Abstract
Introduction:
Evidence of digital interventions that are efficacious among low-income populations is scarce. In a secondary analysis, we determined the efficacy and utilization of an Acceptance and Commitment Therapy (ACT)-based smartphone application (iCanQuit) versus a U.S. Clinical Practice Guidelines (USCPG)-based smartphone application (QuitGuide) for smoking cessation in low-income adults enrolled in the iCanQuit randomized trial.
Methods:
Participants were randomized to receive iCanQuit (n=437) or QuitGuide (n=460) for 12-months. Consistent with the main trial, the primary outcome was self-reported complete-case 30-day point prevalence abstinence (PPA) at 12-months. Secondary outcomes were 7-day PPA, missing-as-smoking and multiple imputation, prolonged abstinence, and cessation of all tobacco products at 12-months. Outcome data retention, utilization, and change in ACT-based processes were compared across arms.
Results:
Participants were recruited from 48 U.S. states. Retention rate was 88% at 12-months and did not differ by arm. At 12-months, iCanQuit was 1.46 times more efficacious than QuitGuide for smoking cessation (27% vs. 20%; OR=1.46 95% CI: 1.04, 2.06). Findings were similar for missing-as-smoking imputation (23% vs. 18%; OR=1.41 95% CI: 1.01, 1.97) and multiple imputation at 12-months (27% vs. 20%; OR=1.51 95% CI: 1.07, 2.14). Treatment utilization was significantly higher among iCanQuit than QuitGuide participants. Increased acceptance of cues to smoke mediated the effect of treatment on cessation.
Conclusions:
The iCanQuit smartphone application was more efficacious and engaging for smoking cessation among low-income adults than a USCPG-based smartphone application. A nationwide dissemination trial of iCanQuit is warranted to determine whether iCanQuit may alleviate cessation-related disparities among low-income adults.
Trial registration: ClinicalTrials.gov Identifier: NCT02724462
Keywords: Acceptance & Commitment Therapy, low-income, iCanQuit, QuitGuide, smartphone applications, smoking cessation
1. Introduction
In the United States (U.S.), cigarette smoking prevalence and the associated burden of disease are disproportionally greater among socioeconomically disadvantaged individuals.1,2 This includes individuals with low incomes who are living close to or below the poverty threshold. Although cigarette smoking has declined to 14.0% in the general adult population, the smoking prevalence among individuals with annual incomes <$35,000 is 21.4%.2 Low-income individuals who smoke are also more likely to have lower levels of education, to report minority race or ethnicity, to be unemployed, and to reside in remote areas, with each additional disadvantage contributing to the smoking-related disparity.3,4
A major reason for this disparity in smoking rates is that low-income individuals have limited access to evidence-based smoking cessation treatments.5 Less than a third of low-income adults who want to quit smoking have access to smoking cessation counseling or medication. Reasons for this limited access include lack of health insurance, living in remote areas, lack of knowledge about existing treatments, or discrimination due to smoking stigma.6–8
One potential way to address accessibility barriers is to offer freely available and remotely delivered smoking cessation treatments. Telephone-delivered interventions, although effective,9–11 are limited by low levels of population reach. For example, state quitlines reach only 1–2% of smokers annually, thereby leaving substantial room for improvement.12–15 For several reasons, digital interventions have the potential to provide an alternative to telephone-delivered approaches, with potential for greater reach and acceptability among low-income populations. First, digital interventions could reach those who have difficulty navigating the medical system, those who are uninsured, and those who experience discrimination due to smoking stigma. Second, the high portability of digital interventions, especially mobile interventions that are available at any time, helps remove barriers related to time and place, and therefore provides users with access to treatments that would not otherwise be feasible. Further, 85% of U.S. adults overall and 76% of adults earning less than $30,000 annually reported that they owned a smartphone in 2021.16 Therefore, smartphone interventions offer a means of increasing treatment access in this population. The potential of smartphones to reach and engage large populations of smokers has been demonstrated in previous efficacy trials of smartphone applications for smoking cessation.17–19 Although promising, little is known about the efficacy and utilization of smartphone applications for smoking cessation in low-income populations.
Low-income populations also face unique challenges to quitting smoking. Cigarette smoking may be used as a way to cope with highly stressful situations, such as unemployment, living in unsafe neighborhoods, financial strain, racial discrimination, and food insecurity.20–24 These factors could reduce motivation to quit and further contribute to poor cessation outcomes.24–28 Therefore, additional efforts to identify efficacious treatments that provide low-income populations with skills to cope with highly stressful environments that cue smoking are needed.
Acceptance and commitment therapy (ACT) is an evidence-based behavioral approach that has shown promise in smoking cessation interventions as evidenced by fifteen randomized clinical trials published that compared ACT to US Clinical Practice Guidelines interventions for smoking cessation,29 and thus could address the need for more efficacious interventions for low-income individuals who smoke.30–32 Through its focus on skills to accept sensations, emotions and thoughts, ACT-based interventions could provide low-income individuals with unique skills to effectively cope with stressors that are known to be associated with poor smoking cessation outcomes. ACT teaches acceptance of internal cues to smoke rather than avoidance, which may be impractical in low-income neighborhoods with high density of tobacco retailers and tobacco advertisements.33
Smartphone applications offer a potentially high impact means of making ACT-based interventions for smoking cessation accessible to low-income populations. The efficacy of an ACT-based smartphone application (iCanQuit) was previously tested against a U.S. Clinical Practice Guidelines (USCPG)-based smartphone application (QuitGuide) in a large two-arm randomized trial among 2,415 daily adult smokers nationwide.34 At 12-months, iCanQuit was 1.5 times more efficacious than QuitGuide for smoking cessation. Moreover, results from this study also showed that the effect of the intervention on smoking cessation was mediated through increase in acceptance of cues to smoke.35 However, the efficacy of iCanQuit for smoking cessation, specifically among low-income adults, has not been evaluated.
Therefore, this study determined the efficacy and utilization of iCanQuit relative to QuitGuide for smoking cessation among low-income adults enrolled in the iCanQuit randomized trial. We hypothesized that, compared with the QuitGuide arm, low-income adults in the iCanQuit arm would have higher quit rates and treatment utilization. We further hypothesized that ACT-based processes, especially acceptance of cues to smoke would mediate the effect of treatment on smoking cessation.
2. Methods
2.1. Design
Data are from the two-arm randomized iCanQuit parent trial that enrolled adult (18 years) daily smokers with smartphone access who wanted to quit smoking.17 Exclusion criteria included being unable to read English, receiving smoking cessation treatment, having used QuitGuide in the past, or having a household member already enrolled in the study. Details of the iCanQuit trial were previously published.17 Briefly, 2,415 adults were randomized 1:1 to receive iCanQuit or QuitGuide for 12-months. All participants were screened for eligibility via online surveys and provided informed consent online. Study procedures were approved by the Fred Hutchinson Cancer Research Center Institutional Review Board. All participants were compensated up to $105 for completing study outcome data collection. No compensation was provided for the utilization of the treatment applications.
