the diagnosis of COVID-19 on mortality and ICU admission rate.
An electronic search of the Pubmed, Embase, Cochrane library databases was conducted from inception to October 2021 with no language restrictions. The following keywords and/or medical subject heading terms searches were applied: (“novel coronavirus” or “2019-nCoV” or “coronavirus disease 2019” or “SARS-CoV-2” or “COVID-19”) and (oral anticoagulation or NOAC or DOAC or directly acting oral anticoagulants or vitamin K antagonists or VKA or warfarin or apixaban or edoxaban or rivaroxaban. We also conducted a manual search for additional articles using references from comparable articles and published reviews to seek potentially relevant citations.
Two independent investigators (YW and JZ) performed the initial screening of titles and abstracts. Full-length articles of identified studies were retrieved. We included studies if they (1) enrolled patients diagnosed with COVID-19 infection; (2) provided a comparison of outcomes of interest between patients with and without OAC use; (3) included patients on oral anticoagulation before admission for COVID-19; (4) availability of a risk ratio (RR) with 95% confidence intervals (CI) for overall survival or relevant clinical events from which it could be calculated. We excluded studies if they were abstracts, conferences, editorials, or reviews. All decisions in terms of eligibility were made according to pre-specified selection criteria. Any differing decision was resolved by a third investigator.
Two main investigators (YW and JZ) independently extracted the data and reached a consensus on all items. The following items were extracted from each study if available: name of the first author, study design, country, number of participants, age of patients, number of male and female participants, type of oral anticoagulation therapy, and outcomes of interest. The endpoint was the effect of chronic oral anticoagulation treatment on rates of ICU admission and mortality of COVID-19. For non-random controlled studies, the risk of bias/quality of studies was assessed using a nine-item Newcastle-Ottawa Scale (NOS) independently by two investigators. If necessary, a third investigator was consulted for any discrepancies. We considered a study of high quality if its score was ≥7, whereas a low-quality study had a score of <7.
We completed meta-analysis and statistical analyses using RevMan 5.3 (Cochrane Collaboration) and Stata 12.0 (StataCorp). Unadjusted and adjusted risk ratios (RRs) with 95% CIs were used as the statistical summary for dichotomous outcomes. Cochrane chi-square test (Q test) and the I2 statistic were calculated to detect heterogeneity. An I2 is less than 25%, 25% to 50%, and greater than 50%, corresponding to low, moderate, and high heterogeneity, respectively. A fixed-effect model was used in case of a low level of heterogeneity (I2 < 50%), otherwise a random-effect model was utilized. Sensitivity analysis was performed to evaluate the influence of a single trial on the overall effect estimate by sequentially excluding one study. If substantial heterogeneity was presented in the meta-analysis, subgroup analysis was conducted based on the countries. P < 0.05 was considered statistically significant.
We retrieved 893 potentially eligible literature by searching electronic databases. Among them, we excluded 132 articles due to duplicated searches. Subsequently, 705 studies were regarded as absolute irrelevant studies by examining titles and abstracts. A full text of 56 studies was reviewed, and 43 records were eliminated because they were abstract, letters, conferences, reviews or with no comparison groups between OAC and no-OAC treatment or with no outcomes reported. Therefore, a total of 13 full-text studies with 1,266,231 patients were incorporated in the final analysis [[8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20]]. The sample size of patients ranged from 467 to 738,423. Two studies were from Turkey, while other studies were from European countries. All included studies were retrospective in design. Three studies reported data on hospitalized and outpatient SARS-CoV-2 infected patients [10,13,18], while other studies enrolled hospitalized patients. Most studies reported that OAC treatment was continued in patients using OAC as long as no clinical condition developed that would limit its use. However, in one study by Gülcü et al. [16], parenteral anticoagulation treatments were given at therapeutic doses to patients who had previously used DOAC. Of note, the in-hospital discontinuation of OAC was considered an exclusion criterion in the Russo et al. [19] study to avoid bias deriving from the out-of-range therapeutic periods caused by in-hospital anticoagulation treatment switching or discontinuation. The characteristics of the study are demonstrated in Table 1 . The overall quality of included studies was high, with NOS scores ≥7. The quality of the included articles is assessed and displayed in Table S1. The meta-analysis of unadjusted results showed no decrease in mortality (OR = 1.31, 95% CI: 0.99 to 1.73, P = 0.059; I2 = 94.6%) (Fig. 1A) or ICU admission rate (OR = 0.71, 95% CI: 0.29 to 1.77, P = 0.46; I2 = 97%) (Fig. 1B) in COVID-19 patients with prior OAC therapy at hospital admission compared to patients without prior use of OAC. Moreover, the meta-analysis of adjusted results showed no lower risk of mortality (OR = 1.08, 95% CI: 0.90 to 1.30, P = 0.415; I2 = 75.5%) (Fig. 1C) or ICU admission (OR = 1.50, 95% CI: 0.72 to 3.12, P = 0.284; I2 = 74.7%) (Fig. 1D) in patients with prior OAC use compared to patients without previous OAC use. The subgroup analysis based on countries significantly reduced the heterogeneity but did not significantly alter the overall results. Additionally, sensitivity analyses by omitting each study at a time did not significantly change the results either.
