ABSTRACT
Background
The dystonias are phenotypically and etiologically heterogenous disorders. Many proposals and a consensus recommendation have been provided for the diagnosis and classification of the dystonias, but these recommendations serve only as general guidelines. Current diagnosis and classification may still depend on clinical judgment causing different opinions.
Objective
To delineate clinical features used by movement disorder specialists in the diagnosis and classification of isolated focal cervical dystonia, and to develop recommendations for a more consistent approach to classification according to anatomical regions involved.
Methods
Cross‐sectional data for subjects diagnosed with isolated dystonia were acquired from the Dystonia Coalition, an international, multicenter collaborative research network. Data from many movement disorder specialists were evaluated to determine how diagnoses of cervical dystonia related to their recorded examinations. Cases were included if they were given a diagnosis of focal cervical dystonia. Cases were also included if they had dystonia of the neck on exam, but were given an alternative diagnosis such as segmental dystonia.
Results
Among 2916 subjects with isolated dystonia, 1258 were diagnosed with focal cervical dystonia. Among these 1258 cases, 28.3% had dystonia outside of the neck region. Regions involved outside of the neck included the shoulder, larynx, and sometimes other regions. Analysis of the results pointed to several factors that may influence specialists' use of current diagnostic guidelines for making a diagnosis of isolated focal cervical dystonia including varied interpretations of involvement of nearby regions (shoulder, larynx, platysma), severity of dystonia across different regions, and occurrence of tremor in different regions.
Conclusions
Although focal cervical dystonia is the most common type of dystonia, a high percentage of subjects given this diagnosis had dystonia outside of the neck region. This observation points to the need for more specific guidelines for defining this common disorder. Such guidelines are proposed here.
Keywords: cervical dystonia, torticollis, diagnosis, diagnostic criteria, classification
The dystonias are a phenotypically and etiologically diverse group of disorders. 1 Over the years, there have been many proposals for how they should be classified into meaningful groups. 2 In 2013, a group of experts provided a consensus statement with recommendations for the diagnosis and classification for all dystonias. 3 These recommendations have been widely adopted and successfully applied to children and adults. 4 , 5 , 6 However, the recommendations were intended to serve only as general guidelines, leaving some room for clinical judgment. As a result, differences of opinion may occur. 6
The body regions affected with dystonia are an important feature for the classification of dystonia. 3 Focal dystonia is defined as involvement of a single body region, and segmental dystonia involves two or more contiguous regions. Multifocal dystonia involves non‐contiguous regions, and generalized dystonia includes the trunk and at least two other body regions. This aspect of classification is simple, intuitive, and both clinically and scientifically valuable. However, this aspect for classification is not consistently used.
Although cervical dystonia (CD) is the most common of all dystonias, 7 there are no widely accepted diagnostic criteria. Some criteria have been proposed, but they have not yet been validated. 8 Furthermore, these criteria do not delineate focal from broader patterns of involvement, such as segmental or multifocal dystonia. The current study provides an in depth analysis of a large multicenter cohort focusing on the diagnosis of CD, and particularly its classification according to body regions affected. Data were methodically collected by numerous specialists in movement disorders internationally and compared to determine how diagnoses related to examination features recorded. The goal was not to establish new criteria or validate recently proposed diagnostic criteria for CD, but to evaluate application of existing guidelines in the diagnosis of focal CD, with a specific focus on classification according to body regions affected. The data are used to generate empirical recommendations for a more consistent approach.
Methods
Data for these analyses were obtained from the Dystonia Coalition, an ongoing multicenter international project aimed at delineating the clinical features and evolution of isolated dystonias. Methodological details for this study, 9 and clinical details for portions of this cohort have been presented elsewhere. 10 , 11 , 12 Briefly, subjects were recruited by 57 different specialists in movement disorders at 40 sites across North America, Europe, and Australia. All investigators were trained to use the same examination and data recording protocols, with a centralized database. All subjects gave written informed consent at the recruiting site according to the Declaration of Helsinki and The Common Rule. For the current study, the analysis of de‐identified aggregate data was further approved by the Emory University Human Subjects Review Board. All data from March 2, 2011 to April 19, 2019 were used. For individuals with multiple evaluations over time, only data from the first visit were used.