2.2. Population, recruitment, and enrollment
For this analysis, iCanQuit trial participants who reported gross household annual incomes of <$20,000 (863/2415, 35.7%) were selected, which was the lowest of three possible income response options on the baseline study survey (Less than $20,000, $20,000 - $54,999, and $55,000 or more). This level of income is significantly below the median income for family households in the U.S. ($41,232/year in 2019);36 and is also below the $35,000/year threshold which is used by the Centers for Disease Control and Prevention to reflect the lowest level of income when comparing cigarette smoking prevalence across income levels.37 Low-income trial participants were recruited via Facebook ads (735/863, 85%), a survey sampling company (91/863, 11%), search engine (18/863, 2%), and word of mouth (20/863, 2%). Although, recruitment was not tailored to low-income populations, the design of the study was intentional in including specific parameters in the Facebook ads that were tailored to low-income interests and employers that tend to pay lower wages, like retail, food service, construction, and manufacturing (e.g., Walmart, McDonald’s, GMC). Participants were enrolled between May 2017 and September 2018. Participants were given access to their assigned application with a unique access code, from the moment of randomization. E-mails with a unique link to an online survey were sent to participants at 3, 6 and 12-month follow-ups. Follow-up data collection was between August 2017 and December 2019 via the online survey platform.
2.3. Interventions
2.3.1. iCanQuit
The iCanQuit smartphone application (version 1.2.1) teaches ACT skills for coping with smoking urges, staying motivated, and preventing relapse.17 The content is delivered in eight levels, including on-demand help with coping with smoking urges, daily tracking of cigarettes smoked, and urges experienced without smoking. The program is self-paced, and content is unlocked in a sequential manner. If a participant lapses, the program encourages (but does not require) them to set a new quit date and return to the first five levels for preparation. iCanQuit targeted two core processes of ACT: acceptance and values. The acceptance component of the application teaches skills to accept sensations, emotions, and thoughts that trigger smoking via distancing from thoughts about smoking, mindfulness, and perspective taking. This teaching of acceptance is conceptually distinct from USCPG-based standard approaches that teach avoidance of internal cues to smoke. The values component of the application teaches skills for determining the core life domains that motivate quitting smoking (e.g., family, health, spirituality) and taking repeated small actions within these domains (e.g., playing with grandchildren) to develop a smoke-free life. This focus on motivation by appealing to values is conceptually distinct from USCPG-based standard approaches that motivate by focusing on reasons for change.
2.3.2. QuitGuide
The USCPG-based QuitGuide smartphone application (version 1.2.2) focuses on increasing motivation to quit by using reason and logic and providing information on the health consequences of smoking. The application helps users develop a quit plan, identify smoking behaviors, triggers, and reasons for being smoke-free, and to identify sources of social support for quitting. It teaches skills for avoiding situations that lead to cravings to smoke, staying smoke-free, and coping with slips. More details on the similarities and differences of the two smartphone applications have been previously published.17 No incentives, coaching, or other interventions were provided in either arm. Similar to real-world use of smartphone applications, participants could reach out to our staff for technical support though this occurred very rarely. Both interventions provided information on U.S. Food and Drug Administration-approved medications for quitting smoking but did not provide any pharmacotherapy.
2.4. Measures
2.4.1. Baseline measures
Data collected at baseline included socio-demographic characteristics and home zip codes.38–40 Alcohol consumption was assessed via the Quick Drinking Screen.41 Smoking behavior variables included the Fagerström Test for Nicotine Dependence (FTND),42 number of cigarettes smoked per day, years of smoking, use of e-cigarettes in past month, quit attempts during the past 12-months, confidence in quitting smoking (0–100, where 0 indicates not confident at all and 100 indicates extremely confident), and number of close relationships with other smokers.
2.4.2. Smoking cessation
Smoking cessation outcomes were measured at the 3, 6 and 12-month follow-ups. The primary smoking cessation outcome was self-reported complete-case 30-day point-prevalence abstinence (PPA) at 12-months, consistent with the iCanQuit parent trial.17 Secondary smoking cessation outcomes were 7-day PPA, missing-as-smoking imputation, multiple imputation sensitivity analysis, prolonged abstinence defined as no smoking at all in the 9-month period of 3 to 12-months post-randomization, and cessation of all nicotine and tobacco products, including any kind of e-cigarettes or vaping, chewing tobacco, snus, hookahs, cigars, cigarillos, tobacco pipes, and kreteks at 12-months.
2.4.3. ACT-based processes
Acceptance of internal cues to smoke was measured via the Avoidance and Inflexibility Scale (AIS-27 adapted from Gifford et al.)43 which includes three subscales that assess one‟s willingness to experience physical sensations, emotions, and thoughts that cue smoking. The 27 items are rated on a 5-point scale from (1) “Not at all” to (5) “Very willing” and averaged, with higher scores indicating greater acceptance. Valued living was measured via the 10-item Valuing Questionnaire44 designed to assess the extent of personal values (e.g., family, health, spirituality) enactment. Items are intended to capture the quality of life of valued action in everyday language and without reference to specific life domains. Each item is rated on a 7-point scale ranging from (0) “Not at all true” to (6) “Completely true”. Scores were averaged and two distinct factors were derived, progress and obstruction with higher scores indicating either greater progress or greater obstruction toward valued living, respectively. Cronbach‟s alpha (95% CI) for each of the three scales showed good internal consistency: (1) mean acceptance, 0.72 (0.69, 0.77); (2) valued living, progress subscale, 0.88 (0.87, 0.89); and (3) valued living, obstruction subscale 0.87 (0.86, 0.89).
2.4.4. Treatment utilization and satisfaction
Treatment utilization was objectively measured by Google Analytics (number of times the application was opened, the time spent per session, and the number of unique days of use). An 11-item measure of satisfaction with the intervention, adapted from previous research,30,32 was completed at the 3-month follow-up.