Table 1.
Characteristics of included studies.
| Study | Country | Study design | Sample size | Age |
Sex |
Oral anticoagulation therapy used | Outcomes | ||
|---|---|---|---|---|---|---|---|---|---|
| OAC | No-OAC | Male | Female | ||||||
| Arachchillage | United Kingdom | Retrospective | 5883 | NR | NR | 3247 | 2636 | DOACs (rivaroxaban 20 mg, 15 mg or 10 mg daily, apixaban 5 mg or 2.5 mg daily or edoxaban 30 mg or 60 mg daily, dabigatran 110 mg bd or 150 mg bd) or warfarin | Mortality, thrombosis, major bleeding, multi-organ failure |
| Aslan | Turkey | Retrospective | 1710 | 74 (67–81) | 61 (51–70) | 850 | 860 | DOAC | Mortality, need for ICU |
| Denas | Italy | Retrospective | 4697 | NR | NR | 2378 | 2319 | VKAs, rivaroxaban, apixaban, edoxaban, dabigatran | ICU admission, hospital admission, all-cause mortality |
| Flam | Denmark | Retrospective | 496,277 | 73.6 (7.6) | 69.3 (9.6) | 301,549 | 194,728 | DOAC (dabigatran, apixaban, rivaroxaban, edoxaban) | Hospital admission for COVID-19, ICU admission or death due to COVID-19 |
| Fröhlich | Germany | Retrospective | 6637 | 80 (75–85) | 65 (52–79) | 3505 | 3132 | VKA, DOAC | All-cause mortality, need for non-invasive ventilation, need for invasive ventilation, ECMO, ARDS |
| Fumagalli | Italy | Retrospective | 806 | NR | NR | NR | NR | VKA, DOAC | Mortality |
| Iaccarino | Italy | Retrospective | 2377 | 79.35 ± 0.86 | 67.59 ± 0.39 | 1489 | 888 | Warfarin, DOACs | Mortality, ICU admission, combined hard events |
| Gülcü | Turkey | Retrospective | 5575 | 69 (59, 77) | 64 (51, 73) | 2801 | 2774 | Warfarin, DOAC | Mortality |
| Rieder | Germany | Retrospective | 1433 | 77.04 ± 10.31 | 69.56 ± 13.61 | 863 | 570 | VKA or Non-VKA (rivaroxaban, apixaban, edoxaban, dabigatran etexilate) | All-cause mortality, thrombotic events, intracerebral bleeding, death or thrombotic event, death or intracerebral bleeding |
| Russo | Italy | Retrospective | 467 | 73 ± 12 | 65 ± 14 | 293 | 174 | NOACs or VKAs | Mortality, ARDS |
| Caravaca a | United Kingdom | Retrospective | 738,423 | 67.30 ± 15.43 | 45.50 ± 18.10 | 321,960 | 416,463 | NOAC (dabigatran, apixaban, rivaroxaban or edoxaban) | all-cause mortality, hospitalization/re-hospitalization, VTE and ICH, the composite of ischemic stroke/TIA/ SE, the composite of ICH/gastrointestinal bleeding, myocardial infarction, and the composite of any thrombotic or thromboembolic event |
| Caravaca b | Spain | Retrospective | 1002 | NR | NR | 593 | 409 | NOACs or VKAs | All-cause mortality, all-cause mortality or any thromboembolic event, renal failure, respiratory insufficiency, systemic inflammatory response syndrome, heart failure, sepsis |
| Schiavone | Italy | Retrospective | 844 | 76.7 ± 11.6 | 62.3 ± 15.9 | 521 | 323 | NOACs or VKAs | ICU admission, ARDS, all-cause mortality, hospital length of stay, non-invasive ventilation |
DOAC:direct-acting oral anticoagulants;NOAC: novel oral anticoagulants; VKA:vitamin K antagonists; ARDS: acute respiratory distress syndrome; ECMO: extracorporeal membrane oxygenation; VTE: venous thromboembolism; ICH: intracranial hemorrhage; TIA: transient ischemic attack; SE: systemic embolism; ICU: intensive care unit.
