Abstract
On October 2, 2020, the U.S. Food and Drug Administration (FDA) approved nivolumab with ipilimumab as first-line treatment for adult patients with unresectable malignant pleural mesothelioma (MPM). The approval was based on results from Study CA209743 (CHECKMATE-743), an open-label trial of patients with MPM randomized to receive nivolumab and ipilimumab for up to two years (n=303) or six cycles of chemotherapy with cisplatin or carboplatin plus pemetrexed (n=302). Overall survival (OS) was improved for patients who received nivolumab and ipilimumab, with a median OS of 18.1 months (95% CI: 16.8, 21.5) compared to 14.1 months (95% CI: 12.5, 16.2) (HR 0.74; 95% CI: 0.61, 0.89; p = 0.002), for patients who received chemotherapy. The magnitude of benefit was larger for patients with non-epithelioid versus epithelioid histology. Additional clinical pharmacology data supports an alternative dosing regimen of nivolumab than evaluated in the trial, which will reduce the number of required treatment visits. This application was reviewed under FDA’s Project Orbis, in collaboration with Australia’s Therapeutic Goods Administration, Switzerland’s Swissmedic, Health Canada, and Brazil’s National Health Surveillance Agency or ANVISA (Agência Nacional de Vigilância Sanitária). Nivolumab and ipilimumab is the first drug regimen approved by the FDA for MPM since 2004.
Introduction
Malignant pleural mesothelioma (MPM) is an aggressive tumor of the lung pleura that occurs most frequently as a result of asbestos inhalation. In the US, MPM is relatively rare and incidence rates are declining, with approximately 3000 new cases diagnosed annually (1). In Australia, incidence rates are significantly higher at approximately 30 cases per million (2). MPM is associated with significant mortality with a five-year OS of <10% for advanced disease (3). There are three major histologic subtypes of MPM: epithelioid, sarcomatoid, or biphasic (mixed). Epithelioid MPM is the most common subtype, comprising 60% of cases, and is associated with a better prognosis than sarcomatoid MPM (4, 5). Although sarcomatoid MPM has a poor response to chemotherapy, it expresses PD-L1 more frequently than epithelioid MPM, which may have therapeutic implications (6, 7, 8).
Prior to this approval, cisplatin in combination with pemetrexed was the only FDA-approved drug regimen for the treatment of patients with MPM whose disease is unresectable or who are otherwise not candidates for curative surgery (9). For patients who are not candidates for cisplatin, carboplatin plus pemetrexed is an accepted option in clinical practice (10). Bevacizumab with pemetrexed and cisplatin or carboplatin are listed in the National Comprehensive Cancer Network (NCCN) guidelines as treatment options; however, these combinations are not approved for MPM treatment by the FDA or any other international regulatory agency (11).
The combination of nivolumab and ipilimumab is the first FDA-approved drug regimen for MPM in over 15 years. This approval provides a chemotherapy-sparing treatment option and adds to the limited arsenal of available therapies for advanced MPM. We provide a summary of FDA’s review of the marketing application that led to the approval of nivolumab and ipilimumab for previously untreated, unresectable MPM.
Clinical Trials
The approval of nivolumab in combination with ipilimumab for MPM was primarily based on the results of CHECKMATE-743, an open-label, randomized trial of patients with previously untreated, unresectable MPM. Patients were randomized 1:1 to nivolumab 3 mg/kg every two weeks and ipilimumab 1 mg/kg every six weeks or six cycles of platinum-doublet chemotherapy (pemetrexed 500 mg/m2 plus investigator’s choice of cisplatin 75 mg/m2 or carboplatin AUC 5), with randomization stratified by sex and tumor histology (epithelioid vs non-epithelioid). The primary endpoint was OS. Key secondary endpoints included progression-free survival (PFS) and overall response rate (ORR) per RECIST 1.1 according to independent central review. The statistical analysis plan did not include a prespecified plan for hierarchical testing of these secondary endpoints.
The contribution of effect of nivolumab and ipilimumab was supported by data from an investigator-sponsored, randomized, non-comparative, open-label study CA209304 (hereafter referred to as the MAPS2 trial) conducted in France (12). In the MAPS2 trial, 125 patients with MPM whose disease progressed after treatment with one or two prior lines of chemotherapy were randomized 1:1 to nivolumab 3 mg/kg every two weeks and ipilimumab 1 mg/kg every six weeks or nivolumab as a single agent at 3 mg/kg every two weeks.
