Abstract
The COVID-19 outbreak sparked by SARS-CoV-2, begat significant rates of malady worldwide, where children with an abnormal post-COVID ailment called the Multisystem Inflammatory Syndrome (MIS-C), were reported by April 2020. Here we have reviewed the clinical characteristics of the pediatric patients and the prognosis currently being utilized. A vivid comparison of MIS-C with other clinical conditions has been done. We have addressed the probable etiology and fundamental machinery of the inflammatory reactions, which drive organ failure. The involvement of androgen receptors portrays the likelihood of asymptomatic illness in children below adolescence, contributing to the concept of antibody-dependent enhancement.
Keywords: MIS-C, Pediatric patient, SARS-CoV-2, Kawasaki disease, Multiorgan failure, Macrophage and antibody-dependent enhancement (ADE)
1. Introduction
The COVID-19 pandemic generated by Severe acute respiratory syndrome coronavirus 2 has swiftly expanded globally with about 18 million confirmed reports by August 2020, after a multitude of pneumonia occurrences resulting from unexplained causes was formerly detected in Wuhan (China) in December 2019. Children generally account for a tiny percentage of COVID-19 instances. However, there is confusion regarding the real disease risk of adolescents and children, due to asymptomatic illness, inadequate examination of diagnostically quiet or moderate cases, or doubts about the accuracy of existing testing protocols.1 In children, COVID-19 hospitalization was uncommon, contributing to only 0.1% of all fatalities.2 But between April 2020, and July 2020, there has been an upsurge in the incidence of a Kawasaki-like disease in youngsters by 30 times. Pediatricians in the United Kingdom initially declared a group of children having fever, cardiovascular shock, and hyper inflammation in April 2020, with symptoms that were identical to those of Kawasaki Disease, cytokine storm, or toxic shock syndrome on the grounds of clinical studies recorded from United States, United Kingdom, Italy, Switzerland, and France.3 The ailment was named “pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2” by the Royal College of Pediatrics & Child Health. Next, the Centers for Disease Control and Prevention in the United States and the World Health Organization issued their separate case definitions for the ailment, renaming it as a multisystem inflammatory syndrome in children.4 , 5 Scientists have named it profusely like “Kawashocky”, “Coronasacki”, “hyperinflammatory shock in children with COVID-19″, “pediatric COVID-19″, “Pediatric COVID-19 Associated Inflammatory Disorder” and many more because it's a novel illness. Reports have been identified where, 15 children, 2–15 years old in the United States with MIS-C were shifted to the intensive care unit, 102 youths with identical symptoms, were recorded in New York many of whom tested positive for SARS-CoV-2 infection.3 , 6 , 7 4196 MIS-C Patients and 37 MIS-C deaths, both meeting MIS-C case definitions were confirmed to the CDC by June 28, 2021,.8 Some organs and tissues, including the heart, lungs, blood vessels, kidneys, digestive system, nervous system, skin, and eyes, become severely inflamed in children who acquire MIS-C. The signs and symptoms vary depending on which body parts are affected.2 , 3 , 9, 10, 11, 12, 13 It remains evident whether it is a post-sepsis or delayed infectious consequence or is chiefly connected with SARS-CoV-2 infection, although the recent epidemiologic accounts are extremely provocative of a relationship. 13
2. Patient demographics
The demographic analysis deals with the assessment of the population, based on variables such as age, race, and sex. Several studies under the present body of knowledge and close monitoring of MIS-C patients have led to a subjective result of the appearance of this syndrome in children.
-
•
Patients with MIS-C had a median age of 9 years. Between the ages of 5 and 13, half of the children with MIS-C were diagnosed. In other studies, the range of age is from 7 months to 20 years, with the highest proportion occurring in youths under the age of 21. 4 , 8 , 9 , 13
-
•
Early findings showed males may be highly represented, same as KD. MIS-C has yet to demonstrate a definite gender preference, but only a small male preponderance is found in six investigations. Sixty percent of reported patients were male, according to instances reported to the CDC on or before June 28, 2021,.4 , 8 , 14, 15, 16, 17, 18, 19, 20
-
•
Many studies have found that MIS-C has a significant impact on African American, African/Afro-Caribbean, and Hispanic youngsters. African/Afro-Caribbean children constituted the largest fraction of the cases in European research with relevant race/ethnicity data, ranging from 38% to 62% of MIS-C patients. The African American and Hispanic were around 18–40% and 24–45% respectively among the MIS-C affected children, in one of the U.S. reports. And, till June 2020, 62% of all cases confirmed to the CDC consisted of Hispanic children or Latino (1246 cases) or Black, Non-Hispanic (1175 cases).4 , 8 , 10 , 14 , 21, 22, 23, 24, 25
-
•
Cases recorded at CDC till June 2020 show that 99% of MIS-C sufferers tested positive for SARS-CoV-2, the rest 1% of patients might have gotten into touch with a COVID-19 infected patient.8 In a separate US analysis with 577 MIS-C patients, 52% had a positive SARS-CoV-2 Real Time-Polymerase Chain Reaction test result, 45% were solely SARS-CoV-2 antibody positive, 31% were positive for both, and an antibody test was not conducted in 19% of the cases.10 Out of 29 patients in a finding, SARS-CoV-2 Polymerase Chain Reaction tests yielded positive results in 10 cases, while SARS-CoV-2 immunoglobulin G assays yielded positive results in 19 patients.26 In these children, the initial COVID-19 infection is nearly often moderate or asymptomatic.27
-
•
Feldstein et al. spotted that 73% of MIS-C affected patients were priorly healthy in case reports of 186 individuals.16 A vast majority of studies found almost no comorbidities. Obesity and a history of asthma have been the most frequent comorbidities in individuals who did possess past medical issues across studies, with autoimmune illness, long-term lung ailment, diabetes, cancer, congenital heart disease, and neurological disorders as fundamental detections.4 , 14 , 15 , 18 , 21, 22, 23 , 28
2.1. Case definition of MIS-C
The WHO has published the case-definition MIS-C, where the following six criteria are to be fulfilled.
-
1.
Age 0–19 years
-
2.
Fever for ≥3 days
-
3.The clinical indication of the involvement of multiple organ systems (At least 2 of the mentioned manifestations)
-
i.Erythema, bilateral non-purulent conjunctivitis, or mucocutaneous or dermatological inflammation signs on mouth, hands, or feet.
-
ii.Hypotension or shock
-
iii.Cardiac disability, pericardial inflammation, coronary anomalies, or valvulitis (including echocardiographic findings or elevated troponin/brain natriuretic peptide)
-
iv.Presence of coagulopathy (prolonged prothrombin time; amplified D-dimer)
-
v.Acute gastrointestinal symptoms (diarrhoea, vomiting, or abdominal pain)
-
i.
-
4.
Inflammation markers that are elevated (namely, erythrocyte sedimentation rate, C-reactive protein, or procalcitonin).
-
5.
Other microbiological causes of inflammation, like bacterial sepsis and staphylococcal/streptococcal toxic shock syndromes are not identified.
-
6.