Subjects treated with botulinum toxin could not be recruited until overt symptoms returned, usually at least 3 months following treatment, and never less than 2 months following treatment. Concurrent treatment with oral medications was allowed. The current study focused on a subgroup of subjects with CD. These cases could be identified in the database in three different ways; (1) an examination checklist that indicated which body regions were affected with dystonia or tremor; (2) a quantitative score for each body region affected using Global Dystonia Rating Scale (GDRS) 13 ; and (3) overall viewpoint on best clinical diagnostic label. Since widely accepted diagnostic criteria for CD are not available, clinicians were asked to use their expert judgment for making the diagnosis, which typically involved applying the 2013 consensus report for definition and classification of dystonia 3 to the neck region. Cases were included when any of these three sources of information suggested dystonia involving the neck, so results cover the full spectrum of cases with neck dystonia, regardless of the diagnosis. Since all three data elements were to be provided by a site investigator with special expertise in movement disorders, comparing these three different sources of information permitted assessment of how the diagnosis given by the specialist compared to their recorded examination and existing guidelines.
Descriptive statistics are presented as average values ± standard deviations. Group comparisons were conducted using Student's t test or the Kruskal‐Wallis test for non‐parametric measures.
Results
After elimination of 79 cases with incomplete data, information was available for 2916 unique individuals with isolated dystonia (Table 1). Among all 2916 subjects, 1258 (43.1%) had a diagnosis of focal CD. Among these 1258 subjects, 1245 (99%) had neck involvement on the examination checklist, confirming dystonia of the neck. The 13 cases (1%) diagnosed with focal CD but without neck involvement on the examination checklist had non‐zero GDRS scores for neck, suggesting that the examination checklist was incomplete for these cases. In addition, data regarding the site of origin for dystonia was recorded as the neck and/or shoulder for all 13 cases, providing further confirmation for neck involvement. With three independent sources of information suggesting CD (diagnosis, GDRS score, site of origin), all 13 cases were considered to have CD, and included in all further analysis. This preliminary analysis revealed how comparing the three different sources of information for the same issue could be used to confirm diagnoses and identify any potential discrepancies in data entry.
TABLE 1.
Descriptive statistics for 2916 subjects with isolated dystonia
| Exam checklist showing neck only (N = 914) | GDRS scores non‐zero for neck only (N = 763) | Diagnosis = focal cervical dystonia (N = 1258) | |
|---|---|---|---|
| Sex | |||
| Females | 671 (73.4%) | 557 (73.0%) | 928 (73.8%) |
| Males | 243 (26.6%) | 206 (27.0%) | 330 (26.2%) |
| Age | |||
| At recruitment | 58.9 ± 12.2 (18–87) | 59.2 ± 12.0 (18–87) | 59.4 ± 12.1 (18–87) |
| At dystonia onset | 44.8 ± 14.2 (5–82) | 45.0 ± 14.2 (6–82) | 45.4 ± 14.0 (6–82) |
| Duration of illness | 14.1 ± 12.0 (0–63) | 14.2 ± 12.0 (0–63) | 14.0 ± 11.8 (0–65) |
| Race | |||
| White | 863 (94.4%) | 723 (94.8%) | 1186 (94.3%) |
| Black | 12 (1.3%) | 10 (1.3%) | 20 (1.6%) |
| Asian | 14 (1.5%) | 12 (1.6%) | 14 (1.1%) |
| Other | 21 (2.3%) | 14 (1.8%) | 25 (2.0%) |
| Unknown | 4 (0.4%) | 3 (0.4%) | 13 (1.0%) |
Age, age of onset, and disease duration are reported as mean ± SD (range).
Neck Dystonia with Shoulder Involvement
Among the 1258 subjects diagnosed with focal CD, 355 (28.2%) had examination checklists that included the neck, along with at least one other body region (Table 2). This finding implies a substantial portion of cases did not have dystonia limited to a single body region. The most common region affected apart from the neck was the shoulder (N = 169), indicating that many movement disorder specialists consider shoulder involvement to be consistent with a diagnosis of focal CD. This view is consistent with the 2013 consensus report, which acknowledged the frequent convention of including shoulder involvement in the diagnosis of focal CD. 2
TABLE 2.
