Abstract
Objectives
Humoral immunity wanes over time after two-dose BNT162b2 vaccination. Emerging variants of concern, such as the B.1.617.2 (delta) variant, are increasingly responsible for breakthrough infections owing to their higher transmissibility and partial immune escape. Longitudinal data on neutralization against the B.1.617.2 (delta) variant are urgently needed to guide vaccination strategies.
Methods
In this prospective longitudinal observational study, anti-S1 IgG and surrogate neutralizing antibodies were measured in 234 collected samples from 60 health care workers after two-dose vaccination with BNT162b2 at five different time points over an 8-month period. In addition, antibodies against various severe acute respiratory syndrome coronavirus 2 epitopes, neutralization against wild-type, and cross-neutralization against the B.1.617.2 (delta) variant using a live virus assay were measured 6 weeks (second time point) and 8 months (last time point) after first vaccine dose.
Results
Median (interquartile range) anti-S1 IgG, surrogate neutralizing, and receptor-binding domain antibodies decreased significantly from a maximum level of 147 (102–298), 97 (96–98), and 20 159 (19 023–21 628) to 8 (4–13), 92 (80–96), and 15 324 (13 055–17 288) at the 8-month follow-up, respectively (p < 0.001 for all). Neutralization against the B.1.617.2 (delta) variant was detectable in all 36 (100%) participants at 6 weeks and in 50 of 53 (94%) participants 8 months after first vaccine dose. Median (interquartile) ID50 as determined by a live virus assay decreased from 160 (80–320) to 40 (20–40) (p < 0.001).
Discussion
Although humoral immunity wanes over time after two-dose BNT162b2 vaccination in healthy individuals, most individuals still had detectable neutralizing activity against the B.1.617.2 (delta) variant after 8 months.
Keywords: COVID-19, Delta variant, SARS-CoV-2, Vaccination, Variants of concern
Introduction
Since a cluster of pneumonia cases was first reported in Wuhan, Hubei Province, China, in December 2019, coronavirus disease 2019 (COVID-19) has become a global burden, resulting in more than 240 million cases and over 4.9 million deaths worldwide by October 2021 [1]. COVID-19 is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which enters host cells via the glycosylated spike protein [2]. The receptor-binding domain (RBD) of the SARS-CoV-2 spike protein is a major target of neutralizing antibodies that block viral attachment to the host cell via angiotensin converting enzyme type-II (ACE2) receptor binding [3].
Safe and effective vaccines have been developed in an unprecedented timeframe, with BNT16b2 by BioNTech/Pfizer (BNT) being the first vaccine to receive an emergency use validation from the WHO. Recently, follow-up data from the phase 2–3 BNT trial reported a gradual decline in vaccine efficacy from 96% between 7 days and 2 months after the second dose to 84% between 4 and 6 months after the second dose [4]. The decline in vaccine efficacy is caused by a combination of waning humoral immunity and the emergence of variants of concern with partial immune escape [[5], [6], [7], [8]]. Only recently, Liu et al. demonstrated a modest reduction in neutralization against the B.1.617.2 (delta) variant compared to SARS-CoV-2 wild-type strain by BNT162b2-elicited sera taken 2 or 4 weeks after the second vaccination [9].
With a significant increase in breakthrough infections, longitudinal data on cross-neutralization against the B.1.617.2 (delta) variant are urgently needed to guide booster vaccination strategies.
Methods
Study design
This prospective longitudinal cohort study was conducted at the Department of Nephrology of the University Hospital Heidelberg, including 60 health care workers who received at least one BNT162b2 vaccine dose between December 2020 and April 2021.
