Abstract
Background
The coronavirus disease 2019 (COVID-19) is an ongoing pandemic. Invasive mechanical ventilation (IMV) is essential for the management of COVID-19 with acute respiratory distress syndrome (ARDS). We aimed to assess the impact of compliance with a respiratory decision support system on the outcomes of patients with COVID-19-associated ARDS who required IMV.
Methods
In this retrospective, single-center, case series study, patients with COVID-19-associated ARDS who required IMV at Zhongnan Hospital of Wuhan University, China, from January 8th, 2020, to March 24th, 2020, with the final follow-up date of April 20th, 2020, were included. Demographic, clinical, laboratory, imaging, and management information were collected and analyzed. Compliance with the respiratory support decision system was documented, and its relationship with 28-day mortality was evaluated.
Results
The study included 46 COVID-19-associated ARDS patients who required IMV. The median age of the 46 patients was 68.5 years, and 31 were men. The partial pressure of arterial oxygen (PaO2)/fraction of inspired oxygen (FiO2) ratio at intensive care unit (ICU) admission was 104 mmHg. The median total length of IMV was 12.0 (interquartile range [IQR]: 6.0–27.3) days, and the median respiratory support decision score was 11.0 (IQR: 7.8–16.0). To 28 days after ICU admission, 18 (39.1%) patients died. Survivors had a significantly higher respiratory support decision score than non-survivors (15.0 [10.3–17.0] vs. 8.5 (6.0–10.3), P = 0.001). Using receiver operating characteristic (ROC) curve to assess the discrimination of respiratory support decision score to 28-day mortality, the area under the curve (AUC) was 0.796 (95% confidence interval [CI]: 0.657–0.934, P = 0.001) and the cut-off was 11.5 (sensitivity = 0.679, specificity = 0.889). Patients with a higher score (>11.5) were more likely to survive at 28 days after ICU admission (log-rank test, P < 0.001).
Conclusions
For severe COVID-19-associated ARDS with IMV, following the respiratory support decision and assessing completion would improve the progress of ventilation. With a decision score of >11.5, the mortality at 28 days after ICU admission showed an obvious decrease.
Keywords: COVID-19, Acute respiratory distress syndrome (ARDS), Respiratory support decision, Invasive mechanical ventilation, Severe acute respiratory coronavirus 2 (SARS-CoV-2)
Introduction
The coronavirus disease 2019 (COVID-19) caused by severe acute respiratory coronavirus 2 (SARS-CoV-2) in an ongoing pandemic. Although most of the cases were mild, about 20% deteriorated and developed severe pneumonia and acute respiratory distress syndrome (ARDS), which has led to millions of deaths. Patients with COVID-19 pneumonia present with dyspnea, high respiratory drive, and severe hypoxemia, eventually complicated with multiorgan dysfunction and death.[1,2]
Invasive mechanical ventilation (IMV) is considered an essential supportive tool for patients with COVID-19-associated ARDS. Overall, 71–88% of patients with COVID-19-associated ARDS admitted to the intensive care unit (ICU) received IMV.[[3], [4], [5]] While IMV is used to provide respiratory support,[6,7] it may result in lung damage due to barotrauma, volutrauma, and atelectrauma[8,9] lead to ventilator-associated pneumonia.[10] Even with similar IMV management strategies, the incidence of mortality among patients requiring IMV is variable. While high mortality rates were reported from China (81%)[4] and New York City (88.1%),[5] relatively lower mortality was noted in the Lombardy region of Italy (26–35%).[3]
To further clarify these controversial reports, we aimed to assess the impact of a standardized respiratory support decision system with its associated scoring tool on the mortality of patients with COVID-19-associated ARDS requiring IMV.[11]
Methods
Design, setting, and patients
This retrospective study was conducted at the Zhongnan Hospital of Wuhan University, Hubei, China, which was a designated COVID-19 hospital by the Chinese government. The Zhongnan Hospital of Wuhan University ethics committee approved the study (No. 2020011). Given the observational retrospective nature of the study, the need for written informed consent from each patient was waived.
We retrospectively analyzed all consecutive adult patients (≥18 years) with COVID-19-associated ARDS who required IMV from January 8th, 2020, to March 24th, 2020. During the treatment period, COVID-19 was diagnosed by both chest CT scan and real-time reverse transcription-polymerase chain reaction. Patients with COVID-19-associated ARDS were managed according to the previous ARDS and sepsis guidelines, as well as references for ARDS and IMV.[[12], [13], [14]] Patients who stayed in the ICU for <24 h were excluded.
Data collection
We reviewed the clinical electronic medical records and nursing notes for all enrolled patients. The recorded data included demographics, medical history, underlying comorbidities, complications, radiological examinations, vital signs, laboratory test results, medications, need for the high-flow nasal cannula (HFNC) or non-invasive ventilation (NIV), mechanical ventilator settings, use of neuromuscular blockers (NMBAs) or prone positioning, need for extracorporeal membrane oxygenation (ECMO), and the mortality of 28-day after ICU admission (28 d-ICU). The Acute Physiology and Chronic Health Evaluation II (APACHE II) score was calculated on the day of ICU admission, and the Sequential Organ Failure Assessment score (SOFA) was assessed both on the day of ICU admission and at intubation. Lung injury score (LIS) was recorded according to the scoring system proposed by Murray et al.[15] on the day of ICU admission. The date of disease onset was defined as the day when the symptoms of COVID-19 started. ARDS was judged based on the Berlin definition.[16] The total length of non-invasive devices (i.e., HFNC or NIV) before the initiation of IMV was defined as the total duration of HFNC and NIV from the day of use to the time of intubation. The total length of mechanical ventilation was counted from the day of intubation to the day of weaning from the ventilator or death.
Respiratory support decision protocol and compliance scoring
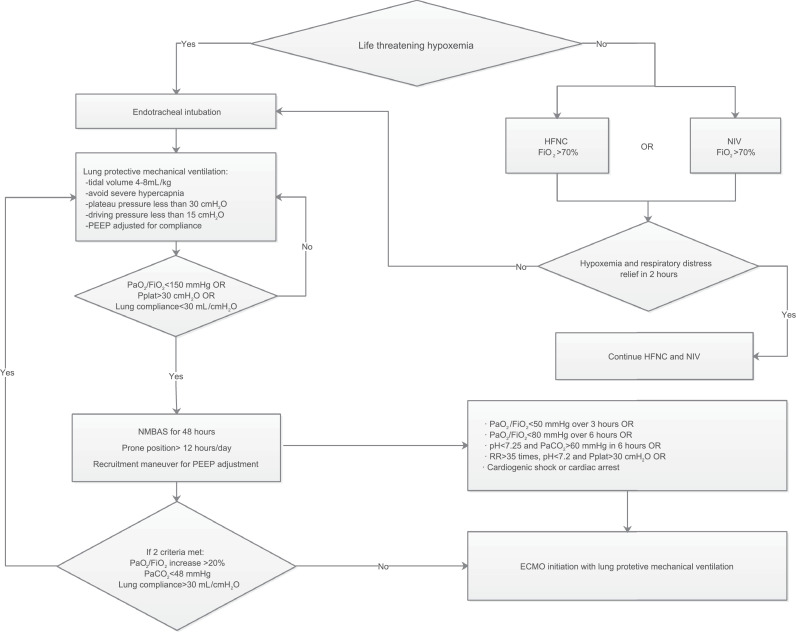
The respiratory support decision protocol was an evidence-based protocol that relied on the Surviving Sepsis Campaign: Guidelines on the Management of Critically Ill Adults with Coronavirus Disease 2019[17] and other current references for ARDS and IMV. Physicians SC, LL, XY, and BH screened the guidelines and references and designed the protocol. Then, the entire team discussed and revised the protocol in three rounds until consensus was achieved. The final protocol is described in Figure 1.
Figure 1.
The respiratory support treatment process of adult severe COVID-19 patients.
COVID-19: Coronavirus disease 2019; ECMO: Extracorporeal membrane oxygenation; FiO2: Fraction of inspired oxygen; HFNC: High-flow nasal cannula; NIV: Non-invasive ventilation; PaCO2: Artery partial pressure of carbon dioxide; PaO2: Partial pressure of arterial oxygen; PEEP: Positive end-expiratory pressure; Pplat: Platform pressure; RR: Respiratory rate; SO2: Oxygen saturation.