Efficacy Results
CHECKMATE-743 randomized a total of 605 patients to receive nivolumab with ipilimumab (n=303) or chemotherapy (n=302). Overall, 75% of patients with MPM had epithelioid histology. Of the 97% patients with quantifiable PD-L1 expression at baseline, 77% had PD-L1 positive (≥1%) tumors (additional key demographic and baseline disease characteristics are shown in Table 1). A pre-specified interim analysis of OS in CHECKMATE-743 demonstrated a statistically significant improvement in OS in the nivolumab with ipilimumab arm compared to the chemotherapy arm (hazard ratio [HR] 0.74; 95% CI: 0.61, 0.89; p-value = 0.002). The median OS in patients randomized to the nivolumab with ipilimumab arm was 18.1 months (95% CI: 16.8, 21.5) versus 14.1 months (95% CI: 12.5, 16.2) in the chemotherapy arm (Table 2; Figure 1).
Table 1.
Demographic and Disease Characteristics of CHECKMATE-743
| Nivolumab and Ipilimumab N=303 |
Chemotherapy N=302 |
|
|---|---|---|
| Age (years) | ||
| Median | 69 | 69 |
| ≥65 (%) | 232 (77) | 206 (68) |
|
| ||
| Race (%) | ||
| White | 266 (88) | 250 (83) |
| Asian | 26 (9) | 39 (13) |
| American Indian or Alaska Native | 2 (0.7) | 4 (1.3) |
| Other | 9 (3.0) | 9 (3.0) |
|
| ||
| ECOG Performance Status (%) | ||
| 0 | 114 (38) | 128 (42) |
| 1 | 189 (62) | 173 (57) |
| 2 | 0 | 1 (0.3) |
|
| ||
| Disease Stage at Study Entry (%) | ||
| I | 12 (4.0) | 20 (7) |
| II | 23 (8) | 22 (7) |
| III | 103 (34) | 106 (35) |
| IV | 160 (53) | 149 (49) |
| Not reported | 5 (1.7) | 5 (1.7) |
|
| ||
| Histology (%) a | ||
| Epithelioid | 229 (76) | 227 (75) |
| Non-epithelioid | 74 (24) | 75 (25) |
|
| ||
| PD-L1 Expression (%) | ||
| Patients with quantifiable baseline expression | 289 (95) | 297 (98) |
| <1% | 57 (20) | 78 (26) |
| ≥1% | 232 (80) | 219 (74) |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; PD-L1, programmed death-ligand 1.
Histology based upon interactive response technology (IRT) assessment.
Source: U.S. FDA BLA Multi-disciplinary Review and Evaluation (sBLA 125554 and sBLA 125377) and Approval Package (ref. 27).
Table 2:
Efficacy Results – ITT population of CHECKMATE-743
| Nivolumab and Ipilimumab N=303 |
Chemotherapy N=302 |
|
|---|---|---|
| OS | ||
| Deaths, n (%) | 200 (66) | 219 (73) |
| Median (months)a (95% CI) | 18.1 (16.8, 21.5) | 14.1 (12.5, 16.2) |
| Hazard ratio (95% CI)b | 0.74 (0.61, 0.89) | |
| Stratified log-rank p-valuec | 0.002 | |
|
| ||
| PFS per BICR | ||
| Disease Progression or Death, n (%) | 218 (72) | 209 (69) |
| Median (months) (95% CI) | 6.8 (5.6, 7.4) | 7.2 (6.9, 8.1) |
| Hazard ratio (95% CI) | 1.0 (0.82, 1.21) | |
|
| ||
| ORR per BICR | ||
| Responders, n (%) | 120 (40) | 129 (43) |
| (95% CI) | (34, 45) | (37, 49) |
|
| ||
| DOR per BICR | ||
| Median (months) (95% CI) | 11.0 (8.1, 16.5) | 6.7 (5.3, 7.1) |
Abbreviations: BICR, blinded independent central review; CI, confidence interval; DOR, duration of response; ITT, intent-to-treat; ORR, overall response rate; OS, overall survival
Kaplan-Meier estimate.
Based on a stratified Cox proportional hazard model.
p-value is compared with the allocated alpha of 0.0345 for this interim analysis.
Figure 1: Kaplan-Meier Plot for Overall Survival – All Randomized Patients from CHECKMATE-743.
