Abstract
Background:
There is a lack of consensus in the literature about the association between meal patterning during pregnancy and birth outcomes. This study examined whether maternal meal patterning in the week before birth was associated with an increased likelihood of imminent spontaneous labor.
Methods:
Data came from 607 participants in the third phase of the Pregnancy, Infection, and Nutrition Study (PIN3). Data were collected through an interviewer-administered questionnaire after birth, before hospital discharge. Questions included the typical number of meals and snacks consumed daily, during both the week prior to labor onset and the 24-hour period prior to labor onset. A self-matched, case-crossover study design examined the association between skipping one or more meals and the likelihood of spontaneous labor onset within the subsequent 24 hours.
Results:
Among women who experienced spontaneous labor, 87.0% reported routinely eating 3 daily meals (breakfast, lunch, and dinner) during the week before their labor began, but only 71.2% reported eating 3 meals during the 24-hour period before their labor began. Compared with the week before their labor, the odds of imminent spontaneous labor were 5.43 times as high (95% CI: 3.41–8.65) within 24 hours of skipping 1 or more meals. The association between skipping 1 or more meals and the onset of spontaneous labor remained elevated for both pregnant individuals who birthed early (37-<39 weeks) and full-term (≥39 weeks).
Conclusion:
Skipping meals later in pregnancy was associated with an increased likelihood of imminent spontaneous labor, though we are unable to rule out reverse causality.
Keywords: spontaneous labor, case-crossover, pregnancy, maternal diet, meal patterning
INTRODUCTION
During pregnancy, adequate food intake is necessary to support healthy weight gain as well as increased energy and nutrient requirements.1–4 This recommendation comes from the National Academy of Medicine (NAM; formerly the Institute of Medicine), which published pregnancy-related nutrition guidelines for health care providers to use when counseling pregnant individuals.1–4 In addition to recommendations around increasing caloric, macronutrient, and micronutrient intake, limiting caffeine consumption, and avoiding alcohol during pregnancy, the NAM guidelines recommended pregnant women “eat small to moderate-sized meals at regular intervals, and eat nutritious snacks…”3,5
Currently, there is conflicting evidence over the relationship between meal patterning and onset of labor. Frequency and patterning of meals could be an important predictor of pregnancy and birth outcomes. Experimental animal studies have found extended periods of food withdrawal are associated with the onset of uterine contractions6, and may influence the onset of labor,7,8 while some human studies identified a relationship between skipping meals during pregnancy and an increased risk of premature rupture of membranes and preterm birth.9–11 A retrospective analysis of medical records also reported an increase in the number of births during the 24 hour period after Yom Kippur, a Jewish holiday characterized by a 25-hour food and water fast.12 Conversely, a meta-analysis including 22 studies published since 1980, reported religious fasting holidays were not related to the onset of spontaneous labor or preterm birth.13,14 However, prior studies were retrospective, and researchers were often unable to ascertain exactly when labor began.12,14 Methodologically, it is difficult to collect data on maternal diet in the week prior to labor; therefore, most studies to date have been unable to evaluate whether meal patterning may trigger the onset of spontaneous labor.
As such, our study objective was to use a self-matched, case-crossover study design to evaluate the association of skipping meals on the onset of spontaneous labor. We hypothesized that nonadherence to the NAM meal patterning recommendations during pregnancy would be associated with an immediate increased likelihood of spontaneous labor onset within the subsequent 24 hours.
METHODS
Study Population
Data for this analysis come from the third Pregnancy, Infection, and Nutrition (PIN3) prospective pregnancy cohort study, which included data on lifestyle factors known to be associated with preterm birth.15 Between January 2001 and June 2005, 2,006 pregnant participants were recruited from 4 different prenatal care clinics in North Carolina. Women were recruited into the PIN3 study at a prenatal visit if they were less than 20 weeks’ completed gestation, greater than 16 years old with a singleton pregnancy, spoke English, planned to deliver and continue their prenatal care at the study site, and had access to a telephone to complete telephone interviews. Complete details about PIN3 protocol and data collection can be found at the PIN3 Protocols webpage.16
This analysis used demographic data collected during the first telephone interview conducted at 17–22 weeks, birth outcome data abstracted from hospital medical records, as well as data obtained from an interviewer-administered questionnaire that each participant completed in the hospital within a day or two after birth, before being discharged.17 The Institutional Review Board of our institution approved the PIN3 study protocol and this analysis, and all participants provided written informed consent.
