Eukaryotic translation initiation factor 2‐alpha kinases (EIF2AK) are serine–threonine kinases involved in integrated stress response, a cytoprotective pathway which ensures adaptation of mammalian cells to stress conditions. 1 Among the four members of this protein family, EIF2AK2, also known as Protein Kinase R, is activated by double‐stranded RNA (primarily during viral infections), oxidative stress, endoplasmic reticulum (ER) stress, cytokines, and growth factors. 2 By phosphorylating Eukaryotic Translation Initiation Factor 2 Subunit 1 (EIF2S1) in response to cellular stressors, EIF2AK2 negatively regulates mRNA translation and protein synthesis and induces apoptosis. 1 , 2
De novo missense variants in the EIF2AK2 gene were first linked to a complex neurological syndrome characterized by developmental delay, language impairment, various combinations of motor manifestations (including cerebellar, pyramidal, and dystonic features), and brain MRI abnormalities (encompassing dysmyelination, thin corpus callosum, and cerebral and/or vermian atrophy) in nine unrelated children in 2020. 3 Intriguingly, all individuals with EIF2AK2 variants exhibited neurological deterioration in the context of febrile illness or infection. 3
In 2021, Kuipers, Musacchio, and respective colleagues reported 13 individuals from six pedigrees carrying heterozygous (autosomal dominantly inherited or de novo) or homozygous EIF2AK2 missense variants which cause early‐onset, mostly isolated, generalized dystonia likely through a gain‐of‐function mechanism. 4 , 5
In order to replicate the association between EIF2AK2 mutations and isolated dystonia phenotypes, we retrieved our internal database of approximately 18,000 exomes (522 belonging to subjects recruited under the diagnostic category “dystonia”) and the 100,000 Genomes Project repository (1116 participants enrolled using the Human Phenotype Ontology term “dystonia”) searching for EIF2AK2 variants previously found in dystonia patients and other rare variants.
The missense variant NM_001135651(EIF2AK2):c.388G>C (p.Gly130Arg) was detected in the heterozygous state in a 28‐year‐old Algerian male who underwent whole‐exome sequencing (WES) for adolescence‐onset generalized dystonia with leg involvement. 6 He was healthy until age 17, when he presented with dystonic posturing of the first two left fingers. Four years later he developed right hand tremor while performing fine manual tasks. Tremor spread to the head and contralateral hand over few months. At this time, he started experiencing chewing and gait difficulties and abnormal trunk posture on walking, which was alleviated by trunk anteflexion, walking backwards, carrying a heavy load, or running. His past medical history included myopia and scoliosis. There was no history of exposure to dopamine receptor antagonists. He was the eldest of five siblings born to non‐consanguineous parents. His family history was negative for neurological disorders. On examination (Fig. 1A; Video 1), he had dysarthria and generalized dystonia mainly affecting the trunk and arms. Truncal dystonia showed extensor and torsional components on walking. No parkinsonian, pyramidal, cerebellar, or cognitive signs were detected. Serum copper and ceruloplasmin, iron profile, vitamin E, brain MRI and NCS/EMG were unremarkable. Dystonia did not respond to levodopa nor trihexyphenidyl but showed a 40% improvement in severity from baseline with deep brain stimulation (DBS) of the globus pallidus internus (age 24; Video 1) over a 3‐year period. Prior to WES, the patient had been tested negative for TOR1A and THAP1 through single gene testing. On WES, we did not identify any other potential candidate variants in genes linked to monogenic movement disorders (MD). We acknowledge that no quantitative genetic testing was performed to rule out variants in MD‐related genes potentially missed due to next‐generation sequencing intrinsic limitations. Segregation analysis revealed the proband's parents did not carry the EIF2AK2 mutation detected, which is therefore assumed de novo according to the American College of Medical Genetics and Genomics (ACGM) guidelines (maternity and paternity not genetically confirmed; Fig. 1B). 7
FIG. 1.
