Abstract
Background
In occurrence of the coronavirus disease 19 (COVID-19) pandemic, carrying out an efficient large-scale vaccination campaign is vital in order to control the virus. Especially in high prevalence areas of COVID – 19, it is crucial to implement an effective vaccination strategy. In Italy, programming an efficient COVID-19 mass vaccination campaign has been the main target of the Ministry of Health.
Aims
This paper gives a comprehensive overview of how the mass vaccination campaign is performed in Milan, one of the cities that has been mostly affected by the COVID-19 pandemic in Italy. We analyze the vaccination strategy implemented by Fondazione Ca’ Granda – Ospedale Maggiore Policlinico located in Milan. Furthermore, we compare the organization of this campaign in regards of those carried out across EU and UK.
Materials and Methods
The data derive from an analysis of the different vaccination plans implemented across EU and UK from the 27/12/2020 to the 15/06/2020. In addition, we discuss the data collected from the internal data server of IRCCS Fondazione Ca’ Granda – Ospedale Maggiore Policlinico from the 15/02/2021 to the 15/06/2021.The collected data are examined by means of descriptive statistics.
Results
From the analysis of the internal data server, we observe that the modular organization of Fiera Milano City guarantees up to 5000 vaccinations/day. Moreover, the precise flow organization of users and a series of strategies adopted to avoid identification errors or vaccine type administration errors are crucial to reach the aforementioned target.
Conclusions
The institution of mass vaccination centers thanks to the optimization of all the involved processes and the meticulous organization of these structures, allows to avoid crowds and guarantees the administration of elevated amounts of vaccines. All these elements assure a rapid vaccination coverage of the population in Lombardy, with a meaningful increase in daily administration doses.
1. Introduction
On March 11, 2020, the World Health Organization (WHO) declared the spread of Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) to be a pandemic [1]. Since then, great technological and scientific efforts have been made to allow the development and distribution of safe and effectiveness vaccines against coronavirus disease 19 (COVID-19). Never before had a vaccine been developed in such a short time span. In addition, in order to achieve as soon as possible an appropriate vaccine coverage those new vaccines need to be distributed as rapidly as possible. Therefore, organizing a mass vaccination campaign on a global scale is a priority to contain the spread of the virus and allow a gradual resumption of economic and social activities. The purpose of this article is to compare different organizational and management models of vaccination campaigns of some European and non-EU Countries, focusing on the model proposed by the IRCCS-Ca' Granda Fondazione which has become the first mass vaccination center in Lombardy.
The IRCCS-Ca' Granda Fondazione (Fondazione) is the first public hospital for research and scientific productivity in Italy as well as the reference hospital for the city of Milan. As well-known Milan, the capital city of Lombardy region is the most affected area in Italy since the beginning of the COVID-19 pandemic. Given the central location of Fondazione in the city of Milan, the organization of the vaccination center had to overcome both logistical and structural problems in order to be able to ensure adequate volumes of administration.
At this time, two types of vaccine have been approved and marketed: an mRNA and a viral vector; the first phase of the massive vaccination campaign was based exclusively on the administration of mRNA vaccines [2], [3]. Later, thanks to the approval of viral vector vaccines, it has been possible to expand the vaccine offer and to allow a more rapid progression of the vaccination campaign.
2. Material and methods
All data from different EU Countries were collected from official website of European Center of Disease Control (ECDC), a literature review has been conducted using PubMed, Scopus and Web of Science, without language restrictions. We restricted the research to articles that included the words “vaccination”, “vaccine”, “rollout”, “mass vaccination” and “COVID-19 vaccination” in their titles or abstracts. We furthered searched reference lists of identified publications for citations of additional relevant articles. Fondazione data were obtained by internal database.
