Abstract
A new immunochromatographic rapid test, POC PUUMALA (Erilab Ltd., Kuopio, Finland), for detection of acute-phase Puumala virus (PUUV) infection was developed based on a highly purified baculovirus-expressed PUUV nucleocapsid protein antigen and lateral immunodiffusion techniques. After addition of sample (5 μl of serum, plasma, or fingertip blood) and buffer, PUUV-specific immunoglobulin M (IgM) antibodies, if present, together with the gold-conjugated anti-human IgM, formed a specific colored line in 5 min. The sensitivity and specificity of the test were evaluated with 200 serum samples and 30 fingertip blood samples. The reference method for the serum samples was a μ-capture enzyme immunoassay (EIA) for IgM and an immunofluorescence assay (IFA) for IgG antibodies. The analytical sensitivity and specificity of the rapid test were 100 and 99%, respectively, for unfrozen serum samples (n = 103; 12 PUUV IgM-positive samples). When freeze-thawed serum samples were used, the sensitivity and specificity were each 97.1% (n = 70; 35 PUUV IgM-positive samples). The specificity of the test was 96.2% for 27 serum samples with nonspecific IgM antibodies or rheumatoid factor (RF). The fingertip blood samples (n = 30) were negative, but they gave clear positive results when spiked with IgM-positive sera (n = 20). The results were in good agreement with the standard diagnostic methods. The rapid performance, the lack of need for refined laboratory equipment, and the high specificity with fresh serum and fingertip blood samples indicate that the developed POC PUUMALA rapid test is a useful tool for fast diagnosis of acute PUUV infection.
Hantaviruses are rodent-borne RNA viruses that belong to the family Bunyaviridae (28). Their genome is negative stranded and tripartite encoding an RNA-dependent RNA polymerase, two envelope glycoproteins (G1 and G2), and a nucleocapsid protein (N) (24). In humans, hantaviruses cause two distinct diseases, a hemorrhagic fever with renal syndrome (HFRS) and a hantavirus pulmonary syndrome (9, 15, 17). In Europe, two hantaviruses cause HFRS, Puumala virus (PUUV) (5) and Dobrava virus (DOBV) (3).
Nephropathia epidemica (NE) is a mild form of HFRS caused by PUUV. The virus is transmitted to humans from the excreta of the chronically infected carrier rodent, the bank vole (Clethrionomys glareolus) (5, 24), most probably by inhalation. PUUV is the most common hantavirus in Europe, and the bank vole is found all over Europe except in the Mediterranean coastal regions.
PUUV causes a generalized infection with acute onset and variable severity from mild to life-threatening. Common symptoms of NE are fever, abdominal and back pain, headache, myalgia, dizziness, anorexia, vomiting, visual disturbances, and signs of renal dysfunction. Proteinuria, leukocytosis, thrombocytopenia, and elevated serum creatinine levels are typical laboratory findings. NE has an incubation period of 2 to 4 weeks (22, 26, 30, 31). In a series of 126 Finnish NE patients (22), 5% had hemorrhagic complications, 2% had acute perimyocarditis, 6% were treated with hemodialysis, and none died. NE is the most common cause of acute renal failure in Scandinavia (27). Mortality due to PUUV infection in Finland is less than 0.1% (6).
The laboratory diagnosis of PUUV infections is based on serology. Shortly after the onset of illness, immunoglobulin M (IgM) and IgG class antibodies to PUUV become detectable. In rare cases (<2%), PUUV-specific IgM antibodies may remain negative up to 5 days after the onset of illness (14). It has been shown that the N protein is the main antigenic target of the human antibody response, and the early IgG antibody response is directed predominantly toward this protein (16). Positive PUUV IgM or low avidity of specific IgG is diagnostic of acute PUUV infection. Due to the low level of viremia during the acute phase of NE, reverse transcription-PCR of blood or serum for PUUV RNA is positive in fewer than two-thirds of the patients (25).