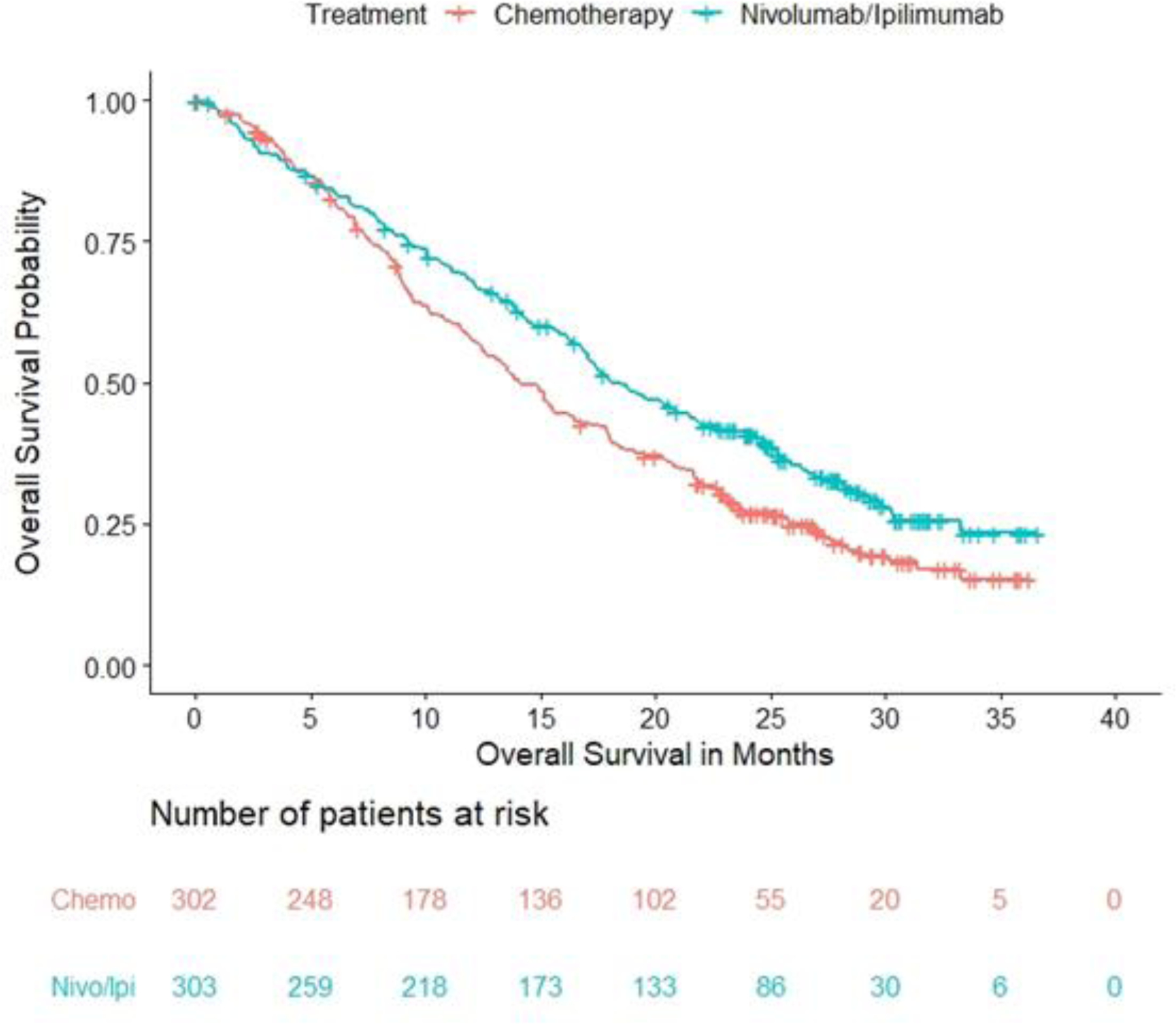
Source: OPDIVO (nivolumab) and YERVOY (ipilimumab) [package insert] (ref. 14 and 15).
Neither PFS nor ORR, both assessed by blinded independent central review (BICR), were improved in patients receiving nivolumab with ipilimumab (Table 2). Median duration of response (DOR), however, was longer for responders in the nivolumab and ipilimumab arm at 11 months (95% CI: 8.1, 16.5) versus 6.7 months (95% CI: 5.3, 7.1) in the chemotherapy arm.