3. Discussion
3.1. Vaccine trend across EU e non-EU Countries
UK has been the first Country in the world to begin the distribution and administration of the SARS-COV2 vaccines on 08/12/2020, followed by USA on 14/12/2020, Israel on 20/12/2020. Meanwhile EU Countries began the distribution and administration of vaccines on 27/12/2020 [2], [3]. Since the beginning of the vaccination campaign, Israel has been the leading nation in terms of doses administered per 100 people. At this time around 59% of the Israelis population has completed the vaccination cycle [4]. The great success of the Israelis vaccination campaign was achieved thanks to a mixture of longstanding characteristics both extrinsic and intrinsic to the Israeli healthcare service. The first strongpoint to highlight is Israel small size in terms of area and population and the relatively young population. Another important characteristic is the centralized national system of government associated with Israel’s experience in implementing large scale responses in case of national emergencies [5], [6]. On the other hand, the most important players that contributed to the campaign success are the organization, logistics and coordination of the four healthcare plans. In fact, these health plans are efficiently coordinated with hospitals, the government and emergency care providers. Moreover, the availability of a preexisting well-functioning framework allowed a fast decision making and mobilization of the resources in order to support the campaign. Another crucial effort to speed up the vaccination process was to acquire a large amount of vaccine compared to the population size and to clearly and easily identify priority criteria at the beginning of the distribution process. Last but not least it is important to point out the creative measures adopted in order to meet the storage requirement as well as to encourage the population to sign up for the vaccination [7].
Meanwhile in USA 42% of the overall population have already completed the vaccination cycle [4]. A peculiar characteristic of the American vaccine campaign is the lack of priority groups. Nevertheless, the strongest point of the USA campaign is the availability of the largest amount of COVID-19 vaccine, as it is the manufacturing Country of the two mRNA vaccines currently marketed as well as the Johnson & Johnson viral vector vaccine [8].
Similarly in UK the amount of people that completed the 2 doses vaccination cycle with either an mRNA vaccine or Vaxzevria is 42% corresponding to around 29 million in a population of 65.637.239 in total [4]. As previously stated, UK became the first Country in the world to approve a COVID-19 vaccine for emergency use early in December. Nevertheless, the groundwork was laid nearly a year earlier, when the Department of Health and Social Care reportedly began planning a mass vaccination program, in January 2020, before first COVID-19 case in the UK was confirmed [9]. At the time, they were already working on a prototype vaccine against the coronavirus that causes Middle East Respiratory Syndrome (MERS) and reasoned that they would be able to adapt the chimpanzee adenovirus vector they were using to confer protection against the SARS-CoV-2. Moreover, another important strategical aspect that contributed to UK success in the vaccination effort was its promptness to strike a deal on vaccine deliveries batches three months before the EU’s. As a result of this, UK’s batches were set in motion earlier and separate to those earmarked for the EU [10].
On the other hand, despite a target value of 70 doses per 100 people EU has currently administered an average of 50 doses per 100 people. At the same time, the number of people that completed the 2 doses vaccination cycle are around 25.8% [11].
EU Countries autonomously organized the vaccination campaign following the guidelines of their own Minister of Health. Across EU Countries we can identify different organizational models, but with similar characteristics. A common aspect of the various organizational models is undoubtedly the introduction of priority groups, defined as clusters of the population to whom the vaccine should firstly be administered. Each Country identified different phases in which different population clusters converge. Ideally at the end of each phase the following phase opens [12], [13].
3.2. Italian vaccine campaign
Comparing Italian data with the European ones available through the ECDC website, Italy is currently in line with the vaccination trend across all Europe.
As a matter of fact, in Italy we have currently administered 66 doses per 100 people corresponding to around 40 million doses in a population of 60.600.590 in total. Around 13.500.000 people (22.3% of the total population) have completed the vaccination cycle with either 2 doses of an mRNA vaccines, Vaxzevria or a single dose of Johnson & Johnson. As of today, the rate of doses distributed to the population is 89,4% of the total number of doses received by the vaccine manufacturers [11], [12], [13], [14], [15], [16].