In the absence of life-threatening hypoxemia and severe respiratory distress, respiratory support was initiated with HFNC or NIV. The use of non-invasive respiratory support continued if hypoxemia and respiratory distress symptoms improved within 2 h (SPO2 > 92%, Vt < 9 mL/kg, respiratory rate [RR] < 30 times/min)[18,19] with fraction of inspired oxygen (FiO2) <70%. Otherwise, endotracheal intubation and IMV were required.
In the presence of life-threatening hypoxemia or severe respiratory distress, emergency endotracheal intubation and IMV were recommended. The IMV setting was based on the lung-protective strategy,[11,20,21] including low tidal volume (TV) (4–8 mL/kg ideal body weight) and inspiratory pressures (plateau pressure ≤30 cmH2O), driving pressure ≤15 cmH2O, as well as positive end-expiratory pressure (PEEP) set based on the lung compliance. The use of NMBAs, prone positioning, recruitment maneuvers (RMs), as well as titrated PEEP according to the best respiratory-system compliance were considered when: (1) partial pressure of arterial oxygen (PaO2) to the fraction of inspired oxygen (FiO2) ratio (PaO2/FiO2) was <150 mmHg, (2) plateau pressure was >30 cmH2O, and (3) lung compliance was <30 mL/cmH2O. ECMO initiation was considered if despite optimized mechanical ventilation, (1) PaO2/FiO2 was <50 mmHg for >3 h; (2) PaO2/FiO2 was <80 mmHg for >6 h; (3) arterial blood gas pH<7.25, partial pressure of carbon dioxide (PaCO2)>60 mmHg for >6 h, or RR >35 breaths/min, respectively; (4) arterial blood gas pH<7.2, or plateau pressure>30 cmH2O, or RR >35 breaths/min, respectively; and (5) respiratory failure was complicated with cardiogenic shock or cardiac arrest.[[22], [23], [24]] Furthermore, ECMO was considered if two of the following criteria were reached within 48 h of optimized mechanical ventilation: (1) PaO2/FiO2 increase by <20%, (2) PaCO2 ≥48 mmHg, and (3) lung compliance ≤30 mL/cmH2O.
To evaluate the quality and completion of the provided respiratory support, we designed a scoring system according to the critical parts of the respiratory support protocol [Table 1].[18,19,22,24,25] Nine items used in this tool provided a range of scores from −6 to 18 points. For each respiratory decision, a certain period of observation and management was permitted before the score was calculated by two groups (FZ and HX vs. HH and JW). If the calculated scores were different between the two groups, SC and BH assigned the final score for each patient.
Table 1.
Evaluation of the quality of respiratory support decision implementation.
| Item | Code | Score | Scoring criteria | Score |
|
|---|---|---|---|---|---|
| Yes | No | ||||
| Prevention of fatal situation and emergency intubation | A | 1 | Avoid fatal hypoxia or hypercapnia (SO2 < 70% or RR < 8 times/min or PaCO2 > 50 mmHg) OR Intubate and IMV in time (no >1 h when fatal hypoxia or hypercapnia happens) |
||
| B | 1 | Avoid fatal respiratory distress (RR > 40 times/min or loss of consciousness or blood lactic acid >4 mmol/L) OR Intubate and IMV in time (no >1 h when fatal respiratory distress happens) |
|||
| Traditional and HFNC oxygen therapy management and intubation timing | C | 1 | FiO2 ≤ 70% to maintain the target SO2 OR Intubate and IMV in <12 h when the FiO2 > 70% |
||
| D | 1 | RR ≤ 35 times/min OR Intubate and IMV in <6 h when RR > 35 times/min |
|||
| NIV management and intubation time | E | 1 | FiO2 ≤ 70% to maintain the target SpO2 OR Intubate and IMV in <12 h when the FiO2 > 70% |
||
| F | 1 | RR ≤ 30 times/min OR Intubate and IMV in <6 h when RR > 30 times/min |
|||
| Lung protective ventilation strategy | G | 1 | Vt ≤ 8 mL/kg (ideal body weight) | ||
| H | 1 | Pplat ≤ 30 cmH2O OR Pplat down to 30 cmH2O within 24 h when Pplat > 30 cmH2O |
|||
| I | 1 | Driving pressure ≤15 cmH2O OR Driving pressure down to 15 cmH2O within 24 h when driving pressure >15 cmH2O |
|||
| NMBAs administration | J | 1 | Avoid PaO2/FiO2 < 150 mmHg OR Using NMBAs for the IMV with PaO2/FiO2 < 150 mmHg in 48 h after intubation for 48–72 h |
||
| Prone position | K | 1 | Avoid PaO2/FiO2 < 100 mmHg OR Prone position >12 h/day for IMV patients with PaO2/FiO2 < 100 mmHg |
||
| Ventilation effect at 48 h after intubation by fine adjustment | L | 1 | Achieved PaO2/FiO2 increases >20% | ||
| M | 1 | Achieved PaCO2 < 48 mmHg | |||
| N | 1 | Achieved Cstat of lungs >30 mL/cmH2O | |||
| ECMO initiation and MV management during ECMO | O | 2 | Avoid ECMO OR Decision of initiating ECMO in time, including PaO2/FiO2 < 50 mmHg over 3 h or PaO2/FiO2 < 80 mmHg over 6 h Arterial blood gas pH < 7.25 and PaCO2 > 60 mmHg over 6 h, as well as RR over 35 times/min RR > 35 times/min, arterial blood gas pH < 7.2 and plateau pressure >30 cmH2O Complicated with cardiogenic shock or cardiac arrest |
||
| P | 1 | Avoid ECMO OR Make right super protective ventilation strategy, including drive pressure ≤15 cmH2O and PEEP ≤ 10 cmH2O |
|||
| Respiratory drive management during IMV | Q | 1 | Avoid RR > 30 times/min lasts >6 h | ||
| Deduction | R | −2 | SO2 < 80% lasts >6 h during hospitalization | ||
| S | −2 | Ineffective RM were performed for more than twice; OR The duration of high PEEP that resulted in decreased lung compliance exceeded 12 h |
|||
| T | −2 | ECMO implementation after 7 days of IMV | |||
Cstat: Static compliance; ECMO: Extracorporeal membrane oxygenation; FiO2: Fraction of inspired oxygen; HFNC: High-flow nasal cannula; IMV: Invasive mechanical ventilation; NIV: Non-invasive ventilation; NMBAs: Neuromuscular blocking agents; PaCO2: Partial pressure of carbon dioxide; PaO2: Partial pressure of arterial oxygen; PEEP: Positive end-expiratory pressure; Pplat: Plat pressure; RM: Recruitment maneuver; RR: Respiratory rate; SO2: Oxygen saturation.
Statistical analysis
Categorical variables were summarized as counts and percentages. Proportions for categorical variables were compared using the chi-squared and Fisher's exact tests, as appropriate. Continuous variables were summarized as mean ± standard deviation when the data were normally distributed, and the groups were compared by Student's t-test; otherwise, median and interquartile range (IQR) were used, and the Mann–Whitney U test was used to examine differences between the groups. Univariate logistic regression analysis was performed for all known factors that could be associated with 28 d-ICU in COVID-19 patients, including demographics, underlying diseases, and decision score. We used a univariate Cox regression analysis for all factors that could be associated with the 28 d-ICU, including demographics, SOFA and APACHE II scores, and the respiratory decision score. A receiver operating characteristic (ROC) curve was generated to detect the best discriminative variable of 28 d-ICU in COVID-19 patients. The area under the curve (AUC) and the Youden index were calculated for the 28 d-ICU. A two-sided α of <0.05 was considered statistically significant. All statistical analyses were performed using the Statistical Package for the Social Sciences (version 26.0, IBM Corporation, Armonk, NY, USA).
Results
Baseline characteristics
The study included 46 COVID-19-associated ARDS patients who required IMV and were admitted to the ICU of Zhongnan Hospital of Wuhan University from January 8th, 2020, to March 24th, 2020. The median age was 68.5 years (IQR: 60.3–79.3), and 31 (67.4%) were men. The mean duration from first symptoms to development of dyspnea, hospital admission, ARDS, and ICU admission were 9.9 ± 6.7 days, 10.7 ± 6.2 days, 14.8 ± 10.2 days, and 14.9 ± 8.9 days, respectively. Of the 46 patients, 38 (82.6%) had coexisting medical conditions. Hypertension (23 [50.0%]), diabetes (8 [17.4%]), cardiovascular disease (11 [23.9%]), and cerebrovascular disease (9 [19.6%]) were the most common coexisting comorbidities. In the 28 days after ICU admission, 28 (60.9%) patients survived, and 18 (39.1%) died. Demographics and comorbidities were similar among non-survivors and survivors [Table 2].
Table 2.