Measures
Spontaneous onset of labor
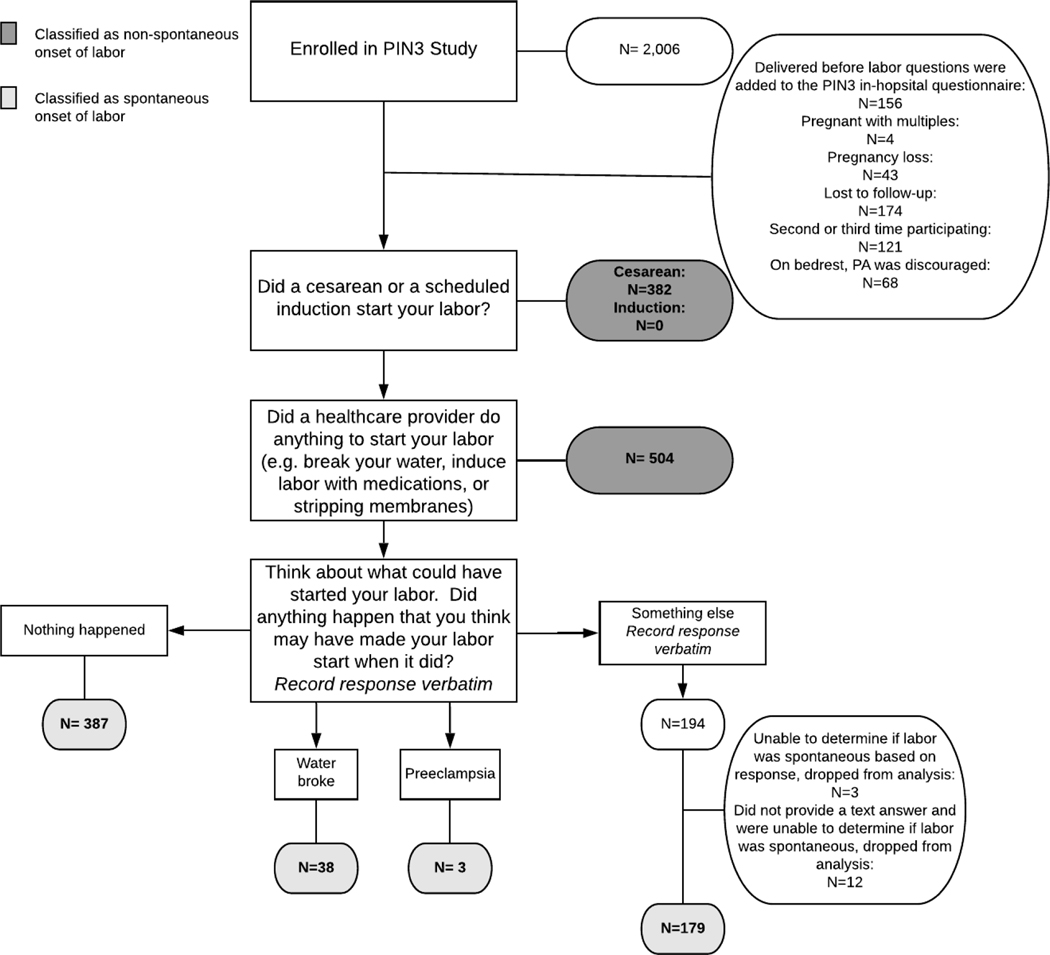
Items from the interviewer-administered, in-hospital questionnaire were used to assess self-reported labor triggers (i.e., proximal causes of labor).18 Participants were asked to report the day and time their labor began and to identify what they believe caused their labor to start. Those who reported a cesarean birth, a scheduled induction, an amniotomy, or having their membranes swept by a provider were considered to have had non-spontaneous labor and were excluded from this analysis.19,20 Participants who reported 2 conflicting causes of labor (i.e., 1 non-spontaneous cause such as an induction and 1 spontaneous cause such as ruptured membranes) were also considered to have had non-spontaneous labor and were excluded from the analysis. Those who reported “nothing” caused their labor to start, who reported “conventional” or at-home methods of inducing labor (i.e., consumption of castor oil, spicy food, or sexual intercourse)21, or who reported their labor started when their membranes ruptured were considered to have experienced spontaneous onset of labor and were included in this analysis (Figure 1). If a study participant provided an answer other than one of these, their response was recorded verbatim and later coded by a researcher (AKN) to determine whether they experienced spontaneous onset of labor. The most commonly reported triggers were walking, spontaneous rupture of membranes, and sexual intercourse.22 The main analysis is limited to those participants who experienced spontaneous onset of labor.
FIGURE 1.
Determination of spontaneous labor using the interviewer administered in-hospital questionnaire from the third phase of the Pregnancy, Infection, and Nutrition (PIN3) Study
Meal patterning
Additional items from the interviewer-administered, in-hospital questionnaire were used to assess meal patterning during two time periods: the week before the onset of labor and, separately, the 24 hours before the onset of labor. First, participants were asked to “think about your meal and snack pattern the last week of your pregnancy, not including the day your labor began/you came to the hospital to have your baby.” They were asked to report if they had routinely eaten breakfast, lunch, dinner, and/or any snacks during that week. Second, participants were asked to “think about the day before your labor began/ you came to the hospital to have your baby.” They were asked to report if they ate breakfast, lunch, dinner, and/or any snacks during that 24-hour period. There were no specified definitions regarding what was considered to be breakfast, lunch, dinner, or a snack.
Based on the distribution of responses, “skipped meal” patterning was coded dichotomously as “yes” if they reported skipping 1 or more daily meals (e.g., breakfast, lunch, or dinner) and “no” if they reported eating all daily meals (e.g., breakfast, lunch, and dinner). Snacks were kept as their own category. As part of the case-crossover design (see below), responses were coded separately for the 24 hours before labor versus the week before labor.