In Italy, similarly to the other EU Countries, different social categories have been identified to be vaccinated in different priority groups [12]:
-
•
Phase 1: health and social health workers, Residential Elderly Facilities guests and staff, over 80 years and people affected by chronic diseases between the ages of 60 and 79.
-
•
Phase 2: law enforcement, teachers and school staff, pharmacists, veterinarians.
-
•
Phase 3: people with comorbidities aged < 60 years without connotation of extreme vulnerability and the remaining cohorts aged < 60 years. The definition of the different priority groups was deemed necessary due to the initial availability of a limited number of doses at the early stage of anti-SARS-CoV-2/COVID-19 vaccination campaign.
In order to maximize the result, the strategical decision was to focus the use of the limited resources available on protecting personnel dedicated to pandemic emergency and on the most fragile subjects (health and social health workers, the staff and guests of residential facilities for the elderly). Thanks to the increase in the availability of vaccines the vaccination process started to be carried out also on other risk categories and then on the rest of the general population.[12], [13] Aspects correlated to procurement and logistics are the responsibility of the Extraordinary Commissioner.
Vaccines requiring storage at temperatures of −20° / −70° are delivered directly by the manufacturing companies in 300 sites appropriately identified by the Regions. On the other hand, vaccines that can be stored at the so-called “standard cold” temperatures (+2 / + 8° C) are distributed according to the “hub and spoke” model (a national storage and delivery center following the second level territorial centers) [17].
According to what was communicated by the Ministry of Health, the agreements provide for the arrival of 469,950 weekly doses in Italy, as of 1 May 2021 over 20,000,000 single doses of vaccine were administered [15], [16].
At the time of writing the age group with the highest vaccine coverage is represented by the over 80 population. In fact, over 94% of this cohort group has already received the first dose. As far as the other age groups are concerned, the vaccination coverage is lower [11], [13], [14].
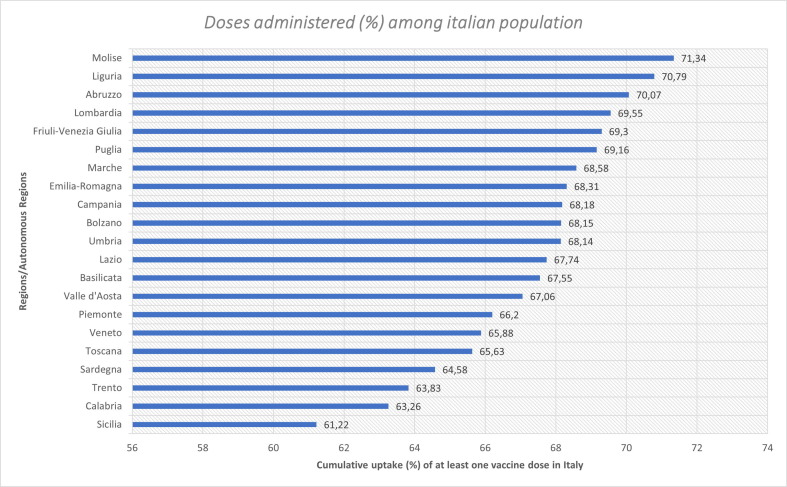
In Italy, the leading region in terms of vaccine doses administered is Molise with 71 doses per 100 people (20.61% of the population has completed the vaccination cycle). On the other hand, the region that has administered the smallest number of doses is Sicily, (Fig. 1 ), with around 61 doses per 100 people (around 19% of the population have completed the vaccination cycle). According to the number of doses administered in the population, Lombardy region is found at the fourth position. In greater detail we see that around 70 doses have been administer every 100 people and 21.31% of the population has completed the vaccination cycle [14].
Fig. 1.
