Abstract
Copper is an essential nutrient whose redox properties make it both beneficial and toxic to the cell. Recent progress in studying transition metal signalling has forged new links between researchers of different disciplines that can help translate basic research in the chemistry and biology of copper into clinical therapies and diagnostics to exploit copper-dependent disease vulnerabilities. This concept is particularly relevant in cancer, as tumour growth and metastasis have a heightened requirement for this metal nutrient. Indeed, the traditional view of copper as solely an active site metabolic cofactor has been challenged by emerging evidence that copper is also a dynamic signalling metal and metalloallosteric regulator, such as for copper-dependent phosphodiesterase 3B (PDE3B) in lipolysis, mitogen-activated protein kinase kinase 1 (MEK1) and MEK2 in cell growth and proliferation and the kinases ULK1 and ULK2 in autophagy. In this Perspective, we summarize our current understanding of the connection between copper and cancer and explore how challenges in the field could be addressed by using the framework of cuproplasia, which is defined as regulated copper-dependent cell proliferation and is a representative example of a broad range of metalloplasias. Cuproplasia is linked to a diverse array of cellular processes, including mitochondrial respiration, antioxidant defence, redox signalling, kinase signalling, autophagy and protein quality control. Identifying and characterizing new modes of copper-dependent signalling offers translational opportunities that leverage disease vulnerabilities to this metal nutrient.
Cell proliferation is a fundamental process that is essential for the development and homeostasis of multicellular organisms and leads to the exponential growth of tissue. In this context, aberrant elevations and/or deficiencies in cell proliferation are major contributors to injury, ageing and disease. Indeed, a prime example of uncontrolled cell proliferation is cancer, in which survival, proliferation and implantation in distant tissues are highly dependent on the ability to acquire adequate oxygen and nutrients within a range of hostile environments1.
Copper is a mineral nutrient that is increasingly implicated in cell proliferation and death pathways. The inherent oxidation–reduction (redox) property of copper makes it both beneficial and potentially toxic to the cell. Cu(II) and Cu(I) are the two physiologically relevant oxidation states of copper, with Cu(I) understood to be the predominant form in the reducing environment of the cell cytosol. Copper is a required cofactor for enzymes that mediate a host of essential cellular functions, including mitochondrial respiration, antioxidant defence and the biosynthesis of hormones, neurotransmitters and pigments, but at the same time dysregulation of copper stores can induce oxidative stress and cytotoxicity2–4. This traditional view of copper as solely an active site cofactor has been challenged by emerging evidence that copper is a dynamic signalling metal and metalloallosteric regulator that governs and coordinates biological activities in response to external stimuli5,6. Rapid progress in the field has forged new links between researchers in different disciplines that can help translate basic research in the chemistry and biology of copper into potential clinical therapies that exploit copper-dependent disease vulnerabilities, particularly in cancer.
To discuss our current understanding of the connection between copper and cancer and to explore how challenges in the field could be addressed, a group of leading researchers with chemical, biological and clinical perspectives on this topic were brought together for an interdisciplinary conference, the Copper Cancer Consortium, organized by Linda Vahdat, Mick Petris and Nick Tonks at the Banbury Center at Cold Spring Harbour Laboratory on 1–4 March 2020. Several common threads emerged from the meeting that are of general interest to the cancer and broader biomedical community. In particular, one unifying theme that crystallized from these discussions is the concept of cuproplasia, a newly recognized form of regulated copper-dependent cell proliferation.
Definition of cuproplasia
‘Cuproplasia’ is defined as copper-dependent cell growth and proliferation. This term encompasses both neoplasia and hyperplasia, describes both primary and secondary effects of copper via signalling pathways and includes both enzymatic and non-enzymatic copper-modulated activities. Cuproplasia can be pharmacologically targeted: copper signalling can be repressed by copper-selective chelators or can be activated with metal ionophores that elevate copper levels or spatially and temporally redistribute copper stores across cellular and subcellular pools. Cuproplasia can also be modulated by genetic and/or pharmacological manipulation of proteins involved in copper homeostasis. We propose that cuproplasia is one example of different forms of metalloplasia, as other metals, such as iron, can also regulate signalling pathways to promote iron-dependent cell proliferation (ferroplasia), akin to iron-dependent cell death (ferroptosis)7,8. Along the same lines, copper can also mediate cell death through cytotoxicity induced by increased mitochondrial-dependent energy metabolism and accumulation of reactive oxygen species (ROS) in a process termed ‘cuproptosis’.
Copper homeostasis
A central tenet regarding elemental nutrients is that they can be neither created nor destroyed, and like all metals, the total pool of intracellular copper is divided into two subsets: a tightly bound protein pool (micromolar) and a bioavailable labile pool (subfemtomolar)9,10. Advances in the biometals field have revealed key molecular pathways that regulate copper acquisition, trafficking, storage and export11–17 (FIG. 1). Key targets in mammalian copper homeostasis include ceruloplasmin as the predominant protein carrier for exchangeable copper in blood plasma, CTR1 (also known as SLC31A1) and related ion transporters for cellular copper uptake, cytoplasmic metallochaperones (ATOX1) and cytoplasmic–mitochondrial metallochaperones (CCS, SCO1, SCO2, COX11 and COX17) for targeted insertion of copper into metalloenzymes and the copper-dependent ATPases ATP7A and ATP7B, which possess both copper export and metallochaperone functions. Metallothionein 1 (MT1) and MT2, two of three isoforms of thiol-rich proteins with high affinity for binding multiple copper ions, are the presumptive analogues of the iron-storing protein ferritin. Together, these proteins maintain appropriate intracellular copper bioavailability and ensure the metallation of copper-dependent enzymes, including cytochrome c oxidase, superoxide dismutase and various oxygenase/oxidase enzymes, including tyrosinase, lysyl oxidase (LOX), dopamine β-hydroxylase and copper amine oxidases (TABLE 1).
Fig. 1 |. Overview of systemic and cellular copper homeostasis.
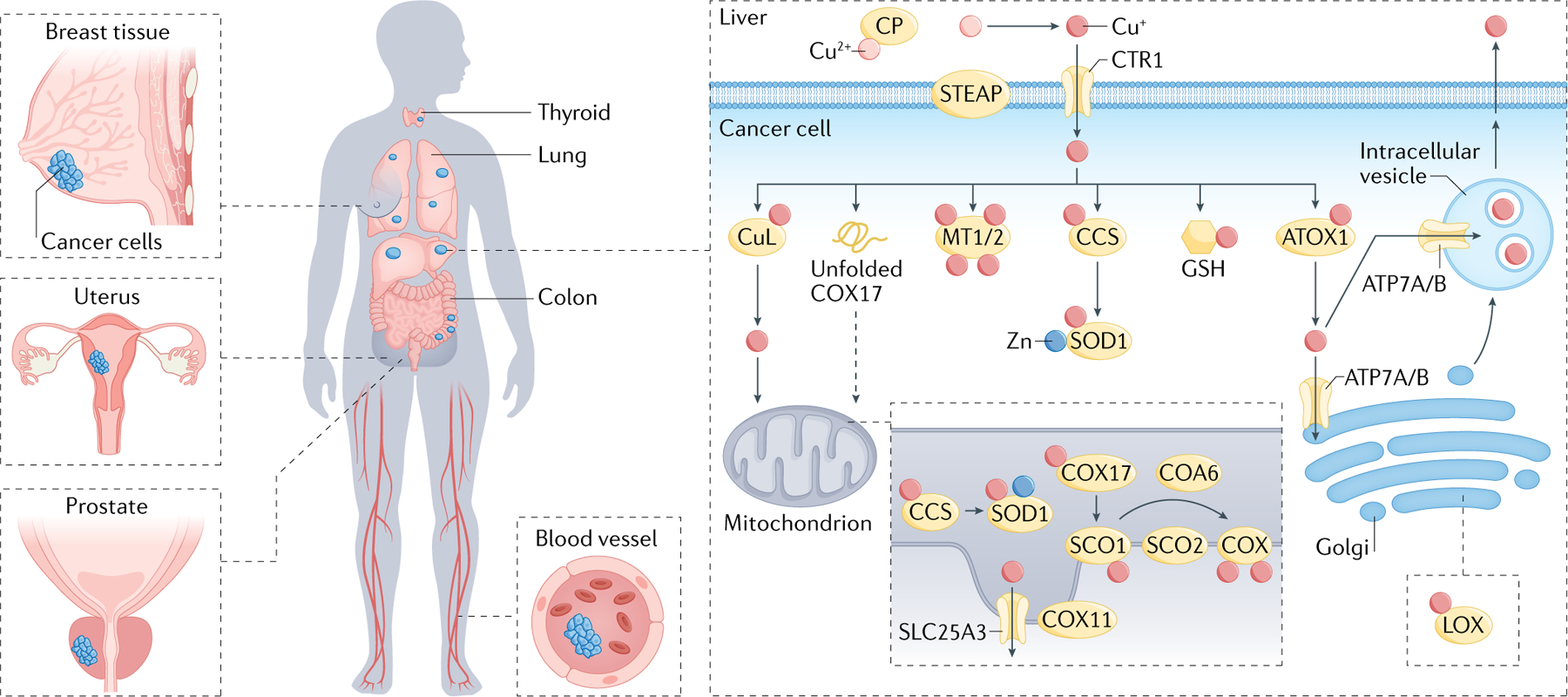
Human copper homeostasis involves a number of key molecular targets. Ceruloplasmin (CP) is the major protein carrier for exchangeable copper in blood plasma for circulation and delivery to organ and tissue systems. At the cellular level, the STEAP family of metalloreductases and copper ion channel copper transporter 1 (CTR1) enable high-affinity copper uptake, with a diverse array of cytoplasmic and mitochondrial metallochaperones (antioxidant protein 1 (ATOX1), copper chaperone for superoxide dismutase (CCS), synthesis of cytochrome oxidase 1 (SCO1), SCO2, copper chaperone for cytochrome c oxidase 11 (COX11), COX17, ATPase 7A (ATP7A) and ATP7B) working in concert to ensure targeted insertion of copper into metalloprotein. The ATP-driven transmembrane copper ion pumps ATP7A and ATP7B perform both copper export and metallochaperone functions. The thiol-rich proteins metallothionein 1 (MT1) and MT2 bind multiple copper ions and can serve as a copper storage reservoir. In addition, the abundant peptide and antioxidant glutathione (GSH) can also participate directly or indirectly in regulating cellular copper pools. Within the mitochondria, cytochrome c oxidase assembly factor 6 (COA6) and SCO2 help maintain the redox balance of SCO1 and in turn its copper binding and delivery to cytochrome c oxidase (COX). Together, these proteins maintain appropriate intracellular copper bioavailability and ensure metallation of copper-dependent enzymes, including COX, superoxide dismutase 1 (SOD1) and oxygenase/oxidase enzymes, including tyrosinase, lysyl oxidase (LOX), dopamine β-hydroxylase (DBH) and copper amine oxidases. Aberrant elevations in copper levels have been reported in tumours or serum of animal models and patients with various cancers, including breast, lung, gastrointestinal, oral, thyroid, gall bladder, gynaecologic and prostate cancers.
