Abstract
Outcome of allogeneic stem cell transplantation (alloSCT) is hampered by substantial non-relapse mortality (NRM). Given its impact on organ function and immune response, the nutritional status has been suggested as relevant for NRM. We aimed to evaluate the association of NRM with nutritional status prior to alloSCT and in the post-SCT course. In a retrospective single-center study, we analyzed 128 alloSCTs. Besides standard characteristics, nutrition-associated parameters BMI, serum total protein, and serum albumin were recorded before conditioning and at various time points after alloSCT. Association with NRM was evaluated by univariate and multivariate survival analysis. The cohort comprised patients with a median BMI of 26 kg/m2 (16.7–46.9 kg/m2), median serum total protein of 59 g/l (41–77 g/l), and serum albumin of 36 g/l (22–46 g/l) before SCT. NRM at d+100 was 14.8% and at 1 year 26.6%. Prior to SCT, only serum albumin deficiency was associated with increased NRM (p = .010) in multivariate analysis. After SCT (d+30 and d+100), all nutrition-associated parameters decreased (p < .002), but no association of deteriorating nutritional status with NRM was found. In multivariate analysis, serum albumin (p = .03) and severe albumin deficiency (p = .02) correlated with NRM at d+30 and d+100, while BMI and serum total protein did not. In our study, albumin deficiency, particularly prior to alloSCT, shows a strong correlation with NRM. This finding may add to monitoring, risk evaluation, and counseling of patients and serve as a rational for interventions to improve the nutritional status in patients undergoing SCT.
Keywords: Allogeneic stem cell transplantation, Nutrition-associated parameters, BMI, Albumin
Introduction
Allogeneic hematopoietic stem cell transplantation (alloSCT) represents a curative therapy for various malignant and non-malignant hematological diseases [1]. However, despite improvement, its success is still limited by its high toxicity. Around 20–32% of the patients die in consequence of non-relapse mortality (NRM) [2–4]. Predominant reasons are lethal infections, organ failure, and both acute and chronic graft-versus-host disease (GvHD) [5, 6]. Established risk factors for NRM include age [4, 7] and comorbidities [8] as well as conditioning intensity in older patients [9, 10]. The most common score used to assess the NRM risk is the HCT-CI score [11]. Despite the fact that alloSCT causes relevant metabolic changes [12] with an estimated increased basal metabolic rate of 130–150% [13] and that a deficient nutritional state is associated with worse outcome [14], the HCT-CI contains only one nutrition-related parameter which is obesity, i.e., a body mass index (BMI) of > 35 kg/m2. However, there is conflicting evidence from different trials on the impact of obesity on alloSCT outcomes and NRM risk, and therefore the relevance of the BMI is still disputed [15–19].
Given its impact on organ function and immune response, a number of studies have explored nutrition-associated parameters in the setting of alloSCT; however, little data exist on their exact role in NRM [20] and no verified and easily accessible parameter for nutritional status monitoring specifically in the alloSCT setting has been established.
Possible parameters include weight as well as BMI or its categories based on WHO standards [21, 22]. Regarding an association of BMI prior to SCT with outcome conflicting results have been reported. Various studies show reduced survival for patients with obesity [15], while other studies did not support this finding [16–18]. There is agreement on a general reduction of BMI post-SCT [23–26], but data on the relevance of BMI change in the post-SCT course have only scarcely been analyzed.
Other parameters related to the nutritional status are serum total protein and serum albumin. For both association with outcome has been reported for different hemato-oncological entities, e.g., peripheral T-/NK-cell lymphomas [27]. A prognostic relevance for SCT has been suggested, but is disputed [28, 29].
A better understanding of nutrition particularly in the post-SCT course and its relevance for NRM may not only aid in assessing prognosis but also in designing interventions to improve outcome. The benefit of such interventions has been discussed, but data remain unclear [30, 31].
Given the sparse data particularly on the relevance of post-SCT nutritional status, we evaluated the association of nutritional status assessed by BMI, serum total protein, and albumin at different time points prior and after alloSCT with NRM-related outcome.
Patients and methods
Patients
A total of 126 adult patients (age ≥ 18 years) who received a first and second (two cases) alloSCT at our center between January 2005 and September 2013 were included. The two patients with a second alloSCT were 11 and 22 months disease-free before relapse leading to their second alloSCT. As this interval was considered sufficient to reconstitute nutritional status, both patients were included and total cohort size was 128 alloSCTs.
Patients received an alloSCT from either an unrelated or related donor. In the absence of an HLA-matched sibling, the search for an unrelated adult donor was conducted in collaboration with the German National Bone Marrow Donor Registry (Zentrales Knochenmarkspender-Register Deutschland, ZKRD).
Nutritional support during alloSCT was performed according to local standards (in-house standard operating procedure) following the recommendations of the European Society of Clinical Nutrition and Metabolism (ESPEN) and the German Society for Nutritional Medicine (DGEM). During neutropenia < 500/µl, a low germ and moist steam sterilized light-balanced diet was offered and patients were encouraged to maintain a sufficient oral food intake for as long as possible. On the basis of DGEM recommendations, indications for initiation of parenteral nutrition were inability to ingest food orally for > 3 days, enteral calorie intake of < 500 kcal per day for > 5 days, > 3 days in patients with severe malnutrition, or < 60% of daily requirement for > 10 days, therapy refractory emesis, diarrhea, ileus, gastroparesis or high-grade mucositis, prolonged inappetence, and prevention of catabolic metabolism.
Data collection and general SCT data
Data were collected retrospectively in our single-center study. Sources for data were inpatient and outpatient documentation. All patients had consented to treatment according to local standards. Data were handled pseudonymized during all stages of collection and analysis. Due to the pseudonymized analysis of retrospectively accrued data, no Institutional Review Board approval was deemed necessary. Patients consented to the collection and analysis of their data. Analyses were in line with the declaration of Helsinki.
Data were collected at the following time points: within 15 days prior to start of conditioning (preCond), the day of SCT ± 5 days (d0) and post-SCT on day 30 ± 5 days (d+30), day 100 ± 20 days (d+100), day 180 ± 10 days (d+180), and the last day of follow-up between day 110 and day 365 ± 10 days (d+365). Conditioning intensity was classified according to established criteria [32]. Patients were censored in case of relapse or disease progression or if treatment for another malignancy was started. Death after SCT without preceding censorship was regarded as non-relapse mortality (NRM). Varying N for different parameters at different time points resulted from censorship, preceding NRM and missing data.