In prespecified exploratory analyses based on histology, median OS in the epithelioid histology subgroups of the nivolumab plus ipilimumab and chemotherapy arms was comparable, however there was a trend illustrating an improvement in the median OS in the subgroup of patients with non-epithelioid histology favoring the nivolumab plus ipilimumab subgroup. Based on information on histology obtained at randomization from interactive response technology (IRT), the OS HR for patients with non-epithelioid histology was 0.46 (95% CI 0.31, 0.70) with median OS of 16.9 months in the nivolumab and ipilimumab arm (n=67) versus 8.8 months in the chemotherapy arm (n=67). For patients with epithelioid histology, the OS HR was 0.85 (95% CI 0.68, 1.06) with median OS of 18.7 months in the combination nivolumab and ipilimumab arm (n=236) versus 16.2 months in the chemotherapy arm (n=235). This apparent difference in the magnitude of OS benefit between histologic subgroups can be attributed to the poor survival outcomes observed in patients with non-epithelioid histology who received chemotherapy, consistent with prior reports (3, 4). While PD-L1 status was not a stratification factor, exploratory subgroup analyses also illustrated a trend toward a larger magnitude of benefit for nivolumab with ipilimumab compared to chemotherapy in patients with tumor PD-L1 expression ≥1% (n=451, OS HR 0.69; 95% CI 0.55, 0.87) versus patients with tumor PD-L1 expression <1% (n=135, OS HR 0.94; 95% CI 0.62, 1.40).
MAPS2 Trial
To assess the contribution of each component to the treatment effect observed with the combination of nivolumab and ipilimumab in CHECKMATE-743, FDA evaluated data from the non-comparative randomized MAPS2 trial. The primary endpoint of the MAPS2 trials was disease control rate (DCR), which FDA generally considers an exploratory endpoint and insufficient to assess efficacy. The study was not powered to compare the two treatment arms statistically. Nonetheless, there were numerical improvements reported in ORR, OS, and PFS in patients randomized to receive nivolumab with ipilimumab compared to those randomized to receive single-agent nivolumab: ORR (25.8% vs 17.5%), median OS (15.93 vs 11.86 months), and median PFS (5.44 vs 3.97 months) (12).
Clinical Pharmacology
The dosage regimen approved for the treatment of MPM (nivolumab 360 mg every 3 weeks and ipilimumab 1 mg/kg every 6 weeks) was different than the dosage regimen of nivolumab 3 mg/kg every 2 weeks and ipilimumab 1 mg/kg every 6 weeks evaluated in CHECKMATE-743. A flat dose of nivolumab 360 mg every 3 weeks was previously approved in combination with ipilimumab and platinum-doublet chemotherapy for the treatment of non-small cell lung cancer (NSCLC) (13). Pharmacokinetic simulation showed a significant overlap of the concentration profiles between nivolumab 360 mg every 3 weeks and 3 mg/kg every 2 weeks at steady-state in patients with MPM. The simulated nivolumab minimum concentration at steady-state with nivolumab 360 mg every 3 weeks is 1.7% lower than that of the 3 mg/kg every 2 weeks. Subgroup analysis of OS across body weight categories demonstrated that patients with greater body weight may have lower nivolumab exposure with flat dosing than with weight-based dosing. However, survival was not compromised in patients with greater body weight [≥80 kg, median OS (95% CI) of 22.8 months (18.6, 28.2)] compared to patients with lower bodyweight [<60 kg, median OS (95% CI) of 15.8 months (10.7, 24.6)]. The clinical safety subgroup analysis suggested that, overall, the rate of Grade 3–4 adverse events (AEs), Grade ≥2 immune-mediated AEs, or serious AEs are not associated with body weight categories in patients with MPM. There was also no association between nivolumab exposure and Grade ≥2 IMAEs in the patients with MPM.