Body regions with dystonia among cases diagnosed with focal CD
| Regions affected | Number of cases affected based on only Exam (%) | Number of cases affected based on only GDRS (%) |
|---|---|---|
| Focal CD with only neck involvement | 890 (70.7) | 757 (60.2) |
| Focal CD with no neck involvement | 13 (1.0) | 9 (0.7) |
| Focal CD with neck involvement and at least one other body region | 355 (28.2) | 492 (39.1) |
| Neck+shoulder only* | 169 (13.4) | 258 (20.5)* |
| Neck+hand only | 33 (2.6) | 22 (1.7) |
| Neck+upper face only | 26 (2.1) | 24 (1.9) |
| Neck+upper arm only* | 15 (1.2) | 258 (20.5)* |
| Neck+larynx only | 14 (1.1) | 16 (1.3) |
| Neck+lower face only | 9 (0.7) | 11 (0.9) |
| Neck+trunk only | 9 (0.7) | 11 (0.9) |
| Neck+jaw+tongue only | 4 (0.3) | 3 (0.2) |
This table shows the body distribution of dystonia based on the exam checklist or the GDRS scores among 1258 cases diagnosed with focal CD.
Exam form lists neck and shoulder separately, however, GDRS form lists shoulder+proximal arm. Therefore, both neck+shoulder only AND neck+upper arm only numbers on exam were compared with neck+shouder+upper arm numbers in GDRS form.
To assess this hypothesis, we reviewed the examination checklist for all 2916 cases where both neck and shoulder were the only regions affected. Among 183 cases meeting these criteria, 19 were excluded because GDRS scores were zero, and three were excluded for data entry errors. Of the remaining 161 cases, 150 (93.2%) had a diagnosis of focal CD. The remaining 11 cases had a diagnosis of segmental dystonia. These results confirm that most movement disorder specialists consider shoulder involvement to be a feature consistent with a diagnosis of focal CD. However, a minority of specialists consider this combination to reflect segmental dystonia.
To determine whether diagnoses of focal CD were given to these 150 cases because neck symptoms dominated over shoulder symptoms, GDRS scores for neck and shoulder were compared. GDRS scores were systematically higher for neck (5.3 ± 2.0; range = 1–10) compared to shoulder (3.2 ± 01.7; range = 1–8) for all 150 cases (P < 0.01). GDRS scores were also higher for neck (4.9 ± 1.6; range = 2–7) compared to shoulder (3.4 ± 1.6; range = 2–7) for the 11 cases diagnosed instead with segmental dystonia (P = 0.03). However, the Kruskal‐Wallis test revealed no significant difference between the focal versus segmental diagnostic groups when comparing the distribution of scores for neck (P = 0.6) versus shoulder (P = 0.70) (Fig. 1). These results imply that movement disorder specialists did not provide a diagnosis of focal CD based only on the more severely affected body region.
FIG. 1.
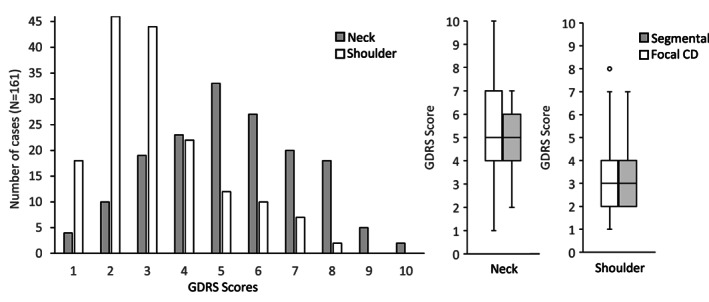
Impact of severity on diagnosis. Panel a shows GDRS scores for neck were higher than shoulder for all cases where both regions were involved. Panel B shows scores were similar for cases diagnosed with focal or segmental dystonia.
Neck Dystonia with Involvement of Other Body Regions
We next investigated how the diagnosis of focal CD was affected by involvement of other body regions that fall in the neck region, such as the larynx. Among all 2916 cases, 69 were reported on the examination checklist to have involvement of only neck and larynx. Of these 69 cases, seven were excluded because GDRS scores were zero, and one was excluded due to incomplete data. Of the remaining 61 cases with neck and larynx involvement only, 40 (65.6%) had a diagnosis of segmental dystonia, 8 (13.1%) focal CD, 11 (18.0%) focal laryngeal dystonia (LD), and 2 (3.3%) multifocal dystonia. These results imply that subjects with both neck and larynx involvement are more often considered to be segmental, not focal dystonia.
