Abstract
Introduction:
To study metabolic signatures can be used to identify predictive biomarkers for a patient's therapeutic response.
Objectives:
We hypothesized that the characterization of a patients’ metabolic profile, utilizing one-dimensional nuclear magnetic resonance (1H-NMR), may predict a response to tocilizumab in patients with rheumatoid arthritis (RA).
Methods:
40 active RA patients meeting the 2010 ACR/EULAR classification criteria initiating treatment with tocilizumab were recruited. Clinical outcomes were determined at baseline, and after six and twelve months of treatment. EULAR response criteria at 6 and 12 months to categorize patients as responders and non-responders. Blood was collected at baseline and after six months of tocilizumab therapy. 1H-NMR was used to acquire a spectra of plasma samples. Chenomx NMR suite 8.5 was used for metabolite identification and quantification. SPSS v.27 and MetaboAnalyst 4.0 were used for statistical and pathway analysis.
Results:
Isobutyrate, 3-hydroxybutyrate, lysine, phenylalanine, sn-glycero-3-phosphocholine, tryptophan and tyrosine were significantly elevated in responders at the baseline. OPLS-DA at baseline partially discriminated between RA responders and non-responders. A multivariate diagnostic model showed that concentrations of 3-hydroxybutyrate and phenylalanine improved the ability to specifically predict responders classifying 77.1% of the patients correctly. At 6 months, levels of methylamine, sn-glycero-3-phosphocholine and tryptophan tended to still be low in non-responders.
Conclusion:
The relationship between plasma metabolic profiles and the clinical response to tocilizumab suggests that 1H-NMR may be a promising tool for RA therapy optimization. More studies are needed to determine if metabolic profiling can predict the response to biological therapies in RA patients.
Keywords: Metabolomic profiling, tocilizumab, therapeutic response, rheumatoid arthritis, NMR
1. INTRODUCTION
Our increasing understanding of the pathogenesis of rheumatoid arthritis (RA) has transformed the therapeutic options available for patients with this disease(McInnes & Schett, 2017). Therapy for RA was revolutionized two decades ago by the introduction of biologic agents, and specifically agents that target the inflammatory cytokine tumor necrosis factor (TNF)(Breedveld et al., 2006; Klareskog et al., 2004; Lipsky et al., 2000). Since the initial approval of etanercept, and shortly thereafter infliximab, three additional TNF-neutralizing antibodies (adalimumab, certolizumab pegol and golimumab) have been approved, as well as anti-T-cell (and/or dendritic cell) therapy, abatacept, the B-cell-depleting antibody, rituximab, and the IL-6 receptor antagonists, tocilizumab (TCZ) (Sebba et al., 2008) and sarilumab(Nam et al., 2017; Rein & Mueller, 2017).
Despite the obvious impact of biologic agents, studies reported that at least 20-40% of the patients were declared non-responders after starting a biologic therapy(Gavrilă et al., 2016; Greenberg et al., 2008). If remission is the goal, there is a clear lack of uniform success with current biologic agents. There could be several reasons for this, including the initiation of therapy too late in the course of a disease, an insufficient duration of the therapy, or an inappropriate biological therapy. If initiating RA therapy with the right biologic earlier in the course of the disease could help to reach the goal of remission, a greater effort should be made to develop tools used to employ 'personalized' medicine, which could be useful to accurately match a patient with the correct therapy.
IL-6 is found in serum and synovial fluid of patients with RA. IL-6 plays a critical role in the pathophysiology of RA via STAT3 pathway activation, including B-cell maturation and production of autoantibodies and Th17 differentiation (Yoshida & Tanaka, 2014). In addition, it contributes to joint destruction by inducing synovitis and osteoclast activation (Yoshida & Tanaka, 2014). IL-6 also regulates glucose and lipid metabolism, IL-6 stimulates glycolysis, lipolysis in adipose tissue, and induces β oxidation via AMPK (Lehrskov & Christensen, 2019). Since only around 35% of the patients achieve remission after 6 months of TCZ, (Huang et al., 2019), biomarkers to identify patients that will respond to TCZ would be of interest.
Given the complexity and heterogeneous nature of RA, it is unlikely that a single cytokine or biomarker will provide a sufficient distinction between patients who will or will not respond to a given drug(Burska et al., 2014). Global biomarker signatures may represent more appropriate approaches for improving RA patient treatment protocols and outcomes. Metabolomics is the science of identifying and quantifying the biochemical state of the body by products of metabolism. The fundamental rationale in metabolomics is that perturbations caused by a disease in a biological system will lead to correlated changes in the concentration of certain metabolites. Since they can be detected in different biofluids, metabolomics is a useful approach for identifying biomarkers and metabolic alterations in patients(Armitage & Ciborowski, 2017; Suman et al., 2018). Metabolomics could also help to differentiate between responders and non-responders in different treatments(Barnes et al., 2016; Sweeney et al., 2016; van Wietmarschen et al., 2012; Young et al., 2013). One-dimensional nuclear magnetic resonance (1H-NMR) allows the identification of polar metabolites in biofluids and tissue with less cost than mass spectrometry, and with very high reproducibility(Emwas, 2015).
The study of metabolic signatures could then be used to identify predictive biomarkers for patients’ treatment responses. The aim of this study is to explore whether pre-treatment plasma metabolomic profiling predicts outcome of TCZ in patients with RA.
2. METHODS
2.1. Subjects
Forty patients (35 women and 5 men) meeting the 2010 ACR/EULAR classification criteria(Aletaha et al., 2010), that initiated treatment with TCZ were recruited from the Rheumatology Outpatient Clinic of the Sant Pau Hospital. The study was approved by the Institutional Board Review (number IIBSP-TOC-2016) and patients signed an informational consent form. All the patients had moderate or high disease activity according to the disease activity score using the erythrocyte sedimentation rate (DAS28-ESR). Demographic and clinical data including the number of swollen joints (SJC), tender joints (TJC), the Health Activity Questionnaire (HAQ), and the DAS-28-ESR, were collected at the baseline, and after six months and 12 months of TCZ treatment (8 mg/kg of TCZ every 4 weeks). Only one patient did not have the 12 months follow up visit. Non-fasting blood samples were collected (at the baseline and 6 months into the TCZ treatment) in 10 mL BD Vacutainer® CPT™ Cell Preparation Tubes with Sodium Heparin tubes for plasma separation. After 30 minutes of incubation at room temperature, the tubes were centrifuged for 20 min at 1800×g. The plasma was transferred into 1.7mL tubes, immediately frozen, and stored at −80 °C until they were analyzed for metabolite quantification. ESR, C-reactive protein (CRP), anti-cyclic citrullinated peptide antibodies (anti-CCP), rheumatoid factor (RF), and interleukin (IL)-6 (soluble and receptor) were also quantified.
2.2. Clinical Outcomes
Patients were classified in good responders. moderate-responders and non-responders, according to EULAR criteria(van Riel & Renskers, 2016). EULAR response criteria classify individual patients as non-, moderate, or good responders, depending on the extent of change and the level of disease activity reached. Since we only had 5 men in our cohort and all were responders, we decided to analyze them separately. Even though prior reports have not found sex to be a biomarker for response to TCZ(Narváez et al., 2016), the metabolic profile between men and women is different(Mittelstrass et al., 2011). Other than that, we did not have missing data.