Safety Results
The safety review focused on CHECKMATE-743 with additional supportive data from the MAPS2 trial. Safety analyses were conducted in all patients who received at least one dose of study therapy. The overall safety profile of nivolumab with ipilimumab in CHECKMATE-743 was generally consistent with the known safety profile of these therapies. Fatal adverse reactions (ARs) occurred in 4 (1.3%) patients who received nivolumab with ipilimumab and included pneumonitis, acute heart failure, sepsis, and encephalitis. Fifty-four percent of patients who received nivolumab and ipilimumab had at least one serious adverse reaction (SAR). Permanent discontinuation and dose delay due to ARs with the immunotherapy combination occurred in 23% and 52% of patients, respectively. Compared to chemotherapy, nivolumab with ipilimumab was associated with higher rates (≥20% difference between arms) of musculoskeletal pain, diarrhea, rash, and pruritis, which are known ARs of immune checkpoint inhibitors. Patients who received chemotherapy had higher rates of nausea and constipation. For lab abnormalities, patients treated with chemotherapy had higher rates (≥20% difference between arms) of anemia, while patients treated with nivolumab and ipilimumab had higher rates of increased lipase, and increased AST or ALT. Immune-mediated ARs of interest including pancreatitis, encephalitis, myositis, myasthenic syndrome, uveitis, and myocarditis were infrequent, while demyelination, Guillain-Barre Syndrome, and rhabdomyolysis did not occur at all.
In general, the incidence and severity of immune‐mediated ARs were comparable to those previously described for the combination of nivolumab and ipilimumab for other tumor types (14). The incidence and severity of immune‐mediated pneumonitis and interstitial lung disease, an AR particularly relevant to patients with thoracic malignancies, was similar for patients with MPM compared to patients with NSCLC treated with nivolumab and ipilimumab in another study (7% vs 9%, respectively). Notably, infusion-related reactions occurred in 12% of patients who received nivolumab and ipilimumab in CHECKMATE-743, which is more than a two-fold increase than previously reported for patients with renal cell carcinoma (RCC, 5%) and colorectal carcinoma (CRC, 4.2%) who received similar combination dosage regimens of nivolumab and ipilimumab in other studies (14, 15).
Regulatory Insights
Following a period of stagnant drug development in MPM, the approval of nivolumab with ipilimumab based upon improved OS provides a new frontline therapy for patients with unresectable disease as summarized in Table 3. Although PFS and ORR were not significantly different between patients treated with nivolumab and ipilimumab and patients treated with chemotherapy, tumor measurements used for PFS and ORR assessment can be imprecise in MPM due to challenges with demarcated margins. The CHECKMATE-743 regimen may be appealing as a chemotherapy-sparing treatment, particularly for those patients with unresectable MPM with non-epithelioid histology. As expected, patients who received nivolumab with ipilimumab had decreased rates of toxicities commonly associated with chemotherapy, such as cytopenias and nausea, but incurred higher rates of immune-related toxicities. The immune-related toxicity incidence was generally consistent with previous reports of nivolumab with ipilimumab for the treatment of other cancer types.
Table 3:
FDA Benefit-Risk Analysis
| Dimension | Evidence and Uncertainties | Conclusions and Reasons |
|---|---|---|
| Analysis of Condition | • MPM is a rare, aggressive tumor of the lung pleura that occurs most frequently due to asbestos exposure. • At time of diagnosis, most patients have unresectable MPM or are ineligible for surgery. Long-term survival is poor. |
Unresectable MPM is a life-threatening condition with poor survival. |
| Current Treatment Options | • Prior to this approval, cisplatin plus pemetrexed was the only FDA-approved drug regimen for unresectable MPM. • Carboplatin plus pemetrexed is an accepted option for patients ineligible to receive cisplatin. |
Nivolumab with ipilimumab provides a second approved and the first chemotherapy-sparing treatment option for unresectable MPM. |
| Benefit | • In CHECKMATE-743, the HR for OS was 0.74 (95% CI: 0.61, 0.89; p = 0.002), favoring nivolumab and ipilimumab (median OS 18.1 months; 95% CI: 16.8, 21.5) compared to chemotherapy (median OS 14.1 months; 95% CI:12.5, 16.2). • The ORR as assessed by BICR in the nivolumab and ipilimumab arm was 40% (95% CI 34, 45) with a median DOR of 11.0 months versus 43% (95% CI 37, 49) with a median DOR of 6.7 months in the chemotherapy arm. • Data from the MAPS2 trial, a randomized study of nivolumab plus ipilimumab or nivolumab alone for patients with previously treated unresectable MPM, supported contribution of components for nivolumab and ipilimumab. |
A statistically significant and robust improvement in OS for nivolumab and ipilimumab over chemotherapy provided evidence of effectiveness. A substantial improvement in DOR favoring nivolumab and ipilimumab was also supportive of clinical benefit. |
| Risk and Risk Management | • The most common (≥20%) adverse reactions to nivolumab and ipilimumab were fatigue, musculoskeletal pain, rash, diarrhea, dyspnea, nausea, decreased appetite, cough, and pruritus. • The incidence and severity of immune‐mediated adverse reactions, including pneumonitis, were consistent with those listed in the current product labeling for the combination of nivolumab and ipilimumab. • The incidence of infusion-related reactions was higher for patients with MPM (12%) treated with nivolumab and ipilimumab in CHECKMATE-743 compared to patients with renal cell carcinoma (5%) and colorectal carcinoma (4.2%) who received similar combinations of nivolumab and ipilimumab in other studies. |
The observed safety profile is acceptable in the context of the treatment of a life‐threatening disease. The increased incidence of infusion-related reactions was added to the Warnings and Precautions section of the prescribing information. No significant safety concerns were identified during the review of the application requiring risk management beyond labeling or warranting consideration for a Risk Evaluation and Mitigation Strategy (REMS) to ensure safe use of the combination. |
Source: U.S. FDA BLA Multi-disciplinary Review and Evaluation (sBLA 125554 and sBLA 125377) and Approval Package (ref. 27).