Reports testing positive for present or past SARS-CoV-2 pathogenesis by RT-PCR, antibody, or antigen test; or interaction with a person infected with COVID-19. 1 , 4 , 11, 12, 13 , 27
CDC also has a separate case definition that focuses on evidence of clinical symptoms involving several organs.4 , 27 , 29
3. Clinical manifestation
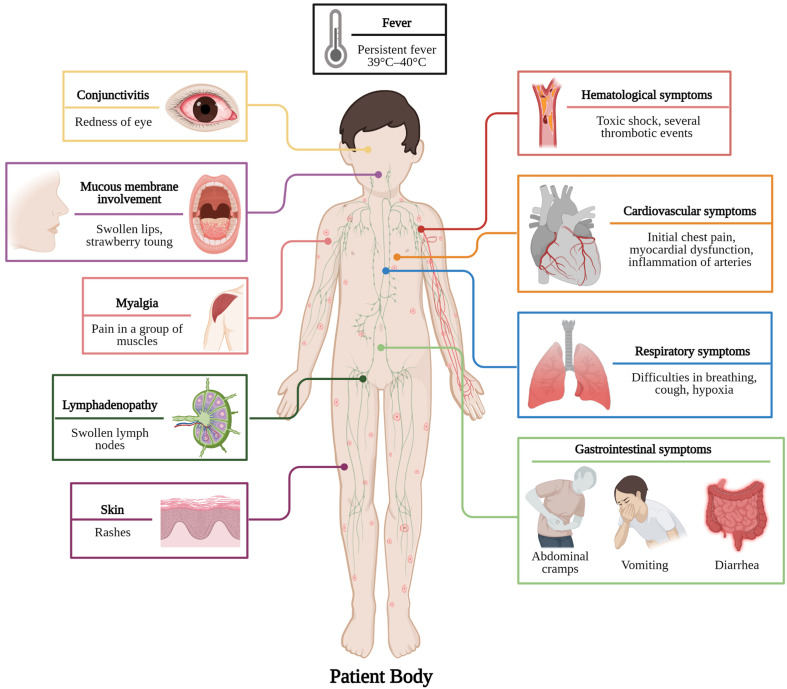
Knowledge revolving around the clinical condition of MIS-C patients is unfolding day by day.11 As a significant percentage of SARS-CoV-2 infections has escaped diagnosis, the overall population of children residing in the danger for MIS-C is unclear, owing to the possibility of asymptomatic or paucisymptomatic infections.4 Based on a temporal link of SARS-CoV-2 invasion with MIS-C, the average time between primary infection and the incidence of MIS-C symptoms, in children with a recorded history of confirmed or suspected COVID-19 infection, is two to six weeks.4 , 29 The establishment of a severe inflammatory state is one of the major symptoms of MIS-C including, spiking and persistent fever (>39°C-40 °C) with severe asthenia for a few couples of days, myalgia, swollen hands or feet, and multisystem damage (Fig. 1 ).3 , 4 , 6 , 9 , 11 , 14 , 27, 28, 29
Fig. 1.
Various types of MIS-C symptoms in pediatric patients. (Created withBioRender.com).
3.1. Cardiovascular symptoms and image finding
Patients initially felt chest pain, with an average delay of 6 days between the outset of clinical symptoms and the outset of heart failure symptoms. They experienced cardiogenic shock upon their entry to the pediatric intensive care unit and were provided with inotropic support.11 , 20 , 30 All of the investigations found cardiac abnormalities using echocardiography or electrocardiography, highlighting the appearance of myocardial dysfunction.4 Echocardiography revealed depressed systolic function, with left ventricular ejection fraction of <55% (moderate dysfunction) and sometimes <30% (severe dysfunction),19 , 21 , 30 pericarditis (pericardial effusion) and myocarditis, atrioventricular valve regurgitation, cardiac dysrhythmia, coronary dilation, or aneurysms with a medial z score range of 2.0–2.8 indicating small aneurysm and rarely giant aneurysm were reported.3 , 4 , 10 , 12, 13, 14 , 21 , 27 , 29, 30, 31 In adolescents with vasodilatory shock, cardiac magnetic resonance imaging (MRI) revealed signs of myocardial edema, necessitating fluid resuscitation.13 , 29 Cardiac involvement is an extensive factor to differentiate MIS-C from COVID-19. 10
3.2. Respiratory symptoms and image findings
Though COVID-19-like respiratory complaints are not often associated with MIS-C, difficulties in breathing like tachypnoea, cough, hypoxia, have been disclosed so far. Chest radiographs showed pulmonary edema, basilar opacities suggestive of atelectasis, either dependent or coercive as a consequence of pleural effusion, pulmonary infiltrates, pneumothorax, pulmonary hemorrhage, and bronchospasm, requiring the utility of bronchodilators continuously. Critical pulmonary infection, such as acute respiratory distress syndrome, was uncommon in children who needed supplemented oxygen or a ventilator for breathing support. 4 , 6 , 10 , 11 , 21 , 27 , 29 , 31 , 32
3.3. Neurological symptoms and image findings
The youngsters have been observed with various neurologic issues. Headaches, hearing & visual problems, amnesia, meningitis, irritability, apathy, and lassitude are some of the symptoms. Encephalopathy, stroke or abrupt intracranial hemorrhage, uveitis, coma, seizures, demyelinating disease, aseptic meningoencephalitis (strengthening pro-inflammatory Central nervous system feedback),31 and brain death were among the profound neurologic findings seen in specific cases. Rare instances reported ischemic brain infarction, acute cerebral edema, and Guillain-Barre syndrome.4 , 10 , 11 , 20 , 27 , 29 , 31
3.4. Gastrointestinal symptoms and image finding
Gastrointestinal involvement was usually the most apparent attribute of MIS-C, reported in maximum patients often resembling abdominal infections.4 , 11 , 12 Abdominal cramps, diarrhoea, and vomiting were among the prominent symptoms.4 , 11 , 12 , 14 , 27 , 29 Abdominal ultrasonography and computed tomography of the abdomen and pelvis disclosed grave results like appendicitis, gall bladder hydrops, ascites, mesenteric adenopathy, pleural effusions, enterocolitis, in certain cases terminal ileitis and colitis, all leading to hypovolemia. The pancreatic images reported pancreatomegaly, and those of the liver reported hepatomegaly, and biliary sludge, while increased renal echogenicity, lead to acute kidney failure.4 , 9 , 10 , 21 , 29 , 32
3.5. Mucocutaneous and dermatological symptoms
The mucocutaneous results were heterogeneous. Morbilliform, urticarial, scarlatiniform, and reticulated forms were among the morphologic features of exanthemas.26 The area of the skin affected also differed where certain individuals were with restricted acrofacial inclusion while others harbored more extensive outbreaks.26 Some studies have also revealed a strong age bias in the advent of symptoms.26 The prevailing cutaneous records were conjunctivitis, hyperemia, periorbital swelling and erythema, and strawberry tongue. A few dermatological findings were whereas malar rashes, facial edema, palmar erythema, lip cracks, and lip hyperemia causing redness and swelling.4 , 10 , 11 , 21 , 26 , 29 , 32 In a special case, a skin biopsy presented lymphocytic infiltrate as the root of skin lesion.26
3.6. Hematological findings
MIS-C patients were found with several thrombotic events where activation of coagulation lead to deep vein thrombosis, intracardiac thrombosis, cerebral venous sinus thrombosis, subarachnoid hemorrhage bringing about ischemic brain death.10 , 13 , 20 , 27 , 33 A prothrombotic coagulopathy may be enhanced by MIS-C's hyperinflammatory condition in conjunction with COVID-19 triggering pulmonary embolism.6 , 33 Additional hematologic abnormalities comprise lymphopenia, neutrophilia, haematolysis, hypoxemia, ischemia, anemia, pancytopenia, and hemolytic uremic syndrome.10 , 11 , 33
3.7. Lymphatic findings
Swollen lymph node often called adenopathy has been noted as a common sign of inflammation in MIS-C-affected children encompassing distinct organs like mesenteric lymphadenitis and mediastinal and hilar lymphadenopathy which have been observed through thoracic imaging.6 , 11 , 32
3.8. Laboratory findings
The common feature found in every MIS-C patient is an extremely elevated level of inflammatory and cardiac indicators.4 Inflammatory indicators like C-reactive protein, Serum interleukin-6, Ferritin, Procalcitonin are significantly raised.4 , 11 , 13 , 27 , 29 , 31 , 34, 35, 36 The elevated values of CRP, Ferritin and Procalcitonin vary as 11.98–27.62 mg/dL, 370.7–1032.5 ng/ml and 8.41–31.96 ng/mL respectively.14 The values of cardiac indicators like Troponin4 and Brain natriuretic peptide4 , 11 vary as 0.03–2.17 ng/mL, 229.5–1778.5 pg/mL respectively.14 Another characteristic feature of MIS-C is raised levels of D-dimer,4 , 13 , 14 , 20 , 30, 31, 32 , 34, 35, 36 Fibrinogen,4 , 13 , 14 , 29 Factor VIII.13 The value varies as 2.42–3.79 μg/mL for D-dimer14 and 468.5–629 mg/mL for Fibrinogen.14 The low percentage of Antithrombin III causes several types of thrombosis in patients.13 Cytokines like Tumour necrosis factor, Interleukin-6, IL-1β are synthesized in excess amounts, which upregulate the inflammatory reaction.13 , 30 MIS-C patients show abnormal Liver function test results having elevated Alanine transaminase and Aspartate transaminase.32 , 34 , 35 The values of ALT and AST vary as 27.73–73.6 U/L and 36.25–56.75 U/L respectively.14 MIS-C patients show a comparatively lower value of Lactate dehydrogenase enzyme than patients having severe COVID-19. 14 A higher Erythrocyte sedimentation rate value is also very common in MIS-C patients.11 ESR value varies as 38–58 mm/hr14 Low blood Sodium14 and Albumin 29 , 35 , 36 36 and a high Creatinine34 value are revealed by laboratory examination in MIS-C patients. According to a recent study, severe COVID-19 instances have a greater neutrophil to lymphocyte ratio.34
4. Comparing MIS-C with other associated diseases
Following the past COVID-19 infection, new publications have revealed that MIS-C possesses symptoms of an array of different disorders namely KD that had originated in Japan in 1967, Toxic Shock Syndrome that had originated in 1978, Secondary Hemophagocytic Lymphohistiocytosis/Macrophage Activation Syndrome, and Severe COVID-19 (Table 1).