We next evaluated how severity of dystonia in the neck or larynx might impact the diagnosis of focal versus segmental dystonia. For the eight subjects diagnosed with focal CD, GDRS scores for neck (average = 4.6 ± 2.0; range = 1–8) trended higher than those for larynx (average = 3.1 ± 1.6; range = 0–8), but the difference did not reach statistical significance (P = 0.2). For the 11 subjects diagnosed with focal LD, GDRS scores were significantly (P = 0.001) higher for larynx (average = 3.7 ± 1.6, range = 2–6) compared to neck (average = 1.7 ± 0.8, range = 1–3). For the group of 40 subjects diagnosed with segmental dystonia, GDRS scores for neck (3.8 ± 1.9; range = 1–7) and larynx (4.2 ± 1.8; range = 1–7) were more closely matched (P = 0.4). Overall, these results imply that diagnoses of focal versus segmental dystonia might be influenced at least in part by the most severely affected area.
Aside from the shoulder and larynx, there were 61 additional cases with dystonia in the neck combined with at least one other body region among all 2916 cases (Table 2). For these cases, 33 were diagnosed with focal dystonia (21 focal CD, 11 focal cranial, 1 focal limb), 27 with segmental dystonia, and 1 multifocal dystonia. Although data entry errors cannot be entirely excluded, evaluation of GDRS scores suggested that some specialists were giving a diagnosis based on the most severely affected region, while others based their diagnosis on the whole distribution of body regions regardless of severity. For example, for all 21 cases diagnosed with focal CD, GDRS scores were higher for neck than any of the accompanying regions. Overall, these data demonstrate significant variations in the practices of movement disorders specialists in diagnosing focal versus broader patterns of involvement in CD.
Neck Dystonia with Tremor
Approximately half of all individuals with CD also have tremor, most commonly affecting the head/neck and/or upper limbs. 14 Whether these tremors should be considered a manifestation of dystonia or a coincidental tremor disorder is not clear. To determine how the presence of tremor might influence diagnoses given by movement disorder specialists, data regarding which body regions were affected with tremor were evaluated. In the Dystonia Coalition database, the presence or absence of tremor is recorded in the examination checklist for individual body regions, although tremor severity scores are not collected.
Among all 1258 cases diagnosed as having focal CD, 930 (73.9%) were recorded to have clinically visible tremor of at least one body area. The most common body areas with tremor included the neck (N = 720, 57.2%), hand (N = 157, 12.5%), upper arm (N = 38, 3.0%), and larynx (N = 21, 1.7%) (Table 3). If tremor is considered part of the spectrum of dystonia, then a significant portion of these subjects diagnosed with focal CD would be re‐classified as having segmental or multifocal dystonia. For example, if hand tremors in CD are considered part of the spectrum of dystonia, then 157 subjects with CD and hand tremors (12.5%) would have segmental or multifocal dystonia, depending on whether or not the arm was also affected. Overall, these results imply that most investigators do not consider the presence of tremor in body regions not affected with dystonia when making determinations of focal, segmental, or multifocal dystonia.
TABLE 3.
Dystonia and tremor in cases diagnosed with focal CD
| Body region affected on exam | Cases with dystonia | Cases with tremor |
|---|---|---|
| Neck | 1245 | 720 |
| Shoulder | 226 | 8 |
| Hand | 69 | 157 |
| Larynx | 36 | 21 |
| Upper face | 51 | 10 |
| Lower face | 37 | 5 |
| Upper arm | 28 | 38 |
| Trunk | 23 | 10 |
| Jaw | 16 | 7 |
| Foot | 5 | 5 |
| Upper leg | 4 | 6 |
| Tongue | 4 | 5 |
| Pelvis | 0 | 0 |
This table shows the body distribution of dystonia or tremor from the examination checklist among 1258 cases diagnosed with focal CD.
Discussion
Although CD is the most common dystonia in most movement disorders clinics, 7 there are no widely accepted diagnostic criteria. 8 There are recommendations for what delineates focal CD versus broader segmental or multifocal patterns of involvement. 3 However, the most important finding from the current study is that these recommendations are not consistently followed. Specifically, there is a high frequency of dystonia outside of the neck region (28.2%) among patients classified by specialists in movement disorders as having focal CD.