Table 1 |.
Genes involved in copper homeostasis and cuproplasia
| Gene | Name | Function |
|---|---|---|
| CP | Ceruloplasmin | Major exchangeable plasma Cu carrier |
| CTR1 (also known as SLC31A1) | Copper transporter 1 | High-affinity Cu importer |
| CTR2 (also known as SLC31A2) | Copper transporter 2 | CTR1 regulator |
| ATOX1 | Antioxidant protein 1 | Cytosolic Cu metallochaperone |
| CCS | Copper chaperone for superoxide dismutase | Cytosolic Cu metallochaperone |
| COX11 | Copper chaperone for cytochrome c oxidase 11 | Mitochondrial Cu metallochaperone |
| COX17 | Copper chaperone for cytochrome c oxidase 17 | Mitochondrial Cu metallochaperone |
| SCO1 | Synthesis of cytochrome oxidase 1 | Mitochondrial Cu metallochaperone |
| SCO2 | Synthesis of cytochrome oxidase 1 | Mitochondrial Cu metallochaperone |
| COA6 | Cytochrome c oxidase assembly factor 6 | Mitochondrial Cu metallochaperone |
| SLC25A3 | Phosphate carrier protein | Mitochondrial Cu importer |
| ATP7A | ATPase 7A | Cu exporter/Golgi apparatus Cu chaperone |
| ATP7B | ATPase 7B | Cu exporter/Golgi apparatus Cu chaperone |
| MT1 and MT2 | Metallothionein | Cu/Zn storage protein |
| COX1 and MT-CO2 | Subunits 1 and 2 of cytochrome c oxidase | Respiratory O2 reduction |
| SOD1 | Superoxide dismutase 1 | Superoxide scavenger |
| TYR | Tyrosinase | Tyrosine oxidation |
| LOXL2 | Lysyl oxidase like-protein 2 | Lysine oxidation |
| DBH | Dopamine β-hydroxylase | Dopamine oxidation |
| AOC3 (also known as VAP1) | Amine oxidase 3 (vascular adhesion protein 1) | Amine oxidation |
| MEK1 and MEK2 (also known as MAP2K1 and MAPK2) | Mitogen-activated protein kinase kinase 1/2 | Protein kinase |
| ULK1/ULK2 | Unc51-like kinase 1/2 | Protein kinase |
| PDK1 | 3-Phosphoinositide dependent protein kinase 1 | Protein kinase |
| PDE3B | Phosphodiesterase 3B | Cyclic AMP degradation |
| UBE2D1, UBE2D2, UBE2D3 and UBE2D4 | E2D ubiquitin conjugating enzyme E2 D1, D2, D3 and D4 | Ubiquitin conjugation to protein target |
| H3C1 and HC14 | Histone H3.1 and Histone H4 in the H3/H4 tetramer | Copper reductase |
| VEGFA | Vascular endothelial growth factor A | Growth factor |
| PD1L1 (also known as CD274) | Programmed cell death 1 Ligand 1 | Immune response control |
Systemic metabolism.
As an essential nutrient, copper is absorbed in the gastrointestinal tract from dietary sources. The recommended daily intake for adults is 0.9 mg, which is typically met or exceeded by the average Western diet18. Foods rich in copper include shellfish, seeds, nuts, organ meats and chocolate19. Dietary copper absorption occurs mainly in the small intestine, where copper uptake across the apical membrane of enterocytes is dependent on CTR1 and copper export across the basolateral membrane requires the protein ATP7A20 (FIG. 1). Within the portal circulation, serum proteins such as albumin deliver copper from the intestine to the liver, where excess copper is stored in hepatocytes by MT1 and MT2. The liver is also the major site of copper removal from the body via hepatobiliary excretion across the bile canalicular membrane of hepatocytes, which occurs via the copper exporter ATP7B21,22. The bulk of copper exported from hepatocytes into the systemic circulation is initially bound to ceruloplasmin, a secreted multicopper oxidase that receives copper via ATP7B-driven copper transport into the secretory pathway23. As ceruloplasmin is rapidly degraded when it is metal-free, its abundance in plasma is a biomarker of systemic copper deficiency24 that has been widely used in clinical trials of therapeutic copper depletion in patients with cancer. However, as ceruloplasmin is also an acute phase reactant, its use as a biomarker of systemic copper may be confounded during inflammatory conditions25. Despite constituting the vast bulk of copper in plasma, copper-bound ceruloplasmin is not essential for copper acquisition by peripheral tissues26. Rather, low molecular weight copper ligands such as amino acids, including cysteine, histidine, methionine, aspartic acid and glutamic acid, are thought to serve as plasma donors of copper to the uptake machinery in tissues25,27. Copper uptake in most tissues is thought to occur via the copper permease CTR1. As CTR1 is highly specific for reduced Cu(I)28,29, CTR1-dependent copper uptake is likely facilitated by the STEAP family of metalloreductases, which maintain copper in the reduced state30. Cellular copper balance is crucial for cellular maintenance and metabolism. This requirement is illustrated by Menkes disease, where mutations in ATP7A prevent the export of dietary copper from enterocytes into the bloodstream, causing a systemic copper deficiency characterized by developmental defects, including stunted growth, hypopigmentation, neurodegeneration and connective tissue defects31. By contrast, mutations in ATP7B cause Wilson disease, which is characterized by progressive hepatic copper overload that may induce liver failure or neuropsychiatric disease secondary to excess copper in the brain32. Although Menkes disease and Wilson diseases are rare, these disturbances in copper metabolism have firmly established the contribution of this metal to critical aspects of cellular pathophysiology.
Copper status in cancer
In the context of cancer, although numerous risk factors for hepatocellular carcinoma have been described, the increased incidence of hepatocellular carcinoma in patients with Wilson disease and animal models of Wilson disease evokes the possibility that aberrant copper accumulation may promote malignant transformation via an unknown mechanism33. Indeed, connections between copper and cancer have been noted for more than a century, with numerous observations pointing to a requirement for higher levels of copper for tumours compared with healthy tissue34.
Once growth and development are complete in mammals, the rates of gastrointestinal absorption and biliary excretion are balanced to maintain systemic copper homeostasis35. However, anabolic states such as pregnancy require significantly higher intakes of dietary copper to meet the metabolic demands for growth and development36. Maternal copper deficiency is known to cause early embryonic death or congenital malformations in mammals, depending on the timing and severity of the deficiency37. This situation is due, in part, to the requirement for copper as a cofactor of mitochondrial cytochrome c oxidase, which is necessary to meet the energy demands of rapidly dividing cells. Cancer cells thus have a higher demand for copper compared with non-dividing cells38. Elevated copper concentrations have been reported in the tumours or serum of animal models and patients with many types of cancers, including breast39–44, lung45–47, gastrointestinal48–53, oral54, thyroid55, gall bladder56, gynaecologic52,53 and prostate57 cancers. Copper imbalance can not only impact mitochondrial respiration but can also lead to changes in glycolysis, insulin resistance and lipid metabolism58–60. Beyond mitochondrial function, copper pathways, for example, the ATOX–ATP7A–LOX pathways, promote metastatic expansion61. In addition, copper regulation of autophagy via ULK1 and ULK2 (REF.62) and/or protein quality control via UBE2D2 (REF.63), provide new copper-dependent targets that can influence tumour growth and progression. Copper is also able to promote blood vessel formation that contributes to tumour initiation, growth and metastasis. This metal nutrient directly activates several proangiogenic factors, including vascular endothelial growth factor (VEGF), fibroblast growth factor 2 (FGF2), tumour necrosis factor (TNF) and interleukin-1 (IL-1).
Emerging mechanisms in cuproplasia
Although decades of research highlight the interplay between copper homeostasis, biology and medicine, the repertoire of known copper-dependent enzymes does not yield a complete molecular explanation for how biological systems convert copper abundance into distinct cellular functions. The ability of cells to initiate and/or maintain cellular processes is governed by signalling networks that facilitate intracellular and intercellular communication and in turn guide cellular responses. Whereas the signalling networks that integrate fluctuations in the abundance of growth factors, nutrients and metabolites are well established, the discovery of signalling molecules capable of mediating similar functions depending on copper availability is rudimentary. An emerging facet in nutrient sensing and protein regulation, termed ‘metalloallostery’6, whereby protein activity is regulated by dynamic copper binding at non-catalytic sites, has expanded our knowledge of the contributions of copper to signalling events and cellular processes. Indeed, identification of targets of copper metalloallostery and other mechanisms of dynamic metal nutrient regulation has given rise to a new field of transition metal signalling6. These advances have, in part, built upon foundational work in the development of copper-responsive fluorescent probes that have enabled identification of rapid changes in labile copper pools in response to biological stimuli across many different cell types64–73.