For serological diagnosis of PUUV infection, traditional immunofluorescence assays (IFA) (5, 13, 29) have been widely used in clinical laboratories. Several enzyme immunoassay (EIA) methods based on recombinant antigens have been developed (7), but only two are commercially available, from Progen (Heidelberg, Germany) and from MRL (Cypress, Calif.). All the currently available serological tests require laboratory facilities with trained personnel and at least a 1-h testing time.
In this article, we introduce an alternative to the conventional methods for the diagnosis of acute PUUV infection.
MATERIALS AND METHODS
Samples.
This study involved four different panels of serum samples. Serum samples in panels 1 and 2 were collected from sera received for the determination of PUUV antibodies at the Zoonosis Unit, Department of Virology, HUCH Laboratory Diagnostics, Helsinki, Finland (a World Health Organization Collaborating Center for Arbovirus and Hemorrhagic Fever Reference and Research). Panel 1 included 103 nonfrozen serum samples from June 2000, which were analyzed by POC PUUMALA and the reference methods at the time they entered the analyzing laboratory. Panel 2 included 70 previously collected and analyzed (by the reference methods) serum samples from February 2000 (35 PUUV IgM positive and 35 PUUV IgM negative) which had been stored at −20°C. Five of the PUUV IgM-negative samples were PUUV IgG positive, representing old immunity. Before analysis by POC PUUMALA, the samples were thawed and recoded.
Fingertip blood samples in panel 3 were collected from 30 healthy volunteers (8 males and 22 females, aged 23 to 53 years). Samples (5 μl) were analyzed immediately at the sampling place. The samples were transferred from the fingertip to the sample wells of the test cassette with an adjustable pipette. The sample donors were interviewed for possible symptoms of PUUV infection during the last year. Other relevant issues such as age, sex, marital status, and other diagnosed diseases were also documented. Two urinary tract infections and one coxsackievirus infection were reported. For artificial positive blood samples, fingertip blood from one individual was spiked (1:2) with different IgM-positive sera (n = 20). Immediately after spiking, 5-μl samples were analyzed by the rapid test.
Serum samples in panel 4 were collected from Kuopio University Hospital, Kuopio, Finland and frozen until tested by POC PUUMALA. Panel 4 (n = 27) included sera with RF (n = 5) or specific IgM antibodies to human parvovirus (n = 1), rubella virus (n = 8), dengue virus (n = 2), Sindbis virus (n = 5), or measles virus (n = 6). Two RF-positive serum samples were also PUUV IgG positive in the reference test. Four RF-positive but POC PUUMALA-negative samples were spiked (1:2) with PUUV-specific IgM positive sera and tested immediately by the rapid test.
Reference methods.
An IFA (13) was used as the reference method for detection of PUUV-specific IgG. This test is based on a mixture of PUUV-infected and uninfected Vero E6 cells which are dropped on slide spots, air dried, acetone fixed, and stored at −70°C until used for the analysis of the serum samples (diluted 1:20), using fluorescein isothiocyanate-conjugated anti-human IgG for detection. For the detection of PUUV-specific IgM antibodies, a μ-capture EIA was used (14, 33). The test is based on a baculovirus-expressed PUUV-N and a peroxidase-conjugated monoclonal antibody (1C12) to PUUV-N.
Production and isolation of recombinant nucleocapsid protein.