Table 1.
Comparison between multisystem inflammatory syndrome in children (MIS-C), Kawasaki disease (KD), toxic shock syndrome (TSS), secondary hemophagocytic lymphohistiocytosis/macrophage activation syndrome (SHLH/MAS), and severe COVID-19.
| Sl. No. | Characters | Multisystem inflammatory syndrome in children (MIS-C) | Other diseases associated with MIS-C |
References | |||||
|---|---|---|---|---|---|---|---|---|---|
| Kawasaki disease (KD) | Toxic Shock Syndrome (TSS) |
Secondary Hemophagocytic lymphohis-tiocytosis/Macrophage activation syndrome (SHLH/MAS) |
Severe COVID-19 in children without MIS-C | Severe COVID-19 in adults | |||||
| 1. | Age of affected persons | Children of age range 8–10 are most commonly affected. | Usually in youngsters of less than five years of age. | Usually in children above the age of ten. | Mostly found in adults. | Adolescents are most commonly affected. | Death rates are increasing as people get older. | 2,4,10,11,29,35,37 | |
| 2. | Differences in gender | Males are mostly affected. | Males are mostly affected. | Females are mostly affected. | Occurs in males as well as females | There is no such differentiation. Both the genders are affected equally. | Males are mostly affected. | 11,37 | |
| 3. | Affected Ethnicity | Hispanic/Latino/African American |
East Asian | No ethnic variation known | No difference | No difference | No difference | 4,10,11,29,37 | |
| 4. | Symptoms | A.Hypotension | May be present or absent. | Generally absent | Almost always present | Generally absent | May be present or absent. | Almost always present | 11,29 |
| B. Rash | Generally present | Generally present | Generally present | Bleeding from the skin is noted in some cases. | May be present or absent. | May be present or absent. | 2,4,38 | ||
| C. Fever | Present | Present | Present | Present | Present | Present | 4,10,11,35,37, 38, 39 | ||
| D. Vomiting/Diarrhoea/or abdominal pain | Almost always present | Rare | Generally present | May be present or absent. | May be present or absent. | May be present or absent. | 11,35,38,39 | ||
| E. Respiratory distress | Generally present | Rare | Almost always present | Generally present | Generally present | Generally present | 11,37 | ||
| F. Mucous Membrane Involvement |
May be present or absent. | Generally present | May be present or absent. | Noted in some cases | Generally present | Generally present | 11,38 | ||
| 5. | Underlying etiology | Assumed to be a post-infectious syndrome; the SARS-CoV-2 antibody test is frequently positive; in seronegative individuals, there is generally a history of exposure to a covid-19 positive individual. | No identifiable cause. | An infection caused by streptococcus or staphylococcus is a regular occurrence. | T-cells and macrophages possess hemophagocytic activity to expand and become highly activated. | There may be underlying comorbidity; SARS-CoV-2 RT-PCR is generally positive. | SARS-CoV-2 RT-PCR is frequently positive; Extreme sickness is frequently caused by pre-existing comorbidity. | 4,29,37,404 | |
| 6. | T Cells | Lymphopenia | Involvement of cytotoxic T cells | Lymphopenia | Activation and proliferation of CD8+ T cells and NK cells, including secretion of IFNγ | Usually, unaltered | Lymphopenia in severe disease | 37,40 | |
| 7. | Comorbidity as risk factors | Immune deficiency states may be present. | Rarely observed when it comes to original immunodeficiency and occasionally in case of acquired immunodeficiency. | Normally, nothing noteworthy | The cytokine storm plays a role in coronavirus infection. COVID-19-associated pneumonia. Some people have minimal or mild lung manifestations, with others having severe pulmonary dysfunction. |
Comorbidity like malignancy, chronic lung disease and neurological disorder is linked to a more severe form of the disease. | Comorbidity like hypertension, diabetes mellitus, chronic heart disease is linked to a more severe form of the disease. | 37,40,41 | |
| 8. | Predominant manifestation | Gastrointestinal signs (abdominal discomfort, diarrhoea) are common, with more than 80% of patients experiencing them. | Symptoms of the gastrointestinal tract are rarely noticeable. | Rash, hypotension. | Unremitting fevers, cytopenia, splenomegaly, hepatitis, coagulopathy, lymphadenitis, and hepatosplenomegaly multisystem organ failure, and death in its most severe form. | Cough, respiratory distress may be present, gastrointestinal symptoms are less common. | Cough, respiratory distress is common. | 37,42,43 | |
| 9. | Management | IVIG; steroids; IL-6 inhibitors IL-1 impeders. | IVIG; steroid; IL-1 blockers | Antibiotics, IVIG | Involvement of particular cytokines in this phenomenon, especially TNF-α, IL-6, and IL-1β |
Antibiotics, antiviral medication, steroids, IVIG, IL-6 inhibitors | HCQS; steroids; IL-6 inhibitors, plasma in remission; antiviral therapies. | 37,41 | |
5. A plausible course of patient management
There exist no definitive therapeutic guidelines for the treatment of MIS-C at this time, but few current administration and therapy options are available. Most of these treatment strategies have yielded a positive result.4 Intravenous immunoglobulin and corticosteroids have been proven to be effective in various studies as a remedy for inflammation, leading to a quick recovery.4 Use of IVIG similar to normal KD therapy and corticosteroids4 , 13 , 15 , 20 , 30 , 44 has been encountered in MIS-C patients.9 , 11 , 13 , 21 , 26 , 32 , 45 , 46 Patients with a low index of suspicion present with some but not all of the MIS-C symptoms should be examined for inflammatory screening, including a complete blood count and CRP, along with SARS-CoV-2 PCR and antibody testing.13
5.1. Hospital treatment
Empiric antibiotic coverage is prescribed in children, who have been assessed for having MIS-C and have been admitted to the hospital, with initial broad-spectrum antibiotics, since symptoms overlap with severe bacterial infections. Ceftriaxone is generally suggested if they are sick to a moderate extent. In cases of severe illness or shock, vancomycin, clindamycin, and cefepime, or vancomycin, meropenem, and gentamicin are recommended.13 , 45 , 47 If redeliver (an antiviral drug with activity against SARS-CoV-2 approved for compassionate use in young children and restricted clinical trials) is available, it must be evaluated, especially for individuals who have been PCR positive and/or have a characteristic COVID-19 presentation.4 , 9 , 13 , 20 , 26 , 45 For children, the current recommended dose is 5 mg/kg IV once (max dose 200 mg) on day 1, then 2.5 mg/kg IV daily for nine days (max dose 100 mg).13 , 45 In case of all children exhibiting KD-like illness and evidence of significant inflammation (CRP >30 g/dL, ferritin >700 ng/mL), cardiac involvement, or multi-fold organ failure, 20–25 mg/kg/dose every 6 h (80–100 mg/kg/day) of aspirin is advised as a medication. However, individual health centers may use different amounts of aspirin. When a patient has been afebrile for 24 h or more, the aspirin dose typically reduces to 3–5 mg/kg as a single daily dose, which will be continued after discharge.9 , 11 , 13 , 30 Anakinra is prescribed at a dose of 2–6 mg/kg/day IV/SQ, with the period of treatment determined with the help of a pediatric rheumatologist or immunologist.4 , 11, 12, 13 , 20 , 21 , 26 , 32 , 48, 49, 50 A major percentage of patients got intravenous steroids, Infliximab, and IL-6 inhibitors (Tociluzimab or Siltuximab) as anti-inflammatory therapy.4 , 11, 12, 13 , 20 , 21 , 45 , 51 , 52 Owing to the involvement of TNF-α in MIS-C, anti-TNF-α medication is useful for the control of auto-inflammatory disorders in which many cytokines are high, implying that anti-TNF-α therapy may stop a cytokine cascade on its own.51 , 53
5.2. ICU treatment
A significant percentage of MIS-C patients are referred to the ICU, frequently requiring respiratory and cardiac assistance. Several studies indicated that about 44–100% of the children were sent to the ICU.30 A major proportion of children also required routine ventilation.18 Mild to medium doses of vasoactive medicines, like vasopressors and inotropes, were regularly administered to MIS-C ICU patients due to shock-induced by myocardial dysfunction (e.g., acute myocarditis) and/or intense vasoplegia.22 , 30
5.3. Discharge norms
Studies have revealed several guidelines that are to be taken care of before patients are discharged off. Some of them include two days without fever, two days out of vasopressors and supplemented oxygen, two to four days of declining inflammatory markers like ferritin, D-dimer, CRP, lowers levels of troponin, standard Electrocardiogram (the German spelling- Elektrokardiogramm) with stable blood pressure.4 , 13 , 20 , 45 , 51 Patients released from the emergency unit must receive particular discharge manuals including a follow-up clinic or telemedicine consultation within 72 h. A repetition of the laboratory tests must be conducted within one week. The interval between the initial echocardiography and the cardiology follow-up should be at least two weeks.13
6. Case study
COVID-19 instances (after COVID as well as current COVID) linked to MIS-C have been discovered all over the world. Some of the occurrences from various nations have been summarised in Table 2 and Table 3 simultaneously.