Recent studies illustrate the capacity of copper to serve as a positive or negative allosteric regulator of enzymatic activity (FIG. 2). One of the first examples of metalloallostery was uncovered in the search for mechanistic features that drive aberrant lipid metabolism in the context of diet-induced copper deficiency or liver copper hyperaccumulation in Wilson disease. Specifically, the level of cyclic AMP (cAMP) generated downstream of G protein-coupled receptor activation was potentiated in response to copper in a 3T3-L1 white adipocyte model67. Mechanistically, copper inhibited cAMP degradation by directly binding to a conserved cysteine residue in the phosphodiesterase 3B (PDE3B), which is responsible for cAMP-dependent breakdown of triglycerides into fatty acids and glycerol, leading to higher cAMP levels and lipolytic activity through copper-dependent PDE3B inhibition. In contrast to allosteric copper-mediated inhibition of enzyme function, ablation of CTR1 (also known as SLC31A1) reduced canonical RAF–mitogen-activated protein kinase kinase (MEK)–ERK signalling output at the level of ERK1/2 phosphorylation in response to proliferative signals in melanoma cell lines. More recently, copper treatment of cell cultures resulted in increased phosphorylation of the receptor tyrosine kinases TRKB, EGFR and MET upstream of MAPK signalling74–76. Epistatic and biochemical analysis revealed that copper acts on MEK1 and MEK2 and allosterically enhances the ability of MEK1 and MEK2 to phosphorylate ERK1 and ERK2 in a dose-dependent manner77. In addition, ablation in flies and mouse cells of Ctr1 (also known as Ctr1A) in flies) to decrease the protein levels or introduction of surface accessible mutations in Mek1 (also known as Map2k1) that disrupt copper binding decreased BrafV600E-driven signalling and tumour growth5,77. Furthermore, targeting the copper-dependent kinase activity of MEK1 and MEK2 with tetrathiomolybdate (TTM), a copper chelator used for the treatment of patients with Wilson disease, dampened the tumorigenesis of treatment-naive and resistant forms of BrafV600E-driven melanoma in mice5. Indeed, copper bioavailability was shown to be a KRAS-dependent vulnerability in colorectal cancer mouse models78. Beyond this first example of copper as a positive regulator of mammalian kinase activity, ULK1 and ULK2, downstream targets of the major nutrient-sensing kinase mechanistic target of rapamycin (mTOR), are also copper-dependent kinases62. Relief of ULK1 and/or ULK2 inhibition by the drug MRT68921 or by TTM or amino acid starvation-induced elevated kinase activity induced autophagy to salvage building blocks for macromolecular biosynthesis and bioenergetics that are scarce due to the nutrient-poor and oxygen-poor tumour microenvironment. Indeed, hepatic copper accumulation in Wilson disease is paralleled by activation of autophagy79. In addition, PDK1 activity is copper dependent, as shown in the colon cancer cell line DLD1 (REF.80). Finally, copper metalloallostery promoted protein degradation by allosteric activation of the E2 conjugating enzyme clade, UBE2D1–UBE2D4 (REF.63). As a result, numerous proteins became ubiquitin-tagged and degraded, notably including p53. Thus, the diminution of p53 by the oversupply of copper, as occurs in malignancy, could play a role in the inability of malignant cells to commit to programmed cell death.
Fig. 2 |. Copper metalloallostery and signalling promotes cell growth/proliferation and autophagy pathways.
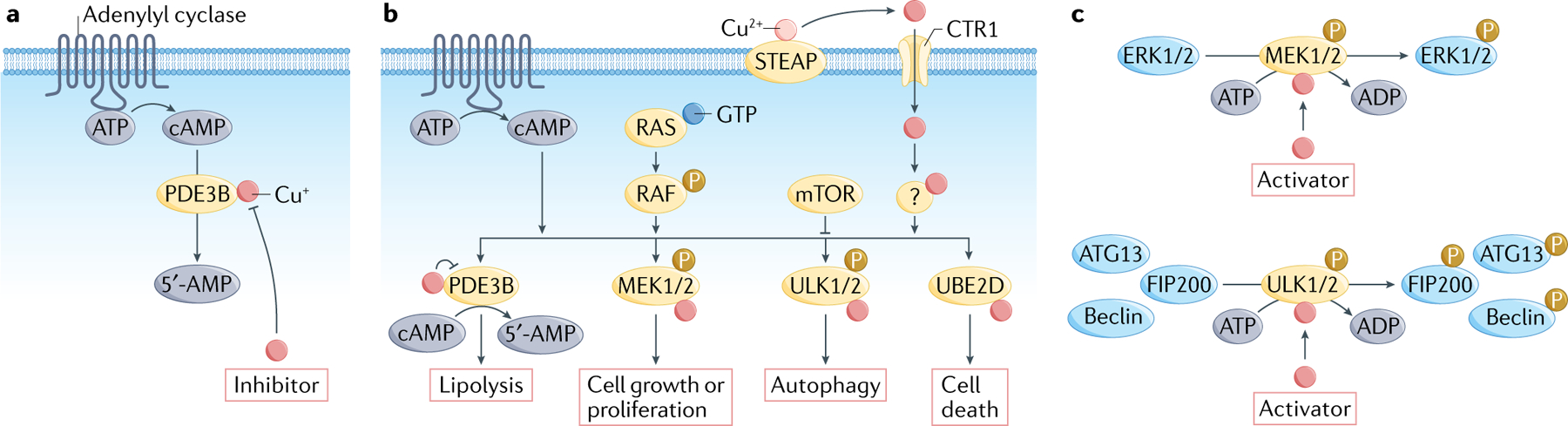
Besides the traditional role of copper as a static cofactor for protein function, emerging evidence shows that copper is able to serve as both a negative allosteric regulator and a positive allosteric regulator of enzyme activity to influence foundational cellular pathways. As an example of negative metalloallostery, copper binds and inhibits phosphodiesterase 3B (PDE3B) to inhibit cyclic AMP (cAMP) degradation (part a) and promote cAMP-dependent lipolysis (part c), the breakdown of triglycerides into fatty acids and glycerol that is essential for fat metabolism. In the context of positive metalloallostery, copper acts on mitogen-activated protein kinase kinase 1 (MEK1) and MEK2 and enhances their ability to phosphorylate extracellular signal-regulated kinase 1 (ERK1) and ERK2) (part b), stimulating RAF–MEK–ERK signalling (part c). Unc51-like kinase 1 (ULK1) and ULK2 provide a second example of copper-dependent kinase regulation, with copper able to relieve ULK1 and ULK2 inhibition and increase kinase activity in response to amino acid starvation (parts b,c). Finally, recent work has identified a role for copper signalling in promotion of protein degradation by positive allosteric activation of the E2 conjugating enzyme clade UBE2D1–UBE2D4 (part c). Therefore, copper-dependent kinase signalling can regulate cell growth/proliferation through MEK1 and MEK2 and autophagy through ULK1and ULK2 (part c). mTOR, mechanistic target of rapamycin.
Copper homeostasis has been shown to influence epigenetic regulators at the level of both chromatin modifications and transcription factors. Exposure of Hep3B human hepatoma cells to Cu2+ resulted in hypoacetylation on histones H3 and H4 via inhibition of histone acetyltransferases81. Excess copper can also damage mitochondria, a defect often observed in Wilson disease, with mitochondrial abnormalities disrupting the production of metabolites used for epigenetic regulation82,83.
Taken together, the discoveries described are beginning to mine the diverse mechanisms of copper sensing in which signalling components are, in part, dependent on copper. This dependency ensures that the activity of associated cellular processes is restricted to optimal environmental conditions17. These findings presage broader roles for metalloallostery and transition metal signalling in the proteome, particularly in the context of cancer.
Potential therapeutic strategies
Current clinical management of inherited disorders of copper dysregulation such as Menke disease, Wilson disease or CTR1 deficiency provides a foundation based on which safe, effective and rational approaches for the pharmacological modulation of cuproplasia can be considered. Coupled with recent advances in characterizing new copper-dependent signalling targets and pathways, there is growing interest in translating therapeutic strategies that can leverage copper-dependent disease vulnerabilities. In the context of cancer, we summarize the two major current approaches: chelators for copper depletion to inhibit cuproplasia and ionophores for copper supplementation to promote cuproptosis. In addition, we also forecast inhibitors and activators of copper metalloallostery as a new class of therapeutics that will emerge from basic science studies. Exploiting disease-dependent vulnerabilities of an endogenous nutrient such as copper for pharmacology has potential advantages for mitigating side effects associated with current chemotherapies. Indeed, chelation therapy is well tolerated for long-term use in chronic genetic diseases of copper misregulation84, whereas copper supplementation strategies can rely on native metal homeostasis pathways to tune therapeutic windows relative to exogenous metallotherapies such as platinum-based therapies85.
Copper chelation.
Copper chelators, which are typically used to lower elevated copper levels in the plasma of patients with Wilson disease, have been evaluated as antitumorigenic drugs in preclinical cancer models and phase I/II clinical trials86–89 (TABLE 2). These drug-repurposing pursuits were attempted following observations that (1) preventing blood vessel development with antiangiogenic agents limits cancer growth as demonstrated in patients90,91, (2) copper supplementation is sufficient to promote angiogenesis in rabbit models92,93 and (3) limiting copper availability diminishes the ability of known angiogenetic factors to stimulate blood vessel development87. Indeed, expression of VEGF is sensitive to copper, presumably through its ability to generate ROS94. On the basis of these findings, copper chelation as an antiangiogenic treatment has been translated in nearly every solid tumour and a few blood cancers86–89,95–101 (TABLE 2).
Table 2 |.
Pharmacological agents for modulating copper
| Therapy | Type | Status | Refs |
|---|---|---|---|
| d-Penicillamine | Chelator | Wilson disease (approved) | 175 |
| Trientine | Chelator | Wilson disease (approved) Melanoma (preclinical) |
84,86 |
| Tetrathiomolybdate | Chelator | Wilson disease (phase II) Breast cancer (phase II) |
87,88,95,176 |
| ATN-224 | Chelator | Wilson disease (phase III) Breast cancer (phase II) |
89,177 |
| Disulfuram | Ionophore | Alcohol abuse (approved) Glioblastoma (phase I/II) |
96,97,100,178 |
| Elesclomol | Ionophore | Melanoma (phase II) | 98,99,101 |
| Diacetylbis(N4-methylthiosemicarbazonato)copper(II) | Ionophore | Amyotrophic lateral sclerosis (phase II) | 179 |
| Glyoxalbis(N4-methyl-3-thiosemicarbazonato)copper(II) | lonophore | PET (preclinical) | 180 |
| Clioquinol | Ionophore | Fungal infection (approved) | 181 |
| 64Cu | Radiotherapy | Melanoma, glioblastoma, prostate cancer (preclinical) | 146,150, 151,182 |
| DC_AC50 | ATOX1/CCS inhibitor |
Melanoma (preclinical) | 168 |
PET, positron emission tomography.
The kinases MEK1 and MEK2 are attractive targets to exploit copper-dependent cancer vulnerability, as the RAF–MEK–ERK signalling cascade is one of the most well defined axes to promote cell proliferation and is mutated in most human cancers. Indeed, 40–50% of melanomas are initiated by mutations in the BRAF gene typically at V600, which constitutively activate the downstream kinases MEK1 and MEK2 to stimulate the MAPK pathway and promote cancer102. Given that the oncogenic effects of BRAF mutations are dependent on copper-stimulated MEK1 and MEK2 kinase activity, combination approaches that target both copper and kinase nodes are attracting interest103 (FIG. 3).