Covariates
During the first telephone interview (17–22 weeks’ gestation), women were asked about their race/ethnicity, marital status, education, parity (live plus still births), number of people living in their household, and household income. From these data we calculated the percent of the 2001 poverty level.23 Information obtained from the medical record included weight and height for the determination of pre-pregnancy body mass index (BMI). The participant’s age at time of conception was determined from the medical record and verified during the telephone interview. Gestational age at birth was estimated using ultrasonography if the test was performed before 22 weeks (>90% of the PIN3 sample). Otherwise, gestational age at birth was estimated using the last menstrual period method.
Statistical Methods
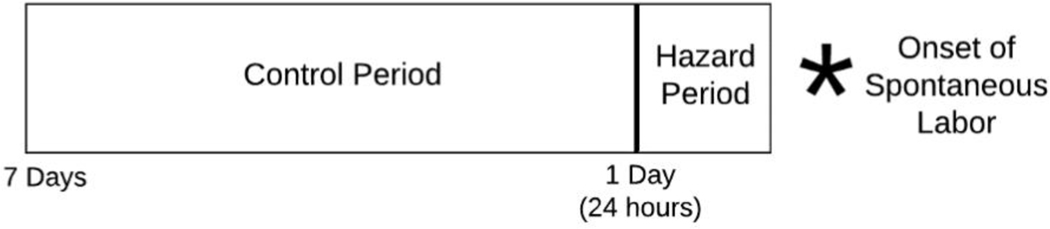
A self-matched, case-crossover design was used to analyze these data. Case-crossover designs are used to assess health event triggers, such as whether severe anger triggers myocardial infarctions.24 The case-crossover method assesses the risk of an acute event (here, onset of spontaneous labor) after exposure to a transient risk factor (skipping one or more meals), comparing within each person a hazard period (24 hours prior to labor onset) to a control period (7 days prior to labor onset).18,25 Thus, each person serves as their own control, eliminating the need to adjust for within-person confounders (e.g., race, weight, sexually transmitted infection, gestational diabetes, etc.)25 (Figure 2).
FIGURE 2.
Self-matched, case-crossover design
Descriptive statistics were used to examine the variable distributions during the control and hazard periods, among participants who had spontaneous labor onset. We then used conditional logistic regression to assess the relationship between meal patterning and onset of spontaneous labor within each person, comparing meal patterns during their hazard period (24 hours prior to labor) to meal patterns during their control period (7 days prior to labor). In these models, the onset of spontaneous labor was the outcome, and the exposure variable was meal patterning during the hazard versus control periods. This yields an estimate of the odds of spontaneous labor for a pregnant person who reported skipping at least one meal during the hazard period but eating 3 daily meals during the control period.
Several sensitivity analyses were conducted to assess the robustness of the findings. First, since a pregnant woman nearing full-term will have a slightly higher probability of spontaneous labor with each passing day, we explored whether the hazard estimates differed among those who delivered preterm (<37 weeks), early term (37-<39 weeks), full term (39-<41 weeks), or post-term (>=41 weeks). However, since the absolute number of post-term births was small (n=45), we collapsed the variable so that women who delivered at full or post term were in the same gestational age category for this analysis. Second, we explored whether categorizing snacks as a “meal” impacted the results. When the in-hospital questionnaire was administered, there was not a definition provided regarding the difference between a meal and a snack. Those who ate numerous daily snacks instead of 3 meals may have been wrongly classified as skipping a meal. Therefore, in this sensitivity analysis, participants were identified as skipping 1 or more meals if they reported consuming a total of less than 3 daily meals, counting each reported snack as a meal.
To assess bias, differences in demographic baseline characteristics between pregnant participants who reported onset of spontaneous labor and those who reported non-spontaneous labor were assessed using paired t-tests for continuous variables and chi-squared tests for categorical variables. Statistical significance was set at P<0.05 and all analyses were conducted using Stata 15 (StataCorp, College Station, TX).
RESULTS
Sample characteristics
Initially, 2,006 individuals agreed to participate in PIN3. Of the 2,006, 47 became ineligible (43 pregnancy losses, 4 pregnant with multiples), 121 were participating for the second or third time, 174 were lost to follow-up, and 156 delivered before the labor questions were added to the PIN3 in-hospital questionnaire, leaving 1,493 participants. Of these, 607 reported spontaneous onset of labor and completed the in-hospital questionnaire necessary for this analysis (Figure 1). The majority of women who went into spontaneous labor were non-Hispanic White, married, and high income (Supplementary Table 1). Over half of the sample were nulliparous, and 62.6% had a normal BMI (18.5 kg/m2≤ BMI<25.0) at conception. The mean gestational age at birth was 38.4 weeks, and 12.9% of infants were born preterm (<37 weeks gestation). Comparisons of characteristics between participants who went into spontaneous and non-spontaneous labor revealed few differences (Supplementary Table 1). ±
Meal patterning and the onset of spontaneous labor
During the control period (one week prior to spontaneous onset of labor), 87% of participants reported eating all 3 daily meals (breakfast, lunch, and dinner); 92%, 96%, 98%, and 83% reported routinely eating breakfast, lunch, dinner, and at least one snack, respectively. During the hazard period (24 hours prior to spontaneous onset of labor), 71% of participants reported eating all 3 daily meals; 90%, 87%, 85%, and 70% reported eating breakfast, lunch, dinner, and at least one snack, respectively (Table 1). Overall, 13% (n=79/607) reported routinely skipping 1 or more daily meals during the control period, while 28% (n=172/607) reported skipping one or more meals during the hazard period (Table 2).