Cumulative uptake in percentage (%) of at least one vaccine dose in Italy [14]
At the time of writing Lombardy region has surpassed the record of 7 million doses administered, corresponding to 92% of the doses received by the manufactures. The region aims at 9 million vaccinated by July with a daily average of 120,000 administrations. At the beginning of the vaccination campaign, the region suffered delays mainly due to the lower availability of vaccine doses and the lack of dedicated staff. Subsequently, thanks to the increased availability of vaccine doses but especially thanks to the construction of mass vaccination centers, Lombardy region continued the vaccination campaign at a faster pace. As a matter of fact, the number of daily doses administered has gone up from about 6000 to about 90,000 in Lombardy. According to the plans of Lombardy region, the booking round for the Lombards will be completed by early June. Finally, even those over 16 will be able to access reservations. Therefore, given the latest development it can be considered a successful vaccination campaign. In addition, whether Lombardy reaches the goal of vaccinating all Lombards with at least one dose by 30 August will only depend on the arrival of the new vaccines.
Focusing on IRCCS Fondazione Ca’ Granda - Ospedale Maggiore Policlinico vaccine administration (Table 1 ), since the last days of December 2020 till the 15th June 2021, 266.608 vaccine doses were administered overall to the population: 195.666 first doses and 70.942 second doses. Vaccine administration trends mirror the increasing performance of the Mass Vaccination Center as well as the opening of vaccination booking for different priority groups (Fig. 2 ): in green Vaxzevria doses, in red Janssen doses, in yellow Moderna doses and in blue Comirnaty doses, all values are vaccine doses administrated per day.
Table 1.
Number of doses administrated distinguished by vaccine types.
| Vaccine Type |
|||||
|---|---|---|---|---|---|
| Number of Dose | COMIRNATY | JANSSEN | MODERNA | VAXZEVRIA | Total Results |
| 1 | 66.730 | 2.193 | 21.108 | 105.635 | 195.666 |
| 2 | 35.689 | 5.730 | 29.523 | 70.942 | |
| Total Results | 102.419 | 2.193 | 26.838 | 135.158 | 266.608 |
Fig. 2.
IRCCS Fondazione Ca’ Granda administration vaccine trend.
3.3. The evolution of the vaccination centers
The Italian Ministry of Health classified the vaccination centers into 3 different categories: hospital vaccination centers (HVC), territorial vaccination centers (TVC) and long-term facilities vaccination centers (LTFVC) [17]. In compliance with the priorities set by the National Strategic Plan of the Ministry of Health, the progressive strengthening of the existing vaccination network, the government suggested to use production sites, large retailers, gyms, schools, structures of the Italian Episcopal Conference as vaccine sites [18]. In addition, it has been postulated a correlation between density population and how many centers were necessary to create in order to cover and assure vaccination to resident people in each area. For a high-density population area such as the one of Milan (more than 2000 people/kmq), Lombardy government proposed a commercial area of more than 13.000 mq of surface for an experimental model of mass vaccination territorial center (MVC): Fiera Milano City, based in Milan city center [19].
-
1.
Organization of the mass vaccination center:
During 2020, healthcare processes were deeply affected by the COVID-19 pandemic. First of all, on the basis of the indications of the competent national and regional authorities, the Fondazione had to reshape its assistance activities in order to face the pandemic waves. In addition to the reconversion of spaces and activities Fondazione was responsible for the set up of the “Ospedale in Fiera”, an intensive care unit to support the Lombard hospital network in the care of COVID-19 patients requiring the highest intensity of care. The authorization to use the first anti SARS-CoV-2 vaccines in December 2020 allowed the launch of a mass vaccination campaign defined within the National Strategic Plan [17]. The Fondazione has set up a first vaccination center in its Ponti Pavilion. Furthermore, following the launch of the campaign, the Fondazione was commissioned to set up a mass vaccination center dedicated to the vaccination of the general population in the spaces available at Fiera Milano City. The idea to set up a mass vaccination center came up following the successful example of the mass vaccination centers at first experimented in the UK. In order to guarantee the best possible performance for the MVC of Fiera Milano City, the Politecnico University of Milan, Fondazione and Ernst&Young Consulting (EY) were entrusted the study and evaluation of different mathematic and statistic tools. The aim of the previously cited study was to define a model for a MVC which could be modulated onto this territory. Therefore, the main points to be addressed were the definition of places, personnel, and requirements. The experimental mass vaccination site chosen for the study was the Fiera Pavilion organized by AREU, the Agency for emergency management, and Fondazione. Realization of the mass vaccination model requires industrialization of activities as well as considering some system constraints, e.g. failure of vaccine delivering, citizen engagement or centralized distribution model. With its 4 units fully active, the vaccination site of Fondazione guarantees 3000/3400 vaccines per day. Since February 2021 more than 200.000 people have been vaccinated [19].
