Abstract
Background:
Since 1971, the annual National Ambient Air Quality Standard (NAAQS) for nitrogen dioxide (NO2) has remained at 53 ppb, the impact of long-term NO2 exposure on mortality is poorly understood.
Objectives:
We examined associations between long-term NO2 exposure (12-month moving average of NO2) below the annual NAAQS and cause-specific mortality among the older adults in the U.S.
Methods:
Cox proportional-hazard models were used to estimate Hazard Ratio (HR) for cause-specific mortality associated with long-term NO2 exposures among about 50 million Medicare beneficiaries living within the conterminous U.S. from 2001–2008.
Results:
A 10 ppb increase in NO2 was associated with increased mortality from all-cause (HR: 1.06; 95% CI: 1.05–1.06), cardiovascular (HR: 1.10; 95% CI: 1.10–1.11), respiratory disease (HR: 1.09; 95% CI: 1.08–1.11), and cancer (HR: 1.01; 95% CI: 1.00–1.02) adjusting for age, sex, race, ZIP code as strata ZIP code- and state-level socio-economic status (SES) as covariates, and PM2.5 exposure using a 2-stage approach. NO2 was also associated with elevated mortality from ischemic heart disease, cerebrovascular disease, congestive heart failure, chronic obstructive pulmonary disease, pneumonia, and lung cancer. We found no evidence of a threshold, with positive and significant HRs across the range of NO2 exposures for all causes of death examined. Exposure-response curves were linear for all-cause, supra-linear for cardiovascular-, and sub-linear for respiratory-related mortality. HRs were highest consistently among Black beneficiaries.
Conclusions:
Long-term NO2 exposure is associated with elevated risks of death by multiple causes, without evidence of a threshold response. Our findings raise concerns about the sufficiency of the annual NAAQS for NO2.
Keywords: air pollution, cardiovascular mortality, respiratory mortality, cancer mortality, pneumonia mortality, congestive heart failure mortality, chronic obstructive pulmonary disease mortality, racial inequality
1. Introduction
Since 1971, the annual National Ambient Air Quality Standard (NAAQS) for nitrogen dioxide (NO2) has remained at 53 ppb. During this period, ambient NO2 concentrations within the US have decreased substantially, with all areas in the US in attainment (U.S. Environmental Protection Agency, 2019). Despite this, several studies have demonstrated associations with mortality at currently observed low annual NO2 levels. For example, in our earlier paper of >14 million Medicare beneficiaries living near EPA monitoring sites (Eum et al. 2019), we showed NO2 exposures below the annual NAAQS to be associated with increased mortality risks, consistent with findings from the Cancer Prevention II (Turner et al. 2016) and the Canadian Census Health and Environment Cohort studies (Crouse et al. 2015). These findings raise questions regarding the sufficiency of the annual NAAQS NO2 standard.
Their findings, however, are limited by their geographic and demographic scope. For example, our recent study of NO2 on mortality was based on a largely urban cohort (89%) (Eum et al. 2019), while the Cancer Prevention Study II included almost entirely White adults (94.6%) (Turner et al. 2016). As such, the generalizability of these findings to other, less studied populations, is not known. Further, prior studies leave unanswered questions regarding whether a threshold level exists below which NO2 exposures pose no harm.
In this paper, we assess the association between long-term NO2 exposure and mortality among a near-complete sample of US Medicare beneficiaries.
2. Materials and methods
2.1. Data Source and Study Population
From the Centers for Medicare and Medicaid Services Medicare Enrollment file, we obtained beneficiary data for ~50 million enrollees living in the conterminous US between 2001 and 2008. For each enrollee, we compiled information on age, sex, race, date of death, and ZIP code of residence. We also obtained cause of death from the National Death Index (NDI) (http://U.S..resdac.org/resconnect/articles/117#cause-of-death). Using International Classification of Disease (ICD–10), we identified deaths from cardiovascular (CVD) and respiratory disease and cancer, which together account for ~74 % of all deaths (Table S1). We also identified deaths from specific subcategories, including ischemic heart disease (IHD), cerebrovascular disease (CBV), congestive heart failure (CHF), chronic obstructive pulmonary disease (COPD), pneumonia, lung cancer, and from aggregate non-accidental causes of mortality. We also classified accidental mortality as a negative control.
2.2. Ambient NO2, PM2.5, Black Carbon Exposures
We estimated 12-month moving average NO2 exposure for beneficiaries based upon their ZIP code of residence using NO2 estimates from Bechle et al. (Bechle et al. 2015), who used land-use regression (LUR) and spatially varying temporal scaling factors to estimate monthly NO2 concentrations on a 100-meter grid across the conterminous U.S. from 2000 to 2008 (Bechle et al. 2015). In brief, monthly average NO2 concentrations were calculated for each of 370 EPA regulatory monitoring stations which met the reliability criterion of ≥75% valid hourly values. These values were scaled using 2006 LUR estimates and through interpolation were used to estimate monthly NO2 along a 100-meter grid. Estimates had high validity and low error, explaining 81% of the spatial (R2 = 0.81), 73% of temporal (R2 = 0.73), and 84% of the spatiotemporal variation (R2 = 0.84) in monthly mean NO2 concentrations from 2000 to 2010, with an absolute average bias of 2.4 ppb. Although the mean error was similar, the model performance was lower in rural (R2 = 0.69) as compared to urban (R2 = 0.80) areas, suggesting greater exposure error for rural ZIP codes.
We adjusted for potential confounding by fine particulate matter (PM2.5) using daily PM2.5 concentrations estimated on a 6×6 km grid across the conterminous U.S. from a set of well-validated spatio-temporal smoothing models (Yanosky et al. 2014). The models predicted PM2.5 concentrations from measured PM2.5 data from the US EPA Air Quality System, meteorological data, geographical factors, and traffic-related PM2.5 estimated from a line-source Gaussian dispersion model. Model performance was strong, with a cross-validation R2 for daily PM2.5 concentrations of 0.76. We averaged monthly NO2 and daily PM2.5 concentrations for each beneficiary to obtain 12-month moving average exposure estimates for each pollutant. We linked estimated PM2.5 concentrations to aggregated beneficiary mortality data by month and ZIP code (using the PM2.5 concentration estimated at the grid point closest to the centroid of each beneficiary’s residential ZIP code), accounting for residential moves.
We adjusted for potential confounding by BC with an aerodynamic diameter less than 2.5 μm using annual BC concentrations for each ZIP code and year from 2000 to 2008 estimated using a combined Geoscience-Statistical Method by van Donkelaar et al. (van Donkelaar et al., 2019). We linked BC estimates to aggregated beneficiary mortality data by calendar year and ZIP code, again accounting for residential moves.