Fig. 3 |. Therapeutic strategies to target cuproplasia in cancer.
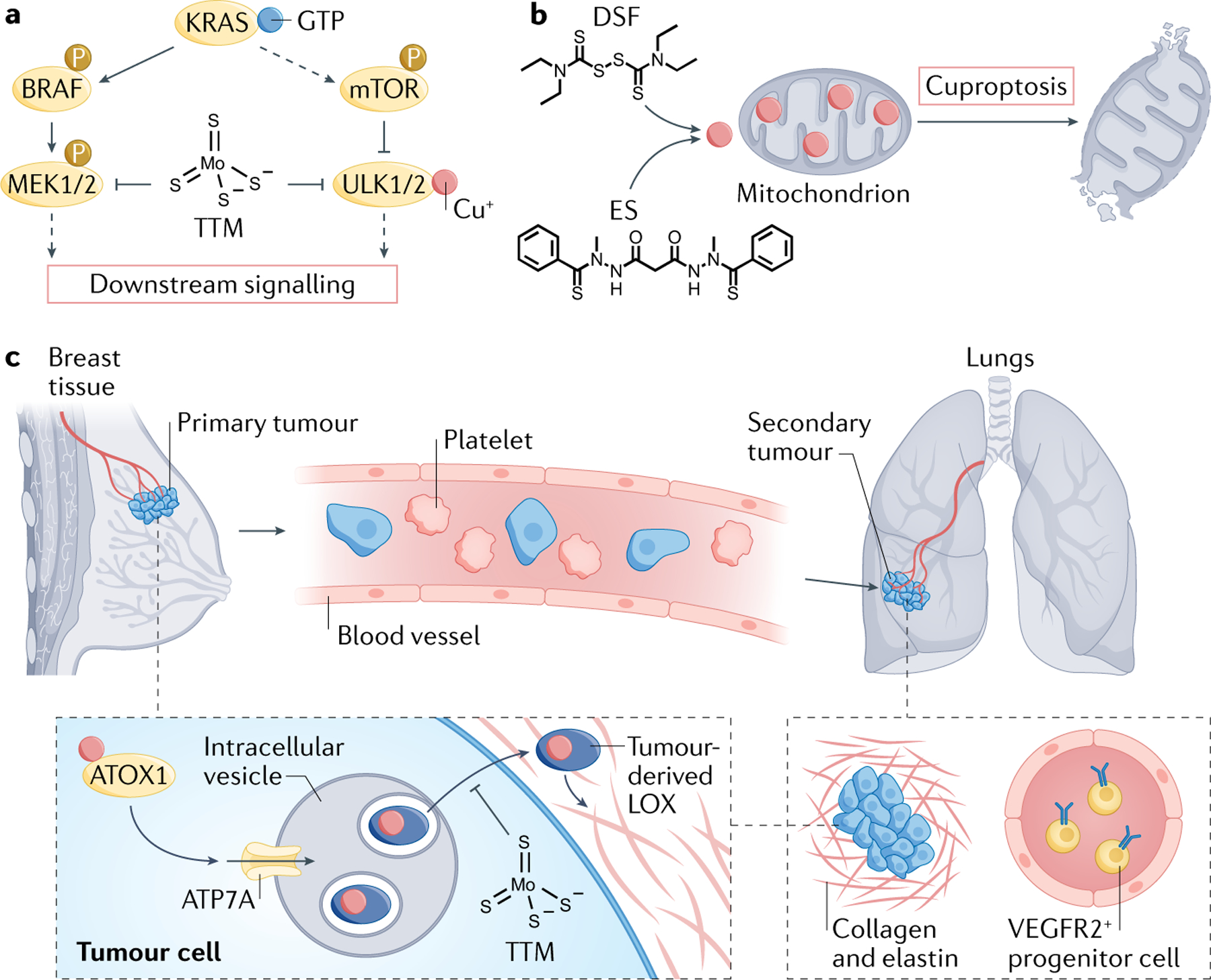
Copper status can be leveraged as a cancer vulnerability, where the two major current treatment approaches targeting this nutrient include Cu(I) chelators to deplete copper pools that drive tumour proliferation and metastasis pathways (part a) or copper ionophores to supplement copper and drive cuproptosis, an oxidative stress-inducing form of cell death triggered by excess copper (part b). a | Copper chelators such as tetrathiomolybdate (TTM) can be used in combination therapy to augment the efficacy of kinase inhibitor drugs for oncogenic signalling pathways, particularly BRAF-driven MAPK signalling. b | Copper ionophores such as disulfiram (DSF) or elesclomol (ES) can be used to induce cuproptosis by inducing oxidative stress by overwhelming native antioxidant systems such as in mitochondria. c | TTM can also be used to deplete copper in the primary tumour and/or the metastatic niche to impede copper-dependent tumour metastasis without impairing the function of healthy tissue, as shown by long-term clinical trials in patients with triple-negative breast cancer. This chelator targets a key antioxidant protein 1 (ATOX1)–ATPase 7A (ATP7A)–lysyl oxidase (LOX) copper nexus that drives invasion and metastasis by impacting collagen deposition and structure and decreases the number of VEGFR2+ endothelial progenitor cells in circulation that prime the metastatic niche. MEK, mitogen-activated protein kinase kinase; mTOR, mechanistic target of rapamycin.
Another copper-dependent cancer vulnerability is autophagy, which in a cancer context salvages building blocks for macromolecular biosynthesis and bioenergetics that are scarce due to the nutrient-poor and oxygen-poor tumour microenvironment104. In both lung adenocarcinoma and pancreatic cancer, oncogenic mutations in KRAS are associated with metabolic reprogramming, including upregulated autophagy that sustains unrestricted tumour growth. Although autophagy inhibitors have had some clinical success for patients harbouring KRAS mutations, success is limited by the lack of potent inhibitors and the onset of resistance105,106. Of significance, traditional MAPK pathway inhibition at the level of KRAS, MEK or ERK results in the upregulation of protective autophagy and signalling in an ULK-dependent fashion in KRAS-mutant and BRAF-mutant cancers. By exploiting the copper dependence of both RAF–MEK–ERK signalling and ULK1-driven and ULK2-driven autophagy, preclinical studies have shown that limiting availability of this metal is an effective strategy for blocking KRAS-driven tumour growth and survival107,108. In addition to kinase pathways, chelators such as DPM-1001 link copper and signalling via inhibition of PTP1B109. These findings chart new ground by defining copper bioavailability as a metabolic Achilles heel that can be exploited to target multiple nodes of vulnerability in oncogenic signalling pathways.
In parallel to preclinical models centred on blocking primary tumour growth, translational efforts in modulating cuproplasia have focused on strategies to achieve copper depletion in metastasis, where modifying the tumour microenvironment by lowering copper levels can create an unwelcoming niche for tumour metastases (FIG. 3). TTM suppressed spontaneous mammary tumour development in mouse models, but tumours returned within weeks of TTM therapy being stopped, suggesting that long-term copper depletion maintains neoplastic tumours cells in a state of dormancy rather than suppressing neoplastic transformation110,111. Speculated mechanisms of action include inhibition of copper enzyme activities, suppression of angiogenesis and inhibition of kinases that function in autophagy and mitogenic signalling5,62,112–115. Clinical work has focused on patients with triple-negative breast cancer (TNBC; lacking all three receptors), who, despite accounting for only 15–20% of all patients with breast cancer, represent 50% of patients with metastatic disease116,117. TNBC systemically generates metastasis-promoting local microenvironments in distant organs referred to as the ‘premetastatic niche’, which provide the optimal infrastructure for disseminated tumour cells to colonize and grow into lethal macrometastases. A long-term phase II clinical trial was undertaken in a population of patients with breast cancer (49% with TNBC) after they had undergone standard chemotherapy including patients with no evidence of disease but high risk of relapse95. By treatment with TTM, patients were copper depleted to a level that impeded copper-dependent tumour-associated processes without impairing the function of healthy tissues. Specifically, TTM-treated patients exhibited a reduction in the levels of circulating VEGFR2+ endothelial progenitor cells and LOX activity, which are both essential for metastatic niche priming. With more than 12,000 weeks of treatment given over a decade, TTM has been safe and well tolerated, with limited severe side effects. A larger randomized phase II clinical trial of copper depletion as a therapeutic strategy in high-risk TNBC is currently in development.
Another attractive target of copper chelation therapy is the immune checkpoint protein programmed cell death 1 ligand 1 (PDL1), which cancer cells overexpress to protect themselves from antitumour immune response. Bioinformatic analyses of pan-cancer gene expression profiles from The Cancer Genome Atlas (TCGA) database compared with normal tissue samples from the Genotype-Tissue Expression (GTEx) database showed strong correlation between expression of CTR1 and PDL1 (also known as CD274) in many cancers, but not in normal tissues. Copper supplementation was shown to enhance PDL1 expression, while copper chelation promoted ubiquitin-mediated degradation of PDL1 in colon cancer DLD1 cell lines. In an immunocompetent transgenic neuroblastoma mouse model, Th-MYCN, treatment with copper-chelating drugs slowed tumour growth and increased survival. These findings suggest that copper chelation therapy could potentially synergize with and enhance antitumour immune response118.
The use of small-molecule copper chelators to treat tumours could be further optimized by use of existing strategies for tissue-specific drug delivery, for example glucose-modified therapeutics for GLUT1 targeting in the brain and pancreas, and prostate-specific membrane antigen (PSMA) for the prostate using glutamate–urea-modified modalities119. However, identifying the most effective clinical settings remains a key barrier in this drug-repurposing strategy and offers future opportunities.
Copper supplementation.
Although copper is essential for cell survival and proliferation and constitutes an exploitable dependency in cancer, excess copper can cause toxicity via production of deleterious ROS120. As certain cancers exhibit constitutively elevated oxidative stress and show dependence on antiapoptotic ROS signalling, they are uniquely susceptible to further increases in ROS levels121–124. Therefore, copper ionophores, such as disulfiram (DSF) and elesclomol, are being pursued as cancer therapeutics to induce cuproptosis, a copper-dependent form of cell death96,125,126 (TABLE 2).