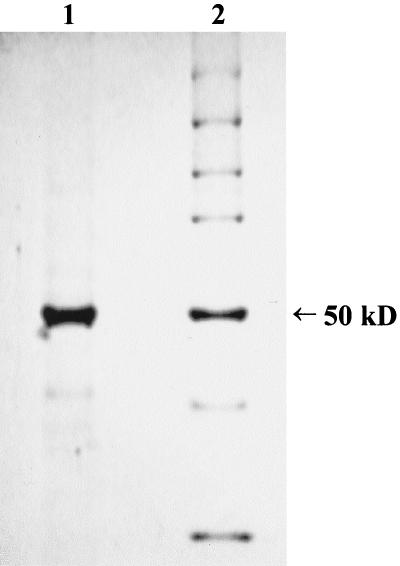
The coding sequence for PUUV Sotkamo strain S segment, cloned in plasmid pACYM1, was used for transfecting Sf9 cells together with wild-type baculovirus DNA, as described previously (33). After repeated infection cycles on monolayer cultures, the cells were mass cultured in stirred suspension cultures. The cells were pelleted by low-speed centrifugation, washed twice with phosphate-buffered saline containing protease inhibitors (Complete protease inhibitor cocktail [Boehringer, Mannheim, Germany), and stored as a 50% (vol/vol) suspension at −70°C. PUUV-N was isolated from the thawed suspension at room temperature by stepwise urea extraction with 3.0 to 8.0 M urea solution. The protease inhibitors were included in all solutions. The cell suspension was washed three times with 3 M urea in 0.05 M Tris-HCl–0.05 M NaCl–1 mM EDTA (pH 8.0) (buffer A), with careful suspension and pipetting between centrifugations. The centrifugations were performed with an RC-5B refrigerated centrifuge (Sorvall, Newtown, Conn.) for 15 min at 15,000 rpm (20,000 × g). After the washes with 3 M urea, the pellets were suspended in 8.0 M urea in buffer A. The volume of the 8.0 M urea was approximately equal to the original volume of the cell suspension. After incubation for 30 min, the suspension was centrifuged as described above. The supernatant, containing the isolated PUUV-N, was recovered and stored as aliquots at −70°C. The purity of N protein was defined by polyacrylamide gel electrophoresis (Fig. 1).
FIG. 1.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis of baculovirus-expressed PUUV-N antigen (lane 1) and molecular mass markers (LMW; Bio-Rad Laboratories, Hercules, Calif.) (lane 2). In this experiment, ∼1 μg of the PUUV antigen was run into a 10% gel under reducing conditions. The gel was stained and destained with the Bio-Rad Coomassie brilliant blue staining system. As estimated from this gel, the level of purity in the antigen preparation is over 90%, and its molecular size is ∼50 kDa, which corresponds to the full-length nucleocapsid protein of PUUV.
POC PUUMALA.
The POC PUUMALA test uses a lateral-flow membrane-based assay technology, in which the baculovirus-expressed highly purified PUUV-N antigen is immobilized on the nitrocellulose membrane. The PUUV-N antigen is purified by urea extraction from Sf9 insect cells (ATCC CRL 1711) as described previously (33). The test result is visualized by using gold-conjugated rabbit anti-human IgM antibodies dried in the conjugate pad.
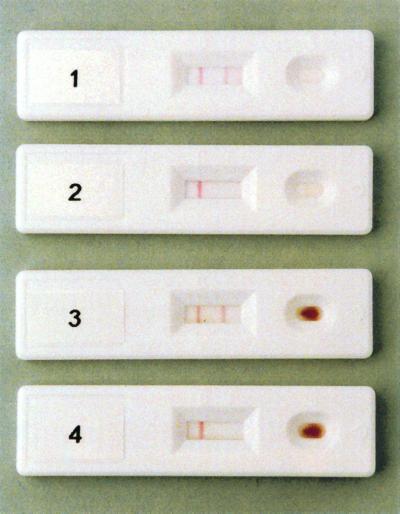
The POC PUUMALA rapid test uses a sample well and a result window (Fig. 2). After addition of the sample to the sample well, 2 drops of the running buffer are added. The result is detected within 5 min after addition of the running buffer by the presence (positive samples) or absence (negative samples) of the red line in the test window location marked T. The red line is formed by precipitation of the PUUV-N-specific IgM and the gold conjugate complex with PUUV-N antigen in the membrane. The control line is formed into the test window location marked C as a consequence of the reaction between the goat anti-rabbit antibody in the control line and the gold-conjugated rabbit anti-human antibody.
FIG. 2.
Typical positive and negative results with IgM-positive serum (row 1), IgM-negative serum (row 2), a fingertip blood sample spiked with IgM-positive serum (row 3), and an IgM-negative fingertip blood sample (row 4) in the POC PUUMALA rapid test. The visible control line (left) and visible test line (right) in the test window indicate a positive result. If only one red line appears in the control window, the test is negative.
Assay conditions.