TABLE 1.
Distribution of participants who experienced spontaneous onset of labor in the Pregnancy, Infection, and Nutrition Study according to meal consumption by hazard and control period (n=607)
| Meal Consumed, % yes | Hazard Period, % (N) | Control Period, % (N) |
|---|---|---|
| All mealsa,b | 71.2 (435) | 87.0 (528) |
|
| ||
| Breakfasta | 89.6 (554) | 91.8 (557) |
|
| ||
| Luncha | 86.7 (526) | 96.1 (583) |
|
| ||
| Dinnera | 85.3 (518) | 98.2 (596) |
|
| ||
| Snacks | ||
| Anya | 69.7 (423) | 82.7 (502) |
| ≥2a | 29.3 (178) | 59.6 (362) |
Statistically significant difference (P < .05) between meal patterning during the hazard and control period. Statistical significance was determined using and a chi-square test for categorical variables.
Indicates participants reported consumption of breakfast, lunch, and dinner during the hazard or control period.
TABLE 2.
Sociodemographic characteristics of participants who experienced spontaneous onset of labor according to meal skipping
| Maternal and household characteristics | Skipped 1 or more meals, % (N) | Did not skip any meals, % (N) | ||
|---|---|---|---|---|
|
| ||||
| 24 hours prior to labor (N=172) | Week prior to labor (N=79) | 24 hours prior to labor (N=435) | Week prior to labor (N=528) | |
| Age, mean (SD)a, b | 27.4 (5.9) | 26.1 (6.1) | 29.7 (5.2) | 29.5 (5.2) |
|
| ||||
| Race, % yesa, b | ||||
| Non-Hispanic, White | 64.5 (111) | 60.8 (48) | 77.2 (336) | 75.6 (399) |
| Non-Hispanic, Black | 25.0 (43) | 25.3 (20) | 12.9 (56) | 15.0 (79) |
| Asian or Pacific Islander | 2.3 (4) | 2.5 (2) | 3.5 (15) | 3.2 (17) |
| Other (not specified) | 8.1 (14) | 11.4 (9) | 6.4 (28) | 6.3 (33) |
|
| ||||
| Marital status, % yesa, b | ||||
| Single | 32.0 (55) | 36.7 (29) | 15.9 (69) | 18.0 (95) |
| Married | 62.8 (108) | 55.7 (44) | 82.3 (358) | 79.9 (422) |
| Separated or Divorced | 5.2 (9) | 7.6 (6) | 1.8 (8) | 2.1 (11) |
|
| ||||
| Income (% of poverty level for 2001), % yesa, b | ||||
| ≤185 | 28.8 (47) | 36.5 (27) | 14.9 (63) | 16.2 (84) |
| 186–350 | 28.2 (46) | 28.4 (21) | 17.3 (73) | 19.1 (98) |
| >350 | 42.9 (70) | 35.1 (26) | 67.9 (287) | 64.7 (331) |
|
| ||||
| Education ≤ 12 years, % yesa, b | 28.0 (48) | 35.4 (28) | 10.6 (46) | 12.5 (66) |
|
| ||||
| Pre-pregnancy BMI (kg/m2), mean (SD)a, b | 25.6 (6.8) | 26.8 (8.3) | 23.9 (5.1) | 24.1 (5.1) |
|
| ||||
| Nulliparous, % yesa | 44.4 (76) | 41.0 (32) | 53.6 (233) | 52.5 (277) |
|
| ||||
| Gestational age (weeks), mean (SD)a | 37.7 (3.4) | 38.1 (2.3) | 38.7 (2.0) | 38.4 (2.5) |
Statistically significant difference (P < .05) between participants who skipped 1 or more meals in the 24-hour period preceding their labor compared to those who did not. Statistical significance was determined using a paired t-test for continuous variables and a chi-square test for categorical variables.
Statistically significant difference (P < .05) between participants who skipped 1 or more meals in the 1-week period preceding their labor compared to those who did not. Statistical significance was determined using a paired t-test for continuous variables and a chi-square test for categorical variables.
Those who reported meal skipping during the control period were more likely to be younger, non-Hispanic Black, non-married, below 350% of the poverty level, have 12 years or less of education, and have a higher pre-pregnancy BMI compared to participants who did not skip any meals during the control period (p<0.05) (Table 2). The same differences were found among participants who reported meal skipping during the hazard period compared to those who did not skip any meals during the hazard period. Additionally, women who reported meal skipping during the hazard period were more likely to be parous and to have given birth at an earlier gestational age compared to those who did not skip any meals during the hazard period (p<0.05).