2.3. Urbanicity, Region, and SES assessment
We classified ZIP codes as urban, micropolitan, and rural using the Rural Health Research Center (RUCA) Categorization B. Specifically, ZIP codes were characterized as ‘urban’ (codes: 1.0, 1.1, 2.0, 2.1, 3.0, 4.1, 5.1, 7.1, 8.1, 10.1), ‘micropolitan,’ (codes: 4.0, 4.2, 5.0, 5.2, 6.0, 6.1), or ‘rural,’ (codes: 7.0, 7.2, 7.3, 7.4, 8.0, 8.2, 8.3, 8.4, 9.0, 9.1, 9.2, 10.0, 10.2, 10.3, 10.4, 10.5, 10.6) (http://depts.washington.edu/uwruca/ruca-uses.php). We classified states into four U.S. Census regions: ‘West’, ‘Midwest’, ‘Northeast’, and ‘South’ (https://www.census.gov/prod/1/gen/95statab/preface.pdf). To estimate ZIP code- and state-level SES, we obtained data from the U.S. Internal Revenue Service (IRS) on the annual mean gross adjusted income for each state and ZIP code. The data were based upon the individual tax returns filed with the IRS (Internal Revenue Service).
2.4. Statistical Analyses
For each month between 2001 and 2008, we computed the number of beneficiaries and deaths for each ZIP code, sex, race, age. For each month, we calculated the number of deaths for each age interval, as year from 65–89 years and as one interval for individuals 90 years and older. Since our Cox proportional hazards model stepped through time every month, we aggregated the number of deaths (by cause) for each month, allowing us to calculate the changes in both the number at risk and number of deaths for each month. This discretized version of the Cox PH model essentially resulted in a coarse grid of times with ties that were broken by adding an extremely small amount of noise to each death time. We used a 12-month moving average of NO2 as our exposure measure, which changes monthly, reflecting the average exposure over the previous 12 months. As our base model, we examined the impact of a 10 ppb increase in 12-month moving average NO2 exposure on cause-specific mortality in age, sex, race, ZIP code-stratified Cox proportional-hazard models, adjusting for ZIP code- and state-level SES. We used the calendar month as a time scale.
We also fit PM2.5-adjusted models to assess potential confounding of our results by PM2.5. To do so, we used a two-stage approach, given the strong correlation between NO2 and PM2.5 concentrations (r= 0.59; Table S2). In the first stage, we regressed 12-month moving average NO2 on a 12-month moving average PM2.5. In the second stage, we used the residuals from this regression as the exposure measure in Cox proportional-hazard models, with the resulting HRs representing the NO2-associated mortality risk, adjusting for PM2.5. In sensitivity analyses, we also fit two-pollutant models that included either NO2 and PM2.5 or NO2 and BC together in the same model.
We fit PM2.5-adjusted Cox models for the conterminous U.S. as well as for each mutually exclusive U.S. region. We examined effect modification using interaction terms for sex, age (age <75, age ≥75), race (White, Black, Asian, Hispanic), urbanicity (urban, micropolitan, rural), and NO2 level (low <7 ppb, medium <12 ppb, high >12 ppb). Given that minority beneficiaries lived predominantly in urban locations, we also examined effect modification by race only for urban beneficiaries. We examined the exposure-response curve using restricted cubic splines with 3 knots, which demonstrated superior model performance compared to 4 and 5 knot models based on BIC criterion (Figure S1). We also assessed the exposure-response curve stratified by the potential effect modifiers. We implemented all Cox models in Java, incorporating linkage, grouping and other data mining techniques to reduce memory needs and improve computational efficiency (Wang et al. 2020). For comparison with previous studies, we rescaled results from prior studies to reflect HRs per 10 ppb increase in NO2 exposure.
3. Results
Our study population included almost 50 million Medicare beneficiaries (65−120 years), with 13.2 million deaths: 96.7% from non-accidental and 2.4% from accidental causes. CVD accounted for 39.9% of all non-accidental mortality, followed by cancer (22.6%), and respiratory mortality (11.2%; Table 1, Table S1). Approximately 78.0%, 11.1%, and 10.9% of beneficiaries lived in urban, micropolitan, and rural areas, respectively. Minority populations lived predominantly in urban areas, with 74.2%, 92.5%, and 85.2% of all Black, Asian, and Hispanic beneficiaries, respectively, while 55.8% of all White beneficiaries lived in urban areas. The annual mean NO2, PM2.5, and BC concentrations were 10.9 ppb, 9.6 μg/m3, and 0.78 μg/m3, respectively, with higher mean concentrations in urban (12.8 ppb, 10.4 μg/m3, 0.87 μg/m3) as compared to micropolitan (6.8 ppb, 8.5 μg/m3, 0.67 μg/m3), and rural areas (5.4 ppb, 7.8 μg/m3, 0.61 μg/m3). The NO2 concentration were higher in the West (14.45 ppb) and Northeast (14.38 ppb) regions than Midwest (9.55 ppb) and South (7.98 ppb) regions (Table 1, Figure S2). Mean 12-month NO2 exposure also differed by race, with Asians (14.3±6.8 ppb), Hispanics (13.6±7.4 ppb), and Black (11.1±6.4 ppb) beneficiaries having higher exposures as compared to White (9.5±5.7 ppb). The interquartile range (IQR) for 1-year moving average NO2 concentrations equaled 7.86 ppb for our entire study population, with IQR values of 8.12 ppb, 3.12 ppb, and 2.46 ppb for participants living in urban, micropolitan, and rural areas, respectively.
Table 1.
