Summary
Ocular drug implants (ODIs) are beneficial for treating ocular diseases. However, the lack of a robust injection approach for small-eyed model organisms has been a major technical limitation in developing ODIs. Here, we present a cost-effective, minimally invasive protocol to deliver ODIs into the mouse vitreous called Mouse Implant Intravitreal Injection (MI3). MI3 provides two alternative surgical approaches (air-pressure or plunger) to deliver micro-scaled ODIs into milli-scaled eyes, and expands the preclinical platforms to determine ODIs’ efficacy, toxicity, and pharmacokinetics.
For complete details on the use and execution of this protocol, please refer to Sun et al. (2021).
Subject areas: Biotechnology and bioengineering, Health Sciences, Model Organisms
Graphical abstract
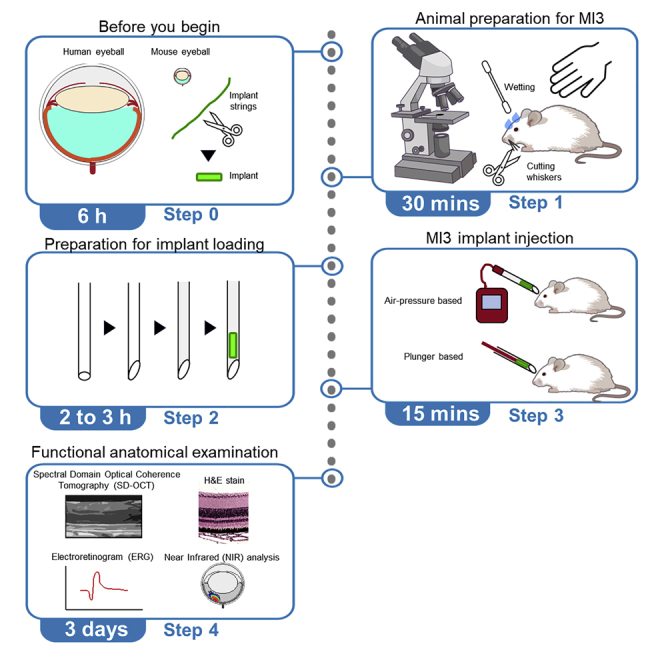
Highlights
-
•
Surgical protocol for intravitreal injection of ocular drug implants into mouse eyes
-
•
Two alternative intravitreal injection options based on implants’ physical properties
-
•
Protocol does not interfere with in vivo examinations for drug efficacy and toxicity
Ocular drug implants (ODIs) are beneficial for treating ocular diseases. However, the lack of a robust injection approach for small-eyed model organisms has been a major technical limitation in developing ODIs. Here, we present a cost-effective, minimally invasive protocol to deliver ODIs into the mouse vitreous called Mouse Implant Intravitreal Injection (MI3). MI3 provides two alternative surgical approaches (air-pressure or plunger) to deliver micro-scaled ODIs into milli-scaled eyes, and expands the preclinical platforms to determine ODIs’ efficacy, toxicity, and pharmacokinetics.
Before you begin
Our Mouse Implant Intravitreal Injection (MI3) method is a robust and effective ocular implant delivery system for small mouse eyes. This method can deliver solid implants of varying sizes and has all the advantages of conventional intravitreal injection methods for small-eyed animal models. The implant-injected mouse eyes using our method can be examined with non-invasive in vivo imaging that monitors the structure and function of the eye, which serve as measures for drug efficacy and toxicity. This protocol is written for researchers who are familiar with conventional intravitreal injections to mouse eyes. Before implementing this protocol, users should familiarize themselves with principles of implant formulation and understand the characteristics of their implant, such as the amount of drug loaded into each implant and their in vitro release profile.
Mice were maintained in the Animal Care Facilities at Stanford University. Animal work in this study was conducted in accordance with protocols approved by the Institutional Animal Care and Use Committees of Stanford University. Male and female BALB/c (Envigo) and C57BL/6J mice (The Jackson Laboratory), aged 4–8 weeks, were used interchangeably in our experiments.
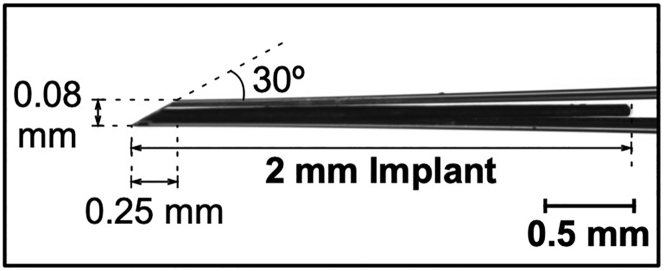
Dimension of an ocular implant
The average volume of the vitreous chamber of an adult mouse is about 5 μL. The length of an implant is limited by the axial length of the mouse eye (about 3.2 mm), and the diameter of an implant is limited by the inner diameter of the intravitreal injection needle (80–100 μm; Figure 1). The MI3 method can inject up to a 2 mm-long flexible implant into the mouse vitreous, yet a smaller implant that provides enough drug may be preferable for better safety and less potential tissue damage.
Figure 1.
Dimensions of a glass capillary needle for mouse eyeballs
An example of implant-loaded glass capillary needle and its recommended dimensions suitable for mouse eyeballs.
Hydrophobic model drug/PLGA implant formulation
Timing: 3.5–4 h
Implant formulation is especially beneficial for hydrophobic drugs since it can protect the drugs from aggregating in hydrophilic vitreous conditions. The protocol below describes the specific steps for formulating micro-scaled implants (50–80 μm in diameter and 500–2,000 μm in length) containing a hydrophobic model drug (cyanine 5.5 [Cy5.5], M.W. 619.23 Da). The implants were made from PLGA (Poly Lactic-co-Glycolic Acid), a biodegradable-polymer widely used for controlled drug release. We have used this protocol for this specific use case and those detailed in our CR Methods paper. Some alternatives and optimizations are required based on the properties of users’ target drugs and experimental goals.
-
1.
Conduct in sterile conditions or in vacuum hood. Sterilize every tool before use.
-
2.
Dissolve PLGA (50:50 LA:GA, MW: 30,000–60,000 Da) in acetone in a 1 to 5 weight-to-volume (w/v) ratio.
-
3.
Create a stock solution of 50 mg/mL Cy5.5 dye in acetone.
-
4.
Add a 1% dye solution (Cy5.5:PLGA, v/v) to the PLGA solution. Mix well and spin down.
Note: Dye quenching effect: 5% dye implant > 1% dye implant.
-
5.
On a hotplate set to 37°C, place a flat metal pan.
CRITICAL: Do not use pans with nonstick coating since the coating material may contaminate implants.
-
6.
Transfer the solution to the pan and prod with tweezers until the consistency is slightly stiff but pliable.
Note: The acetone should be mostly evaporated at this point.
-
7.
Using a pair of tweezers, pinch a section of the mixture mass and pull towards the edge of the pan (Figure 2).
Note: Gently pull the strand downwards against the lip of the pan to detach from the tweezers.
Note: To achieve a thickness of 80 μm, use a thin piece of hair for reference.
-
8.
Leave the formulated implant-string to dry for at least 3 h in the dark.
CRITICAL: Avoid a humid and light-exposed area since these conditions may affect the stability of the PLGA and quench the fluorophore.
Note: For long term storage, place formulated drug strings in a desiccator, protected from light.
-
9.