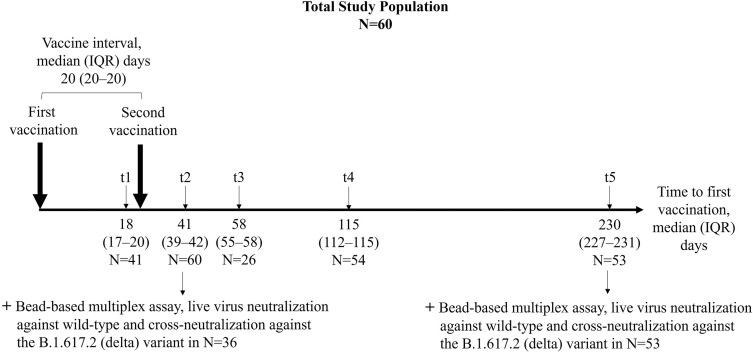
We collected 234 serum samples from 60 individuals at five different time points (t) after the first vaccine dose. Humoral vaccine response was determined after a median (interquartile range (IQR)) of 18 (17–20), 41 (39–42), 58 (55–58), 115 (112–115), and 230 (227–231) days after the first vaccine dose in 41, 60, 26, 54, and 53 participants, respectively (Fig. 1 ). The first (t1) and second (t2) time points were designed to determine maximum humoral immunity 3 weeks after the first (t1) and second (t2) vaccine dose. Time points t3–5 were chosen to determine a detailed kinetics of the humoral response over an 8-month follow-up period.
Fig. 1.
Study design to determine humoral immune responses to BNT162b2 vaccination in health care workers in a longitudinal observational study. In total, 60 participants were included in this study. Anti-S1 IgG and surrogate neutralizing antibodies were determined at five different time points (t1–t5). A bead-based analysis of antibodies against different SARS-CoV-2 target epitopes and a live virus neutralization assay to determine neutralization against wild-type and the B.1.617.2 (delta) variant of concern were performed in a representative subgroup analysis 3 weeks (t2) and 7 months (t5) after second vaccination. IQR, interquartile range.
Anti-spike S1 IgG and SARS-CoV-2–specific surrogate neutralizing antibodies were assessed at all time points in all individuals (Fig. 1). A bead-based analysis of antibodies against different SARS-CoV-2 target epitopes and a live virus neutralization assay to determine neutralization against wild-type and the B.1.617.2 (delta) variant of concern were performed in a subgroup analysis 6 weeks (t2) and 8 months (t5) after first vaccination in 36 and 53 individuals who matched the entire study population in age and sex (Fig. 1). In addition, anti-nucleocapsid antibodies were measured at each study visit to exclude participants with prior SARS-CoV-2 infection or infection during follow-up.
The study is part of an ongoing single-centre study to determine immunogenicity of COVID-19 vaccines (DRKS00024668). The study was approved by the ethics committee of the University of Heidelberg and conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all study participants.
IgG antibodies against SARS-CoV-2 spike S1 and nucleocapsid protein
We used the SARS-CoV-2 Total Assay (Siemens, Eschborn, Germany) to measure the IgG response against the S1 protein with a semi-quantitative index ≥1 defining positivity. This cut-off for detection gives a specificity of 100% with a sensitivity of 89%. IgG against the nucleocapsid protein was measured by the semiquantitative Elecsys anti-SARS-CoV-2 assay (Roche, Mannheim, Germany). Assays were performed according to the manufacturers' instructions.
SARS-CoV-2–specific surrogate neutralizing antibodies
We used a surrogate virus neutralization test (Medac, Wedel, Germany) to detect surrogate neutralizing antibodies in a sample, as described previously by us and others [[10], [11], [12], [13], [14]]. The test mimics the interaction between the virus and the host cell by direct protein–protein interaction using purified RBD protein from the viral spike protein and the host cell receptor ACE2 [14]. With a cut-off of ≥30% inhibition of RBD:ACE2 binding, the test achieves 99.9% specificity with 95% to 100% sensitivity in detecting surrogate neutralizing antibodies.
IgG antibodies against different SARS-CoV-2 target epitopes
To identify IgG antibodies against different SARS-CoV-2 target epitopes, a multiplex bead-based assay for the Luminex platform (LabScreen COVID Plus, One Lambda Inc., West Hill, CA, USA) was performed [15]. The assay detects antibodies against the SARS-CoV-2 nucleocapsid protein and against four distinct fragments of the SARS-CoV-2 spike protein, namely the full spike protein, spike S1, spike S2, and the receptor-binding domain of the spike protein. The mean fluorescence intensity was analyzed on a Luminex 200 device (Luminex Corporation, Noord-Brabant, The Netherlands). Cut-off values are given in Table S2.