Fig. 1.
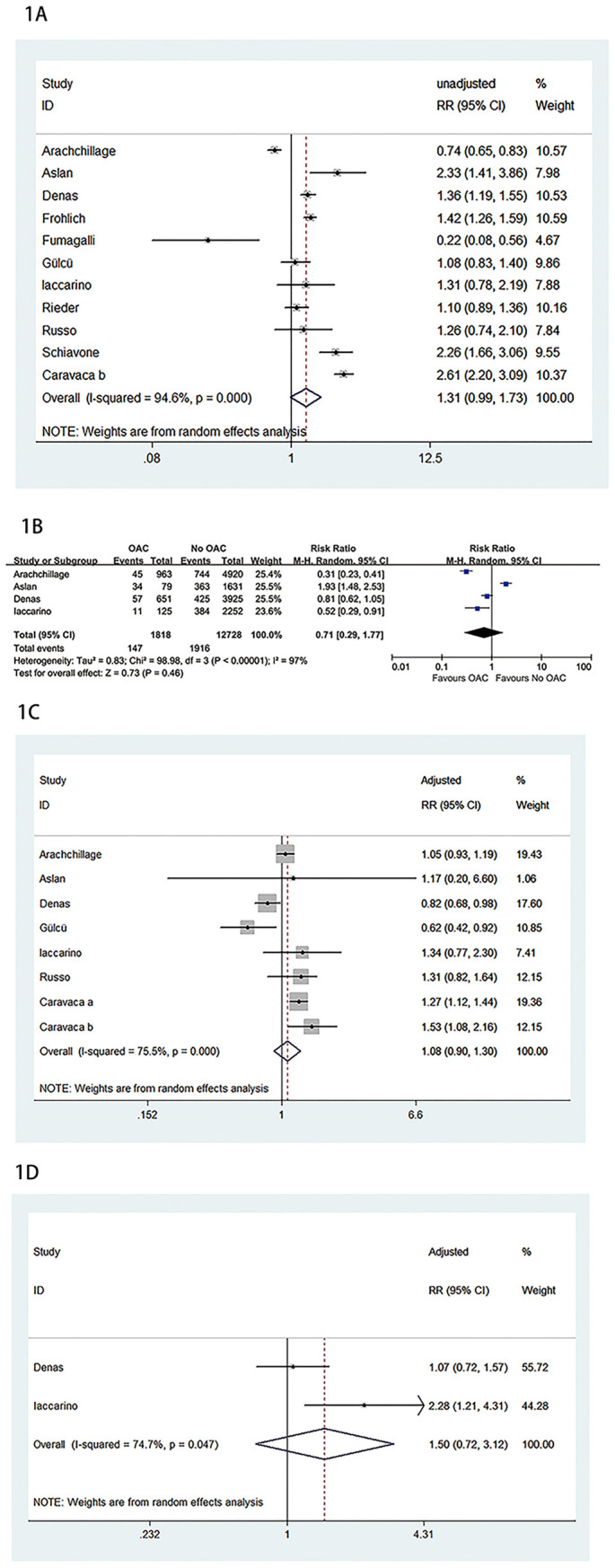
A. Meta-analysis of unadjusted results of association between oral anticoagulation and mortality. B. Meta-analysis of unadjusted results of association between oral anticoagulation and ICU admission. C. Meta-analysis of adjusted results of association between oral anticoagulation and mortality. D. Meta-analysis of adjusted results of association between oral anticoagulation and ICU admission.