2.5. Statistical analysis
Baseline characteristics for low-income participants are described overall and by treatment arm. Zip codes were tied to geographic location using the R library „zipcode‟45 and were categorized as urban or rural using Rural-Urban Commuting Area (RUCA) codes.46 RUCA codes of 1–3 were considered urban, while RUCA codes of 4–10 were considered rural.47–50 Logistic regression models were used to compare binary smoking cessation outcomes and outcome data retention rates between arms at all timepoints, as well as binary satisfaction outcomes. Outcome data retention rates (%) were calculated as the number of participants who completed study data collection at each follow-up time point (3, 6 and 12-months) out of the total number of participants included in the imputed missing-as-smoking analysis (see Figure 1). As a sensitivity analysis, multiple imputation was used to estimate missing 30-day PPA at 12-months. Effect sizes and standard errors from ten imputed datasets were pooled using Rubin‟s rules51 to generate a single OR and 95% confidence interval. Generalized linear models were used to compare changes from baseline to 3-months in ACT-based processes and utilization data. Full utilization data up to 12-months was not available due to a technical error by Google Analytics. For this reason, we reported utilization for participants with full 6-months of data. Right-skewed count outcomes were compared using negative binomial models. All models were adjusted for factors used in stratified randomization52 including daily smoking frequency (20 vs.21 cigarettes/day), minority race/ethnicity, education level (high school vs. some college), and positive screening for depression (CESD-20 score15 vs.16).38 Hayes‟ PROCESS macro for SAS was used to test the potential mediation of the effect of treatment on cessation at 12-months by changes in acceptance and valued living at 3-months.53 Indirect effects were estimated with 5,000 bootstrapped samples and were considered statistically significant when bias-corrected 95% confidence intervals did not include zero. All statistical tests were 2-sided, with α=.05. Regression analyses were completed using R, version 4.0.3, library „MASS‟ for negative binomial regression, and library „mice‟ for multiple imputation.54–56
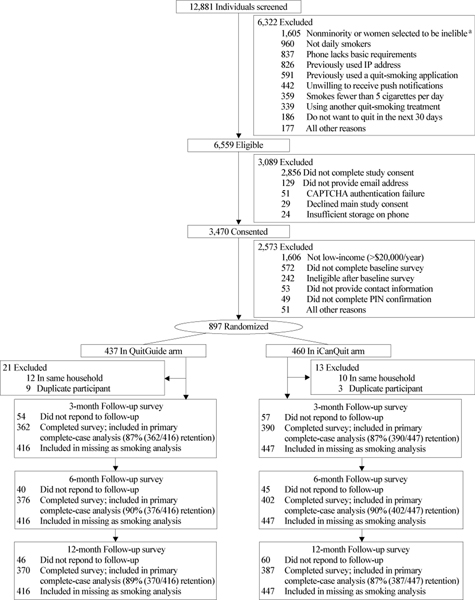
Figure 1.
CONSORT Diagram
aTo increase enrollment of racial/ethnic minorities and men, some nonminorities and women who were eligible for study enrollment were randomly selected to be excluded. Retention rates (%) were calculated as the number of participants who completed study data collection at each follow-up time point (3, 6 and 12-months) out of the total number of participants included in the imputed missing as smoking analysis. The missing as smoking analysis assumes that data are missing not at random, and that those who were lost to follow-up failed to quit.
3. Results
3.1. Enrollment and outcome data retention
A total of 12,881 individuals were screened, 6,559 were eligible and 3,470 consented to participate in the iCanQuit parent trial (Figure 1). The main reason for ineligibility was not providing consent (22%). For this analysis, all 897 randomized participants with annual incomes of <$20,000 were included. These participants were randomly assigned to receive QuitGuide (n=437) or iCanQuit (n=460) application for 12-months. Of the 897 randomized, 34 (4%) were excluded because another household member was already enrolled in the study, or they enrolled twice. The retention rates were 87%, 90%, and 88% at the 3, 6, and 12-month follow-ups, respectively, with no differential retention rate by arm at any follow-up time point (all p‟s>.05).
3.2. Participant characteristics
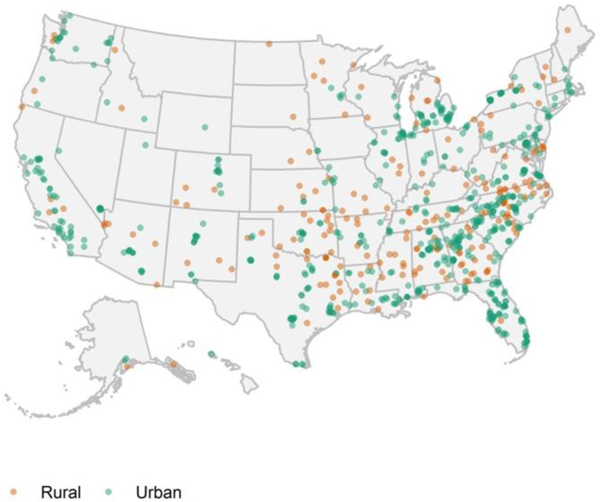
Participants were an average 37 years old, 28% male, 39% from racial minority groups in the US (Black or African American, American Indian or Alaska Natives, Asian, Native Hawaiian or Pacific Islander, or more than one race), and 10% Hispanic or Latino (Table 1). More than half (55%) had a high school diploma or lower education attainment, 24% were disabled and 19% were unemployed. The majority were long-time smokers (79% smoked 10 years) and had high nicotine dependence (65% FTND score 6). Figure 2 shows the geographic location and rural (24%) vs. urban (74%) residence of low-income participants included in this analysis that were recruited from 48 U.S. states.
Table 1.
Baseline socio-demographic characteristics of low-income trial participants
| No. (%) or Mean (SD) | ||||
|---|---|---|---|---|
|
| ||||
| Characteristic | n | Total (N = 863) | QuitGuide (n = 416) | iCanQuit (n = 447) |
| Age, mean (SD) | 863 | 36.9 (11.1) | 36.7 (11.2) | 37.2 (11.1) |
| Male | 863 | 244 (28%) | 128 (31%) | 116 (26%) |
| Race, n=846 | ||||
| White | 846 | 519 (61%) | 246 (60%) | 273 (62%) |
| Black or African American | 846 | 232 (27%) | 117 (29%) | 115 (26%) |
| Multiracial | 846 | 67 (8%) | 32 (8%) | 35 (8%) |
| American Indian or Alaska Native | 846 | 25 (3%) | 10 (2%) | 15 (3%) |
| Native Hawaiian or Pacific Islander | 846 | 2 (<1%) | 1 (<1%) | 1 (<1%) |
| Asian | 846 | 1 (<1%) | 1 (<1%) | 0 (0%) |
| Hispanic or Latino ethnicity | 830 | 83 (10%) | 45 (11%) | 38 (9%) |
| Education | ||||
| Less than GED or high school education | 863 | 125 (14%) | 64 (15%) | 61 (14%) |
| GED | 863 | 127 (15%) | 56 (13%) | 71 (16%) |
| High school diploma | 863 | 222 (26%) | 110 (26%) | 112 (25%) |
| Some college, no degree | 863 | 279 (32%) | 139 (33%) | 140 (31%) |
| College degree or higher | 863 | 110 (13%) | 47 (11%) | 63 (14%) |
| Employment status | ||||
| Employed | 863 | 342 (40%) | 164 (39%) | 178 (40%) |