The lack of consistency may reflect varied interpretation of the 2013 expert consensus panel recommendations, which state that only one body region may be affected to qualify as focal dystonia, while at the same time recognizing the common convention of allowing shoulder involvement in focal CD. 3 Uncertainty regarding the exception for shoulder as part of focal CD may come in part from commonly used dystonia rating scales, where the Toronto Western Spasmodic Torticollis Rating Scale for CD includes the shoulder, but the shoulder is listed as a body region separate from the neck in the GDRS. 13 Results from the current study imply additional factors that may lead to variations in the diagnosis and classification of CD as a focal dystonia. For example, some specialists appear to base their overall diagnosis on the most severely affected region, even though other body regions may also be affected to a lesser degree. Another reason for the varied use of diagnostic labels is that some investigators may carry forward the initial diagnosis despite progression over time, or they may use only the diagnosis being treated, even though there may be other body regions involved. Data to evaluate these possibilities were not available. Another factor that may contribute to variations in the diagnosis and classification of focal CD is the lack of consensus regarding tremor as an intrinsic or accompanying feature of dystonia. Whatever the reasons, the results point to the need for more specific guidelines that delineate focal CD from broader distributions.
In accordance with current guidelines, 3 we propose that the diagnosis and classification of focal CD be reserved for individuals with involvement of muscles that move the neck, with or without neck tremor. It is important to recognize that several muscles that move the neck can also move the shoulder. For example, the trapezius or levator scapula causes laterocollis when the shoulder is fixed. These same muscles cause shoulder elevation when the neck is fixed. Therefore, we recommend that the combination of neck and shoulder dystonia be classified as focal CD, in keeping with the 2013 expert consensus recommendation. 3 However, additional involvement of the upper arm should be labeled segmental dystonia, even when it is mild (Table 4).
TABLE 4.
Recommendations for diagnosis of cervical dystonia according to body regions affected
| Diagnosis | Body regions involved |
|---|---|
| Focal cervical dystonia | Neck only |
| Neck plus shoulder | |
| Neck plus platysma | |
| Segmental dystonia with neck involvement | Neck plus shoulder and upper arm |
| Neck plus shoulder and whole arm/hand | |
| Neck plus jaw/tongue | |
| Neck plus lower face | |
| Neck plus larynx | |
| Neck plus trunk | |
| Multifocal dystonia with neck involvement | Neck plus hand (excluding upper arm) |
| Neck plus upper face (not including lower face, jaw or tongue) | |
| Neck plus lower limb | |
| Generalized dystonia with neck involvement | Neck plus trunk plus at least one other body region |
Most investigators classified the combination of cervical and laryngeal dystonia as segmental, not focal dystonia. Although the larynx is obviously located in the neck, it has substantially different muscle control, innervation, and developmental origins. Therefore, a diagnosis of segmental dystonia seems more appropriate when there is cervical combined with laryngeal dystonia. This recommendation is consistent with results from the current study, where specialists more often classified the combination of cervical and laryngeal dystonia as segmental, not focal dystonia. Dystonia can also affect the platysma, which was not separately evaluated in the current studies because it is not included as part of the examination checklist or GDRS. Although the innervation and developmental origins of the platysma are more closely aligned with the face, we recommend that the combination of dystonia in the neck and platysma be labeled focal CD, because the platysma can contribute to antecollis. Therefore, we suggest that involvement of the platysma is consistent with a diagnosis of focal CD. A summary of recommendations for CD based on all potential body regions affected is provided in Table 4.
Tremor is another issue that needs to be considered when classifying dystonias according to the body region affected. Oscillatory movements of a body region simultaneously affected by dystonia are sometimes considered part of the spectrum of dystonia. However, the relevance of tremors in body regions unaffected by dystonia is less clear. Some investigators consider these tremors to be an independent co‐existing movement disorder. Others prefer to consider them part of the spectrum of dystonia, especially if they have a jerky and irregular quality. The inclusion of tremor in classifying dystonias according to body region affected remains a major challenge, because of the lack of consensus regarding how dystonic tremors should be defined, along with uncertainty regarding whether dystonic tremor can be discriminated from other tremors by bedside exam alone. 14 Until additional data are available for further guidance when considering the role of tremor in subjects with dystonia, we recommend continuing the current tradition used by the vast majority of specialists that does not include tremor occurring in body regions unaffected by dystonia when classifying dystonia according to body region affected.
