Abstract
Our previous studies demonstrate the therapeutic efficacy against bovine diseases of an anti-bovine programmed death-ligand 1 (PD-L1) chimeric antibody. In humans, PD-1 and PD-L1 antibodies are more effective when combined with an antibody targeting cytotoxic T lymphocyte antigen 4 (CTLA-4) and these combination therapies are therefore clinically used. Here we generated an anti-bovine CTLA-4 chimeric antibody (chAb) to enhance the therapeutic efficacy of the PD-L1 antibody. We further analyzed the effects of dual blockade of CTLA-4 and PD-1 pathways on T-cell responses. The established anti-bovine CTLA-4 chAb showed comparable blocking activity on the binding of bovine CTLA-4 to CD80 and CD86 as the anti-bovine CTLA-4 mouse monoclonal antibody. Anti-bovine CTLA-4 chAb also significantly increased IL-2 production from bovine peripheral blood mononuclear cells (PBMCs). Further, the combination of anti-CTLA-4 chAb with anti-PD-L1 chAb significantly upregulated IL-2 production by PBMCs. These results suggest that the combination of antibodies have higher potential to enhance immune responses against pathogens compared with single administration.
Keywords: cattle, chimeric antibody, cytotoxic T lymphocyte antigen 4, interleukin-2, programmed death-ligand 1
The immune system protects the host from viruses, bacteria, and tumor cells. T cells, which mediate cellular immunity, are activated through antigen presentation by cells such as macrophages and dendritic cells. In contrast, prolonged activation of T cells may damage adjacent normal tissues. The immune checkpoint molecule designated cytotoxic T lymphocyte antigen 4 (CTLA-4), which is expressed by activated T cells and regulatory T cells, inhibits T-cell activation. CTLA-4, the homolog of the costimulatory molecule CD28, binds to CD80/CD86 [4]. The binding avidity of CTLA-4 for CD80/CD86 is >10-times higher compared with that of CD28, which competitively inhibits the binding of CD28 to CD80/CD86 [4].
The binding of CTLA-4 to CD80/86 transmits an inhibitory signal that suppresses T-cell functions [3, 31, 32]. Indoleamine 2,3-dioxygenase, which is induced through the binding of CTLA-4 to CD80/86 in antigen presenting cells, inhibits the functions of effector T cells as well [15]. Moreover, CTLA-4 physically removes CD80/CD86 through trans-endocytosis and reduces the number of CD80/CD86 molecules on antigen presenting cells [25]. Thus, CTLA-4 plays a critical role in the maintenance of host homeostasis mediated by these inhibitory activities.
Upregulation of CTLA-4 expression occurs during chronic infections and cancers of humans. The increased expression of CTLA-4 on T cells correlates with disease progression during human immunodeficiency virus infection [12] as well as with the viral load in hepatitis B virus-infected patients [33]. Further, the upregulated expression of CTLA-4 is associated with shorter survival rates of patients with nasopharyngeal carcinoma [8].
Other immune checkpoint molecules such as programmed cell death-1 (PD-1) and its ligand PD-ligand 1 (PD-L1) are associated with chronic infections and cancers of humans [5, 36]. Overexpression of immune checkpoint molecules serves as a mechanism through which infectious agents and tumor cells evade host immunity. Antibody therapy targeting immune checkpoint molecules is widely used to effectively treat human cancers [26, 27, 38]. Further, a clinical trial of an anti-human CTLA-4 antibody (tremelimumab) against chronic hepatitis C achieves a significant decrease in viral load [30].
We previously reported the upregulation of PD-1, PD-L1, and CTLA-4 in cattle chronically infected with bovine leukemia virus (BLV) [9, 10, 24, 34]. Moreover, we performed several clinical studies using an anti-bovine PD-L1 rat-bovine chimeric antibody (chAb) or anti-bovine PD-1 rat-bovine chAb administered to BLV-infected cattle [18, 23, 29]. The administration of these chAbs decreased the BLV provirus load in cattle [18, 23]. However, certain BLV-infected cattle did not respond to this treatment [29], indicating the importance of identifying a novel molecular target for immunotherapy to obtain a stronger therapeutic effect.
We therefore focused on different immune checkpoint molecules, including CTLA-4, for use in combination with the anti-PD-L1 antibody to treat BLV-infected cattle. We previously demonstrated the immune inhibitory function of bovine CTLA-4 and established an anti-bovine CTLA-4 monoclonal antibody (mAb) designated 4G2-A3 [37]. However, 4G2-A3 did not enhance cytokine production when combined with the anti-PD-L1 antibody [37]. The insufficient effect of 4G2-A3 on immune activation is presumably explained by its partial ability to block binding of CTLA-4 to CD80/CD86 [37].
Therefore, here we attempted to establish anti-bovine CTLA-4 mouse mAbs with stronger blocking and immune activating abilities by employing a modified method to screen hybridomas. The resultant mAb (4C2-D9) was tested for its abilities to bind bovine CTLA-4, block the binding of bovine CTLA-4 to CD80/CD86, and enhance the activation of antiviral immunity. Further, we developed an anti-bovine CTLA-4 mouse-bovine chimeric antibody (chAb) (Boch4C2), which comprises variable regions of 4C2-D9 and constant regions of bovine IgG1 and Igλ.
MATERIALS AND METHODS
Development of blocking mAbs against bovine CTLA-4
Recombinant bovine CTLA-4, CD80, and CD86 immunoglobulin fusion proteins (CTLA-4-Ig, CD80-Ig, and CD86-Ig) were generated using a mammalian-cell expression system [37]. A hybridoma library of anti-bovine CTLA-4 mouse mAbs was previously established [37]. To isolate a blocking antibody targeting bovine CTLA-4, the hybridoma library was rescreened as follows. The reactivity of antibodies in culture supernatants of the hybridomas was screened using an enzyme-linked immuno-sorbent assay (ELISA) to detect CTLA-4-Ig. Flow cytometry was used next to screen supernatants for their ability to block the binding of CTLA-4 to CD80/CD86. Briefly, bovine CHO-DG44 cells that express CTLA-4-EGFP (CTLA-4-EGFP-expressing cells [37]) were incubated in phosphate buffered saline (PBS) containing 10% goat serum (Sigma–Aldrich, St. Louis, MO, USA) at room temperature for 15 min to suppress nonspecific binding to Fc receptors. The cells were incubated with the culture supernatants or mouse IgG (Sigma–Aldrich) for 30 min at 37°C, washed twice, and incubated with 0.2 μg/ml of CD80-Ig or CD86-Ig for 30 min at 37°C. After washing twice, the cells were incubated with Alexa Fluor 647-conjugated anti-rabbit IgG (H+L) goat IgG (Thermo Fisher Scientific, Waltham, MA, USA), and the binding of CD80-Ig or CD86-Ig was analyzed by FACSVerse (BD Biosciences, San Jose, CA, USA). The selected anti-bovine CTLA-4 mAb (designated 4C2-D9) was purified from supernatants of the hybridoma cultures.
Confirmation of the reactivity of purified anti-CTLA-4 mAbs
The reactivity of the purified anti-CTLA-4 mAbs was measured using flow cytometry of CTLA-4-EGFP-expressing cells, which were incubated in PBS containing 10% goat serum (Sigma–Aldrich) for 15 min at room temperature. The cells were incubated with anti-CTLA-4 mAbs (10 µg/ml) (4G2-A3 [37] and 4C2-D9) or a mouse IgG1 isotype control (Southern Biotech, Birmingham, AL, USA) for 30 min at 37°C. The cells were washed twice, incubated with Alexa Fluor 647-conjugated anti-mouse IgG (H+L) F (ab’)2 (Thermo Fisher Scientific) for 30 min at 37°C, washed twice, and analyzed using a FACSVerse.