Demographics and baseline characteristics of patients with severe COVID-19 and ARDS.
| Characteristics | Total (n = 46) | Non-survival (n = 18) | Survival (n = 28) | t/χ2/Z | P-value |
|---|---|---|---|---|---|
| Age (years) | 68.5 (60.3–79.3) | 76.0 (60.3–81.3) | 68.0 (58.3–76.3) | 0.957 | 0.339 |
| Male sex | 31 (67.4) | 13 (72.2) | 18 (64.3) | 0.314 | 0.575 |
| Comorbidities | |||||
| Hypertension | 23 (50.0) | 11 (61.1) | 12 (42.9) | 1.460 | 0.227 |
| Diabetes | 8 (17.4) | 3 (16.7) | 5 (17.9) | 0.010 | 0.917 |
| Cardiovascular disease | 11 (23.9) | 5 (27.8) | 6 (21.4) | 0.243 | 0.662 |
| Cerebrovascular disease | 9 (19.6) | 3 (16.7) | 6 (21.4) | 0.158 | 0.691 |
| COPD | 2 (4.3) | 1 (5.6) | 1 (3.6) | 0.104 | 0.747 |
| CKD | 2 (4.3) | 1 (5.6) | 1 (3.6) | 1.104 | 0.747 |
| Chronic liver disease | 1 (2.2) | 1 (5.6) | 0 (0) | 1.590 | 0.207 |
| Malignancy | 4 (8.7) | 1 (5.6) | 3 (10.7) | 0.367 | 0.545 |
| Onset of illness (days) | |||||
| To hospital admission | 10.7 ± 6.2 | 11.7 ± 7.3 | 10.1 ± 5.5 | 0.984 | 0.327 |
| To dyspnea | 9.9 ± 6.7 | 8.1 ± 8.0 | 11.1 ± 5.6 | 0.270 | 0.606 |
| To ARDS | 14.8 ± 10.2 | 14.8 ± 11.3 | 14.8 ± 9.8 | 0.789 | 0.379 |
| To ICU admission | 14.9 ± 8.9 | 14.9 ± 11.0 | 14.8 ± 7.6 | 2.271 | 0.103 |
Data presented as median (interquartile range), n (%), and mean ± standard deviation.
ARDS: Acute respiratory distress syndrome; CKD: Chronic kidney disease; COPD: Chronic obstructive pulmonary disease; COVID-19: Coronavirus disease 2019; ICU: Intensive care unit.
Vital signs and laboratory parameters
At ICU admission, the heart rate (HR) (99.0[IQR: 84.8–109.8] beats/min) and RR (24.5[IQR: 20.0–30.8] beats/min) were higher than the normal range. The PaO2/FiO2 ratio was low (104 [IQR: 72–154] mmHg), while PaCO2 was within the normal range (39.0 [IQR: 31.8–42.5] mmHg). Although the total white blood cell count was normal (9.7 [IQR: 5.3–14.7] × 109/L), the lymphocyte count was low (0.64 [IQR: 0.37–0.89] × 109/L). d-dimer (1855 [IQR: 496–8418] mg/L), and lactate dehydrogenase (476.5 [IQR: 370.3–598.5] U/L) levels were elevated. Interleukin-6 (IL-6) level (82.7[IQR: 20.6–203.3] pg/mL) was higher than the normal range (0–7 pg/mL). APACHE II and SOFA scores were 19.0 (IQR: 14.8–25.0) and 7.0 (IQR: 5.0–9.0), respectively. The Murray LIS was 3.25 (IQR: 3.00–3.50), and all patients showed bilateral infiltrations in the chest radiograph. At the time of intubation, in comparison with ICU admission, there were no significant changes in most of the parameters, except PaCO2 (47.4 [IQR: 39.7–58.6] mmHg) and d-dimer (2541 [IQR: 1179–7672] mg/L) elevation [Table 3].
Table 3.
Vital signs and laboratory parameters of patients with severe COVID-19 and ARDS.
| Items | ICU admission |
Intubation |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total (n = 46) | Non-survival (n = 18) | Survival (n = 28) | t/χ2/Z | P-value | Total (n = 46) | Non-survival (n = 18) | Survival (n = 28) | t/χ2/Z | P-value | |
| Heart rate (beats/min) | 99.0 (84.8–109.8) | 95.5 (83.3–105.0) | 102.0 (86.0–117.3) | 1.126 | 0.260 | 108.0 (98.3–130.0) | 104.5 (95.5–126.0) | 109.0 (99.0–130.0) | 0.405 | 0.685 |
| Respiratory rate (times/min) | 24.5 (20.0–30.8) | 22.5 (20.0–30.8) | 25 (20.0–32.3) | 0.034 | 0.973 | 25.0 (20.0–32.25) | 24.5 (20.0–33.25) | 25.0 (20.25–29.5) | 0.305 | 0.760 |
| MAP (mmHg) | 90.0 (80.0–101.0) | 89.0 (80.0–101.0) | 92.0 (81.0–104.0) | 0.248 | 0.804 | 84.0 (72.75–97.3) | 84.5 (76.0–97.0) | 84.0 (70.0–96.0) | 0.541 | 0.589 |
| PH | 7.41 (7.37–7.46) | 7.41 (7.32–7.48) | 7.41 (7.38–7.46) | 0.087 | 0.931 | 7.30 (7.20–7.10) | 7.29 (7.20–7.40) | 7.30 (7.30–7.40) | 1.135 | 0.256 |
| Lactate (mmol/L) | 1.60 (1.20–2.30) | 1.80 (1.10–2.50) | 1.50 (1.30–2.30) | 0.298 | 0.765 | 1.55 (1.20–2.13) | 1.50 (1.20–2.15) | 1.60 (1.20–2.10) | 0.039 | 0.969 |
| PaO2 (mmHg) | 68.4 (49.9–81.9) | 72.9 (54.9–84.6) | 67.0 (47.0–80.2) | 0.944 | 0.345 | 80.1 (57.2–94.0) | 80.0 (57.1–90.9) | 80.1 (56.4–95.0) | 0.185 | 0.853 |
| PaO2/FiO2 ratio (mmHg) | 104 (72–154) | 103 (73–149) | 106 (71–160) | 0.199 | 0.842 | 107 (71–173) | 99 (60–166) | 127 (87–191) | 1.495 | 0.135 |
| PaCO2 (mmHg) | 39.0 (31.8–42.5) | 37.5 (28.6–44.1) | 39.3 (32.1–42.6) | 0.754 | 0.456 | 47.4 (39.7–58.6) | 47.7 (38.8–61.0) | 47.4 (40.9–58.0) | 0.197 | 0.844 |
| White blood cell count (× 109/L) | 9.7 (5.3–14.7) | 10.7 (6.8–14.1) | 8.7 (4.8–16.1) | 0.563 | 0.574 | 10.9 (7.1–15.6) | 10.9 (7.2–16.0) | 10.5 (5.8–16.1) | 0.630 | 0.529 |
| Lymphocyte count (× 109/L) | 0.64 (0.37–0.89) | 0.60 (0.43–0.79) | 0.69 (0.36–0.91) | 0.563 | 0.574 | 0.61 (0.31–0.87) | 0.55 (0.28–0.76) | 0.69 (0.33–0.97) | 1.182 | 0.237 |
| Platelets count (× 109/L) | 177 (115–236) | 166 (111–210) | 180 (116–252) | 0.675 | 0.499 | 169 (110–217) | 126 (100–188) | 180 (122–236) | 1.688 | 0.091 |
| Prothrombin time (s) | 13.6 (12.7–14.9) | 13.5 (12.5–16.4) | 13.8 (12.8–14.3) | 0.135 | 0.892 | 13.4 (12.8–14.9) | 14.3 (13.0–16.2) | 13.2(12.7–13.9) | 1.521 | 0.128 |
| Activated partial, second thromboplastin time (s) | 32.2 (28.3–36.8) | 30.3 (26.5–42.6) | 32.5 (29.6–36.3) | 0.957 | 0.339 | 31.5 (27.1–36.2) | 28.6 (26.5–37.8) | 32.6 (28.3–36.3) | 0.901 | 0.368 |
| d-dimer (mg/L) | 1855 (496–8418) | 2461 (856–8838) | 1367 (252–8228) | 0.945 | 0.344 | 2541 (1179–7672) | 5637 (1225–9021) | 2110 (1153–7377) | 0.855 | 0.392 |
| Hypersensitive troponin I (pg/mL) | 30.5 (11.0–140.4) | 27.0 (11.0–98.9) | 35.5 (11.3–182.4) | 0.630 | 0.528 | 42.5 (11.0–247.8) | 48.0 (11.0–157.7) | 41.0 (11.0–733.0) | 0.369 | 0.725 |
| Creatine kinase (U/L) | 130.0 (60.0–224.0) | 154.5 (59.8–314.5) | 109.0 (59.0–195.0) | 0.689 | 0.491 | 121.0 (54.0–278.0) | 139.0 (58.5–260.8) | 103.0 (45.4–309.5) | 0.505 | 0.630 |
| Lactate dehydrogenase (U/L) | 476.5 (370.3–598.5) | 517.5 (413.3–600.3) | 425.0 (288.0–588.0) | 1.272 | 0.204 | 479.5 (346.5–637.0) | 528.5 (458.5–637.0) | 446.0 (263.0–721.8) | 0.195 | 0.204 |