2.3. Sample Preparation for Metabolic Extraction
The samples were thawed at room temperature for 30 minutes, and subsequently 150 μL of each sample was transferred to a deep well plate. The plates were spun at 2000xg at 4°C for 1 min (Eppendorf 5804 R centrifuge, A-2-DWP rotor). Methanol was taken directly from a −20°C freezer and 750 μL was added into each well while shaking using an Agilent Bravo 96-channel liquid handling robot. The plates were shaken at 850 rpm, 12°C for 30 min (Eppendorf Thermomixer Comfort), and then centrifuged at 2250 x g, 4°C for 60 min. After that, 600 μL of the supernatant was transferred to a new deepwell plate with the Agilent Bravo and dried overnight (Labconco CentriVap lyophilizer set to 20°C). Each dried pellet was washed with 50 μL of methanol-d4, shaken at 850 rpm, 12°C for 10 min before drying again for 1 hr at 20°C. To finish, 200 μL of buffer (37.5 mM sodium phosphate pD 6.95, 0.02% w/v sodium azide, 0.747 mM TSP-d4) was added and it was shaken at 850 rpm, 20°C for 1 h. 180 μL of each sample was transferred to a 3 mm SampleJet NMR tube with a Bruker SamplePro Tube L robot.
2.4. 1H-NMR Acquisition, Processing, Metabolic Identification and Quantification
A 600 MHz Bruker Avance III spectrometer equipped with a cooled SampleJet and a 5 mm, room temperature BBI-probe was used for data acquisition. 1D 1H spectra were acquired using the pulse sequence ‘zgespe’, encompassing water suppression through excitation, sculpting, and including a perfect echo sequence. The acquisition time was 2.04s, the relaxation delay was of 2s, the receiver gain 181 and 128 scans were collected into 64k points. A 0.3Hz exponential line broadening was applied before Fourier transformation and zero filling to 128k points. Spectra were phased, baseline-corrected, and referenced to TSP-d4, all performed in TopSpin3.5pl7 (Bruker BioSpin). Samples in the SampleJet carousel were kept at 6°C before and after insertion in the magnet. A daily quality assurance procedure was performed before sample data acquisition, involving temperature calibration checks, shim and water suppression quality, and consistent quantification using a reference sample, all according to the Bruker In Vitro Diagnostics for research (IVDr) SOP.
The metabolites detected in the 1D 1H-NMR were identified and quantified using the software Chenomx NMR suite 8.5 professional (Chenomx Inc., Edmonton, Canada), which contains a library that matches compounds’ peaks according to the chemical shift. Metabolite concentration was normalized to the TSP-d4. Forty-seven metabolites were quantified and 2-phenylpropionate was removed because it was detected in less than 50% of the patients. Metabolite concentrations were reported in micromoles (μM). Metabolomic data was submitted at Metabolights (Haug et al., 2019) with the URL: www.ebi.ac.uk/metabolights/MTBLS3013,
2.5. Statistical Analysis
Metabolites were normalized by sum, log transformed and averaged, using MetaboAnalyst version 4.0, which is an open resource for metabolomics analysis(Chong et al., 2018; Chong et al., 2019). For predicting models that are based in algorithmic analysis, we used Partial Least Square Discriminant Analysis (PLS-DA), considered the gold standard for binary classification, to obtain the variable importance of projection and to determine the importance of individual metabolites in group discrimination(Mendez et al., 2019). We also used Orthogonal Partial Least Square Discriminant Analysis (OPLS-DA) to observe the difference between responders and non-responders. Pathway analysis was evaluated with the enrichment analysis tool available on MetaboAnalyst, using the set KEGG 2019, which contains 84 metabolite sets(Kanehisa et al., 2017).
Continuous variables were expressed as mean ± standard deviation (SD) and the categorical variables as a percentage. Differences between means for the continuous variables were assessed using paired and unpaired t-test analysis, differences between proportions using the chi-square test, and analysis of variance (ANOVA) Dunnett’s post hoc tests for multiple group comparisons. Pearson's correlation coefficient was used to assess the association between variables. Linear regression was performed between each clinical characteristic/cytokine-metabolite pair, and age, diabetes and dyslipidemia status were controlled by including these factors as covariates in the model. We performed a binary logistic regression adjusted by age, anti-CCP, diabetes and dyslipidemia as dichotomic variables, interleukin 6 (its receptor and the ratio) as well as the metabolites with a p<0.20 in the binary analysis, to predict the relationship between the variables included and the response. We also performed a receiver operating characteristic (ROC) curve to evaluate the performance of the classification model using response as the variable of prediction. The value p<0.05 was considered statistically significant. The analysis was performed using SPSS v.27 (IBM Corp. Released 2019 v27.0. Armonk, NY: IBM Corp.).
3. RESULTS
3.1. Patient demographics and disease characteristics at baseline
Thirty-five women with active RA were recruited. Patient demographics, along with the disease characteristics and co-morbidities are summarized in Table 1. The average of age was 53 years old (± 10), and the average number of years since diagnosis was 11.8 years (±7.2). TJC and SJC were 9.26 (± 6.23) and 6.00 (± 4.08) respectively, with an average DAS28-ESR 5.77 (± 0.99). Prior to TCZ treatment, 34% of the patients were biological disease-modifying antirheumatic drug (bDMARD)- naïve. Patients had failed an average of 2 conventional synthetic DMARDs and an average of 1.33 bDMARDs. During TCZ treatment (8mg/kg), 31.4% of patients received TCZ as monotherapy, 42.9% received TCZ in combination with methotrexate (15.5 ± 8.36 mg/week), 5.7% with sulfasalazine (1.5 ± 0.7 mg/day), 22.9% with leflunomide (16.25 ± 5.17 mg/day) and, 68% with steroids (7.58 ± 4.92 mg/day). Clinical parameters at baseline and 6 months are shown in supplementary table S1 and demonstrate the efficiency of TCZ.
Table 1.
Characteristics of RA patients (women) included in the study.
| Variable | Baseline |
|---|---|
| Age, years | 53 ± 10 |
| RF positive, n (%) | 23 (65.7) |
| Anti-CCP positive % | 23 (65.7) |
| Seropositive, n (%) | 28 (80.0) |
| ESR | 52.29 ± 31.44 |
| CRP | 1.98 ± 2.69 |
| SJC | 6.00 ± 4.08 |
| TJC | 9.26 ± 6.23 |
| HAQ, score | 1.41 ± 0.68 |
| DAS28-ESR | 5.77 ± 0.99 |
| SDAI, score | 29.53 ± 11.41 |
| CDAI, score | 27.54 ± 10.54 |
| Tobacco, n (%) | 9 (22.9) |
| Diabetes, n (%) | 3 (8.6) |
| Hypertension, n (%) | 8 (22.9) |
| Dyslipidemia | 7 (20) |
Continuous variables expressed in mean ± standard deviation; Categorical variables expressed in percentage. RF: rheumatoid factor; Anti-CCP: anti-cyclic citrullinated peptide; ESR: erythrocyte sedimentation rate; CRP: C-reactive protein; SJC: Swollen joint count; TJC: Tender joint count; HAQ: health assessment questionnaire; DAS-28: disease activity score in base of 28 joints; SDAI: simple disease activity index; CDAI: clinical disease activity index
3.2. Metabolic Profiling in responders vs non-responders at baseline
We first classified the 35 women in 2 groups according to their response to TCZ, determined by EULAR classification criteria. Patients with a >1.2 reduction of DAS28-ESR after 6 months of treatment and a DAS28-ESR ≤ 3.2 were considered responders (n=17). Patients with a DAS28-ESR reduction of ≤1.2 after 6 months of TCZ and a DAS28-ESR of > 3.2 were considered non-responders (14, moderate responders and 4 non-responders according to EULAR response, total n=18). No significant differences in clinical variables were observed at the baseline between groups, except for ESR which was higher in non-responders (39.67 ± 16.75 responders vs 61.75 ± 36.63 non-responders; p<0.002) (supplementary table S2).