Under a microscope, trim the formulated string into properly sized implants (Figure 3A).
Note: To ensure the density of the implants, we highly recommend measuring the weight of the implants.
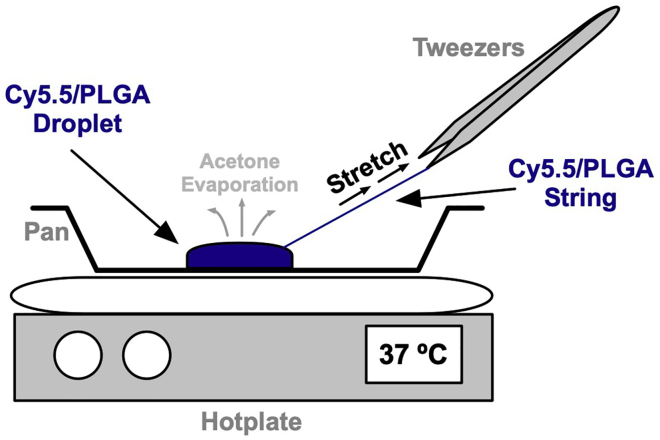
Figure 2.
Cy5.5/PLGA implant stretching procedure
A drop of Cy5.5/PLGA suspended in acetone is applied to a pan on a hotplate set to 37°C. Using a pair of tweezers, pinch a section of the mixture mass and pull towards the edge of the pan as acetone evaporates.
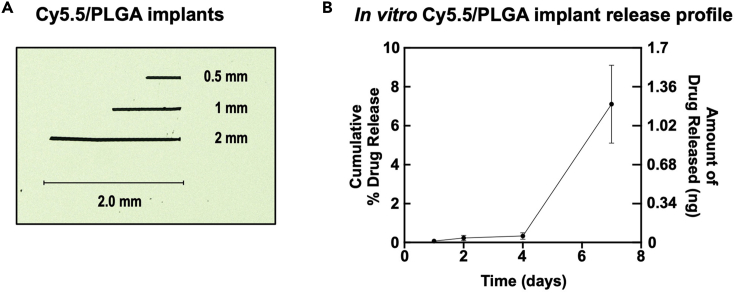
Figure 3.
In vitro characterization of implants
(A) Cy5.5/PLGA implants used in this specific use case.
(B) In vitro release profile of Cy5.5 from implant (data displayed as mean ± SEM; n = 3) in PBS supplemented with bovine serum albumin (0.1%, w/v) over 7 days reveals the sustained drug release. At 1 week, 0.6 ng of dye was detected in the buffer, for a cumulative release percentage of 3.4% (based on initial calculation of 18 ng Cy5.5 in intact implant). See also Figure S1.
Quantification of Cy5.5 in a loaded implant
Timing: 1 h
-
10.Standard solution preparation.
-
a.Dissolve Cy5.5 powder in DMSO (Dimethyl Sulfoxide) for a concentration of 310 μg/mL (5.16 mM).
-
b.Create a 0.1% (w/v, 1 mg/mL) BSA in 1X PBS solution (Bovine Serum Albumin; Phosphate-Buffered Serum).
-
c.Filter the 0.1% BSA/PBS solution using a 10 mL plastic syringe and a 0.2 μm polyether sulfone membrane filter in the laminar flow hood and keep the solution at 4°C.
-
d.Dilute the 310 μg/mL Cy5.5 dye solution in a 0.1% BSA/PBS solution for a starting concentration of 5 μg/mL.
-
e.Perform seven 2-fold serial dilutions (0, 0.039, 0.078, 0.156, 0.3125, 0.625, 1.25, 2.5, 5 μg/mL).
-
a.
-
11.Preparation of an implant for a measurement
-
a.Dissolve a Cy5.5/PLGA implant in 10 μL of DMSO and mix vigorously (troubleshooting 1).
-
b.Dilute the dissolved implant in DMSO in 90 μL of 0.1% BSA-PBS.
-
a.
-
12.Measurement
-
a.In a black bottomed 96 well plate, add 90 μL of each solution.
-
b.Measure the fluorescent intensity using a fluorometric plate reader.
-
i.λex = 650 nm
-
ii.λem = 714 nm
-
iii.Gain: 100
-
iv.Z-position: 2,000 μm
-
i.
-
c.Determine the concentration of Cy5.5 in an implant by read-signal and plotting it against the standard curve (Figure S1; troubleshooting 1).
-
a.
In vitro release profiling of a model drug implant (Cy5.5/PLGA)
Timing: 1 week
-
13.
Suspend one Cy5.5/PLGA implant in 60 μL of PBS supplemented with a bovine serum albumin (BSA/PBS, 0.1%, w/v) to prevent the precipitation of hydrophobic free Cy5.5 during the test.
Note: Keep the test tubes at 37°C.
-
14.
At 1-, 2-, 4-, and 7-days, briefly vortex the tubes, spin down, remove 30 μL of supernatant, and replace with 30 μL of fresh BSA/PBS solution.
Note: Store the collected supernatant at −20°C before the measurement.
-
15.After the last collection, dissolve the remaining implants by adding DMSO at an equivalent volume of remaining BSA/PBS (final solvent composition is 1:1 = DMSO:BSA/PBS).
-
a.Vortex thoroughly.
-
b.Incubate samples at 20°C–25°C for 10 min.
-
a.
Note: These steps are to ensure that the implants are fully dissolved.
Note: The amount of remaining dye in the implants is determined with a standard curve of free dye dissolved in DMSO:BSA/PBS cosolvent.
-
16.
Measure the fluorescent intensity (λex = 650 nm; λem = 714 nm) of the collected supernatants in a 384 flat black well plate to determine the Cy5.5 concentration at each time point.
-
17.
To produce a cumulative release profile, determine the dye amount in nmol released into the buffer at each time point.
Note: Quantification equation
-
18.
Plot the amount of Cy5.5 dye released (nmol) v. time (days) (Figure 3B).
Note: For a cumulative percentage release profile, divide individual values by the total amount of dye determined to be in the intact implant.
Note: Equation for cumulative percentage release profile
Make an implant-loaded microcapillary needle
Timing: 1 h
Conventionally, commercially available small-gauge stainless steel needles or glass capillary needles made in laboratories were utilized for intravitreal injection of drugs to mouse eyes. Compared to small-gauge stainless steel needles, glass capillary needles may be advantageous for the intravitreal injection of implants. The dimensions and properties of glass capillary needles can be easily adjusted and modified depending on the properties of the implants under different experimental setups. The transparency of glass capillary needles also allows easy visual examination of the implants in the needles. Thus, we built on previously established methods of intravitreal injection in mice, which utilize glass capillary needles, to load an implant (Chan et al., 2020; Wang et al., 2014; Wert et al., 2012). For the intravitreal implant injection method, needles must be manually customized to coordinate with the implant size. To penetrate the posterior segment of the eye, the needle needs special features. The following is the step-by-step procedure for tube fabrication, needle polishing, and implant loading into fabricated needles.
-
19.
Microcapillary tube fabrication.
To fabricate a microcapillary tube injection without relying on traditional microfabrication preformed in a clean room, we used a capillary fabrication procedure with a micropipette puller and a borosilicate glass capillary with an inner and outer diameter of 0.58 and 1.00 mm, respectively (Figure 4) (Chan et al., 2020; Wang et al., 2014; Wert et al., 2012). This procedure allows local stretch at the site of heating (the middle section of the glass capillary) without impacting the cross sections of both glass capillary ends. For ODI injection purposes, an inner diameter of ∼50 μm is optimal for the pulled glass capillaries. The following is the step-by-step procedure for microcapillary pulling:-
a.Turn the micropipette puller
-
b.Set the heating temperature to 67°C (Figure 4A).