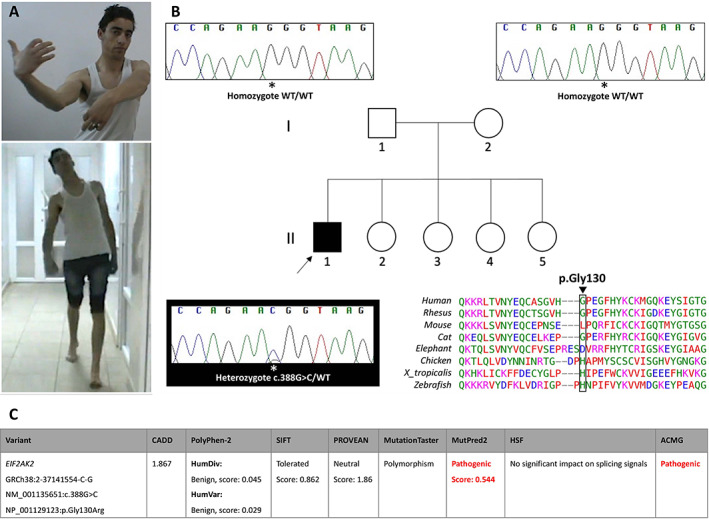
(A) Video frames showing the proband with prominent upper limb and truncal dystonia, the latter having extensor and torsional components on walking. (B) Family tree and segregation analysis revealing the NM_001135651(EIF2AK2):c.388G>C (p.Gly130Arg) missense variant occurred in the heterozygous state only in the proband (II‐1). The DNA region of interest was amplified bidirectionally using the following primers (5′→3′): F‐catggggaattacataggcct and R‐gtggcaccctgtactctctt, with an amplicon size of 385 base pairs. Electropherograms were analyzed using the Sequencher software package. Arrow: proband. WT: wild type. Bottom right. Interspecies alignment showing lack of evolutionary conservation of the amino acid involved by the variant. (C) Functional analysis of the EIF2AK2 variant herein reported. ACMG: American College of Medical Genetics and Genomics; CADD: Combined Annotation Dependent Depletion (https://cadd.gs.washington.edu/snv); HSF: Human Splicing Finder (https://hsf.genomnis.com/home); MutationTaster (http://www.mutationtaster.org/); MutPred2 (http://mutpred.mutdb.org); PolyPhen‐2: Polymorphism Phenotyping v2 (http://genetics.bwh.harvard.edu/pph2/); PROVEAN: Protein Variation Effect Analyzer (http://provean.jcvi.org/index.php); SIFT: Sorting Intolerant From Tolerant (http://sift.bii.a‐star.edu.sg).
Video 1.
First segment. The proband (age 23) presented with dysarthria and dystonia mainly affecting the trunk and arms. Truncal dystonia worsened on walking, with extensor and torsional components. Dystonic posturing of the upper limbs while writing. Second segment. The proband (age 24) with truncal dystonia. Third segment. The proband (age 27) after undergoing DBS of the globus pallidus internus at age 24 showed improvement of dysarthria, upper limb and truncal dystonia, and gait.
The EIF2AK2 variant herein reported consists in a novel nucleotide change (NM_001135651:c.388G>C) causing the same amino acid substitution p.Gly130Arg previously reported in 10 affected individuals from four pedigrees who however carried a guanosine‐to‐adenosine substitution at the same position (NM_001135651:c.388G>A), which is due to codon redundancy. 4 , 5 The mutation is absent in the population database gnomAD (https://gnomad.broadinstitute.org/) and predicted benign/tolerated by most in silico tools, including a low Combined Annotation Dependent Depletion (CADD) score (Fig. 1C). This likely reflects intrinsic limitations of pathogenicity prediction algorithms when examining regions with poor evolutionary conservation, as is the case of this amino acid residue (Fig. 1B). 4 The variant is pathogenic according to ACGM guidelines. 7 No other rare EIF2AK2 variants associated with isolated dystonia phenotypes were identified by screening the above‐mentioned datasets (Supplementary File S1).
Our case further supports the inclusion of EIF2AK2 mutation analysis in the diagnostic workup of early‐onset isolated generalized dystonia, including sporadic cases. 4 Furthermore, it confirms that DBS is an effective treatment for EIF2AK2‐associated dystonia. 4 , 5 Although triggers or precipitating factors were not identifiable in our patient's history and clinical course, the association between EIF2AK2 variants and dystonia might directly link inflammatory or infectious events and phenotypic expression of dystonia in patients with causative (incompletely penetrant) or predisposing genetic makeup, thereby unveiling one of the molecular underpinnings of gene‐environmental interaction in dystonia pathogenesis. 8 Defective EIF2S1 signaling pathway is the shared pathobiological mechanism on which not only EIF2AK2 mutations but also other monogenic causes of dystonia, including DYT‐TOR1A, DYT‐THAP1, DYT‐PRKRA, and DYT‐SGCE converge, either directly or indirectly. 4 , 10 In addition, since the EIF2S1 pathway plays a role in regulating neuronal long‐term synaptic plasticity, EIF2AK2 variants might represent a direct link between ER stress and aberrant synaptic plasticity, which is a well‐recognized pathophysiological mechanism of dystonia. 10 Finally, we speculate that EIF2AK2(NM_001135651) nucleotide 388 might be critical for EIF2AK2‐related dystonia since a variant at this level segregates with the phenotype in five out of seven kindreds hitherto reported, including the present one. Further evidence is warranted to establish whether the corresponding codon or, more broadly, the second double‐stranded RNA binding motif of EIF2AK2 might represent a mutational hotspot for EIF2AK2‐associated dystonia. 4
Author Roles
(1) Research project: A. Conception, B. Organization, C. Execution; (2) Data Analysis: A. Design, B. Execution, C. Review and Critique; (3) Manuscript Preparation: A. Writing of the first draft, B. Review and Critique.