-
2.
General organization aspects in Fiera Milano City MVC:
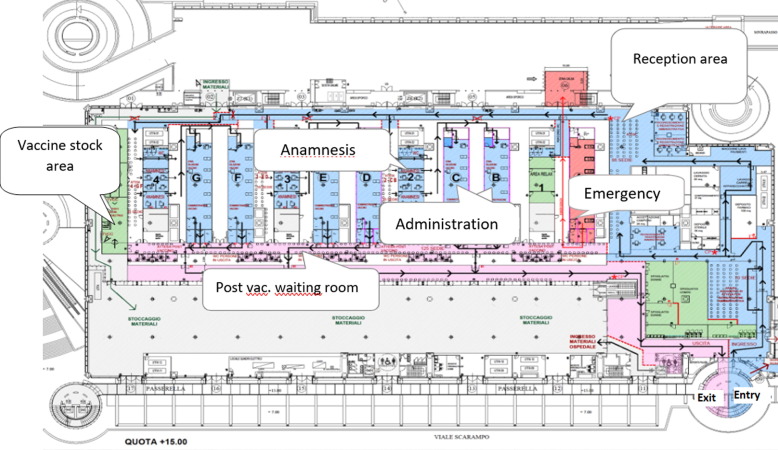
In Fiera Milano City MVC vaccine administration is managed through four different process lines: reception, anamnestic area, administration area, supplying and stock areas and an emergency department area to manage vaccination criticalities. In the emergency area, a specialized medical team composed of an anesthetist and a nurse is present during each vaccination session. Each process line is autonomous but nevertheless integrated with other process lines, owns a person in charge and can be isolated in case of necessity. Integration among different process lines is translated in a modular organization model. Modular organization means that each area in MVC interacts with other areas to reach the best optimization processes and in this way, it could be possible to guarantee a huge vaccination number per day. A module is so composed by reception desks, an anamnestic box with two correlated inoculation areas and a post-vaccination common area for waiting time (Fig. 3 ). Currently, in Fiera Milano City MVC four modules operates to guarantee up to 5000 vaccines per day, with more or less 30–36 medical doctors for anamnestic processes and 24 nurses for vaccine administrations; 16 reception desks address to anamnestic box vaccinating people assisted by volunteers. Before entering in Fiera Milano City MVC, convocation process plays a crucial role.
-
a)
People convocation:
Fig. 3.
Planimetry of Fiera Milano City Pavilion.
Integration with Regional Health Services is mandatory for an effective convocation process. As a matter of fact, in order to assure an effective and well-organized convocation plan/program based on a voluntary adherence to the vaccination campaign, Lombardy region decided to implement the use of an online portal service. Lombardy region schedules reservations exploiting a computerized system. Scheduled volumes are then transmitted to Fiera Milano City reception area that manages reservation slots based on transmitted volumes.
-
b)
Flow organization of the Fiera Pavilion:
Once at the vaccination center, the user is directed along an obligatory path, designed to avoid the mixing of flows. To guarantee this, voluntary support staff are deployed throughout the course to direct users and constantly check flows.