Analyzed nutrition-associated parameters
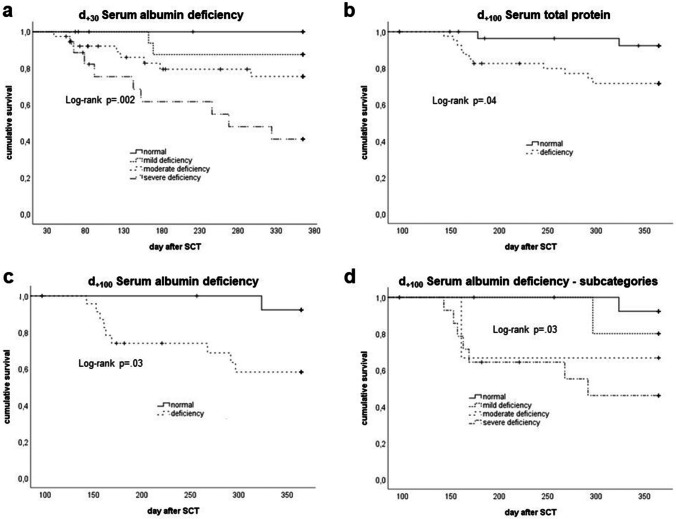
Patients’ body weight and height were derived from patient records. BMI (kg/m2) was calculated using body weight (in kg) divided by the square of the height (in m). For cross-sectional analyses, BMI was categorized according to WHO standards (BMI < 18.5 underweight, 18.5–24.9 normal weight, 25.0–29.9 overweight, ≥ 30.0 obesity). For analyses of longitudinal changes, an alteration in BMI of > 0.1 kg/m2 was classified as increase or of ≤ 0.1 kg/m2 as reduction. For serum albumin, a value of < 35 g/l was considered deficient with the following subcategories emerging as relevant during the analysis: mild deficiency 32–34.9 g/l, moderate deficiency 28–31.9 g/l, severe deficiency < 28 g/l, whereas serum albumin level ≥ 35 g/l was defined as normal. Subgrouping was initially based on current guidelines suggesting albumin levels < 32 g/l as a marker for cachexia [33]. Further analysis revealed a particular worse outcome for patients with albumin < 28 g/l (Fig. 1), and thus the present subgroups were analyzed. Regarding serum total protein, a level < 60 g/l was regarded as deficiency.
Fig. 1.
Analysis of survival (Kaplan–Meier) according to nutrition-associated parameter levels and distribution prior to alloSCT. a Serum total protein deficiency, b serum albumin deficiency, c categories of serum albumin deficiency; log rank testing according to Kaplan–Meier method
Statistical analysis
All statistical analyses were conducted using software SPSS (version 25, IBM). Survival analysis was performed using the Kaplan–Meier method with log rank testing. Unifactorial associations of parameters with NRM were assessed using chi2-test. T-test for dependent variables was used when comparing parameters at different time points. Multivariate analysis was performed by binary logistic regression model with stepwise backward likelihood inclusion for parameters showing an association in univariate analysis and relevant cofactors as stated. Results are shown as odds ratio (OR) with 95% confidence interval (CI). All reported p-values are explorative.
Results
Patient characteristics and nutritional data at pre-SCT
General patient characteristics are summarized in Table 1. Upon start of conditioning patients had a median weight of 77.4 kg (minimum 46 kg, maximum 157 kg; SD 18.9 kg) and a median BMI of 26 kg/m2 (minimum 16.7 kg/m2, maximum 46.9 kg/m2, SD ± 5.2 kg/m2). The distribution of WHO-based BMI categories is shown in Table 1. Median serum total protein was 59 g/l (minimum 41 g/l, maximum 77 g/l; SD ± 7.1 g/l) and median serum albumin was 36 g/l (minimum 22 g/l, maximum 46 g/l; SD ± 4.7 g/l). This grouped patients according to normal or protein/albumin deficiency as shown in Table 1.
Table 1.
Patient characteristics pre-SCT (N = 128)
| Characteristic | Specificity | Values |
|---|---|---|
| Gender, N (%) | Female | 50 (39.1) |
| Male | 78 (60.9) | |
| Age, years | median: 56.4, range: 18–72 | |
| Karnofsky index, % | median: 90%, range: 40–100% | |
| Diagnosis, N (%) | AML | 58 (45.3) |
| Lymphoma | 35 (27.3) | |
| Myeloma | 19 (14.8) | |
| Other 1 | 16 (12.5) | |
| Disease status, N (%) | CR | 56 (43.8) |
| PR | 34 (26.6) | |
| SD | 28 (21.9) | |
| PD | 8 (6.3) | |
| NA | 2 (1.6) | |
| Conditioning regimen, N (%) | MA | 39 (30.5) |
| RIC/NMA | 89 (69.5) | |
| Graft source, N (%) | Peripheral blood | 124 (96.9) |
| Bone marrow | 4 (3.1) | |
| Death, N (%) | Until d+100 | 23 (18.0) |
| Cumulative until 1y | 53 (41.4) | |
| Cause of death, N (%) (cumulative until d+100 / until 1y) | Relapse | 4 (3.1) / 19 (14.8) |
| NRM | 19 (14.8) / 34 (26.6) | |
| Lethal sepsis as cause of NRM | 16 (12.5) / 31 (24.2) | |
| Pre-SCT BMI, N (%) | Obesity | 22 (17.2) |
| Overweight | 55 (43.0) | |
| Normal weight | 46 (35.9) | |
| Underweight | 5 (3.9) | |
| Pre-SCT total protein, N (%) | Normal | 57 (44.5) |
| Deficiency | 60 (46.9) | |
| NA | 11 (8.6) | |
| Pre-SCT albumin, N (%) | Normal | 58 (58.6) |
| Mild deficiency | 24 (24.2) | |
| Moderate deficiency | 9 (7.0) | |
| Severe deficiency | 8 (6.3) | |
| NA | 29 (22.7) |
1Includes acute lymphatic leukemia, chronic myelogenous leukemia, osteomyelofibrosis, aplastic anemia; AML, acute myeloid leukemia; MA, myeloablative; MM, multiple myeloma; NA, unknown; NMA, non-myeloablative; NRM, non-relapse mortality; CR, complete remission; PD, progressive disease; PR, partial remission; RIC, reduced intensity conditioning; SCT, stem cell transplantation; SD, stable disease
As shown in Table 1, NRM at d+100 was 14.8% and at 1 year (1y) 26.6%. The main cause of NRM was infection with sepsis, accounting for 84.2% of NRM at d+100 and 91.1% of NRM at 1y.
Association of pre-SCT nutritional status with post-SCT NRM
Survival analysis did not reveal an association of pre-SCT BMI with NRM and showed an increased NRM for patients with pre-SCT protein deficiency (Fig. 1a, log rank p = 0.041). Especially pre-SCT albumin deficiency was associated with worse NRM (Fig. 1b, log rank p = 0.010). Analysis for albumin deficiency subgroups revealed a particularly increased NRM for patients with pre-SCT severe albumin deficiency (Fig. 1c, log rank p = 0.001).