Compared with the week before labor (control period), the immediate odds of spontaneous labor were 5.43 times as high (95% CI: 3.41–8.65) within 24 hours of skipping 1 or more meals (Table 3). The association between skipping 1 or more meals and the onset of spontaneous labor remained significant across all gestational age categories. Including snacks as a “meal” did not change any of the results shown in Table 3 (data not shown).
TABLE 3.
The odds of spontaneous labor by meal skipping on term birth for participants in the Pregnancy, Infection, and Nutrition Study
| Gestational age at birth | Sample Size | Skipped 1 or more meals | Odds Ratio (95% CI) | ||
|---|---|---|---|---|---|
|
| |||||
| Hazard Only | Control Only | Both | |||
| Overall | 607 | 114 | 21 | 58 | 5.43 (3.41–8.65) |
| Preterm (<37 weeks gestation) | 78 | 21 | 0 | 12 | --a |
| Early term (37-<39 weeks gestation) | 151 | 28 | 7 | 20 | 4.00 (1.75–9.16) |
| Full or post term (≥39 weeks gestation) | 378 | 65 | 14 | 26 | 4.64 (2.61–8.27) |
Unable to compute odds ratio and 95% confidence interval for participants who delivered preterm due to 0 occurrences in the control period.
DISCUSSION
Using a case-crossover design, we found that the odds of spontaneous labor were 5.43 times as high during the 24 hours after skipping a meal, compared to when 3 or more daily meals or snacks were consumed. Based on previous studies that have reported a relationship between fasting and preterm birth or premature rupture of membranes9,10,12, we hypothesized that skipping one or more daily meals during pregnancy would result in an immediate, possibly transient, increased likelihood of spontaneous labor within the subsequent 24 hours. None of the individuals in this analysis, when asked an open-ended question, attributed the onset of their labor to meal patterning22, yet we found a strong association. To our knowledge, this is the first study to assess the relationship between meal patterning and imminent likelihood of spontaneous labor at term. Previous research around meal patterning explored its associations with preterm birth and birthweight.13,26–29
In the broader population, skipping meals may not always be a choice as approximately 10% of pregnant people experience prenatal food insecurity;30 whereas, in this sample the majority of participants reported regularly eating all 3 meals during the control period. The prevalence of skipping a meal during the week and the 24 hours prior to labor onset was greater among non-Hispanic Black participants. Meal skipping during pregnancy has been previously associated with preterm birth. In a descriptive comparative study, non-Hispanic Black women who experienced preterm labor had higher odds of skipping meals during their pregnancy.27 The self-matched design of our study did not enable us to stratify the associations between meal patterning and labor onset by race. However, given that non-Hispanic Black women are at an increased risk of preterm birth31, and that our findings showed meal patterning in the final week of pregnancy varied by race/ethnicity, future studies should consider the interaction between meal patterning and race/ethnicity in order to address this possible disparity. In our sample, higher pre-pregnancy BMI was also associated with meal skipping during the hazard and control period. This finding supports previous studies that have found pregnant people with high BMI are more likely to skip meals during the second and third trimester.27,32 Lastly, we found meal skipping in the last week of pregnancy was more frequent among participants of low socioeconomic status (i.e., less than 12 years of education or household income 350% below the poverty line). Studies have identified similar associations between socioeconomic status and diet, finding that low educational attainment and low income are associated with inadequate dietary intake during pregnancy.33
In terms of a potential biological mechanism, skipping a meal induces the release of corticotropin releasing hormone (CRH) signaling adrenocorticotropic hormone to be released from the pituitary gland, stimulating the production and release of cortisol from the adrenal gland.34 During pregnancy, increased levels of maternal cortisol increase production of placental CRH which aids in labor onset through dilation of the uterus and stimulation of smooth muscle contractions.35–37 Therefore, it is possible that if a pregnant person’s body has already started preparing for birth by increasing production of hormones like CRH, skipping a meal may further increase placental CRH, accelerating the timing of spontaneous labor onset.
There are two possible alternative explanations for the pattern of results we observed. First, as a woman nears full-term, their probability of labor slowly but steadily increases naturally.38,39 It is possible that the large odds ratios we observed for the hazard period versus the control period are merely an artefact of gestational age: by definition, the 24 hours before labor begins occurs later in a pregnancy than does the entirety of the week before labor begins. We were able to stratify our results by gestational age and observed the odds of spontaneous labor immediately after meal skipping were increased regardless of gestational age category. However, there is no way to control for exact gestational age in the model using a case-crossover design.
The second possible alternative explanation is reverse causality. Late in pregnancy, the digestive tract alterations necessary to accommodate the large uterus may cause a pregnant person to feel nauseous40, or otherwise uninterested in eating, much as occurs during labor itself.41 In either of these scenarios, the imminent labor would be the cause of the woman deciding to skip a meal, rather than the skipped meal leading to labor onset. In order to conduct sensitivity analyses to address the potential timing bias inherent within a case crossover design, it would be worthwhile for future studies to collect data on additional exposures that are not expected to be related to the outcome (e.g., milk consumption).