-
c.Adjust the pulling-weight applied to the pulling module.Note: As long as the glass capillary can be partially pulled and meets the inner diameter of 50 μm, the applied pulling-weight can be flexible.
-
d.Place a borosilicate glass microcapillary on the pulling clamps.Note: Position the microcapillary so the desired pulling-part is in the middle of the heating ring.
-
e.Turn the heater (heating ring) of the micropipette puller on.
-
f.As the microcapillary heats up, it will be stretched by the weight of the pulling-weight (Figure 4A).
-
g.Cut the narrowest part of the pulled microcapillary by using a clean forceps to make the needle (Figure 4B).Note: Typically, the narrowest part of the microcapillary is located near the bottom end of the heating ring.
-
h.Adjust the needle length to about 3–4 mm using a clean forceps (Figures 4C and 4E).Note: Perform this step using a dissecting microscope.
-
a.
-
20.
Needle polishing.
For the intravitreal injection, it is necessary to insert the needle into the posterior segment of the eye rather than the anterior segment. This allows the drug to be delivered directly to the retina without dosing the anterior segment. The mouse posterior segment has an average thickness of approximately 180 μm (Horio et al., 2001; Schmucker and Schaeffel, 2004). Its outer layer consists of multiple layers of tissue (i.e., sclera, choroid, and retina), and its inner chamber is filled with vitreous humor, a semi-viscous fluid, that helps to maintain the spherical shape of the eye. These anatomical features generate strong enough resistance against trauma during penetration of the injection needle. A sharpened needle is necessary to perform a good intravitreal injection to deliver an implant into the vitreous chamber. To sharpen the injection needle, we ground the tip of the needle using a micropipette grinder. The following is the step-by-step procedure for needle polishing:-
a.Turn the micropipette grinder on (Figure 4F).
-
b.Set the motor speed to 2000 rpm.
-
c.Place the length-adjusted needle in the micromanipulator.
-
d.Load double distilled H2O to the wheel cleaner syringe.
-
e.Turn on the valve of the wheel cleaner and soak the whetstone.Note: This will prevent overheating of the needle and remove ground debris.
-
f.Set the angle of micromanipulator to 30°.Note: This makes a 30° slanted needle, which is optimized for sclera penetration.
-
g.Adjust the x- and y-axis positions of the micromanipulator so the needle touches the whetstone (Figure 4F).
-
h.Stop grinding when the needle opening reaches about 80–100 μm (Figure 4G).Note: Final needle tip dimensions are shown in Figure 1 (troubleshooting 2).
-
i.Sterilize the needle by autoclaving.
-
a.
-
21.
Implant loading.
The mechanical properties of polymeric implants used for the ODI formulations are sturdy enough to retain their shape and size for microcapillary needle injections (e.g., biodegradable polymers: poly(lactic-co-glycolic acid) (PLGA), polylactic acid (PLA), polyglycolic acid (PGA), and polycaprolactones (PCL); non-biodegradable polymers: ethylene vinyl acetate (EVA), silicone, and polyimide/polyvinyl alcohol (PVA) (Maya-Vetencourt et al., 2020; Shmueli et al., 2013). The following is the step-by-step procedure for implant loading:-
a.Sterilize the tools with 70% ethanol.
-
b.Under a dissecting microscope, align the implant with the tip of a needle and move the implant into the needle bevel using a fine forceps (Figure 4H).
-
c.Gently load the implant into the needle using the forceps until the implant is fully in the needle (Figure 4I; troubleshooting 3).Note: To prevent damage to the needle, avoid contacting the sharpened needle tip with the forceps.
-
d.Using a pipette loaded with a gel-loading tip, load 1 μL of PBS into the needle (Figures 4J and 4K).Note: The PBS should fill the needle cavity around the implant.Note: The PBS is loaded to prevent the introduction of air into the vitreous chamber during the injection.
-
a.
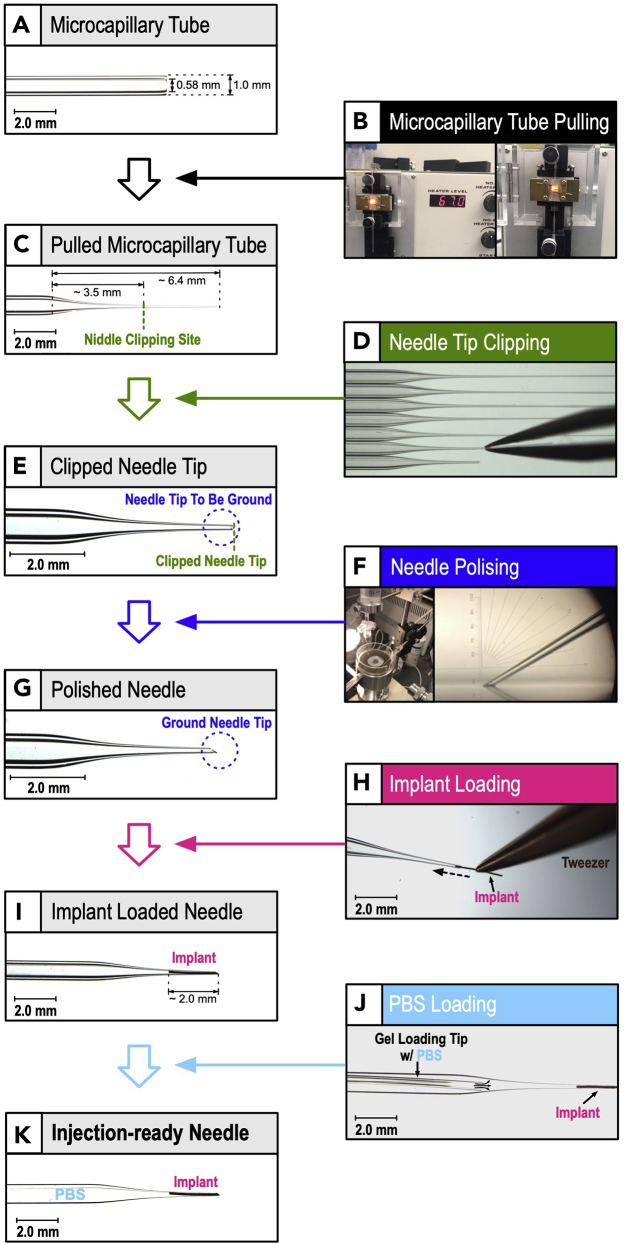
Figure 4.
Production schematics of implant-loaded intravitreal implant injection needle for mouse
(A) A borosilicate glass capillary, with inner and outer diameters of 0.58 and 1.00 mm respectively, was used.
(B) The microcapillary tube is pulled using a micropipette puller.
(C) General dimensions of pulled microcapillary tube.
(D and E) By using a clean forceps, the needle length is pulled to approximately 3–4 mm (needle clipping site in panel C) using clean forceps.
(F and G) The clipped needle tip is polished with the micropipette grinder to create a needle opening with a width of 80–100 μm and needle slant angle of 30°.
(H and I) 2 mm long implant is loaded to the needle using clean forceps.