These results were confirmed by multivariate regression analysis for continuous levels of pre-SCT BMI, serum total protein, serum albumin and including covariates sex, age, and conditioning intensity. Only pre-SCT serum albumin but not BMI or serum total protein was independently associated with NRM as shown in Table 2 (stating the last step of the stepwise backward likelihood inclusion). Multivariate regression analysis stated a lower NRM with increasing pre-SCT serum albumin values (OR 0.82; 95%CI 0.73 to 0.92, p = 0.001). Furthermore, serum total protein deficiency and severe albumin deficiency were associated with clinically relevant increased NRM (OR 3.7; 95%CI 1.2–11.3 or OR 13.3; 95%CI 2.2–81.7) (Table 2).
Table 2.
Results of multivariate analysis on association of nutrition-associated factors with NRM
| Variable | p-value | OR [(Exp(B)] | 95% CI |
|---|---|---|---|
| pre-SCT a | |||
| Serum albumin | .001 | .82 | .73-.92 |
| Protein deficiency | .02 | 3.72 | 1.23–11.29 |
| Severe albumin deficiency | .005 | 13.28 | 2.16–81.72 |
| d+30 b | |||
| Serum albumin | .004 | .80 | .69–.93 |
| Severe albumin deficiency | .005 | 6.30 | 1.75–22.71 |
| d+100 b | |||
| Serum albumin | .03 | .90 | .81–.99 |
| Severe albumin deficiency | .02 | 15.00 | 1.63–138.16 |
aCovariates at pre-SCT: age, sex, disease leading to SCT, conditioning intensity (MAC vs. others) and linear (BMI, albumin, protein) or categorized (BMI 4 categories, protein deficiency, 4 categories albumin) nutrition-associated parameters
bCovariates at d+30 and d+100: age, sex, conditioning intensity (MAC vs. others) and linear (BMI, albumin, protein) or categorized (BMI 4 categories, protein deficiency, 4 categories albumin) nutrition-associated parameters
Longitudinal alterations of nutrition-associated parameters in the post-SCT course
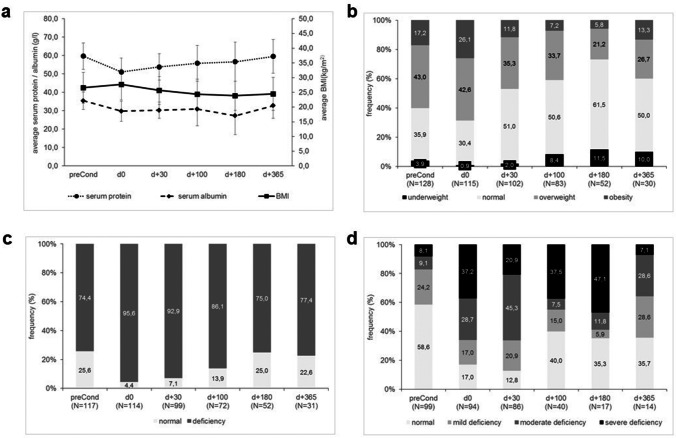
Average levels of all parameters—BMI, serum total protein and serum albumin—decreased post-SCT (Fig. 2a). Given the expected alterations in fluid status and metabolism during conditioning further analysis was performed for levels at d+30 and d+100 but not at d0.
Fig. 2.
Distribution of nutrition-associated parameters in the course from prior to SCT (preCond) until 1 year post-SCT. a Mean BMI (right Y-axis), serum total protein and serum albumin (left Y-axis), error bars represent standard deviation, b BMI categories, c categories of serum total protein deficiency, d categories of serum albumin deficiency
Compared to pre-SCT, on d+30 and d+100, a decrease in mean BMI (27.1 vs. 25.6 kg/m2 and 24,4 kg/m2, p < 0.0001 for both), mean serum total protein (60.4 g/l vs. 53.9 g/l and 55.8 g/l, p < 0.0001 for both), and mean serum albumin (35.9 g/l vs. 30.5 g/l and 31.7 g/l, p < 0.0001 and p = 0.001 respectively) was observed (t-test for dependent variables).
This corresponded to a relevant change (non-parametric testing for dependent variables) in the proportion of BMI subgroups and in the number of patients with a deficiency of serum total protein and serum albumin (for all p < 0.0001) on d+30 compared to pre-SCT (Fig. 2b–d). For d+100, a difference (non-parametric testing for dependent variables) was seen for the proportion of BMI subgroups (p < 0.0001) with a continuous increase of underweight and decrease of obese patients as well as in the number of patients with deficient serum albumin (p < 0.02). However, no difference was seen for the number of patients with serum total protein deficiency (p = 0.22).
Association of NRM with longitudinal post-SCT alterations of nutrition-associated parameters
Given the evident changes in BMI status as well as in serum total protein and serum albumin on d+30 and d+100 (see Fig. 2), we analyzed whether the changes from pre-SCT status to d+30 and d+100 status correlated with NRM.
The occurrence of a decrease in serum total protein from pre-SCT to d+30 (log rank p = 0.81) and to d+100 (log rank p = 0.06) did not correlate with NRM (data not shown). Similarly, for serum albumin a decrease from pre-SCT to d+30 (log rank p = 0.39) and to d+100 (log rank p = 0.24) did not correlate with NRM-related survival (data not shown).
Interestingly, while a decrease in BMI from pre-SCT to d+30 did not correlate with NRM (log rank p = 0.24; data not shown), a decrease from pre-SCT to d+100 correlated with better NRM-related survival (log rank p = 0.006; Fig. 3), suggesting a better survival for patients with a decrease in BMI from pre-SCT to d+100 compared to patients with a stable or increasing BMI.
Fig. 3.
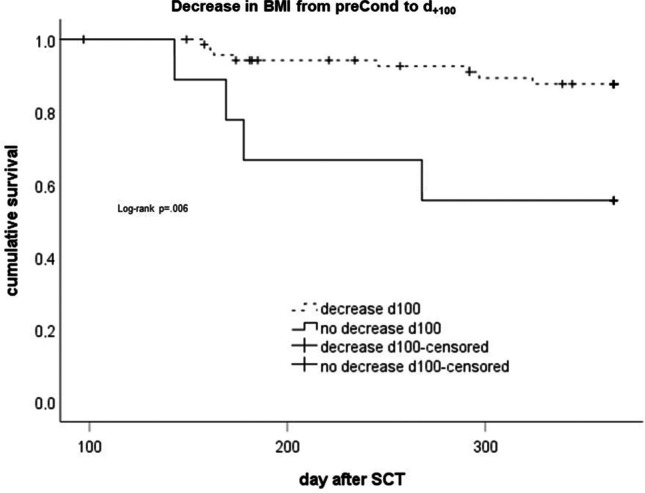
Analysis of survival (Kaplan–Meier) according to occurrence of a decrease in BMI from prior to SCT to d+100. Log rank testing according to Kaplan–Meier method
However, in multivariate regression analysis (binary logistic as well as Cox), BMI decrease from pre-SCT to d+100 showed no significant association (p > 0.1, data not shown) with NRM. Thus, it was not included in further multivariate analyses.