Assessment of blocking ability of purified anti-CTLA-4 mAbs
CTLA-4-EGFP-expressing cells were incubated in PBS containing 10% goat serum (Sigma–Aldrich) at room temperature for 15 min. CTLA-4-EGFP-expressing cells were then incubated with the purified anti-CTLA-4 mAbs (4G2-A3 and 4C2-D9) (0, 1.25, 2.5, 5, 10, and 20 μg/ml) or a mouse IgG1 isotype control (Southern Biotech) for 30 min at 37°C. The cells were then washed twice and incubated with 0.2 μg/ml CD80-Ig or 0.2 μg/ml CD86-Ig for 30 min at 37°C. CD80-Ig or CD86-Ig was labeled with Alexa Fluor 647-conjugated anti-rabbit IgG (H+L) goat IgG (Thermo Fisher Scientific) and analyzed using a FACSVerse. Three independent experiments were performed. The relative binding of CD80-Ig or CD86-Ig to CTLA-4-expressing cells was calculated using the value of the mean fluorescence intensity (MFI) of a sample preincubated with each antibody vs that of cells preincubated without antibody (MFI=1).
Preparation of blood cells and cell cultures
Blood samples were collected from healthy cattle housed at dairy farms in Hokkaido, Japan. The Animal Experiment Committee of Hokkaido University approved the experimental procedures (approval #17-0024). Informed consent was obtained from the farmers. Peripheral blood mononuclear cells (PBMCs) from blood samples were purified using density-gradient centrifugation through Percoll (GE Healthcare, Little Chalfont, UK). PBMCs (1 × 106 cells/0.2 ml) in 96-well plates (Corning Inc., Corning, NY, USA) were cultured in RPMI 1640 medium (Sigma-Aldrich) containing 10% heat-inactivated fetal bovine serum (Thermo Fisher Scientific), 100 U/ml penicillin, 100 μg/ml streptomycin, and 2 mM L-glutamine (Thermo Fisher Scientific) at 37°C in an atmosphere containing 5% CO2.
Immune activation assay
To evaluate the immune activation efficacy of the anti-CTLA-4 mAb, bovine PBMCs were cultured with 10 μg/ml of anti-CTLA-4 mAb (4C2-D9) or mouse IgG (Sigma-Aldrich) in the presence of 0.1 μg/ml of Staphylococcus aureus enterotoxin B (SEB) (Sigma-Aldrich). The culture medium was harvested after 3 days, and interferon-γ (IFN-γ) concentrations in duplicate wells were measured using an ELISA (Mabtech, Nacka Strand, Sweden) according to the manufacturer’s protocol.
Immune activation assay employing purified anti-CTLA-4 mAb combined with anti-bovine PD-L1 mAb
Bovine PBMCs were cultured in the presence of 10 μg/ml anti-CTLA-4 mAb (4C2-D9), 10 µg/ml anti-PD-L1–rat mAb (4G12 [11]), and 0.1 µg/ml SEB. Isotype-specific mouse and rat IgGs (Sigma–Aldrich) were used as negative controls. After 3 days, the culture medium was harvested, and the concentrations of IFN-γ and IL-2 in duplicate wells were measured using an ELISA (Mabtech) according to the manufacturer’s protocol.
Cloning of a cDNA encoding the variable region of the anti-bovine CTLA-4 mAb
TRIzol reagent (Thermo Fisher Scientific) was used to extract RNA from the hybridoma expressing anti-bovine CTLA-4 mAb (4C2-D9). A cDNA encoding the variable regions of the anti-bovine CTLA-4 mAb was synthesized using the 5′-Rapid Amplification of cDNA Ends System Version 2.0 (Thermo Fisher Scientific). Gene-specific primers RACEMOG1 (5′-TAT GCA AGG CTT ACA ACC ACA -3′) (heavy chain) and MOCKFOR (5′-CTC ATT CCT GTT GAA GCT CTT GAC AAT -3′) (light chain) were used to synthesize the cDNAs representing the variable regions of heavy and light chains, respectively [2]. The original mRNA template was removed using RNase, and cDNA purification was performed using a S.N.A.P column (Thermo Fisher Scientific). The 3′-end of the purified cDNA was tailed with dCTP and amplified using a forward primer AAP (5′-GGC CAC GCG TCG ACT AGT ACG GGI IGG GII GGG IIG -3′) and a reverse primer MOCG12FOR (5′- CTC AAT TTT CTT GTC CAC CTT GGT GC -3′) (heavy chain) or CKMOsp (5′-CTC ATT CCT GTT GAA GCT CTT GAC AAT GGG -3′) (light chain) [2]. The PCR product, which was purified using a FastGene Gel/PCR extraction Kit (Nippon Genetics, Tokyo, Japan), was inserted into the T-Vector pMD20 (Takara Bio, Kusatsu, Japan). The plasmid clones were sequenced using a GenomeLab GeXP Genetic Analysis System (SCIEX, Framingham, MA, USA).
Expression of anti-bovine CTLA-4 mouse-bovine chAb
The nucleotide sequences of the variable regions of the heavy and light chains of 4C2-D9 were combined with the constant regions of bovine IgG1 (GenBank accession number X62916) and bovine Igλ (GenBank accession number X62917), respectively. The sequences were optimized according to the codon usage of the Chinese hamster genome and were synthesized by Eurofins Genomics, Tokyo, Japan). The amplicons were inserted into the pDC62c5-U533 expression vector [35]. The plasmid encoding anti-bovine CTLA-4–mouse-bovine chAb (Boch4C2) was extracted and purified using a NucleoBond Xtra Midi Kit (Macherey-Nagel, Düren, Germany). Boch4C2 was transiently expressed using the ExpiCHO Expression System (Thermo Fisher Scientific). Boch4C2 was affinity-purified from the culture supernatant of transfected cells using an Ab-Capcher ExTra (ProteNova, Higashi-Kagawa, Japan) according to the manufacturer’s protocol. The buffer was then exchanged with PBS through size-exclusion chromatography using a PD-10 Desalting Column (GE Healthcare). The purity of Boch4C2 was determined using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) under reducing and non-reducing conditions using a SuperSep Ace 5–20% gradient polyacrylamide gel (Fujifilm Wako Pure Chemical, Osaka, Japan) and 2 × Laemmli Sample Buffer (Bio-Rad, Hercules, CA, USA). The Precision Plus Protein Dual Color Standard (Bio-Rad) was used as a molecular-mass marker, and the proteins were visualized using Quick-CBB (Fujifilm Wako Pure Chemical). The concentration of Boch4C2 was measured using a BCA Protein Assay Kit (Thermo Fisher Scientific).
Analysis of the reactivity of Boch4C2 with bovine CTLA-4
The reactivity of Boch4C2 with bovine CTLA-4 was confirmed using flow cytometric analysis of CTLA-4-EGFP-expressing cells, which were incubated in PBS containing 10% goat serum (Sigma-Aldrich) for 15 min at room temperature. The cells were incubated with 10 µg/ml Boch4C2 or bovine IgG (Sigma-Aldrich) for 30 min at 37°C, washed twice, incubated with Alexa Flour 647-conjugated anti-bovine IgG (H+L) goat IgG (Jackson ImmunoResearch, West Grove, PA, USA) for 30 min at 37°C, washed twice, and analyzed using a FACSVerse.