A total of 46 polar metabolites were identified using 1H-NMR spectroscopy and included in the analysis. We identified several amino acids, ketone bodies and several glycolytic intermediates among other metabolites. The 1H-NMR spectra showed visible differences in the concentration of some metabolites between responders and non-responders at baseline (Supplementary Table S3 and Figure 1A).
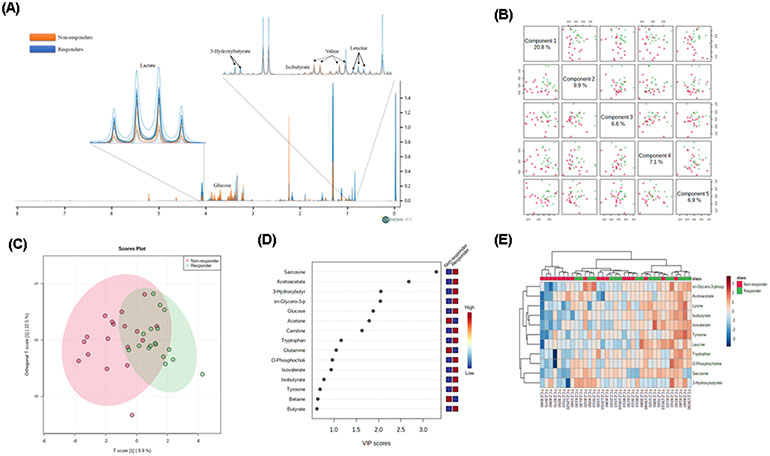
Fig 1.
Plasma metabolic profile of patients with RA discriminate responders and non-responders. A) 1H-NMR of plasma from 12 patients (6 responders and 6 non-responders) with RA showing differences of metabolites intensities between responders and non-responders at baseline. The variation of polar metabolite concentrations indicates a characteristic metabolic profile in patients who achieve or not response after 6 months of treatment with TCZ. B) Pairwise score plots between the 5 principal components showing a total explained variance is 51.3%. C) Orthogonal PLS-DA between non-responders and responders at baseline. D) Important features identified by PLS-DA. E) Concentration of metabolites in format Heatmap based on Euclidean distances considering the 11 metabolites observed in figure 1D VIP>1, shows clustering between responders and non-responders according to metabolites concentration.
After classifying RA patients according to EULAR response criteria, we performed a multivariate OPLS-DA with all the metabolites observing a partial discrimination between the groups. Principal components (1-5) explained 51.3% of the variance of the 1H-NMR data. With a cutoff value of PLS-DA Variables Importance in Projection (VIP) > 1.0, we identified sarcosine, acetoacetate, 3-hydroxybutyrate, sn-glycero-3-phophocholine, glucose, acetone, carnitine, tryptophan, glutamine and o-phosphocholine as the key metabolites to differentiate between groups (Figure 1B, C, D). Heat map analysis clustered the groups according to the metabolic profile but was not able to fully classify them (Figure 1E).
Since our group previously found that the IL6/IL6R ratio was important to identify responders to TCZ(Diaz-Torne et al., 2018), we also evaluated if the addition of these parameters would better distinguish the groups (supplementary figure S1). Yet, IL-6, sIL-6R and the ratio did not achieve the cutoff value and did not improve the classification between responders and non-responders.
It is important to note that OPLS-DA did not show good discrimination when analyzing the subjects by age (young <55 years, old ≥ 55 years), DAS28-ESR (high activity vs moderate activity), RF (positive>25 IU/mL vs negative ≤25 IU/mL), ESR (High ≥ 30 mm/h vs normal < 30 mm/h), or CRP (negative <2.0 mg/L vs positive ≥2.0 mg/L) (Supplementary figure S2A and Supplemental material). However, based on the results of OPLS-DA, several metabolites discriminated between positive (>20 U/mL) and negative anti-CCP (≤20 U/mL) at baseline (Supplementary figure S2B and Supplemental material).
3.3. Significantly different enriched pathways and metabolites between responders and non-responders at baseline
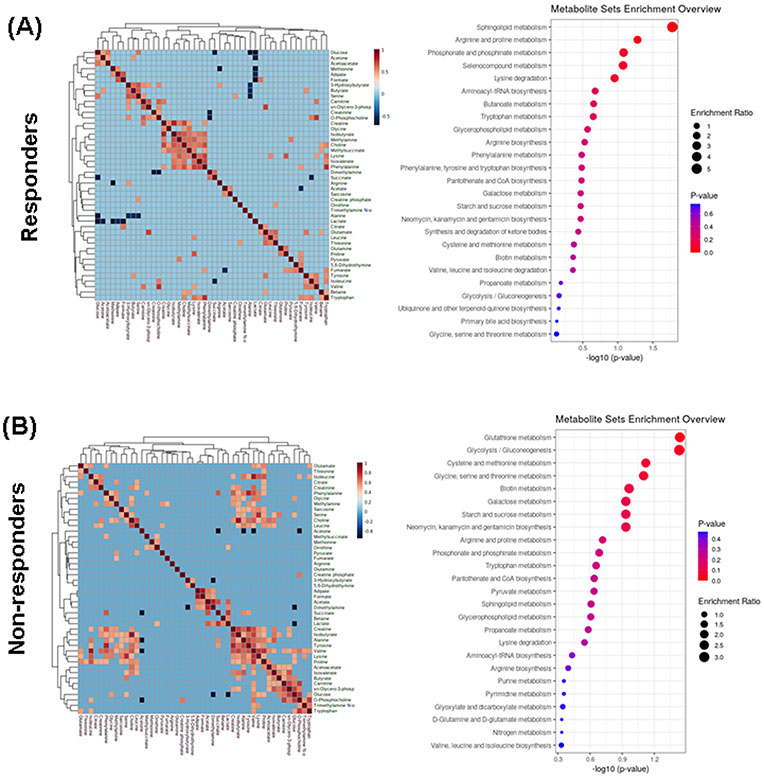
We analyzed whether blood metabolites had a different metabolic fingerprint in both groups at baseline. We had some significant observations of different associations between metabolites at baseline in responders compared to non-responders (Figure 2A). In addition, when the metabolites were mapped to known metabolic pathways using MetaboAnalyst V.4.0, enriched pathways were significantly different in both groups (Figure 2B). In responders, sphingolipid and arginine/proline metabolism were the most enriched metabolic pathways, while glutathione metabolism and glycolysis/ gluconeogenesis pathways were the most important enrichment pathways in non-responders. These differences of metabolic enrichment pathways before treatment could condition the patient’s response to TCZ.
Fig 2.
Left. Heatmap and hierarchical cluster analysis indicating relationships between polar metabolites identified by 1H-NMR in plasma from RA patients before treatment with TCZ. A) responders, B) non-responders. Pearson correlation with a Cutoff value of 0.5. The orange color indicates a positive correlation >0.5 and the dark blue indicates negative correlation <−0.5. Right. Summary of bar graphs for quantitative pathway enrichment analysis showing the significant changes on metabolic pathway involve before treatment with TCZ in A) responders and B) non-responders.