Neutralization against wild-type and the B.1.617.2 (delta) variant of concern
Neutralization titres were determined in titration experiments on VeroE6 cells, as described previously [13,16]. SARS-CoV-2 virus stocks were produced by either amplification of the BavPat1/2020 strain (European Virus Archive) or isolation and amplification of the B.1.617.2 (delta) variant from nasopharyngeal and oropharyngeal swabs of PCR-confirmed SARS-CoV-2-positive patients, as previously described by us and others [13,17]. For the B.1.617.2 (delta) variant, next-generation sequencing (NGS) sequencing was performed, and lineage, variant assignment, and clade classification of the SARS-CoV-2 sequence was carried out using Pangolin and Nextclade as part of the Nextstrain framework. Virus was amplified in VeroE6 cells, and virus titres of stocks produced in this cell line were determined by plaque assay. Stocks were stored at –80°C until use.
For titration experiments, two-fold serial dilutions of vaccine sera were incubated with 104 plaque-forming unit of wild-type and the B.1.617.2 (delta) variant. After 1 hour at 37°C, the mixture was added to VeroE6 cells, and cells were fixed in the plates with 5% formaldehyde 24 hours later. Virus replication was determined by immunostaining for the viral nucleocapsid protein using an in-cell ELISA. Values were normalized to those obtained with cells infected in the absence of patient serum (100% infection) and noninfected cells (0% infection), the latter determining the assay background. The ID50 equates the reciprocal of the serum dilution that reduces infection of cells by 50%. The cut-off for detection of this immunodetection assay is at a neutralization titre of 1:10.
Statistics
Data are given as median and IQR or number and percent. Continuous variables were compared using the Mann-Whitney U test or the Kruskal-Wallis test with Dunn's post-test. Categorical data were compared using Fisher's exact test or the χ2 test. Spearman's rho as a nonparametric measure of rank correlation was calculated to describe the relationship between two different tests analyzing humoral immunity. Statistical significance was assumed at a p value of <0.05. The statistical analysis was performed using GraphPad Prism version 9.0.0 (GraphPad Software, San Diego CA, USA).
Results
Study population
From December 29, 2020 to September 17, 2021, we prospectively enrolled 60 health care workers who had received BNT162b2 SARS-CoV-2 vaccination. The vaccination interval was a median (IQR) of 20 (20–20) days. The median (IQR) age of the full study cohort was 46 (35–57) years, and 44 (73%) participants were female. Median (IQR) age and sex of the participants did not significantly differ from the full study cohort at any time point (t1–t5) (p = 0.90 and p = 0.75, respectively; Table S1).
Kinetics of SARS-CoV-2–specific antibodies over an 8-month follow-up period after first vaccination with BNT162b2
After the first vaccine dose, anti-S1 IgG and surrogate neutralizing antibodies were detectable above predefined thresholds for detection in 40 of 41 (98%) and 39 of 41 (95%) study participants, respectively. After the second vaccine dose and at all follow-up time points, anti-S1 IgG and surrogate neutralizing antibodies remained above the threshold for detection in all study participants.