| Unemployed | 863 | 166 (19%) | 75 (18%) | 91 (20%) |
| Disabled | 863 | 204 (24%) | 101 (24%) | 103 (23%) |
| Out of labor force | 863 | 151 (17%) | 76 (18%) | 75 (17%) |
| Rural residence | 863 | 207 (24%) | 103 (25%) | 101 (23%) |
| Married | 863 | 163 (19%) | 87 (21%) | 76 (17%) |
| LGBT | 863 | 166 (19%) | 78 (19%) | 88 (20%) |
|
| ||||
| Alcohol use | ||||
|
| ||||
| Heavy drinker3 | 833 | 115 (14%) | 52 (13%) | 63 (15%) |
| No. of drinks/drinking day, mean (SD) | 833 | 1.8 (4.0) | 1.7 (4.0) | 1.8 (4.1) |
|
| ||||
| Smoking behavior | ||||
|
| ||||
| FTND score, mean (SD) | 863 | 6.1 (1.9) | 6.2 (1.9) | 6.1 (2.0) |
| High nicotine dependence (FTND score > 6) | 863 | 560 (65%) | 266 (64%) | 294 (66%) |
| Smokes more than one-half pack/d | 863 | 617 (71%) | 308 (74%) | 309 (69%) |
| Smokes more than 1 pack/d | 863 | 168 (19%) | 78 (19%) | 90 (20%) |
| First cigarette within 5 min of waking | 863 | 533 (62%) | 266 (64%) | 267 (60%) |
| Smoked for >10 years | 863 | 679 (79%) | 329 (79%) | 350 (78%) |
| Used e-cigarettes at least once in past month | 863 | 188 (22%) | 92 (22%) | 96 (21%) |
| Quit attempts in past 12 months, mean (SD) | 819 | 1.3 (2.7) | 1.4 (3.0) | 1.2 (2.4) |
| Confidence to quit smoking, mean (SD)b | 863 | 65.8 (27.8) | 66.7 (27.6) | 65.0 (27.9) |
| Friend and partner smoking | ||||
| Close friends who smoke, mean (SD) | 863 | 2.9 (1.8) | 2.9 (1.8) | 3.0 (1.7) |
| No. of housemates who smoke, mean (SD) | 863 | 1.4 (0.8) | 1.4 (0.9) | 1.4 (0.8) |
| Living with partner who smokes | 863 | 274 (32%) | 129 (31%) | 145 (32%) |
|
| ||||
| ACT theory-based measures | ||||
|
| ||||
| Acceptancê mean (SD) | ||||
| Physical sensations | 851 | 3.0 (0.6) | 3.0 (0.6) | 3.0 (0.6) |
| Emotions | 856 | 2.8 (0.4) | 2.8 (0.5) | 2.8 (0.4) |
| Thoughts | 858 | 2.8 (0.4) | 2.8 (0.4) | 2.7 (0.4) |
| Acceptance mean score | 849 | 2.9 (0.4) | 2.9 (0.4) | 2.9 (0.4) |
|
| ||||
| Valued livingd, mean (SD) | ||||
|
| ||||
| Progresse | 852 | 18.5 (8.1) | 18.9 (8.1) | 18.1 (8.1) |
| Obstructionf | 851 | 13.1 (8.6) | 13.0 (8.5) | 13.3 (8.7) |
Abbreviations: ACT, Acceptance and Commitment Therapy; FTND, Fagerström Test for Nicotine Dependence; GED, General Education Development; LGBT, lesbian, gay, bisexual, or transgender; PTSD, Posttraumatic Stress Disorder.
Heavy drinking is defined as 4 or more drinks on a typical drinking day for women and 5 or more drinks on a typical drinking day for men within the past 30 days.
Range, 0–100, where 0 indicates not at all confident and 100 indicates extremely confident.
Avoidance and Inflexibility Scale. Range is 1 to 5. Higher scores indicate greater acceptance.
Valuing Questionnaire.
Range is 0 to 30. Higher scores indicate greater progression towards one‟s values.
Range is 0 to 30. Higher scores indicate greater obstruction of one‟s values.
Figure 2.
Geographic locations of low-income trial participants
3.3. Smoking cessation
The self-reported complete-case 30-day PPA was 27% (105/387) for iCanQuit vs. 20% (74/370) for QuitGuide at 12-months (OR=1.46 95% CI: 1.04, 2.06), 25% vs. 14% at 6-months (OR=2.15 95% CI: 1.48, 3.12), and 17% vs. 11% at 3-months (OR=1.67 95% CI: 1.10, 2.56) (Table 2). The missing-as-smoking imputed 30-day PPA at 12-months was 23% for iCanQuit vs. 18% for QuitGuide (OR=1.41; 95% CI: 1.01, 1.97). Rates of prolonged abstinence at 12-months were 12% for iCanQuit vs. 8% for QuitGuide (OR=1.43; 95% CI: 0.83, 2.45). The 30-day PPA for cessation from all nicotine and tobacco products, including e-cigarettes and vaping, was 25% for iCanQuit vs. 16% for QuitGuide at 12-months (OR=1.71 95% CI: 1.19, 2.46). The 7-day PPA was 33% for iCanQuit vs. 27% for QuitGuide at 12-months (OR=1.29 95% CI: 0.94, 1.77), 35% vs. 24% at 6-months (OR=1.69 95% CI: 1.23, 2.32), and 29% vs. 17% at 3-months (OR=1.96 95% CI: 1.38, 2.78). Multiple imputation 30-day PPA at 12-months resulted in quit rates of 27% for iCanQuit vs. 20% for QuitGuide (OR=1.51 95% CI: 1.07, 2.14).
Table 2.
Smoking cessation outcomes by follow-up time pointb
| No. (%) or Mean (SD) | |||||
|---|---|---|---|---|---|
|
| |||||
| Variable | Overall (N = 863) | QuitGuide (n = 416) | iCanQuit (n = 447) | OR (95% CI) | p value |
| 12-months outcomes | |||||
| 30-d PPA | 179/757 (24%) | 74/370 (20%) | 105/387 (27%) | 1.46 (1.04, 2.06) | 0.030 |
| 30-d PPA, missing-as- smokingc | 179/863 (21%) | 74/416 (18%) | 105/447 (23%) | 1.41 (1.01, 1.97) | 0.046 |
| 30-d PPA, multiple imputationd | 2035/8630 (24%) | 823/4160 (27%) | 1212/4470 (20%) |
1.51 (1.07, 2.14) | 0.020 |
| 7-d PPA | 226/757 (30%) | 100/370 (27%) | 126/387 (33%) | 1.29 (0.94, 1.77) | 0.116 |
| Prolonged abstinencee | 60/598 (10%) | 25/296 (8%) | 35/302 (12%) | 1.43 (0.83, 2.45) | 0.199 |
| 30-d PPA of all tobacco productsf | 157/758 (21%) | 60/371 (16%) | 97/387 (25%) | 1.71 (1.19, 2.46) | 0.004 |
|
| |||||
| 6-months outcomes | |||||
|
| |||||
| 30-d PPA | 153/778 (20%) | 51/376 (14%) | 102/402 (25%) 139/402 (35%) | 2.15 (1.48, 3.12) | <0.001 |
| 7-d PPA | 229/778 (29%) | 90/376 (24%) | 139/402 (35%) | 1.69 (1.23, 2.32) | 0.001 |
|
| |||||
| 3-months outcomes | |||||
|
| |||||
| 30-d PPA | 107/752 (14%) | 40/362 (11%) | 67/390 (17%) | 1.67 (1.10, 2.56) | 0.017 |
| 7-d PPA | 176/752 (23%) | 63/362 (17%) | 113/390 (29%) | 1.96 (1.38, 2.78) | <0.001 |
Abbreviations: OR, odds ratio; PPA, point prevalence abstinence
All models include the following covariates: education (high school diploma or less), heavy smoking (>20 cigs/day), minority race or ethnicity and depression symptoms (CESD-2016).
All outcomes are complete case (i.e., exclusion of participants lost to follow-up) was specified a priori as the primary outcome, except where noted.