The current systematic review and meta-analysis revealed that OAC use prior to admission was not associated with lower risks of mortality or ICU admission. This finding could be explained by the fact that patients on OAC therapy are older, more susceptible to COVID-19 complications, and have more comorbidities. In addition, comorbid diseases and advanced age are associated with morbidity and mortality in COVID-19 infection. Thus, those patients should be hospitalized and followed up more closely after the diagnosis of COVID-19 infection. Akiyama et al. indicated that microvascular thrombosis rather than classical pulmonary embolism could lead to hypoperfusion in COVID-19 infection. Thus, directly acting oral anticoagulants (DOAC) therapy has no protective effect on leukocyte-related thrombosis and prevention of severe COVID-19 infection [21,22]. In addition, recent evidence associated the use of heparin and low-molecular-weight heparin (LMWH) with various non-anticoagulant effects, including antiviral, anti-inflammatory/immunomodulatory properties [23,24]. Anti-inflammatory properties of heparin, inhibition of NF-κB transcription factor can potentially reduce the activation of inflammatory molecules and regulate the expression and production of proinflammatory cytokines, chemokines, and adhesion molecules [25]. The antiviral and anti-inflammatory/immunomodulatory effects indicated a potential role of heparin and LMWH in the treatment of COVID-19 infection [26]. Due to a certain proportion of patients who previously took OAC switching their in-hospital antithrombotic treatment to heparin following the local attending physician criteria, parenteral anticoagulant therapy may be a serious confounding factor on outcomes. Our results should be interpreted with caution. All of the studies included were retrospective in design, which could be subject to selection bias and potential confounders. Data on duration, type, the dose of OAC, and other clinical outcomes were insufficient in most incorporated studies; hence, they cannot be further analyzed. In conclusion, the results of this study revealed that the use of OAC prior to hospital admission appeared to be ineffective in reducing the risk of intensive care need and mortality in COVID-19 patients. Randomized controlled trials are needed to evaluate and optimize the use of OAC in the course of the COVID-19.
The following are the supplementary data related to this article.
Study quality assessment using the Newcastle-Ottawa Scale.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ajem.2022.01.059.
Funding/Acknowledgments
None.
Declaration of Competing Interest
None.
References
- 1.Guan W., Ni Z., Hu Y., Liang W.H., Ou C.Q., He J.X., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clerkin K.J., Fried J.A., Raikhelkar J., Sayer G., Griffin J.M., Masoumi A., et al. Coronavirus disease 2019 (COVID-19) and cardiovascular disease. Circulation. 2020;141(20):1648–1655. doi: 10.1161/CIRCULATIONAHA.120.046941. [DOI] [PubMed] [Google Scholar]
- 3.Cui S., Chen S., Li X., Liu S., Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. 2020;18:1421–1424. doi: 10.1111/jth.14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lodigiani C., Iapichino G., Carenzo L., Cecconi M., Ferrazzi P., Sebastian T., et al. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan. Italy Thromb Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kruse J.M., Magomedov A., Kurreck A., Munch F.H., Koerner R., Kamhieh-Milz J., et al. Thromboembolic complications in critically ill COVID-19 patients are associated with impaired fibrinolysis. Crit Care. 2020;24(1):676. doi: 10.1186/s13054-020-03401-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hunt B.J., De Paula E.V., McLintock C., Dumantepe M. Prophylactic anticoagulation for patients in hospital with covid-19. BMJ. 2021;372 doi: 10.1136/bmj.n487. [DOI] [PubMed] [Google Scholar]
- 7.Tang N., Bai H., Chen X., Gong J., Li D., Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18:1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arachchillage D.J., Rajakaruna I., Zain O., Thambiah C.C., Nicolson P.L.R., Roberts L.N., et al. Clinical outcomes and the impact of prior oral anticoagulant use in patients with coronavirus disease 2019 admitted to hospitals in the UK - a multicentre observational study. Br J Haematol. 2021 doi: 10.1111/bjh.17787. [Online ahead of print] [DOI] [PubMed] [Google Scholar]