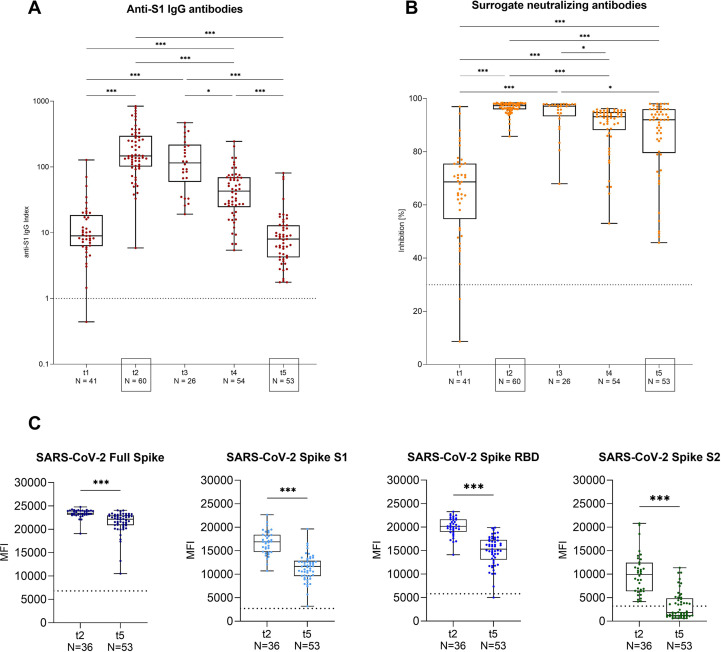
Median (IQR) anti-S1 IgG levels increased significantly from 9 (6–19) after the first vaccination (t1) to 147 (102–298) 3 weeks after the second vaccination (t2; p < 0.001). Subsequently, anti-S1 IgG levels decreased to 115 (59–218), 43 (24–70), and 8 (4–13) 8 weeks (t3), 4 months (t4), and 8 months (t5) after the first vaccination, respectively (Fig. 2 A). Median (IQR) inhibition for surrogate neutralizing antibodies increased significantly from 69% (55%–76%) after first vaccination (t1) to 97% (96%–98%) after second vaccination (t2; p < 0.001). Surrogate neutralizing antibody levels remained high at all follow-up time points, with a median (IQR) inhibition of 97% (93%–98%) at 8 weeks (t3), 93% (88%–95%) at 4 months (t4), and 92% (80%–96%) at 8 months (t5) after the first vaccination, respectively (Fig. 2B). During the first 8 weeks after first vaccination, anti-S1 IgG and surrogate neutralizing antibody levels did not decrease significantly, whereas anti-S1 IgG levels and surrogate neutralizing antibodies were significantly lower 4 months (t4) and 8 months (t5) after first vaccination when compared to maximum levels 3 weeks (t2) after second vaccination (p < 0.001 for all; Figs. 2A and B).
Fig. 2.
Anti-S1 IgG, surrogate neutralizing, full spike, spike S1, spike receptor-binding domain, and spike S2 antibodies in health care workers at different time points after BNT162b2 vaccination. (A) SARS-CoV-2 IgG antibodies were determined by a chemiluminescent immunoassay at five different time points after BNT162b2 vaccination. The x-axis displays the different time points (t1–t5), and the y-axis shows the anti-S1 IgG index, represented logarithmically. The dashed black line indicates the cut-off for detection. A semiquantitative index ≥1 was classified as positive. (B) Surrogate neutralizing antibodies as determined by a surrogate virus neutralization test at five different time points after BNT162b2 vaccination. The x-axis displays the different time points (t1–t5), and the y-axis shows the percent binding inhibition. The dashed black line indicates the cut-off for detection with a cut-off of ≥30% defining positivity. (C) Antibodies against different SARS-CoV-2 target epitopes, namely the SARS-CoV-2 full spike, spike S1, spike receptor-binding domain (RBD) and spike S2 protein 3 weeks (t2) and 7 months (t5) after second vaccination in a representative subgroup as determined by a bead-based multiplex assay. The dashed black line indicates the cut-off for detection for each respective target. Cut-offs are given in the Supplementary Data. MFI, mean fluorescence intensity; RBD, receptor-binding domain; t, time point; ∗∗∗p < 0.001; ∗∗p < 0.01; ∗p < 0.05.
Antibodies against various SARS-CoV-2 target epitopes were assessed in 36 and 53 individuals who had received two-dose BNT16b2 vaccination at 6 weeks (t2) and 8 months (t5) after first vaccination, respectively. Median (IQR) mean fluorescence intensity decreased from 23 439 (23 119–23 977) to 22 157 (20 881–22 987) for the full spike, 16 876 (14 732–18 409) to 11 670 (9616–12 855) for the spike S1, 20 159 (19 023–21 628) to 15 324 (13 055–17 288) for the RBD of the spike S1 protein, and 9933 (6399–12 431) to 1834 (1114–4869) for the spike S2 protein (p < 0.001 for all; Figs. 2C and Fig. S1). Antibodies against the nucleocapsid protein were not detectable during follow-up in any individual, confirming no prior SARS-CoV-2 infection or infection during follow-up.