General Characteristics of the Study Population
| Population | N (%) or Mean ± SD | |||
|
| ||||
| Persons | 49,712,702 | |||
| Deaths | 13,213,500 | |||
| Person-months | 3,119,343,105 | |||
|
| ||||
| Pollution concentration | Mean ± SD | Median (25th, 75th) | Range | |
| NO2, ppb | 10.90 ± 6.44 | 9.14 (6.13, 13.98) | 1.03 – 47.04 | |
| PM2.5, μg/m3 | 9.59 ± 3.12 | 9.44 (7.56, 11.45) | 0.70 – 25.79 | |
| Black Carbon, μg/m3 | 0.78 ± 0.38 | 0.70 (0.53,0.95) | 0.00 – 3.23 | |
| Individual characteristics | N (%) | NO2, ppb Mean ± SD | PM2.5, μg/m3 Mean ± SD | Black Carbon, μg/m3 Mean ± SD |
| Sex | ||||
| Male | 22,032,670 (44.32) | 10.84 ± 6.43 | 9.55 ± 3.13 | 0.77 ± 0.38 |
| Female | 27,680,032 (55.68) | 10.94 ± 6.45 | 9.62 ± 3.12 | 0.78 ± 0.38 |
| Race | ||||
| White | 43,220,223 (86.94) | 9.51 ± 5.65 | 9.17 ± 3.00 | 0.72 ± 0.33 |
| Black | 4,518,885 (9.9) | 11.13 ± 6.40 | 10.61 ± 2.74 | 0.88 ± 0.35 |
| Asian | 512,041 (1.03) | 14.33 ± 6.75 | 10.54 ± 3.33 | 0.94 ± 0.48 |
| Hispanic | 805,346 (1.62) | 13.61 ± 7.36 | 9.85 ± 3.55 | 0.85 ± 0.50 |
| Native American | 656,208 (1.32) | 9.30 ± 5.79 | 7.75 ± 3.30 | 0.61 ± 0.35 |
| Neighborhood characteristics | ||||
| Urban | 38,775,908 (78.00) | 12.8 ± 6.5 | 10.4 ± 3.1 | 0.87 ± 0.41 |
| Micropolitan | 5,518,110 (11.10) | 6.8 ± 2.3 | 8.5 ± 2.6 | 0.67 ± 0.27 |
| Rural | 5,418,685 (10.90) | 5.4 ± 1.9 | 7.8 ± 2.5 | 0.61 ± 0.27 |
| Geological regions | ||||
| West | 9,296,275 (18.70) | 14.45 ± 7.48 | 8.39 ± 4.53 | 0.81 ± 0.61 |
| Midwest | 10,489,380 (21.10) | 9.55 ± 5.25 | 9.72 ± 2.83 | 0.66 ± 0.23 |
| South | 11,985,732 (24.11) | 7.98 ± 3.75 | 10.07 ± 2.29 | 0.80 ± 0.28 |
| Northeast | 17,936,343 (36.08) | 14.38 ± 7.18 | 9.79 ± 2.71 | 0.88 ± 0.33 |
3.1. Associations of Long-term NO2 and Specific Causes of Death
In base models, we found 12-month moving average NO2 to be significantly associated with increased mortality for all examined causes of death, with increased risks ranging from 4% for lung cancer (HR: 1.04; 95% CI: 1.02–1.07) to 33% for pneumonia (HR: 1.33; 95% CI 1.29–1.37) (Table 2). While attenuated, HRs remained significant and positive for all causes of death after adjusting for PM2.5. PM2.5-adjusted HRs were highest for CVD-related mortality (HR: 1.10; 95% CI: 1.10–1.11), with 1.12 (95% CI: 1.11–1.13), 1.08 (95% CI: 1.06–1.10), and 1.10 (95% CI: 1.06–1.13) times the risk of death for IHD, CBV, and CHF, respectively (Table 2). For all respiratory mortality, we observed a HR of 1.09 (95% CI: 1.08–1.11); risks were higher for pneumonia (HR: 1.23; 95% CI: 1.19–1.27) and lower for COPD (HR: 1.03; 95% CI: 1.01–1.05). While lower, NO2-associated HRs (HR: 1.03; 95% CI: 1.01–1.05) were positive and significant for lung cancer mortality and were marginally significant for all cancer mortality (HR: 1.01; 95% CI: 1.00–1.02). Associations between NO2 and accidental mortality were null for all examined models, supporting the validity of the analysis. When we additionally adjusted for year and month (Table S4), we found similar patterns of association. We found greater associations between NO2 and cause-specific mortality in models that did not adjust for ZIP- and state-SES (Table S5), or that used the two-pollutant models (versus our two-stage approach) to control for confounding by PM2.5 or BC exposure (Table 2).
Table 2.
Mortality Hazard Ratios (95% CI) Associated with a 10 ppb Increase in 12-month Moving Average NO2 Exposure: by Cause of Death in Base and PM2.5- and BC-Adjusted Models
| Cause of Death | HR a | PM2.5-Adjusted HR |
BC-Adjusted HR d | |
|---|---|---|---|---|
| 2-stageb | 2-pollutant c | |||
|
| ||||
| All-Cause | 1.14 (1.13,1.15) | 1.06 (1.05,1.06) | 1.14 (1.13,1.15) | 1.14 (1.13,1.15) |
| Non-Accidental | 1.14 (1.14,1.15) | 1.06 (1.06,1.07) | 1.15 (1.14,1.15) | 1.15 (1.14,1.15) |
| Accidental | 0.99 (0.95,1,03) | 0.98 (0.95,1.02) | 0.98 (0.94,1,02) | 0.98 (0.94,1,02) |
| All cardiovascular | 1.23 (1.22,1.24) | 1.10 (1.10,1.11) | 1.24 (1.23,1.25) | 1.24 (1.23,1.26) |
| IHD | 1.26 (1.24,1.27) | 1.12 (1.11,1.13) | 1.27 (1.25,1.29) | 1.28 (1.27,1.30) |
| CBV | 1.25 (1.22,1.28) | 1.08 (1.06,1.10) | 1.24 (1.21,1.27) | 1.28 (1.25,1.30) |
| CHF | 1.16 (1.12,1.21) | 1.10 (1.06,1.13) | 1.19 (1.15,1.24) | 1.19 (1.14,1.23) |
| All respiratory | 1.17 (1.15,1.19) | 1.09 (1.08,1.11) | 1.19 (1.17,1.21) | 1.17 (1.15,1.19) |
| COPD | 1.06 (1.03,1.08) | 1.03 (1.01,1.05) | 1.06 (1.04,1.09) | 1.06 (1.03,1.08) |
| Pneumonia | 1.33 (1.29,1.37) | 1.23 (1.19,1.27) | 1.41 (1.36,1.47) | 1.36 (1.31,1.40) |
| All Cancer | 1.04 (1.03,1.06) | 1.01 (1.00,1.02) | 1.04 (1.03,1.05) | 1.04 (1.03,1.06) |
| Lung Cancer | 1.04 (1.02,1.07) | 1.03 (1.01,1.05) | 1.05 (1.03,1.08) | 1.04 (1.02,1.07) |
Base models with strata for sex (male, female), race (White, non-White), age (1 year age categories with 90+ years old as one category), ZIP code and adjusted for ZIP code- and state-level SES by including the two SES variables as covariates.
Base models with additional adjustment for 12-month PM2.5 using a two-stage approach.
Base models with additional adjustment for PM2.5 using 2-pollutant modeling approach.
Base models with additional adjustment for annual BC using a 2-pollutant modeling approach.