In detail the user waits for his turn in one of the pre-acceptances waiting rooms where he is provided an information note and an informed consent to fill it in. The user is received in the administrative front office where the appointment reservation is checked, and the acceptance is carried out. He is then directed to one of the anamnestic modules where he awaits the interview with the medical doctor who confers eligibility for the vaccination. The steps following the vaccination eligibility differ on the basis of the chosen vaccine. In fact, for subjects eligible for an mRNA vaccine it was foreseen a recognition system: the mRNA vaccine candidate could be either dressed in a jacket or the medical doctor gives him a colored sheet. Those who are eligible for viral vector vaccines are directed by volunteers to the appropriate box, identified by the specific name of the vaccine affixed at the entrance After the anamnesis is completed, volunteers direct the candidate to an mRNA vaccine administration box where the vaccine is inoculated by the nursing staff. Following the inoculation, the nursing staff releases a detailed certificate of proven vaccination. After the administration, he proceeds to the waiting room dedicated to the observation where he stops for a period defined during the interview with the doctor (15–30-45–60 min). The vaccinated candidate then leaves the vaccination center via a parallel but separate path from that of the entrance.
At all stages of the process, the user is identified by optical reading of the electronic identity card or the personal National Service Card. The details of the vaccination (type of vaccine, lot number, administration site, name of the health professionals involved) are recorded electronically through a special application that feeds the user's Electronic Health Record.
-
c)
Professional figures involved:
Different professional figures are involved in Fiera Milano City vaccination center: medical doctors, nurses, volunteers, receptionists. Referring to the medical process line, doctors can assume two different roles, either supervisor or vaccinator doctor. The first one provides coordination of the different activities and clinical support of medical staff. On the other hand, the second one evaluates the vaccination eligibility of a person. Due to the high turnover of staff a continuous training of the personnel is required. Therefore, different training activities through different modalities such as meetings, remote lessons, and training session together with other doctors, are mandatory to assures a correct and safe vaccine administration that is in accordance to the latest operative instructions.
As far as nurses are concerned, starting of activities is preceded by training sessions with nurses already employed in Fiera Milano City Vaccination Center and studying some informative documents. The aforementioned activities are managed by a chief nurse for the process line of competence. Finally, receptionist staff is inserted after completing a training period after supervision of operation management staff, logistic aspects are managed by Civil Defense volunteers and vaccine stocking is managed by pharmacy department of Fondazione.
-
3.
Experimental massive vaccination process: Fiera Pavilion
Due to the coordination between AREU volunteers and Fondazione personnel, Politecnico of Milan and EY Consulting have realized the first experimental mass vaccination site in Lombardy. During the two evaluation days, 16th and 17th January, the medium vaccine inoculation time was 3'30''. Meanwhile the whole process since the volunteer arrival in the receptionist area to the release of the certification post-vaccine was 15’. The major critical point was the anamnestic area due to the high variability in terms of operator anamnestic time. Each vaccination unit at first employed 13 medical doctors and 26 nurses. The vaccination rate with 2 vaccine units was 125/h. After an optimization process by Politecnico of Milan, increasing the number of medical doctors to 24 units instead of 13 had determined a huge increase of the vaccination rate with 2 vaccine units. As a matter of fact, the vaccine rate went up from 125/h to 330/h [19].
Another interesting aspect which has been highlighted from this optimization and simulation process is the importance of monitoring the arrival rate of people. According to this model the arrival rate should be set at a maximum of 86 people/15 min in order to guarantee an acceptable waiting time to avoid crowds. This experimentation has laid the foundations of the MVC model that is now adopted in all Lombardy [19].
-
4.
MVC opening to population and increasing of vaccine lines:
On 6th and 7th February 2500 AREU volunteers completed their vaccine cycle with their second dose. As seen in the previous paragraph this was an operating simulation to optimize and calculate the timing of the vaccination process since entering the Fiera Pavilion to the inoculation of the vaccine, as well as to highlight the critical issues and planning the opening to the rest of the population.