Itent-to-treat missing-as-smoking analysis was specified a priori as a secondary outcome.
Multiple imputation sensitivity analysis was used to estimate missing 30-day PPA at 12-months. Effect sizes and standard errors from ten imputed datasets were pooled using Rubin‟s rules51 to generate a single OR and 95% confidence interval.
Defined as no smoking since 3-months post-randomization, using self-reported data of last cigarette.
Including any kind of e-cigarettes or vaping, chewing tobacco, snus, hookahs, cigars, cigarillos, tobacco pipes, and kreteks.
3.4. Change of ACT-based process mediators
Indirect effects of the treatment on cessation through ACT-based processes are shown in Table 3. Increases in acceptance of sensations (p<0.001), emotions (p=0.001), and thoughts (p=0.001) that cue smoking between baseline and 3-months were significantly greater among iCanQuit than QuitGuide participants. Change in progress and obstruction of valued living did not differ between arms (p>0.05). Baseline to 3-month increases in mean acceptance (indirect effect: 0.27; 95% CI: 0.13, 0.45) mediated the relationship between treatment and cessation at 12-months. In contrast, baseline to 3-month changes in the progress and obstruction measures of valued living did not mediate this relationship. Further analysis showed that baseline to 3-months increases in acceptance of emotions that cue smoking, but not in acceptance of sensations or thoughts that cue smoking mediated the relationship between treatment and cessation at 12-months (Supplementary Table 1).
Table 3.
Change in ACT-theory based processes from baseline to 3-months as mediators of the effect of treatment on the primary cessation outcomea,b
| Change from baseline to 3-months | |||||||
|---|---|---|---|---|---|---|---|
| Mean (SD) | |||||||
| Mediator | n | Overall (N = 863) | QuitGuide (n = 416) | iCanQuit (n = 447) | Point estimate for difference (95% CI) | P value | Estimate of mediation effect (95% CI) |
| Acceptance to internal cues to smoke Mean Acceptance Scorec | 710 | 0.2 (0.6) | 0.1 (0.5) | 0.2 (0.6) | 0.15 (0.08, 0.23) | <0.001 | 0.27 (0.13, 0.45)* |
| Valued livingd Progress | 726 | −0.3 (8.3) | −0.5 (8.4) | −0.2 (8.2) | −0.1 (−1.2, 0.9) | 0.828 | 0.00 (−0.03, 0.02) |
| Obstruction | 725 | −0.4 (8.7) | 0.1 (8.3) | −0.9 (9.0) | −0.9 (−2.0, 0.2) | 0.104 | 0.00 (−0.03, 0.02) |
Abbreviations: ACT, Acceptance and Commitment Therapy; PPA, point prevalence abstinence
All models include the following covariates: education (high school diploma or less), heavy smoking (>20 cigs/day), minority race or ethnicity and depression symptoms (CESD-2016).
All changes in acceptance scores calculated as follow-up minus baseline. Negative score indicates measure was higher at baseline.
Avoidance and Inflexibility Scale. Mean acceptance score includes the three subscales of acceptance, including acceptance of sensations, emotions, and thoughts that cue smoking. Range is −4 to 4. Positive scores indicate higher acceptance at follow-up.
Valuing Questionnaire. Range is −30 to 30. Positive scores indicate higher subscale scores at follow-up.
p<0.05.
3.5. Treatment utilization and satisfaction
The effects of treatment group assignment on treatment utilization and satisfaction are presented in Table 4. Compared with QuitGuide participants, iCanQuit participants opened the application on nearly three times more occasions over a period of 6-months (25.2 vs. 8.8 times, p<0.001), spent nearly two times longer using the application (4.6 vs. 2.5 minutes per session, p<0.001), and used the application on nearly three times more days (16.8 vs. 5.9 days, p<0.001). Overall treatment satisfaction did not differ between arms (86% iCanQuit vs. 82% QuitGuide, p=0.121). Compared with QuitGuide participants, iCanQuit participants found the application more useful for quitting (81% vs. 74%, p=0.031), they were more likely to recommend the application (84% vs. 77%, p=0.016), and they were more likely to report that they felt like the application was “made for them” (81% vs. 72%, p=0.005).
Table 4.
Treatment utilization and satisfaction of the assigned smartphone applicationa
| Mean (SD) or No. (%) | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Variable | n | Overall (N = 863) | QuitGuide (n = 416) | iCanQuit (n = 447) | IRR, point estimate or Odds Ratio (95% CI) | p value |
| Utilization at 6 monthsb | ||||||
|
| ||||||
| No. of times opened, mean (SD) | 850 | 17.3 (42.7)c | 8.8 (37.6)d | 25.2 (45.5)e | IRR: 3.02 (2.50, 3.64) | <0.001 |
| Time spent per session, mean (SD), min | 761 | 3.6 (4.5) | 2.5 (2.2) | 4.6 (5.7) | Point estimate: 2.0 (1.4, 2.6) | <0.001 |
| No. of unique days of use, mean (SD) | 850 | 11.6 (22.2) | 5.9 (10.2) | 16.8 (28.3) | IRR: 2.85 (2.39, 3.39) | <0.001 |
|
| ||||||
| Satisfaction at 3 months, No. (%) | ||||||
|
| ||||||
| Satisfied with assigned application | 712 | 599/712 (84%) | 284/346 (82%) | 315/366 (86%) | OR: 1.38 (0.92, 2.08) | 0.121 |
| Application was useful for quitting | 711 | 552/711 (78%) | 255/343 (74%) | 297/368 (81%) | OR: 1.49 (1.04, 2.14) | 0.031 |
| Would recommend assigned application | 734 | 589/734 (80%) | 273/356 (77%) | 316/378 (84%) | OR: 1.58 (1.09, 2.29) | 0.016 |
| Felt application was made for me | 697 | 535/697 (77%) | 242/335 (72%) | 293/362 (81%) | OR: 1.67 (1.16, 2.39) | 0.005 |
Abbreviations: IRR, incident rate ratio; OR, odds ratio; PE, point estimate.
All models include the following covariates: education (high school diploma or less), heavy smoking (>20 cigs/day), minority race or ethnicity and depression symptoms (CESD-2016).
A full 6 months of utilization data from Google Analytics were available for n=850/863, 98%.
median = 5
median = 4
median = 8
4. Discussion
Using data from a full-scale randomized trial with long-term follow-up, this study demonstrated that, among low-income adults, the iCanQuit smartphone application was more efficacious for smoking cessation than the USCPG-based QuitGuide smartphone application. The self-reported complete-case 30-day PPA for cessation at 12-months was 27% for iCanQuit vs. 20% for QuitGuide participants. Findings were similar for missing-as-smoking imputation and for the multiple imputation analysis. Results in this study were also comparable with those found in the main iCanQuit trial (28% vs. 21%; OR=1.49 95% CI: 1.22, 1.83).17 Outcome data retention rate was 88% at 12-months and did not differ by arm.