(J and K) PBS is loaded to the needle using a pipette with gel loading tip.
(K) ODI needle ready for injection.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER | |
|---|---|---|---|
| Chemicals, peptides, and recombinant proteins | |||
| Acetone ACS reagent, ≥99.5% | Sigma-Aldrich Inc, St. Louis, MO, USA | Cat. #: 179124 | |
| AnaSed xylazine injection | Akorn Inc, Lake Forest, IL, USA | NDC: 59399-110-20 | |
| Bovine Serum Albumin (BSA) | Fisher BioReagents, Waltham, MA, USA | Cat. #: BP9700100 | |
| Cyanine5.5 carboxylic acid | Lumiprobe Corporation, Hunt Valley, MD, USA | Cat. #: 47090 | |
| Dimethyl Sulfoxide (DMSO) | Santa Cruz Biotechnology, Dallas, TX, USA | Cat. #: sc-358801 | |
| Eosin Y | Fisher Scientific, Hampton, NH, USA | Item #: 17372-87-1 | |
| Gill III Hematoxylin | Mercedes Scientific, Lakewood Ranch, FL, USA | Item #: MER 347961GL | |
| Phloxine B | Electron Microscopy Sciences, Hatfield, PA, USA | Item #: 19350 | |
| 10X Phosphate-Buffered Serum | Invitrogen, Waltham, MA, USA | Cat. #: AM9624 | |
| Poly(D,L-lactide-co-glycolide) | Sigma-Aldrich Inc, St. Louis, MO | SKU: P2191 | |
| Poly(lactide-co-glycolide)-Fluorescein | Sigma-Aldrich Inc, St. Louis, MO | SKU: 908649-50MG | |
| Refresh Liquigel Lubricant Eye Gel | Allergan, Irvine, CA, USA | NDC: 0023-9205 | |
| Refresh Tears Lubricant Eye Drops | Allergan, Irvine, CA, USA | NDC: 0023-0798-01 | |
| Tissue-Tek O.C.T. Compound | VWR International, LLC, Radnor, PA, USA | Cat. #: 4583 | |
| Tropicamide ophthalmic solution (1%) | Akorn Inc, Lake Forest, IL, USA | NDC: 17478-102-12 | |
| VetaKet CIII (ketamine hydrochloride injection, USP) | Akorn Inc, Lake Forest, IL, USA | NDC: 59399-114-10 | |
| Experimental models: Organisms/strains | |||
| Strain: WT BALB/c mouse Gender: male/female Age: 4–8 weeks |
Envigo, USA | N/A | |
| Strain: WT C57BL/6J mouse Gender: male/female Age: 4–8 weeks |
The Jackson Laboratory, USA | Stock #: 000664 | |
| Software and algorithms | |||
| Aura imaging software | Spectral Instruments Imaging, Tucson, AZ, USA | https://spectralinvivo.com/ | |
| GraphPad Prism 8 | GraphPad Software, Inc. | https://www.graphpad.com/ | |
| ImageJ | National Institutes of Health (NIH) | https://imagej.nih.gov/ij/index.html | |
| Leica Application Suite X | Leica Microsystems, San Francisco, CA, USA | https://www.leica-microsystems.com/products/microscope-software/p/leica-las-x-ls/ | |
| Other | |||
| Black Arkansas stone | Dan's Whetstone Company Inc., AR, USA | N/A | |
| Borosilicate glass capillaries | World Precision Instruments, FL, USA | Cat. #: 1B100-4 | |
| Capillary holder | Eppendorf, Hamburg, Germany | Cat #: 920007392 | |
| Customized +10D contact lens (3.0 mm diameter, 1.6 mm BC, PMMA clear) | Advanced Vision Technologies, CO, USA | N/A | |
| Diagnosys ERG Celeris | Diagnosys LLL, Littleton, MA, USA | Celeris Model #: D430 | |
| Dual-Stage Glass Micropipette Puller | Narishige international USA, NY, USA | Model PC-10 | |
| Femtojet Express Electronic microinjector | Eppendorf, Hamburg, Germany | Cat #: 920010521 | |
| Foot control pedal | Eppendorf, Hamburg, Germany | Cat #: 920005098 | |
| Heating pad | K&H Manufacturing, CO, USA | Model HM10 | |
| Heidelberg OCT Spectralis | Heidelberg Engineering, Germany | N/A | |
| Leica DM4000 B LED automated upright microscope system | Leica Microsystems, San Francisco, CA | N/A | |
| Leica M165 FC fluorescent stereo microscope | Leica Microsystems, San Francisco, CA | N/A | |
| Leica MZ6 modular stereomicroscope | Leica Microsystems, San Francisco, CA | N/A | |
| Micropipette Grinder | Narishige international USA, NY, USA | Model EG-401 | |
| Near-infrared machine | Spectral Instruments Imaging, Tucson, AZ, USA | Lago X system | |
| BD Precisionglide needles, 20 gauge | Sigma-Aldrich Inc, St. Louis, MO, USA | Cat #: Z192511 | |
| BD Hypodermic Syringe, polypropylene, 10 mL | Sigma-Aldrich Inc, St. Louis, MO, USA | Cat #: Z192023 | |
| Whatman PURADISC 25, Polyether Sulfone Membrane Filter, 0.2 μm | GE Healthcare, Chicago, IL, USA | Cat #: WHA67802502 | |
| Positioning aids | Eppendorf, Hamburg, Germany | Cat #: 920005829 | |
| Spark plate reader | Tecan, Morrisville, NC, USA | N/A | |
| Syringe cleaning wire | Hamilton Company, Reno, NV, USA | Part/REF #: 18300 | |
| VWR micro cover glass | VWR International, LLC, Radnor, PA, USA | Cat. # :48393-081 | |
| VWR Superfrost Plus Micro Slide | VWR International, LLC, Radnor, PA, USA | Cat. #: 48311-703 | |
Materials and equipment
A model drug implant (Cy5.5/PLGA) formulation
PLGA Solution
| Reagent | Final concentration | Amount |
|---|---|---|
| PLGA | 200 mg/mL | 200 mg |
| Acetone | n/a | 1 mL |
| Total | PLGA:Acetone = 1:5 (w/v) | 1 mL |
Use immediately due to rapid solvent evaporation.
CRITICAL: Acetone is flammable and toxic.
Cy5.5 Stock
| Reagent | Final concentration | Amount |
|---|---|---|
| Cy5.5 | 50 mg/mL | 50 mg |
| Acetone | n/a | 1 mL |
| Total | n/a | 1 mL |
Use immediately due to rapid solvent evaporation.
CRITICAL: Acetone is flammable and toxic.
Cy5.5/PLGA Solution
| Reagent | Final concentration | Amount |
|---|---|---|
| Cy5.5 Stock | 1% | 10 μL |
| PLGA Solution | n/a | 1 mL |
| Total | Cy5.5:PLGA = 1:100 (v/v) | 1,010 μL |
Use immediately due to rapid solvent evaporation.
CRITICAL: Acetone is flammable and toxic.
Standard preparation for Cy5.5 quantification
Cy5.5 Standard Stock
| Reagent | Final concentration | Amount |
|---|---|---|
| Cy5.5 | 310 μg/mL (5.16 mM) | 310 μg |
| DMSO | n/a | 1 mL |
| Total | n/a | 1 mL |
Store full volume at −20°C for 9–12 months.