Analysis of the blocking ability of Boch4C2
The ability of Boch4C2 to block the binding of bovine CTLA-4 to CD80/CD86 was assessed using an ELISA. MaxiSorp Immuno Plates (Thermo Fisher Scientific) were coated with 1 μg/ml CD80-Ig or CD86-Ig in PBS for 30 min at 37°C and then blocked with PBS containing 1% bovine serum albumin (Sigma-Aldrich) for 30 min at 37°C. Biotin-conjugated CTLA-4-Ig was preincubated with Boch4C2, bovine IgG (Sigma-Aldrich), 4C2-D9, or mouse IgG1 (Southern Biotech) at molar ratios (CTLA-4-Ig:antibody)=1:0, 1:0.1, 1:0.5, 1:1, 1:2, 1:5, and 1:10 for 30 min at 37°C. These reagents were added to the microplate and incubated for 30 min at 37°C. Binding of biotin-conjugated CTLA-4-Ig to CD80-Ig or CD86-Ig was detected using the NeutrAvidin Horseradish Peroxidase Conjugate (Thermo Fisher Scientific) for 30 min at 37°C. TMB One Component Substrate (Bethyl Laboratories, Montgomery, TX, USA) was then added to the microplate, and the reaction was stopped using 0.18 M H2SO4. Absorbance at 450 nm was measured using an MTP-900 microplate reader (Corona Electric, Hitachinaka, Japan). Three duplicate independent experiments were performed. The relative binding of CTLA-4-Ig to CD80-Ig or CD86-Ig was calculated from the absorbance of samples preincubated with each antibody, and the absorbance of the sample preincubated without antibody, which was defined as 100%.
Immune activation assay using Boch4C2
Bovine PBMCs were cultured with Boch4C2 or bovine IgG (10 μg/ml each) in the presence of 0.1 µg/ml of SEB. After 3 days, the culture supernatant was harvested, and the concentrations of IFN-γ and IL-2 in duplicate samples were measured using an ELISA according to the manufacturer’s protocol.
Immune activation assay of Boch4C2 combined with Boch4G12
Bovine PBMCs were cultured with Boch4C2 and anti-bovine PD-L1–rat bovine chAb (Boch4G12 [18]) (10 μg/ml each) in the presence of 0.1 µg/ml of SEB. Bovine IgG served as a negative control. After 3 days, the culture supernatants were harvested, and the concentrations of IFN-γ and IL-2 in duplicate samples were measured using an ELISA according to the manufacturer’s protocol.
Statistical analysis
The Wilcoxon matched-pairs test was used to compare paired groups; and Tukey’s test and the Steel-Dwass test were used to compare three or more groups. P values <0.05 were considered significant.
RESULTS
Confirmation of binding and blocking abilities of the anti-bovine CTLA-4 mAb
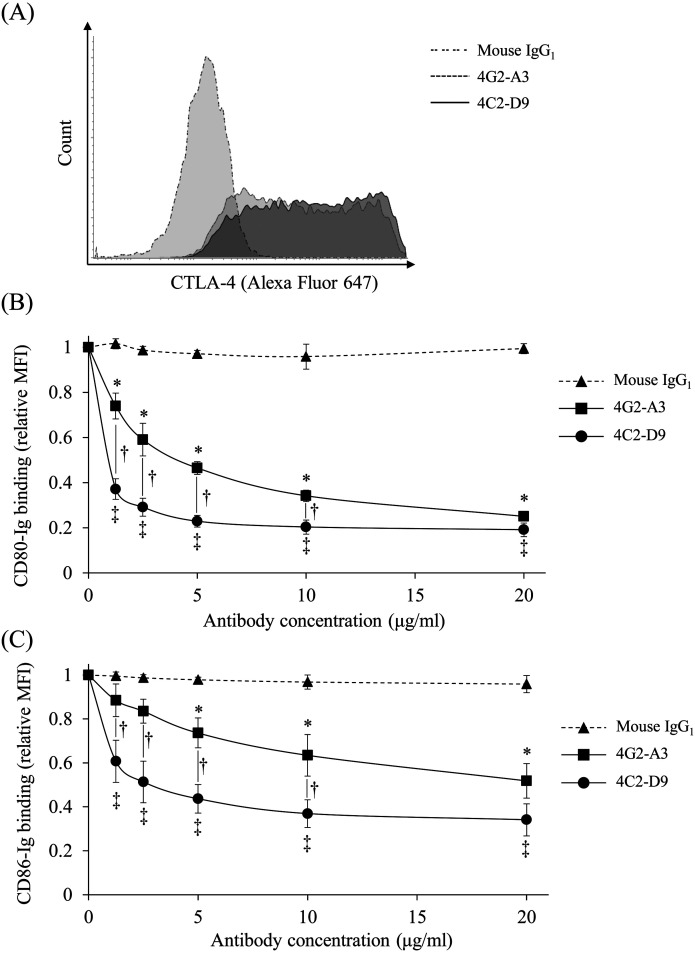
The newly established anti-bovine CTLA-4 mAb (4C2-D9) was tested for its binding and blocking abilities were compared with the previously established anti-bovine CTLA-4 mAb (4G2-A3, [37]). The ability of 4C2-D9 to bind bovine CTLA-4 was comparable with that of 4G2-A3 (Fig. 1A). The mAbs 4C2-D9 and 4G2-A3 blocked the binding of CTLA-4 to CD80/CD86 compared with the mouse IgG1 control (Fig. 1B and 1C). Further, the blocking ability of 4C2-D9 was significantly stronger compared with that of 4G2-A3 (1.25, 2.5, 5, and 10 µg/ml) (Fig. 1B and 1C). Thus, we successfully established an anti-bovine CTLA-4 mAb with strong blocking activity.
Fig. 1.
The binding and blocking abilities of anti-bovine cytotoxic T lymphocyte antigen 4 (CTLA-4) mAb (4C2-D9). Bovine CTLA-4-EGFP-expressing cells was incubated with anti-bovine CTLA-4 mAbs (4C2-D9 and 4G2-A3) to assess binding and blocking ability. (A) The binding ability of anti-bovine CTLA-4 mAbs was measured using flow cytometry. (B and C) Concentration-dependent blocking effect of the binding of anti-bovine CTLA-4 mAbs (4C2-D9 and 4G2-A3) to CTLA-4/CD80 (B) and CTLA-4/CD86 (C). CTLA-4-EGFP-expressing cells were preincubated with 4C2-D9, 4G2-A3, or a mouse IgG1 control (1.25, 2.5, 5, 10, and 20 μg/ml). Ig binding was detected using flow cytometry. Each point indicates the mean value of the relative MFI of three independent experiments (relative to the control without antibody; error bar, SEM). Tukey’s test was used for statistical analysis (*P<0.05) between groups treated with the same concentration of 4G2-A3 and mouse IgG1. ‡P<0.05, between the groups treated with the same concentration of 4C2-D9 and mouse IgG1. †P<0.05, between the groups with the same concentration of 4C2-D9 and 4G2-A3.