To determine the significantly different metabolites between responders and non-responders, metabolites were analyzed by univariate analysis. Seven metabolites were significantly different between groups: 3-hydroxybutyrate (p= 0.01), isobutyrate (p=0.02), lysine (p= 0.05), sn-glycero-3-phosphocholine (p= 0.02), phenylalanine (p=0.02), tryptophan (p=0.01) and tyrosine (p= 0.03). Each of these displayed higher levels in responders than non-responders (Figure 3A and supplementary table S3). We did observe tendencies in the levels of these metabolites when analyzed between moderate responders and non-responders according to EULAR response (Figure 3B).
Fig 3.
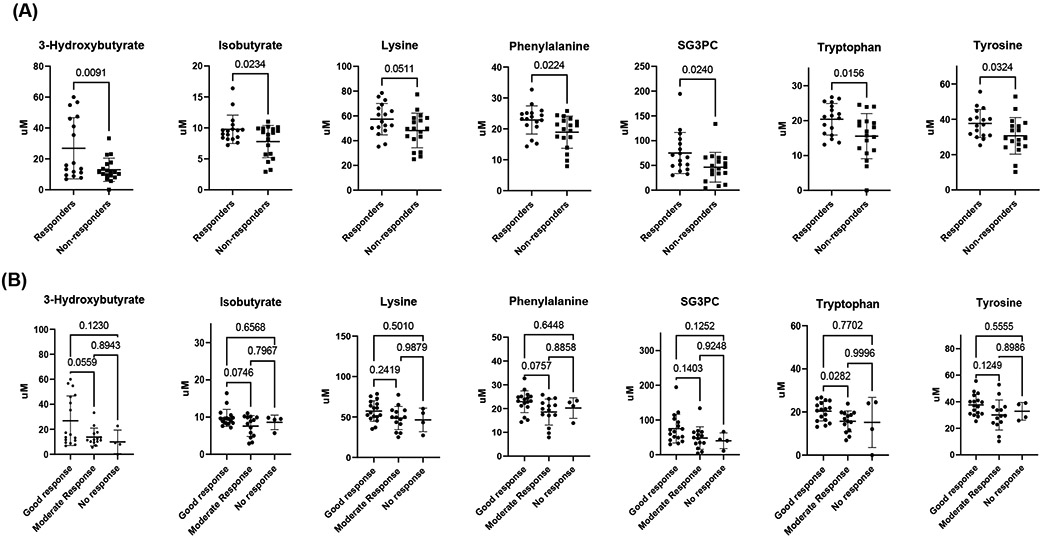
A) Concentrations of specific polar metabolites at baseline that are different between TCZ responders and non-responders measured by 1H-NMR before treatment with TCZ. These metabolites represent a group of potential biomarkers for predicting and assessing patient response to TCZ treatment. B) Concentrations of the same specific polar metabolites at baseline after dividing the non-responders between moderate-responder and non-responders at 6 months. SG3PC: Sn-glycero-3-phosphocholine. p values are shown in the graphics.
We also determined whether some of these metabolites were associated with clinical variables. Linear regression was performed between each clinical characteristic-metabolite pair; age, diabetes and dyslipidemia status were controlled by including these factors as covariates in the model. The regression coefficients for each clinical outcome-metabolite pair were used to form a clustered heatmap to lend insight into which clinical characteristics were correlated with which groups of metabolites. An important find was that ESR had a negative correlation with the levels of several amino acids (p<0.05). Some metabolites also correlated with swollen joints (p<0.05) (supplementary figure S3). We also performed linear regression between levels of IL-6, sIL-6R, and IL-6/IL-6R ratio and each metabolite pair adjusted as above (supplementary figure S4).
3.4. Multivariate analysis identifies 3-hydroxybutyrate and phenylalanine as predictors of response.
A binary logistic regression analysis, including the metabolites with p<0.20 in the univariate model after adjusting by age, anti-CCP positivity, diabetes, dyslipidemia, IL-6, sIL-6R, and IL-6/IL-6R ratio, showed that RA patients with elevated 3-hydroxybutyrate and phenylalanine at baseline had 1.5 times more chances of a response to treatment with TCZ, overall classifying 77.1% of the patients correctly. The classification model for response to TCZ at baseline showed a sensitivity of 94% and specificity of 66% with an AUC of 0.899 and a p<0.001 (Figure 4).
Fig 4.
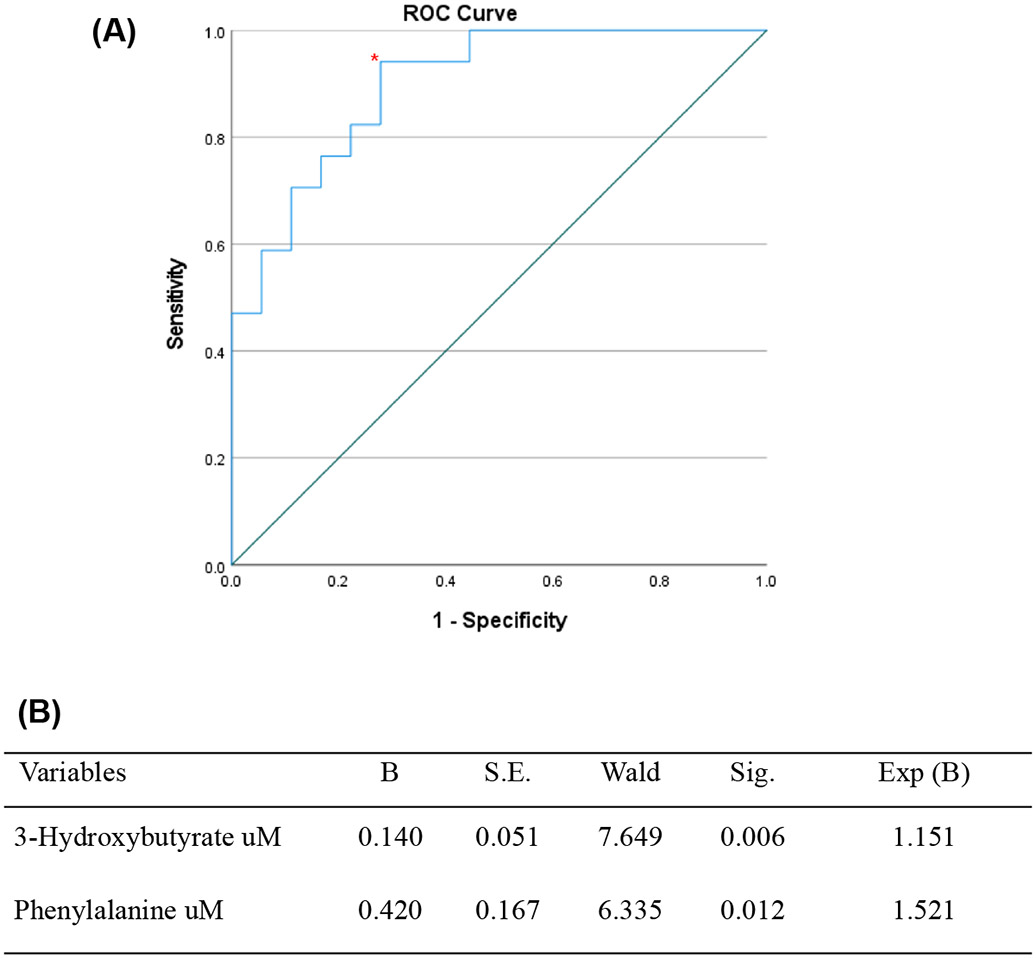
A) Receiver operating characteristic (ROC) curve for the model probability for responders before treatment based on binary logistic regression. The area under the curve (AUC) was 0.899, the sensitivity of 94% and the specificity of 66% with a p<0.001. The patients with elevated phenylalanine and 3-hydroxybutyrate had 1.5 chances to respond to TCZ. B) Binary logistic regression analysis adjusted by age, anti-CCP positivity and interleukins. The variables considered for the logistic regression analysis were metabolites with p<0.20 in the bivariate model. The model correctly categorized 77.8% of non-responders and 76.5% of responders.