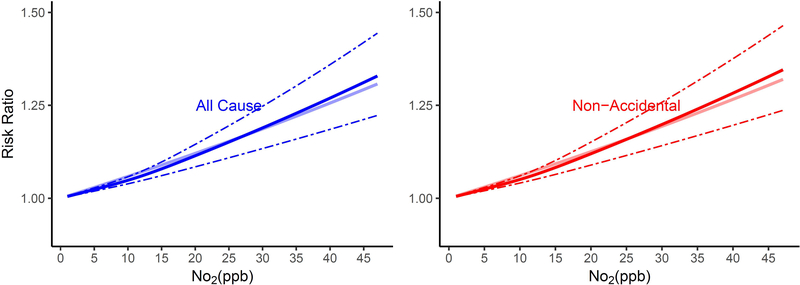
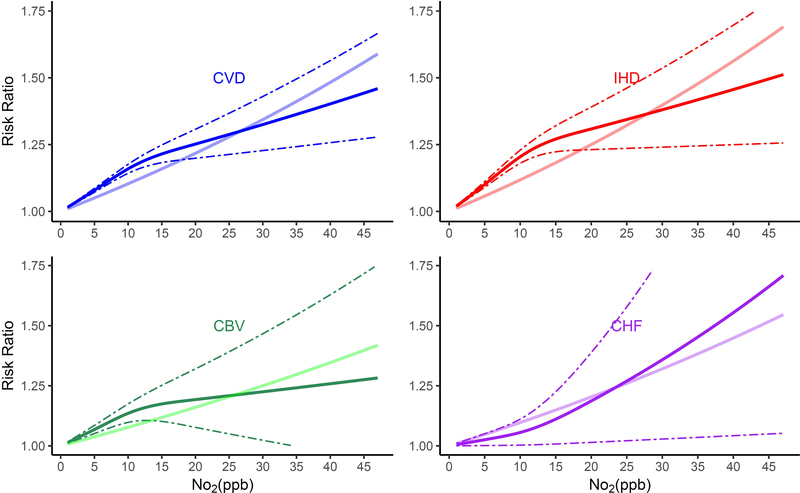
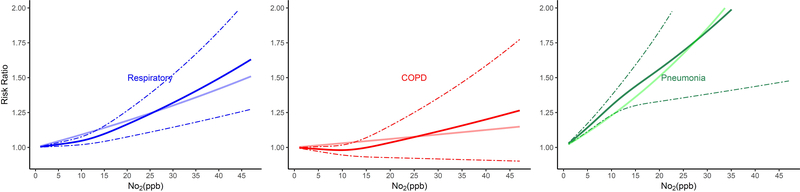
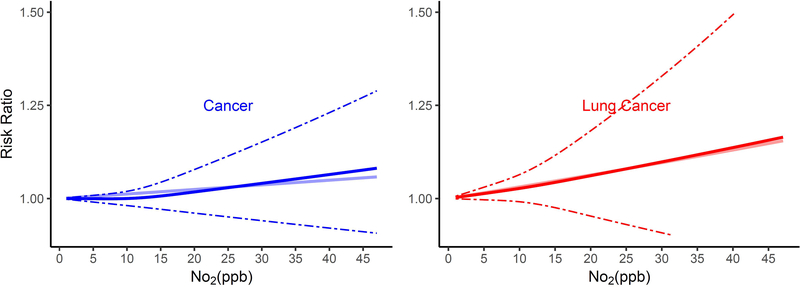
The shape of the exposure-response curves varied by cause of death (Figure 1). Associations of long-term NO2 exposure and non-accidental and lung cancer mortality were linear, while those for CVD, IHD, CVB, and pneumonia mortality were supra-linear, with higher HRs when NO2 exposures were lower as compared to higher than ~12 ppb. In contrast, exposure-response curves for respiratory mortality were sub-linear in shape, with lower risks when NO2 exposures were less than ~12 ppb.
Figure 1.
Non-linear Relationship of Mortality Hazard Ratio (95% CI) for Cause-Specific Mortality and 10 ppb Increase in 12-month Moving Average NO2. All analyses were conducted using Cox Proportional Hazard models with strata for sex (male, female), race (White, non-White), age (1 year age categories with 90+ years old as one category), and ZIP code, and including ZIP code- and state-level SES as model covariates. Models were also adjusted for 12-month moving average PM2.5 using a 2-stage approach. The bold solid line represents the non-linear relationship estimated using restricted cubic spline (3 knots); the dotted line represents the 95% C.I.s for the non-linear association, and the muted solid line represents the linear association for (A) All-cause and non-accidental mortality, (B) Cardiovascular-related mortality, (C) Respiratory-related mortality, (D) Cancer and lung cancer mortality
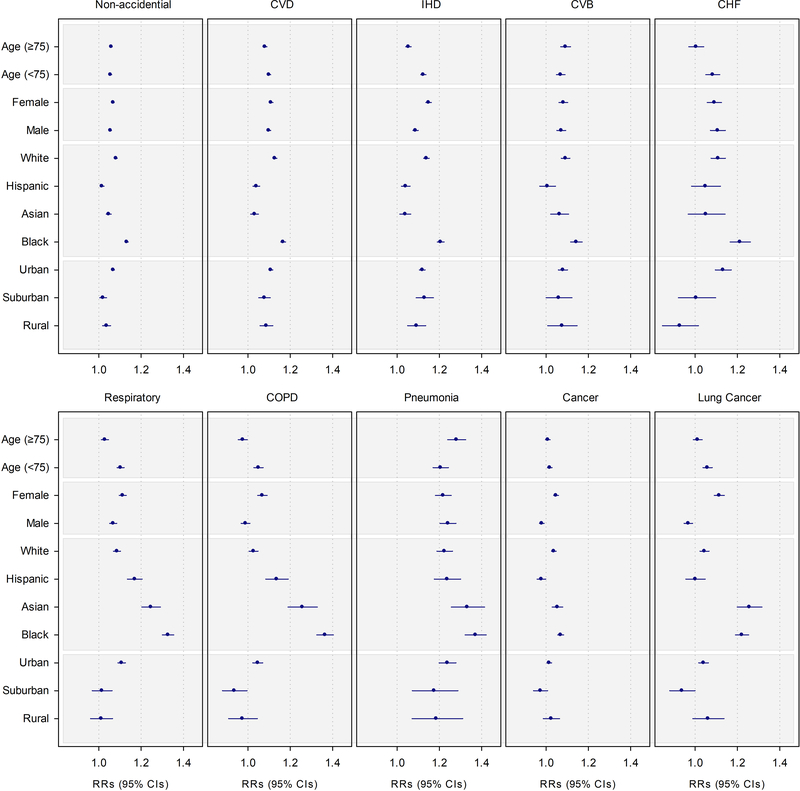
3.2. Effect Modification
Of the examined effect modifiers, race had the greatest impact on NO2-associated HRs (Figure 2, Table S6). PM2.5-adjusted HRs for Black, White, and Asian beneficiaries were positive and significant for most causes of death. Black beneficiaries had the highest NO2-associated risks of death, with HRs 1.3 to 1.9 times higher for CVD-related diseases, 3.7 to 12 times higher for respiratory-related disease, and 7 times higher for cancer and lung cancer, as compared to White beneficiaries (p-values for differences < 0.05). Likewise, Asian and Hispanic beneficiaries also had higher risks of respiratory and COPD mortality as compared to White beneficiaries (p-values for differences < 0.05). NO2-associated HRs for Black, Asian, and Hispanic, but not White beneficiaries, were higher for respiratory- as compared to CVD-related mortality. NO2-associated risks of lung cancer were highest for Black and Asian, and while lower, were also significant and positive for White beneficiaries. NO2-associated risks of lung cancer for Hispanic beneficiaries were null. When we limited our analyses to beneficiaries living in urban ZIP codes, we found similar pattern of associations by race (Table S7).
Figure 2.
Mortality Hazard Ratio (95% CI) Associated with a 10 ppb Increase in 12-month Moving Average NO2 Exposure by Beneficiary Characteristics. Analyses were based on Cox proportional hazard models with strata for sex (male, female), race (White, non-White), age (1 year age categories with 90+ years old as one category), and ZIP code, and including ZIP code- and state-level SES as model covariates. Models were also adjusted for 12-month moving average PM2.5 using a 2-stage approach.