Association of post-SCT nutrition-associated parameters with NRM
Given the altered nutrition-associated parameters in the post-SCT course, we analyzed NRM-related survival according to nutritional status on d+30 and d+100.
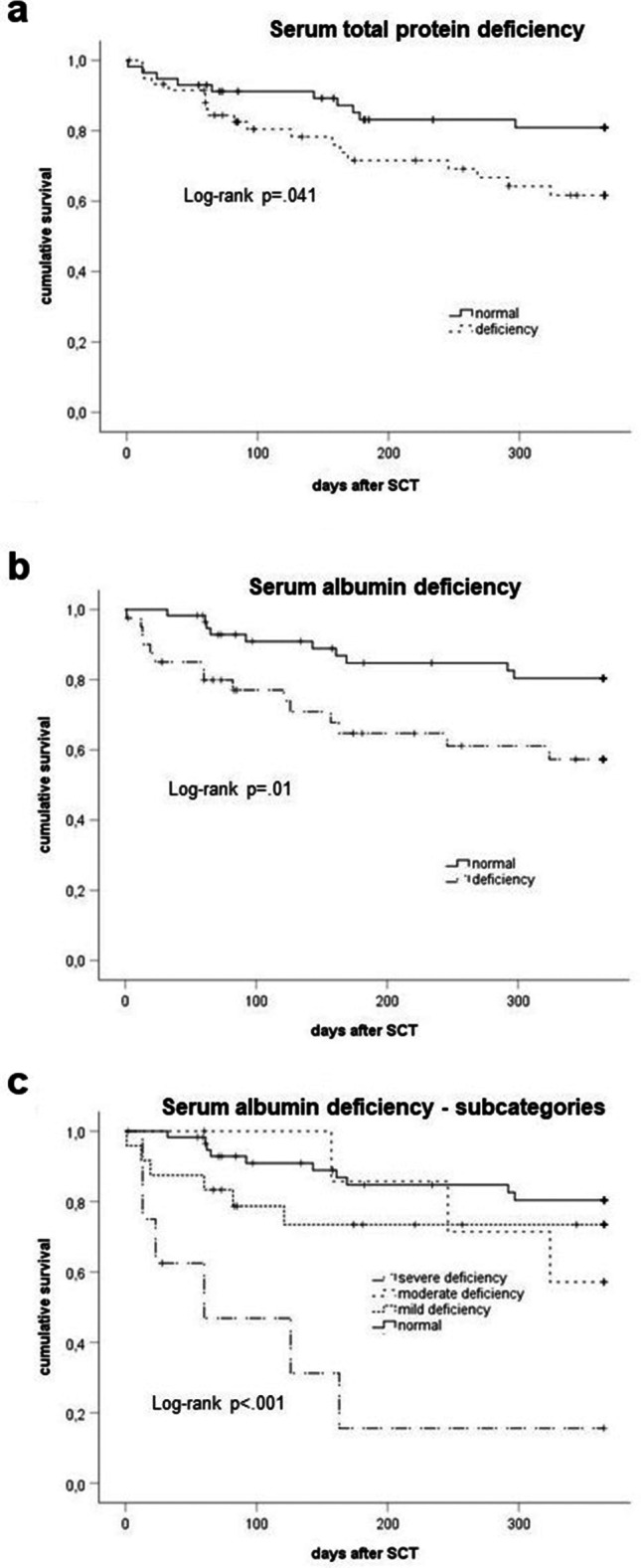
For d+30 status, according to the 4 BMI subgroups (log rank p = 0.75) as well as deficiency in serum total protein (log rank p = 0.11) and serum albumin (log rank p = 0.06) did not correlate with NRM (data not shown). However, severe serum albumin deficiency showed a strong correlation with very low NRM-related survival (log rank p = 0.002; Fig. 4a).
Fig. 4.
Analysis of survival (Kaplan–Meier) according to nutrition-associated parameter levels at the time post-alloSCT. a serum albumin levels at d+30 post-SCT, b serum total protein deficiency at d+100, c serum albumin deficiency at d+100, d subcategories of serum albumin deficiency at d+100; log rank testing according to Kaplan–Meier method
Similarly, for d+100, BMI subgroups (log rank p = 0.71) showed no correlation with NRM. However, patients with low serum total protein (log rank p = 0.04, Fig. 4b), low serum albumin (log rank p = 0.03; Fig. 4c), and particularly severe albumin deficiency (log rank p = 0.03; Fig. 4d) at d+100 had a low NRM-related survival.
As shown in Table 2, multivariate regression analysis confirmed a significant impact of serum albumin levels and of severe albumin deficiency on d+30 and d+100 on NRM. In contrast, neither BMI and BMI categories nor serum total protein or deficiency in serum total protein were associated with NRM at these time points.
Discussion
Our present study belongs to the small number of studies analyzing nutrition-related data not only prior to alloSCT but also in the post-SCT course and in specific relation to NRM rather than overall survival (OS). In our single-center cohort, which comprised a typical distribution of donor, recipient, and transplant characteristics, we observed no association of BMI or its alterations in the post-SCT course with NRM. In contrast, serum albumin deficiency prior to SCT as well as at d+30 and d+100 post-SCT was consistently associated with NRM in multivariate analysis. While BMI, serum total protein, and serum albumin decreased in the post-SCT course, this decrease did not correlate with NRM.
Concerning the association between pre-SCT BMI and the outcome of allogeneic SCT, current data are inconsistent and comparability is hindered by different cut-offs of categorized BMI. Our finding of pre-SCT BMI being not associated with NRM is supported by other studies [16, 19], suggesting that neither underweight nor obesity prior to allogeneic SCT effect patients’ outcome. Due to the rising prevalence of adiposity, many studies focused on the impact of obesity on safety and efficacy of allogeneic SCT. In a retrospective analysis of CIBMTR data, Navarro et al. reported a comparable outcome of AML patients with a BMI ≥ 30 kg/m2, including OS [18]. These results were confirmed in obese (BMI ≥ 30 kg/m2) elderly patients (≥ 60 years) who underwent allogeneic SCT for myeloid malignancies and whose outcome including OS and progression-free survival were not affected by obesity [34].