DSF is a member of the dithiocarbamate family and was approved by the FDA in 1951 as the first drug to treat alcohol dependence through inhibition of aldehyde dehydrogenase (ALDH)96,127,128. Many studies over the past 30 years have shown that DSF in combination with cupric ion (Cu(II)) has potential to treat various human cancers96. In acidic environments such as the stomach, DSF is reduced to diethyldithiocarbamate (DDTC), a potent chelator of divalent transition metal ions, including Cu(II)129. DDTC–Cu(II) complexes have been reported to possess strong anticancer activities130–133 with multiple identified potential mechanisms of action, including inhibition of the ubiquitin protein pathway, suppression of nuclear factor-κB, ROS generation, activation of the MAPK pathway, inhibition of superoxide dismutase activity, alterations in non-protein thiols and chemosensitization, in addition to irreversible inhibition of ALDH134–137. In this context, ALDH+ cancer stem cell populations harbour strong self-renewal and tumour-initiating capabilities that develop resistance to chemotherapy and radiotherapy138–140. Preclinical studies revealed that the combination of DSF and Cu(II) selectively targets and kills these cancer stem cells, contributing to the inhibition of tumour recurrence138–140. As an FDA-approved drug, DSF has well-studied pharmacokinetics and an excellent safety profile. Clinical trials confirm the anticancer and/or chemosensitizing effects of DSF or Cu–DSF127,137, particularly in glioblastomas owing to the penetrance of DSF across the blood–brain barrier. A phase I clinical trial (NCT01907165) showed that DSF in combination with temozolomide had an acceptable safety profile and produced increased progression-free survival in patients with glioblastoma97, and a recent phase II study (NCT03034135) showed that addition of Cu–DSF to temozolomide for treatment of patients with otherwise-resistant glioblastoma appears well tolerated but requires further efforts to select responsive populations97.
Elesclomol is another copper-binding compound originally identified through a cell-based phenotypic screen for small molecules that enhance the antitumour activity of paclitaxel141. Elesclomol is a bis(thiohydrazide) amide compound that forms a 1:1 complex with Cu(II)142. Initial in vitro experiments showed that elesclomol strongly induced ROS in cancer cells, leading to unmitigated oxidative stress and cell death125. However, recent studies aimed at uncovering small-molecule vulnerabilities in the context of proteasome inhibitor-resistant multiple myelomas that exhibit elevated mitochondrial metabolism provided new mechanistic insights into elesclomol and DSF efficacy. Specifically, a high-throughput screen of small molecules in cancer cells with reduced 19S subunit expression or cultured in galactose to establish mitochondrial metabolism dependence revealed selective sensitivity to either elesclomol treatment or DSF treatment. Elesclomol generates ROS by selectively transporting Cu(II) from the extracellular environment into mitochondria, where it is reduced to Cu(I)143. Subsequent mechanistic studies that used a CRISPR–Cas9 deletion screen revealed that the mitochondrial protein ferredoxin 1 is responsible for reduction of elesclomol-bound Cu(II) to Cu(I) and cancer cell sensitivity to elesclomol126. Reaction of Cu(I) released in mitochondria with molecular oxygen is expected to yield superoxide that would dismutate to produce H2O2, which can further react with Cu(I) to generate the even more damaging and highly reactive hydroxyl radical120. Therefore, tumours relying on mitochondrial metabolism are likely to be particularly susceptible to elesclomol. Elesclomol in combination with paclitaxel has been evaluated in a number of clinical trials mostly targeting advanced-stage melanomas98,99,144. A phase I clinical trial showed that the combination of elesclomol and paclitaxel was well tolerated with a toxicity profile similar to that of paclitaxel alone144. A phase II study showed that addition of elesclomol to paclitaxel yielded a doubling of median progression-free survival, a 41.7% risk reduction for disease progression/death and increased overall survival rates98. Although in a large, randomized, double blind, phase III study of patients with chemotherapy-naive advanced melanoma, elesclomol and paclitaxel combination therapy did not achieve its progression-free end point, a prospectively defined subgroup analysis revealed a statistically significant improvement in patients with normal baseline levels of lactate dehydrogenase99. As increased lactate dehydrogenase activity is associated with tumour hypoxia, this finding is consistent with the requirement of active mitochondrial respiration for the action of elesclomol126. Taken together, the findings suggest that ionophore-mediated copper delivery to intracellular compartments could be a promising therapeutic strategy for a subset of tumours.
Copper imaging and replacement.
Finally, in addition to chelation and ionophore therapies, the general propensity of tumours to accumulate elevated copper levels relative to adjacent tissue has been exploited to develop copper-based targeted anticancer agents. Of note, following this strategy, the radioisotope 64Cu has been applied for in vivo tumour imaging and therapy (TABLE 2). With a half-life of 12.7 hours and decay characteristics that enable imaging by positron emission tomography (PET), 64Cu shows significant promise for the detection and treatment of primary cancer and its metastases145. Unlike most conventional radiopharmaceuticals, which require complexation between a radioisotope and a targeting ligand, 64Cu in the form of simple 64CuCl2 has theranostic potential as both a radiotracer for PET and a targeted radiotherapeutic agent145. PET of mouse cancer models after intravenous injection of 64CuCl2 has been successfully used to detect different types of tumours, including melanoma, breast cancer, prostate cancer, glioblastoma, colorectal cancer, ovarian cancer, lung cancer, and head and neck cancer146–149. At elevated doses, 64CuCl2 was shown to exhibit radiotherapeutic activity in various mouse models of cancer, including melanoma146, glioblastoma150 and prostate cancer151. In humans, PET of 64CuCl2 has been used in the staging of prostate cancer152, and was recently found to outperform 18F-labelled choline in detecting relapse of prostate cancer in patients with low levels of prostate-specific antigen153. In a study of patients with glioblastoma, PET of intravenously administered 64CuCl2 readily identified brain lesions with excellent agreement with standard diagnostic magnetic resonance images154. Importantly, 64CuCl2 may be able to detect metastatic brain lesions associated with other primary cancers, such as lung and breast cancer154.
An important number of copper-based compounds have been studied as experimental anticancer agents in vitro, and in some cases in vivo155–157. Thus, various families of Cu(I) and Cu(II) complexes have been designed and have shown promising pharmacological effects against different cancer types155,158–161. While the mechanisms of cytotoxic action of these metallodrugs have not been fully elucidated and may also involve effects triggered by the Cu(I)/Cu(II) coordinating ligands, the results obtained point towards a multimodal spectrum of biological activity, involving intracellular redox reactions triggered by the metal centre.
Overall, the aforementioned examples of either copper chelators or copper-based compounds further demonstrate that the selective accumulation of copper by cancer cells has immense potential in nuclear medicine for the development of diagnostic and therapeutic interventions.
Assessing clinical copper status
As therapeutics targeting copper and copper-dependent signalling pathways are developed and tested, a parallel need is to assess functional ‘copper status’ in patients to minimize side effects and any impact on essential biological processes and to determine whether therapeutic goals are reached. The potential for unintended adverse events and consequences of perturbing copper balance includes alterations in physiologic processes and metabolism that are dependent on patient age and/or the stage of development.
As clinical signs and symptoms of altered copper homeostasis often appear later than biochemical evidence for copper deficiency or excess, there is a need to establish best methods and practices to evaluate a patient’s ‘copper status’ to prevent clinically significant complications. Standard testing measures total copper concentration in the circulation (serum or plasma) and in the urine32. Less frequently performed is measurement of tissue copper content due to the more invasive nature of obtaining samples for measurement from organs. Indirect estimation of copper status can be accomplished by measurement of the activity of cuproenzymes as a reduction in enzymatic activity can occur in copper-depleted states. Indirect detection of copper depletion is possible on routine blood counts, because copper depletion is reflected in a decreased count of white blood cells (mainly neutrophils). Platelet counts may also be lower and a sideroblastic anaemia may occur. In copper-deficient states, ceruloplasmin may not acquire its full complement of copper. Due to the short plasma half-life of ceruloplasmin without copper (apoceruloplasmin), ceruloplasmin detection by immunoassay or by oxidase activity is lower in copper-deficient states. In untreated patients with Wilson disease, there may be elevation of ‘non-ceruloplasmin-bound copper’ (NCC) deposition in other tissues, in particular in the liver and the central nervous system, where it causes tissue injury. Treatment goals for Wilson disease include reduction of NCC levels using copper chelation or with zinc, which indirectly inhibits copper absorption across the gut. Methods are in development that directly measure the copper in ceruloplasmin, permitting a more accurate determination of NCC that should help with treatment monitoring. Alternatively, administration of radiolabelled copper and measurement of its accumulation in tumours and/or secondary appearance in the circulation as radiolabelled ceruloplasmin offers another companion diagnostic approach. As new agents that target copper are developed for treatment of cancer and other disorders, determination of which biomarker of ‘copper status’ provides the most accurate and reliable therapeutic target will be needed in addition to its measurement for safety monitoring.
Conclusions and future prospects
Mounting observations connect copper signalling to cell proliferation as well as tumour growth and metastasis in cancer. However, more foundational mechanistic information is needed to establish causal rather than correlative relationships and further link copper-dependent targets and pathways to copper-dependent disease vulnerabilities. In particular, technical approaches to measuring and manipulating the actions of copper within biological systems produce data that are often difficult to interpret and require continued development of new chemical probes to monitor labile copper, particularly in terms of subcellular resolution and oxidation state specificity, as well as the ability for comparable profiling of the total and labile copper status of different cell populations. Indeed, in vivo imaging of copper and other metals will help advance the field162–164. The diverse chemical properties of metals offer several features to consider in the design of metal-specific sensors, ionophores and chelators, including their abundance, binding affinities and complexes formed with other competing ligands in the cell. For detection, the emergence of activity-based sensing70,165, which relies on reactivity rather than recognition, can provide a complementary approach to binding-based approaches to achieve selectivity and sensitivity. Challenges remain in distinguishing between relevant oxidation states of copper69,71, as well as achieving specificity for copper over more abundant alkali and alkaline earth metals and other transition metals in biological environments. In this regard, thioethers72,166 and phosphine sulfides167 offer privileged ligand donors for copper-selective binders.
Likewise, the discovery and characterization of the first examples of metalloallostery, as shown by copper as a negative allosteric regulator of PDE3B and a positive allosteric regulator of MEK1, MEK2, the kinases ULK1 and ULK2 and the E2 ligase UBE2D2, provide motivation for studies to provide a more comprehensive picture of the metalloproteome and copper-sensitive signalling targets, as well as basic biochemical mechanisms of how allosteric metal binding regulates protein activity and function. Indeed, nutrient sensing of copper and the intersection between copper and cancer metabolism warrant further studies in various aspects of cancer biology, including tumour initiation, growth and metastasis, particularly in stem cell niches and inflammatory response. Bringing to bear advanced sequencing, proteomics, metabolomics and other analyses will uncover new metallobiology relevant to cancer and other diseases.