| Alanine aminotransferase (U/L) | 29.0 (20.0–53.5) | 41.5 (23.8–64.5) | 25.0 (16.0–46.5) | 1.869 | 0.062 | 29.0 (19.0–75.5) | 43.0 (26.5–96.5) | 25.0 (16.3–57.5) | 2.061 | 0.039 |
| Aspartate aminotransferase (U/L) | 41.0 (29.8–63.3) | 43.5 (30.8–79.3) | 40.5 (27.5–57.3) | 0.822 | 0.411 | 41.0 (30.5–77.0) | 62.0 (36.0–127.0) | 40.0 (28.3–58.8) | 1.686 | 0.092 |
| Total bilirubin (μmol/L) | 13.6 (9.0–22.9) | 14.9 (11.4–25.8) | 11.5 (7.1–18.7) | 1.778 | 0.075 | 13.5 (9.4–21.9) | 18.4 (10.5–25.3) | 12.5 (7.8–17.9) | 2.025 | 0.043 |
| Blood urea nitrogen (mmol/L) | 6.7 (4.7–11.0) | 7.9 (6.3–11.4) | 5.9 (4.6–8.8) | 1.677 | 0.094 | 7.3 (5.7–13.7) | 7.3 (6.3–13.2) | 7.3 (5.1–16.1) | 0.193 | 0.847 |
| Creatinine (μmol/L) | 75.7 (60.6–108.2) | 81.3 (62.4–112.9) | 70.9 (57.4–90.9) | 0.968 | 0.333 | 76.3 (57.4–117.6) | 77.5 (63.3–113.0) | 70.4 (54.5–121.0) | 0.494 | 0.621 |
| Procalcitonin (ng/mL) | 0.36 (0.11–1.50) | 0.35 (0.19–1.89) | 0.36 (0.07–1.15) | 0.730 | 0.465 | 0.74 (0.16–2.13) | 0.55 (0.32–2.04) | 0.77 (0.07–3.22) | 0.527 | 0.625 |
| IL-6 (pg/mL) | 82.7 (20.6–203.3) | 94.1 (51.5–281.2) | 68.5 (18.2–204.3) | 0.608 | 0.564 | NA | NA | NA | NA | NA |
| Murray LIS | 3.25 (3.00–3.50) | 3.29 (3.0–3.5.0) | 3.25 (3.00–3.5.0) | 0.479 | 0.632 | NA | NA | NA | NA | NA |
| Bilateral involvement of chest radiographs | 46 (100.0) | 18 (100.0) | 28 (100.0) | 0.000 | 1.000 | NA | NA | NA | NA | NA |
| APACHE II score | 19.0 (14.8–25.0) | 19.0 (13.5–22.3) | 20.0 (15.0–25.0) | 0.654 | 0.513 | NA | NA | NA | NA | NA |
| SOFA score | 7.0 (5.0–9.0) | 7.0 (5.0–8.5) | 7.0 (4.3–9.0) | 0.329 | 0.742 | 7.5 (5.0–9.0) | 8.0 (7.0–9.0) | 6.5 (4.0–9.0) | 1.248 | 0.212 |
Data expressed as median (interquartile range) and n (%).
APACHE II: Acute Physiology and Chronic Health Evaluation II; ARDS: Acute respiratory distress syndrome; COVID-19: Coronavirus disease 2019; FiO2: Fraction of inspired oxygen; ICU: Intensive care unit; IL-6: Interleukin-6; LIS: Lung injury score; MAP: Mean arterial pressure; NA: Not available; PaCO2: Partial pressure of carbon dioxide; PaO2: Partial pressure of arterial oxygen; SOFA: Sequential Organ Failure Assessment.
Compare to non-surviving patients at 28 days after ICU admission, the survivors had similar vital signs (including HR, RR, and mean arterial pressure[MAP]) and APACHE II and SOFA scores, both at the time of ICU admission and intubation. In laboratory findings, the differences between the survival and non-survival groups were minimal, except alanine aminotransferase (25.0 [16.3–57.5] U/L vs. 43.0 [26.5–96.5] U/L, P = 0.039) and total bilirubin (12.5 [7.8–17.9] μmol/L vs. 18.4 [10.5–25.3] μmol/L, P = 0.043) that at the timing of intubation were lower in survivors [Table 3].
Main interventions and organ dysfunctions
Thirty (65.2%) patients received HFNC, and 26 (56.5%) individuals were on NIV before intubation. The length of HFNC and NIV before intubation was 53 (IQR: 0–180) h, and the time from hospital admission to intubation was 4.0 (IQR: 1.5–9.5) days. After intubation, the initial IMV settings were FiO2 75% (IQR: 50–100%), tidal volume (Vt) 6.0 (IQR: 5.8–6.0) mL/kg of ideal body weight, and PEEP 10 (IQR: 8–10) cmH2O. The median PaO2/FiO2 ratio after intubation was 113.0 (IQR: 83.9–177.7) mmHg, and the static compliance (Cstat) after intubation was 28 (IQR: 17–36) mL/cmH2O. The total length of IMV was 12.0 (IQR: 6.0–27.3) days. During the IMV period, 35 (76.1%) patients received NMBAs, 28.0 (60.9%) received prone positioning, and 11 (23.9%) received ECMO [Table 4].
Table 4.
Main interventions and organ dysfunctions in patients with severe COVID-19 and ARDS.
| Items | Total (n = 46) | Non-survival (n = 18) | Survival (n = 28) | t/χ2/Z | P-value |
|---|---|---|---|---|---|
| HFNC before IMV | 30 (65.2) | 13 (72.2) | 17 (60.7) | 0.640 | 0.424 |
| NIV before IMV | 26 (56.5) | 10 (55.6) | 16 (57.1) | 0.011 | 0.916 |
| Interval between hospital admission and mechanical ventilation (days) | 4.0 (1.5–9.5) | 8.0 (2.5–12.0) | 4.0 (1.0–7.0) | 1.511 | 0.131 |
| Total length of HFNC + NIV before IMV (h) | 53 (0–180) | 72 (0–180) | 48 (0–200) | 0.213 | 0.831 |
| Total length of mechanical ventilation (days) | 12.0 (6.0–27.3) | 9.5 (6.5–12.3) | 16.5 (6.0–32.8) | 1.714 | 0.087 |
| Vt after intubation (mL/kg) | 6.00 (5.8–6.0) | 6.00 (5.9–6.0) | 6.00 (5.8–6.0) | 0.000 | 1.000 |
| FiO2 after intubation (%) | 75.0 (50.0–100.0) | 85.0 (57.5–100.0) | 75.0 (46.3–100.0) | 0.854 | 0.393 |
| PaO2/FiO2 ratio after intubation (mmHg) | 113.0 (83.9–177.7) | 101.5 (89.8–153.8) | 128.6 (71.8–192.8) | 0.984 | 0.325 |
| PEEP after intubation (cmH2O) | 10 (8–10) | 10 (7–10) | 10 (8–10) | 0.327 | 0.744 |
| Cstat after intubation (mL/cmH2O) | 28 (17–36) | 22 (17–34) | 29 (17–40) | 1.422 | 0.162 |
| NMBAs | 35 (76.1) | 14 (77.8) | 21 (75.0) | 0.046 | 0.829 |
| Prone position ventilation | 28 (60.9) | 9 (50.0) | 19 (67.9) | 1.467 | 0.226 |
| ECMO | 11 (23.9) | 3 (16.6) | 8 (28.6) | 1.963 | 0.161 |
| Respiratory support decision score | 11.0 (7.8–16.0) | 8.5 (6.0–10.3) | 15.0 (10.3–17.0) | 3.366 | 0.001 |
| Medication | |||||
| Lopinavir/ritonavir (Kaletra) | 4 (8.7) | 2 (11.1) | 2 (7.1) | 0.217 | 0.641 |
| Umifenovir (Arbidol) | 11 (23.9) | 3 (16.7) | 8 (28.6) | 0.853 | 0.356 |
| Glucocorticoid therapy | 37 (80.4) | 15 (83.3) | 22 (78.6) | 0.158 | 0.691 |
| Anti-Gram-negative bacteria drug | 44 (95.7) | 18 (100.0) | 26 (92.9) | 1.344 | 0.246 |
| Anti-Gram-positive bacteria drug | 43 (93.5) | 18 (100.0) | 25 (89.3) | 2.063 | 0.151 |
| Antifungal drug | 18 (39.1) | 4 (22.2) | 14 (50.0) | 3.549 | 0.060 |
| Complications | |||||
| Shock | 42 (91.3) | 18 (100.0) | 24 (85.7) | 2.816 | 0.093 |
| Acute cardiac injury | 23 (50.0) | 10 (55.6) | 13 (46.4) | 0.365 | 0.564 |
| Arrhythmia | 25 (54.3) | 10 (55.6) | 15 (53.6) | 0.017 | 0.895 |
| AKI | 17 (37.0) | 7 (38.9) | 10 (35.7) | 0.047 | 0.828 |
Data expressed as median (interquartile range) and n (%).