The exposure-response curves for CVD, IHD, and CBV mortality were again supra-linear for all racial groups (Figure S3, S3a). However, the curves for Hispanic and Asian beneficiaries were positive and significant only until ~66th percentile of exposure (12 ppb), after which the associations were non-significant. The associations for lung cancer mortality were largely linear and positive for Asian and Black beneficiaries, but as in the linear analysis, were null for Hispanics. Conversely, the curves for all races for CHF, respiratory, and COPD mortality were sub-linear, with lower associations at lower levels of exposure. For cancer, NO2 exposures were significantly associated with mortality in all races other than Hispanics, for whom associations were null. (Table S6, Figure 2, S3, S3a).
HRs also varied by age, sex, and urbanicity. Associations for CVD, IHD, CHF, respiratory, COPD, and lung cancer were higher for older as compared to younger beneficiaries (Figure 2, Table S6). The curves for each age group largely mirrored those for the whole population (Figure S4, S4a). Associations for both sexes were significant and positive, except for COPD, cancer, and lung cancer mortality in men. HRs for men and women were comparable for all-CVD, CBV, and CHF, and all respiratory mortality. For other causes of death, HRs were higher for women as compared to men (Table S6, Figure 2). Exposure-response curves were similar to those we observed by age (Figure S5, S5a).
NO2-associated mortality risks were higher among beneficiaries living in urban, as compared to non-urban ZIP codes for non-accidental, CHF, and respiratory-related causes of death (Table S6, Figure 2). In contrast, HRs for CVD, IHD, CHF, cancer, and lung cancer mortality were similar regardless of urbanicity. As with age and sex, exposure-response curves were largely linear for all-cause, lung cancer, and pneumonia, supra-linear for CVD, IHD, and CBV, and sub-linear for CHF, respiratory, and COPD mortality (Figure S6, S6a).
4. Discussion
In our cohort of Medicare beneficiaries, we found that long-term exposure to NO2 was associated with increased mortality from all causes as well as from specific causes related to CVD, respiratory diseases, and cancer. Significant associations remained after adjusting for PM2.5 and BC, suggesting the adverse effect of NO2 is independent of that from PM2.5 and BC. Risks varied by beneficiary characteristics, including race, age, urbanicity, and to a lesser extent by sex. Importantly, we found no evidence of a threshold in NO2-associated HRs on mortality from non-accidental, CVD, respiratory and lung cancer mortality, with significant and positive associations across the range of examined NO2 exposures.
Our findings of increased mortality risk are consistent with those from previous studies (Beelen et al. 2008; Crouse et al. 2015; Cesaroni et al. 2013; Eum et al. 2019; Filleul et al. 2005; Fischer et al. 2015; Gehring et al. 2006; Hart et al. 2011; Heinrich et al. 2013; Katanoda et al. 2011; Krewski et al. 2000; Schikowski et al. 2007; Turner et al. 2016; Zhang et al. 2011), including our earlier study of Medicare beneficiaries living near air pollution monitoring sites (Eum et al. 2019) (Figure S7). As in our prior study, we found an increased risk of non-accidental mortality, CVD, IHD, CBV, cancer, respiratory disease, and pneumonia (Eum et al. 2019; Figure S7), with similar HRs as found previously for non-accidental mortality (HR: 1.06 vs. 1.04), CVD (HR: 1.10 vs. 1.11), CBV (HR: 1.08 vs. 1.05), all cancer (HR: 1.01 vs. 1.02), pneumonia (HR: 1.23 vs. 1.29), and respiratory disease (HR: 1.09 vs. 1.03). In contrast, we found statistically significant increased risk of COPD (HR: 1.03; 95% CI: 1.01–1.05) and lung cancer (HR: 1.03; 95% CI: 1.01–1.05) mortality in our present study, for which associations were null in our prior study, possibly due to its smaller sample size. Similarly, other studies (Figure S7) also found the increased risk of non-accidental mortality (or all cause mortality), CBV, respiratory disease, COPD, or lung cancer (Beelen et al. 2008; Crouse et al. 2015; Cesaroni et al. 2013; Filleul et al. 2005; Fischer et al. 2015; Gehring et al. 2006; Hart et al. 2011; Heinrich et al. 2013; Katanoda et al. 2011; Krewski et al. 2000; Schikowski et al. 2007; Turner et al. 2016; Zhang et al. 2011). We also newly found a significant 10% increased risk of CHF mortality (HR: 1.10; 95% CI: 1.06–1.13), an association which has only rarely been explored in prior literature, given that CHF comprises only a small proportion (3%) of overall deaths. It is also notable that adjustment for PM2.5 resulted in attenuated effect estimates across all mortality outcomes, and the impact of this adjustment was greater in rural environments than in urban environments (Table S6, S8). This may be due to differences in the correlation of NO2 and PM2.5 (Beelen et al. 2014; Monn et al. 1995; Putaud et al. 2010), in PM2.5 composition (Kulshreshtha et al. 2014), and/or mortality outcome ascertainment (Kulshreshtha et al. 2014) between urban and non-urban environments.
Our findings were insensitive to model specifications, with similar patterns of association in models additionally adjusting for year or month (Table S4), although confounding by weather remains possible, given that both mortality and NO2 are associated with weather (Marti-Soler and Marques-Vidal 2015; Braga et al. 2002), which warrants further study.
We showed the shape of the exposure-response curve for NO2 and mortality to vary by aggregate cause of death groupings (e.g. cardiovascular, respiratory), but to be generally consistent within these groupings. For example, we found linear associations for non-accidental and lung cancer mortality, supra-linear associations for all CVD, IHD, and CBV mortality, and sub-linear (but still significant and positive) associations for all respiratory and COPD mortality. These findings add substantially to the scientific literature, as to date only two studies have examined the shapes of the exposure-response curves for NO2 and mortality, with mixed results. As in our study, Cesaroni et al. (Cesaroni et al. 2013) found associations to be linear for non-accidental and lung cancer mortality and supra-linear for IHD mortality, but showed a linear association for CVD mortality. In contrast, in a Canadian cohort, Crouse et al. (Crouse et al. 2015) reported a supra-linear pattern of association for all-cause mortality, with higher risks below as compared to above approximately 10 ppb. Lack of adjustment for PM2.5 in the previous studies may explain the different findings (Crouse et al. 2015; Cesaroni et al. 2013). Neither study examined the impact of NO2 on other causes of death, or the shape of the exposure-response curve for different sub-populations based on demographic characteristics or place of residence.