Table 2.
Table showing the case studies of the individual patients having Post COVID-19 MIS-C.
| Sl. No. | Region | Schedule of patient admission | Patient's description | Symptoms and image findings | Laboratory findings | Similarities with | Treatment | Reference |
|---|---|---|---|---|---|---|---|---|
| 1. | Atlanta, Georgia | June 2020 | A 25-year-old woman | Exhaustion, dyspnea, mild cough and low-grade fevers, vomiting, sore throat, diarrhoea, slight hypotension (blood pressure 98/56 mmHg) and usual blood oxygen level in indoor air, cervical lymphadenopathy; notable conjunctival injection; red and cracked lips; left lower abdominal tremble. Echocardiogram: Enlarged inferior vena cava, cardiac dysfunction. CT of abdomen/pelvis: Stranding of peri-pancreatic fat; pancreatitis, indefinite bilateral perinephric fat stranding. |
Troponin-I was discovered at 0.06 ng/mL; high levels of creatinine (7.74 mg/dL), BNP (378 pg/ml), d-dimer (960 ng/ml), ferritin (798 ng/ml). | KD | To minimize the chances of thromboembolic and nephrotoxicity, IVIG (2 g/kg) was administered in uniform dosages on the second and third day of the hospital admission, along with aspirin (325 mg) for 7 days, and redeliver. | 9 |
| 2. | New York | May 2020 | An 11-year-old female | Initial: sore throat, uneasiness, low appetite, leg and abdominal pain, pruritus skin rash, fever (39.3 °C), tachycardia (126beats/minute), hypotension, slight dehydration, erythematous palm with a widespread reticular, non-blanching papular rash across the belly and bilateral upper extremities. Echocardiogram: Decreased systolic function of the left ventricle. Electrocardiogram: Showed sinus tachycardia and S1Q3T3 indicating strain on the right side of the heart. |
Uplifted levels of troponin (0.112 ng/mL) and BNP (8718 pg/mL). White blood cell count increased to 14.18 causing lymphopenia. PTT yielded an increased value of 1.9 along with the raised levels of IL-6 (0.0–15.5 pg/mL), ferritin (13.00–150.00 ng/mL), D-dimer (0–243 ng/mL, procalcitonin (0.00–0.50 ng/mL), CRP (0.10–2.80 mg/L) and normal level of creatinine (0.53–0.79 mg/dL). | TSS, septic shock, cytokine storm, KD, SHLH | Furosemide along with antibiotics like clindamycin, ceftaroline, and piperacillin-tazobactam was administered. Enoxaparin was started as a comprehensive anticoagulant. Vitamin K was employed to improve elevated INR and PT. An IL-6 blocker, tocilizumab, was progressed along with convalescent plasma therapy and remdesivir. | 45 |
| 3. | Not found | Not found | A 14-year-old boy | Fever, tachycardia and inflamed maculopapular rash on the face, abdominal sensitivity, as well as a perianal injurydischarging pus. A 28-cm ileitis, a 2.3-cm perianal pustule, and a fistula were diagnosed on magnetic resonance enterography. | Initially, tests revealed a normal ESR rate (0–5 mg/L) and normal levels of CRP (0–15 mm/h). Increased serum amounts of IL-6 (73.4 pg/mL), IL-8 (21.8 pg/mL), IL-1β (0.4 pg/mL), TNF-α (97.8 pg/mL) were noticed in the cytokine profile up to eight days of hospitalization, which later on declined till the tenth day on treatment with infliximab. | KD | Azithromycin and hydroxychloroquine were used for SARS-CoV-2 infection, intravenous piperacillin/tazobactam was used to cure perianal abscess, and enoxaparin was utilized for the prevention of venous thromboembolism, along with intravenous fluid therapy. | 51 |
| 4. | Kerala, India | April 2020 | A 5-year-old boy | Fever of high intensity, abdominal cramps and watery stools, pyuria, bulbar conjunctivitis without pus and non-pitting edema of the feet and hands, tachycardia (130 beats per min), vasoplegia. Echocardiogram: Comprehensive left ventricular hypokinesia with medium systolic dysfunction (EF = 35%) and myocarditis was discovered. Chest X-ray: Disclosed cardiomegaly. |
Inflammatory cytokines in blood serum-like CRP, ferritin, creatinine and liver enzymes were found to be upraised. The results of a complete blood count revealed neutrophilic leucocytosis. | KD | Pulmonary support using a high flow nasal cannula with a 2 L/kg flow rate was attempted, as well as inotropic support was provided with adrenaline; Ceftriaxone, an injectable antibiotic, immunoglobulins, diuretic drugs, enalapril and methylprednisolone pulse (30 mg/kg/d for 3 d), were some of the remedies. | 47 |
Table 3.
Table showing the case studies of the cohorts having Post COVID-19 MIS-C.
| Study | Region | Schedule of patient admission | Description of patients (number, age/interquartile range [IQR]) | Number of Patients detected positive | Symptoms and Image findings | Laboratory findings | Similarities with | Treatment | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Trevor K. Young et al.(2020) | New York | April 1 to July 14, 2020 | A cohort of a patient (total = 56) IQR = 0.7–17 years |
PCR: 10/56 IgG Tests: 19/56 Mucocutaneousfindings: 27/56 |
Fever for 1–2 days, mild cough. Major mucocutaneous findings (in 21) included strawberry tongue (in 8), lip crack (in 13), conjunctivitis (in 21), erythmateous hands and feets (in 13), cheek (in 6) and orbit of the eye (in7). Eruptions of several types, i.e., mobiliform (in 3), reticulate (in 3), scarletiniform (in 5) and urtecarial (in 5). Gastrointestinal and cardiac trouble. | D-dimer, BNP, and troponin levels were all enhanced. | KD | Injectable immunoglobulin, corticosteroids, Aspirin, Remdesivir, Anakinra. | 26 |
| Blumfieldet al. (2020) | New York | April 21- May 22, 2020 | A cohort of patients (total = 16) IQR = 20 months to 20 years. |
RT-PCR: 3/16 IgG Tests: 10/16 Both RT-PCR and IgG Tests: 1/16 |
Fever (in 16), erythema (in 10), emesis (in 12), diarrhoea (in 7), abdominal discomfort (in 11), conjunctivitis (in 8), headache (in 6), and hoarseness (in 5) were the first symptoms, followed by breathing issues and congestion (in 1), hypotension (in 10), and ischemia (in 7). Echocardiography: Systolic myocardial abnormality (in 10), ectatic coronary arteries (in 4), and pericardial effusion (in 2) were discovered on echocardiography. Chest radiography: Megalocardia (in 10), cardiogenic pulmonary edema (in 9), and a modest pleural discharge (in 7) were seen on chest radiographs, with only a few patients developing pneumonia (in 1) and acute respiratory distress syndrome (in 2). CT scan of abdomen and pelvis: Abdominal fluid build-up (in 6), hepatomegaly (in 6), mesenteric lymphadenitis (in 2), and thickening of the urinary (in 1) and gall bladder (in 3) walls were all seen on abdominal radiography. |