A precise diagnosis is important for identifying clearly defined populations for many studies. It is important for treatment studies that involve patient‐reported outcomes or quality of life for CD, because dystonia outside of the neck region may contribute to disability and therefore increase experimental variance when evaluating the effect of an intervention. A precise diagnosis is even more important for genetic studies, because a single incorrect diagnosis can dramatically alter outcomes. The results of the current study highlight several sources of inconsistency and provide recommendations to address them.
Author Roles
(1) Research Project: A. Conception, B. Organization, C. Execution; (2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique; (3) Manuscript Preparation: A. Writing of the first draft, B. Review and Critique.
G.K.B.:1B, 1C, 2A, 2B, 3A
S.P.R., 1C, 3B
J.S.P.: 1C, 3B
M.H.: 1B, 3B
C.K.: 1C, 3B
A.W.S.: 1C, 3B
I.A.M.: 1C, 3B
S.G.R.: 1C, 3B
B.D.B.: 1C, 3B
J.F.: 1C, 3B
M.V.: 1C, 3B
E.R.: 1C, 3B
J.J.: 1C, 3B
A.M.: 1C, 3B
A.J.E.: 1C, 3B
R.L.B.: 1C, 3B
M.S.L.: 1C, 3B
A.P.: 1C, 3B
S.F.: 1C, 3B
N.S.: 1C, 3B
A.B.: 1C, 3B
J.L.K.: 1C, 3B
G.D.: 1C, 3B
S.A.N.: 1C, 3B
H.A.J.: 1A, 1B, 1C, 2A, 3A
Disclosures
Ethical Compliance Statement
Data for these analyses were obtained from the Dystonia Coalition, an ongoing multicenter international project aimed at delineating the clinical features and evolution of all isolated dystonias. All subjects gave written informed consent at the recruiting site according to the Declaration of Helsinki and The Common Rule. For the current study, the analysis of de‐identified aggregate data was further approved by the Emory University Human Subjects Review Board. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Funding Sources and Conflicts of Interest
This work was supported by grants to The Dystonia Coalition (NS065701, TR001456, NS116025) which is part of the National Institutes of Health (NIH) Rare Disease Clinical Research Network (RDCRN), supported by the Office of Rare Diseases Research (ORDR) at the National Center for Advancing Translational Science (NCATS), and the National Institute of Neurological Diseases and Stroke (NINDS). TD also was supported in part by training grant NINDS T32‐NS007480 at Emory University. The authors have no conflicts of interest to report.
Financial Disclosures for Previous 12 Months
AB has no additional disclosures to report. AJE has received grant support from the NIH and the Michael J Fox Foundation; personal compensation as a consultant/scientific advisory board member for Abbvie, Neuroderm, Neurocrine, Amneal, Acadia, Acorda, Kyowa Kirin, Sunovion, Lundbeck, and USWorldMeds; honoraria from Acadia, Sunovion, Amneal, USWorldMeds; and publishing royalties from Lippincott Williams & Wilkins, Cambridge University Press, and Springer. AM has received funding from the Dystonia Medical Research Foundation, Sunflower Parkinson's disease foundation and Parkinson's Foundation for work outside the submitted work. He reports no conflicts of interest. AP has been supported by the National Institutes of Health. He has served as a consultant for US WorldMeds and MedRhythms, Inc. AWS reports grants from the NIH and has received grant support from Benign Essential Blepharospasm Research foundation, Dystonia coalition, Dystonia Medical Research foundation, National Organization for Rare Disorders and grant support from NIH (KL2 and K23 NS092957‐01A1). BDB, in the last 24 months, has received research grant support from the Dystonia Coalition (receives the majority of its support through NIH grant NS065701 from the Office of Rare Diseases Research in the National Center for Advancing Translational Science and National Institute of Neurological Disorders and Stroke), Parkinson's Foundation, and the VCU School of Medicine. He is on the medical advisory board of the Benign Essential Blepharospasm Research Foundation and the National Spasmodic Torticollis Association. CK serves as a consultant to Centogene for genetic testing reports in the fields of movement disorders and dementia, excluding Parkinson's disease, and is part of the Scientific