There are some limitations to this analysis. First, the PIN3 study took place in a teaching hospital and required participants to be receiving prenatal care at an affiliated clinic in order to participate. To the extent that people choosing an academic medical center for care are not representative of the entire pregnant population, we may have a non-representative sample, though whether the underlying mechanisms, if any, would also vary by these characteristics is unclear. Additionally, data were collected from 2001 through 2005, before the Affordable Care Act legislation in 2012. Lack of access to early prenatal care (participants had to enroll by week 20) secondary to lack of health insurance was thus much more of an issue, and disproportionately affected low-income individuals.
Second, both meal patterning and spontaneous labor onset were self-reported. Validity of these measures was not assessed, so we are unable to estimate measurement error, though as participants were not informed of the hypothesis for this analysis, it is likely that any resulting misclassification is non-differential. Without access to medical records data, we were unable to determine whether participants and their health care providers had differing views on what initiated their labor increasing the risk for outcome misclassification. To assess meal patterning during the control period, participants were asked to report “meals and snacks they routinely ate in the week leading up to their birth (not including the day their labor began).” Therefore, if there was any variation during the week, participants were required to give their best estimate, and thus details were lost. Additionally, there were no prespecified definitions for a meal or snack, which may also increase the risk of misclassification bias. It also would have been beneficial to collect detailed daily caloric intake during the control and hazard periods. Lastly, because of the retrospective data collection after birth and design of the questions, we were only able to compare two time periods (24 hours before birth versus 1 week before birth). Prospective collection of meal and snack consumption, including details on caloric and macronutrient intake, during mid-to-late pregnancy would enable researchers to examine the influence of meal patterning within multiple hazard periods, potentially providing a stronger understanding of whether it relates to spontaneous onset of labor.
CONCLUSION
Participants who reported skipping 1 or more daily meals in late pregnancy had a five-fold higher likelihood of spontaneous labor within the subsequent 24 hours compared to participants who ate at least 3 daily meals, though whether this is causal requires further investigation. It is plausible that a lack of appetite resulting in irregular meal patterning may be a potential sign that labor is approaching, rather than the skipped meal cause the labor per se. We are not advocating for pregnant women to skip meals to induce their labor, though perinatal care providers might hear questions about this practice from pregnant clients. Additionally, if the reverse causality scenario proves correct, it is possible that disinterest in meals could signal imminent labor. This could prove an important clinical tool for self-monitoring by pregnant individuals near term.
Supplementary Material
Abbreviations:
- NAM
National Academy of Medicine
- PIN3
the third Pregnancy, Infection, and Nutrition cohort study
- BMI
body mass index
- CRH
corticotropin releasing hormone
Contributor Information
Alison K Nulty, Department of Anthropology, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina.
Marit L Bovbjerg, Epidemiology Program, Oregon State University, Corvallis, Oregon.
Amy H Herring, Department of Statistical Science, Duke University, Durham, North Carolina; Duke Global Health Institute, Duke University, Durham, North Carolina; Department of Biostatistics and Bioinformatics, Duke University, Durham, North Carolina.
Anna Maria Siega-Riz, Department of Nutrition, School of Public Health and Health Services, University of Massachusetts, Amherst, Massachusetts.
John M Thorp, Department of Obstetrics and Gynecology, University of North Carolina School of Medicine, Chapel Hill, North Carolina.
Kelly R Evenson, Department of Epidemiology, Gillings School of Global Public Health, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina.