All the analyses were done under conditions recommended by the manufacturer, namely, 5 μl of sample, 2 drops of running buffer, and a 5-min running time at room temperature. Additionally, assay tolerances for different sample volumes (1, 2, 5, and 20 μl), different temperatures (room temperature and 37°C), and different reading times (1 to 10 mins) were defined.
Interpretation of the test results.
Coded serum samples were tested and interpreted independently by four analysts who judged the results as negative, weak positive, or positive. The final results were scored as negative, very weak reactivity, weak positive, or positive by combining the results of the four analysts according to the following rules: (i) negative, when the interpretation of all four readers was negative (−, −, −, −); (ii) very weak reactivity, when two or three readers interpret the result as negative but one or two interpret it as weak positive, (e.g., −, −, −, +/− or −, −, +/−, +/−); (iii) weak positive, when three or four readers interpret the result as weak positive and none or one as positive (e.g., +/−, +/−, +/−, +/− or +/−, +/−, +/−, +); and (iv) positive, when all readers interpret the result as positive, or two or three readers interpret it as positive and the rest interpret it as weak positive (e.g., +, +, +, + or +, +, +, +/−, or +, +, +/−, +/−)
The fingertip blood samples were obtained, tested, and interpreted as negative or positive by a laboratory technician who was trained to perform the test.
RESULTS
Assay tolerances.
Testing of deviant assay conditions showed that the test produces equivalent results to those obtained under the optimal assay conditions with as little as 1 μl of sample, a 3-min assay time, and an assay temperature of 37°C. In rare cases, some of the negative samples produced a “ghost line,” which caused difficulties in interpretation, when the running time was extended to 10 min or longer. Sample volumes exceeding 20 μl may also cause this problem due to reduced liquid flow.
Comparison of the rapid test to reference methods.
The nonfrozen serum samples (panel 1, n = 103) were analyzed by the POC PUUMALA rapid test on the day they arrived at the HUCH Laboratory Diagnostics for the determination of PUUV antibodies. Of the 12 IgM-positive reference samples in panel 1, 11 were judged as clear positives and 1 was judged as a weak positive. Of 86 IgG- and IgM-negative samples, 2 gave a faint ghost line in the rapid test and were interpreted as very weak reactivity. All four samples representing old immunity (PUUV IgG positive, IgM negative) were clearly negative. One sample gave a borderline absorbance value in the IgM EIA as the reference method and was weakly positive by the rapid test. A subsequent sample from this patient, obtained 1 month later, was clearly PUUV IgM and IgG negative.
When calculating the test sensitivities and specificities, both the negative and very-weak-reactivity results in the rapid test were scored as negative whereas the weak-positive results were grouped with the positive results and scored as positive. All 12 nonfrozen reference test-positive serum samples also scored positive in the rapid test, and 86 of 87 reference test-negative samples also scored negative in the rapid test. For the POC PUUMALA rapid test with nonfrozen sera, this results in a sensitivity of 100% and a specificity of 99%.
The freeze-thawed serum samples (panel 2, n = 70) were previously analyzed by reference methods in the diagnostic routine at HUCH Laboratory Diagnostics. Of 35 reference-positive PUUV IgM samples tested by the POC PUUMALA rapid test, 26 (74.3%) were interpreted as positive and 8 (22.9%) were interpreted as weak positive. One (2.9%) of the samples showed very weak reactivity, and none of the samples were interpreted as clearly negative. Of the 30 PUUV IgM- and IgG-negative samples, 1 (3.3%) was interpreted as weak positive with the rapid test and two (6.6%) were interpreted as very weak reactivity, while the other 27 (90%) were clearly negative. All of the PUUV IgG-positive and IgM-negative samples (n = 5) gave a clearly negative result with the rapid test. Considering that the majority of the analysts interpreted 34 of 35 confirmed negative samples as being negative and 34 of 35 confirmed positive samples as being positive, the specificity and sensitivity of the POC PUUMALA rapid test with freeze-thawed samples were each 97.1%.