0.1% BSA/PBS Solution
| Reagent | Final concentration | Amount |
|---|---|---|
| BSA | 1 mg/mL | 310 μg |
| 10X PBS solution | n/a | 100 μL |
| ddH2O | n/a | 900 μL |
| Total | BSA:PBS = 1:1000 (w/v) | 1 mL |
Store full volume after filter-sterilization at 4°C for 6 months.
CRITICAL: Filter-sterilize the solution using a 10 mL plastic syringe and a 0.2 μm polyether sulfone membrane filter.
Fluorometric plate reader setup for Cy5.5 quantification
Aura imaging software was used to operate a fluorometric plate reader. The following are the parameters used for Cy5.5 quantification:
-
•
λex = 650 nm
-
•
λem = 714 nm
-
•
Gain: 100
-
•
Z-position: 2,000 μm
Step-by-step method details
This section provides detailed instructions for injecting an implant by using air-pressure based microinjector (part 2a) and injecting an implant by using a plunger (part 2b). Based on the physical properties of the implants (size, weight, density, and surface property), either an air-pressure based microinjector or a plunger can be used. The air-pressure based microinjector method (part 2a) is easier to use than the plunger-based method, but smaller, lighter, and/or less surface-resistant implants are preferred. The plunger-based method (part 2b) is less restricted by the physical properties of the implants. For experienced users, we recommend testing the air-pressure based microinjector method (part 2a) first due to the simple surgical setup and straightforward procedure.
Part 1. Rodent handling
Timing: 30 min
The mouse was anesthetized using ketamine and xylazine based on its body weight according to the Administrative Panel of Laboratory Animal Care (APLAC) and the Institutional Animal Care and Use Committee (IACUC)-approved protocols at Stanford University.
-
1.
Weigh the animal and calculate the correct dosage of the ketamine and xylazine injection mixture.
Note: 0.08 mg ketamine/g and 0.01 mg xylazine/g
-
2.
Using one hand, restrain the mouse with the abdomen facing up.
-
3.
Perform the intraperitoneal injection with the ketamine and xylazine mixture using a 20 G needle.
-
4.
Place the mouse on the heating pad.
-
5.
Apply 1% tropicamide to dilate the pupil.
-
6.
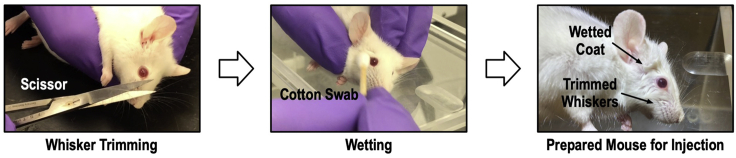
Trim the whiskers to prevent microscope examination disruption (Figure 5; left panel).
-
7.
Wet around the eye with clean 1X PBS (Figure 5; middle panel).
-
8.
Wait for 5 min for the mouse to become anesthetized (Figure 5; right panel).
-
9.
Apply lubricant eye drops to the eye to prevent the cornea surface from drying out.
Note: Apply one drop per eye every 5 min.
Figure 5.
Mouse preparation for the implant surgery
The whiskers and the hairs may interfere with the implant injection procedure by blocking the eye. Mouse whiskers are trimmed using scissors (left panel). The coat around the mouse eye is wetted to keep fur together and away from the eyeball (middle panel). An injection ready mouse is presented (right panel).
Part 2a. Implant injection by air-pressure
Timing: 10 min for each surgery
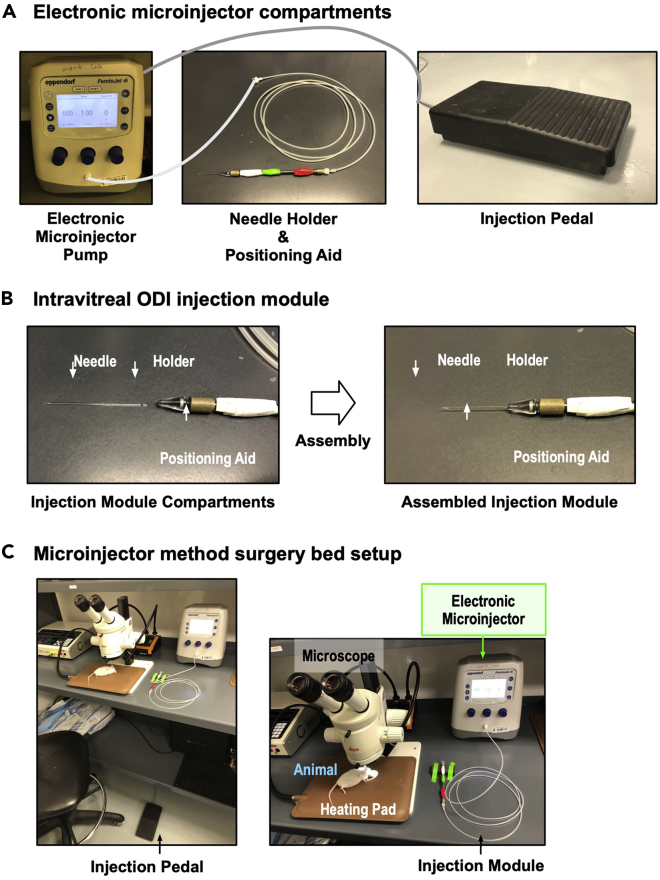
To inject an implant by air-pressure, an electronic microinjector commonly used for intravitreal injection of fluids was utilized. The following is the step-by-step procedure for the electronic microinjector setup, the surgery bed setup, and the surgical process for an implant injection by air-pressure:
-
10.The electronic microinjector setup.
-
a.Turn on the electronic microinjector and run a self-test.
-
b.Assemble the needle with the capillary holder and positioning aids (Figures 6A and 6B).
-
c.Connect the injection device to the electronic microinjector.
-
d.Set up a working parameter for the electronic microinjector: 500 hPa injection pressure, 1.0 s injection time.
-
a.
-
11.Surgery bed setup (Figure 6C).
-
a.Set a heating pad on the surgery bed to maintain the body temperature of the mouse.Note: The preset of the heating pad used was 37°C.
-
b.Transfer an anesthetized animal to the surgery bed for surgery.
-
c.Apply lubricant eye drops to the eyes to prevent the cornea surface from drying out.
-
a.
-
12.Implant injection.
-
a.Sterilize the needle and capillary holder with 70% ethanol.
-
b.Load the needle to a capillary holder attached to a positioning aid.
-
c.Hold and adjust the animal’s head using the non-dominant hand, and perform the injection using the dominant-hand.
-
d.Gently insert the needle into the eyeball.Note: The recommended needle entry-position is 2 mm posterior to the limbus (troubleshooting 4) in order to reach the vitreous cavity directly with minimal possible damage to the lens, central retina, or optic nerve.Note: The needle is perpendicular to the eye surface.Note: The recommended depth of the needle head is 2–3 mm.
-
e.Press the foot control pedal to engage the electronic microinjector.
-
f.Once the implant is delivered, slowly remove the needle from the eyeball.
-
g.Apply the antibiotic ophthalmic solution to the eye to prevent potential infections.
-
h.Place the post-surgical mouse on a heating pad until it wakes up.Note: Throughout the procedure, keep applying one lubricant eye drop every 5 min to prevent the cornea surface from drying out.
-
a.
Figure 6.