Conversely, other authors reported an association of pre-SCT BMI with NRM-related survival [15, 17, 35, 36]. In a large cohort of 2503 adult patients, Doney et al. showed that both underweight (BMI ≤ 18.5 kg/m2) and very obese (BMI ≥ 35.0 kg/m2) patients had an increased NRM and underweight patients also had an elevated relapse and overall mortality rate [15]. In the retrospective analysis performed by Fuji et al. with registry data of 12,050 patients, NRM was significantly higher in the overweight and obese group (BMI ≥ 25.0 kg/m2) compared with the normal BMI group, mainly due to an increased GvHD- and infection-related NRM. However, this did not translate into a decreased overall survival for this patient group due to an increased risk of relapse in patients with a BMI ≤ 18.5 kg/m2 [35]. The study of Gleimer et al. showed the same association with a higher NRM in obese patients (BMI ≥ 30.0 kg/m2), yet no difference in OS due to a lower incidence of relapse in the obese patient group. NRM was mainly caused by acute and chronic GvHD, but despite a trend towards a higher incidence of both acute and chronic GvHD in obese patients, no difference compared to normal weight patients was shown [36]. Given the limited size of our cohort, we did not include GvHD as an additional covariate in our analysis.
The data reporting a higher NRM for obese patients are reflected in the addition of obesity defined by a BMI > 35 kg/m2 to the relevant comorbidities in the HCT-CI score, which is used for predicting transplant-related mortality risk prior to allogeneic SCT [11].
In a longitudinal analysis, we showed similar to other authors a relevant weight loss represented by a decrease of BMI over the course of alloSCT. Due to the intensity of the treatment, this finding is expected and has been confirmed in other studies [25, 26, 37]. The retrospective study by Rieger et al. compared patients’ weight at the time of hospital admission for alloSCT, discharge (median 41 days after alloSCT), and at the end of follow-up, which was 873 ± 361 days after transplant [37]. There was no difference between the medium BMI at these time points, providing a comparable cohort to our study with a medium follow-up of 1 year. Surprisingly, in our study, patients without a reduction in BMI after d+30 showed an increased NRM; however, this finding did not remain significant in multivariate analysis and may thus be related to other factors. For instance, other studies showed an association of GvHD with a BMI reduction [24, 26]. This was confirmed by Rieger et al., observing no meaningful difference in overall survival between patients with or without substantial weight loss. This data is contradicted by a study of Fuji et al., reporting a worse outcome (NRM and OS) of patients with a weight loss ≥ 10% after alloSCT [38].
Overall, the effect of pre-SCT BMI and its course after SCT on the outcome of SCT remains unclear. Different institutional practices of chemotherapy dose modifications according to actual or adjusted body weight as well as internal guidelines on nutritional support further reduce the comparability of the data. In addition, BMI might not represent a suitable marker for describing the nutritional status of a patient, since it does not differentiate between excess body fat, muscle mass, or surplus water. Due to the retrospective design of our study, no further analyses of patients’ body composition, e.g., by bioelectric impedance analysis, which allows an estimation of human body composition, especially body fat and muscle mass, were possible. Yet, since weight remains one of the few patient-related factors that can be influenced by reasonable interventions prior to SCT, further studies are needed to evaluate its impact.
One parameter that may represent the nutritional status of the patients more accurately is serum albumin. In our cohort, serum albumin pre-SCT as well as at 30 and 100 days after SCT was significantly associated with NRM and particularly a severe albumin deficiency (< 28 g/l) correlated strongly with a higher NRM risk. Various studies have shown that serum albumin levels both prior to SCT [39, 40] and in the post-SCT course [41, 42] correlate with outcome of SCT. Although different classifications and cut-offs for hypoalbuminemia limit the comparability of these studies, low serum albumin has been consistently associated to an increased NRM, and therefore the addition of this parameter to the HCT-CI score (“Augmented HCT-CI”) has been suggested and retrospectively validated [43, 44]. A possible explanation for this observation is the higher susceptibility towards and worse outcome of complications after allogeneic SCT in patients with hypalbuminemia, such as invasive fungal infections [45] and acute GvHD [46, 47].
Additionally, our present study for the first time to our knowledge shows that the further decrease of albumin levels in the post-SCT course is not relevant for NRM. We therefore reason that primarily albumin deficiency prior to SCT is a prognostic factor predicting increased NRM risk. This is relevant, as many other factors common in the SCT and post-SCT course influence albumin levels, e.g., hydration and sepsis [48, 49]. While low serum albumin levels are well-recognized markers of both nutritional status and liver function, it also serves as a surrogate parameter for chronic inflammation processes [50]. Among other laboratory indicators, lower albumin is associated with disease activity in patients with chronic GvHD [51]. In patients undergoing an allogeneic SCT, and therefore an intensive immunotherapeutic treatment characterized by extensive immune and inflammatory processes in the course of engraftment and further follow-up, this emphasizes the clinical relevance of pre-SCT albumin levels and underlines the difficulty of evaluating albumin levels during the course of the treatment.
In contrast to others [42], we have not found an association of serum total protein levels with NRM. In a multivariate analysis in a cohort of patients surviving 100 days after alloSCT, Wojnar et al. identified low serum total protein (< 60 g/l) on day + 100 after allogeneic SCT as an independent risk factor for NRM. The authors suggested that low serum total protein may reflect most of the complications common about 3 months after SCT, including impaired liver function as part of both acute and chronic GvHD as well as infections, malabsorption, and low immunoglobulin levels, which may additionally contribute to an elevated risk of potentially lethal infections [42]. Additionally, in a retrospective analysis by Ferreira et al., a positive correlation between serum total protein level at discharge and survival time after alloSCT was shown; yet, the influence on NRM was not analyzed [52].
In our cohort, both low serum total protein pre-SCT and on d+100 after SCT were associated with increased NRM in univariate analysis. However, this correlation was not confirmed in multivariate analysis. These findings are supported by Dietrich et al., who showed that low serum total protein (< 70 g/l) is a strong predictor of relapse in patients undergoing allogeneic SCT for AML, but has no impact on NRM [28].
Overall, data on the impact of pre-SCT serum total protein on the outcome of SCT is very limited, possibly due to its limited ability in reflecting patients’ nutritional status as it comprises many different serum proteins, including acute phase proteins and other short-lived proteins. In our cohort, only pre-SCT albumin, but not serum total protein was confirmed as a predictive marker of NRM in multivariate analysis. Yet, when assessing the nutritional status of patients, nutrition-associated blood parameters are influenced by diverse (individual) factors and are subject to their own restrictions. In the setting of allogeneic SCT especially, edema needs to be considered for BMI evaluation and stress, infections, organ dysfunctions, and gastrointestinal symptoms for evaluation of blood parameters [25, 48, 49, 53]. Therefore, nutrition-associated parameters must always be interpreted in the clinical context of the individual patient.