The fingertip blood samples (panel 3, n = 30) collected from the healthy volunteers gave negative results in the rapid test, whereas the serum-spiked fingertip blood samples gave clear positive results in all cases. Spiking was done with moderately (n = 10) and highly (n = 20) PUUV IgM-positive serum samples (according to EIA) by mixing serum 1:2 with the fresh fingertip blood from one volunteer.
In panel 4, which included five RF-positive sera, two of which were also PUUV IgG positive, 26 of 27 serum samples gave clear negative results in the rapid test. One serum sample, positive for both RF and PUUV-specific IgG, gave a positive result. Thus, the calculated specificity of the rapid test with panel 4 was 96.2%. After being spiked with PUUV-specific IgM serum, the four RF-positive sera gave clearly positive results in the rapid test.
DISCUSSION
PUUV is the most common cause of HFRS in Europe. According to previous studies, PUUV seroprevalence is approximately 5% in Finland, 5 to 9% in Northern Sweden, and 2% in Estonia (1, 6, 20). PUUV infections have also been diagnosed in Russia (where outbreaks involving thousands of cases occur), Norway, Denmark, Germany, Belgium, France, The Netherlands, and Austria (12, 26) as well as in many regions of Eastern Europe and the Balkans, where another hantavirus, DOBV, causes a more severe form of HFRS. DOBV cases have been reported in Albania, Greece, Russia, Estonia, Slovenia, Bosnia-Herzegovina, and Germany (2, 4, 11, 18, 19, 21). Hantavirus antibodies have also been found in Northern Italy and Spain by epidemiological studies (23).
The risk of acquiring PUUV infection is dependent on the geographic location and season and is highest among farmers and forestry workers. In Finland, the incidence is highest in late autumn to early winter, although a smaller incidence peak in August is due to the urban population acquiring the disease during their summer holidays. By comparison of the seroprevalence (5%) and disease incidence (19/100,000) figures, it has been estimated that currently only 13% of the PUUV infections in Finland are diagnosed, probably due to a mild or atypical course of the illness or lack of clinical alert (6, 32). Since the risk of contracting PUUV infection is higher in the countryside than in urban areas, this emphasizes the need for a simple decentralized test system, which enables the laboratory diagnosis of PUUV infections at point-of-care settings.
PUUV infection causes a lifelong immunity, and the IgG antibodies can be detected for decades after the infection. In some rural areas of Finland, among elderly men the seroprevalence may exceed 50% (6). The POC PUUMALA rapid test is very specific for PUUV IgM antibodies, thus providing a feasible diagnostic tool for acute NE cases in a population with endemic infection.
According to this first evaluation of the POC PUUMALA rapid test, nonfrozen serum samples gave better results than the freeze-thawed samples did. The other viral diseases tested did not interfere with the test result. One serum sample from a patient with RF caused a positive result, however, it should be noted that this sample was also PUUV-IgG positive. The usual problem with the freeze-thawed samples was the formation of a ghost line, which in some cases led to erroneous results. The reason for the appearance of the ghost line remains unknown since we could not point out any clinical or technical factor. It was probably caused by nonspecific binding of some serum proteins to the antigen, leading to partial blocking of liquid flow. The very-weak-reactivity result could be distinguished from the weak-positive result based on the color and shape of the test line. When the test result is truly positive, although weak, the test line is clearly red and even throughout, whereas the ghost line is uneven and nebulous.
The quality of the antigen or other critical materials or the optimization of the assay configuration may affect the specificity and sensitivity of rapid tests (10). The sensitivity (97 to 100%) and specificity (97 to 99%) of POC PUUMALA are comparable to those of other available assays based on EIA for detection of PUUV IgM. The most reliable method has been μ-capture IgM EIA with either native viral antigen or full-length recombinant PUUV-N protein expressed in Escherichia coli or in insect cells, produced either by baculovirus expression in Sf9 cells or by stable expression in Drosophila melanogaster (DES-PUU-N) cells (7, 8, 14). All the full-length N antigens show 100% sensitivity and specificity values in the IgM test evaluations (7, 8); however, in IgG tests only insect cell-expressed antigens have given optimal sensitivity and specificity. In POC PUUMALA, high specificity and sensitivity were achieved by using very homogeneous recombinant protein as antigen (Fig. 1). Reducing the purity of this preparation resulted in markedly lower analytical performance (data not shown). According to the present data, the POC PUUMALA rapid test meets the analytical requirements for the diagnosis of acute PUUV infections. The new immunochromatography-based method is cost-effective, rapid, and convenient, and its diagnostic performance is equal to that of the other methods developed for this purpose. In addition, the ability to use fingertip blood, ease of performance, and lack of need for specialized equipment or personnel suggest that POC PUUMALA is also applicable in smaller laboratories and doctors' offices.