- 9.Aslan B., Akyüz A., Işık F., Çap M., İnci Ü., Kaya İ., et al. The effect of chronic DOAC treatment on clinical outcomes of hospitalized patients with COVID-19. Int J Clin Pract. 2021;75(9) doi: 10.1111/ijcp.14467. [Epub 2021 Jun 22] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caravaca J.M.R., Buckley B.J.R., Harrison S.L., Eynullayeva E.F., Underhill P., Marín F., et al. Direct-acting oral anticoagulants use prior to COVID-19 diagnosis and associations with 30-day clinical outcomes. Thromb Res. 2021;205:1–7. doi: 10.1016/j.thromres.2021.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caravaca J.M.R., Gil I.J.N., Vivas D., Llamas M.C.V., Uribarri A., Muñoz V.M.B., et al. Clinical profile and prognosis in patients on oral anticoagulation before admission for COVID-19. Eur J Clin Invest. 2021;51(1):e13436. doi: 10.1111/eci.13436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Denas G., Gennaro N., Ferroni E., Fedeli U., Lorenzoni G., Gregori D., et al. Reduction in all-cause mortality in COVID-19 patients on chronic oral anticoagulation: a population-based propensity score matched study. Int J Cardiol. 2021;15(329):266–269. doi: 10.1016/j.ijcard.2020.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flam B., Wintzell V., Ludvigsson J.F., Martensson J., Pasternak B. Direct oral anticoagulant use and risk of severe COVID-19. J Intern Med. 2021;289(3):411–419. doi: 10.1111/joim.13205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fröhlich G.M., Jeschke E., Eichler U., Thiele H., Alhariri L., Reinthaler M., et al. Impact of oral anticoagulation on clinical outcomes of COVID-19: a nationwide cohort study of hospitalized patients in Germany. Clin Res Cardiol. 2021;110(7):1041–1050. doi: 10.1007/s00392-020-01783-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fumagalli S., Trevisan C., Signore S.D., Pelagalli G., Volpato S., Gareri P., et al. COVID-19 and atrial fibrillation in older patients: does Oral anticoagulant therapy provide a survival benefit?-an insight from the GeroCovid registry. Thromb Haemost. 2021 doi: 10.1055/a-1503-3875. [DOI] [PubMed] [Google Scholar]
- 16.Gülcü O., Aksakal E., Aydemir S., Doğan R., Saraç I., Aydın S.S., et al. Association between previous anticoagulant use and mortality among hospitalized patients with COVID-19. J Thromb Thrombolysis. 2021:1–8. doi: 10.1007/s11239-021-02489-1. [Online ahead of print.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iaccarino G., Grassi G., Borghi C., Grassi D., Mancusi C., Muiesan M.L., et al. Preexisting oral anticoagulant therapy ameliorates prognosis in hospitalized COVID-19 patients. Front Cardiovasc Med. 2021;8:633878. doi: 10.3389/fcvm.2021.633878. [eCollection 2021] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rieder M., Gauchel N., Kaier K., Jakob C., Borgmann S., Classen A.Y., et al. Pre-medication with oral anticoagulants is associated with better outcomes in a large multinational COVID-19 cohort with cardiovascular comorbidities. Clin Res Cardiol. 2021:1–11. doi: 10.1007/s00392-021-01939-3. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Russo V., Bottino R., D’Andrea A., Silverio A., Maio M.D., Golino P., et al. 2021. Cardiovasc drugs Ther. Chronic oral anticoagulation and clinical outcome in hospitalized COVID-19 patients; pp. 1–8. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schiavone M., Gasperetti A., Mancone M., Curnis A., Mascioli G., Mitacchione G., et al. Oral anticoagulation and clinical outcomes in COVID-19: an Italian multicenter experience. Int J Cardiol. 2021;323:276–280. doi: 10.1016/j.ijcard.2020.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akiyama S., Hamdeh S., Micic D., Sakuraba A. Prevalence and clinical outcomes of COVID-19 in patients with autoimmune diseases: a systematic review and meta-analysis. Correspondence Ann Rheum Dis. 2020 doi: 10.1136/annrheumdis-2020-218946. annrheumdis-2020-218946. [Online ahead of print] [DOI] [PubMed] [Google Scholar]
- 22.Gremese E., Ferraccioli G. The pathogenesis of microthrombi in COVID-19 can not be controlled by DOAC: NETosis should be the target. J Intern Med. 2021;289(3):420–421. doi: 10.1111/joim.13228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Litov L., Petkov P., Rangelov M., Ilieva N., Lilkova E., Todorova N., et al. Molecular mechanism of the anti-inflammatory action of heparin. Int J Mol Sci. 2021;22:10730. doi: 10.3390/ijms221910730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mousavi S., Moradi M., Khorshidahmad T., Motamedi M. Antiinflammatory effects of heparin and its derivatives: a systematic review. Adv Pharmacol Sci. 2015;2015 doi: 10.1155/2015/507151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Antonio V., Francesco F. Low molecular weight heparin, anti-inflammatory/immunoregulatory and antiviral effects, a short update. Cardiovasc Drugs Ther. 2021:1–5. doi: 10.1007/s10557-021-07251-6. [Online ahead of print] [DOI] [Google Scholar]
- 26.Hippensteel J.A., LaRiviere W.B., Colbert J.F., Langouët-Astrié C.J., Schmidt E.P. Heparin as a therapy for COVID-19: current evidence and future possibilities. Am J Physiol Lung Cell Mol Physiol. 2020;319:L211–L217. doi: 10.1152/ajplung.00199.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Study quality assessment using the Newcastle-Ottawa Scale.