Immune checkpoint inhibitors, such as tremelimumab and pembrolizumab, have shown limited efficacy as single agents for the treatment of unresectable MPM (16, 17). There is scientific rationale for the nivolumab and ipilimumab combination for MPM treatment. Nivolumab and ipilimumab target distinct but complementary immune checkpoint proteins (18). The clinical benefit of nivolumab and ipilimumab has also been demonstrated across multiple tumor types, and the combination is approved for the treatment of melanoma, RCC, hepatocellular carcinoma (HCC), microsatellite instable-high or mismatch repair deficient CRC, and NSCLC.
The CHECKMATE-743 study design did not characterize the individual contributions of nivolumab and ipilimumab to the effects observed with the combination. However, external data from the MAPS2 trial support the clinical benefit of the nivolumab and ipilimumab combination over nivolumab as a single agent. The MAPS2 trial reported improvements across all efficacy outcomes, including OS, PFS, and ORR, for nivolumab with ipilimumab compared to nivolumab as a single agent. Of note, CHECKMATE-743 evaluated treatment-naïve patients while the MAPS2 trial enrolled a more refractory population. The totality of data including a biologic rationale for the immunotherapy combination, demonstration of clinical efficacy of nivolumab with ipilimumab in other tumor types, and strength of the OS results of the combination in MPM, support the contribution of components for this approval.
The nivolumab with ipilimumab regimen is approved for an unselected population of patients with unresectable MPM, regardless of histology and PD-L1 status; however, exploratory analyses of data from CHECKMATE-743 suggest that patients with non-epithelioid histology and PD-L1-positive tumors may derive the most benefit from the combination of nivolumab with ipilimumab. These subgroup analyses are limited by the small sample sizes of patients with non-epithelioid histology. The interpretation of OS based on PD-L1 expression warrants even greater caution as patients were not stratified by PD-L1 status in CHECKMATE-743. Furthermore, non-epithelioid MPM is more frequently associated with higher levels of PD-L1 expression compared to epithelioid MPM (5). The degree to which immunological and other host- and tumor-related factors such as PD-L1 expression and features of the tumor microenvironment influence the degree of clinical benefit conferred by nivolumab and ipilimumab remains to be determined (19). Stratifying patients by both tumor histology and PD-L1 expression in future clinical trials of MPM will be important to elucidate which patients are most likely to benefit from immunotherapy regimens.
Noting the reduced number of patient visits required with an alternative dose of nivolumab, FDA requested submission of additional clinical pharmacology data to support its approval for the MPM application. The data from CHECKMATE-743 along with PK simulation results indicated that both dosage regimens have a similar benefit-risk profile for patients with MPM. Amidst the COVID-19 pandemic, minimizing in-person clinical encounters, when possible and safe, may help protect especially vulnerable groups such as patients with cancer. The FDA Oncology Center of Excellence (OCE) has taken measures to help reduce risk for patients with cancer due to COVID-19, including multiple approvals of alternative dosing regimens (20, 21, 22).
The approval of nivolumab and ipilimumab for MPM was conducted under Project Orbis, an FDA OCE initiative established to support concurrent submission and review of oncology drug applications by multiple international health agencies with a goal of delivering expedited access to therapies for patients globally (23). Under Project Orbis, FDA reviewed this application in collaboration with the Australian TGA, the Switzerland Swissmedic, Health Canada, and the Brazilian ANVISA. Participating in Project Orbis did not cause any delays with the FDA review timeline and FDA approved this application nearly five months ahead of schedule based on the Priority Review time clock. The application also utilized the Real-Time Oncology Review (RTOR), Assessment Aid (AAid), and Summary Review programs to maximize efficiency of the drug review process. The Summary Review program relies on qualified data summaries from the applicant to support approval of a supplemental application, without independent verification by the FDA of all data analyses (24).