Blockade of CTLA-4/PD-L1 pathway induces an enhanced T-helper (Th1) cell response
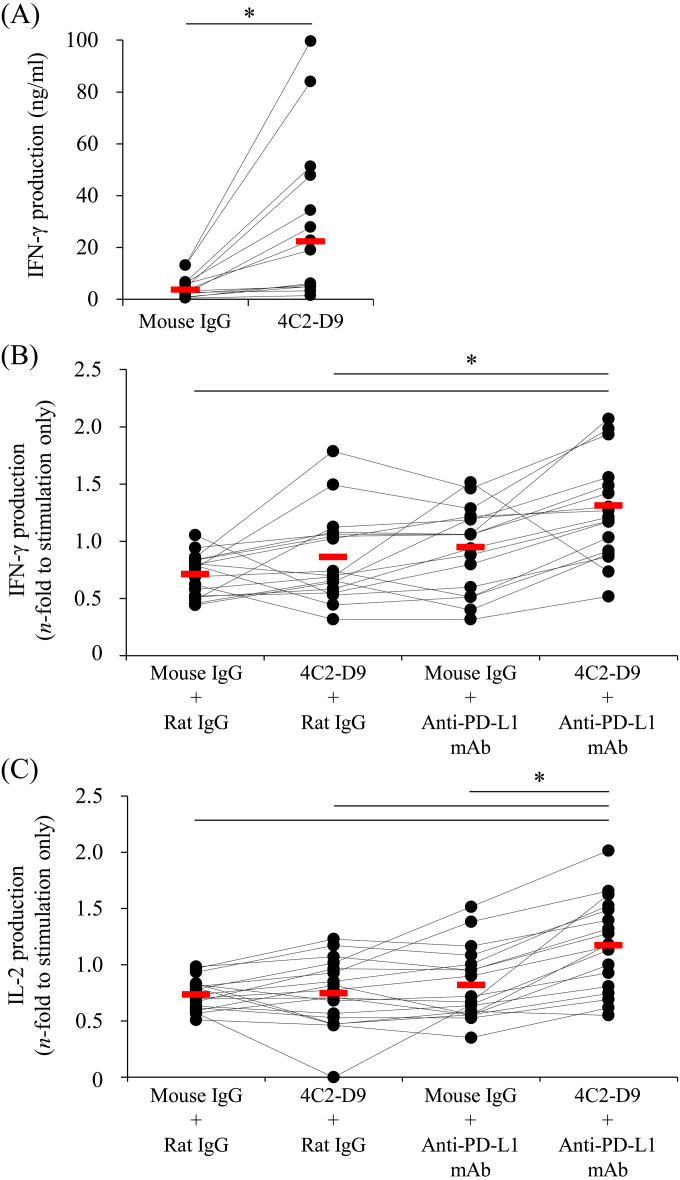
The immune activation efficacy of 4C2-D9 was assessed in vitro using PBMCs isolated from healthy cattle. The mAb 4C2-D9 significantly upregulated IFN-γ production by PBMCs compared with the mouse IgG control (Fig. 2A). Further, the immune activation efficacy of 4C2-D9 combined with the anti-bovine PD-L1 rat mAb was similarly evaluated. Combination treatment increased production of IFN-γ compared with IgG controls or 4C2-D9 treatment alone (Fig. 2B). Moreover, IL-2 production induced by combination treatment was significantly higher compared with that using 4C2-D9 or anti-bovine PD-L1 mAb alone (Fig. 2C). Thus, dual blockade of CTLA-4 and PD-L1 may serve as a new strategy to enhance the ability of cattle to mount a Th1 response.
Fig. 2.
The immune activation efficacy of 4C2-D9 alone or combined with the anti-programmed death-ligand 1 (PD-L1) antibody. Peripheral blood mononuclear cells (PBMCs) were isolated from healthy cattle and cultured with (A) 4C2-D9 alone or (B) 4C2-D9 and anti-PD-L1 mAb 4G12 in the presence of Staphylococcus aureus enterotoxin B (SEB). After 3 days, the supernatant was harvested. (A and B) The interferon-γ (IFN-γ) concentration in the supernatant was measured using an ELISA (a, n=14; b, n=17). (C) The interleukin-2 (IL-2) concentration in the supernatant was measured using an ELISA (n=18). The bar indicates the median value of each group. Comparisons between each group were performed using the Wilcoxon matched-pairs test (A) and the Steel-Dwass test (B and C). P<0.05 (*) indicates a significant difference.
Establishment of an anti-bovine CTLA-4 mouse-bovine chAb
The mAb 4C2-D9 achieved significantly higher blocking ability and induced an enhanced immune response alone or when combined with the anti-bovine PD-L1 mAb. However, 4C2-D9 comprises mouse IgG sequences, which are highly immunogenic in cattle. We reasoned therefore that anti-4C2-D9 neutralizing antibodies might cause adverse effects such as increased risk of an allergic reaction (e.g. anaphylactic shock) and shorter half-life of 4C2-D9 in blood, all of which may reduce clinical efficacy.
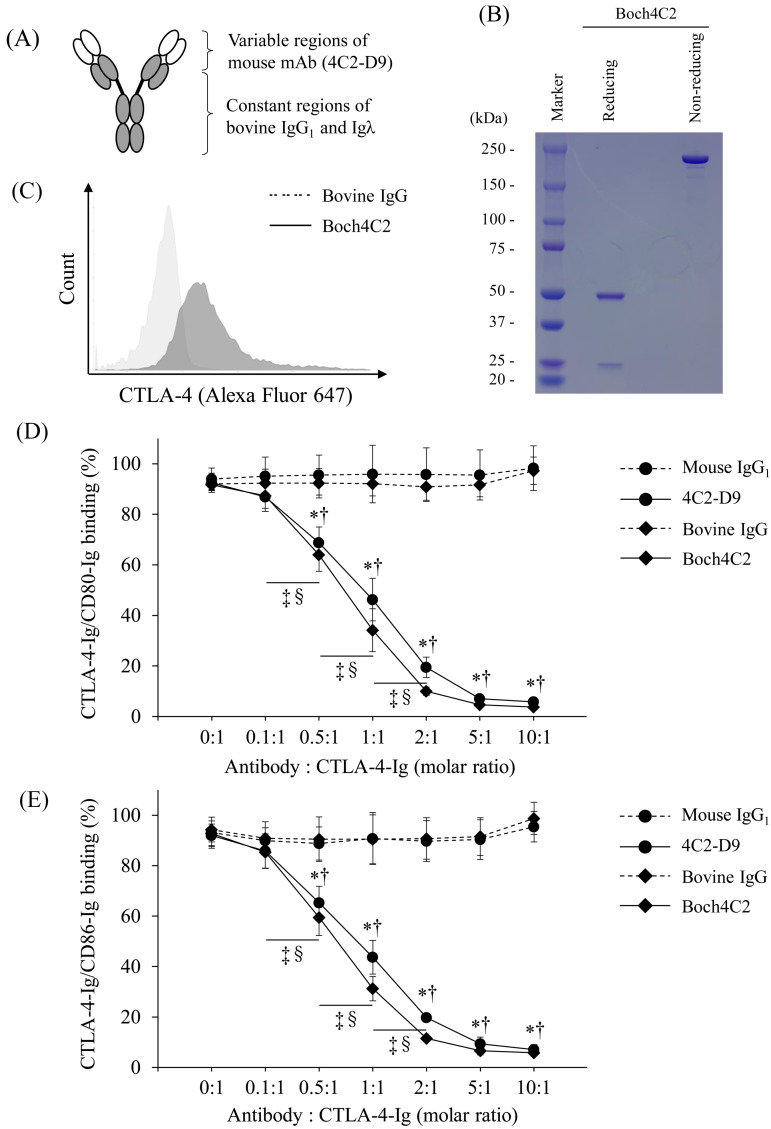
Therefore, we developed a mouse-bovine chAb by combining the variable regions of 4C2-D9 with the constant regions of bovine IgG1 and Igλ (Fig. 3A). The chAb, designated Boch4C2, was expressed and purified as documented in the Methods section (Fig. 3B). SDS-PAGE analysis revealed that the heavy and light chains of Boch4C2 migrated at positions corresponding to approximately 50 kDa and 25 kDa, respectively (Fig. 3B). Under nonreducing conditions, Boch4C2 was detected at a position corresponding to approximately 200 kDa (Fig. 3B). Thus, Boch4C2 has been successfully established with sufficient production efficiency and purity.