Setup for microinjector-based implant injection
(A) Electronic microinjector compartments. A positioning aid loaded with an injection needle (injection module) and an injection pedal are connected to an electronic microinjector. By pressing the injection pedal, the electronic microinjector will send compressed air to the needle through the injection module.
(B) Compartments of the injection module and their assembly are shown.
(C) Overview of the microinjector method surgery platform. A mouse and heating pad are set under the dissecting microscope, and the injection module is attached to the electronic microinjector and the injection pedal is placed near the microscope.
Part 2b. Implant injection by plunger
Timing: 50 min for surgery tool production/10 min for each surgery
To inject an implant by plunger, a micromanipulator and a syringe-cleaning wire were utilized. The following is the step-by-step procedure to make the plunger, assemble the injection module, and set up the micromanipulator and the surgery bed. The surgical process for an implant injection by plunger is also detailed:
-
10.
Implant plunger making.
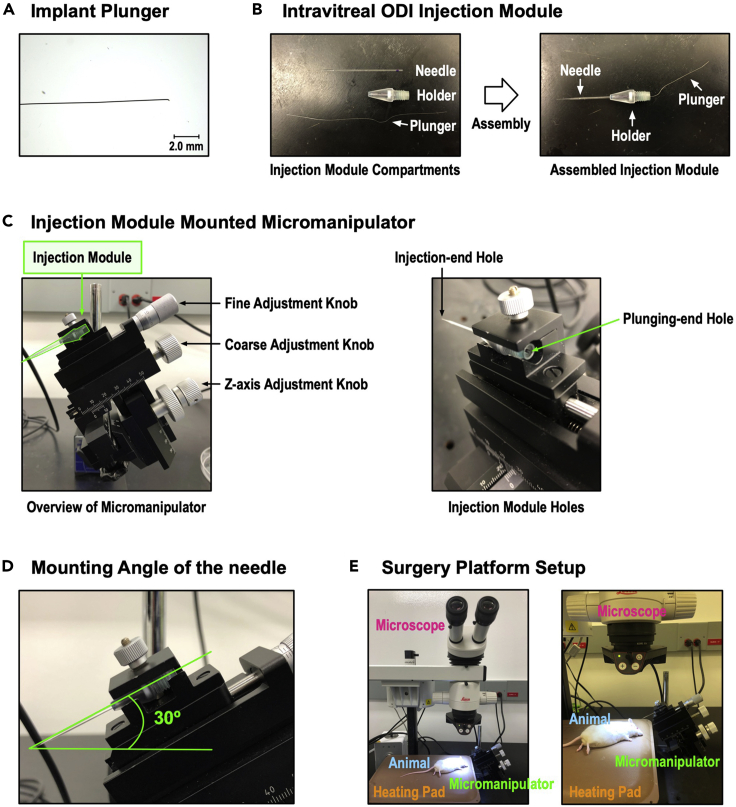
To generate an implant plunger, a syringe cleaning wire was used. To adjust the thickness of the implant plunger to fit into the needle, one end of the syringe cleaning wire was ground using a Black Arkansas stone until the diameter of the wire was 50 μm. The following is the step-by-step procedure for making the implant plunger:-
a.Apply a syringe-cleaning wire on the Black Arkansas stone.
-
b.Using your pointer finger, press and move one end of the cleaning wire back and forth.
-
c.After 10 repetitions, check the width under a dissecting microscope.
-
d.Repeat step c. until the diameter of the wire reaches 50 μm (Figure 7A).Optional: By banding the non-ground end of the cleaning wire, make a handle-like structure that can be used to easily manipulate the plunger during surgery.
-
a.
-
11.
Injection module assembly.
An injection module was assembled to mount the implant loaded needle to the micromanipulator (Figure 7B). The following is the step-by-step procedure for the injection module assembly:-
a.Sterilize the needle and capillary holder with 70% ethanol.
-
b.Assemble the implant loaded needle to a capillary holder.Note: This step should be done with care so that the implant does not fall out of the needle.Note: Parafilm the needle and the capillary holder together to provide a more stable assembly.
-
c.Insert the plunger into the hole at the opposite end of the needle tip.
-
d.Keep the plunger at the halfway point of the implant loaded needle.Note: The plunger should not be touching the implant.
-
a.
-
12.
Micromanipulator and surgery bed setup.
For the intravitreal injection, stability of the microcapillary during the surgery is very important. Since the mouse eye is small and fragile, any unnecessary movement or vibration from the surgical hand may damage or tear the eyeball during injection. There are several commercially available micromanipulators with angle specific adjustable arms. In our MI3 method, utilizing a micromanipulator provides a precise and stable position to fine-tune the needle holder angle. Also, it secures the needle position and supports a smooth injection when using the plunger. The following is the recommended setup for the micromanipulator and the surgical platform for the intravitreal injection of ODIs:-
a.Install the micromanipulator right next to the animal surgical platform.
-
b.Set the angle of the needle holder on the micromanipulator to 30°.
-
c.Raise the surgical bed to meet the height limitation of the micromanipulator.
-
d.Place the heating pad on top of the surgical bed.
-
e.Set the heat to maintain the body temperature of the mouse.Note: The preset of the heating pad used was 37°C.
-
f.Place the implant and plunger loaded needle on the micromanipulator (Figures 7C and 7D).
-
g.Adjust the micromanipulator location, making the needle and eye visible under the dissecting microscope’s examination stage (Figure 7E).
-
a.
-
13.Implant injection (Methods video S1).
-
a.Using the micromanipulator’s x-axis adjustment knob, move the needle closer to the injection site.
-
b.Apply lubricant eye drops to the eye to prevent the eye from drying.
-
c.Slowly adjust the x-axis adjustment knob until the needle is inserted into the eye.Note: The recommended needle entry-position is 2 mm posterior to the limbus (troubleshooting 4).
-
d.Insert the needle until the tip of the needle can be seen in the eye under the dissecting microscope.Note: The recommended depth of the needle head is 2–3 mm.
-
e.Move the plunger from the bottom of the microcapillary until its end touches the implant (troubleshooting 5).
-
f.Gently push the implant into the eye.
-
g.When the implant is fully inserted, slowly remove the needle by adjusting the x-axis adjustment knob.
-
h.Apply an antibiotic ophthalmic solution to the eye to prevent potential infections.
-
i.Place the post-surgical mouse on a heating pad until it wakes up.Note: Throughout the procedure, keep applying one lubricant eye drop every 5 min to prevent the cornea surface from drying out.
-
a.
Figure 7.
Tools and surgery platform setup for plunger-based implant injection
(A) Implant plunger before sharpening (left panel) and after sharpening (right panel).
(B) Injection module before (left panel) and after (right panel) sharpening and assembly.
(C) Injection module mounted on micromanipulator, with needle at 30° angle (magenta). Plunging-end hole is available for plunger loading and manipulation.
(D) Overview of the plunger method surgery platform. A mouse and heating pad are set under the dissecting microscope, and the micromanipulator is placed nearby to be seen under the microscope.
(E) Overview of the plunger-based method surgery platform. A mouse and heating pad are set under the dissecting microscope, and the micromanipulator with assembled plunger-module placed near the microscope.