We found both demographic characteristics and place of residence to be important modifiers of the NO2-mortality association. Of these factors, we found race to have the largest impact on NO2-associated HRs, with minority, especially Black beneficiaries being particularly susceptible to the impacts of NO2. Higher HRs for minority may result from their higher NO2 exposures, with Asians having the highest exposures, followed by Hispanic and Black beneficiaries, and finally White beneficiaries, consistent with previous studies (Clark et al. 2014; Grineski et al. 2007; Jones et al. 2014; Su et al. 2011). For respiratory mortality, higher NO2 exposures for minority beneficiaries corresponded to higher mortality risks, as evidenced by its sub-linear curve, with higher HRs for NO2 exposures above 12 ppb. Correspondingly, we found lower HRs for CVD mortality among Asian and Hispanic as compared to White beneficiaries despite their higher overall exposure, which is consistent with our findings of lower mortality risks at higher NO2 exposures for CVD mortality. Notably, Black beneficiaries had the highest CVD mortality risks of all racial groups, possibly reflecting the fact that their average NO2 exposures, while higher than that for Whites, were below the inflection point of our exposure-response curves, for which HRs were higher. Alternatively, high CVD mortality risks may also be result from the influence of other, unmeasured factors. While ours is the first study to examine modification of the NO2-mortality association by race, our findings are indirectly supported by prior studies showing Black persons to have higher PM2.5-related mortality risks (Di et al. 2017; Wang et al. 2017), as found in our study of PM2.5 and cause-specific mortality in the Medicare cohort (Wang et al. 2020). We note that our findings of effect modification by race does not control for individual level socioeconomic status, which is closely tied with race/ethnicity in the United States (Tibuakuu et al. 2018). Since individual-level race/ethnicity data were not available in our study, our effect modification analyses controlled for ZIP code-level SES and were restricted to beneficiaries living in urban areas, given that the majority of non-White beneficiaries lived in urban areas. However, given the lack of control for individual level SES, our findings of effect modification by race may reflect the combined impacts of both race/ethnicity and SES, or as suggested by Gwynn and Thurston (Gwynn and Thurston 2001) and Grineski et al. (Grineski et al. 2010), modification by SES instead. Future analyses should control or conduct stratified analyses using individual-level SES data to better understand whether and how NO2-associated mortality impacts differ separately or jointly by race and SES.
Associations of NO2 and mortality were also significantly modified by urbanicity. Urban beneficiaries showed generally greater mortality risks than their rural counterparts, consistent with their higher mean NO2 exposures (Table 1) and higher proportion of minorities (18.75%, 9.22%, 8.4% of Black, Asian and Hispanic among urban population versus 9.09%, 1.03%, 1.62% among the whole study population). There findings are consistent with a previous Minnesota study, which found minorities and lower SES urban populations to be exposed to higher levels of traffic-related air pollution and to be at higher health risk, than those living in rural environments (Want et al. 2017). Together, our findings suggest that the impact of NO2 on mortality vary by both race and urbanicity, underpinning the importance of estimating risk among representative sub-populations.
While our study adds to the literature showing significant and positive associations between NO2 and mortality for several causes of death, the biological mechanism through which long- term NO2 exposures may independently cause mortality is not well understood. It is possible, however, that observed NO2-associated sub-clinical effects contribute over long time periods to increased mortality. NO2 exposures, for example, have been shown to cause lipid peroxidation in cell membranes and damage to structural and functional molecules by the release of free radicals (Sandström, 1995). Consistent with this, NO2 exposures have been associated with pulmonary inflammation, bronchial hyperresponsiveness, and increased risk of respiratory infection in toxicological studies (Koenig, 2000), while epidemiological findings show associations between NO2 exposure and increased systemic inflammation and blood pressure and decreased pulmonary function (Gao et al. 2020; Lepeule et al. 2014). Importantly, these NO2-associated sub-clinical impacts have been shown to be independent of PM2.5.
It is possible that these NO2-associated adverse effects contribute over long time periods to increased mortality, as has been observed in ours and other epidemiological studies. Importantly, our findings suggest independent impacts of NO2 on mortality, given that NO2 remained significantly and positively associated with mortality even after adjustment for PM2.5 and BC. However, it is possible that some unmeasured confounding by PM2.5 and/or BC remains.
Our study has several limitations. First, while we used ambient NO2 and BC estimates based upon validated models, exposure misclassification is likely (Bechle et al. 2015; Yanosky et al. 2014). As stated in Bechle et al. (Bechle et al. 2015), exposure error was similar in areas near versus far from EPA monitoring sites and was not dependent on urbanicity, which was associated with mortality in our study and in others (House et al. 2000). Since the vast majority of our cohort lived in urban areas, exposure misclassification in our study was likely non-differential, and any bias in effect sizes in our study would likely be towards the null (Hart et al. 2015a, Zeger et al. 2000). Support for this is provided by previous epidemiological studies that corrected for measurement error using calibration factors that accounted for differences between personal exposures and corresponding ambient concentrations, finding an increase in the association’s magnitude when the exposure measurement error corrections were made (Avery et al. 2010a; Avery et al. 2010b; Hart et al. 2015b). Consistent with this, when we additionally adjusted for urbanicity, associations were similar, supporting the robustness of the findings among the whole population. We note, however, that since the error associated with NO2 estimates in rural areas was relatively larger, our associations of 1-year NO2 exposures and cause-specific mortality for rural populations may be more vulnerable to exposure misclassification. Second, while our analyses controlled for individual covariates, including age, sex, race, and residential ZIP code, and ZIP code- and state-level SES, we were unable to adjust for individual-level SES or behavioral confounders, given our reliance on Medicare data, which lack this information. Despite this, confounding by individual-level SES or behavioral characteristics is unlikely to explain our findings, given results from Krewski et al. (Krewski et al. 2009) which found little change in air pollution-associated mortality risk estimates after adjustment for individual-level characteristics. Nonetheless, residual confounding by unmeasured covariates, such as individual-level SES remains a possibility (Tibuakuu et al. 2018). Third, our data included only older adults living in the U.S. between 2000 and 2008, limiting generalizability to younger or non-U.S. populations and to a lesser extent more recent time periods. While the gender, race and age composition of the US older adult population has remained largely unchanged from 2008 to now (U.S Census Bureau, 2008; U.S Census Bureau, 2019), annual NO2 concentrations have decreased in the US across this same time period. As a result, NO2-associated mortality risks at the high end of the NO2 concentration distribution may be less applicable to the current US older adult population, given that fewer US older adults experience long-term NO2 exposures of this magnitude. Our findings for lower NO2 exposures, however, are generalizable to today’s US older adults, as evidenced by our exposure-response curves, which show 1-year moving average NO2 exposures to be associated with higher mortality risks at concentrations below 12 ppm with very low associated uncertainty. Fourth, we used a two-stage model to adjust for PM2.5 given collinearity between ambient PM2.5 and NO2 concentrations, which may overcontrol for PM2.5 concentrations and underestimate NO2-associated mortality risks. Support for this theory is provided by our findings from a two-pollutant model that includes both NO2 and PM2.5, which showed greater effects for NO2 as compared to that found in our two-stage model. These findings are consistent with an independent effect of long-term NO2 exposures on mortality. While our findings may also reflect the ability of NO2 to serve as a better proxy of combustion-related PM2.5, as has been found in previous studies (Pope et al. 1995), our finding of similar HRs for NO2 in single and BC-adjusted models suggests this to not be the case.
These limitations are balanced by our study’s substantial strengths, including its large size and its ZIP code-specific pollution estimates, which provide ample statistical power to estimate associations and exposure-response curves in understudied populations. Our study provides strong evidence of an association between NO2 and increased all-cause and cause-specific mortality in U.S. older adults, especially for racial minorities and older adults living in urban areas. Black beneficiaries, those living in urban environments, and those >75 years were found to be most susceptible. Given that NO2 exposures were well below the annual NAAQS, our findings suggest that its current annual NAAQS does not sufficiently protect public health.