3.5. Metabolic profiling in men
5 men with active RA were recruited. Patient demographics, along with the disease characteristics and co-morbidities compared with woman are summarized in Supplemental material. We did not observe differences in disease characteristics and comorbidities. Yet, the metabolic profile was different between men and women (Supplementary figure S5A, B). When we analyzed women and men together (n=40), we observed only slight differences in number of metabolites and metabolites predictors of response compared to only women (Supplementary figure S5C). The classification model for response to TCZ at baseline showed a sensitivity and specificity of 80% with an AUC of 0.886 and a p<0.001 (Supplementary figure S5D).
3.6. Metabolomic profile after treatment
Plasma collected after 6-month of TCZ treatment was also used for metabolomics analysis. Polar metabolite quantification at 6 months revealed several metabolites with increased concentration after treatment including) choline (p=0.008), creatine (p= 0.01), glutamine (0.006), glycine (p=0.04), lactate (p=0.006), leucine (p=0.05), lysine (p=0.006), proline (p=0.006), serine (0.002), threonine (p=0.04), and valine (0.02) (supplementary table S4). When comparing changes in metabolites before and after treatment in responders and non-responders, we observed that the concentration of metabolites did not change considerably in responders, while the concentrations of the metabolites increased until reaching responders’ metabolite levels in non-responders, except for the acetone (Supplementary figure S6 and supplementary material).
We then classified the group of patients classified as non-responders at 6 months, in late responders (patient that did not respond at 6 months but did reach good response at 12 months according to the EULAR classification; n=8), and non-responders (patient that at 12 months were still non-responders or moderate responders; n=9). One patient was lost for the 12 months follow-up. Of interest we observed that patients who did not respond had levels of sG3PC, tryptophan and methylamine still downregulated, suggesting an alternative activated metabolic pathway that is not dependent of IL-6 (Figure 5 and supplementary material).
Fig 5.
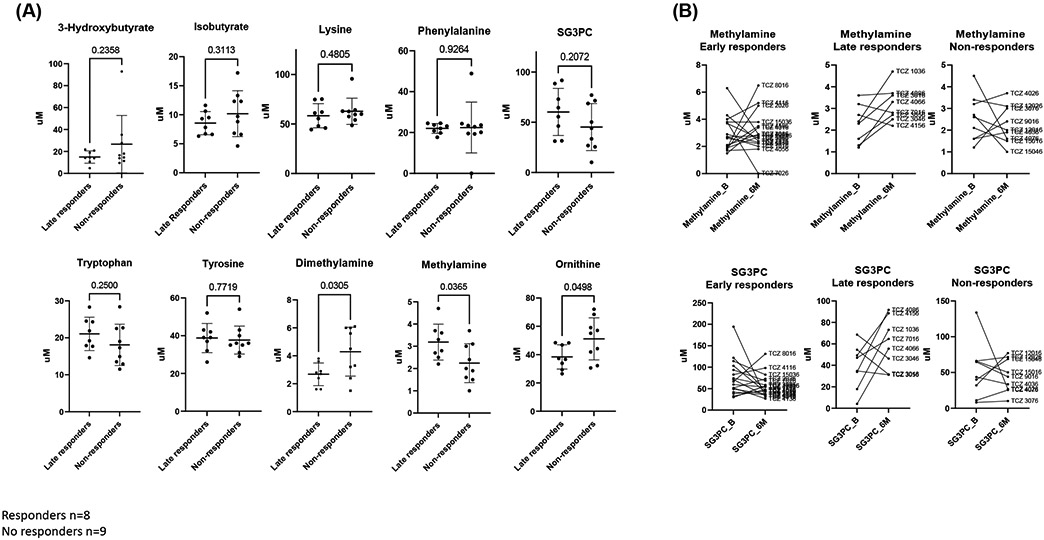
A) Concentrations of specific polar metabolites at 6 months that are different between late TCZ responders and non-responders measured by 1H-NMR. p values are shown in the graphics B) Metabolite trajectories of SG3PC and methylamine between baseline (B) and 6 months (6M) in early responders, late responders, and non-responders.
4. DISCUSSION
Predictive biomarkers for targeted therapies are an unmet clinical need since not all patients on these therapies will respond; around 50% of patients, for instance, will not achieve ACR50(Nishimoto et al., 2004). Global and targeted metabolomic studies using 1H-NMR and/or mass spectrometry can uncover a set of metabolites associated with prognosis and response. In the present study, we successfully identified a set of polar metabolites in plasma that differentiate responders from non-responders to TCZ in RA patients using 1H-NMR. These metabolites were higher in responders compared to non-responders. Of interest, some of these metabolites reached similar concentration after 6 months in both groups with the exception of SG3PC, tryptophan and methylamine, which were still downregulated in non-responders. Concentrations of circulating metabolites that belong to deregulated metabolic pathways can be either upregulated or downregulated. These upregulated or downregulated metabolites are not necessarily pathogenic, but a biomarker of an altered metabolic pathway. The fact that the late responders also normalize their concentration prior to their response, suggest that these metabolic pathways were IL-6 dependent in these patients, but an alternative activated metabolic pathway that is not dependent of IL-6 was still dysregulated in non-responders. The metabolites that strongly identified TCZ responders were 3-hydroxybutyrate and phenylalanine.
Prior studies have described metabolic signatures that classify responders to several therapies(Kapoor et al., 2013; Priori et al., 2015; Sweeney et al., 2016; Takahashi et al., 2019; Teitsma et al., 2018). For instance, Priori et al observed higher levels of isoleucine, leucine, valine, alanine, glutamine, tyrosine, and glucose, as well as lower levels of 3-hydroxybutyrate(Priori et al., 2015) in etanercept responders. In our study, we observed a higher level of 3-hydroxybutyrate and isobutyrate in responders. 3-hydroxybutyrate is synthesized in the liver from acetyl-CoA and can be used as an energy source when blood glucose is low. IL-6 is crucial for hepatocyte homeostasis(Schmidt-Arras & Rose-John, 2016). Of note, an association between levels of IL-6 and ketosis was described(Jain et al., 2003), hence, levels of this metabolite could be reflecting IL-6 pathway activation in the liver. In addition, 3-hydroxybutyrate also has an anti-inflammatory role by blocking NOD-like receptor protein 3 (NLRP3) inflammasome(Møller, 2020; Newman & Verdin, 2017). Isobutyrate is a short chain fatty acid derived from the gut (Petrognani et al., 2020). Differences in levels of this metabolite could be associated with different microbiome between R and NR. Of note, prior studies have suggested an effect of variations within the human gut microbiome on bioavailability, clinical efficacy and toxicity of a wide array of drugs through direct and indirect mechanisms (Scher et al., 2020). Thus, levels of this metabolite can reflect the presence of microbiome more adequate for a TCZ response.