Neutralizing antibody activity against the B.1.617.2 (delta) variant 8 months after first vaccination with BNT16b2
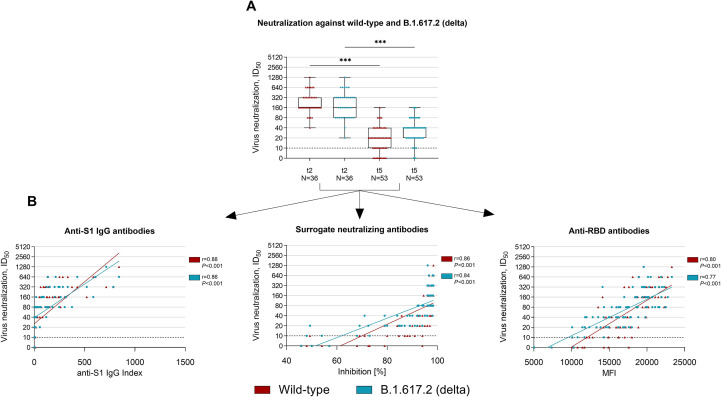
Live virus neutralization against wild-type and the B.1.617.2 (delta) variant was assessed in 36 and 53 individuals with two-dose BNT162b2 vaccination 6 weeks (t2) and 8 months (t5) after first vaccination, respectively. The median (IQR) ID50, which corresponds to the serum dilution that inhibits 50% of infectivity, decreased significantly from 160 (160–320) to 20 (10–40) for neutralization against wild-type and from 160 (80–320) to 40 (20–40) for neutralization against the B.1.617.2 (delta) variant (p < 0.001 for both; Fig. 3 A). The cut-off for detection (1:10) of neutralizing antibody activity against wild-type and the B.1.617.2 (delta) variant was exceeded in all 36 (100%) individuals 6 weeks after the first vaccination (t2). Eight months after first vaccination (t5), 10 of 53 (19%) showed no neutralizing activity against wild-type and 3 of 53 (6%) showed no cross-neutralizing activity against the B.1.617.2 (delta) variant.
Fig. 3.
Live virus neutralization against wild-type and the B.1.617.2 (delta) variant of concern 6 weeks and 8 months after first vaccination in a representative subgroup of health care workers. (A) Neutralization against wild-type and the B.1.617.2 (delta) variant was determined in a SARS-CoV-2 infection assay using VeroE6 target cells and serial two-fold dilutions of sera from 36 and 53 health care workers at 6 weeks (t2) and 8 months (t5) after first vaccination. The ID50 is defined as the serum dilution that inhibits 50% of the infectivity. The dashed black line indicates the cut-off for detection. (B) Spearman's rank coefficient of correlation between anti-S1 IgG index (left panel), surrogate neutralizing antibodies (middle panel), and anti-receptor binding domain (RBD) antibodies (right panel) with the live virus neutralization of wild-type (red) and the B.1.617.2 (delta) variant (turquoise). MFI, mean fluorescence intensity; RBD, receptor-binding domain; t, time point; ∗∗∗p < 0.001.
Correlation between live virus neutralization and anti-S1 IgG, surrogate neutralizing, and anti-receptor-binding domain antibodies
We correlated the results of commercially available assays obtained at t2 and t5 to the neutralizing activity against wild-type and the B.1.617.2 (delta) variant as determined by live virus neutralization. Spearman's rank correlation was 0.88 and 0.86 for anti-S1 IgG, 0.86 and 0.84 for surrogate neutralizing, and 0.80 and 0.77 for anti-RBD antibodies to neutralization against wild-type and the B.1.617.2 (delta) variant, respectively (Fig. 3B).
Discussion
Waning humoral immunity after COVID-19 vaccination and emerging variants of concern such as the B.1.617.2 (delta) variant with partial immune escape increasingly lead to breakthrough infections [[4], [5], [6], [7], [8]]. Longitudinal data on cross-neutralization against the B.1.617.2 (delta) variant are urgently needed to determine the need and strategies for booster vaccinations.