During the early stages of the vaccination campaign in the vaccination centers there was only one type of vaccine. This has inevitably led to the presence of ineligible candidates for that specific vaccine not to be vaccinated that day. This critical point has been overcome since different types of vaccines have now been made available in the center. This last point is one of the positive aspects of massive vaccination centers in which multiple vaccine types are available.
On the other hand, vaccine management has become even more complicated since the simultaneous administration of first and second doses of different vaccines has begun.
-
5.
Critical issues:
In order to reach an appropriate result in terms of the amount of vaccine administered, it is crucial to take into account both the critical issues that may derive from the vaccination process and the relative measures taken to cope with them.
First of all, the high personnel turnover requires a learning system which promptly adopts and embraces the Ministry of Health and AIFA notes. To avoid crowds correlated to personnel training, supervisors adopted several strategies such as organizing online meetings with videocall platforms and distance learning tests, regular email sending of informative material with the Ministry and AIFA latest provisions concerning vaccine administrations. After completing an initial phase of distance learning, a support and training phase with more experienced staff was organized. Personnel follow operative instructions contained in a manual, continuously updated and easy to understand.
Another criticism is the slowdown of the administration process due, for example, to increasing people flow in a module from reception area to anamnestic area and then to administration boxes: different modules may have a different process speed in each of their area. To solve this critical point, volunteers could address people to modules less congested without blocking the whole MVC.
Authorities directives are rapidly changing, and this requires the adoption of coping measures to avoid the error of administration. The first action taken to achieve this goal is to perform a first screening in the reception area in order to discriminate if the user requires a first or second dose of the vaccine. Up to now, it is not allowed to mix different vaccine types in a vaccination cycle. Once the anamnesis ends, a second check point is established to avoid that people enter the wrong administration box: medical doctors or volunteers hand over a jacket or a colored sheet to mRNA vaccination users. Another point that needs attention is the age of the user: each age group requires a specific vaccine type approved by EMA and AIFA and it is mandatory for doctors to know user’s age. In order to avoid mistakes the medical and nursing staff repeatedly check the coherence among the vaccine administered and the user’s age. In case an error occurs, the staff is asked to put down a report to analyze how the error occurred and how to avoid repeating it in the future.
Finally, allergies can be another critical issue important to take into account. In fact, sometimes users can’t be vaccinated in their reservation days because of pre-existing severe allergic reactions that require an allergic evaluation from specialists. This incident could delay the administration of the first vaccine dose and expose the allergic user to COVID-19 infection. In addition, an allergic reaction occurring after the first dose it may lead to exceed the given time limit between two doses. In Fiera Milano City a priority line was organized for people who require a novel reservation. Allergic subjects may be evaluated in Fondazione to decide whether they are eligible to be vaccinated. In addition those requiring it may have their vaccine administered in a secure environment in the intensive care unit in Fondazione.
4. Conclusions
The introduction of the massive vaccination centers to increase rapidly the vaccination coverage of the population, as an alternative organizational model, has made it possible for Lombardy to readily recover vaccine administration region chart positions. After an initial phase of adjustment, Fiera Milano City MCV by Fondazione became the landmark for all MVC in Lombardy and an organizational model for an effective vaccination planning in Italy. Despite the unavoidable critical issue, optimizing processes and modular management determined avoiding crowds and a rapid administration of elevated amounts of different type vaccines, after a careful process requiring different staff groups, from receptionists to doctors, nurses and pharmacists.