We recently identified 7 polar metabolites including phenylalanine and tyrosine that predicted response to rituximab(Sweeney et al., 2016). Yet, the levels of these metabolites were lower in responders instead of elevated. Interestingly, phenylalanine and tyrosine work closely with IL-6R which interacts with IL-6(Blanchetot et al., 2016). Phenylalanine levels were also found to vary after infusion of recombinant IL-6 and returned to control’s levels when the infusion stopped suggesting a relationship between levels of IL-6 and phenylalanine(van Hall et al., 2008), and that the levels of IL-6 or ratio between IL-6/IL-6R might alter the concentration of phenylalanine. Tryptophan is another amino acid that has been associated with the IL-6 pathway, which is also elevated in responders to TCZ treatment, and it has antioxidant and anti-inflammatory properties through kynurenine, one of its metabolites. The 5-hydroxytryptophan, another product of tryptophan metabolism, reduces proinflammatory cytokines levels, such as IL-6 in animal models(Yang et al., 2015). In our study, the levels of several amino acids significantly changed after treatment, highlighting the important role of the IL-6 pathway in their metabolism.
Sphingolipid and arginine/proline metabolism were the most enriched metabolic pathways in responders, while glutathione metabolism and glycolysis/ gluconeogenesis pathways were the most important enrichment pathways in non-responders. Metabolic reprogramming, specifically the increment in glycolysis, has been implicated in immune and inflammation processes, as well as RA pathogenesis(Li et al., 2020; Mouton et al., 2020). Garcia-Carbonell showed that in RA fibroblast-like synoviocytes (FLS) compared to osteoarthritis FLS, the oxidative phosphorylation shifts to glycolysis, increasing the expression of transporters of glucose such as GLUT1(Garcia-Carbonell et al., 2016). Moreover, the inhibition of glycolysis improves the clinical parameters of RA by decreasing activation of neutrophils and monocytes, as well as reducing the expression of cells associated with inflammation(Abboud et al., 2018). The fact that the glycolysis pathway was increased in non-responders could reflect higher levels of inflammatory cytokines which enhanced glycolysis. Even though, in our cohort, DAS28-ESR and CRP was not different between responders and non-responders, serum metabolites could identify more subtle changes than CRP or clinical outcomes.
In this study we were not able to identify lipids in our samples, but serine is critical for sphingolipid metabolism. Teitsma et al described sphingolipid metabolism also being a pathway that predicted response to TCZ(Teitsma et al., 2018) and suggested that sphinganine and sphingosine were also associated with IL-6 levels. Interestingly, several metabolites differentiate patients with and without anti-CCP antibodies. Methylamine, creatine, carnitine, and glycine were higher in patients who were anti-CCP positive. Of note, these metabolites merge into the urea cycle at different points likely impacting the levels of citrulline(Chandrasekharan et al., 2018; Dimitroulas et al., 2017). However, the significant increase of some metabolites in patients with anti-CCP positive was not associated with their response to TCZ. This observation is supported by Jonsson et al, who found no differences of anti-CCP and therapeutic response with methotrexate (Jonsson et al., 2018) or TCZ (Narváez et al., 2016). Finally, there is little data describing the correlation between metabolites and CRP in early rheumatoid arthritis. Young et al, showed in patients with early arthritis that choline, lactate, acetylglycine, urea, glucose, taurine, threonine, and fatty acids among other, correlated with CRP levels (Young et al., 2013). In our study, we only observed a negative correlation of CRP with isoleucine. Since our cohort have long-standing disease, these differences should be viewed with caution.
5. CONCLUSIONS
In conclusion, the relationship between plasma metabolic profiles and the clinical response to TCZ suggests that 1H-NMR may be a promising tool for RA therapy optimization. Although the results are promising, our study has several limitations, not only the small sample size and the small male population in our cohort, but also the difficulty to control the number of variables involved in metabolite levels in plasma, including other co-morbidities and diet (Coras et al., 2020). More studies are needed to determine if metabolic profiling can predict response to biological therapies in RA patients.
Supplementary Material
Funding:
This work was supported by the National Institutes of Health (R01AR073324 to M.G., T32AR064194 to JDM-S and RC).
Footnotes
Compliance with ethical standards: The authors declare no conflict of interest and all the humans included in the study singed an inform consent.
Conflict of interest/Competing interest: All the authors declare no conflict of interest. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Ethics approval: The study was approved by the Institutional Board Review at Clinic of the Sant Pau Hospital. All procedures performed in this study were in accordance with the ethical standards of the Institutional Board Review at Clinic of the Sant Pau Hospital and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Consent to participate: Informed consent was obtained from all individual participants included in the study.
Availability of data and material: The datasets generated during the current study are available from the corresponding author on reasonable request.
7 REFERENCES
- Abboud G, Choi SC, Kanda N, Zeumer-Spataro L, Roopenian DC, & Morel L (2018). Inhibition of Glycolysis Reduces Disease Severity in an Autoimmune Model of Rheumatoid Arthritis. Front Immunol, 9, 1973. 10.3389/fimmu.2018.01973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO 3rd, Birnbaum NS, Burmester GR, Bykerk VP, Cohen MD, Combe B, Costenbader KH, Dougados M, Emery P, Ferraccioli G, Hazes JM, Hobbs K, Huizinga TW, Kavanaugh A, Kay J, Kvien TK, Laing T, Mease P, Ménard HA, Moreland LW, Naden RL, Pincus T, Smolen JS, Stanislawska-Biernat E, Symmons D, Tak PP, Upchurch KS, Vencovský J, Wolfe F, & Hawker G (2010). 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum, 62(9), 2569–2581. 10.1002/art.27584 [DOI] [PubMed] [Google Scholar]
- Armitage EG, & Ciborowski M (2017). Applications of Metabolomics in Cancer Studies. In Sussulini A (Ed.), Metabolomics: From Fundamentals to Clinical Applications (pp. 209–234). Springer International Publishing. 10.1007/978-3-319-47656-8_9 [DOI] [PubMed] [Google Scholar]