These results are a major advance over the existing body of literature on smartphone applications, which has consisted of single-arm designs and feasibility pilot trials.57–59 For example, Hébert et al.59 conducted a randomized pilot trial on the use of an automated smartphone-based application (Smart-T2) compared with QuitGuide and in-person usual care among 81 low-income adults who smoked. The 7-day PPA rates at the 3-month follow-up were 22% for Smart-T2, 15% for QuitGuide and 15% for usual care. Although user engagement was high, higher quit rates in the Smart-T2 arm did not reach statistical significance. Compared to the broader literature of digital interventions for smoking cessation among socioeconomically disadvantaged populations (e.g., low education, unemployed or manual occupation) with 6-month or longer follow-ups, studies have tested text messages,60,61 interactive websites,62 or a combination thereof.63 Quit rates have ranged between 10.7 to 19.9% for text messages alone and between 7.3 to 9.0% for interactive websites or video-based interventions plus text messages, and thus the higher quit rates for the iCanQuit application show great promise.
To understand why iCanQuit was efficacious, acceptance and valued living measures were explored as potential mediators. These analyses showed that ACT-based processes help low-income adults quit smoking via increases in acceptance of internal cues to smoke, but not via valued living measures. Stress and social disadvantage are strong triggers to smoke and consistent triggers of relapse among low-income individuals who may use smoking as a way to cope with highly stressful situations.28,64 Our results suggest that providing low-income adults with skills to increase their willingness to experience cravings to smoke without trying to control them in potentially stressful situations could be a key process underlying abstinence. Future studies should further explore these key mediators in this group.
This study showed much higher utilization of iCanQuit than the QuitGuide application among low-income adults, suggesting that smartphone applications for smoking cessation are engaging in this population. While the specific reasons why iCanQuit was more engaging are beyond the scope of this paper, the evaluation of predictors of utilization of smartphone interventions for smoking cessation in this population is a worthwhile topic for future research. And although overall satisfaction was high for both treatment arms, iCanQuit participants were significantly more likely to report that they felt the application was “made for them”.
There are several strengths of this study. First, the study was successful in recruiting a racially/ethnically and geographically diverse sample (39% minority race/ethnicity, 24% rural residence) from 48 U.S. states, thereby demonstrating potential for broad reach. Second, outcome data retention rates were high, with 88% of study participants retained at 12-months among one of the largest populations of low-income adults enrolled in a digital intervention for smoking cessation. Participant recruitment methods to increase diversity and reduce attrition are described elsewhere.65 Third, participants were not compensated for the use of the smartphone applications. Lastly, iCanQuit‟s high cessation rates were achieved without provision of any pharmacotherapy or coaching,9 which makes the intervention lower cost and logistically easier to disseminate. Rates of outside pharmacotherapy use, or coaching did not differ by treatment arm (results not shown).
The study also has limitations. First, the results are from a secondary analysis of a two-arm randomized parent trial and as such, the results are exploratory, rather than definitive. Second, the trial and interventions were not tailored to low-income individuals. However, a review of smoking cessation interventions among socioeconomically disadvantaged individuals concluded that there were no added benefits of tailoring approaches in this group when compared with non-tailored approaches.66 Third, smoking status was not biochemically-verified. The self-reported outcome was prespecified based on methodological problems with remote biochemical verification in remote population-based studies: (1) high attrition, (2) difficulty with identifying the person providing the sample, and (3) high-cost relative to the prospect of falsifying abstinence in low-touch interventions.67,68 Previous studies have demonstrated strong agreement between self-reported and biochemically verified smoking status,69,70 while others showed evidence of significant discordance.71,72 Therefore, the external validity of the self-reported smoking status in this trial is not known. However, given the double blinding of the trial, we see no compelling reason the false reporting rate would be higher in one intervention arm versus the other, and thus there is no strong rationale for a bias in the odds ratios. Lastly, full utilization data up to 12-months was not available due to a technical error by Google Analytics. Because participants were unaware of the error, the missing data after 6 months is unlikely to change the validity of the results.73
4.1. Conclusions
In a racially diverse sample with high outcome data retention and treatment utilization, this study showed that, among low-income adults, the iCanQuit smartphone application was more efficacious for smoking cessation than a USCPG-based smartphone application. A nationwide dissemination trial of iCanQuit is warranted to determine whether the iCanQuit application may alleviate cessation-related disparities among low-income adults.
Supplementary Material
Highlights.
Smartphone interventions can help reduce smoking disparities in low-income adults.
iCanQuit was more efficacious than QuitGuide for cessation in low-income adults.
iCanQuit application was more engaging than QuitGuide in low-income adults.
Acceptance of cues to smoke mediated the effect of treatment on smoking cessation.
Acknowledgements
We appreciate the tireless contributions of the entire study staff, most notably, Eric Meier, Eric Strand, Carolyn Ehret, Alanna Boynton, the design services of Ayogo, Inc., and the development services of Moby, Inc. We are very appreciative of the study participants.
Author Disclosures
Role of Funding Source
This study was funded by grant R01CA192849, awarded to Dr. Bricker, from the National Cancer Institute and registered in ClinicalTrials.gov (NCT02724462).
Abbreviations:
- ACT
Acceptance and Commitment Therapy
- CI
95% confidence interval
- FTND
Fagerström Test for Nicotine Dependence
- GED
General Education Development
- LGBT
lesbian, gay, bisexual, or transgender
- OR
odds ratio
- PPA
point-prevalence abstinence
- PTSD
posttraumatic stress disorder
- RCT
randomized clinical trial
- RUCA
Rural-Urban Commuting Area
- USCPG
United States Clinical Practice Guidelines
- U.S.