Fig. 3.
Establishment of Boch4C2 and assessment of its binding and blocking abilities. (A) Structure of Boch4C2 showing the variable regions of 4C2-D9 (white) and constant regions of bovine IgG1 and Igλ (black). (B) ExpiCHO-S cells were transfected with a plasmid expressing Boch4C2, and Boch4C2 was purified from the culture supernatant. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analyses to determine the purity of Boch4C2 were performed under reducing and nonreducing conditions. (C) The binding ability of Boch4C2 was measured using flow cytometry of CTLA-4-EGFP-expressing cells. The binding of Boch4C2 was detected using an anti-bovine IgG secondary antibody. (D and E) The concentration-dependent blocking effect of Boch4C2 and 4C2-D9 on the binding of cytotoxic T lymphocyte antigen 4 (CTLA-4)/CD80 (D) and CTLA-4/CD86. (E) Biotin-conjugated CTLA-4-Ig was preincubated with Boch4C2, 4C2-D9 or a control antibody at the molar ratios as follows: (antibody:CTLA-4-Ig) 0:1, 0.1:1, 0.5:1, 1:1, 2:1, 5:1, and 10:1). Each point indicates the mean value of the relative OD value of three independent experiments (relative to the control without antibody; error bar, SEM). Tukey’s test was used for statistical analysis (*P<0.05, between the groups with Boch4C2 and bovine IgG at same molar ratio. ‡P<0.05, between the groups with different molar ratios of Boch4C2. †P<0.05, between groups with 4C2-D9 and mouse IgG1 at same molar ratio. §P<0.05, between the groups with different molar ratios of 4C2-D9.
We next asked whether the conformation of the chimeric antibody compromised its binding and blocking abilities. We found that Boch4C2 bound bovine CTLA-4 (Fig. 3C) and significantly blocked the binding of CTLA-4 to CD80/CD86 in a concentration-dependent manner (Fig. 3D and 3E). Moreover, the chimeric structure of the antibody molecule did not reduce its blocking ability compared with that of 4C2-D9 (Fig. 3D and 3E).
Boch4C2 combined with anti-bovine PD-L1–rat-bovine chAb enhances IL-2 production by PBMCs
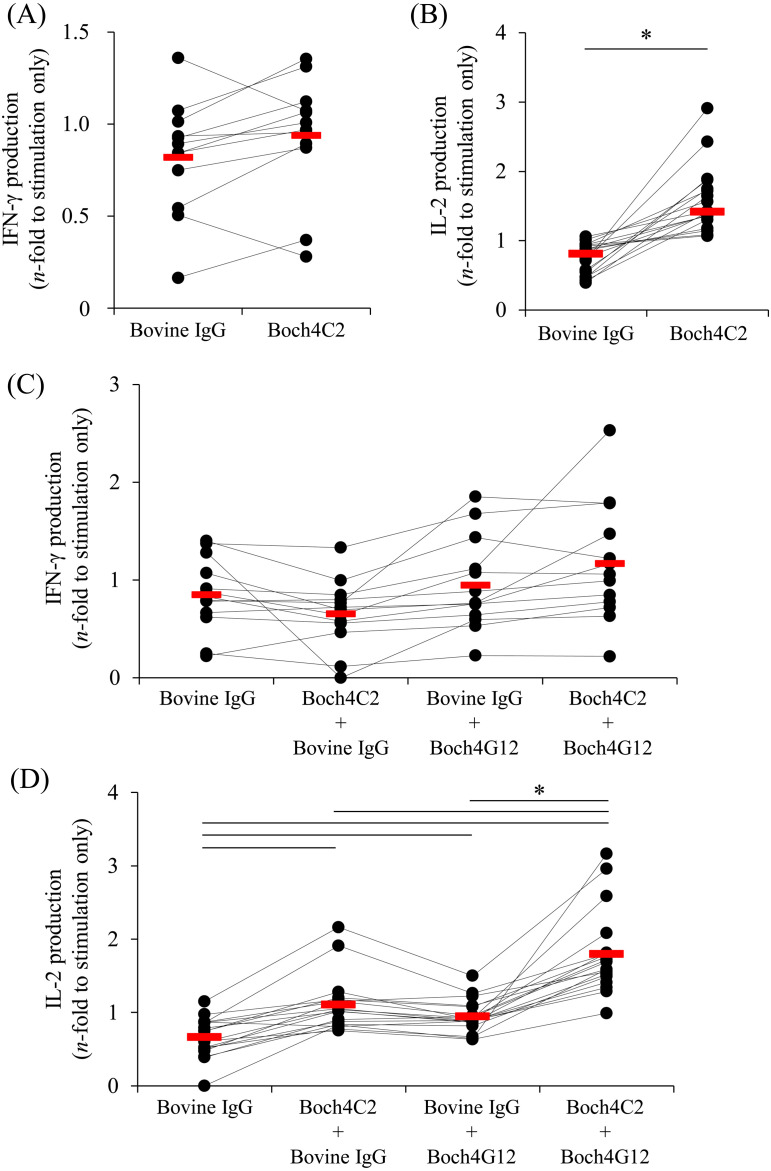
The chimeric structure of Boch4C2 did not impair its blocking ability. Further single treatment of PBMCs with Boch4C2 significantly enhanced IL-2 production (Fig. 4B). Moreover, Boch4C2, combined with Boch4G12, significantly increased the production of IL-2 from PBMCs compared with each antibody alone (Fig. 4D). In contrast, IFN-γ production was not altered when PBMCs were treated with Boch4C2 alone or Boch4C2 combined with Boch4G12 (Fig. 4A and 4C). The finding indicate that these blocking antibodies represent to be novel candidates for regulation of the immune response in cattle.
Fig. 4.
Efficacies of immune activation of peripheral blood mononuclear cells (PBMCs) of healthy cattle in response to treatment with Boch4C2 alone or combined with Boch4G12. PBMCs were isolated from healthy cattle and cultured for three days in the presence of Staphylococcus aureus enterotoxin B (SEB). (A and B) 10 μg/ml Boch4C2 or bovine IgG was added to the medium, and the concentrations of interferon-γ (IFN-γ) (A) and interleukin-2 (IL-2) (B) were measured using an ELISA (A, n=12; B, n=18). (C and D) Media contained 10 μg/ml each of Boch4C2 or bovine IgG and 10 μg/ml each of Boch4G12 or bovine IgG. The concentrations of IFN-γ (C) and IL-2 (D) were measured using an ELISA (C, n=13; D, n=18). The bar indicates the median value of each group. The comparison between each group was performed using the Wilcoxon matched-pairs test (B) and the Steel-Dwass test (D). P<0.05 (*) indicates a significant difference.