This is one of the first studies to determine live virus neutralizing antibodies against the B.1.617.2 (delta) variant over an 8-month follow-up period. We demonstrate a steady decline in anti-S1 IgG, surrogate neutralizing, and anti-RBD antibodies during the 8-month follow-up after two-dose BNT162b2 vaccination. At peak levels, neutralization against wild-type and cross-neutralization against the B.1.617.2 (delta) variant was detectable in all participants with high ID50 levels. However, at the 8-month follow-up, 19% and 6% did not show neutralization against wild-type or cross-neutralization against the B.1.617.2 (delta) variant, respectively. Our results for live virus neutralization at peak levels after second vaccination are in concordance with the current literature, showing only a slightly impaired vaccine-induced humoral response to the B.1.617.2 (delta) variant after two-dose vaccination [9,18,19]. Poorer cross-neutralization is seen in variants of concern with mutations resulting in amino acid substitutions K417N, E484K, and N501Y in the receptor-binding site as demonstrated for example for the immune-escaping B.1.351 (beta) variant [20,21].
Understanding the protection achieved through vaccination is crucial to efficiently determine the extent of population protection and to adapt booster vaccination strategies [22]. To help define humoral or cellular cut-off values that confer protective immunity, Khoury et al. [23] modelled SARS-CoV-2 immune protection by analyzing the relationship between in vitro neutralization levels and the observed protection from SARS-CoV-2 infection across different convalescent and vaccine studies. They estimated a “50% protective neutralization level” at an in vitro neutralization titre (ID50) between 1:10 and 1:30, which best predicted protection against severe COVID-19 [23]. Feng et al. recently reported a strong correlation between higher anti-spike IgG, anti-RBD IgG, and neutralizing antibody titres and a lower risk of symptomatic disease [24]. Because no breakthrough infections were detected in our study cohort, we were unable to estimate correlates of protection in our cohort. Considering the recent literature, our data on live virus neutralization suggest better protection from severe COVID-19 infection with higher antibody levels 3 weeks after second vaccination compared to the lower levels at 8-month follow-up. Because commercially available tests correlate strongly with neutralizing activity determined by live virus neutralization, these tests may aid in clinical decision-making regarding additional booster vaccinations.
Booster vaccinations are one strategy to counteract waning humoral immunity against COVID-19. Falsey et al. recently reported an increase in the magnitude and breadth of neutralization against the B.1.617.2 (delta) variant with a third BNT162b2 vaccine dose [18]. Furthermore, Bar-On et al. reported 5.4- and 11.3-times lower rates of confirmed infections and of severe illness among participants who had received a booster BNT162b2 vaccination compared to those with only two vaccine doses [26]. However, when discussing additional booster vaccinations in high-income countries, we should not forget the ongoing global disparity in COVID-19 vaccination, prolonging the pandemic not least because of the emergence of new variants of concern [27].
A limitation of our study is possible selection bias that may have been introduced in this longitudinal study design because serum was not available for all study participants at all time points. We reduced this bias by only including individuals with ≥3 sera available during follow-up. Another limitation is the lack of data on vaccine-induced cellular immunity and the effect of the B.1.617.2 (delta) variant on T-cell response. However, Cassaniti et al. showed that SARS-CoV-2 T-cell response 3 weeks after a second BNT162b2 vaccine dose is not significantly affected by mutations in the B.1.617.2 (delta) variant [28]. Furthermore, Woldemeskel et al. showed only a modest decline in T-cell frequency 6 months after mRNA vaccination [29].
Although humoral immunity wanes after two-dose BNT162b2 vaccination, our results show that most healthy individuals still exhibit vaccine-induced neutralizing activity against the B.1.617.2 (delta) variant after 8 months. Commercially available tests may help to guide additional booster vaccinations.
Transparency declaration
None.
Funding
Funding for this study has been received by the Dietmar Hopp Stiftung. Louise Benning is funded by the Rahel Goitein-Strauss Program of the Heidelberg Faculty of Medicine. Ralf Bartenschlager is supported by the program for surveillance and control of SARS-CoV-2 mutations of the State of Baden-Württemberg, the German Federal Research Network Applied Surveillance and Testing (BFAST) within the Network University Medicine, the DKFZ@fightCOVID initiative, and Helmholtz Association's Initiative and Networking Fund Project “Virological and immunological determinants of COVID-19 pathogenesis—lessons to get prepared for future pandemics (KA1-Co-02 “CoViPa”)”. Claudius Speer is funded by the Physician Scientist Program of the Heidelberg Faculty of Medicine.