Conclusions
The approval of nivolumab and ipilimumab provides a chemotherapy-sparing option for the treatment of patients with previously untreated, unresectable MPM. Results from the pivotal trial CHECKMATE-743 demonstrate a favorable benefit-risk profile and support approval. The combination of nivolumab and ipilimumab marks the first approval of a drug regimen for patients with MPM in over 15 years. Despite this milestone, treatment options are still limited, and effective and durable therapies for patients with advanced MPM remain an area of significant unmet need. Future directions for MPM therapy could involve combining cytotoxic therapies with immune checkpoint inhibitors, and studies to determine whether this leads to improved clinical outcomes are ongoing (25–26).
Footnotes
This is a U.S. Government work. There are no restrictions on its use. M. Biable has the left FDA and started working at DataRevive US LLC in February 2021. H. Qosa has left the FDA and started working at Bristol Myers Squibb in January 2021. Their work on the article was done during their employment at the FDA.
Disclosure of Potential Conflicts of Interest: The authors report no financial interests or relationships with the commercial sponsors of any products discussed in this report.
References:
- 1.Robinson BWS, Musk AW, Lake RA. Malignant mesothelioma. Lancet 2005. Jul 30-Aug 5;366(9483):397–408. [DOI] [PubMed] [Google Scholar]
- 2.Robinson BM. Malignant pleural mesothelioma: an epidemiological perspective. Ann Cardiothorac Surg 2012;1(4):491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Howlader N, Noone AM, Krapcho M, Miller D, Brest A, Yu M, et al. SEER Cancer Statistics Review, 1975–2016. Natl. Cancer Inst 2019.
- 4.Vigneswaran WT, Kircheva DY, Ananthanarayanan V, Watson S, Arif Q, Celauro AD, et al. Amount of Epithelioid Differentiation Is a Predictor of Survival in Malignant Pleural Mesothelioma. Ann Thorac Surg 2017. Mar;103(3):962–966 [DOI] [PubMed] [Google Scholar]
- 5.Brosseau S, Danel C, Scherpereel A, Mazières J, Lantuejoul S, Margery J, et al. Shorter Survival in Malignant Pleural Mesothelioma Patients With High PD-L1 Expression Associated With Sarcomatoid or Biphasic Histology Subtype: A Series of 214 Cases From the Bio-MAPS Cohort. Clin Lung Cancer 2019. Sep;20(5):e564–e575. [DOI] [PubMed] [Google Scholar]
- 6.Kindler HL, Ismaila N, Armato SG, Bueno R, Hesdorffer M, Jahan T, et al. Treatment of malignant pleural mesothelioma: American society of clinical oncology clinical practice guideline. J. Clin. Oncol May 1;36(13):1343–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Forest F, Patoir A, Dal Col P, Sulaiman A, Camy F, Laville D, et al. Nuclear grading, BAP1, mesothelin and PD-L1 expression in malignant pleural mesothelioma: prognostic implications. Pathology 2018. Oct;50(6):635–641. [DOI] [PubMed] [Google Scholar]
- 8.Cedrés S, Ponce-Aix S, Zugazagoitia J, Sansano I, Enguita A, Navarro-Mendivil A, et al. Analysis of expression of programmed cell death 1 ligand 1 (PD-L1) in malignant pleural mesothelioma (MPM). PLoS One 2015. Mar 16;10(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vogelzang NJ, Rusthoven JJ, Symanowski J, Denham C, Kaukel E, Ruffie P, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol 2003. Jul 15;21(14):2636–44. [DOI] [PubMed] [Google Scholar]
- 10.Ceresoli GL, Zucali PA, Favaretto AG, Grossi F, Bidoli P, Del Conte G, et al. Phase II study of pemetrexed plus carboplatin in malignant pleural mesothelioma. J Clin Oncol 2006. Mar 20;24(9):1443–8. [DOI] [PubMed] [Google Scholar]
- 11.Zalcman G, Mazieres J, Margery J, Greillier L, Audigier-Valette C, Moro-Sibilot D, et al. Bevacizumab for newly diagnosed pleural mesothelioma in the Mesothelioma Avastin Cisplatin Pemetrexed Study (MAPS): A randomised, controlled, open-label, phase 3 trial. Lancet 2016. Apr 2;387(10026):1405–1414. [DOI] [PubMed] [Google Scholar]
- 12.Scherpereel A, Mazieres J, Greillier L, Lantuejoul S, Dô P, Bylicki O, et al. Nivolumab or nivolumab plus ipilimumab in patients with relapsed malignant pleural mesothelioma (IFCT-1501 MAPS2): a multicentre, open-label, randomised, non-comparative, phase 2 trial. Lancet Oncol 2019. Feb;20(2):239–253. [DOI] [PubMed] [Google Scholar]
- 13.Bi Y, Liu J, Furmanski B, Zhao H, Yu J, Osgood C, et al. Model-informed drug development approach supporting approval of the 4-week (Q4W) dosing schedule for nivolumab (Opdivo) across multiple indications: A regulatory perspective. Ann Oncol 2019. Apr 1;30(4):644–651. [DOI] [PubMed] [Google Scholar]
- 14.Nivolumab (Opdivo), BMS, USPI. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/125554s089lbl.pdf.