ACKNOWLEDGMENTS
This work was supported by the National Technology Agency, TEKES (project 40566/98).
We thank Raija Leveelahti, Tytti Manni, Johanna Myllynen, Hilppa Nykyri, Leena Kostamovaara, and Kirsi Lehikoinen for expert technical assistance.
REFERENCES
- 1.Ahlm C, Linderholm M, Juto P, Stegmayr B, Settergren B. Prevalence of serum IgG antibodies to Puumala virus (haemorrhagic fever with renal syndrome) in Northern Sweden. Epidemiol Infect. 1994;113:129–136. doi: 10.1017/s0950268800051542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antoniadis A, Stylianakis A, Papa A, et al. Direct genetic detection of Dobrava virus in Greek and Albanian patients with haemorrhagic fever with renal syndrome. J Infect Dis. 1996;174:407–410. doi: 10.1093/infdis/174.2.407. [DOI] [PubMed] [Google Scholar]
- 3.Avsic-Zupanc T, Xiao S-Y, Stojanovic R, Gligic A, van der Groen G, LeDuc J W. Characterisation of Dobrava virus: a hantavirus from Slovenia. J Med Virol. 1992;38:132–137. doi: 10.1002/jmv.1890380211. [DOI] [PubMed] [Google Scholar]
- 4.Avsic-Zupanc T, Petrovec M, Furlan P, Kaps R, Elgh F, Lundkvist Å. Haemorrhagic fever with renal syndrome (HFRS) in the Dolensjska region of Slovenia— a ten-year survey. Clin Infect Dis. 1999;28:860–865. doi: 10.1086/515185. [DOI] [PubMed] [Google Scholar]
- 5.Brummer-Korvenkontio M, Vaheri A, Hovi T, von Bonsdorff C H, Vuorimies J, Manni T, Penttinen K, Oker-Blom N, Lähdevirta J. Nephropathia epidemica: detection of antigen in bank voles and serologic diagnosis of human infection. J Infect Dis. 1980;141:131–134. doi: 10.1093/infdis/141.2.131. [DOI] [PubMed] [Google Scholar]
- 6.Brummer-Korvenkontio M, Vapalahti O, Henttonen H, Koskela P, Kuusisto P, Vaheri A. Epidemiological study of nephropathia epidemica in Finland 1989–96. Scand J Infect Dis. 1999;31:427–435. doi: 10.1080/00365549950163941. [DOI] [PubMed] [Google Scholar]
- 7.Brus Sjölander K B, Elgh F, Kallio-Kokko H, Vapalahti O, Hägglund M, Palmcrantz V, Juto P, Vaheri A, Niklasson B, Lundkvist Å. Evaluation of serological methods for diagnosis of Puumala hantavirus infection (nephropathia epidemica) J Clin Microbiol. 1997;35:3264–3264. doi: 10.1128/jcm.35.12.3264-3268.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brus Sjölander K, Golovljova I, Plyusnin A, Lundkvist Å. Diagnostic potential of Puumala virus nucleocapsid protein expressed in Drosophila melanogaster cells. J Clin Microbiol. 2000;38:2324–2329. doi: 10.1128/jcm.38.6.2324-2329.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duchin J S, Koster F T, Peters C J, Simpson G L, Tempest B, Zaki S R, Ksiazek T G, Rollin P, Nichol S, Umland E, Moolenaar R L, Reef S E, Nolte K B, Gallaher M M, Butler J C, Breiman R F the Hantavirus Study Group. Hantavirus pulmonary syndrome: a clinical description of 17 patients with a newly recognized disease. N Engl J Med. 1994;330:949–955. doi: 10.1056/NEJM199404073301401. [DOI] [PubMed] [Google Scholar]
- 10.Faigel D O, Magaret N, Corless C, Lieberman D A, Fennerty M B. Evaluation of rapid antibody tests for the diagnosis of Helicobacter pylori infection. Am J Gastroenterol. 2000;95:72–77. doi: 10.1111/j.1572-0241.2000.01702.x. [DOI] [PubMed] [Google Scholar]