Supplementary Material
Highlights.
Long-term NO2 exposure was associated with increased nonaccidental mortality, as well as mortality from cardiovascular disease, respiratory disease, and cancer.
NO2-mortality associations were without a lower threshold, suggesting that any increase in long-term NO2 exposures is associated with elevated mortality risk.
The increased mortality associated with NO2 exposure was considerably higher among the Black beneficiaries than other races.
Funding:
This work was supported by NIEHS grant R01ES022657-01A1 and Electric Power Research Institute grant (EPRI 00-10003095).
Abbreviations
- NAAQS
National Ambient Air Quality Standard
- NO2
Nitrogen dioxide
- PM2.5
Particulate matter with an aerodynamic diameter less than 2.5 μm
- HR
Hazard ratio
- CI
Confidence interval
- ICD
International Classification of Disease
- CVD
Cardiovascular disease
- IHD
Ischemic heart disease
- CBV
Cerebrovascular disease
- CHF
Congestive heart failure
- COPD
Chronic obstructive pulmonary disease
- LUR
land-use regression
- RUCA
Rural health research center
- IRS
Internal Revenue Service
- SD
standard deviation
Footnotes
Conflict of Interest Disclosures: The authors declare that they have no known competing financial interests or personal relationships that could appeared to influence the work reported in the paper.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Human subjects research committee approvals: This study was approved by the Institutional Review Boards of Northeastern University and Tufts University.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Avery CL, Mills KT, Williams R, et al. 2010a. Estimating error in using residential outdoor pm2.5 concentrations as proxies for personal exposures: A meta-analysis. Environ Health Perspect. 118:673–678. 10.1289/ehp.0901158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery CL, Mills KT, Williams R, et al. 2010b. Estimating error in using ambient pm2.5 concentrations as proxies for personal exposures: A review. Epidemiology 21:215–223. 10.1097/ede.0b013e3181cb41f7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechle MJ, Millet DB, Marshall JD, 2015. National spatiotemporal exposure surface for NO2: Monthly scaling of a satellite-derived land-use regression, 2000–2010. Environ Sci Technol. 49(20):12297–12305. 10.1021/acs.est.5b02882 [DOI] [PubMed] [Google Scholar]
- Beelen R, Hoek G, van den Brandt PA, et al. , 2008. Long-term effects of traffic-related air pollution on mortality in a Dutch cohort (NLCS-AIR study). Environ Health Perspect. 116(2):196–202. 10.1289/ehp.10767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beelen R, Raaschou-Nielsen O, Stafoggia M, et al. , 2014. Effects of long-term exposure to air pollution on natural-cause mortality: An analysis of 22 European cohorts within the multicentre ESCAP project. Lancet 383(9919):785–795. 10.1016/s0140-6736(13)62158-3 [DOI] [PubMed] [Google Scholar]
- Braga AL, Zanobetti A, Schwartz J, 2002. The effect of weather on respiratory and cardiovascular deaths in 12 U.S. cities. Environ Health Perspect. 110(9):859–63 10.1289/ehp.02110859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesaroni G, Badaloni C, Gariazzo C, et al. , 2013. Long-term exposure to urban air pollution and mortality in a cohort of more than a million adults in Rome. Environ Health Perspect. 121(3):324–331. 10.1289/ehp.1205862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark LP, Millet DB, Marshall JD, 2014. National patterns in environmental injustice and inequality: Outdoor NO2 air pollution in the United States. PLoS One 9:e94431. 10.1371/journal.pone.0094431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouse DL, Peters PA, Hystad P, et al. , 2015. Ambient PM2.5, O3, and NO2 exposures and associations with mortality over 16 years of follow-up in the canadian census health and environment cohort (CanCHEC). Environ Health Perspect. 123(11):1180–1186. 10.1289/ehp.1409276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Q, Wang Y, Zanobetti A, et al. , 2017. Air pollution and Mortality in the Medicare Population. N Engl J Med. 376(26):2513–2522. 10.1056/nejmoa1702747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eum KD, Kazemiparkouhi F, Wang B, et al. , 2019. Long-term NO2 exposures and cause-specific mortality in american older adults. Environ Int. 124:10–15. 10.1016/j.envint.2018.12.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filleul L, Rondeau V, Vandentorren S, et al. , 2005. Twenty five year mortality and air pollution: Results from the French PAARC survey. Occup Environ Med. 62:453–460. 10.1136/oem.2004.014746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer PH, Marra M, Ameling CB, et al. , 2015. Air Pollution and Mortality in Seven Million Adults: The Dutch Environmental Longitudinal Study (DUELS). Environ Health Perspect. 123(7):697–704. doi: 10.1289/ehp.1408254. Epub 2015 Mar 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring U, Heinrich J, Krämer U, et al. , 2006. Long-term exposure to ambient air pollution and cardiopulmonary mortality in women. Epidemiology. 17(5):545–51. doi: 10.1097/01.ede.0000224541.38258.87. [DOI] [PubMed] [Google Scholar]
- Gao N, Xu W, Ji J, et al. , 2020. Lung function and systemic inflammation associated with short-term air pollution exposure in chronic obstructive pulmonary disease patients in Beijing, China. Environ Health. 19(1):12. doi: 10.1186/s12940-020-0568-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grineski S, Bolin B, Boone C, 2007. Criteria air pollution and marginalized populations: Environmental inequity in metropolitan Phoenix, Arizona. Soc Sci Quart. 88(2):535–554. 10.1111/j.1540-6237.2007.00470.x [DOI] [Google Scholar]
- Grineski SE, Staniswalis JG, Peng Y, et al. , Children’s asthma hospitalizations and relative risk due to nitrogen dioxide (NO2): effect modification by race, ethnicity, and insurance status. Environ Res. 2010;110(2):178–188. doi: 10.1016/j.envres.2009.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwynn RC, Thurston GD. 2001. The burden of air pollution: impacts among racial minorities. Environ Health Perspect. 109 Suppl 4(Suppl 4):501–506. doi: 10.1289/ehp.01109s4501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart JE, Garshick E, Dockery DW, et al. , 2011. Long-term ambient multipollutant exposures and mortality. Am J Respir Crit Care Med. 183(1):73–78. 10.1164/rccm.200912-1903oc [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart JE, Liao X, Hong B, et al. , 2015a. The association of long-term exposure to pm2.5 on all-cause mortality in the nurses’ health study and the impact of measurement-error correction. Environ Health. 14:38. 10.1186/s12940-015-0027-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart JE, Spiegelman D, Beelen R, et al. , 2015b. Long-term ambient residential traffic-related exposures and measurement error-adjusted risk of incident lung cancer in the netherlands cohort study on diet and cancer. Environ Health Perspect. 123:860–866. 10.1289/ehp.1408762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich J, Thiering E, Rzehak P, et al. , 2013. Long-term exposure to NO2 and PM10 and all-cause and cause-specific mortality in a prospective cohort of women. Occup Environ Med. 70:179–186. 10.1136/oemed-2012-100876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- House JS, Lepkowski JM, Williams DR, et al. , 2000. Excess mortality among urban residents: how much, for whom, and why? Am J Public Health. 90(12):1898–1904. 10.2105/ajph.90.12.1898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Internal Revenue Service, SOI Tax Stats - Individual Income Tax Statistics - ZIP Code Data (SOI), accessed at https://www.irs.gov/statistics/soi-tax-stats-individual-income-tax-statistics-zip-code-data-soi on 23 June 2021.