AKI: Acute kidney injury; ARDS: Acute respiratory distress syndrome; COVID-19: Coronavirus disease 2019; Cstat: Static compliance; ECMO: Extracorporeal membrane oxygenation; FiO2: Fraction of inspired oxygen; HFNC: High-flow nasal cannula; IMV: Invasive mechanical ventilation; NIV: Non-invasive ventilation; NMBAs: Neuromuscular blocking agents; PaCO2: Partial pressure of carbon dioxide; PaO2: Partial pressure of arterial oxygen; PEEP: Positive end-expiratory pressure; Vt: Tidal volume.
Only a small proportion of patients received antiviral therapy for SARS-CoV-2 (umifenovir, 11 [23.9%]; lopinavir/ritonavir, 4 [8.7%]) following ICU admission, and nearly all patients received antibacterial therapy (coverage against Gram-negative bacteria, 44 [95.7%]; Gram-positive bacteria, 43 [93.5%]; and fungal infection, 18 [39.1%]). Glucocorticoid therapy was used in 37 [80.4%] patients [Table 4].
The most common complications during the illness period included shock (42 [91.3%]), arrhythmia (25 [54.3%]), acute cardiac injury (23 [50%]), and acute kidney injury (AKI) (17 [37.0%]) [Table 4].
Compare to non-survivors at 28-day after ICU admission, survivors had the following non-significant trends: (1) a shorter time on HFNC and NIV (48 [0–200] h vs. 72h [0–180] h, P = 0.831); (2) a shorter time interval between hospital admission and IMV initiation (4.0[1.0-7.0] days vs. 8 [2.5–12.0] days, P = 0.131); and (3) a higher static lung compliance after intubation (29[17-40] mL/cmH2O vs. 22[17-34] mL/cmH2O , P = 0.162) [Table 4].
Respiratory support decision score
Among all included patients, the median respiratory support decision score was 11.0 (IQR, 7.8–16.0). At 28 daysafter ICU admission, the decision score was significantly higher in survival group than non-survival group (15.0 [10.3–17.0] vs. 8.5 (6.0–10.3), P = 0.001) [Table 4]. Based on univariate logistic regression analysis, a higher respiratory support decision score was associated with lower 28 d-ICU (odds ratio [OR]: 0.763; 95% confidence interval [CI]: 0.640–0.909, P = 0.002) [Table 5].
Table 5.
Risk factors associated with all-cause 28d-ICU mortality in patients with severe COVID-19 and ARDS according to univariate logistic regression analysis.
| Risk factors | OR | 95% CI | P-value |
|---|---|---|---|
| Age (years) | 1.012 | 0.970–1.057 | 0.573 |
| Murray LIS | 1.365 | 0.314–5.927 | 0.678 |
| ICU admission | |||
| White blood cell count (× 109/L) | 1.035 | 0.947–1.131 | 0.447 |
| Leukocyte count (× 109/L) | 0.597 | 0.131–2.710 | 0.504 |
| Platelets count (× 109/L) | 0.997 | 0.990–1.004 | 0.360 |
| Prothrombin time (s) | 0.975 | 0.812–1.172 | 0.790 |
| d-dimer (mg/L) | 1.000 | 1.000–1.000 | 0.307 |
| Hypersensitive troponin I (pg/mL) | 0.999 | 0.997–1.001 | 0.290 |
| Creatine kinase-MB (U/L) | 1.016 | 0.994–1.038 | 0.159 |
| Lactate dehydrogenase (U/L) | 1.002 | 0.999–1.005 | 0.259 |
| Alanine aminotransferase (U/L) | 1.003 | 0.996–1.010 | 0.338 |
| Aspartate aminotransferase (U/L) | 1.002 | 0.997–1.007 | 0.363 |
| Bilirubin (μmol/L) | 1.050 | 0.989–1.115 | 0.108 |
| After intubation | |||
| White blood cell count (× 109/L) | 1.047 | 0.950–1.154 | 0.353 |
| Leukocyte count (× 109/L) | 0.373 | 0.092–0.151 | 0.167 |
| Platelets count (× 109/L) | 0.994 | 0.986–1.002 | 0.144 |
| Prothrombin time (s) | 01.288 | 0.947–1.752 | 0.106 |
| Hypersensitive troponin I (pg/mL) | 0.999 | 0.997–1.001 | 0.174 |
| Bilirubin (μmol/L) | 1.060 | 0.994–1.129 | 0.076 |
| Acute cardiac injury | 1.442 | 0.439–4.741 | 0.546 |
| Arrhythmia | 1.083 | 0.330–3.561 | 0.895 |
| AKI | 1.145 | 0.337–3.891 | 0.828 |
| Glucocorticoid therapy | 1.364 | 0.294–6.319 | 0.692 |
| Interval between hospital admission and mechanical ventilation (days) | 1.051 | 0.973–1.136 | 0.209 |
| Total length of HFNC + NIV before IMV (days) | 0.999 | 0.994–1.004 | 0.604 |
| Respiratory support decision score | |||
| Code C | 0.212 | 0.059–0.760 | 0.017 |
| Code H | 0.136 | 0.036–0.517 | 0.003 |
| Code I | 0.214 | 0.059–0.775 | 0.019 |
| Code J | 0.212 | 0.059–0.760 | 0.017 |
| Code N | 0.125 | 0.024–0.648 | 0.013 |
| Code O | 0.255 | 0.073–0.891 | 0.032 |
| Code P | 0.174 | 0.045–0.665 | 0.011 |
| Code R | 3.680 | 0.962–14.075 | 0.057 |
| Code S | 5.000 | 0.852–29.349 | 0.075 |
| Total decision score | 0.763 | 0.640–0.909 | 0.002 |
28 d-ICU: 28-day mortality after ICU admission; AKI: Acute kidney injury; ARDS: Acute respiratory distress syndrome; CI: Confidence interval; COVID-19: Coronavirus disease 2019; HFNC: High-flow nasal cannula; ICU: Intensive care unit; IMV: Invasive mechanical ventilation; LIS: Lung injury score; NIV: Non-invasive ventilation; OR: Odds ratio.
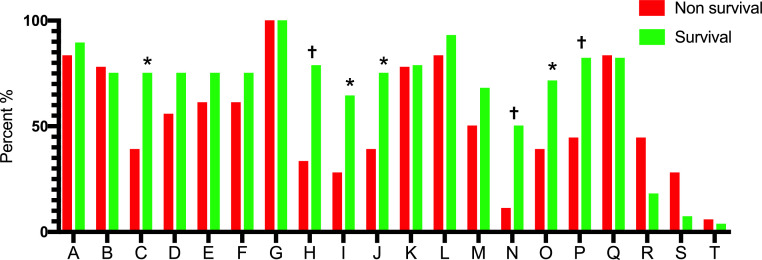
Code N (achieving static lungs compliance of >30 mL/cmH2O after 48 h of IMV by fine adjustment) was noted only in 16 (34.8%) patients, Code I (driving pressure <15 cmH2O within 24 h of IMV) was seen in 23 (50.0%) patients, and Code O (decision process for ECMO initiation) was observed in 27 (58.7%) patients. Code R (SO2 <80% lasts for >6 h during hospitalization) was present in 13 (28.3%) patients and associated with deduction in total score [Supplementary Table 1].