In sum, it is apparent that nutritional parameters and status influence the outcome after allogeneic SCT. Since the nutritional status can be modified through nutritional support, the implementation of nutritional guidelines for patients during and after allogeneic SCT should be an essential component of clinical practice. In a survey among German, Swiss, and Austrian transplant centers, all centers stated that they had established nutritional guidelines for their patients undergoing alloSCT; yet, the clinical implementation of nutritional support was very heterogenous [54]. Therefore, more data are required to facilitate the development and implementation of evidence-based nutrition guidelines for patients undergoing alloSCT.
Our present single-center cohort study was limited by its retrospective design. Moreover, its heterogeneous cohort with a relatively small number of patients limited the interpretation of the results, especially in the subgroup analyses. Given our findings, larger studies and prospective trials are needed to confirm the adverse effects of pretransplant malnutrition and determine whether improving the nutritional status prior to alloSCT by an adequate pretransplant nutritional support would lead to an improved outcome.
Our findings of serum albumin deficiency both prior to SCT as well as post-SCT being associated with NRM after allogeneic SCT may not only add to the risk evaluation and counseling of patients but may also serve as a rational for early monitoring and interventions to improve the nutritional status in at-risk patients starting prior to SCT.
Author contribution
Dr. med. Judith Schaffrath: original draft preparation, validation; Dr. rer. nat. Tanja Diederichs: research design, methodology, acquisition, analysis, and interpretation of data, formal analysis; PD Dr. rer. nat. Susanne Unverzagt: formal analysis, validation; Dr. med. Maxi Wass: resources, review and editing; Dr. med. Ulrike Glaeser: data collection; Dr. med. Thomas Weber: review and editing; Prof. Dr. med. Mascha Binder: supervision, review and editing; Prof. Dr. med. Carsten Müller-Tidow: resources, supervision; apl. Prof. Dr. med. Lutz P. Müller: research design, methodology, formal analysis, supervision.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval
Since all patients had consented to treatment according to local standards and data were handled pseudonymised during all stages of accrual and analysis, no reactive consent or institutional review board approval was deemed necessary.
Consent to participate
Patients consented to the collection and analysis of their data. Analyses were in line with the declaration of Helsinki.
Conflict of interest
The authors declare no competing interests.
Footnotes
Judith Schaffrath and Tanja Diederichs have contributed equally
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Majhail NS, Farnia SH, Carpenter PA, Champlin RE, Crawford S, Marks DI, et al. Indications for autologous and allogeneic hematopoietic cell transplantation: guidelines from the American Society for blood and marrow transplantation. Biol Blood Marrow Transplant. 2015;21(11):1863–1869. doi: 10.1016/j.bbmt.2015.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gooley TA, Chien JW, Pergam SA, Hingorani S, Sorror ML, Boeckh M, et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. N Engl J Med. 2010;363(22):2091–2101. doi: 10.1056/NEJMoa1004383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McDonald GB, Sandmaier BM, Mielcarek M, Sorror M, Pergam SA, Cheng G-S, et al. Survival, nonrelapse mortality, and relapse-related mortality after allogeneic hematopoietic cell transplantation: comparing 2003–2007 versus 2013–2017 cohorts. Ann Intern Med. 2020 doi: 10.7326/M19-2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carré M, Porcher R, Finke J, Ehninger G, Koster L, Beelen D, et al. Role of age and hematopoietic cell transplantation-specific comorbidity index in myelodysplastic patients undergoing an allotransplant: a retrospective study from the chronic malignancies Working Party of the European Group for blood and marrow transplantation. Biol Blood Marrow Transplant. 2019 doi: 10.1016/j.bbmt.2019.10.015. [DOI] [PubMed] [Google Scholar]
- 5.Barrett AJ, Battiwalla M. Relapse after allogeneic stem cell transplantation. Expert Rev Hematol. 2010;3(4):429–441. doi: 10.1586/ehm.10.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tanaka Y, Kurosawa S, Tajima K, Tanaka T, Ito R, Inoue Y, et al. Analysis of non-relapse mortality and causes of death over 15 years following allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2016;51(4):553–559. doi: 10.1038/bmt.2015.330. [DOI] [PubMed] [Google Scholar]
- 7.Sorror ML, Storb RF, Sandmaier BM, Maziarz RT, Pulsipher MA, Maris MB, et al. Comorbidity-age index: a clinical measure of biologic age before allogeneic hematopoietic cell transplantation. J Clin Oncol. 2014;32(29):3249–3256. doi: 10.1200/JCO.2013.53.8157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.ElSawy M, Storer BE, Pulsipher MA, Maziarz RT, Bhatia S, Maris MB, et al. Multi-centre validation of the prognostic value of the haematopoietic cell transplantation- specific comorbidity index among recipient of allogeneic haematopoietic cell transplantation. Br J Haematol. 2015;170(4):574–583. doi: 10.1111/bjh.13476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gilleece MH, Labopin M, Savani BN, Yakoub-Agha I, Socié G, Gedde-Dahl T, et al. Allogeneic haemopoietic transplantation for acute myeloid leukaemia in second complete remission: a registry report by the Acute Leukaemia Working Party of the EBMT. Leukemia. 2020;34(1):87–99. doi: 10.1038/s41375-019-0527-4. [DOI] [PubMed] [Google Scholar]
- 10.McLornan D, Szydlo R, Koster L, Chalandon Y, Robin M, Wolschke C, et al. Myeloablative and reduced-intensity conditioned allogeneic hematopoietic stem cell transplantation in myelofibrosis: a retrospective study by the chronic malignancies Working Party of the European Society for blood and marrow transplantation. Biol Blood Marrow Transplant. 2019;25(11):2167–2171. doi: 10.1016/j.bbmt.2019.06.034. [DOI] [PubMed] [Google Scholar]