DISCUSSION
Immune therapy targeting immune checkpoint molecules using blocking antibodies has become one of the main therapies for human cancers. For example, administration of an anti-PD-1 antibody, or an anti-CTLA-4 antibody, increases the rates of overall survival, progression-free survival, and overall response rates of patients with melanoma or those with advanced non-small cell lung cancer [6, 19]. However, most such patients do not respond to treatment with an anti-PD-1/PD-L1 antibody or to an anti-CTLA-4 antibody. In contrast, treatment with an anti-CTLA-4 antibody combined with an anti-PD-1 antibody achieves significantly improved efficacy for treating melanoma and recurrent small cell lung cancer [1, 13]. Moreover, the 5-year overall survival rates of patients with melanoma treated with an anti-PD-1 antibody combined with an anti-CTLA-4 antibody, an anti-PD-1 antibody, or an anti-CTLA-4 antibody are 52%, 44%, and 26%, respectively [14]. Moreover, hepatitis C virus (HCV)-specific CD8+ T cells are activated by the blockade of PD-1 and CTLA-4 activities in the PBMCs in patients infected with HCV [17]. Thus, targeting immune checkpoint molecules represents a novel strategy to enhance the host’s immune response to cancer cells as well as to infectious agents.
Effective vaccines or other therapeutics are unavailable for treating cattle infected with BLV. BLV persistently infects B cells and causes enzootic bovine leukemia (EBL) after a long latent period. In Japan, the overall prevalence of BLV infection of cattle was 35.2% from 2009–2011 [16]. Along with the nationwide expansion of BLV infection, the number of reported cases of EBL increased approximately 40-fold within the last 20 years in Japan, inflicting great economic losses on the cattle industry. Therefore, developing a novel therapeutic strategy is required to control BLV infection in Japanese farms.
We previously established an anti-bovine PD-L1 rat-bovine chAb, designated Boch4G12, which we applied in clinical studies of BLV-infected cattle [18, 29]. Treatment with Boch4G12 achieved an antiviral effect in cattle with a low proviral load, although not when the proviral load was high [18, 29]. Therefore, other therapeutic targets must be engaged to achieve effective immunotherapy of BLV infection.
We previously found that the expression level of CTLA-4 is upregulated in CD4+ T cells and correlates with the expression level of transforming growth factor (TGF)-β during BLV infection [34]. TGF-β dramatically inhibits the Th1 immune response and is associated with the progression of BLV infection [20]. We therefore hypothesized that blocking the PD-1/PD-L1 and CTLA-4 signaling pathways will serve as an effective method to enhance the Th1 response in cattle.
Here we employed a modified method to screen hybridomas, which led to the establishment of an anti-bovine CTLA-4 mAb (4C2-D9) with stronger blocking and immune activating abilities. As we predicted, the CTLA-4 blockade induced by 4C2-D9 administered in combination with an anti-bovine PD-L1 mAb, enhanced the production of IFN-γ and IL-2 by bovine PBMCs. Thus, dual blockade of PD-L1 and CTLA-4 represents a promising strategy to activate the Th1 response in cattle.
Moreover, here we developed an anti-CTLA-4 chAb (Boch4C2) for clinical application. Thus, Boch4C2 formed a stable IgG molecule with biological activity equivalent to the original mAb 4C2-D9. Moreover, IL-2 production by bovine PBMCs treated with Boch4C2 and Boch4G12 was significantly enhanced. However, there was a larger variation in IFN-γ production among individuals, and the activating effect of treatment with the chAbs was not pronounced. Further experiments are therefore required to demonstrate the immune activation efficacy of combination chAb therapy administered to cattle. For example, the levels of other Th1 cytokines such as TNF-α and cytotoxic proteins such as granzyme and perforin will be determined.
We previously detected upregulated expression of CTLA-4 during BLV infection [34]. Other immune checkpoint molecules such as PD-1 and PD-L1 are upregulated in immune cells, for example, in cattle with Johne’s disease, anaplasmosis, or mycoplasmosis [7, 21, 22, 28]. However, we only determined the expression levels of CTLA-4 during BLV infection. We will therefore employ mAb 4C2-D9 to analyze CTLA-4 levels in chronic infections of cattle with the aim of identifying diseases amenable to CTLA-4-targeted therapy. Further, we will conduct clinical studies of the therapeutic effects of Boch4C2 combined with Boch4G12 on cattle suffering from these target diseases.
This is the first study, to our knowledge, to establish an anti-bovine CTLA-4 mouse-bovine chAb and to demonstrate its immune activation efficacy in cattle through targeting CTLA-4 and PD-L1. These results suggest that combination of an anti-bovine CTLA-4 antibody with an anti-bovine PD-L1 antibody will serve as a novel strategy to enhance the immune response in cattle. A clinical study of the chAb established here is required to delineate its therapeutic effects on chronic infections of cattle.
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
Acknowledgments
We are grateful to Dr. Yasuyuki Mori, Dr. Hideyuki Takahashi and Dr. Tomio Ibayashi for valuable advice and discussions. This work was supported by JSPS KAKENHI grant number 19KK0172 [to S.K.], 19K15993 [to T.O.], grants from the Project of the NARO, Bio-oriented Technology Research Advancement Institution (Research Program on Development of Innovative Technology 26058 BC [to S.K.] and Special Scheme Project on Regional Developing Strategy, Grant 16817557 [to S.K.]), regulatory research projects for food safety, animal health and plant protection (JPJ008617.17935709) funded by the Ministry of Agriculture, Forestry and Fisheries of Japan, and AMED under grant number JP20am0101078 [to Y.K.]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
REFERENCES
- 1.Antonia S. J., López-Martin J. A., Bendell J., Ott P. A., Taylor M., Eder J. P., Jäger D., Pietanza M. C., Le D. T., de Braud F., Morse M. A., Ascierto P. A., Horn L., Amin A., Pillai R. N., Evans J., Chau I., Bono P., Atmaca A., Sharma P., Harbison C. T., Lin C. S., Christensen O., Calvo E.2016. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol. 17: 883–895. doi: 10.1016/S1470-2045(16)30098-5 [DOI] [PubMed] [Google Scholar]
- 2.Bradbury A.2010. Cloning hybridoma cDNA by RACE. pp. 15–20. In: Antibody Engineering Vol. 1, 2nd ed. (Kontermann, R. and Dübel, S. eds.), Springer, Berlin. [Google Scholar]