United States
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.U.S. Department of Health and Human Services. Smoking Cessation: A Report of the Surgeon General—Executive Summary. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health: 2020. [Google Scholar]
- 2.Cornelius ME, Wang TW, Jamal A, Loretan CG, Neff LJ. Tobacco product use among adults—United States, 2019. MMWR Morb Mortal Wkly Rep. 2020;69:1736–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Okoro CA, Zhao G, Fox JB, Eke PI, Greenlund KJ, Town M. Surveillance for Health Care Access and Health Services Use, Adults Aged 18–64 Years - Behavioral Risk Factor Surveillance System, United States, 2014. MMWR Surveill Summ 2017;66:1–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hiscock R, Bauld L, Amos A, Fidler JA, Munafo M. Socioeconomic status and smoking: a review. Ann N Y Acad Sci 2012;1248:107–23. [DOI] [PubMed] [Google Scholar]
- 5.Babb S, Malarcher A, Schauer G, Asman K, Jamal A. Quitting smoking among adults—United States, 2000–2015. MMWR Morb Mortal Wkly Rep. 2017;65:1457–64. [DOI] [PubMed] [Google Scholar]
- 6.Hammett PJ, Fu SS, Burgess DJ, et al. Treatment barriers among younger and older socioeconomically disadvantaged smokers. Am J Manag Care 2017;23:e295–e302. [PMC free article] [PubMed] [Google Scholar]
- 7.van Wijk EC, Landais LL, Harting J. Understanding the multitude of barriers that prevent smokers in lower socioeconomic groups from accessing smoking cessation support: A literature review. Prev Med 2019;123:143–51. [DOI] [PubMed] [Google Scholar]
- 8.Smith CE, Hill SE, Amos A. Impact of population tobacco control interventions on socioeconomic inequalities in smoking: a systematic review and appraisal of future research directions. Tob Control 2020:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vidrine DJ, Frank-Pearce SG, Vidrine JI, et al. Efficacy of Mobile Phone-Delivered Smoking Cessation Interventions for Socioeconomically Disadvantaged Individuals: A Randomized Clinical Trial. JAMA Intern Med 2019;179:167–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fu SS, van Ryn M, Nelson D, et al. Proactive tobacco treatment offering free nicotine replacement therapy and telephone counselling for socioeconomically disadvantaged smokers: a randomised clinical trial. Thorax 2016;71:446–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haas JS, Linder JA, Park ER, et al. Proactive tobacco cessation outreach to smokers of low socioeconomic status: a randomized clinical trial. JAMA Intern Med 2015;175:218–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention. Best Practices for Comprehensive Tobacco Control Programs—2014. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2014. [Google Scholar]
- 13.Marshall LL, Zhang L, Malarcher AM, Mann NH, King BA, Alexander RL. Race/EthnicVariations in Quitline Use Among US Adult Tobacco Users in 45 States, 2011–2013. Nicotine Tob Res 2017;19:1473–81. [DOI] [PubMed] [Google Scholar]
- 14.Vijayaraghavan M, Dove MS, Stewart SL, et al. Racial/Ethnic Differences in the Response to Incentives for Quitline Engagement. Am J Prev Med 2018;55:S186–S95. [DOI] [PubMed] [Google Scholar]
- 15.Colston DC, Simard BJ, Xie Y, et al. The Association between Quitline Characteristics and Smoking Cessation by Educational Attainment, Income, Race/Ethnicity, and Sex. Int J Environ Res Public Health 2021;18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pew Research Center. 2021. Mobile Fact Sheets: Internet and Broadband. URL: https://www.pewresearch.org/internet/fact-sheet/mobile/ [accessed 2021-06-25].
- 17.Bricker JB, Watson NL, Mull KE, Sullivan BM, Heffner JL. Efficacy of Smartphone Applications for Smoking Cessation: A Randomized Clinical Trial. JAMA Intern Med 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Danaher BG, Tyler MS, Crowley RC, Brendryen H, Seeley JR. Outcomes and Device Usage for Fully Automated Internet Interventions Designed for a Smartphone or Personal Computer: The MobileQuit Smoking Cessation Randomized Controlled Trial. Journal of medical Internet research 2019;21:e13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.BinDhim NF, McGeechan K, Trevena L. Smartphone Smoking Cessation Application (SSC App) trial: a multicountry double-blind automated randomised controlled trial of a smoking cessation decision-aid ‘app’. BMJ Open 2018;8:e017105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma P, Businelle MS, Balis DS, Kendzor DE. The influence of perceived neighbourhood disorder on smoking cessation among urban safety net hospital patients. Drug Alcohol Depend 2015;156:157–61. [DOI] [PubMed] [Google Scholar]
- 21.Kendzor DE, Reitzel LR, Mazas CA, et al. Individual- and area-level unemployment influence smoking cessation among African Americans participating in a randomized clinical trial. Soc Sci Med 2012;74:1394–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kendzor DE, Businelle MS, Costello TJ, et al. Financial strain and smoking cessation among racially/ethnically diverse smokers. Am J Public Health 2010;100:702–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kendzor DE, Businelle MS, Reitzel LR, et al. The influence of discrimination on smoking cessation among Latinos. Drug Alcohol Depend 2014;136:143–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim-Mozeleski JE, Seligman HK, Yen IH, Shaw SJ, Buchanan DR, Tsoh JY. Changes in Food Insecurity and Smoking Status over Time: Analysis of the 2003 and 2015 Panel Study of Income Dynamics. Am J Health Promot 2019;33:698–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guillaumier A, Twyman L, Paul C, Siahpush M, Palazzi K, Bonevski B. Financial Stress and Smoking within a Large Sample of Socially Disadvantaged Australians. Int J Environ Res Public Health 2017;14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parnia A, Siddiqi A. Socioeconomic disparities in smoking are partially explained by chronic financial stress: marginal structural model of older US adults. J Epidemiol Community Health 2020;74:248–54. [DOI] [PubMed] [Google Scholar]
- 27.Shagiwal SS, Schop-Etman A, Bergwerff I, Vrencken W, Denktas S. The BeHealthyR Study: a randomized trial of a multicomponent intervention to reduce stress, smoking and improve financial health of low-income residents in Rotterdam. BMC Public Health 2018;18:891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Widome R, Joseph AM, Hammett P, et al. Associations between smoking behaviors and financial stress among low-income smokers. Prev Med Rep 2015;2:911–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bricker JB (In press). Smoking cessation. In Twohig MP, Levin ME, & Petersen JM(Eds.), The Oxford Handbook of Acceptance and Commitment Therapy. Oxford University Press. [Google Scholar]
- 30.Bricker JB, Bush T, Zbikowski SM, Mercer LD, Heffner JL. Randomized trial of telephone-delivered acceptance and commitment therapy versus cognitive behavioral therapy for smoking cessation: a pilot study. Nicotine Tob Res 2014;16:1446–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bricker JB, Mull KE, Kientz JA, et al. Randomized, controlled pilot trial of a smartphone app for smoking cessation using acceptance and commitment therapy. Drug Alcohol Depend 2014;143:87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bricker JB, Mull KE, McClure JB, Watson NL, Heffner JL. Improving quit rates of web delivered interventions for smoking cessation: full-scale randomized trial of WebQuit.org versus Smokefree.gov. Addiction 2018;113:914–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hayes SC, Levin ME, Plumb-Vilardaga J, Villatte JL, Pistorello J. Acceptance and commitment therapy and contextual behavioral science: examining the progress of a distinctive model of behavioral and cognitive therapy. Behav Ther 2013;44:180–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bricker JB, Watson NL, Mull KE, Sullivan BM, Heffner JL. Efficacy of Smartphone Applications for Smoking Cessation: A Randomized Clinical Trial. JAMA Intern Med 2020;180:1472–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bricker JB, Levin M, Lappalainen R, Mull K, Sullivan B, Santiago-Torres M. Mechanisms of Smartphone Apps for Cigarette Smoking Cessation: Results of a Serial Mediation Model From the iCanQuit Randomized Trial. JMIR mHealth and uHealth 2021;9:e32847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Semega J, Kollar M, Shrider EA, Creamer J. U.S. Census Bureau, Current Population Reports, P60–270, Income and Poverty in the United States: 2019. U.S. Govertment Publishing Office, Washington, DC: 2019. [Google Scholar]
- 37.Creamer MR, Wang TW, Babb S, et al. Tobacco product use and cessation indicators among adults—United States, 2018. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas 1977;1:385–401. [Google Scholar]
- 39.Stein MB, Roy-Byrne PP, McQuaid JR, et al. Development of a brief diagnostic screen for panic disorder in primary care. Psychosom Med 1999;61:359–64. [DOI] [PubMed] [Google Scholar]
- 40.Lang AJ, Wilkins K, Roy-Byrne PP, et al. Abbreviated PTSD Checklist (PCL) as a guide to clinical response. Gen Hosp Psychiatry 2012;34:332–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roy M, Dum M, Sobell LC, et al. Comparison of the quick drinking screen and the alcohol timeline followback with outpatient alcohol abusers. Subst Use Misuse 2008;43:2116–23. [DOI] [PubMed] [Google Scholar]
- 42.Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict 1991;86:1119–27. [DOI] [PubMed] [Google Scholar]
- 43.Gifford EV, Kohlenberg BS, Hayes SC, et al. Acceptance-based treatment for smoking cessation. Behavior Therapy 2004;35:689–705. [Google Scholar]
- 44.Smout M, Davies M, Burns N, Christie A. Development of the Valuing Questionnaire (VQ). J Contextual Behav Sci 2014;3:164–72. [Google Scholar]
- 45.Breen J. zipcode: U.S. ZIP Code database for geocoding. R package version 1.0. URL: https://CRAN.R-project.org/package=zipcode. 2012.