Erythrocyte sedimentation rate (in 12), CRP (in16), D-dimer (in 16), troponin (in six), and pro-BNP (in 15) values were all raised. High white blood cell count (in 13) leading to leucocytosis and hypoalbuminaemia (in 16) were encountered too. | Kawasaki Disease (KD) | Intravenous corticosteroids Intravenous immunoglobulin and Anakinra | 32 |
| Belhadjer et al.(2020) | France and Switzerland | March 22 to April 30, 2020. | A cohort of a patient (total = 35) IQR = 2–16 years. |
Nasopharyngeal swab PCR: 12/35 Fecal PCR: 2/35 Antibody Tests: 30/35 |
All of the children had a fever and weakness, and 80% of them had gastrointestinal issues (in 29) such as abdominalache, diarrhoea, and vomiting. Runny nose (in 15), skin rashes (in 20), meningism (in 11), angina (in 6) mesenteric and cervical lymphadenopathy (in 21) were among the additional symptomatology. Echocardiography: It denoted impaired left ventricular systolic activity, with an EF of 30–50%, resulting in left ventricular hypokinesia (EF<45%) in 31 individuals. |
Heightened IL- 6, D-dimer, troponin, CRP and BNP. | KD | Inotropic support, Immunoglobulin infusion, Intravenous corticosteroids, IL-1 inhibitor and therapeutic dose of heparin. | 28 |
| Whittekar E. et al. (2020) | England | March 23 to May 16, 2020. | A cohort of a patient (total = 58) IQR = 3 months-17 years. |
PCR: 15/58 IgG Test: 40/58 |
Every single patient had a continuous fever for 3–19 days, as well as a variety of conditions such as pharyngitis (in 6), headache (in 15), abdomen ache (in 31) and lymphadenitis (in 9). Manifestations of the mucosa included distended hands and feet (in 9), erythema (in 30), conjunctival injection (in 26), reddish cracked lips (in 17). They also exhibited renal injury (in 13) and cardiac shock (in 27). Echocardiography: Malfunctioning of the left ventricle. |
All of the patients exhibited a significant inflammatory response in terms of elevated levels of CRP, troponin, ferritin, N-terminal pro-BNP and neutrophilia. | PIMS-TS and Kawasaki Disease (KD) shock syndrome. | Intravenous immunoglobulin (in 41), Corticosteroids (in 37), Anakinra (in 3) and Infliximab (in 8). | 21 |
| Kaushik et al. (2020) | New York | April 23 to May 23, 2020 | A cohort of a patient (total = 33) IQR = 6–13 years. |
RT-PCR: 11/33 Antibody Test: 27/33 Both test: 6/33 |
Major portion of the patients had fever (Avg. temperature of about 39.4C°) (in 31) and other symptoms like uneasiness of the stomach/vomiting (in 23), diarrhoea (in 16), dyspnoea (in 11), vertigo (in 3), low blood pressure (in 21), peritoneal pain (in 21), mucocutaneous involvement (in 7) like conjunctivitis (in 12) and dermatological symptoms like rashes (in 14), and also neurological involvement (in 4). Echocardiogram: Depressed LVEF with various range of EF was observed (in 21). Chest Radiograph: Megacardia (in 10) and in addition bilateral pulmonary opacities were noted (in 11). |
Inflammatory indicators like CRP, procalcitonin, D-dimer, ferritin, ESR, and fibrinogen were found to be increased. There were also heightened markers of aberrant cardiac state like, troponin, N-proBNP, and BNP. | Toxic shock | Intravenous immunoglobulin (in 18), Corticosteroids (in 17), Tocilizumab (in 12), Remdesivir (in 7), Anakinra (in 4), Convalescent plasma therapy (in1), Norepinephrine (in 10) and Dopamine (in 9). | 3,4 |
7. The most feasible mechanism of the build-out of MIS-C
Pediatric patients distressed with MIS-C exhibit large amounts of SARS-CoV-2 antibodies in their serum but test negative for the virus through RT-PCR, indicating that certified reports of COVID-19 are relatively few in children or they might have had a prior infection.1 , 3 The feedback from antibodies in children was unique from those of the adults stating that the induction of adaptive immune reaction to SARS-CoV-2 virus in the former corresponds with the onset of inflammatory symptoms and is not influenced by viral attack.1
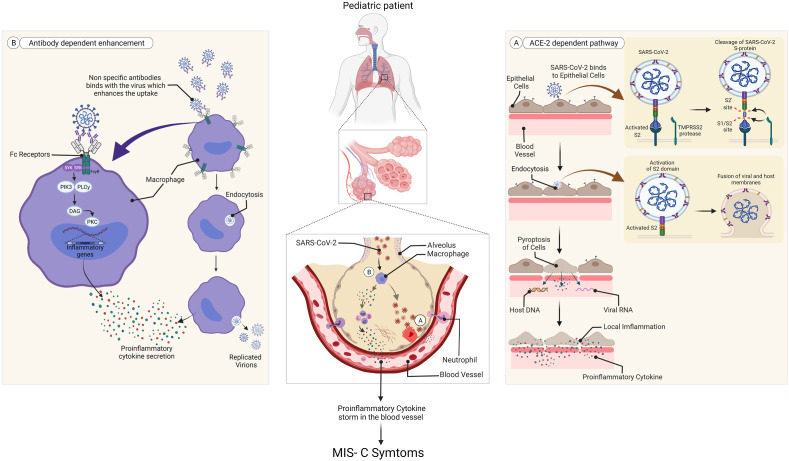
The dominant receptor for the ingress of the virus inside the human body is the Angiotensin-Converting Enzyme-2 receptor, which renders its activity along with Transmembrane protease, serine 2 cell surface protein, representing a type II Transmembrane Serine Protease, preferably in the alveolar pneumocytes.3 , 37 , 54, 55, 56, 57, 58 Mainly TMPRSS2 sunders the S- protein of SARS-CoV-2 utilizing its protease activity, into two parts S1 and S2, which facilitate binding of the virus and its unification with the target cell respectively (Fig. 2 A).3 , 37 , 59 , 60 The gene encoding for TMPRSS2 protein has been spotted in chromosome 21 of humans, whose transcription is modulated by allosomal androgen receptor transcription factor.54 , 55 , 58 , 61 , 62 Sex-steroid hormones such as testosterone reactive promoter sequence existing upstream of the gene, thereby deploy AR's activity through several signaling systems.54 , 55 Though hints of estrogen affecting the task of TMPRSS2 have been obtained, nevertheless male sex hormones form the chief regulator of TMPRSS2 bringing about a lower rate of AR activity in females in contrast to males, featuring the varying levels of extremity and mortality due to COVID-19 disease in different genders.3 , 54 , 55 , 63 , 64 Alongside lower amounts of androgens in prepubescent children conceals TMPRSS2 activity in their lung cells which contributes to the reduced prevalence and extremity of COVID-19 related inflammation in pediatric patients.3 , 41 , 54 , 55 , 65 , 66 Thereupon, adrenarche is an essential milestone that describes the reason for the greater vulnerability of children 10–12 years or above, to MIS-C, signifying that those children have entered the adrenarche stage that enhances androgen output. This age-dependent revelation of ACE2 and TMPRSS2 eases viral entry in adolescents causing pronounced pathogenesis and MIS-C symptoms, while curbing viral access in preadolescents minimizing their symptoms.3 , 54 , 55 , 67 , 68
Fig. 2.
The most likely mechanism for MIS-C expansion in pediatric patients.1,3A. ACE-2 dependent pathway B. Antibody-dependent enhancement. (Created withBioRender.com).
[ACE-2 = Angiotensin-converting enzyme 2. DAG = diacylglycerol. FcyR = Fc-gamma receptor. MIS-C = multisystem inflammatory syndrome in children. PIK3 = phosphoinositide 3 kinase. PKC = protein kinase C. PLC-y = phospholipase C gamma. SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2. SYK = tyrosine protein kinase SYK. TMPRS52 = transmembrane serine protease 2.].