- 3.Chuang E., Fisher T. S., Morgan R. W., Robbins M. D., Duerr J. M., Vander Heiden M. G., Gardner J. P., Hambor J. E., Neveu M. J., Thompson C. B.2000. The CD28 and CTLA-4 receptors associate with the serine/threonine phosphatase PP2A. Immunity 13: 313–322. doi: 10.1016/S1074-7613(00)00031-5 [DOI] [PubMed] [Google Scholar]
- 4.Collins A. V., Brodie D. W., Gilbert R. J., Iaboni A., Manso-Sancho R., Walse B., Stuart D. I., van der Merwe P. A., Davis S. J.2002. The interaction properties of costimulatory molecules revisited. Immunity 17: 201–210. doi: 10.1016/S1074-7613(02)00362-X [DOI] [PubMed] [Google Scholar]
- 5.Day C. L., Kaufmann D. E., Kiepiela P., Brown J. A., Moodley E. S., Reddy S., Mackey E. W., Miller J. D., Leslie A. J., DePierres C., Mncube Z., Duraiswamy J., Zhu B., Eichbaum Q., Altfeld M., Wherry E. J., Coovadia H. M., Goulder P. J., Klenerman P., Ahmed R., Freeman G. J., Walker B. D.2006. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature 443: 350–354. doi: 10.1038/nature05115 [DOI] [PubMed] [Google Scholar]
- 6.Gettinger S., Rizvi N. A., Chow L. Q., Borghaei H., Brahmer J., Ready N., Gerber D. E., Shepherd F. A., Antonia S., Goldman J. W., Juergens R. A., Laurie S. A., Nathan F. E., Shen Y., Harbison C. T., Hellmann M. D.2016. Nivolumab monotherapy for first-line treatment of advanced non-small-cell lung cancer. J. Clin. Oncol. 34: 2980–2987. doi: 10.1200/JCO.2016.66.9929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goto S., Konnai S., Okagawa T., Nishimori A., Maekawa N., Gondaira S., Higuchi H., Koiwa M., Tajima M., Kohara J., Ogasawara S., Kato Y., Suzuki Y., Murata S., Ohashi K.2017. Increase of cells expressing PD-1 and PD-L1 and enhancement of IFN-γ production via PD-1/PD-L1 blockade in bovine mycoplasmosis. Immun. Inflamm. Dis. 5: 355–363. doi: 10.1002/iid3.173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang P. Y., Guo S. S., Zhang Y., Lu J. B., Chen Q. Y., Tang L. Q., Zhang L., Liu L. T., Zhang L., Mai H. Q.2016. Tumor CTLA-4 overexpression predicts poor survival in patients with nasopharyngeal carcinoma. Oncotarget 7: 13060–13068. doi: 10.18632/oncotarget.7421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ikebuchi R., Konnai S., Shirai T., Sunden Y., Murata S., Onuma M., Ohashi K.2011. Increase of cells expressing PD-L1 in bovine leukemia virus infection and enhancement of anti-viral immune responses in vitro via PD-L1 blockade. Vet. Res. (Faisalabad) 42: 103. doi: 10.1186/1297-9716-42-103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ikebuchi R., Konnai S., Okagawa T., Yokoyama K., Nakajima C., Suzuki Y., Murata S., Ohashi K.2013. Blockade of bovine PD-1 increases T cell function and inhibits bovine leukemia virus expression in B cells in vitro. Vet. Res. (Faisalabad) 44: 59. doi: 10.1186/1297-9716-44-59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ikebuchi R., Konnai S., Okagawa T., Yokoyama K., Nakajima C., Suzuki Y., Murata S., Ohashi K.2014. Influence of PD-L1 cross-linking on cell death in PD-L1-expressing cell lines and bovine lymphocytes. Immunology 142: 551–561. doi: 10.1111/imm.12243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaufmann D. E., Kavanagh D. G., Pereyra F., Zaunders J. J., Mackey E. W., Miura T., Palmer S., Brockman M., Rathod A., Piechocka-Trocha A., Baker B., Zhu B., Le Gall S., Waring M. T., Ahern R., Moss K., Kelleher A. D., Coffin J. M., Freeman G. J., Rosenberg E. S., Walker B. D.2007. Upregulation of CTLA-4 by HIV-specific CD4+ T cells correlates with disease progression and defines a reversible immune dysfunction. Nat. Immunol. 8: 1246–1254. doi: 10.1038/ni1515 [DOI] [PubMed] [Google Scholar]
- 13.Larkin J., Chiarion-Sileni V., Gonzalez R., Grob J. J., Cowey C. L., Lao C. D., Schadendorf D., Dummer R., Smylie M., Rutkowski P., Ferrucci P. F., Hill A., Wagstaff J., Carlino M. S., Haanen J. B., Maio M., Marquez-Rodas I., McArthur G. A., Ascierto P. A., Long G. V., Callahan M. K., Postow M. A., Grossmann K., Sznol M., Dreno B., Bastholt L., Yang A., Rollin L. M., Horak C., Hodi F. S., Wolchok J. D.2015. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N. Engl. J. Med. 373: 23–34. doi: 10.1056/NEJMoa1504030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Larkin J., Chiarion-Sileni V., Gonzalez R., Grob J. J., Rutkowski P., Lao C. D., Cowey C. L., Schadendorf D., Wagstaff J., Dummer R., Ferrucci P. F., Smylie M., Hogg D., Hill A., Márquez-Rodas I., Haanen J., Guidoboni M., Maio M., Schöffski P., Carlino M. S., Lebbé C., McArthur G., Ascierto P. A., Daniels G. A., Long G. V., Bastholt L., Rizzo J. I., Balogh A., Moshyk A., Hodi F. S., Wolchok J. D.2019. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N. Engl. J. Med. 381: 1535–1546. doi: 10.1056/NEJMoa1910836 [DOI] [PubMed] [Google Scholar]
- 15.Munn D. H., Sharma M. D., Mellor A. L.2004. Ligation of B7-1/B7-2 by human CD4+ T cells triggers indoleamine 2,3-dioxygenase activity in dendritic cells. J. Immunol. 172: 4100–4110. doi: 10.4049/jimmunol.172.7.4100 [DOI] [PubMed] [Google Scholar]
- 16.Murakami K., Kobayashi S., Konishi M., Kameyama K., Tsutsui T.2013. Nationwide survey of bovine leukemia virus infection among dairy and beef breeding cattle in Japan from 2009–2011. J. Vet. Med. Sci. 75: 1123–1126. doi: 10.1292/jvms.12-0374 [DOI] [PubMed] [Google Scholar]
- 17.Nakamoto N., Cho H., Shaked A., Olthoff K., Valiga M. E., Kaminski M., Gostick E., Price D. A., Freeman G. J., Wherry E. J., Chang K. M.2009. Synergistic reversal of intrahepatic HCV-specific CD8 T cell exhaustion by combined PD-1/CTLA-4 blockade. PLoS Pathog. 5: e1000313. doi: 10.1371/journal.ppat.1000313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nishimori A., Konnai S., Okagawa T., Maekawa N., Ikebuchi R., Goto S., Sajiki Y., Suzuki Y., Kohara J., Ogasawara S., Kato Y., Murata S., Ohashi K.2017. In vitro and in vivo antivirus activity of an anti-programmed death-ligand 1 (PD-L1) rat-bovine chimeric antibody against bovine leukemia virus infection. PLoS One 12: e0174916. doi: 10.1371/journal.pone.0174916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O’Day S. J., Maio M., Chiarion-Sileni V., Gajewski T. F., Pehamberger H., Bondarenko I. N., Queirolo P., Lundgren L., Mikhailov S., Roman L., Verschraegen C., Humphrey R., Ibrahim R., de Pril V., Hoos A., Wolchok J. D.2010. Efficacy and safety of ipilimumab monotherapy in patients with pretreated advanced melanoma: a multicenter single-arm phase II study. Ann. Oncol. 21: 1712–1717. doi: 10.1093/annonc/mdq013 [DOI] [PubMed] [Google Scholar]