Author contributions
LB and CSp analyzed and interpreted the data and drafted the manuscript. LB, MR, MT, CN, FK, PR, MS, KK and CSp collected and managed the data. LB, MT, PS, CS and CSp performed experiments on humoral response. MB and RB performed experiments on live virus neutralization. CM, PS, MZ, CS and RB supervised the project and revised the manuscript. All the authors critically reviewed the manuscript.
Acknowledgements
We thank Iris Arnold and Sabine Bönisch at the Department of Nephrology, Verena Backendorf and Tina Hildenbrand at the Department of Immunology, and Heeyoung Kim at the Department of Infectious Diseases, Molecular Virology (all at Heidelberg University Hospital) for their technical support.
Editor: L. Kaiser
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2022.01.011.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.WHO COVID-19 dashboard. World Health Organization; Geneva: 2021. https://covid19.who.int/table [Google Scholar]
- 2.Wrapp D., Wang N., Corbett K.S., Goldsmith J.A., Hsieh C.-L., Abiona O., et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367:1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deshpande A., Harris B.D., Martinez-Sobrido L., Kobie J.J., Walter M.R. Epitope classification and RBD binding properties of neutralizing antibodies against SARS-CoV-2 variants of concern. Front Immunol. 2021;12:691715. doi: 10.3389/fimmu.2021.691715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thomas S.J., Moreira E.D., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine through 6 months. New Engl J Med. 2021;385:1761–1773. doi: 10.1056/nejmoa2110345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robilotti E.V., Whiting K., Lucca A., Poon C., Guest R., McMillen T., et al. Clinical and genomic characterization of SARS CoV-2 infections in mRNA vaccinated health care personnel in New York City. Clin Infect Dis. 2021 doi: 10.1093/cid/ciab886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kustin T., Harel N., Finkel U., Perchik S., Harari S., Tahor M., et al. Evidence for increased breakthrough rates of SARS-CoV-2 variants of concern in BNT162b2-mRNA-vaccinated individuals. Nat Med. 2021;27:1379–1384. doi: 10.1038/s41591-021-01413-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bergwerk M., Gonen T., Lustig Y., Amit S., Lipsitch M., Cohen C., et al. Covid-19 breakthrough infections in vaccinated health care workers. New Engl J Med. 2021;385:1474–1484. doi: 10.1056/nejmoa2109072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levin E.G., Lustig Y., Cohen C., Fluss R., Indenbaum V., Amit S., et al. Waning immune humoral response to BNT162b2 Covid-19 vaccine over 6 months. New Engl J Med. 2021;385:e84. doi: 10.1056/nejmoa2114583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu J., Liu Y., Xia H., Zou J., Weaver S.C., Swanson K.A., et al. BNT162b2-elicited neutralization of B.1.617 and other SARS-CoV-2 variants. Nature. 2021;596:273–275. doi: 10.1038/s41586-021-03693-y. [DOI] [PubMed] [Google Scholar]
- 10.Speer C., Göth D., Benning L., Buylaert M., Schaier M., Grenz J., et al. Early humoral responses of hemodialysis patients after COVID-19 vaccination with BNT162b2. Clin J Am Soc Nephrol. 2021;16:1073–1082. doi: 10.2215/cjn.03700321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Speer C., Morath C., Töllner M., Buylaert M., Göth D., Nusshag C., et al. Humoral responses to single-dose BNT162b2 mRNA vaccination in dialysis patients previously infected with SARS-CoV-2. Front Med. 2021;8:721286. doi: 10.3389/fmed.2021.721286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benning L., Töllner M., Hidmark A., Schaier M., Nusshag C., Kälble F., et al. Heterologous ChAdOx1 nCoV-19/BNT162b2 prime-boost vaccination induces strong humoral responses among health care workers. Vaccines. 2021;9:857. doi: 10.3390/vaccines9080857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Speer C., Benning L., Töllner M., Nusshag C., Kälble F., Reichel P., et al. Neutralizing antibody response against variants of concern after vaccination of dialysis patients with BNT162b2. Kidney Int. 2021;100:700–702. doi: 10.1016/j.kint.2021.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tan C.W., Chia W.N., Qin X., Liu P., Chen M.I.-C., Tiu C., et al. A SARS-CoV-2 surrogate virus neutralization test based on antibody-mediated blockage of ACE2–spike protein–protein interaction. Nat Biotechnol. 2020;38:1073–1078. doi: 10.1038/s41587-020-0631-z. [DOI] [PubMed] [Google Scholar]