At 28 days after ICU admission, survival group had a significantly higher proportion of Code C (HFNC oxygen concentration and intubation timing, 75.0% vs. 38.9%, P = 0.014); Code H (Plat pressure [Pplat] ≤ 30 cmH2O within 24 h of IMV, 78.6% vs. 33.3%, P = 0.002); Code I (driving pressure <15 cmH2O within 24 h of IMV, 64.3% vs. 27.8%, P = 0.016); Code J (using of NMBAs, 75.0% vs. 38.9%, P = 0.014); Code N (achieved Cstat of lungs >30 mL/cmH2O after 48 h IMV by fine adjustment, 50.0% vs. 11.1%, P = 0.007); Code O (avoid ECMO or decision of initiating ECMO in time, 71.4% vs. 38.9%, P = 0.029); and Code P (avoid ECMO or make right super protective ventilation strategy during ECMO, 82.1% vs. 44.4%, P = 0.008) achievement than the non-survivorsal group [Figure 2; Supplementary Table 1]. Based on the univariate logistic regression analysis, the 28 d-ICU was lower when the following codes were met (Code C: OR = 0.212, 95% CI: 0.059–0.760, P = 0.017; Code H: OR = 0.136, 95% CI: 0.036–0.517, P = 0.003; Code I: OR = 0.214, 95% CI: 0.059–0.775, P = 0.019; Code J: OR = 0.212, 95% CI: 0.059–0.760, P = 0.017; Code N: OR = 0.125, 95% CI: 0.024–0.648, P = 0.013; Code O: OR: 0.255, 95% CI = 0.073–0.891, P = 0.032; cCode P: OR = 0.174, 95% CI: 0.045–0.665, P = 0.011) [Table 5].
Figure 2.
The completed proportion of each respiratory support decision item in IMV patients with severe COVID-19 and ARDS.
A-T as per the Code A-T in Table 1.
ARDS: Acute respiratory distress syndrome; COVID-19: Coronavirus disease 2019; IMV: Invasive mechanical ventilation.
Compared with non-survival, *P < 0.05, †P < 0.01.
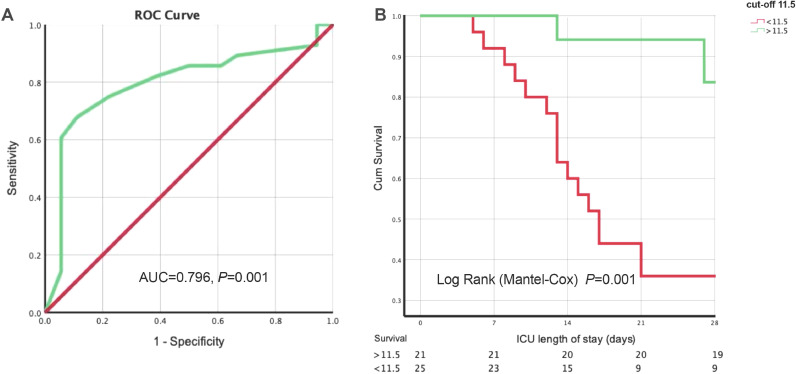
Using the ROC curve to assess the performance of respiratory support decision score in prediction of 28 d-ICU, the AUC was 0.796 (95% CI: 0.657–0.934, P = 0.001) and the cut-off was 11.5 (sensitivity = 0.679, specificity = 0.889) [Figure 3A]. We divided the entire cohort into two groups based on the respiratory support decision score cut-off of 11.5 (>11.5 group included 21 patients, and <11.5 group included 25 patients). The >11.5 group had lower 28 d-ICU than the <11.5 group (9.5% vs. 64.0%, P < 0.001), but had non-significant differences in clinical characteristics (age, APACHE II, SOFA, and LIS) and intervention (total length of HFNC + NIV, total length of IMV, interval between hospital admission, and IMV) [Supplementary Table 2]. Patients with a higher score (>11.5) were more likely to survive 28 days after ICU admission (log-rank test, P < 0.001) than those with a lower score (<11.5) [Figure 3B].
Figure 3.
ROC curve of respiratory support decision score to 28-day mortality and surviving analysis with different decision score range (<11.5 and >11.5). A: ROC curve. The AUC was 0.796 (95% CI: 0.657–0.934, P = 0.001) and the cut-off was 11.5 (sensitivity = 0.679, specificity = 0.889). B: Surviving analysis. Using the log-rank test, patients with a higher score (>11.5) had a higher proportion of survival than those with a score of <11.5 (P = 0.001).
AUC: Area under the curve; CI: Confidence interval; ROC: Receiver operating characteristic.
Discussion
In this retrospective study, we assessed the quality of implementation of a respiratory support decision system and provided an assessment score for evaluating such quality in patients with COVID-19-associated ARDS who required IMV. For these patients, compliance with the respiratory support decision and implementation of the assessment score was associated with improvement in the ventilation process.
IMV is usually considered as a supportive management strategy and is often life-saving for patients with respiratory failure. However, the risk associated with IMV, especially ventilator-induced lung injury (VILI)[[26], [27], [28]] and right heart strain resulting from acute cor-pulmonale secondary to ARDS[29,30] should be considered.
In this study, we reported the implementation of a respiratory support decision system based on the current level of evidence and best practices for the management of respiratory failure in COVID-19-associated ARDS.[[31], [32], [33]] IMV should be implemented as a rapid and efficient respiratory support method for life-threatening respiratory failure. Protective ventilation strategy including low Vt (4–8 mL/kg),[34,35] restricted plateau pressure (≤30 cmH2O),[36] a driving pressure (<15 cmH2O), and appropriate PEEP for the static lung compliance was applied in the IMV management to minimize VILI among patients enrolled in our study.[37] Alveolar salvage therapies, the use of NMBAs,[38] and prone positioning[[39], [40], [41]] should be considered for the patients with PaO2/FiO2 ≤ 150 mmHg and in the early stages of ARDS. Because RM may directly over distend aerated lung units and could, paradoxically, lead to increased VILI or alter hemodynamic status, the use of RM should be only limited to appropriate cases among ARDS patients.[42] In a randomized clinical trial, the authors showed that in patients with moderate-to-severe ARDS, the 28-day all-cause mortality was higher in lung recruitment and titrated PEEP group in comparison with the low PEEP group.[43] Assessment of lung recruitability should be performed before RM, so RM and titrated PEEP can only be used among those with recruitment potential. Extracorporeal lung support therapies are also viable options to protect the lungs, while the key question is how to balance the trade-offs between patient self-inflicted lung injury (P-SILI), the complications of extracorporeal circuits, and the side effects of IMV.[6] When the optimal lung-protective strategy and alveolar salvage therapy are found ineffective, ECMO should be considered.[44]
The prolonged use of HFNC or NIV in the presence of strong respiratory drive may lead to P-SILI among COVID-19-associated ARDS patients.[45] Frat et al.[19] found that under standard oxygen therapy, patients with a RR ≥30 breaths/min were more likely to need intubation than patients with a RR <30 breaths/min. For NIV, a PaO2/FiO2 ratio <200 mmHg and a TV of >9 mL/kg of predicted body weight were independent predictors of intubation.[19]
To assess compliance with the respiratory support decision system, an evaluation score tool was designed based on respiratory support and cardiopulmonary protection principles. The items in the tool were chosen to assess the effect of different oxygen supplement therapies, controlling respiratory drive, intubation timing, and initiation of ECMO. Limitations in resources may delay the implementation of the respiratory support decision system and its assessment, as observed in our study (6–12 h delay in the implementation of the support system).
A large number of patients achieved Codes A (87.0%), B (76.1%), D (67.4%), E (69.6%), F (69.6%), G (100%), K (78.3%), L (89.1%), and Q (82.6%), and there were no differences between the survivors and non-survivors [Figure 2; Supplementary Table 1]. This may indicate that clinical providers considered these codes as clinically critical. While nearly 40% of patients were at risk of acute cor-pulmonale due to hypercarbia (Code M),[44] it was not an independent risk factor for death in this cohort. The proportion of patients who achieved Codes C (60.9%), H (60.9%), I (50.0%), J (60.9%), N (34.8%), O (58.7%), and P (67.4%) were relatively low in all the patients, and yet there were statistical differences between the survivors and non-survivors [Figure 2; Supplementary Table 1; Table 5]. Maintaining SpO2 by using high oxygen concentration (FiO2 > 0.7) may lead to hyperoxic acute lung injury[46] (Code C). In a recent study, in patients with ARDS, early exposure to a conservative-oxygenation strategy with an SPO2 between 88% and 92% did not increase the 28 d-ICU, when compared with maintaining the SPO2 ≥ 96%.[47] In the lung-protective mechanical ventilation strategy, although low TV ventilation was implemented for all the patients, the plateau pressure was >30 cmH2O (Code H) in 50% of patients, and >60% of the patients had driving pressure >15 cmH2O (Code I). In other studies, similar relationships have been reported.[48,49] Only 34.8% of patients had lung compliance of >30 mL/cmH2O after 48 h of IMV (Code N). As the median PaO2/FiO2 ratio of these patients was only 113 (IQR: 83.9–177.7) mmHg, all patients with a PaO2/FiO2 ratio <150 mmHg received NMBAs, yet 39.1% of the cases did not achieve Code J due to late initiation of NMBAs. In patients with poor lung compliance, conventional alveolar salvage therapy did not protect the lungs; thus, initiating ECMO (Code O) and resting lung during ECMO (Code P) were found to be independently associated with a lower risk of death.