Moreover, the higher concentration of serum antibodies in pediatric patients portrays the possible operation of antibody-dependent enhancement mechanism3 in provoking MIS-C, which is more certain to arise as an outcome of acquired immune response and not due to enhanced multiplication of virus.1 , 3 , 69 Certain viral disorders, like dengue and Zika virus infections, have well-documented ADE pathways.3 , 69 Reports on MIS-C patients producing neutralizing70 and non-neutralizing (binding) antibodies3 as feedback to the spike protein of SARS-CoV-2 have been obtained. Neutralizing antibodies confers sterilizing immunity by negating the pathogenic effect of the virus while the non-neutralizing one attaches to the virus but doesn't possess the potential to nullify its virulence.3 , 41 , 70 It is thought that when children are first exposed to the SARS-CoV-2 virus, their immune system produces both of these antibodies. Later on, the youngsters overridden with neutralizing antibodies are likely to suffer from asymptomatic sickness but, virus attack and critical multisystem inflammation are boosted in them with prevalent binding antibodies via ADE.3 Non-neutralizing antibodies or inadequate quantities of neutralizing antibodies bound to the epitopes of SARS-CoV-2, in the patient's blood, promote its intake inside the host tissue which is described as ADE. This machinery is unassociated with the ACE2 pathway and involves uniting of the complex of virus epitope and virus-specific non-neutralizing antibody by dint of the FC domain of immunoglobulin to the immune cell's membrane harboring IgG Fc receptor (FcR).3 , 71 This interaction activates macrophages, natural killer cells, lymphocytes, and monocytes causing cellular endocytosis3 , 41 (Fig. 2B). Endocytic Toll-like receptors such as TLR3, TLR7 detect the viral RNA and thus make the macrophages operational, inducing a surge of pro-inflammatory cytokines like TNF-α, IL-6, IL-18, IL-16, IL-1β occasionally by the NF-κβ route.3 , 37 , 41 , 71, 72, 73 This originates a cytokine storm mimicking the provocation of macrophages as seen in hemophagocytic lymphohistiocytosis.3 , 74 CD68+, CD169+ macrophages3 aid in viral dispersion and induce pyroptosis via inflammation41 , 75 (Fig. 2A). Pyroptosis indicates cell death linked to the NLR family pyrin domain containing 3 inflammasome activation systems. The cellular damage thus instigates the surrounding macrophages to generate chemokines and cytokines3 furthermore indicators of inflammation can also help macrophages to engage T-cells in the infection area.41 , 72 , 76 , 77 Elevated levels of IL-1β in blood serum evince the occurrence of pyroptosis.41 A probable role of non-specific antibodies3 has been put forward that justifies the genesis of MIS-C through ADE, in seropositive patients where non-specific antibodies unite with the virus aiding its intake by the immune cells. Gruber et al. (2020)78 have also studied the role of auto-antibodies found against endothelial and gastrointestinal cells in MIS-C patients, which fails to distinguish between self and non-self cells, ultimately attacking a patient's native tissues.1 , 78 Thus, it can be inferred that localized inflammation and the build-up of pathogenic macrophage congregations in body tissues are two especially common factors that cause MIS-C syndrome and more analysis is needed to illustrate the role of macrophages further.3
8. Conclusion
MIS-C is generally curable and rarely happens, but a certain lack of knowledge could make it severe in the long term aspect.4 As it is a rare condition, most children who have it improve with medical treatment. However, some children swiftly deteriorate to the point where their lives are jeopardized. As the number of MIS-C cases related to COVID-19 is increasing incessantly, it can be clearly stated that COVID-19 is not only just a respiratory disease,6 further elaborate research is needed to know more about the etiology of MIS-C associated with COVID-19, as it is still unknown how the risk factor for MIS-C varies among child community.4 Children develop COVID-19, unlike adults, by ADE due to a lack of androgens,3 , 54 which directly regulates the TMPRSS2 receptor.55 , 66 Therefore, to prevent the transmission of COVID-19 in this age group, parents should be more careful of their children in surroundings with a high population density.4 Precautions and safety measures such as social distancing, use of face masks, frequent washing of hands, use of alcohol-based disinfectants, should be followed in places like schools,4 parks, crèche, etc. Parents, babysitters, teachers, and school officials should primarily be cognizant of the indications and signs of both COVID-19 and MIS-C so that proper treatment is provided before its late.
Authors’ contributions
Conceptualization: [Joy Sarkar, Suchismita Kumar]; Formal analysis and investigation: [Anusrita Kundu, Joy Sarkar]; Writing – original draft preparation: [Anusrita Kundu], [Swagata Maji], [Suchismita Kumar], [Shreya Bhattacharya], [Pallab Chakraborty]; Image Preparation: [Pallab Chakraborty]; Writing – review and editing: [Joy Sarkar]; Funding acquisition: [N/A]; Resources: [N/A]; Supervision: [Joy Sarkar].
Availability of data and material
Not applicable.
Code availability
Not applicable.
Ethics approval
Not applicable.
Funding
We don't have any funding support from any organizational or institutional level.
Permission to reproduce material from other sources
Not applicable.
Consent to participate
All the authors mutually agree to participate in this work.
Consent for publication
All the authors mutually agree to submit the manuscript for publication.
Declaration of competing interest
On behalf of all listed authors, the corresponding author declares that there is not any sort of financial and non-financial conflict of interest in the subject materials mentioned in this manuscript.
Acknowledgments
We do not have any funding support from any organizational or institutional level. The authors acknowledge the immense help received from the scholars whose articles are cited and included in references of this manuscript. The authors are also grateful to authors/editors/publishers of all those articles, journals, and books from where the literature for this article has been reviewed and discussed.
References
- 1.Jiang L., Tang K., Levin M., et al. COVID-19 and multisystem inflammatory syndrome in children and adolescents. Lancet Infect Dis. 2020;20(11):e276–e288. doi: 10.1016/S1473-3099(20)30651-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vella L.A., Giles J.R., Baxter A.E., et al. Deep immune profiling of MIS-C demonstrates marked but transient immune activation compared to adult and pediatric COVID-19. Science Immunology. 2021;6(57):1–19. doi: 10.1126/SCIIMMUNOL.ABF7570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rothan H.A., Byrareddy S.N. The potential threat of multisystem inflammatory syndrome in children during the COVID-19 pandemic. Pediatr Allergy Immunol. 2021;32(1):17–22. doi: 10.1111/pai.13361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rafferty M.S., Burrows H., Joseph J.P., Leveille J., Nihtianova S., Amirian E.S. Multisystem inflammatory syndrome in children (MIS-C) and the coronavirus pandemic: current knowledge and implications for public health. J Infect Public Health. 2021;14(4):484–494. doi: 10.1016/j.jiph.2021.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Riphagen S., Gomez X., Gonzalez-Martinez C., Wilkinson N., Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020;395(10237):1607–1608. doi: 10.1016/S0140-6736(20)31094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Winant A.J., Blumfield E., Liszewski M.C., Kurian J., Foust A.M., Lee E.Y. Thoracic imaging findings of multisystem inflammatory syndrome in children associated with COVID-19: what radiologists need to know now. Radiology: Cardiothorac Imag. 2020;2(4) doi: 10.1148/ryct.2020200346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Verdoni L., Mazza A., Gervasoni A., et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020;395(10239):1771–1778. doi: 10.1016/S0140-6736(20)31103-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Health Department-Reported Cases of Multisystem Inflammatory Syndrome in Children (MIS-C) in the United States. 2021. [Google Scholar]