- 11.Golovljova I, Vasilenko V, Prükk T, Brus Sjölander K, Plyusnin A, Lundkvist Å. Puumala and Dobrava hantaviruses cause hemorrhagic fever with renal syndrome in Estonia. Eur J Clin Microbiol Infect Dis. 2000;19:968–969. doi: 10.1007/s100960000405. [DOI] [PubMed] [Google Scholar]
- 12.Groen J, Gerding M N, Jordans J G, Clement J P, Nieuwebhuijs J H, Osterhaus A D. Hantavirus infections in the Netherlands: epidemiology and disease. Epidemiol Infect. 1995;114:373–383. doi: 10.1017/s0950268800058003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hedman K, Vaheri A, Brummer-Korvenkontio M. Rapid diagnosis of hantavirus disease with an IgG-avidity assay. Lancet. 1991;338:1353–1356. doi: 10.1016/0140-6736(91)92235-t. [DOI] [PubMed] [Google Scholar]
- 14.Kallio-Kokko H, Vapalahti O, Lundkvist Å, Vaheri A. Evaluation of Puumala virus IgG and IgM enzyme immunoassays based on recombinant baculovirus-expressed nucleocapsid protein for early nephropathia epidemica diagnosis. Clin Diagn Virol. 1998;10:83–90. doi: 10.1016/s0928-0197(97)10019-8. [DOI] [PubMed] [Google Scholar]
- 15.Kanerva M, Mustonen J, Vaheri A. Pathogenesis of Puumala and other hantavirus infections. Rev Med Virol. 1998;8:67–86. doi: 10.1002/(sici)1099-1654(199804/06)8:2<67::aid-rmv217>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 16.Lundkvist Å, Hörling J, Niklasson B. The humoral response to Puumala virus infection (nephropathia epidemica) investigated by viral protein specific immunoassays. Arch Virol. 1993;130:121–130. doi: 10.1007/BF01319001. [DOI] [PubMed] [Google Scholar]
- 17.Lundkvist Å, Niklasson B. Haemorrhagic fever with renal syndrome and other hantavirus infections. Rev Med Virol. 1994;4:177–184. [Google Scholar]
- 18.Lunkvist Å, Apekina N, Myasnikov Y, Vapalahti O, Vaheri A, Plyusnin A. Dobrava hantavirus outbreak in Russia. Lancet. 1997;350:781–182. doi: 10.1016/S0140-6736(05)62565-2. [DOI] [PubMed] [Google Scholar]
- 19.Lundkvist Å, Hukic M, Hörling J, Gilljam M, Nichol S, Niklasson S. Puumala and Dobrava viruses cause haemorrhagic fever with renal syndrome in Bosnia-Herzegovina: evidence of highly cross-neutralising antibody responses in early patient sera. J Med Virol. 1997;53:51–59. [PubMed] [Google Scholar]
- 20.Lundkvist Å, Vasilenko V, Golovljova I, Plyusnin A, Vaheri A. Human Dobrava hantavirus infections in Estonia. Lancet. 1998;352:369. doi: 10.1016/S0140-6736(05)60467-9. [DOI] [PubMed] [Google Scholar]
- 21.Meisel H, Lundkvist Å, Ganzer K, Bär W, Sibold C, Krüger D H. First clinical case of infection by hantavirus Dobrava in Germany. Eur J Clin Microbiol Infect Dis. 1998;17:884–885. doi: 10.1007/s100960050214. [DOI] [PubMed] [Google Scholar]
- 22.Mustonen J, Brummer-Korvenkontio M, Hedman K, Pasternack A, Pietilä K, Vaheri A. Nephropathia epidemica in Finland: a retrospective study of 126 cases. Scand J Infect Dis. 1994;26:7–13. doi: 10.3109/00365549409008583. [DOI] [PubMed] [Google Scholar]