- Jones MR, Diez-Roux AV, Hajat A, et al. , 2014. Race/ethnicity, residential segregation, and exposure to ambient air pollution: The Multi-Ethnic Study of Atherosclerosis (MESA). Am J Public Health. 104(11):2130–2137. 10.2105/ajph.2014.302135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katanoda K, Sobue T, Satoh H, et al. , 2011. An association between long-term exposure to ambient air pollution and mortality from lung cancer and respiratory diseases in japan. J Epidemiol. 21:132–143. 10.2188/jea.je20100098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig JQ 2000. Health Effects of Nitrogen Dioxide. In: Health Effects of Ambient Air Pollution. Springer, Boston, MA. 10.1007/978-1-4615-4569-9_12 [DOI] [Google Scholar]
- Krewski D, Burnett RT, Goldberg M, et al. , 2000. Reanalysis of the Harvard six-cities study and the American Cancer Society study of air pollution and mortality, phase II: sensitivity analysis. Res Rep Health Eff Inst. 295 [Google Scholar]
- Krewski D, Jerrett M, Burnett RT, et al. , 2009. Extended follow-up and spatial analysis of the American Cancer Society study linking particulate air pollution and mortality. Res Rep Health Eff Inst. 5–114; discussion 115–136. [PubMed] [Google Scholar]
- Kulshreshtha A, Goyal A, Dabhadkar K, et al. , 2014. Urban-rural differences in coronary heart disease mortality in the United States: 1999–2009. Public Health Rep. 129:19–29. 10.1177/003335491412900105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepeule J, Bind MA, Baccarelli AA, et al. , 2014. Epigenetic influences on associations between air pollutants and lung function in elderly men: the normative aging study. Environ Health Perspect. 122(6):566–72. doi: 10.1289/ehp.1206458. Epub 2014 Mar 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marti-Soler H, Marques-Vidal P, 2015. Weather and cardiovascular mortality. Heart 101:1941–1942. 10.1136/heartjnl-2015-308613 [DOI] [PubMed] [Google Scholar]
- Monn CH, Braendli O, Schaeppi G, et al. , 1995. Particulate matter <10 mu m (PM10) and total suspended particulates (TSP) in urban, rural and alpine air in Switzerland. Atmos Environ. 29(19):2565–2573. https://ui.adsabs.harvard.edu/link_gateway/1995AtmEn..29.2565M/doi:10.1016/1352-2310(95)94999-U [Google Scholar]
- Pope CA 3rd, Thun MJ, Namboodiri MM, et al. , 1995. Particulate air pollution as a predictor of mortality in a prospective study of U.S. adults. Am J Respir Crit Care Med. 151(3 Pt 1):669–74. doi: 10.1164/ajrccm/151.3_Pt_1.669 [DOI] [PubMed] [Google Scholar]
- Putaud JP, Van Dingenen R, Alastuey A, et al. , 2010. A European aerosol phenomenology-3: Physical and chemical characteristics of particulate matter from 60 rural, urban, and kerbside sites across europe. Atmos Environ. 44(10):1308–1320. 10.1016/j.atmosenv.2009.12.011 [DOI] [Google Scholar]
- Sandström T 1995. Respiratory effects of air pollutants: experimental studies in humans. Eur Respir J. 8(6):976–95. [PubMed] [Google Scholar]
- Schikowski T, Sugiri D, Ranft U, et al. , 2007. Does respiratory health contribute to the effects of long-term air pollution exposure on cardiovascular mortality? Resp Res 8(1):20. 10.1186/1465-9921-8-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su JG, Jerrett M, de Nazelle A, et al. , 2011. Does exposure to air pollution in urban parks have socioeconomic, racial or ethnic gradients? Environ Res. 111(3):319–328. 10.1016/j.envres.2011.01.002 [DOI] [PubMed] [Google Scholar]
- Tibuakuu M, Michos ED, Navas-Acien A, et al. , Air Pollution and Cardiovascular Disease: A Focus on Vulnerable Populations Worldwide. Curr Epidemiol Rep. 2018. Dec;5(4):370–378. doi: 10.1007/s40471-018-0166-8. Epub 2018 Sep 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner MC, Jerrett M, Pope CA, et al. , 2016. Long-term ozone exposure and mortality in a large prospective study. Am J Respir Crit Care Med. 193(10):1134–1142. 10.1164/rccm.201508-1633oc [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Census Bureau, Current Population Survey, Annual Social and Economic Supplement, 2019. Accessed at https://www.census.gov/topics/population/race/data/tables.2019.html on 23 June 2021.
- U.S. Census Bureau, Current Population Survey, Annual Social and Economic Supplement, 2008. Accessed at https://www.census.gov/topics/population/race/data/tables.2008.html on 23 June 2021.
- U.S. Environmental Protection Agency, 2019. Applying or Implementing Nitrogen Dioxide Standards. Accesed at https://www.epa.gov/no2-pollution/applying-or-implementing-nitrogen-dioxide-standards on 28 October 2019.
- van Donkelaar A, Martin RV, Li C, Burnett RT. Regional Estimates of Chemical Composition of Fine Particulate Matter Using a Combined Geoscience-Statistical Method with Information from Satellites, Models, and Monitors. Environ Sci Technol. 2019. Mar 5;53(5):2595–2611. doi: 10.1021/acs.est.8b06392. Epub 2019 Feb 12. [DOI] [PubMed] [Google Scholar]
- Wang B, Eum KD, Kazemiparkouhi F, et al. , 2020. The impact of long-term PM2.5 exposure on specific causes of death: Exposure-response curves and effect modification among 53 million u.S. Medicare beneficiaries. Environ Health. 19(1):20. 10.1186/s12940-020-00575-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Shi L, Lee M, et al. , 2017. Long-term exposure to PM2.5 and mortality among older adults in the Southeastern US. Epidemiology 28(2):207–214. 10.1097/ede.0000000000000614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanosky JD, Paciorek CJ, Laden F, et al. , 2014. Spatio-temporal modeling of particulate air pollution in the conterminous united states using geographic and meteorological predictors. Environ Health. 13(1):63. 10.1186/1476-069x-13-63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeger SL, Thomas D, Dominici F, et al. , 2000. Exposure measurement error in time-series studies of air pollution: Concepts and consequences. Environ Health Perspect 108:419–426. 10.1289/ehp.00108419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Dong G, Sun B, et al. , 2011. Long-term exposure to ambient air pollution and mortality due to cardiovascular disease and cerebrovascular disease in Shenyang, China. PLoS One 6(6):e20827. 10.1371/journal.pone.0020827 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.