REFERENCES
- 1.Institute of Medicine (US) Committee on Nutritional Status During Pregnancy and Lactation. Nutrition during Pregnancy: Part I Weight Gain: Part II Nutrient Supplements. Washington (DC): National Academies Press; (US: ); 1990. doi: 10.17226/1451 [DOI] [PubMed] [Google Scholar]
- 2.Institute of Medicine (US) and National Research Council (US) Committee to Reexamine IOM Pregnancy Weight Guidelines. Weight Gain during Pregnancy: Reexamining the Guidelines. (Rasmussen KM, Yaktine AL, eds.). Washington (DC): National Academies Press; (US: ); 2009. doi: 10.17226/12584 [DOI] [PubMed] [Google Scholar]
- 3.Nutrition during Pregnancy and Lactation: An Implementation Guide. Washington, D.C.: National Academies Press; 1992. doi: 10.17226/1984 [DOI] [Google Scholar]
- 4.Otten JJ, Hellwig J pitzi, meyers LD, eds. Dietary Reference Intakes: The Essential Guide to Nutrient Requirements. Washington, D.C.: National Academies Press; 2006:144. doi: 10.17226/11537 [DOI] [Google Scholar]
- 5.Fowles ER. What’s a pregnant woman to eat? A review of current USDA dietary guidelines and mypyramid. J Perinat Educ. 2006;15(4):28–33. doi: 10.1624/105812406X151394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Binienda Z, Massmann A, Mitchell MD, Gleed RD, Figueroa JP, Nathanielsz PW. Effect of food withdrawal on arterial blood glucose and plasma 13,14-dihydro-15-keto-prostaglandin F2 alpha concentrations and nocturnal myometrial electromyographic activity in the pregnant rhesus monkey in the last third of gestation: a model for preterm labor? Am J Obstet Gynecol. 1989;160(3):746–750. doi: 10.1016/s0002-9378(89)80073-0 [DOI] [PubMed] [Google Scholar]
- 7.Silver M, Fowden AL. Uterine prostaglandin F metabolite production in relation to glucose availability in late pregnancy and a possible influence of diet on time of delivery in the mare. J Reprod Fertil Suppl. 1982;32:511–519. [PubMed] [Google Scholar]
- 8.Fowden AL, Silver M. The effect of the nutritional state on uterine prostaglandin F metabolite concentrations in the pregnant ewe during late gestation. Q J Exp Physiol. 1983;68(3):337–349. doi: 10.1113/expphysiol.1983.sp002729 [DOI] [PubMed] [Google Scholar]
- 9.Siega-Riz AM, Herrmann TS, Savitz DA, Thorp JM. Frequency of eating during pregnancy and its effect on preterm delivery. Am J Epidemiol. 2001;153(7):647–652. doi: 10.1093/aje/153.7.647 [DOI] [PubMed] [Google Scholar]
- 10.Hernández-Díaz S, Boeke CE, Romans AT, et al. Triggers of spontaneous preterm delivery--why today? Paediatr Perinat Epidemiol. 2014;28(2):79–87. doi: 10.1111/ppe.12105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herrmann TS, Siega-Riz AM, Hobel CJ, Aurora C, Dunkel-Schetter C. Prolonged periods without food intake during pregnancy increase risk for elevated maternal corticotropin-releasing hormone concentrations. Am J Obstet Gynecol. 2001;185(2):403–412. doi: 10.1067/mob.2001.115863 [DOI] [PubMed] [Google Scholar]
- 12.Kaplan M, Eidelman AI, Aboulafia Y. Fasting and the precipitation of labor. The Yom Kippur effect. JAMA. 1983;250(10):1317–1318. [PubMed] [Google Scholar]
- 13.Glazier JD, Hayes DJL, Hussain S, et al. The effect of Ramadan fasting during pregnancy on perinatal outcomes: a systematic review and meta-analysis. BMC Pregnancy Childbirth. 2018;18(1):421. doi: 10.1186/s12884-018-2048-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lurie S, Baider C, Boaz M, Sulema V, Golan A, Sadan O. Fasting does not precipitate onset of labour. J Obstet Gynaecol. 2010;30(1):35–37. doi: 10.3109/01443610903249455 [DOI] [PubMed] [Google Scholar]
- 15.Gillings School of Global Public Health. Design and Overview of PIN3 and PIN3plus | PIN — Pregnancy, Infection, and Nutrition Study. Design and Overview of PIN3 and PIN3plus. http://epidpin.web.unc.edu/pin3-and-pin3plus/design-and-overview-of-pin3-and-pin3plus/. Published 2020. Accessed July 20, 2020. [Google Scholar]
- 16.Gillings School of Global Public Health. Protocols for PIN3 and PIN3plus | PIN — Pregnancy, Infection, and Nutrition Study. PIN- Pregnancy, Infection, and Nutrition Study. http://epidpin.web.unc.edu/pin3-and-pin3plus/protocols-for-pin3-and-pin3plus/. Published 2020. Accessed July 4, 2020. [Google Scholar]
- 17.Gillings School of Global Public Health. Documentation for PIN3 and PIN3plus | PIN — Pregnancy, Infection, and Nutrition Study. Pregnancy, Infection, and Nutrition Study. http://epidpin.web.unc.edu/pin3-and-pin3plus/documentation-for-pin3-and-pin3plus/. Published 2020. Accessed August 3, 2020. [Google Scholar]
- 18.Maclure M, Mittleman MA. Should we use a case-crossover design? Annu Rev Public Health. 2000;21:193–221. doi: 10.1146/annurev.publhealth.21.1.193 [DOI] [PubMed] [Google Scholar]