Expected outcomes
In vivo imaging of ODI injected mouse eyes
Immediately following the implant injection (within a minute), the ODI-injected mouse eyeball was examined. The injected fluorescein-PLGA implant was clearly observable in the live image and the enucleated eye image (cleared by PACT method (Treweek et al., 2015)) taken under a fluorescence microscope. No sign of severe trauma (e.g., intravitreal hemorrhages, cataract, or sclera damage) was observed under the microscope. We further examined anatomical structure of the eyes and retinas to address any complications through spectral-domain optical coherence tomography (SD-OCT) and hematoxylin and eosin (H&E) histology at 6-weeks post injection. Compared to the control eyes, implant-injected eyes did not show any significant structural or thickness changes in the retinal layers, and there was no cataract, vitreous hemorrhage, intraocular inflammation, or retinal cell death. To address whether our surgery damages the function of the mouse eyes, we performed an electroretinography (ERG). The weekly ERG measures of the control (no injection) eyes and the implant-injected eyes were taken for 6 weeks after injection. The ERG responses stayed stable and normal for 6-weeks post implant-injection. Taken together, these results suggest that our surgery method does not damage the retinal function nor anatomical structure.
Validation of sustained release of model drug-implant in mouse eyes: Infrared imaging and model drug quantification in mouse eyes
At 3-days post injection of Cy5.5/PLGA implant, the eyes were enucleated and the remaining intraocular drug was measured by non-invasive near-infrared (NIR) image analysis. A strong NIR fluorescent signal was detected in the implant injected eyes, while no signals were detectable in both the free-Cy5.5 injected and control eyes. Therefore, there was rapid clearance of free-Cy5.5 while the implant formulation prolonged drug retention time in the mouse eyes. The implant-formulation strategy retained its pharmacokinetic advantages in mice.
Quantification and statistical analysis
Quantification of the Cy5.5 model drug
The Cy5.5 dye concentration was determined by measuring fluorescent intensities (λex 650 nm/λem 714 nm) using a standard curve with free dye dissolved in a DMSO/digestion buffer. Standard curve was linearly fitted using GraphPad Prism software. The amount of Cy5.5 was summarized as a mean and standard error of the mean (SEM) for three biological replicates.
Retinal thickness in OCT images
Retinal thickness was measured with a Heidelberg Spectralis built in thickness measurement where the thickness of each layer was defined as the average thickness in the circular scans centered on the optic nerve head (ONH). Four thickness measurements were evaluated: the whole retina thickness, defined as the distance from the retinal nerve fiber layer (RNFL) to the retinal pigment epithelium (RPE); the ganglion cell complex (GCC), defined as the thickness of RNFL, ganglion cell layer (GCL) and the inner plexiform layer (IPL); the photoreceptor (RP) thickness, defined as distance from the outer plexiform layer (OPL) to the outer segment (OS); and the RPE thickness. The thickness data were summarized as means and standard error of the means (SEM) for six biological replicates. Paired t-tests were used for comparison of the different layer thickness of the control eye and the PLGA injected eye using GraphPad Prism software.
ERG measurements
Mice were dark-adapted for 16–18 h, manipulations were conducted under dim light illumination, and recordings were made using Espion ERG Diagnosys equipment (Diagnosys LLL, Littleton, MA). Electrodes were placed on the corneas, and refresh lubricant eye gel (Allergan, Irvine, CA) was applied to each eye. Both eyes were recorded simultaneously. Paired t tests were used for comparison of ERG signals (a-waves and b-waves) of the control eyes and the implant-injected eyes using GraphPad Prism software.
Limitations
Our method allows for the testing of small molecule implants in a broad range of ocular disease mouse models to determine efficacy, toxicity, and desired drug dose and release rates, which can serve as target reference parameters when developing human ocular implants. However, the implant’s dimensions are not directly scalable from micron-sized mouse implants to millimeter-sized human implants, considering that a smaller particle leads to a faster release rate due to the surface-area-to-volume ratio. Thus, based on the efficacious dose, release rate, and toxicity parameters identified in mouse models, implants may require further formulation optimization such as modifications to implant surfaces or using different polymer compositions to obtain suitable drug release profiles in human eyes (Chen et al., 2018; Hines and Kaplan, 2013).
Troubleshooting
Problem 1
Poor solubility of Cy5.5 after dissolving of implant
While dissolving a Cy5.5/PLGA implant in DMSO, the Cy5.5 in an implant may aggregate and may not be detected (Before you begin: Quantification of Cy5.5 in a loaded implant; step 11, step 12).
Potential solution
If aggregation of Cy5.5 is detected, further dilution in 0.1% BSA/PBS should be used. Note that BSA/PBS should be used instead of PBS to prevent attachment of the dye molecules to the plastic and suspension of the dye after spinning down. Where lower concentrations are not detectable, take the lowest detectable concentration, perform several more serial dilutions in 0.1% BSA/PBS, increase the gain, and remeasure.
Problem 2
Microcapillary needles not suitable for use
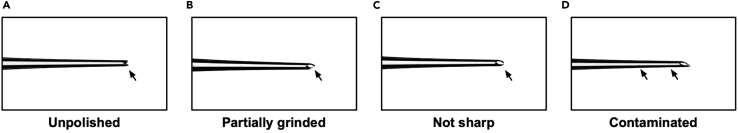
Since sclera is thick and tight in its tissue structure, microcapillary glass needles should be sharpened and polished properly for successful injections. Performing injection with inappropriate needles can cause unnecessary damage to the eyeball. In parallel, implants may not be properly loaded into poorly made needles, which may result in implants being stuck inside the needle during surgery. (Before you begin: make an implant-loaded microcapillary needle; step 20-h).
Potential solution
Here, we show several examples of poorly made needles (Figure 8). In many cases, further grinding the needles to become sharper in every dimension may result in usable needles. If you observe any debris inside (Figure 8D), do not use the needle. The debris may become a contaminant or interfere in proper loading of an implant.
Figure 8.
Examples of microcapillary needles that are not suitable for surgery
(A) An example of unpolished needle. Needle tip is clipped by forceps, but it is not grinded. This needle is hard to penetrate sclera and can damage eyeballs.
(B) An example of partially grinded needle. Only the one side of the needle tip is grinded. This needle is hard to load an implant.
(C) An example of not sharp enough needle. This needle may cause unnecessary damage on the eyeball as it requires more force to penetrate sclera.
(D) An example of contaminated needle. The needle tip may be sharp enough, yet several debris (contaminants) inside the needle may induce inflammation and interfere in proper loading of an implant.
Problem 3
Implant cannot be loaded into the needle
Like a human hair, implants for mouse eyes are very small, thin, light, and can be static depending on the materials used for formulation. When loading an implant into the needle, it is hard to keep the implant stay steady, and implants can be crushed or deformed (Before you begin: make an implant-loaded microcapillary needle; step 21-c).
Potential solution
Before attempting to load implants to needles, check the dimensions of fabricated glass capillary needles. The inner diameter of the needle tip is critical for the implant loading. Wearing a mask during the implant loading can prevent the implant from being blown by users’ breath. Gentle manipulation of the forceps can help implants to stay intact while loading. Lightly wetting and brush drying your personal protection equipment and tools (e.g., gloves, lab coats, and forceps) may help reduce static charge. Do not wet the biodegradable implants because the water triggers the degradation of implants.
Problem 4
Potential damage to mouse lens, central retina, or optic nerve during needle insertion to eyes
Compared to human eyes, mouse eyes have a relatively larger lens volume, and their vitreous cavity is comparatively smaller. Thus, an inappropriate needle entry site or needle entry-angle can damage lens, central retina, or optic nerve. Ultimately, this can result in an unsuccessful implant delivery to the vitreous chamber and may increase the chances of damage to ocular structures, trigger intraocular inflammation, or cause a retinal detachment (Step-by-step method details: Implant injection by air-pressure; step 12-d, step-by-step method details: part 2b. Implant injection by plunger; step 13-c).