In this research, we used three codes (i.e., R, S, and T) that were deducted from the total score. Thirteen patients (five survivors and eight non-survivors) had a history of SO2 < 80% for >6 h during hospitalization, and seven patients (two survivors and five non-survivors) had non-effective RM or improper PEEP level during IMV. A randomized clinical trial showed that in patients with moderate-to-severe ARDS, a strategy with lung recruitment and titrated PEEP compared with low PEEP increased the 28-day all-cause mortality.[43] The absence of statistical differences in our study might be because of the small sample size.
As shown in Table 2, Table 3, Table 4, Table 5, there were no differences in most parameters between survivors and non-survivors except for some respiratory decision support system scores. The patients included in this study, regardless of survival status, were generally older and critically ill when admitted to ICU. As shown in Table 3, all patients had a Murray LIS of >3, with median APACHE II and SOFA scores of 20 and 7, respectively, for survivors, and median APACHE II and SOFA scored of 19 and 7 for non-survivors, respectively. In the early period of the pandemic, uncertainty and non-standard treatment were common; therefore, the completion of some critical details in the respiratory decision support system was crucially important and might be able to influence patient's clinical outcomes.
According to the ROC curve and respiratory support decision score with a cut-off of 11.5 as an independent risk factor of the 28 d-ICU, complying with the protocolized respiratory support decision system process to improve the quality of respiratory support could potentially reduce the risk of death among patients with COVID-19-associated ARDS who require IMV, particularly in resource-limited settings.
Our study has some limitations. This was a single-center retrospective study with a small sample size, which may limit its generalizability and conclusions. For the same reason, we are not able to imply any causal relationship as our findings are solely associations. Hence, prospective studies with large sample sizes are required to verify our results. Because SARS-CoV-2 is a relatively new virus, some items of our standard scoring system were mostly based on experts’ opinions and lacked evidence-based medical support.
Conclusions
For patients with COVID-19-associated ARDS who required IMV, complying with and implementing the respiratory support decision system can likely improve the ventilation process. The mortality at 28 days after ICU admission was lower with a decision score of >11.5 than with a decision score <11.5.
Funding
This work was supported by the Chinese Medical Information and Big Data Association (Bo Hu, No. Z-2019-1-003) and by the Translational Medicine and Interdisciplinary Research Joint Fund of Zhongnan Hospital of Wuhan University (Bo Hu, No. ZNJC202011), and the key project of the Ministry of Science and Technology of China (Zhiyong Peng, No. 2020YFC0841300).
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
None.
Managing Editor: Jingling Bao
Footnotes
Given his role as Editorial Board Member, Prof. Kianoush B. Kashani had no involvement in the peer-review of this article and has no access to information regarding its peer-review. Prof. Dechang Chen who is the co-editor-in-chief took the responsibility for peer-review progress and made the final decision.
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jointm.2021.12.003.
Contributor Information
Bo Hu, Email: hobbier1979@163.com.
Zhiyong Peng, Email: pengzy5@hotmail.com.
Appendix. Supplementary materials
References
- 1.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grasselli G., Zangrillo A., Zanella A., Antonelli M., Cabrini L., Castelli A., et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323(16):1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H., et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Richardson S., Hirsch J.S., Narasimhan M., Crawford J.M., McGinn T., Davidson K.W., et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area. JAMA. 2020;323(20):2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brochard L., Slutsky A., Pesenti A. Mechanical ventilation to minimize progression of lung injury in acute respiratory failure. Am J Respir Crit Care Med. 2017;195(4):438–442. doi: 10.1164/rccm.201605-1081CP. [DOI] [PubMed] [Google Scholar]
- 7.Spinelli E., Mauri T., Beitler J.R., Pesenti A., Brodie D. Respiratory drive in the acute respiratory distress syndrome: pathophysiology, monitoring, and therapeutic interventions. Intensive Care Med. 2020;46(4):606–618. doi: 10.1007/s00134-020-05942-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yoshida T., Amato M., Kavanagh B.P., Fujino Y. Impact of spontaneous breathing during mechanical ventilation in acute respiratory distress syndrome. Curr Opin Crit Care. 2019;25(2):192–198. doi: 10.1097/MCC.0000000000000597. [DOI] [PubMed] [Google Scholar]
- 9.Schmidt M., Banzett R.B., Raux M., Morélot-Panzini C., Dangers L., Similowski T., et al. Unrecognized suffering in the ICU: addressing dyspnea in mechanically ventilated patients. Intensive Care Med. 2014;40(1):1–10. doi: 10.1007/s00134-013-3117-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.François B., Cariou A., Clere-Jehl R., Dequin P.F., Renon-Carron F., Daix T., et al. Prevention of early ventilator-associated pneumonia after cardiac arrest. N Engl J Med. 2019;381(19):1831–1842. doi: 10.1056/NEJMoa1812379. [DOI] [PubMed] [Google Scholar]
- 11.Dondorp A.M., Hayat M., Aryal D., Beane A., Schultz M.J. Respiratory support in COVID-19 patients, with a focus on resource-limited settings. Am J Trop Med Hyg. 2020;102(6):1191–1197. doi: 10.4269/ajtmh.20-0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levy M.M., Evans L.E., Rhodes A. The surviving sepsis campaign bundle: 2018 update. Crit Care Med. 2018;46(6):997–1000. doi: 10.1097/CCM.0000000000003119. [DOI] [PubMed] [Google Scholar]
- 13.Griffiths M., McAuley D.F., Perkins G.D., Barrett N., Blackwood B., Boyle A., et al. Guidelines on the management of acute respiratory distress syndrome. BMJ Open Respir Res. 2019;6(1) doi: 10.1136/bmjresp-2019-000420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fan E., Del Sorbo L., Goligher E.C., Hodgson C.L., Munshi L., Walkey A.J., et al. An official american thoracic society/European society of intensive care medicine/society of critical care medicine clinical practice guideline: mechanical ventilation in adult patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2017;195(9):1253–1263. doi: 10.1164/rccm.201703-0548ST. [DOI] [PubMed] [Google Scholar]
- 15.Murray J.F., Matthay M.A., Luce J.M., Flick M.R. An expanded definition of the adult respiratory distress syndrome. Am Rev Respir Dis. 1988;138(3):720–723. doi: 10.1164/ajrccm/138.3.720. [DOI] [PubMed] [Google Scholar]
- 16.Ranieri V.M., Rubenfeld G.D., Thompson B.T., Ferguson N.D., Caldwell E., Fan E., et al. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307(23):2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 17.Alhazzani W., Møller M.H., Arabi Y.M., Loeb M., Gong M.N., Fan E., et al. Surviving sepsis campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19) Intensive Care Med. 2020;46(5):854–887. doi: 10.1007/s00134-020-06022-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roca O., Caralt B., Messika J., Samper M., Sztrymf B., Hernández G., et al. An index combining respiratory rate and oxygenation to predict outcome of nasal high-flow therapy. Am J Respir Crit Care Med. 2019;199(11):1368–1376. doi: 10.1164/rccm.201803-0589OC. [DOI] [PubMed] [Google Scholar]
- 19.Frat J.P., Ragot S., Coudroy R., Constantin J.M., Girault C., Prat G., et al. Predictors of intubation in patients with acute hypoxemic respiratory failure treated with a noninvasive oxygenation strategy. Crit Care Med. 2018;46(2):208–215. doi: 10.1097/CCM.0000000000002818. [DOI] [PubMed] [Google Scholar]
- 20.World Health Organization . World Health Organization; 2020. Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected: interim guidance, 13 March 2020. [Google Scholar]; https://apps.who.int/iris/handle/10665/331446.