- 11.Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106(8):2912–2919. doi: 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martin-Salces M, de Paz R, Canales MA, Mesejo A, Hernandez-Navarro F. Nutritional recommendations in hematopoietic stem cell transplantation. Nutrition. 2008;24(7–8):769–775. doi: 10.1016/j.nut.2008.02.021. [DOI] [PubMed] [Google Scholar]
- 13.Zatarain L, Savani BN. The role of nutrition and effects on the cytokine milieu in allogeneic hematopoietic stem cell transplantation. Cell Immunol. 2012;276(1–2):6–9. doi: 10.1016/j.cellimm.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 14.El-Ghammaz AMS, Ben Matoug R, Elzimaity M, Mostafa N. Nutritional status of allogeneic hematopoietic stem cell transplantation recipients: influencing risk factors and impact on survival. Support Care Cancer. 2017;25(10):3085–3093. doi: 10.1007/s00520-017-3716-6. [DOI] [PubMed] [Google Scholar]
- 15.Doney K, McMillen K, Buono L, Deeg HJ, Gooley T. Impact of body mass index on outcomes of hematopoietic stem cell transplantation in adults. Biol Blood Marrow Transplant. 2019;25(3):613–620. doi: 10.1016/j.bbmt.2018.10.006. [DOI] [PubMed] [Google Scholar]
- 16.Hadjibabaie M, Tabeefar H, Alimoghaddam K, Iravani M, Eslami K, Honarmand H, et al. The relationship between body mass index and outcomes in leukemic patients undergoing allogeneic hematopoietic stem cell transplantation. Clin Transplant. 2012;26(1):149–155. doi: 10.1111/j.1399-0012.2011.01445.x. [DOI] [PubMed] [Google Scholar]
- 17.Le Blanc K, Ringdén O, Remberger M. A low body mass index is correlated with poor survival after allogeneic stem cell transplantation. Haematologica. 2003;88(9):1044–1052. [PubMed] [Google Scholar]
- 18.Navarro WH, Agovi M-A, Logan BR, Ballen K, Bolwell BJ, Frangoul H, et al. Obesity does not preclude safe and effective myeloablative hematopoietic cell transplantation (HCT) for acute myelogenous leukemia (AML) in adults. Biol Blood Marrow Transplant. 2010;16(10):1442–1450. doi: 10.1016/j.bbmt.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aplenc R, Zhang M-J, Sung L, Zhu X, Ho VT, Cooke K, et al. Effect of body mass in children with hematologic malignancies undergoing allogeneic bone marrow transplantation. Blood. 2014;123(22):3504–3511. doi: 10.1182/blood-2013-03-490334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hirose EY, de Molla VC, Gonçalves MV, Pereira AD, Szor RS, da Fonseca ARBM, et al. The impact of pretransplant malnutrition on allogeneic hematopoietic stem cell transplantation outcomes. Clin Nutr ESPEN. 2019;33:213–219. doi: 10.1016/j.clnesp.2019.05.005. [DOI] [PubMed] [Google Scholar]
- 21.Keys A, Fidanza F, Karvonen MJ, Kimura N, Taylor HL. Indices of relative weight and obesity. J Chronic Dis. 1972;25(6):329–343. doi: 10.1016/0021-9681(72)90027-6. [DOI] [PubMed] [Google Scholar]
- 22.Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:i-xii, 1–253. [PubMed]
- 23.Iestra JA, Fibbe WE, Zwinderman AH, van Staveren WA, Kromhout D. Body weight recovery, eating difficulties and compliance with dietary advice in the first year after stem cell transplantation: a prospective study. Bone Marrow Transplant. 2002;29(5):417–424. doi: 10.1038/sj.bmt.1703375. [DOI] [PubMed] [Google Scholar]
- 24.Jacobsohn DA, Margolis J, Doherty J, Anders V, Vogelsang GB. Weight loss and malnutrition in patients with chronic graft-versus-host disease. Bone Marrow Transplant. 2002;29(3):231–236. doi: 10.1038/sj.bmt.1703352. [DOI] [PubMed] [Google Scholar]
- 25.Kyle UG, Chalandon Y, Miralbell R, Karsegard VL, Hans D, Trombetti A, et al. Longitudinal follow-up of body composition in hematopoietic stem cell transplant patients. Bone Marrow Transplant. 2005;35(12):1171–1177. doi: 10.1038/sj.bmt.1704996. [DOI] [PubMed] [Google Scholar]
- 26.Urbain P, Birlinger J, Lambert C, Finke J, Bertz H, Biesalski H-K. Longitudinal follow-up of nutritional status and its influencing factors in adults undergoing allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2013;48(3):446–451. doi: 10.1038/bmt.2012.158. [DOI] [PubMed] [Google Scholar]
- 27.Watanabe T, Kinoshita T, Itoh K, Yoshimura K, Ogura M, Kagami Y, et al. Pretreatment total serum protein is a significant prognostic factor for the outcome of patients with peripheral T/natural killer-cell lymphomas. Leuk Lymphoma. 2010;51(5):813–821. doi: 10.3109/10428191003721359. [DOI] [PubMed] [Google Scholar]
- 28.Dietrich S, Radujkovic A, Stölzel F, Falk CS, Benner A, Schaich M, et al. Pretransplant metabolic distress predicts relapse of acute myeloid leukemia after allogeneic stem cell transplantation. Transplantation. 2015;99(5):1065–1071. doi: 10.1097/TP.0000000000000471. [DOI] [PubMed] [Google Scholar]
- 29.Artz AS, Logan B, Zhu X, Akpek G, Bufarull RM, Gupta V, et al. The prognostic value of serum C-reactive protein, ferritin, and albumin prior to allogeneic transplantation for acute myeloid leukemia and myelodysplastic syndromes. Haematologica. 2016;101(11):1426–1433. doi: 10.3324/haematol.2016.145847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sommacal HM, Gazal CHA, Jochims AMK, Beghetto M, Paz A, Silla LMdR, et al. Clinical impact of systematic nutritional care in adults submitted to allogeneic hematopoietic stem cell transplantation. Rev Bras Hematol Hemoter. 2012;34(5):334–8. doi: 10.5581/1516-8484.20120088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Skaarud KJ, Hjermstad MJ, Bye A, Veierød MB, Gudmundstuen AM, Lundin KEA, et al. Effects of individualized nutrition after allogeneic hematopoietic stem cell transplantation following myeloablative conditioning; a randomized controlled trial. Clin Nutr ESPEN. 2018;28:59–66. doi: 10.1016/j.clnesp.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 32.Bacigalupo A, Ballen K, Rizzo D, Giralt S, Lazarus H, Ho V, et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant. 2009;15(12):1628–1633. doi: 10.1016/j.bbmt.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Evans WJ, Morley JE, Argilés J, Bales C, Baracos V, Guttridge D, et al. Cachexia: a new definition. Clin Nutr. 2008;27(6):793–799. doi: 10.1016/j.clnu.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 34.Voshtina E, Szabo A, Hamadani M, Fenske TS, D'Souza A, Chhabra S, et al. Impact of obesity on clinical outcomes of elderly patients undergoing allogeneic hematopoietic cell transplantation for myeloid malignancies. Biol Blood Marrow Transplant. 2019;25(1):e33–e38. doi: 10.1016/j.bbmt.2018.08.031. [DOI] [PubMed] [Google Scholar]