- Barnes S, Benton HP, Casazza K, Cooper SJ, Cui X, Du X, Engler J, Kabarowski JH, Li S, Pathmasiri W, Prasain JK, Renfrow MB, & Tiwari HK (2016). Training in metabolomics research. I. Designing the experiment, collecting and extracting samples and generating metabolomics data. Journal of mass spectrometry : JMS, 51(7), 461–475. 10.1002/jms.3782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchetot C, De Jonge N, Desmyter A, Ongenae N, Hofman E, Klarenbeek A, Sadi A, Hultberg A, Kretz-Rommel A, Spinelli S, Loris R, Cambillau C, & de Haard H (2016). Structural Mimicry of Receptor Interaction by Antagonistic Interleukin-6 (IL-6) Antibodies. J Biol Chem, 291(26), 13846–13854. 10.1074/jbc.M115.695528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breedveld FC, Weisman MH, Kavanaugh AF, Cohen SB, Pavelka K, van Vollenhoven R, Sharp J, Perez JL, & Spencer-Green GT (2006). The PREMIER study: A multicenter, randomized, double-blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive rheumatoid arthritis who had not had previous methotrexate treatment. Arthritis Rheum, 54(1), 26–37. 10.1002/art.21519 [DOI] [PubMed] [Google Scholar]
- Burska A, Boissinot M, & Ponchel F (2014). Cytokines as biomarkers in rheumatoid arthritis. Mediators Inflamm, 2014, 545493. 10.1155/2014/545493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekharan UM, Wang Z, Wu Y, Wilson Tang WH, Hazen SL, Wang S, & Elaine Husni M (2018). Elevated levels of plasma symmetric dimethylarginine and increased arginase activity as potential indicators of cardiovascular comorbidity in rheumatoid arthritis. Arthritis research & therapy, 20(1), 123–123. 10.1186/s13075-018-1616-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong J, Soufan O, Li C, Caraus I, Li S, Bourque G, Wishart DS, & Xia J (2018). MetaboAnalyst 4.0: towards more transparent and integrative metabolomics analysis. Nucleic acids research, 46(W1), W486–W494. 10.1093/nar/gky310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong J, Wishart DS, & Xia J (2019). Using MetaboAnalyst 4.0 for Comprehensive and Integrative Metabolomics Data Analysis. Curr Protoc Bioinformatics, 68(1), e86. 10.1002/cpbi.86 [DOI] [PubMed] [Google Scholar]
- Coras R, Murillo-Saich JD, & Guma M (2020). Circulating Pro- and Anti-Inflammatory Metabolites and Its Potential Role in Rheumatoid Arthritis Pathogenesis. Cells, 9(4). 10.3390/cells9040827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Torne C, Ortiz MDA, Moya P, Hernandez MV, Reina D, Castellvi I, De Agustin JJ, Fuente D, Corominas H, Sanmarti R, Zamora C, Cantó E, & Vidal S (2018). The combination of IL-6 and its soluble receptor is associated with the response of rheumatoid arthritis patients to tocilizumab. Semin Arthritis Rheum, 47(6), 757–764. 10.1016/j.semarthrit.2017.10.022 [DOI] [PubMed] [Google Scholar]
- Dimitroulas T, Hodson J, Sandoo A, Smith J, & Kitas GD (2017). Endothelial injury in rheumatoid arthritis: a crosstalk between dimethylarginines and systemic inflammation. Arthritis research & therapy, 19(1), 32–32. 10.1186/s13075-017-1232-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emwas AH (2015). The strengths and weaknesses of NMR spectroscopy and mass spectrometry with particular focus on metabolomics research. Methods Mol Biol, 1277, 161–193. 10.1007/978-1-4939-2377-9_13 [DOI] [PubMed] [Google Scholar]
- Fiehn O, Robertson D, Griffin J, van der Werf M, Nikolau B, Morrison N, Sumner LW, Goodacre R, Hardy NW, Taylor C, Fostel J, Kristal B, Kaddurah-Daouk R, Mendes P, van Ommen B, Lindon JC, & Sansone S-A (2007). The metabolomics standards initiative (MSI). Metabolomics, 3(3), 175–178. 10.1007/s11306-007-0070-6 [DOI] [Google Scholar]
- Garcia-Carbonell R, Divakaruni AS, Lodi A, Vicente-Suarez I, Saha A, Cheroutre H, Boss GR, Tiziani S, Murphy AN, & Guma M (2016). Critical Role of Glucose Metabolism in Rheumatoid Arthritis Fibroblast-like Synoviocytes. Arthritis & rheumatology (Hoboken, N.J.), 68(7), 1614–1626. 10.1002/art.39608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavrilă BI, Ciofu C, & Stoica V (2016). Biomarkers in Rheumatoid Arthritis, what is new? J Med Life, 9(2), 144–148. [PMC free article] [PubMed] [Google Scholar]
- Greenberg JD, Kishimoto M, Strand V, Cohen SB, Olenginski TP, Harrington T, Kafka SP, Reed G, & Kremer JM (2008). Tumor necrosis factor antagonist responsiveness in a United States rheumatoid arthritis cohort. Am J Med, 121(6), 532–538. 10.1016/j.amjmed.2008.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haug K, Cochrane K, Nainala V,Williams M, Chang J, Jayaseelan K, O'Donovan C (2019) MetaboLights: a resource evolving in response to the needs of its scientific community. Nucleic Acids Research, 48(D1), D440–D444. 10.1093/nar/gkz1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Cen H, Zhou L, Wang T-H, Qin W, Xie B-H, Xiao D-M, Wu X-D, & Wu H-X (2019). Body Mass Index and Clinical Response to Tocilizumab in Patients With Rheumatoid Arthritis. Archives of rheumatology, 34(4), 406–413. 10.5606/ArchRheumatol.2019.7146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain SK, Kannan K, Lim G, Matthews-Greer J, McVie R, & Bocchini JA Jr. (2003). Elevated blood interleukin-6 levels in hyperketonemic type 1 diabetic patients and secretion by acetoacetate-treated cultured U937 monocytes. Diabetes Care, 26(7), 2139–2143. 10.2337/diacare.26.7.2139 [DOI] [PubMed] [Google Scholar]
- Jonsson MK, Hensvold AH, Hansson M, Aga A-B, Sexton J, Mathsson-Alm L, Cornillet M, Serre G, Lillegraven S, Fevang B-TS, Catrina AI, & Haavardsholm EA (2018). The role of anti-citrullinated protein antibody reactivities in an inception cohort of patients with rheumatoid arthritis receiving treat-to-target therapy. Arthritis research & therapy, 20(1), 146–146. 10.1186/s13075-018-1635-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanehisa M, Furumichi M, Tanabe M, Sato Y, & Morishima K (2017). KEGG: new perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res, 45(D1), D353–d361. 10.1093/nar/gkw1092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor SR, Filer A, Fitzpatrick MA, Fisher BA, Taylor PC, Buckley CD, McInnes IB, Raza K, & Young SP (2013). Metabolic profiling predicts response to anti-tumor necrosis factor α therapy in patients with rheumatoid arthritis. Arthritis Rheum, 65(6), 1448–1456. 10.1002/art.37921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klareskog L, van der Heijde D, de Jager JP, Gough A, Kalden J, Malaise M, Martín Mola E, Pavelka K, Sany J, Settas L, Wajdula J, Pedersen R, Fatenejad S, & Sanda M (2004). Therapeutic effect of the combination of etanercept and methotrexate compared with each treatment alone in patients with rheumatoid arthritis: double-blind randomised controlled trial. Lancet, 363(9410), 675–681. 10.1016/s0140-6736(04)15640-7 [DOI] [PubMed] [Google Scholar]
- Lehrskov LL, & Christensen RH (2019). The role of interleukin-6 in glucose homeostasis and lipid metabolism. Semin Immunopathol, 41(4), 491–499. 10.1007/s00281-019-00747-2 [DOI] [PubMed] [Google Scholar]
- Li W, Xu M, Li Y, Huang Z, Zhou J, Zhao Q, Le K, Dong F, Wan C, & Yi P (2020). Comprehensive analysis of the association between tumor glycolysis and immune/inflammation function in breast cancer. J Transl Med, 18(1), 92. 10.1186/s12967-020-02267-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsky PE, van der Heijde DM, St Clair EW, Furst DE, Breedveld FC, Kalden JR, Smolen JS, Weisman M, Emery P, Feldmann M, Harriman GR, & Maini RN (2000). Infliximab and methotrexate in the treatment of rheumatoid arthritis. Anti-Tumor Necrosis Factor Trial in Rheumatoid Arthritis with Concomitant Therapy Study Group. N Engl J Med, 343(22), 1594–1602. 10.1056/nejm200011303432202 [DOI] [PubMed] [Google Scholar]