- 20.Ohira K., Nakahara A., Konnai S., Okagawa T., Nishimori A., Maekawa N., Ikebuchi R., Kohara J., Murata S., Ohashi K.2016. Bovine leukemia virus reduces anti-viral cytokine activities and NK cytotoxicity by inducing TGF-β secretion from regulatory T cells. Immun. Inflamm. Dis. 4: 52–63. doi: 10.1002/iid3.93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Okagawa T., Konnai S., Nishimori A., Ikebuchi R., Mizorogi S., Nagata R., Kawaji S., Tanaka S., Kagawa Y., Murata S., Mori Y., Ohashi K.2015. Bovine immunoinhibitory receptors contribute to suppression of mycobacterium avium subsp. paratuberculosis-specific T-cell responses. Infect. Immun. 84: 77–89. doi: 10.1128/IAI.01014-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Okagawa T., Konnai S., Deringer J. R., Ueti M. W., Scoles G. A., Murata S., Ohashi K., Brown W. C.2016. Cooperation of PD-1 and LAG-3 contributes to T-cell exhaustion in Anaplasma marginale-infected cattle. Infect. Immun. 84: 2779–2790. doi: 10.1128/IAI.00278-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okagawa T., Konnai S., Nishimori A., Maekawa N., Ikebuchi R., Goto S., Nakajima C., Kohara J., Ogasawara S., Kato Y., Suzuki Y., Murata S., Ohashi K.2017. Anti-bovine programmed death-1 rat-bovine chimeric antibody for immunotherapy of bovine leukemia virus infection in cattle. Front. Immunol. 8: 650. doi: 10.3389/fimmu.2017.00650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Okagawa T., Konnai S., Nishimori A., Maekawa N., Goto S., Ikebuchi R., Kohara J., Suzuki Y., Yamada S., Kato Y., Murata S., Ohashi K.2018. Cooperation of PD-1 and LAG-3 in the exhaustion of CD4+ and CD8+ T cells during bovine leukemia virus infection. Vet. Res. (Faisalabad) 49: 50. doi: 10.1186/s13567-018-0543-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qureshi O. S., Zheng Y., Nakamura K., Attridge K., Manzotti C., Schmidt E. M., Baker J., Jeffery L. E., Kaur S., Briggs Z., Hou T. Z., Futter C. E., Anderson G., Walker L. S., Sansom D. M.2011. Trans-endocytosis of CD80 and CD86: a molecular basis for the cell-extrinsic function of CTLA-4. Science 332: 600–603. doi: 10.1126/science.1202947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ready N., Farago A. F., de Braud F., Atmaca A., Hellmann M. D., Schneider J. G., Spigel D. R., Moreno V., Chau I., Hann C. L., Eder J. P., Steele N. L., Pieters A., Fairchild J., Antonia S. J.2019. Third-line nivolumab monotherapy in recurrent SCLC: checkmate 032. J. Thorac. Oncol. 14: 237–244. doi: 10.1016/j.jtho.2018.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robert C., Long G. V., Brady B., Dutriaux C., Di Giacomo A. M., Mortier L., Rutkowski P., Hassel J. C., McNeil C. M., Kalinka E. A., Lebbé C., Charles J., Hernberg M. M., Savage K. J., Chiarion-Sileni V., Mihalcioiu C., Mauch C., Arance A., Cognetti F., Ny L., Schmidt H., Schadendorf D., Gogas H., Zoco J., Re S., Ascierto P. A., Atkinson V.2020. Five-year outcomes with nivolumab in patients with wild-type BRAF advanced melanoma. J. Clin. Oncol. 38: 3937–3946. doi: 10.1200/JCO.20.00995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sajiki Y., Konnai S., Okagawa T., Nishimori A., Maekawa N., Goto S., Ikebuchi R., Nagata R., Kawaji S., Kagawa Y., Yamada S., Kato Y., Nakajima C., Suzuki Y., Murata S., Mori Y., Ohashi K.2018. Prostaglandin E2 induction suppresses the Th1 immune responses in cattle with Johne’s disease. Infect. Immun. 86: e00910–e00917. doi: 10.1128/IAI.00910-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sajiki Y., Konnai S., Okagawa T., Nishimori A., Maekawa N., Goto S., Watari K., Minato E., Kobayashi A., Kohara J., Yamada S., Kaneko M. K., Kato Y., Takahashi H., Terasaki N., Takeda A., Yamamoto K., Toda M., Suzuki Y., Murata S., Ohashi K.2019. Prostaglandin E2-induced immune exhaustion and enhancement of antiviral effects by anti-PD-L1 antibody combined with COX-2 inhibitor in bovine leukemia virus infection. J. Immunol. 203: 1313–1324. doi: 10.4049/jimmunol.1900342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sangro B., Gomez-Martin C., de la Mata M., Iñarrairaegui M., Garralda E., Barrera P., Riezu-Boj J. I., Larrea E., Alfaro C., Sarobe P., Lasarte J. J., Pérez-Gracia J. L., Melero I., Prieto J.2013. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J. Hepatol. 59: 81–88. doi: 10.1016/j.jhep.2013.02.022 [DOI] [PubMed] [Google Scholar]
- 31.Schneider H., Mandelbrot D. A., Greenwald R. J., Ng F., Lechler R., Sharpe A. H., Rudd C. E.2002. Cutting edge: CTLA-4 (CD152) differentially regulates mitogen-activated protein kinases (extracellular signal-regulated kinase and c-Jun N-terminal kinase) in CD4+ T cells from receptor/ligand-deficient mice. J. Immunol. 169: 3475–3479. doi: 10.4049/jimmunol.169.7.3475 [DOI] [PubMed] [Google Scholar]
- 32.Schneider H., Smith X., Liu H., Bismuth G., Rudd C. E.2008. CTLA-4 disrupts ZAP70 microcluster formation with reduced T cell/APC dwell times and calcium mobilization. Eur. J. Immunol. 38: 40–47. doi: 10.1002/eji.200737423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schurich A., Khanna P., Lopes A. R., Han K. J., Peppa D., Micco L., Nebbia G., Kennedy P. T., Geretti A. M., Dusheiko G., Maini M. K.2011. Role of the coinhibitory receptor cytotoxic T lymphocyte antigen-4 on apoptosis-Prone CD8 T cells in persistent hepatitis B virus infection. Hepatology 53: 1494–1503. doi: 10.1002/hep.24249 [DOI] [PubMed] [Google Scholar]
- 34.Suzuki S., Konnai S., Okagawa T., Ikebuchi R., Nishimori A., Kohara J., Mingala C. N., Murata S., Ohashi K.2015. Increased expression of the regulatory T cell-associated marker CTLA-4 in bovine leukemia virus infection. Vet. Immunol. Immunopathol. 163: 115–124. doi: 10.1016/j.vetimm.2014.10.006 [DOI] [PubMed] [Google Scholar]
- 35.Suzuki Y., Nakagawa M., Kameda Y., Konnai S., Okagawa T., Maekawa N., Goto S., Sajiki Y., Ohashi K., Murata S., Kitahara Y., Yamamoto K.2020. Novel vector and use thereof. US patent application No. 17/054,936.
- 36.Wang X., Teng F., Kong L., Yu J.2016. PD-L1 expression in human cancers and its association with clinical outcomes. OncoTargets Ther. 9: 5023–5039. doi: 10.2147/OTT.S105862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Watari K., Konnai S., Maekawa N., Okagawa T., Suzuki Y., Murata S., Ohashi K.2019. Immune inhibitory function of bovine CTLA-4 and the effects of its blockade in IFN-γ production. BMC Vet. Res. 15: 380. doi: 10.1186/s12917-019-2082-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wolchok J. D., Neyns B., Linette G., Negrier S., Lutzky J., Thomas L., Waterfield W., Schadendorf D., Smylie M., Guthrie T., Jr., Grob J. J., Chesney J., Chin K., Chen K., Hoos A., O’Day S. J., Lebbé C.2010. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol. 11: 155–164. doi: 10.1016/S1470-2045(09)70334-1 [DOI] [PubMed] [Google Scholar]