- 21.Alhazzani W., Møller M.H., Arabi Y.M., Loeb M., Gong M.N., Fan E., et al. Surviving sepsis campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19) Intensive Care Med. 2020;46(5):854–887. doi: 10.1007/s00134-020-06022-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brodie D., Slutsky A.S., Combes A. Extracorporeal life support for adults with respiratory failure and related indications: a review. JAMA. 2019;322(6):557–568. doi: 10.1001/jama.2019.9302. [DOI] [PubMed] [Google Scholar]
- 23.van Diepen S., Katz J.N., Albert N.M., Henry T.D., Jacobs A.K., Kapur N.K., et al. Contemporary management of cardiogenic shock: a scientific statement from the American Heart Association. Circulation. 2017;136(16):e232–e268. doi: 10.1161/CIR.0000000000000525. [DOI] [PubMed] [Google Scholar]
- 24.Brooks S.C., Anderson M.L., Bruder E., Daya M.R., Gaffney A., Otto C.W., et al. Part 6: alternative techniques and ancillary devices for cardiopulmonary resuscitation: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2015;132(18 Suppl 2):S436–S443. doi: 10.1161/CIR.0000000000000260. [DOI] [PubMed] [Google Scholar]
- 25.Dondorp A.M., Hayat M., Aryal D., Beane A., Schultz M.J. Respiratory support in COVID-19 patients, with a focus on resource-limited settings. Am J Trop Med Hyg. 2020;102(6):1191–1197. doi: 10.4269/ajtmh.20-0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tremblay L., Valenza F., Ribeiro S.P., Li J., Slutsky A.S. Injurious ventilatory strategies increase cytokines and c-fos m-RNA expression in an isolated rat lung model. J Clin Invest. 1997;99(5):944–952. doi: 10.1172/JCI119259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dreyfuss D., Saumon G. Ventilator-induced lung injury: lessons from experimental studies. Am J Respir Crit Care Med. 1998;157(1):294–323. doi: 10.1164/ajrccm.157.1.9604014. [DOI] [PubMed] [Google Scholar]
- 28.Slutsky A.S., Ranieri V.M. Ventilator-induced lung injury. N Engl J Med. 2013;369(22):2126–2136. doi: 10.1056/NEJMra1208707. [DOI] [PubMed] [Google Scholar]
- 29.Moloney E.D., Evans T.W. Pathophysiology and pharmacological treatment of pulmonary hypertension in acute respiratory distress syndrome. Eur Respir J. 2003;21(4):720–727. doi: 10.1183/09031936.03.00120102. [DOI] [PubMed] [Google Scholar]
- 30.Mekontso Dessap A., Boissier F., Charron C., Bégot E., Repessé X., Legras A., et al. Acute cor pulmonale during protective ventilation for acute respiratory distress syndrome: prevalence, predictors, and clinical impact. Intensive Care Med. 2016;42(5):862–870. doi: 10.1007/s00134-015-4141-2. [DOI] [PubMed] [Google Scholar]
- 31.Price S., Singh S., Ledot S., Bianchi P., Hind M., Tavazzi G., et al. Respiratory management in severe acute respiratory syndrome coronavirus 2 infection. Eur Heart J Acute Cardiovasc Care. 2020;9(3):229–238. doi: 10.1177/2048872620924613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilcox S.R. Management of respiratory failure due to COVID-19. BMJ. 2020;369:m1786. doi: 10.1136/bmj.m1786. [DOI] [PubMed] [Google Scholar]
- 33.Marini J.J., Gattinoni L. Management of COVID-19 respiratory distress. JAMA. 2020;323(22):2329–2330. doi: 10.1001/jama.2020.6825. [DOI] [PubMed] [Google Scholar]
- 34.Brower R.G., Matthay M.A., Morris A., Schoenfeld D., Thompson B.T., Wheeler A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 35.Walkey A.J., Goligher E.C., Del Sorbo L., Hodgson C.L., Adhikari N., Wunsch H., et al. Low tidal volume versus non-volume-limited strategies for patients with acute respiratory distress syndrome. A systematic review and meta-analysis. Ann Am Thorac Soc. 2017;14(Supplement_4):S271–S279. doi: 10.1513/AnnalsATS.201704-337OT. [DOI] [PubMed] [Google Scholar]
- 36.Laffey J.G., Bellani G., Pham T., Fan E., Madotto F., Bajwa E.K., et al. Potentially modifiable factors contributing to outcome from acute respiratory distress syndrome: the LUNG SAFE study. Intensive Care Med 2016;42(12):1865–76. doi: 10.1007/s00134-016-4571-5. [DOI] [PubMed]
- 37.Vieillard-Baron A., Matthay M., Teboul J.L., Bein T., Schultz M., Magder S., et al. Experts’ opinion on management of hemodynamics in ARDS patients: focus on the effects of mechanical ventilation. Intensive Care Med 2016;42(5):739–49. doi: 10.1007/s00134-016-4326-3. [DOI] [PubMed]
- 38.Papazian L., Forel J.M., Gacouin A., Penot-Ragon C., Perrin G., Loundou A., et al. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med. 2010;363(12):1107–1116. doi: 10.1056/NEJMoa1005372. [DOI] [PubMed] [Google Scholar]
- 39.Taccone P., Pesenti A., Latini R., Polli F., Vagginelli F., Mietto C., et al. Prone positioning in patients with moderate and severe acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2009;302(18):1977–1984. doi: 10.1001/jama.2009.1614. [DOI] [PubMed] [Google Scholar]
- 40.Munshi L., Del Sorbo L., Adhikari N., Hodgson C.L., Wunsch H., Meade M.O., et al. Prone position for acute respiratory distress syndrome. A systematic review and meta-analysis. Ann Am Thorac Soc. 2017;14(Supplement_4):S280–S288. doi: 10.1513/AnnalsATS.201704-343OT. [DOI] [PubMed] [Google Scholar]
- 41.Scholten E.L., Beitler J.R., Prisk G.K., Malhotra A. Treatment of ARDS with prone positioning. Chest. 2017;151(1):215–224. doi: 10.1016/j.chest.2016.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Constantin J.M., Grasso S., Chanques G., Aufort S., Futier E., Sebbane M., et al. Lung morphology predicts response to recruitment maneuver in patients with acute respiratory distress syndrome. Crit Care Med. 2010;38(4):1108–1117. doi: 10.1097/CCM.0b013e3181d451ec. [DOI] [PubMed] [Google Scholar]
- 43.Cavalcanti A.B., Suzumura É.A., Laranjeira L.N., Paisani D.M., Damiani L.P., Guimarães H.P., et al. Effect of lung recruitment and titrated positive end-expiratory pressure (PEEP) vs low PEEP on mortality in patients with acute respiratory distress syndrome: a randomized clinical trial. JAMA. 2017;318(14):1335–1345. doi: 10.1001/jama.2017.14171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Paternot A., Repessé X., Vieillard-Baron A. Rationale and description of right ventricle-protective ventilation in ARDS. Respir Care. 2016;61(10):1391–1396. doi: 10.4187/respcare.04943. [DOI] [PubMed] [Google Scholar]
- 45.Cinesi Gómez C., Peñuelas Rodríguez Ó., Luján Torné M., Egea Santaolalla C., Masa Jiménez J.F., García Fernández J., et al. Clinical consensus recommendations regarding non-invasive respiratory support in the adult patient with acute respiratory failure secondary to SARS-CoV-2 infection. Arch Bronconeumol. 2020;56:11–18. doi: 10.1016/j.arbres.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kallet R.H., Matthay M.A. Hyperoxic acute lung injury. Respir Care. 2013;58(1):123–141. doi: 10.4187/respcare.01963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barrot L., Asfar P., Mauny F., Winiszewski H., Montini F., Badie J., et al. Liberal or conservative oxygen therapy for acute respiratory distress syndrome. N Engl J Med. 2020;382(11):999–1008. doi: 10.1056/NEJMoa1916431. [DOI] [PubMed] [Google Scholar]
- 48.Chang S.Y., Dabbagh O., Gajic O., Patrawalla A., Elie M.C., Talmor D.S., et al. Contemporary ventilator management in patients with and at risk of ALI/ARDS. Respir Care. 2013;58(4):578–588. doi: 10.4187/respcare.01755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Newell C.P., Martin M.J., Richardson N., Bourdeaux C.P. Protective mechanical ventilation in United Kingdom critical care units: a multicentre audit. J Intensive Care Soc. 2017;18(2):106–112. doi: 10.1177/1751143716683712. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.