CRediT authorship contribution statement
Francesco Oliani: Conceptualization, Writing – original draft, Visualization, Writing – review & editing. Antonella Savoia: Conceptualization, Writing – original draft, Visualization, Writing – review & editing. Giulia Gallo: Conceptualization, Writing – original draft, Visualization, Writing – review & editing. Navpreet Tiwana: Conceptualization, Data curation, Resources. Matteo Letzgus: Conceptualization, Data curation, Resources. Flaminia Gentiloni: Data curation, Resources. Alessandra Piatti: Conceptualization, Data curation, Resources. Laura Chiappa: Conceptualization, Resources. Alberto Bisesti: Resources, Data curation. Dario Laquintana: Resources, Data curation. Silvana Castaldi: Conceptualization, Methodology, Supervision, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.WHO. WHO Director-General’s opening remarks at the media briefing on COVID-19 - 11 March 2020 [Internet]. Available from: https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-COVID-19---11-march-2020
- 2.EMA. COVID-19 vaccines [Internet]. Available from: https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-COVID-19/treatments-vaccines/COVID-19-vaccines
- 3.AIFA. Vaccini COVID-19 [Internet]. Available from: https://www.aifa.gov.it/vaccini-COVID-19
- 4.Gedi Visual. Coronavirus, le vaccinazioni nel mondo.
- 5.Adini B., Goldberg A., Cohen R., Laor D., Bar-Dayan Y. Evidence-based support for the all- hazards approach to emergency preparedness. Isr J Heal Policy Res. 2012;1(1) doi: 10.1186/2045-4015-1-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adini B., Peleg K. On constant alert: lessons to be learned from Israel’s emergency response to mass-casualty terrorism incidents. Heal Aff. 2013;32(12):2179–2185. doi: 10.1377/hlthaff.2013.0956. [DOI] [PubMed] [Google Scholar]
- 7.Rosen B., Waitzberg R., Israeli A. Israel ’s rapid rollout of vaccinations for. Isr J Health Policy Res. 2021;6:1–14. doi: 10.1186/s13584-021-00440-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.CDC. Different COVID-19 Vaccines [Internet]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines.html
- 9.Manthorpe R. COVID-19: rejected contracts and a Hollywood movie—how UK struck deal to guarantee vaccine supply.No Title. Sky News. 2021 Feb 1;
- 10.Baraniuk C. COVID-19: How the UK vaccine rollout delivered success, so far. BMJ. 2021 doi: 10.1136/bmj.n421. [DOI] [PubMed] [Google Scholar]
- 11.ecdc. ecdc vaccine tracker [Internet]. Available from: https://vaccinetracker.ecdc.europa.eu/public/extensions/COVID-19/vaccine-tracker.html
- 12.ecdc. Overview of the implementation of COVID-19 vaccination strategies and vaccine deployment plans in the EU / EEA. 2021.
- 13.ecdc. Overview of the implementation of COVID-19 vaccination strategies and vaccine deployment plans in the EU / EEA Key findings. 2021.
- 14.Gedi Visual. Coronavirus, le vaccinazioni in Italia regione per regione [Internet]. Available from: https://lab.gedidigital.it/gedi-visual/2021/report-vaccini-anti-covid-aggiornamento-vaccinazioni-italia/
- 15.Ministero della salute, Presidenza del Consiglio dei Ministri, Commissario Straordinario COVID-19. Report Vaccini Anti COVID-19 [Internet]. Available from: https://www.governo.it/it/cscovid19/report-vaccini/
- 16.Presidenza del Consiglio dei Ministri, Commissario Straordinario COVID-19. Opendata [Internet]. Available from: https://github.com/italia/covid19-opendata-vaccini
- 17.Ministero della salute. Raccomandazioni per l’organizzazione della campagna vaccinale contro SARS-CoV-2/COVID-19 e procedure di vaccinazione. 2020.
- 18.Presidenza del Consiglio dei Ministri, COVID-19 CS per l’attuazione e il coordinamento delle misure sanitarie di contenimento e contrasto dell’emergenza epidemiologica. Piano vaccinale anticovid. 2021.
- 19.AREU, ARIA, Fondazione IRCCS Ca’Granda, Fondazione Fiera Milano, Politecnico di Milano EC. Regione Lombardia Realizzazione polo vaccinale massivo. 2021.