In terms of pharmacology, increasing the specificity of therapeutics to target cuproplasia is an important next challenge to address. The possibility to directly target the copper homeostasis machinery, as illustrated by lead candidates that block the copper metallochaperone machinery168,169, or leverage gene therapy as has been shown in treatment of genetic disorders of copper dysregulation such as Menkes disease16,170 show promises in this regard. Delivery of therapeutic agents to specific cell populations and even subcellular compartments (for example, by targeted ionophore metal supplementation for liver-specific copper delivery171 and lipid nanoformulations for the delivery of Cu(II) complexes to the tumour site172,173), organelle-specific copper depletion (for example, with nanoparticles for lowering mitochondrial copper levels164) and biochemically and environmentally sensitive reagents to uncage copper-sensitive reagents174 offer but a few strategies to increase specificity and reduce off-target effects. Indeed, the data show that both depleting copper and supplementing copper to toxic levels are viable strategies to block cuproplasia pathways.
In parallel to developing new therapeutic strategies, dissecting the metal and metalloproteome landscape may help stratify patients in clinical trials and predict response to targeted therapy. Clinical trials point to the promise of copper depletion on the metastatic microenvironment by rendering the ‘soil’ of distant tissues less congenial to ‘seeds’ of primary tumours. Also, new ways to reliably measure total copper inside cells and in plasma and/or bioavailable, labile forms of this metal (for example, non-ceruloplasmin pools in serum), along with identifying and validating new copper-dependent biomarkers, will give rise to companion diagnostics that can identify populations most amenable to copper-responsive therapies. Cuproplasia offers a new lens to view an ancient vulnerability that cancer is, and efforts to target copper-dependent signalling and cell proliferation have the potential to change the natural history of cancer and other diseases. We predict that cuproplasia is the first example of a broader range of potential metalloplasias and provides a starting point to join basic and translational efforts for connecting metal signalling, metabolism and disease.
Acknowledgements
The authors thank the following sources for funding: NIH (GM79465 and GM139245 to C.J.C., GM124749 to D.C.B., R01-GM084176 to K.J.F., GM120211 to P.A.C., GM111672 to V.M.G., R21-GM129592 to M.R., R01-NS109307 to P.Y., CA190265 and DK116859 to M.J.P., Z01 HD008768 and Z01 HD008892 to S.G.K., CA53840 and DK124907 to N.K.T. and R01 - GM101502, R01 - DK117396 and R01-DK071865 to S.L.), the US National Cancer Institute (R21CA184788 to Q.P.D.), the Welch Foundation (A-1810 to V.M.G.), Pew Charitable Trusts (Pew Scholars Program in Biomedical Science award no. 50350 to D.C.B.)), AIRC Italy (IG 17118 to R.P.), the Florida Department of Health Bankhead-Coley Cancer Research Program (9BC07 to G.M.D.), the Breast Cancer Research Foundation, Susan G. Komen Greater New York City (L.T.V.), the US Department of Army (W81XH-20-1-0754 to L.V.A.), the CSHL Cancer Centre Support Grant (CA45508 to N.K.T.) and the V Foundation Scholar Award (3C59 8ABS 3424 3BDA to D.C.B.) V.M. was supported by the Center on the Physics of Cancer Metabolism through award number U54CA210184 from the National Cancer Institute, and also by National Cancer Institute award R01 CA257254-01A1 (V.M. and L.T.V.). S.G. received support from NIDDK R01-DK-071111 and NIDDK Center grants, P30-DK-41296 and P30-DK-020541 and NCI Center grant P30-CA-13330. A.I.B. is funded by the National Health and Medical Research Council of Australia. C.J.C. is a CIFAR Fellow.
Footnotes
Competing interests
N.K.T. is a member of the Scientific Advisory Board of DepYmed Inc. V.M.G. is listed as an inventor on the patent application PCT/US2019/041571 submitted by Texas A&M University entitled “Compositions for the treatment of copper deficiency and methods of use”. D.C.B. holds ownership in Merlon Inc. A.I.B. holds equity in Alterity Biotechnology Ltd, Cogstate Ltd, Mesoblast Ltd and Collaborative Medicinal Development LLC and is a paid consultant for Collaborative Medicinal Development Pty Ltd. L.T.V. is a consultant for Berg Pharma, Osmol Therapeutics and Sema4, serves on the advisory board of Seattle Genetics and Immunomedics/Gilead, and receives research funding from Genentech, Arvinas, and Oncotheraphy Sciences. E.J.G, A.C., P.A.C., J.R.C, G.M.D., Q.P.D., K.J.F., S.G., S.G.K., S.L., V.M., M.J.P., R.P., M.R., M.L.S., L.V.A., D.X., P.Y. and C.J.C. declare no competing interests.
References
- 1.Hanahan D & Weinberg RA Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011). [DOI] [PubMed] [Google Scholar]
- 2.Lippard SJ, Berg JM Principles of bioinorganic chemistry. vol. xvii, 411 p (University Science Books; 1994). [Google Scholar]
- 3.Solomon EI, Sundaram UM & Machonkin TE Multicopper oxidases and oxygenases. Chem. Rev 96, 2563–2606 (1996). [DOI] [PubMed] [Google Scholar]
- 4.Que EL, Domaille DW & Chang CJ Metals in neurobiology: probing their chemistry and biology with molecular imaging. Chem. Rev 108, 1517–1549 (2008). [DOI] [PubMed] [Google Scholar]
- 5.Brady DC et al. Copper is required for oncogenic BRAF signalling and tumorigenesis. Nature 509, 492–496 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang CJ Searching for harmony in transition-metal signaling. Nat. Chem. Biol 11, 744–747 (2015). [DOI] [PubMed] [Google Scholar]
- 7.Dixon SJ et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149, 1060–1072 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang WS et al. Regulation of ferroptotic cancer cell death by GPX4. Cell 156, 317–331 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ackerman CM, Lee S & Chang CJ Analytical methods for imaging metals in biology: from transition metal metabolism to transition metal signaling. Anal. Chem 89, 22–41 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hare DJ, New EJ, de Jonge MD & McColl G Imaging metals in biology: balancing sensitivity, selectivity and spatial resolution. Chem. Soc. Rev 44, 5941–5958 (2015). [DOI] [PubMed] [Google Scholar]
- 11.Rosenzweig A Structure and chemistry of the copper chaperone proteins. Curr. Opin. Chem. Biol 4, 140–147 (2000). [DOI] [PubMed] [Google Scholar]
- 12.Thiele DJ & Gitlin JD Assembling the pieces. Nat. Chem. Biol 4, 145–147 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cobine PA, Moore SA & Leary SC Getting out what you put in: copper in mitochondria and its impacts on human disease. Biochim. Biophys. Acta Mol. Cell Res 1868, 118867 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gudekar N et al. Metallothioneins regulate ATP7A trafficking and control cell viability during copper deficiency and excess. Sci. Rep 10, 7856 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gupta A & Lutsenko S Evolution of copper transporting ATPases in eukaryotic organisms. Curr. Genomics 13, 124–133 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaler SG ATP7A-related copper transport diseases-emerging concepts and future trends. Nat. Rev. Neurol 7, 15–29 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ackerman CM & Chang CJ Copper signaling in the brain and beyond. J. Biol. Chem 293, 4628–4635 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prohaska JR Copper. In: Present Knowledge in Nutrition 10 (eds. Erdman JWMIA, Zeisel SH) p. 540–553 (Wiley-Blackwell; 2012). [Google Scholar]
- 19.Institute of Medicine (US) Panel on Micronutrients. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc (National Academies Press, 2001). [PubMed] [Google Scholar]
- 20.Wang Y et al. Maternofetal and neonatal copper requirements revealed by enterocyte-specific deletion of the Menkes disease protein. Am. J. Physiol. Gastrointest. Liver Physiol 303, G1236–G1244 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roelofsen H et al. Copper-induced apical trafficking of ATP7B in polarized hepatoma cells provides a mechanism for biliary copper excretion. Gastroenterology 119, 782–793 (2000). [DOI] [PubMed] [Google Scholar]
- 22.Polishchuk EV et al. Wilson disease protein ATP7B utilizes lysosomal exocytosis to maintain copper homeostasis. Dev. Cell 29, 686–700 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saroli Palumbo C & Schilsky ML Clinical practice guidelines in Wilson disease. Ann. Transl. Med 7, S65 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harvey LJ, Ashton K, Hooper L, Casgrain A & Fairweather-Tait SJ Methods of assessment of copper status in humans: a systematic review. Am. J. Clin. Nutr 89, 2009S–2024S (2009). [DOI] [PubMed] [Google Scholar]
- 25.Linder MC Ceruloplasmin and other copper binding components of blood plasma and their functions: an update. Metallomics 8, 887–905 (2016). [DOI] [PubMed] [Google Scholar]
- 26.Meyer LA, Durley AP, Prohaska JR & Harris ZL Copper transport and metabolism are normal in aceruloplasminemic mice. J. Biol. Chem 276, 36857–36861 (2001). [DOI] [PubMed] [Google Scholar]
- 27.Gray LW et al. Urinary copper elevation in a mouse model of Wilson’s disease is a regulated process to specifically decrease the hepatic copper load. PLoS ONE 7, e38327 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee J, Pena MM, Nose Y & Thiele DJ Biochemical characterization of the human copper transporter Ctr1. J. Biol. Chem 277, 4380–4387 (2002). [DOI] [PubMed] [Google Scholar]
- 29.Ren F et al. X-ray structures of the high-affinity copper transporter Ctr1. Nat. Commun 10, 1386 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ohgami RS, Campagna DR, McDonald A & Fleming MD The Steap proteins are metalloreductases. Blood. 108, 1388–1394 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaler SG, DiStasio AT ATP7A-related copper transport disorders. In: (eds Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Mirzaa G et al.) (GeneReviews, 1993). [PubMed] [Google Scholar]
- 32.Czlonkowska A et al. Wilson disease. Nat. Rev. Dis. Prim 4, 21 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gunjan D et al. Hepatocellular carcinoma: an unusual complication of longstanding Wilson disease. J. Clin. Exp. Hepatol 7, 152–154 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blockhuys S et al. Defining the human copper proteome and analysis of its expression variation in cancers. Metallomics 9, 112–123 (2017). [DOI] [PubMed] [Google Scholar]