- 46.US Department of Agriculture, Economic Research Service, 2020. URL:https://www.ers.usda.gov/data-products/rural-urban-commuting-area-codes/ [accessed 2021-02-21].
- 47.Larson EH, Andrilla CHA, Garberson LA, Evans DV. Geographic Access to Health Care for Rural Medicare Beneficiaries in Five States: An Update. Policy Brief. WWAMI Rural Health Research Center, University of Washington; April 2021. [Google Scholar]
- 48.Ratcliffe M, Burd C, Holder K, Fields A. Defining Rural at the U.S. Census Bureau, ACSGEO-1, U.S. Census Bureau, Washington, DC, 2016. [Google Scholar]
- 49.Chen X, Orom H, Hay JL, et al. Differences in Rural and Urban Health Information Access and Use. J Rural Health 2019;35:405–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Unger JM, Moseley A, Symington B, Chavez-MacGregor M, Ramsey SD, Hershman DL. Geographic Distribution and Survival Outcomes for Rural Patients With Cancer Treated in Clinical Trials. JAMA Netw Open 2018;1:e181235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rubin DB. Multiple imputation for nonresponse in surveys. New York;: Wiley; 1987. [Google Scholar]
- 52.Kernan WN, Viscoli CM, Makuch RW, Brass LM, Horwitz RI. Stratified randomization for clinical trials. J Clin Epidemiol 1999;52:19–26. [DOI] [PubMed] [Google Scholar]
- 53.Hayes AF. Introduction to mediation, moderation, and conditional process analysis: a regression-based approach. 2nd ed. New York, NY: Guilford Press.2018. [Google Scholar]
- 54.R Core Team (2020). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL: https://www.R-project.org/. [Google Scholar]
- 55.Venables WN, Ripley BD. Modern Applied Statistics with S. 4th ed. Springer; 2002. doi: 10.1007/978-0-387-21706-2. [DOI] [Google Scholar]
- 56.van Buuren S, G-O K. mice: multivariate imputation by chained equations in R. J Stat Softw. 2011:1–67. [Google Scholar]
- 57.Businelle MS, Ma P, Kendzor DE, Frank SG, Vidrine DJ, Wetter DW. An Ecological Momentary Intervention for Smoking Cessation: Evaluation of Feasibility and Effectiveness. Journal of medical Internet research 2016;18:e321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kendzor DE, Businelle MS, Waring JJC, et al. Automated Mobile Delivery of Financial Incentives for Smoking Cessation Among Socioeconomically Disadvantaged Adults: Feasibility Study. JMIR mHealth and uHealth 2020;8:e15960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hebert ET, Ra CK, Alexander AC, et al. A Mobile Just-in-Time Adaptive Intervention for Smoking Cessation: Pilot Randomized Controlled Trial. Journal of medical Internet research 2020;22:e16907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Free C, Knight R, Robertson S, et al. Smoking cessation support delivered via mobile phone text messaging (txt2stop): a single-blind, randomised trial. Lancet 2011;378:49–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Abroms LC, Boal AL, Simmens SJ, Mendel JA, Windsor RA. A randomized trial of Text2Quit: a text messaging program for smoking cessation. American journal of preventive medicine 2014;47:242–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brown J, Michie S, Geraghty AW, et al. Internet-based intervention for smoking cessation (StopAdvisor) in people with low and high socioeconomic status: a randomised controlled trial. Lancet Respir Med 2014;2:997–1006. [DOI] [PubMed] [Google Scholar]
- 63.Stanczyk N, Bolman C, van Adrichem M, Candel M, Muris J, de Vries H. Comparison of text and video computer-tailored interventions for smoking cessation: randomized controlled trial. Journal of medical Internet research 2014;16:e69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rogers E, Palacios J, Vargas E, et al. Financial Hardship, Motivation to Quit and Post-Quit Spending Plans among Low-Income Smokers Enrolled in a Smoking Cessation Trial. Subst Abuse 2019;13:1178221819878765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Watson NL, Mull KE, Heffner JL, McClure JB, Bricker JB. Participant Recruitment and Retention in Remote eHealth Intervention Trials: Methods and Lessons Learned From a Large Randomized Controlled Trial of Two Web-Based Smoking Interventions. Journal of medical Internet research 2018;20:e10351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kock L, Brown J, Hiscock R, Tattan-Birch H, Smith C, Shahab L. Individual-level behavioural smoking cessation interventions tailored for disadvantaged socioeconomic position: a systematic review and meta-regression. Lancet Public Health 2019;4:e628–e44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Herbec A, Brown J, Shahab L, West R. Lessons learned from unsuccessful use of personal carbon monoxide monitors to remotely assess abstinence in a pragmatic trial of a smartphone stop smoking app - A secondary analysis. Addict Behav Rep 2019;9:100122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Thrul J, Meacham MC, Ramo DE. A novel and remote biochemical verification method of smoking abstinence: Predictors of participant compliance. Tob Prev Cessat 2018;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.van der Aalst CM, de Koning HJ. Biochemical verification of the self-reported smoking status of screened male smokers of the Dutch-Belgian randomized controlled lung cancer screening trial. Lung Cancer 2016;94:96–101. [DOI] [PubMed] [Google Scholar]
- 70.Wong SL, Shields M, Leatherdale S, Malaison E, Hammond D. Assessment of validity of self-reported smoking status. Health Rep 2012;23:47–53. [PubMed] [Google Scholar]
- 71.Pineiro B, Vidrine DJ, Wetter DW, et al. Implementation of Ask-Advise-Connect in a safety net healthcare system: quitline treatment engagement and smoking cessation outcomes. Transl Behav Med 2020;10:163–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Patrick DL, Cheadle A, Thompson DC, Diehr P, Koepsell T, Kinne S. The validity of self-reported smoking: a review and meta-analysis. Am J Public Health 1994;84:1086–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Graham JW. Missing data analysis: making it work in the real world. Annu Rev Psychol 2009;60:549–76. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.