- 15.Bray R.A., Lee J.-H., Brescia P., Kumar D., Nong T., Shih R., et al. Development and validation of a multiplex, bead-based assay to detect antibodies directed against SARS-CoV-2 proteins. Transplantation. 2020;105:79–89. doi: 10.1097/tp.0000000000003524. [DOI] [PubMed] [Google Scholar]
- 16.Tönshoff B., Müller B., Elling R., Renk H., Meissner P., Hengel H., et al. Prevalence of SARS-CoV-2 infection in children and their parents in Southwest Germany. Jama Pediatr. 2021;175:586–593. doi: 10.1001/jamapediatrics.2021.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mallm J.-P., Bundschuh C., Kim H., Weidner N., Steiger S., Lander I., et al. Local emergence and decline of a SARS-CoV-2 variant with mutations L452R and N501Y in the spike protein. Medrxiv. 2021:2021. doi: 10.1101/2021.04.27.21254849. [DOI] [Google Scholar]
- 18.Falsey A.R., Frenck R.W., Jr., Walsh E.E., Kitchin N., Absalon J., Gurtman A., et al. SARS-CoV-2 neutralization with BNT162b2 vaccine dose 3. New Engl J Med. 2021;385:1627–1629. doi: 10.1056/nejmc2113468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arora P., Kempf A., Nehlmeier I., Graichen L., Sidarovich A., Winkler M.S., et al. Delta variant (B.1.617.2) sublineages do not show increased neutralization resistance. Cell Mol Immunol. 2021;18:2557–2559. doi: 10.1038/s41423-021-00772-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu Y., Liu J., Xia H., Zhang X., Fontes-Garfias C.R., Swanson K.A., et al. Neutralizing activity of BNT162b2-elicited serum. New Engl J Med. 2021;384:1466–1468. doi: 10.1056/nejmc2102017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garcia-Beltran W.F., Lam E.C., St Denis K., Nitido A.D., Garcia Z.H., Hauser B.M., et al. Multiple SARS-CoV-2 variants escape neutralization by vaccine-induced humoral immunity. Cell. 2021;184:2372–2383. doi: 10.1016/j.cell.2021.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krammer F. A correlate of protection for SARS-CoV-2 vaccines is urgently needed. Nat Med. 2021;27:1147–1148. doi: 10.1038/s41591-021-01432-4. [DOI] [PubMed] [Google Scholar]
- 23.Khoury D.S., Cromer D., Reynaldi A., Schlub T.E., Wheatley A.K., Juno J.A., et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27:1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 24.Feng S., Phillips D.J., White T., Sayal H., Aley P.K., Bibi S., et al. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat Med. 2021;27:2032–2040. doi: 10.1038/s41591-021-01540-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bar-On Y.M., Goldberg Y., Mandel M., Bodenheimer O., Freedman L., Kalkstein N., et al. Protection of BNT162b2 vaccine booster against Covid-19 in Israel. New Engl J Med. 2021;385:1393–1400. doi: 10.1056/nejmoa2114255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Asundi A., O’Leary C., Bhadelia N. Global COVID-19 vaccine inequity: the scope, the impact, and the challenges. Cell Host Microbe. 2021;29:1036–1039. doi: 10.1016/j.chom.2021.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cassaniti I., Bergami F., Percivalle E., Gabanti E., Sammartino J.C., Ferrari A., et al. Humoral and cell-mediated response elicited by SARS-CoV-2 mRNA vaccine BNT162b2 e in healthcare workers: a longitudinal observational study. Clin Microbiol Infec. 2022;28:301.e1–301.e8. doi: 10.1016/j.cmi.2021.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Woldemeskel B.A., Garliss C.C., Blankson J.N. mRNA vaccine-elicited SARS-CoV-2-specific T cells persist at 6 months and recognize the delta variant. Clin Infect Dis. 2021:ciab915. doi: 10.1093/cid/ciab915. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.