- 35.Turnlund JR, Keyes WR, Anderson HL & Acord LL Copper absorption and retention in young men at three levels of dietary copper by use of the stable isotope 65Cu. Am. J. Clin. Nutr 49, 870–878 (1989). [DOI] [PubMed] [Google Scholar]
- 36.Prohaska JR Impact of copper deficiency in humans. Ann. N. Y. Acad. Sci 1314, 1–5 (2014). [DOI] [PubMed] [Google Scholar]
- 37.Gambling L, Kennedy C & McArdle HJ Iron and copper in fetal development. Semin. Cell Dev. Biol 22, 637–44 (2011). [DOI] [PubMed] [Google Scholar]
- 38.Lopez J, Ramchandani D & Vahdat L Copper depletion as a therapeutic strategy in cancer. Met. Ions Life Sci 10.1515/9783110527872-018 (2019). [DOI] [PubMed] [Google Scholar]
- 39.Ding X et al. Analysis of serum levels of 15 trace elements in breast cancer patients in Shandong, China. Env. Sci. Pollut. Res. Int 22, 7930–7935 (2015). [DOI] [PubMed] [Google Scholar]
- 40.Adeoti ML, Oguntola AS, Akanni EO, Agodirin OS & Oyeyemi GM Trace elements; copper, zinc and selenium, in breast cancer afflicted female patients in LAUTECH Osogbo, Nigeria. Indian J. Cancer 52, 106–109 (2015). [DOI] [PubMed] [Google Scholar]
- 41.Kuo HW, Chen SF, Wu CC, Chen DR & Lee JH Serum and tissue trace elements in patients with breast cancer in Taiwan. Biol. Trace Elem. Res 89, 1–11 (2002). [DOI] [PubMed] [Google Scholar]
- 42.Pavithra V et al. Serum levels of metal ions in female patients with breast cancer. J. Clin. Diagn. Res 9, BC25–BC27 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Feng JF et al. Serum total oxidant/antioxidant status and trace element levels in breast cancer patients. Int. J. Clin. Oncol 17, 575–583 (2012). [DOI] [PubMed] [Google Scholar]
- 44.Zowczak M, Iskra M, Torlinski L & Cofta S Analysis of serum copper and zinc concentrations in cancer patients. Biol. Trace Elem. Res 82, 1–8 (2001). [DOI] [PubMed] [Google Scholar]
- 45.Diez M, Cerdan FJ, Arroyo M & Balibrea JL Use of the copper/zinc ratio in the diagnosis of lung cancer. Cancer 63, 726–730 (1989). [DOI] [PubMed] [Google Scholar]
- 46.Jin Y et al. Combined effects of serum trace metals and polymorphisms of CYP1A1 or GSTM1 on non-small cell lung cancer: a hospital based case-control study in China. Cancer Epidemiol. 35, 182–187 (2011). [DOI] [PubMed] [Google Scholar]
- 47.Oyama T et al. Efficiency of serum copper/zinc ratio for differential diagnosis of patients with and without lung cancer. Biol. Trace Elem. Res 42, 115–127 (1994). [DOI] [PubMed] [Google Scholar]
- 48.Stepien M et al. Pre-diagnostic copper and zinc biomarkers and colorectal cancer risk in the European prospective investigation into cancer and nutrition cohort. Carcinogenesis 38, 699–707 (2017). [DOI] [PubMed] [Google Scholar]
- 49.Sohrabi M et al. Trace element and heavy metal levels in colorectal cancer: comparison between cancerous and non-cancerous tissues. Biol. Trace Elem. Res 183, 1–8 (2018). [DOI] [PubMed] [Google Scholar]
- 50.Ribeiro SM et al. Copper-Zinc ratio and nutritional status in colorectal cancer patients during the perioperative period. Acta Cir. Bras 31, 24–28 (2016). [DOI] [PubMed] [Google Scholar]
- 51.Nayak SB, Bhat VR, Upadhyay D & Udupa SL Copper and ceruloplasmin status in serum of prostate and colon cancer patients. Indian. J. Physiol. Pharmacol 47, 108–110 (2003). [PubMed] [Google Scholar]
- 52.Margalioth EJ, Schenker JG & Chevion M Copper and zinc levels in normal and malignant tissues. Cancer 52, 868–872 (1983). [DOI] [PubMed] [Google Scholar]
- 53.Yaman M, Kaya G & Yekeler H Distribution of trace metal concentrations in paired cancerous and non-cancerous human stomach tissues. World J. Gastroenterol 13, 612–618 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Khanna SS & Karjodkar FR Circulating immune complexes and trace elements (Copper, Iron and Selenium) as markers in oral precancer and cancer: a randomised, controlled clinical trial. Head Face Med. 2, 33 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Baltaci AK, Dundar TK, Aksoy F & Mogulkoc R Changes in the serum levels of trace elements before and after the operation in thyroid cancer patients. Biol. Trace Elem. Res 175, 57–64 (2017). [DOI] [PubMed] [Google Scholar]
- 56.Basu S et al. Heavy and trace metals in carcinoma of the gallbladder. World J. Surg 37, 2641–2646 (2013). [DOI] [PubMed] [Google Scholar]
- 57.Saleh SAK, Adly HM, Abdelkhaliq AA & Nassir AM Serum levels of selenium, zinc, copper, manganese, and iron in prostate cancer patients. Curr. Urol 14, 44–49 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ishida S, Andreux P, Poitry-Yamate C, Auwerx J & Hanahan D Bioavailable copper modulates oxidative phosphorylation and growth of tumors. Proc. Natl Acad. Sci. USA 110, 19507–19512 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wooton-Kee CR et al. Metabolic dysregulation in the Atp7b (−/−) Wilson’s disease mouse model. Proc. Natl Acad. Sci. USA 117, 2076–2083 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang H et al. Copper-dependent amino oxidase 3 governs selection of metabolic fuels in adipocytes. PLoS Biol. 16, e2006519 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shanbhag V et al. ATP7A delivers copper to the lysyl oxidase family of enzymes and promotes tumorigenesis and metastasis. Proc. Natl Acad. Sci. USA 116, 6836–41 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tsang T et al. Copper is an essential regulator of the autophagic kinases ULK1/2 to drive lung adenocarcinoma. Nat. Cell Biol 22, 412–24 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Opazo CM et al. Copper signaling promotes proteostasis and animal development via allosteric activation of ubiquitin E2D conjugases. Preprint at bioRxiv 10.1101/2021.02.15.431211 (2021). [DOI] [Google Scholar]
- 64.Dodani SC et al. Calcium-dependent copper redistributions in neuronal cells revealed by a fluorescent copper sensor and X-ray fluorescence microscopy. Proc. Natl Acad. Sci. USA 108, 5980–5985 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dodani SC et al. Copper is an endogenous modulator of neural circuit spontaneous activity. Proc. Natl Acad. Sci. USA 111, 16280–16285 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hong-Hermesdorf A et al. Subcellular metal imaging identifies dynamic sites of Cu accumulation in Chlamydomonas. Nat. Chem. Biol 10, 1034–1042 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Krishnamoorthy L et al. Copper regulates cyclic-AMP-dependent lipolysis. Nat. Chem. Biol 12, 586–592 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Morgan MT et al. Ratiometric two-photon microscopy reveals attomolar copper buffering in normal and Menkes mutant cells. Proc. Natl Acad. Sci. USA 116, 12167–12172 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chung CY et al. Activity-based ratiometric FRET probe reveals oncogene-driven changes in labile copper pools induced by altered glutathione metabolism. Proc. Natl Acad. Sci. USA 116, 18285–18294 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bruemmer KJ, Crossley SWM & Chang CJ Activity-based sensing: a synthetic methods approach for selective molecular imaging and beyond. Angew. Chem. Int. Ed 59, 13734–13762 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lee S et al. Activity-based sensing with a metal-directed acyl imidazole strategy reveals cell type-dependent pools of labile brain copper. J. Am. Chem. Soc 142, 14993–15003 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cotruvo JA Jr., Aron AT, Ramos-Torres KM & Chang CJ Synthetic fluorescent probes for studying copper in biological systems. Chem. Soc. Rev 44, 4400–4414 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Aron AT, Ramos-Torres KM, Cotruvo JA Jr. & Chang CJ Recognition- and reactivity-based fluorescent probes for studying transition metal signaling in living systems. Acc. Chem. Res 48, 2434–2442 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hwang JJ, Park MH & Koh JY Copper activates TrkB in cortical neurons in a metalloproteinase-dependent manner. J. Neurosci. Res 85, 2160–2166 (2007). [DOI] [PubMed] [Google Scholar]
- 75.Michniewicz F et al. Copper: an intracellular Achilles’ heel allowing the targeting of epigenetics, kinase pathways, and cell metabolism in cancer therapeutics. ChemMedChem 16, 2315–2329 (2021). [DOI] [PubMed] [Google Scholar]
- 76.He F et al. Copper (II) ions activate ligand-independent receptor tyrosine kinase (RTK) signaling pathway. Biomed. Res. Int 2019, 4158415 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Turski ML et al. A novel role for copper in Ras/mitogen-activated protein kinase signaling. Mol. Cell. Biol 32, 1284–1295 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Aubert L et al. Copper bioavailability is a KRAS-specific vulnerability in colorectal cancer. Nat. Commun 11, 3701 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Polishchuk EV et al. Activation of autophagy, observed in liver tissues from patients with Wilson disease and from ATP7B-deficient animals, protects hepatocytes from copper-induced apoptosis. Gastroenterology 156, 1173–89 e5 (2019). [DOI] [PubMed] [Google Scholar]
- 80.Guo J et al. Copper promotes tumorigenesis by activating the PDK1-AKT oncogenic pathway in a copper transporter 1 dependent manner. Adv. Sci 8, e2004303 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kang J, Lin C, Chen J & Liu Q Copper induces histone hypoacetylation through directly inhibiting histone acetyltransferase activity. Chem. Biol. Interact 148, 115–123 (2004). [DOI] [PubMed] [Google Scholar]
- 82.Kieffer DA & Medici V Wilson disease: at the crossroads between genetics and epigenetics-a review of the evidence. Liver Res. 1, 121–130 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Itoh S et al. Novel role of antioxidant-1 (Atox1) as a copper-dependent transcription factor involved in cell proliferation. J. Biol. Chem 283, 9157–9167 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Walshe JM Treatment of Wilson’s disease with trientine (triethylene tetramine) dihydrochloride. Lancet 319, 643–647 (1982). [DOI] [PubMed] [Google Scholar]
- 85.Ishida S, McCormick F, Smith-McCune K & Hanahan D Enhancing tumor-specific uptake of the anticancer drug cisplatin with a copper chelator. Cancer Cell 17, 574–583 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.University D. A Pilot Study of Trientine with Vemurafenib for the Treatment BRAF Mutated Metastatic Melanoma https://ClinicalTrials.gov/show/NCT02068079 (2014).