F.M.: 1A, 1B, 1C, 2B, 3A
D.M.: 1B, 1C, 3B
M.T.: 1B, 1C, 3B
L.A.P.: 1B, 1C, 3B
A.V.: 3B
K.P.B.: 1C, 2C, 3B
R.M.: 1A, 1B, 1C, 2C, 3B
H.H.: 1C, 2C, 3B
Disclosures
Ethical Compliance Statement: We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines. The authors confirm that the approval of an institutional review board was not required for this work. We confirm that we have obtained the patient consent for genetic testing on a research basis as well as for video acquisition and publication.
Funding Sources and Conflicts of Interest: Biological samples from the family here reported were collected as part of the SYNaPS Study Group collaboration funded by The Wellcome Trust and strategic award (Synaptopathies) funding (WT093205 MA and WT104033AIA) and research was conducted as part of the Queen Square Genomics group at University College London, supported by the National Institute for Health Research University College London Hospitals Biomedical Research Centre. The authors declare that there are no conflicts of interest relevant to this work.
Financial Disclosures for the Previous 12 Months: Francesca Magrinelli is supported by the Edmond J Safra Foundation and by the research grant “Fondo Gianesini” in collaboration with UniCredit Foundation and University of Verona, Italy. Kailash P. Bhatia has received grant support from Welcome/MRC, NIHR, Parkinson's UK and EU Horizon 2020. He receives royalties from publication of the Oxford Specialist Handbook Parkinson's Disease and Other Movement Disorders (Oxford University Press, 2008), of Marsden's Book of Movement Disorders (Oxford University Press, 2012), and of Case Studies in Movement Disorders–Common and uncommon presentations (Cambridge University Press, 2017). He has received honoraria/personal compensation for participating as consultant/scientific board member from Ipsen, Allergan, Merz and honoraria for speaking at meetings and from Allergan, Ipsen, Merz, Sun Pharma, Teva, UCB Pharmaceuticals and from the American Academy of Neurology and the International Parkinson's Disease and Movement Disorders Society. Henry Houlden is funded by The MRC (MR/S01165X/1, MR/S005021/1, G0601943), The National Institute for Health Research University College London Hospitals Biomedical Research Centre, Rosetree Trust, Ataxia UK, MSA Trust, Brain Research UK, Sparks GOSH Charity, Muscular Dystrophy UK (MDUK), Muscular Dystrophy Association (MDA USA).
Supporting information
Supplementary File S1 Rare EIF2AK2 variants identified by screening our internal exome database and the rare disease sub‐cohort of the 100,000 Genomes Project.
Acknowledgments
The authors would like to thank the patient and his family for participating in this study. The Authors are grateful to Clarissa Rocca and David Murphy (UCL Queen Square Institute of Neurology, University College London, London, United Kingdom) for their valuable bioinformatic support as well as to Dr Eoin Mulroy (UCL Queen Square Institute of Neurology, University College London, London, United Kingdom) for his kind support with post‐DBS assessment.
Relevant disclosures and conflicts of interest are listed at the end of this article.
References
- 1. Costa‐Mattioli M, Walter P. The integrated stress response: from mechanism to disease. Science 2020;368(6489):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Krishna KH, Kumar MS. Molecular evolution and functional divergence of eukaryotic translation initiation factor 2‐alpha kinases. PLoS One 2018;13(3):e0194335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mao D, Reuter CM, Ruzhnikov MRZ, et al. De novo EIF2AK1 and EIF2AK2 variants are associated with developmental delay, leukoencephalopathy, and neurologic decompensation. Am J Hum Genet 2020;106(4):570–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kuipers DJS, Mandemakers W, Lu CS, et al. EIF2AK2 missense variants associated with early onset generalized dystonia. Ann Neurol 2021;89(3):485–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Musacchio T, Zech M, Reich MM, Winkelmann J, Volkmann J. A recurrent EIF2AK2 missense variant causes autosomal‐dominant isolated dystonia. Ann Neurol 2021;89(6):1257–1258. [DOI] [PubMed] [Google Scholar]
- 6. Köhler S, Gargano M, Matentzoglu N, et al. The human phenotype ontology in 2021. Nucleic Acids Res 2021;49(D1):D1207–D1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Albanese A, Bhatia K, Bressman SB, et al. Phenomenology and classification of dystonia: a consensus update. Mov Disord 2013;28(7):863–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 2015;17(5):405–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Magrinelli F, Balint B, Bhatia KP. Challenges in clinicogenetic correlations: one gene ‐ many phenotypes. Mov Disord Clin Pract 2021;8(3):299–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gonzalez‐Latapi P, Marotta N, Mencacci NE. Emerging and converging molecular mechanisms in dystonia. J Neural Transm (Vienna) 2021;128(4):483–498. [DOI] [PubMed] [Google Scholar]
- 11. Balint B, Mencacci NE, Valente EM, et al. Dystonia. Nat Rev Dis Primers 2018;4(1):25. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary File S1 Rare EIF2AK2 variants identified by screening our internal exome database and the rare disease sub‐cohort of the 100,000 Genomes Project.