- 35.Fuji S, Takano K, Mori T, Eto T, Taniguchi S, Ohashi K, et al. Impact of pretransplant body mass index on the clinical outcome after allogeneic hematopoietic SCT. Bone Marrow Transplant. 2014;49(12):1505–1512. doi: 10.1038/bmt.2014.178. [DOI] [PubMed] [Google Scholar]
- 36.Gleimer M, Li Y, Chang L, Paczesny S, Hanauer DA, Frame DG, et al. Baseline body mass index among children and adults undergoing allogeneic hematopoietic cell transplantation: clinical characteristics and outcomes. Bone Marrow Transplant. 2015;50(3):402–410. doi: 10.1038/bmt.2014.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rieger CT, Wischumerski I, Rust C, Fiegl M. Weight loss and decrease of body mass index during allogeneic stem cell transplantation are common events with limited clinical impact. PLoS One. 2015;10(12):e0145445. doi: 10.1371/journal.pone.0145445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fuji S, Mori T, Khattry N, Cheng J, Do YR, Yakushijin K, et al. Severe weight loss in 3 months after allogeneic hematopoietic SCT was associated with an increased risk of subsequent non-relapse mortality. Bone Marrow Transplant. 2015;50(1):100–105. doi: 10.1038/bmt.2014.228. [DOI] [PubMed] [Google Scholar]
- 39.Sivgin S, Baldane S, Ozenmis T, Keklik M, Kaynar L, Kurnaz F, et al. The impact of pretransplant hypoalbuminemia on survival in patients with leukemia who underwent allogeneic hematopoietic stem cell transplantation (alloHSCT): a nutritional problem? Transplant Proc. 2013;45(9):3371–3374. doi: 10.1016/j.transproceed.2013.02.144. [DOI] [PubMed] [Google Scholar]
- 40.Shouval R, de Jong CN, Fein J, Broers AEC, Danylesko I, Shimoni A, et al. Baseline renal function and albumin are powerful predictors for allogeneic transplantation-related mortality. Biol Blood Marrow Transplant. 2018;24(8):1685–1691. doi: 10.1016/j.bbmt.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 41.Kharfan-Dabaja MA, Chavez JC, Yu D, Zhu W, Fernandez-Vertiz EI, Perkins J, et al. Severe hypoalbuminemia at day 90 predicts worse nonrelapse mortality and overall survival after allogeneic hematopoietic stem cell transplantation for acute myelogenous leukemia and myelodysplastic syndrome. Biol Blood Marrow Transplant. 2011;17(3):384–393. doi: 10.1016/j.bbmt.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 42.Wojnar J, Giebel S, Holowiecka-Goral A, Markiewicz M, Wylezol I, Kopera M, et al. Prediction of nonrelapse mortality for patients surviving 100 days after allogeneic hematopoietic stem cell transplantation. Transplant Proc. 2007;39(10):3375–3379. doi: 10.1016/j.transproceed.2007.03.111. [DOI] [PubMed] [Google Scholar]
- 43.Vaughn JE, Storer BE, Armand P, Raimondi R, Gibson C, Rambaldi A, et al. Design and validation of an augmented hematopoietic cell transplantation-comorbidity index comprising pretransplant ferritin, albumin, and platelet count for prediction of outcomes after allogeneic transplantation. Biol Blood Marrow Transplant. 2015;21(8):1418–1424. doi: 10.1016/j.bbmt.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.ElSawy M, Storer BE, Milano F, Sandmaier BM, Delaney C, Salit RB, et al. Prognostic performance of the augmented hematopoietic cell transplantation-specific comorbidity/age index in recipients of allogeneic hematopoietic stem cell transplantation from alternative graft sources. Biol Blood Marrow Transplant. 2019;25(5):1045–1052. doi: 10.1016/j.bbmt.2018.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Corzo-León DE, Satlin MJ, Soave R, Shore TB, Schuetz AN, Jacobs SE, et al. Epidemiology and outcomes of invasive fungal infections in allogeneic haematopoietic stem cell transplant recipients in the era of antifungal prophylaxis: a single-centre study with focus on emerging pathogens. Mycoses. 2015;58(6):325–336. doi: 10.1111/myc.12318. [DOI] [PubMed] [Google Scholar]
- 46.Kharfan-Dabaja MA, Sheets K, Kumar A, Murthy HS, Nishihori T, Tsalatsanis A, et al. Hypoalbuminaemia segregates different prognostic subgroups within the refined standard risk acute graft-versus-host disease score. Br J Haematol. 2018;180(6):854–862. doi: 10.1111/bjh.15105. [DOI] [PubMed] [Google Scholar]
- 47.Ayuk F, Bussmann L, Zabelina T, Veit R, Alchalby H, Wolschke C, et al. Serum albumin level predicts survival of patients with gastrointestinal acute graft-versus-host disease after allogeneic stem cell transplantation. Ann Hematol. 2014;93(5):855–861. doi: 10.1007/s00277-013-1957-0. [DOI] [PubMed] [Google Scholar]
- 48.Arfons LM, Lazarus HM. Total parenteral nutrition and hematopoietic stem cell transplantation: an expensive placebo? Bone Marrow Transplant. 2005;36(4):281–288. doi: 10.1038/sj.bmt.1705039. [DOI] [PubMed] [Google Scholar]
- 49.Hess CT. Monitoring laboratory values: protein and albumin. Adv Skin Wound Care. 2009;22(1):48. doi: 10.1097/01.ASW.0000343728.99179.99. [DOI] [PubMed] [Google Scholar]
- 50.Soeters PB, Wolfe RR, Shenkin A. Hypoalbuminemia: pathogenesis and clinical significance. JPEN J Parenter Enteral Nutr. 2019;43(2):181–193. doi: 10.1002/jpen.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grkovic L, Baird K, Steinberg SM, Williams KM, Pulanic D, Cowen EW, et al. Clinical laboratory markers of inflammation as determinants of chronic graft-versus-host disease activity and NIH global severity. Leukemia. 2012;26(4):633–643. doi: 10.1038/leu.2011.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ferreira EE, Guerra DC, Baluz K, de Resende Furtado W, da Silva Bouzas LF. Nutritional status of patients submitted to transplantation of allogeneic hematopoietic stem cells: a retrospective study. Rev Bras Hematol Hemoter. 2014;36(6):414–419. doi: 10.1016/j.bjhh.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stratton RJ, Green CJ, Elia M. Disease-related malnutrition: an evidence-based approach to treatment. Wallingford: CABI; 2003. [Google Scholar]
- 54.Toenges R, Greinix H, Lawitschka A, Halter J, Baumgartner A, Simon A, et al. Current practice in nutrition after allogeneic hematopoietic stem cell transplantation - results from a survey among hematopoietic stem cell transplant centers. Clin Nutr. 2021;40(4):1571–1577. doi: 10.1016/j.clnu.2021.02.030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.