- 15.Ipilimumab (Yervoy), BMS, USPI. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/125377s119lbl.pdf.
- 16.Maio M, Scherpereel A, Calabrò L, Aerts J, Perez SC, Bearz A, et al. Tremelimumab as second-line or third-line treatment in relapsed malignant mesothelioma (DETERMINE): a multicentre, international, randomised, double-blind, placebo-controlled phase 2b trial. Lancet Oncol 2017. Sep;18(9):1261–1273. [DOI] [PubMed] [Google Scholar]
- 17.Popat S, Curioni-Fontecedro A, Dafni U, Shah R, O’Brien M, Pope A, et al. A multicentre randomised phase III trial comparing pembrolizumab versus single-agent chemotherapy for advanced pre-treated malignant pleural mesothelioma: the European Thoracic Oncology Platform (ETOP 9–15) PROMISE-meso trial. Ann Oncol 2020. Dec;31(12):1734–1745. [DOI] [PubMed] [Google Scholar]
- 18.Buchbinder EI, Desai A. CTLA-4 and PD-1 Pathways: Similarities, Differences, and Implications of Their Inhibition. Am J Clin Oncol 2016;39(1):98–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reuss JE, Forde PM. Immunotherapy for mesothelioma: Rationale and new approaches. Clin Adv Hematol Oncol 2020. Sep;18(9):562–572. [PubMed] [Google Scholar]
- 20.Gao JJ, Pazdur R. FDA Oncology Center of Excellence During COVID-19—Working for Patients With Cancer. JAMA Oncol. JAMA Oncol 2020. Dec 23. Epub ahead of print. [DOI] [PubMed]
- 21.Pembrolizumab (Keytruda), Merck & Co., USPI. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/125514s088lbl.pdf.
- 22.FDA. FDA Guidance on Conduct of Clinical Trials of Medical Products during COVID-19 Public Health Emergency: Guidance for Industry, Investigators, and Institutional Review Boards. Guid. Doc (2020).
- 23.de Claro RA, Gao JJ, Kim T, Kluetz PG, Theoret MR, Beaver JA, et al. U.S. Food and Drug Administration: Initial Experience with the Real-Time Oncology Review Program. Clin Cancer Res 2021. Jan 1;27(1):11–14. [DOI] [PubMed] [Google Scholar]
- 24.21st Century Cures Act Section 3031 “Summary Level Review. https://www.congress.gov/114/plaws/publ255/PLAW-114publ255.pdf.
- 25.Forde PM, Nowak AK, Kok P-S, Brown C, Sun Z, Anagnostou V, et al. DREAM3R: Durvalumab with chemotherapy as first-line treatment in advanced pleural mesothelioma—A phase 3 randomized trial. J Clin Oncol 2021; 39_15_suppl. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Gooijer CJ, Borm FJ, Scherpereel A, Baas P. Immunotherapy in Malignant Pleural Mesothelioma. Front Oncol 2020;10:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.U.S. Food and Drug Administration. BLA Multi-disciplinary Review and Evaluation (sBLA 125554 and sBLA 125377) and Approval Package: OPDIVO (nivolumab) and YERVOY (ipilimumab)