Advisory Board of Retromer Therapeutics. ER received research support from Merz‐Pharma, Orkyn, Elivie, Ipsen, Allergan, Everpharma, Fondation Desmarest, AMADYS, Fonds de Dotation Brou de Laurière, Société Française de Médecine Esthétique, ADCY5.org, Agence Nationale de la Recherche. GD has no additional disclosures to report. GKB has no additional disclosures to report. HAJ has active or recent grant support from the US government (National Institutes of Health), private philanthropic organizations (Cure Dystonia Now), and industry (Revance Therapeutics, Inc.). Dr. Jinnah has also served on advisory boards or as a consultant for Addex, Allergan, CoA Therapeutics, Cavion Therapeutics, EnePharmaceuticals, Ipsen, Retrophin, Revance, and Takaha Pharmaceuticals. He has received honoraria or stipends for lectures or administrative work from the International Parkinson's Disease and Movement Disorders Society. Dr. Jinnah serves on the Scientific Advisory Boards for several private foundations including the Benign Essential Blepharospasm Research Foundation, Cure Dystonia Now, the Dystonia Medical Research Foundation, the Tourette Association of America, and Tyler's Hope for a Cure. He also is principle investigator for the Dystonia Coalition, which has received the majority of its support through the NIH (grants NS116025, NS065701 from the National Institutes of Neurological Disorders and Stroke and TR001456 from the Office of Rare Diseases Research at the National Center for Advancing Translational Sciences). The Dystonia Coalition has received additional material or administrative support from industry sponsors (Allergan Inc. and Merz Pharmaceuticals) as well as private foundations (The Benign Essential Blepharospasm Foundation, Cure Dystonia Now, The Dystonia Medical Research Foundation, and The National Spasmodic Dysphonia Association). IAM has participated in research funded by the Parkinson Foundation, Tourette Association, Dystonia Coalition, AbbVie, Boston Scientific, Eli Lilly, Neuroderm, Prilenia, Revance, Teva but has no owner interest in any pharmaceutical company. She has received travel compensation or honoraria from the Tourette Association of America, Parkinson Foundation, Medscape, and Cleveland Clinic, and royalties from Robert Rose publishers. JF has no additional disclosures to report. JJ has received research or training grants from AbbVie Inc; Acadia Pharmaceuticals; Cerevel Therapeutics; CHDI Foundation; Dystonia Coalition; Emalex Biosciences, Inc; F. Hoffmann‐La Roche Ltd; Huntington Study Group; Medtronic Neuromodulation; Merz Pharmaceuticals; Michael J Fox Foundation for Parkinson Research; National Institutes of Health; Neuraly, Inc.; Neurocrine Biosciences; Parkinson's Foundation; Parkinson Study Group; Prilenia Therapeutics; Revance Therapeutics, Inc; Teva Pharmaceutical Industries Ltd. Dr. Jankovic has served as a consultant for Aeon BioPharma; Allergan, Inc; Revance Therapeutics; Teva Pharmaceutical Industries Ltd. Dr. Jankovic has received royalties from Cambridge; Elsevier; Medlink: Neurology; Lippincott Williams and Wilkins; UpToDate; Wiley‐Blackwell. JLK has no additional disclosures to report. JSP was supported by NIH (NINDS/NIA) NS075321, the American Parkinson Disease Association (APDA), the Greater St. Louis Chapter of the APDA, the Barnes Jewish Hospital Foundation (Elliot Stein Family Fund), the Oertli Fund, the Murphy Fund and the Paula and Rodger Riney Fund. MH is an inventor of patents held by NIH for an immunotoxin for the treatment of focal movement disorders and the H‐coil for magnetic stimulation; in relation to the latter, he has received license fee payments from the NIH (from Brainsway). He is on the Medical Advisory Boards of CALA Health and Brainsway (both unpaid positions). He is on the Editorial Board of approximately 15 journals and receives royalties and/or honoraria from publishing from Cambridge University Press, Oxford University Press, Springer, Wiley, Wolters Kluwer, and Elsevier. He has research grants from Medtronic, Inc. for a study of DBS for dystonia and CALA Health for studies of a device to suppress tremor. MH is supported by