- 23.Nuti M, Amaddeo D, Crovatto M, et al. Infections in an Alpine environment: antibodies to hantaviruses, leptospira, rickettsiae, and Borrelia burgdorferi in defined Italian populations. Am J Trop Med Hyg. 1993;48:20–25. doi: 10.4269/ajtmh.1993.48.20. [DOI] [PubMed] [Google Scholar]
- 24.Plyusnin A, Vapalahti O, Vaheri A. Hantaviruses: genome structure, expression and evolution. J Gen Virol. 1996;77:2677–2687. doi: 10.1099/0022-1317-77-11-2677. [DOI] [PubMed] [Google Scholar]
- 25.Plyusnin A, Hörling J, Kanerva M, Mustonen J, Cheng Y, Partanen J, Vapalahti O, Kukkonen S K, Niemimaa J, Henttonen H, Niklasson B, Lundkvist Å, Vaheri A. Puumala hantavirus genome in patients with nephropathia epidemica: correlation of PCR positivity with HLA haplotype and link to viral sequences in local rodents. J Clin Microbiol. 1997;35:1090–1096. doi: 10.1128/jcm.35.5.1090-1096.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rollin P E, Coudrier D, Sureau P. Hantavirus epidemic in Europe, 1993. Lancet. 1994;343:115–116. [PubMed] [Google Scholar]
- 27.Saha H, Mustonen J, Pietilä K, Pasternack A. Metabolism of calcium and vitamin D3 in patients with acute tubulointerstitial nephritis: a study of 41 patients with nephropathia epidemica. Nephron. 1993;63:159–163. doi: 10.1159/000187175. [DOI] [PubMed] [Google Scholar]
- 28.Schmaljohn C S, Hasty S E, Dalrymple J M, LeDuc J W, Lee H W, von Bonsdorff C H, Brummer-Korvenkontio M, Vaheri A, Tsai T F, Regnery H L, Goldgaber D, Lee P W. Antigenic and genetic properties of viruses linked to hemorrhagic fever with renal syndrome. Science. 1985;227:1041–1044. doi: 10.1126/science.2858126. [DOI] [PubMed] [Google Scholar]
- 29.Settergren B, Juto P, Wadell G. Detection of specific serum immunoglobulin M in nephropathia epidemica (Scandinavian epidemic nephropathy) by biotin-avidin-amplified immunofluorescence method. J Clin Microbiol. 1987;25:1134–1136. doi: 10.1128/jcm.25.6.1134-1136.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Settergren B, Juto P, Trollfors B, Wadell G, Norrby S R. Hemorrhagic complications and other clinical findings in nephropathia epidemica in Sweden: a study of 355 serologically verified cases. J Infect Dis. 1988;157:380–382. doi: 10.1093/infdis/157.2.380. [DOI] [PubMed] [Google Scholar]
- 31.Settergren B. Clinical aspects of nephropathia epidemica (Puumala virus infection) in Europe: a review. Scand J Infect Dis. 2000;32:125–132. doi: 10.1080/003655400750045204. [DOI] [PubMed] [Google Scholar]
- 32.Vapalahti K, Paunio M, Brummer-Korvenkontio M, Vaheri A, Vapalahti O. Puumala virus infections in Finland: increased occupational risk for farmers. Am J Epidemiol. 1999;149:1142–1151. doi: 10.1093/oxfordjournals.aje.a009769. [DOI] [PubMed] [Google Scholar]
- 33.Vapalahti O, Lundkvist Å, Kallio-Kokko H, Paukku K, Julkunen I, Lankinen H, Vaheri A. Antigenic properties and diagnostic potential of Puumala virus nucleocapsid protein expressed in insect cells. J Clin Microbiol. 1996;34:119–125. doi: 10.1128/jcm.34.1.119-125.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]