Potential solution
To avoid eye damage during needle insertion, we recommend inserting the needle at 2 mm posterior to the limbus. The inner eye structure of this site is the peripheral retina, which is physically distant from the core region for visual function of an eye (e.g., central retina and optic nerve). When a needle enters this site in a posterior direction with an entry angle of 45° from the tangent line of entry site, the needle can avoid the lens and directly reach the vitreous cavity. Thus, needle entry site and entry-angle should be carefully controlled to avoid damaging lens, central retina, and optic nerve. This can significantly increase the success rate of implant injection and reduce potential retina damage.
Problem 5
Plunger is hard to move
For the plunger-based implant injection, the plunger may not move smoothly. This may increase the chance to disturb the mouse eye during implant injection (step-by-step method details: part 2b. Implant injection by plunger; step 13-e).
Potential solution
If the movement of the plunger is not smooth, thoroughly grind the plunger in every dimension. Before surgery, test the movement of the plunger with an empty needle.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to the lead contact, Vinit B. Mahajan (vinit.mahajan@stanford.edu). All stable reagents generated in this study are available from the lead contact with a completed Materials Transfer Agreement.
Materials availability
This study did not generate new unique reagents.
Acknowledgments
We thank Dr. Jeffrey Goldberg in the department of ophthalmology at Stanford University for allowing us to use his micromanipulator for plunger-based implant injections. We thank Drs. Michael Kapiloff and Jinliang Li in the department of ophthalmology at Stanford University for training us and allowing us to use their microscope to collect histology images. We thank Drs. Liang Li, Gabriella Maria Fernandes Cunha, and David Myung in the department of ophthalmology at Stanford University for helpful discussions. We also thank Dr. Jill Helms in the department of surgery at Stanford University for allowing us to use her teaching microscope to collect movies and images for the procedure.
V.B.M. is supported by NIH grants (R01EY026682, R01EY024665, R01EY025225, R01EY024698, and P30EY026877), Stanford ChEM-H IMA, the Stanford Center for Optic Disc Drusen, and Research to Prevent Blindness, New York, New York. S.W. is supported by American Diabetes Association (1-16-INI-16), NIH grants (R01NS109990 and 1R01EY03258501), and P30 to Stanford Ophthalmology. Y.J.S. is supported by BrightFocus Foundation’s Macular Degeneration Research program. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the sponsor.
Author contributions
Study concept and design: V.B.M. Acquisition of data, C.H.L., Y.J.S., S.H.L., C.R.K., M.R.W., and J.Y.; Data analysis and interpretation: C.H.L., Y.J.S., S.H.L., C.R.K., M.R.W., J.Y., S.W., and V.B.M.; Drafting of the manuscript and video, C.H.L., Y.J.S., S.H.L., E.M.M., C.R.K., M.R.W., J.Y., Y.S.J., S.W., and V.B.M.; Critical revision of the manuscript, C.H.L., Y.J.S., S.H.L., E.M.M., C.R.K., M.R.W., B.C., S.W., and V.B.M.; Funding Acquisition, S.W. and V.B.M. Administrative, technical, and material support, V.B.M.; Study supervision, V.B.M.
Declaration of interests
The authors declare no competing interests.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.xpro.2022.101143.
Contributor Information
Sui Wang, Email: suiwang@stanford.edu.
Vinit B. Mahajan, Email: vinit.mahajan@stanford.edu.
Supplemental information
Data and code availability
All data generated in this paper will be shared by the lead contact by request.
This paper does not report original code.
Any additional information required to reanalyze the data reported in this work paper is available from the lead contact upon request.
References
- Chan C.S.Y., Lonfat N., Zhao R., Davis A.E., Li L., Wu M.-R., Lin C.-H., Ji Z., Cepko C.L., Wang S. Cell type- and stage-specific expression of Otx2 is regulated by multiple transcription factors and cis-regulatory modules in the retina. Development. 2020;147:dev187922. doi: 10.1242/dev.187922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W., Yung B.C., Qian Z., Chen X. Improving long-term subcutaneous drug delivery by regulating material-bioenvironment interaction. Adv. Drug Deliv. Rev. 2018;127:20–34. doi: 10.1016/j.addr.2018.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines D.J., Kaplan D.L. Poly(lactic-co-glycolic) acid−controlled-release systems: experimental and modeling insights. 2013;30:257–276. doi: 10.1615/CritRevTherDrugCarrierSyst.2013006475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horio N., Kachi S., Hori K., Okamoto Y., Yamamoto E., Terasaki H., Miyake Y. Progressive change of optical coherence tomography scans in retinal degeneration slow mice. Arch. Ophthalmol. 2001;119:1329–1332. doi: 10.1001/archopht.119.9.1329. [DOI] [PubMed] [Google Scholar]
- Maya-Vetencourt J.F., Manfredi G., Mete M., Colombo E., Bramini M., Di Marco S., Shmal D., Mantero G., Dipalo M., Rocchi A., et al. Subretinally injected semiconducting polymer nanoparticles rescue vision in a rat model of retinal dystrophy. Nat. Nanotechnol. 2020;15:698–708. doi: 10.1038/s41565-020-0696-3. [DOI] [PubMed] [Google Scholar]
- Schmucker C., Schaeffel F. A paraxial schematic eye model for the growing C57BL/6 mouse. Vis. Res. 2004;44:1857–1867. doi: 10.1016/j.visres.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Shmueli R.B., Ohnaka M., Miki A., Pandey N.B., Lima e Silva R., Koskimaki J.E., Kim J., Popel A.S., Campochiaro P.A., Green J.J. Long-term suppression of ocular neovascularization by intraocular injection of biodegradable polymeric particles containing a serpin-derived peptide. Biomaterials. 2013;34:7544–7551. doi: 10.1016/j.biomaterials.2013.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y.J., Lin C.-H., Wu M.-R., Lee S.H., Yang J., Kunchur C.R., Mujica E.M., Chiang B., Jung Y.S., Wang S., et al. An intravitreal implant injection method for sustained drug delivery into mouse eyes. Cell Reports Methods. 2021;1:100125. doi: 10.1016/j.crmeth.2021.100125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treweek J.B., Chan K.Y., Flytzanis N.C., Yang B., Deverman B.E., Greenbaum A., Lignell A., Xiao C., Cai L., Ladinsky M.S., et al. Whole-body tissue stabilization and selective extractions via tissue-hydrogel hybrids for high-resolution intact circuit mapping and phenotyping. Nat. Protoc. 2015;10:1860–1896. doi: 10.1038/nprot.2015.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Sengel C., Emerson M.M., Cepko C.L. A gene regulatory network controls the binary fate decision of rod and bipolar cells in the vertebrate retina. Dev. Cell. 2014;30:513–527. doi: 10.1016/j.devcel.2014.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wert K.J., Skeie J.M., Davis R.J., Tsang S.H., Mahajan V.B. Subretinal injection of gene therapy vectors and stem cells in the perinatal mouse eye. JoVE. 2012:e4286. doi: 10.3791/4286. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated in this paper will be shared by the lead contact by request.
This paper does not report original code.
Any additional information required to reanalyze the data reported in this work paper is available from the lead contact upon request.