the NINDS Intramural Program. MSL has been supported by the Dystonia Medical Research Foundation, Benign Essential Blepharospasm Research Foundation, Revance Therapeutics, PharmaTwoB, Michael J Fox Foundation, National Institutes of Health, Cerevel, Aeon, Neurocrine, and Teva Pharmaceutical Industries. He has served as a consultant for US WorldMeds, and speaker for US WorldMeds, Supernus, Amneal, Acadia Pharmaceuticals, Teva Pharmaceutical Industries, Kyowa Kirin, and Acorda Therapeutics. MV has no additional disclosures to report. NS has no additional disclosures to report. RLB has received grant support from the Dystonia Coalition, Revance, and Vaccinex; serves on an advisory board for Acorda, Allergan, Oscine Corporation, and Revance; receives honoraria from Neurology Clinical Practice; and contractual payment from Visual Dx. SAN has active or recent grant support from the US government (National Institutes of Health) and Dystonia Medical Research Foundation. He serves on the Dystonia Medical Research Foundation Medical And Scientific Advisory Council. SF has no additional disclosures to report. SPR has no additional disclosures to report. SR has received research support from the NINDS; served as a consultant for the MDS and Best Doctors; served as reviewer for UpToDate; served as the chair of the Data Safety Monitoring Board of Enterin; and has received book royalties from Springer and Oxford.
Relevant disclosures and conflicts of interest are listed at the end of this article.
References
- 1. Jinnah HA. The dystonias. Continuum 2019;25:976–1000. [DOI] [PubMed] [Google Scholar]
- 2. Jinnah HA, Albanese A. The new classification for the dystonias: why was it needed and how was it accomplished? Mov Disord Clin Pract 2014;1:280–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Albanese A, Bhatia K, Bressman SB, et al. Phenomenology and classification of dystonia: a consensus update. Mov Disord 2013;28:863–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sasikumar S, Albanese A, Krauss JK, Fasano A. Implementation of the current dystonia classification from 2013 to 2018. Mov Disord Clin Pract 2019;6:250–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lumsden DE, Gimeno H, Lin JP. Classification of dystonia in childhood. Parkinsonism Relat Disord 2016;33:138–141. [DOI] [PubMed] [Google Scholar]
- 6. van Egmond ME, Contarino MF, Lugtenberg CHA, et al. Variable interpretation of the dystonia consensus classification items compromises its solidity. Mov Disord 2019;34(3):317–320. [DOI] [PubMed] [Google Scholar]
- 7. Ortiz R, Scheperjans F, Mertsalmi T, Pekkonen E. The prevalence of adult‐onset isolated dystonia in Finland 2007–2016. PLoS One 2018;13:e0207729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Defazio G, Albanese A, Pellicciari R, et al. Expert recommendations for diagnosing cervical, oromandibular, and limb dystonia. Neurol Sci 2019;40:89–95. [DOI] [PubMed] [Google Scholar]
- 9. Kilic‐Berkmen G, Wright LJ, Perlmutter JS, et al. The dystonia coalition: a multicenter network for clinical and translational studies. Front Neurol 2021;12:660909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yan L, Hicks M, Winslow K, et al. Secured web‐based video repository for multicenter studies. Parkinsonism Relat Disord 2015;21:366–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Norris SA, Jinnah HA, Espay AJ, et al. Clinical and demographic characteristics related to onset site and spread of cervical dystonia. Mov Disord 2016;31:1874–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Berman BD, Groth CL, Sillau SH, et al. Risk of spread in adult‐onset isolated focal dystonia: a prospective international cohort study. J Neurol Neurosurg Psychiatry 2019;91:314–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Comella CL, Leurgans S, Wuu J, Stebbins GT, Chmura T. Rating scales for dystonia: a multicenter trial. Mov Disord 2003;18:303–312. [DOI] [PubMed] [Google Scholar]
- 14. Shaikh AG, Beylergil SB, Scorr L, et al. Dystonia & tremor: a cross‐sectional study of the dystonia coalition cohort. Neurology 2021;96:e563–e574. [DOI] [PMC free article] [PubMed] [Google Scholar]