- 19.Finucane EM, Murphy DJ, Biesty LM, et al. Membrane sweeping for induction of labour. Cochrane Database Syst Rev. 2020;2:CD000451. doi: 10.1002/14651858.CD000451.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bricker L, Luckas M. Amniotomy alone for induction of labour. Cochrane Database Syst Rev. 2000;(4):CD002862. doi: 10.1002/14651858.CD002862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mozurkewich EL, Chilimigras JL, Berman DR, et al. Methods of induction of labour: a systematic review. BMC Pregnancy Childbirth. 2011;11:84. doi: 10.1186/1471-2393-11-84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bovbjerg ML, Evenson KR, Bradley C, Thorp JM. What started your labor? Responses from mothers in the third pregnancy, infection, and nutrition study. J Perinat Educ. 2014;23(3):155–164. doi: 10.1891/1058-1243.23.3.155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Proctor BD, Dalaker J. Poverty in the United States: 2001. U.S. Government Printing Office; 2002. [Google Scholar]
- 24.Möller J, Hallqvist J, Diderichsen F, Theorell T, Reuterwall C, Ahlbom A. Do episodes of anger trigger myocardial infarction? A case-crossover analysis in the Stockholm Heart Epidemiology Program (SHEEP). Psychosom Med. 1999;61(6):842–849. doi: 10.1097/00006842-199911000-00019 [DOI] [PubMed] [Google Scholar]
- 25.Mittleman MA, Maclure M, Robins JM. Control sampling strategies for case-crossover studies: an assessment of relative efficiency. Am J Epidemiol. 1995;142(1):91–98. doi: 10.1093/oxfordjournals.aje.a117550 [DOI] [PubMed] [Google Scholar]
- 26.Baron R, Te Velde SJ, Heymans MW, Klomp T, Hutton EK, Brug J. The Relationships of Health Behaviour and Psychological Characteristics with Spontaneous Preterm Birth in Nulliparous Women. Matern Child Health J. 2017;21(4):873–882. doi: 10.1007/s10995-016-2160-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hennessy MD, Volpe SL, Sammel MD, Gennaro S. Skipping meals and less walking among African Americans diagnosed with preterm labor. J Nurs Scholarsh. 2010;42(2):147–155. doi: 10.1111/j.1547-5069.2010.01345.x [DOI] [PubMed] [Google Scholar]
- 28.Englund-Ögge L, Birgisdottir BE, Sengpiel V, et al. Meal frequency patterns and glycemic properties of maternal diet in relation to preterm delivery: Results from a large prospective cohort study. PLoS One. 2017;12(3):e0172896. doi: 10.1371/journal.pone.0172896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salunkhe A, Pratinidhi A, Kakade SV, Mohite V. Frequency and Nutrient Content of Meals of the Mothers and the Birth Weight and. Journal of Krishna Institute of Medical Sciences University. 2018;7(2):33–41. [Google Scholar]
- 30.Dinour LM, Rivera Rodas EI, Amutah-Onukagha NN, Doamekpor LA. The role of prenatal food insecurity on breastfeeding behaviors: findings from the United States pregnancy risk assessment monitoring system. Int Breastfeed J. 2020;15(1):30. doi: 10.1186/s13006-020-00276-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Manuck TA. Racial and ethnic differences in preterm birth: A complex, multifactorial problem. Semin Perinatol. 2017;41(8):511–518. doi: 10.1053/j.semperi.2017.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ainscough KM, Kennelly MA, Lindsay KL, et al. An observational analysis of meal patterns in overweight and obese pregnancy: exploring meal pattern behaviours and the association with maternal and fetal health measures. Ir J Med Sci. 2020;189(2):585–594. doi: 10.1007/s11845-019-02099-0 [DOI] [PubMed] [Google Scholar]
- 33.Doyle I-M, Borrmann B, Grosser A, Razum O, Spallek J. Determinants of dietary patterns and diet quality during pregnancy: a systematic review with narrative synthesis. Public Health Nutr. 2017;20(6):1009–1028. doi: 10.1017/S1368980016002937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Le T, Bhushan V. Endocrine. In: First Aid for the USMLE Step 1 2019, Twenty-Ninth Edition. 29th ed. McGraw-Hill Professional Publishing; 2018:329. [Google Scholar]
- 35.Petraglia F, Imperatore A, Challis JRG. Neuroendocrine mechanisms in pregnancy and parturition. Endocr Rev. 2010;31(6):783–816. doi: 10.1210/er.2009-0019 [DOI] [PubMed] [Google Scholar]
- 36.Smith R. Parturition. N Engl J Med. 2007;356(3):271–283. doi: 10.1056/NEJMra061360 [DOI] [PubMed] [Google Scholar]
- 37.Weiss G. Endocrinology of parturition. J Clin Endocrinol Metab. 2000;85(12):4421–4425. doi: 10.1210/jcem.85.12.7074 [DOI] [PubMed] [Google Scholar]
- 38.Bergsjø P, Denman DW, Hoffman HJ, Meirik O. Duration of human singleton pregnancy. A population-based study. Acta Obstet Gynecol Scand. 1990;69(3):197–207. doi: 10.3109/00016349009028681 [DOI] [PubMed] [Google Scholar]
- 39.Kieler H, Axelsson O, Nilsson S, Waldenström U. The length of human pregnancy as calculated by ultrasonographic measurement of the fetal biparietal diameter. Ultrasound Obstet Gynecol. 1995;6(5):353–357. doi: 10.1046/j.1469-0705.1995.06050353.x [DOI] [PubMed] [Google Scholar]
- 40.Soma-Pillay P, Nelson-Piercy C, Tolppanen H, Mebazaa A. Physiological changes in pregnancy. Cardiovasc J Afr. 2016;27(2):89–94. doi: 10.5830/CVJA-2016-021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blackburn S. Maternal, Fetal, & Neonatal Physiology: A Clinical Perspective (Maternal Fetal and Neonatal Physiology). 5th ed. Saunders; 2017. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.