- 9.Kofman A.D., Sizemore E.K., Detelich J.F., Albrecht B., Piantadosi A.L. A young adult with COVID-19 and multisystem inflammatory syndrome in children (MIS-C)-like illness: a case report. BMC Infect Dis. 2020;20(1):1–4. doi: 10.1186/s12879-020-05439-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feldstein L.R., Tenforde M.W., Friedman K.G., et al. Characteristics and outcomes of US children and adolescents with multisystem inflammatory syndrome in children (MIS-C) compared with severe acute COVID-19. JAMA - J Am Med Assoc. 2021;325(11):1074–1087. doi: 10.1001/jama.2021.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakra N., Blumberg D., Herrera-Guerra A., Lakshminrusimha S. Multi-system inflammatory syndrome in children (MIS-C) following SARS-CoV-2 infection: review of clinical presentation, hypothetical pathogenesis, and proposed management. Children. 2020;7(7):69. doi: 10.3390/children7070069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Radia T., Williams N., Agrawal P., et al. Multi-system inflammatory syndrome in children & adolescents (MIS-C): a systematic review of clinical features and presentation. Paediatr Respir Rev. 2021;38(xxxx):51–57. doi: 10.1016/j.prrv.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hennon T.R., Penque M.D., Abdul-Aziz R., et al. COVID-19 associated multisystem inflammatory syndrome in children (MIS-C) guidelines; a western New York approach. Prog Pediatr Cardiol. 2020;57 doi: 10.1016/j.ppedcard.2020.101232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reiff D.D., Mannion M.L., Samuy N., Scalici P., Cron R.Q. Distinguishing active pediatric COVID-19 pneumonia from MIS-C. Pediatr Rheumatol. 2021;19(1):1–9. doi: 10.1186/s12969-021-00508-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dufort E.M., Koumans E.H., Chow E.J., et al. Multisystem inflammatory syndrome in children in New York state. N Engl J Med. 2020;383(4):347–358. doi: 10.1056/nejmoa2021756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feldstein L.R., Rose E.B., Horwitz S.M., et al. Multisystem inflammatory syndrome in U.S. Children and adolescents. N Engl J Med. 2020;383(4):334–346. doi: 10.1056/nejmoa2021680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee P.Y., Day-Lewis M., Henderson L.A., et al. Distinct clinical and immunological features of SARS–CoV-2–induced multisystem inflammatory syndrome in children. J Clin Invest. 2020;130(11):5942–5950. doi: 10.1172/JCI141113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moraleda Cinta, Serna-Pascual Miquel, Soriano-Arandes Antoni, et al. Multi-inflammatory syndrome in children related to SARS-CoV-2 in Spain. Clin Infect Dis. 2020;72(9) doi: 10.1093/cid/ciaa1042. e397–e401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramcharan T., Nolan O., Lai C.Y., et al. Paediatric inflammatory multisystem syndrome: temporally associated with SARS-CoV-2 (PIMS-TS): cardiac features, management and short-term outcomes at a UK tertiary paediatric hospital. Pediatr Cardiol. 2020;41(7):1391–1401. doi: 10.1007/s00246-020-02391-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaushik S., Aydin S.I., Derespina K.R., et al. Multisystem inflammatory syndrome in children (MIS-C) associated with SARSCoV- 2 infection: a multi-institutional study from New York city. J Pediatr. 2020;224:24–29. doi: 10.1016/j.jpeds.2020.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Whittaker E., Bamford A., Kenny J., et al. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA - J Am Med Assoc. 2020;324(3):259–269. doi: 10.1001/jama.2020.10369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller J., Cantor A., Zachariah P., Ahn D., Martinez M., Margolis K.G. Gastrointestinal symptoms as a major presentation component of a novel multisystem inflammatory syndrome in children that is related to coronavirus disease 2019: a single center experience of 44 cases. Gastroenterology. 2020;159(4):1571–1574. doi: 10.1053/j.gastro.2020.05.079. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheung E.W., Zachariah P., Gorelik M., et al. Multisystem inflammatory syndrome related to COVID-19 in previously healthy children and adolescents in New York city. JAMA - J Am Med Assoc. 2020;324(3):294–296. doi: 10.1001/jama.2020.10374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pouletty M., Borocco C., Ouldali N., et al. Paediatric multisystem inflammatory syndrome temporally associated with SARS-CoV-2 mimicking Kawasaki disease (Kawa-COVID-19): a multicentre cohort. Ann Rheum Dis. 2020;79(8):999–1006. doi: 10.1136/annrheumdis-2020-217960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Toubiana J., Poirault C., Corsia A., et al. Kawasaki-like multisystem inflammatory syndrome in children during the covid-19 pandemic in Paris, France: prospective observational study. BMJ (Clinical research ed) 2020;369 doi: 10.1136/bmj.m2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Young T.K., Shaw K.S., Shah J.K., et al. Mucocutaneous manifestations of multisystem inflammatory syndrome in children during the COVID-19 pandemic. JAMA Dermatology. 2021;157(2):207–212. doi: 10.1001/jamadermatol.2020.4779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vogel T.P., Top K.A., Karatzios C., et al. Multisystem inflammatory syndrome in children and adults (MIS-C/A): case definition & guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine. 2021;39(22):3037–3049. doi: 10.1016/j.vaccine.2021.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Belhadjer Z., Méot M., Bajolle F., et al. Acute heart failure in multisystem inflammatory syndrome in children in the context of global SARS-CoV-2 pandemic. Circulation. 2020;142(5):429–436. doi: 10.1161/CIRCULATIONAHA.120.048360. [DOI] [PubMed] [Google Scholar]
- 29.F Son Mary Beth, MDKevin Friedman M. 2021. COVID-19: Multisystem Inflammatory Syndrome in Children (MIS-C) Clinical Features, Evaluation, and Diagnosis.https://www.uptodate.com/contents/covid-19-multisystem-inflammatory-syndrome-in-children-mis-c-clinical-features-evaluation-and-diagnosis Published. [Google Scholar]
- 30.Belhadjer Z., Méot M., Bajolle F., et al. Acute heart failure in multisystem inflammatory syndrome in children in the context of global SARS-CoV-2 pandemic. Circulation. 2020;142(5):429–436. doi: 10.1161/CIRCULATIONAHA.120.048360. [DOI] [PubMed] [Google Scholar]
- 31.De Paulis M., Oliveira D.B.L., Vieira R.P., et al. Multisystem inflammatory syndrome associated with covid-19 with neurologic manifestations in a child: a brief report. Pediatr Infect Dis J. 2020;39(10):E321–E324. doi: 10.1097/INF.0000000000002834. [DOI] [PubMed] [Google Scholar]
- 32.Flors L. Imaging findings in multisystem inflammatory syndrome in children (MIS-C) associated with coronavirus disease (COVID-19) Am J Roentgenol. 2021;216(2):518. doi: 10.2214/AJR.20.24423. [DOI] [PubMed] [Google Scholar]
- 33.Whitworth H., Sartain S.E., Kumar R., et al. Rate of thrombosis in children and adolescents hospitalized with COVID-19 or MIS-C. Blood. 2021;138(2):190–198. doi: 10.1182/blood.2020010218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ragab D., Salah Eldin H., Taeimah M., Khattab R., Salem R. The COVID-19 cytokine storm; what we know so far. Front Immunol. 2020;11(June):1–4. doi: 10.3389/fimmu.2020.01446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sokolovsky S., Soni P., Hoffman T., Kahn P., Scheers-Masters J. COVID-19 associated Kawasaki-like multisystem inflammatory disease in an adult. AJEM (Am J Emerg Med) 2021;39:253. doi: 10.1016/j.ajem.2020.06.053. e1-253.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sullivan C. 2021. Prolonged Pediatric Fever and Evaluating Patients for Kawasaki Disease, Toxic Shock Syndrome, and Multi-Inflammatory Syndrome of Children . emDocs.http://www.emdocs.net/prolonged-pediatric-fever-and-evaluating-patients-for-kawasaki-disease-toxic-shock-syndrome-and-multi-inflammatory-syndrome-of-children/ Published. [Google Scholar]
- 37.Kabeerdoss J., Pilania R.K., Karkhele R., Kumar T.S., Danda D., Singh S. Severe COVID-19, multisystem inflammatory syndrome in children, and Kawasaki disease: immunological mechanisms, clinical manifestations and management. Rheumatol Int. 2021;41(1):19–32. doi: 10.1007/s00296-020-04749-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li C feng, He X hu, Kuang W ying, Han T xin, Zhou Y fang. Macrophage activation syndrome in Chinese children with systemic onset juvenile idiopathic arthritis. Zhonghua er ke za zhi = Chinese journal of pediatrics. 2006;44(11):806–811. [PubMed] [Google Scholar]
- 39.Ebina-Shibuya R., Namkoong H., Shibuya Y., Horita N. Multisystem inflammatory syndrome in children (MIS-C) with COVID-19: insights from simultaneous familial kawasaki disease cases. Int J Infect Dis. 2020;97:371–373. doi: 10.1016/j.ijid.2020.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schulert G.S., Grom A.A. Pathogenesis of macrophage activation syndrome and potential for cytokine-directed therapies. Annu Rev Med. 2015;66(October 2014):145–159. doi: 10.1146/annurev-med-061813-012806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Otsuka R., Seino K.I. Macrophage activation syndrome and COVID-19. Inflamm Regen. 2020;40(1) doi: 10.1186/s41232-020-00131-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bose K., Saha S., Saha P., Mondal P. Macrophage activation syndrome: a potentially fatal complication of kawasaki disease. Archives of Rheumatology. 2015;30(2):178–180. doi: 10.5606/ArchRheumatol.2015.5186. [DOI] [Google Scholar]