- McInnes IB, & Schett G (2017). Pathogenetic insights from the treatment of rheumatoid arthritis. Lancet, 389(10086), 2328–2337. 10.1016/s0140-6736(17)31472-1 [DOI] [PubMed] [Google Scholar]
- Mendez KM, Reinke SN, & Broadhurst DI (2019). A comparative evaluation of the generalised predictive ability of eight machine learning algorithms across ten clinical metabolomics data sets for binary classification. Metabolomics, 15(12), 150. 10.1007/s11306-019-1612-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittelstrass K, Ried JS, Yu Z, Krumsiek J, Gieger C, Prehn C, Roemisch-Margl W, Polonikov A, Peters A, Theis FJ, Meitinger T, Kronenberg F, Weidinger S, Wichmann HE, Suhre K, Wang-Sattler R, Adamski J, & Illig T (2011). Discovery of sexual dimorphisms in metabolic and genetic biomarkers. PLoS Genet, 7(8), e1002215. 10.1371/journal.pgen.1002215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Møller N (2020). Ketone Body, 3-Hydroxybutyrate: Minor Metabolite - Major Medical Manifestations. J Clin Endocrinol Metab, 105(9). 10.1210/clinem/dgaa370 [DOI] [PubMed] [Google Scholar]
- Mouton AJ, Li X, Hall ME, & Hall JE (2020). Obesity, Hypertension, and Cardiac Dysfunction: Novel Roles of Immunometabolism in Macrophage Activation and Inflammation. Circ Res, 126(6), 789–806. 10.1161/circresaha.119.312321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam JL, Takase-Minegishi K, Ramiro S, Chatzidionysiou K, Smolen JS, van der Heijde D, Bijlsma JW, Burmester GR, Dougados M, Scholte-Voshaar M, van Vollenhoven R, & Landewé R (2017). Efficacy of biological disease-modifying antirheumatic drugs: a systematic literature review informing the 2016 update of the EULAR recommendations for the management of rheumatoid arthritis. Ann Rheum Dis, 76(6), 1113–1136. 10.1136/annrheumdis-2016-210713 [DOI] [PubMed] [Google Scholar]
- Narváez J, Magallares B, Díaz Torné C, Hernández MV, Reina D, Corominas H, Sanmartí R, Llobet JM, Rodriguez de la Serna A, & Nolla JM (2016). Predictive factors for induction of remission in patients with active rheumatoid arthritis treated with tocilizumab in clinical practice. Seminars in Arthritis and Rheumatism, 45(4), 386–390. 10.1016/j.semarthrit.2015.07.001 [DOI] [PubMed] [Google Scholar]
- Newman JC, & Verdin E (2017). β-Hydroxybutyrate: A Signaling Metabolite. Annual review of nutrition, 37, 51–76. 10.1146/annurev-nutr-071816-064916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimoto N, Yoshizaki K, Miyasaka N, Yamamoto K, Kawai S, Takeuchi T, Hashimoto J, Azuma J, & Kishimoto T (2004). Treatment of rheumatoid arthritis with humanized anti-interleukin-6 receptor antibody: a multicenter, double-blind, placebo-controlled trial. Arthritis Rheum, 50(6), 1761–1769. 10.1002/art.20303 [DOI] [PubMed] [Google Scholar]
- Priori R, Casadei L, Valerio M, Scrivo R, Valesini G, & Manetti C (2015). ¹H-NMR-Based Metabolomic Study for Identifying Serum Profiles Associated with the Response to Etanercept in Patients with Rheumatoid Arthritis. PLoS One, 10(11), e0138537. 10.1371/journal.pone.0138537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rein P, & Mueller RB (2017). Treatment with Biologicals in Rheumatoid Arthritis: An Overview. Rheumatol Ther, 4(2), 247–261. 10.1007/s40744-017-0073-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scher JU, Nayak RR, Ubeda C, Turnbaugh PJ, & Abramson SB (2020). Pharmacomicrobiomics in inflammatory arthritis: gut microbiome as modulator of therapeutic response. Nat Rev Rheumatol, 16(5), 282–292. 10.1038/s41584-020-0395-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Arras D, & Rose-John S (2016). IL-6 pathway in the liver: From physiopathology to therapy. J Hepatol, 64(6), 1403–1415. 10.1016/j.jhep.2016.02.004 [DOI] [PubMed] [Google Scholar]
- Sebba A (2008). Tocilizumab: the first interleukin-6-receptor inhibitor. Am J Health Syst Pharm, 65(15), 1413–1418. 10.2146/ajhp070449 [DOI] [PubMed] [Google Scholar]
- Suman S, Sharma RK, Kumar V, Sinha N, & Shukla Y (2018). Metabolic fingerprinting in breast cancer stages through (1)H NMR spectroscopy-based metabolomic analysis of plasma. J Pharm Biomed Anal, 160, 38–45. 10.1016/j.jpba.2018.07.024 [DOI] [PubMed] [Google Scholar]
- Sweeney SR, Kavanaugh A, Lodi A, Wang B, Boyle D, Tiziani S, & Guma M (2016). Metabolomic profiling predicts outcome of rituximab therapy in rheumatoid arthritis. RMD Open, 2(2), e000289. 10.1136/rmdopen-2016-000289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi S, Saegusa J, Onishi A, & Morinobu A (2019). Biomarkers identified by serum metabolomic analysis to predict biologic treatment response in rheumatoid arthritis patients. Rheumatology (Oxford), 58(12), 2153–2161. 10.1093/rheumatology/kez199 [DOI] [PubMed] [Google Scholar]
- Teitsma XM, Yang W, Jacobs JWG, Pethö-Schramm A, Borm MEA, Harms AC, Hankemeier T, van Laar JM, Bijlsma JWJ, & Lafeber F (2018). Baseline metabolic profiles of early rheumatoid arthritis patients achieving sustained drug-free remission after initiating treat-to-target tocilizumab, methotrexate, or the combination: insights from systems biology. Arthritis Res Ther, 20(1), 230. 10.1186/s13075-018-1729-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hall G, Steensberg A, Fischer C, Keller C, Møller K, Moseley P, & Pedersen BK (2008). Interleukin-6 Markedly Decreases Skeletal Muscle Protein Turnover and Increases Nonmuscle Amino Acid Utilization in Healthy Individuals. The Journal of Clinical Endocrinology & Metabolism, 93(7), 2851–2858. 10.1210/jc.2007-2223 [DOI] [PubMed] [Google Scholar]
- van Riel PL, & Renskers L (2016). The Disease Activity Score (DAS) and the Disease Activity Score using 28 joint counts (DAS28) in the management of rheumatoid arthritis. Clin Exp Rheumatol, 34(5 Suppl 101), S40–s44. [PubMed] [Google Scholar]
- van Wietmarschen HA, Dai W, van der Kooij AJ, Reijmers TH, Schroën Y, Wang M, Xu Z, Wang X, Kong H, Xu G, Hankemeier T, Meulman JJ, & van der Greef J (2012). Characterization of rheumatoid arthritis subtypes using symptom profiles, clinical chemistry and metabolomics measurements. PLoS One, 7(9), e44331–e44331. 10.1371/journal.pone.0044331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang TH, Hsu PY, Meng M, & Su CC (2015). Supplement of 5-hydroxytryptophan before induction suppresses inflammation and collagen-induced arthritis. Arthritis Res Ther, 17, 364. 10.1186/s13075-015-0884-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida Y, & Tanaka T (2014). Interleukin 6 and rheumatoid arthritis. BioMed research international, 2014, 698313–698313. 10.1155/2014/698313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young SP, Kapoor SR, Viant MR, Byrne JJ, Filer A, Buckley CD, Kitas GD, & Raza K (2013). The impact of inflammation on metabolomic profiles in patients with arthritis. Arthritis Rheum, 65(8), 2015–2023. 10.1002/art.38021 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.