- 43.Weaver L.K., Behrens E.M. Hyperinflammation, rather than hemophagocytosis, is the common link between macrophage activation syndrome and hemophagocytic lymphohistiocytosis. Curr Opin Rheumatol. 2014;26(5):562–569. doi: 10.1097/BOR.0000000000000093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.De Graeff N., Groot N., Ozen S., et al. European consensus-based recommendations for the diagnosis and treatment of Kawasaki disease-the SHARE initiative. Rheumatology. 2019;58(4):672–682. doi: 10.1093/rheumatology/key344. [DOI] [PubMed] [Google Scholar]
- 45.Greene A.G., Saleh M., Roseman E., Sinert R. Toxic shock-like syndrome and COVID-19: multisystem inflammatory syndrome in children (MIS-C) AJEM (Am J Emerg Med) 2020;38(11):2492. doi: 10.1016/j.ajem.2020.05.117. e5-2492.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Giamarellos-Bourboulis E.J., Netea M.G., Rovina N., et al. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe. 2020;27(6):992–1000. doi: 10.1016/j.chom.2020.04.009. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rauf A., Vijayan A., John S.T., Krishnan R., Latheef A. Multisystem inflammatory syndrome with features of atypical kawasaki disease during COVID-19 pandemic. Indian J Pediatr. 2020;87(9):745–747. doi: 10.1007/s12098-020-03357-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Halyabar O., Chang M.H., Schoettler M.L., et al. Calm in the midst of cytokine storm: a collaborative approach to the diagnosis and treatment of hemophagocytic lymphohistiocytosis and macrophage activation syndrome. Pediatr Rheumatol. 2019;17(1):1–12. doi: 10.1186/s12969-019-0309-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McCrindle B.W., Rowley A.H., Newburger J.W., et al. vol. 135. 2017. (Diagnosis, Treatment, and Long-Term Management of Kawasaki Disease: A Scientific Statement for Health Professionals from the American Heart Association). [DOI] [PubMed] [Google Scholar]
- 50.Rajasekaran S., Kruse K., Kovey K., et al. Therapeutic role of anakinra, an interleukin-1 receptor antagonist, in the management of secondary hemophagocytic lymphohistiocytosis/sepsis/multiple organ dysfunction/macrophage activating syndrome in critically Ill children. Pediatr Crit Care Med. 2014;15(5):401–408. doi: 10.1097/PCC.0000000000000078. [DOI] [PubMed] [Google Scholar]
- 51.Dolinger M.T., Person H., Smith R., et al. Pediatric crohn disease and multisystem inflammatory syndrome in children (MIS-C) and COVID-19 treated with Infliximab. J Pediatr Gastroenterol Nutr. 2020;71(2):153–155. doi: 10.1097/MPG.0000000000002809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Michot J.M., Albiges L., Chaput N., et al. Tocilizumab, an anti-IL-6 receptor antibody, to treat COVID-19-related respiratory failure: a case report. Ann Oncol. 2020;31(7):961–964. doi: 10.1016/j.annonc.2020.03.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Feldmann M., Maini R.N., Woody J.N., et al. Trials of anti-tumour necrosis factor therapy for COVID-19 are urgently needed. Lancet. 2020;395(10234):1407–1409. doi: 10.1016/S0140-6736(20)30858-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Younis J.S., Skorecki K., Abassi Z. The double edge sword of testosterone's role in the COVID-19 pandemic. Front Endocrinol. 2021;12(March):1–8. doi: 10.3389/fendo.2021.607179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mihalopoulos M., Levine A.C., Marayati N.F., et al. The resilient child: sex-steroid hormones and COVID-19 incidence in pediatric patients. J Endocr Soc. 2020;4(9) doi: 10.1210/jendso/bvaa106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wan Y., Shang J., Graham R., Baric R.S., Li F. Receptor recognition by the novel coronavirus from wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J Virol. 2020;94(7) doi: 10.1128/jvi.00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhou P., Lou Yang X., Wang X.G., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lucas J.M., Heinlein C., Kim T., et al. The androgen-regulated protease TMPRSS2 activates a proteolytic cascade involving components of the tumor microenvironment and promotes prostate cancer metastasis. Cancer Discov. 2014;4(11):1310–1325. doi: 10.1158/2159-8290.CD-13-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Glowacka I., Bertram S., Muller M.A., et al. Evidence that TMPRSS2 activates the severe acute respiratory syndrome coronavirus spike protein for membrane fusion and reduces viral control by the humoral immune response. J Virol. 2011;85(9):4122–4134. doi: 10.1128/jvi.02232-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hoffmann M., Kleine-Weber H., Schroeder S., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280. doi: 10.1016/j.cell.2020.02.052. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rahman F., Christian H.C. Non-classical actions of testosterone: an update. Trends Endocrinol Metabol. 2007;18(10):371–378. doi: 10.1016/j.tem.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 62.Mikkonen L., Pihlajamaa P., Sahu B., Zhang F.P., Jänne O.A. Androgen receptor and androgen-dependent gene expression in lung. Mol Cell Endocrinol. 2010;317(1-2):14–24. doi: 10.1016/j.mce.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 63.Yu J., Yu J., Mani R.S., et al. An integrated network of androgen receptor, polycomb, and TMPRSS2-ERG gene fusions in prostate cancer progression. Cancer Cell. 2010;17(5):443–454. doi: 10.1016/j.ccr.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McCrohon J.A., Death A.K., Nakhla S., et al. Androgen receptor expression is greater in macrophages from male than from female donors. Circulation. 2000;101(3):224–226. doi: 10.1161/01.CIR.101.3.224. [DOI] [PubMed] [Google Scholar]
- 65.Kim J.B., Lee J.J., Park J.C., et al. Integrated analyses of single-cell atlases reveal age, gender, and smoking status associations with cell type-specific expression of mediators of SARS-CoV-2 viral entry and highlights inflammatory programs in putative target cells. Angew Chem Int Ed. 2018;6(11):951–952. 12(3):351-376. [Google Scholar]
- 66.Kashon M.L., Hayes M.J., Shek P.P., Sisk C.L. Regulation of brain androgen receptor immunoreactivity by androgen in prepubertal male ferrets. Biol Reprod. 1995;52(5):1198–1205. doi: 10.1095/biolreprod52.5.1198. [DOI] [PubMed] [Google Scholar]
- 67.Denison M.R. Severe acute respiratory syndrome coronavirus pathogenesis, disease and vaccines: an update. Pediatr Infect Dis J. 2004;23(11 SUPPL):207–214. doi: 10.1097/01.inf.0000144666.95284.05. [DOI] [PubMed] [Google Scholar]
- 68.Liu Y., Yan L.M., Wan L., et al. Viral dynamics in mild and severe cases of COVID-19. Lancet Infect Dis. 2020;20(6):656–657. doi: 10.1016/S1473-3099(20)30232-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rothan H.A., Bidokhti M.R.M., Byrareddy S.N. Current concerns and perspectives on Zika virus co-infection with arboviruses and HIV. J Autoimmun. 2018;89:11–20. doi: 10.1016/j.jaut.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ho M.S., Chen W.J., Chen H.Y., et al. Neutralizing antibody response and SARS severity. Emerg Infect Dis. 2005;11(11):1730–1737. doi: 10.3201/eid1111.040659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang S.F., Tseng S.P., Yen C.H., et al. Antibody-dependent SARS coronavirus infection is mediated by antibodies against spike proteins. Biochem Biophys Res Commun. 2014;451(2):208–214. doi: 10.1016/j.bbrc.2014.07.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Feng Z., Diao B., Wang R., et al. The novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) directly decimates human spleens and lymph nodes. medRxiv. 2020;2:1–18. doi: 10.1101/2020.03.27.20045427. [DOI] [Google Scholar]
- 73.Santoro M.G., Rossi A., Amici C. NF-κB and virus infection: who controls whom. EMBO J. 2003;22(11):2552–2560. doi: 10.1093/emboj/cdg267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.D'Antiga L. Coronaviruses and immunosuppressed patients: the facts during the third epidemic. Liver Transplant. 2020;26(6):832–834. doi: 10.1002/lt.25756. [DOI] [PubMed] [Google Scholar]
- 75.Rothan H.A., Byrareddy S.N. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun. 2020;109(February) doi: 10.1016/j.jaut.2020.102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yang M. SSRN Electronic Journal; 2020. Cell Pyroptosis, a Potential Pathogenic Mechanism of 2019-nCoV Infection. Published online. [DOI] [Google Scholar]
- 77.Malmgaard L., Melchjorsen J., Bowie A.G., Mogensen S.C., Paludan S.R. Viral activation of macrophages through TLR-dependent and -independent pathways. J Immunol. 2004;173(11):6890–6898. doi: 10.4049/jimmunol.173.11.6890. [DOI] [PubMed] [Google Scholar]
- 78.Gruber C.N., Patel R.S., Trachtman R., et al. Mapping systemic inflammation and antibody responses in multisystem inflammatory syndrome in children (MIS-C) Cell. 2020;183(4):982–995. doi: 10.1016/j.cell.2020.09.034. e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.