- 87.Brewer GJ et al. Treatment of metastatic cancer with tetrathiomolybdate, an anticopper, antiangiogenic agent: phase I study. Clin. Cancer Res 6, 1–10 (2000). [PubMed] [Google Scholar]
- 88.Center MSKC, University WMCoC. Phase II Study of Tetrathiomolybdate (TM) in Patients With Breast Cancer https://ClinicalTrials.gov/show/NCT00195091 (2003).
- 89.UK CR, Institute NC. Exemestane With or Without ATN-224 in Treating Postmenopausal Women With Recurrent or Advanced Breast Cancer https://ClinicalTrials.gov/show/NCT00674557 (2008).
- 90.Folkman J Anti-angiogenesis: new concept for therapy of solid tumors. Ann. Surg 175, 409–416 (1972). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Folkman J Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat. Med 1, 27–31 (1995). [DOI] [PubMed] [Google Scholar]
- 92.Parke A, Bhattacherjee P, Palmer RM & Lazarus NR Characterization and quantification of copper sulfate-induced vascularization of the rabbit cornea. Am. J. Pathol 130, 173–8 (1988). [PMC free article] [PubMed] [Google Scholar]
- 93.Raju KS, Alessandri G, Ziche M & Gullino PM Ceruloplasmin, copper ions, and angiogenesis2. J. Natl Cancer Inst 69, 1183–1188 (1982). [PubMed] [Google Scholar]
- 94.Sen CK et al. Copper-induced vascular endothelial growth factor expression and wound healing. Am. J. Physiol. Heart Circ. Physiol 282, H1821–H1827 (2002). [DOI] [PubMed] [Google Scholar]
- 95.Chan N et al. Influencing the tumor microenvironment: a phase II study of copper depletion using tetrathiomolybdate in patients with breast cancer at high risk for recurrence and in preclinical models of lung metastases. Clin. Cancer Res 23, 666–676 (2017). [DOI] [PubMed] [Google Scholar]
- 96.Jiao Y, Hannafon BN & Ding WQ Disulfiram’s anticancer activity: evidence and mechanisms. Anticancer. Agents Med. Chem 16, 1378–1384 (2016). [DOI] [PubMed] [Google Scholar]
- 97.Huang J et al. A phase I study to repurpose disulfiram in combination with temozolomide to treat newly diagnosed glioblastoma after chemoradiotherapy. J. Neurooncol 128, 259–66 (2016). [DOI] [PubMed] [Google Scholar]
- 98.O’Day S et al. Phase II, randomized, controlled, double-blinded trial of weekly elesclomol plus paclitaxel versus paclitaxel alone for stage IV metastatic melanoma. J. Clin. Oncol 27, 5452–5458 (2009). [DOI] [PubMed] [Google Scholar]
- 99.O’Day SJ et al. Final results of phase III SYMMETRY study: randomized, double-blind trial of elesclomol plus paclitaxel versus paclitaxel alone as treatment for chemotherapy-naive patients with advanced melanoma. J. Clin. Oncol 31, 1211–1218 (2013). [DOI] [PubMed] [Google Scholar]
- 100.Sahlgrenska University Hospital S, Hospital SO, Hospital LU, Hospital KU, University Hospital L, County RÖ, et al. Disulfiram in Recurrent Glioblastoma https://ClinicalTrials.gov/show/NCT02678975 (2017).
- 101.Gohil VM Repurposing elesclomol, an investigational drug for the treatment of copper metabolism disorders. Expert. Opin. Investig. Drugs 30, 1–4 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ascierto PA et al. The role of BRAF V600 mutation in melanoma. J. Transl. Med 10, 85 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Brady DC, Crowe MS, Greenberg DN & Counter CM Copper chelation inhibits BRAF(V600E)-driven melanomagenesis and counters resistance to BRAF(V600E) and MEK1/2 inhibitors. Cancer Res. 77, 6240–6252 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Amaravadi R, Kimmelman AC & White E Recent insights into the function of autophagy in cancer. Genes. Dev 30, 1913–1930 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Piffoux M, Eriau E & Cassier PA Autophagy as a therapeutic target in pancreatic cancer. Br. J. Cancer 124, 333–344 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yang H, Liang SQ, Schmid RA & Peng RW New horizons in KRAS-mutant lung cancer: dawn after darkness. Front. Oncol 9, 953 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bryant KL et al. Combination of ERK and autophagy inhibition as a treatment approach for pancreatic cancer. Nat. Med 25, 628–640 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Krishnan N, Felice C, Rivera K, Pappin DJ & Tonks NK DPM-1001 decreased copper levels and ameliorated deficits in a mouse model of Wilson’s disease. Genes. Dev 32, 944–952 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Krishnan N, Konidaris KF, Gasser G & Tonks NK A potent, selective, and orally bioavailable inhibitor of the protein-tyrosine phosphatase PTP1B improves insulin and leptin signaling in animal models. J. Biol. Chem 293, 1517–1525 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pan Q et al. Copper deficiency induced by tetrathiomolybdate suppresses tumor growth and angiogenesis. Cancer Res. 62, 4854–4859 (2002). [PubMed] [Google Scholar]
- 111.Pan Q, Rosenthal DT, Bao L, Kleer CG & Merajver SD Antiangiogenic tetrathiomolybdate protects against Her2/neu-induced breast carcinoma by hypoplastic remodeling of the mammary gland. Clin. Cancer Res 15, 7441–7446 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lee K et al. The copper chelator ATN-224 induces peroxynitrite-dependent cell death in hematological malignancies. Free. Radic. Biol. Med 60, 157–167 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Glasauer A, Sena LA, Diebold LP, Mazar AP & Chandel NS Targeting SOD1 reduces experimental non-small-cell lung cancer. J. Clin. Invest 124, 117–128 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Juarez JC et al. Copper binding by tetrathiomolybdate attenuates angiogenesis and tumor cell proliferation through the inhibition of superoxide dismutase 1. Clin. Cancer Res 12, 4974–4982 (2006). [DOI] [PubMed] [Google Scholar]
- 115.Tsang T et al. Copper is an essential regulator of the autophagic kinases ULK1/2 to drive lung adenocarcinoma. Preprint at bioRxiv 22, 412–424 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lin NU et al. Sites of distant recurrence and clinical outcomes in patients with metastatic triple-negative breast cancer: high incidence of central nervous system metastases. Cancer 113, 2638–2645 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kennecke H et al. Metastatic behavior of the breast cancer subtypes. J. Clin. Oncol 28, 3271–3277 (2010). [DOI] [PubMed] [Google Scholar]
- 118.Voli F et al. Intratumoral copper modulates PD-L1 expression and influences tumor immune evasion. Cancer Res. 80, 4129–4144 (2020). [DOI] [PubMed] [Google Scholar]
- 119.Zhao Z, Ukidve A, Kim J & Mitragotri S Targeting strategies for tissue-specific drug delivery. Cell 181, 151–167 (2020). [DOI] [PubMed] [Google Scholar]
- 120.Halliwell B & Gutteridge JM Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem. J 219, 1–14 (1984). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Trachootham D, Alexandre J & Huang P Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat. Rev. Drug. Discov 8, 579–591 (2009). [DOI] [PubMed] [Google Scholar]
- 122.Schumacker PT Reactive oxygen species in cancer cells: live by the sword, die by the sword. Cancer Cell 10, 175–176 (2006). [DOI] [PubMed] [Google Scholar]
- 123.Cabello CM, Bair WB 3rd & Wondrak GT Experimental therapeutics: targeting the redox Achilles heel of cancer. Curr. Opin. Investig. Drugs 8, 1022–1037 (2007). [PubMed] [Google Scholar]
- 124.Fruehauf JP & Meyskens FL Jr. Reactive oxygen species: a breath of life or death? Clin. Cancer Res 13, 789–794 (2007). [DOI] [PubMed] [Google Scholar]
- 125.Kirshner JR et al. Elesclomol induces cancer cell apoptosis through oxidative stress. Mol. Cancer Ther 7, 2319–2327 (2008). [DOI] [PubMed] [Google Scholar]
- 126.Tsvetkov P et al. Mitochondrial metabolism promotes adaptation to proteotoxic stress. Nat. Chem. Biol 15, 681–689 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Viola-Rhenals M et al. Recent advances in Antabuse (disulfiram): the importance of its metal-binding ability to its anticancer activity. Curr. Med. Chem 25, 506–524 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wattenberg LW Inhibition of dimethylhydrazine-induced neoplasia of the large intestine by disulfiram. J. Natl Cancer Inst 54, 1005–1006 (1975). [DOI] [PubMed] [Google Scholar]
- 129.Sunderman FW Efficacy of sodium diethyldithiocarbamate (dithiocarb) in acute nickel carbonyl poisoning. Ann. Clin. Lab. Sci 9, 1–10 (1979). [PubMed] [Google Scholar]
- 130.Victoriano LI The reactivity of metal species towards thiuram sulfides: an alternative route to the syntheses of metal dithiocarbamates. Coord. Chem. Rev 196, 383–398 (2000). [Google Scholar]
- 131.Lewis DJ, Deshmukh P, Tedstone AA, Tuna F & O’Brien P On the interaction of copper(II) with disulfiram. Chem. Commun 50, 13334–13337 (2014). [DOI] [PubMed] [Google Scholar]
- 132.Cvek B, Milacic V, Taraba J & Dou QP Ni(II), Cu(II), and Zn(II) diethyldithiocarbamate complexes show various activities against the proteasome in breast cancer cells. J. Med. Chem 51, 6256–6258 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tawari PE et al. The cytotoxic mechanisms of disulfiram and copper(II) in cancer cells. Toxicol. Res 4, 1439–1442 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chen D, Cui QC, Yang H & Dou QP Disulfiram, a clinically used anti-alcoholism drug and copper-binding agent, induces apoptotic cell death in breast cancer cultures and xenografts via inhibition of the proteasome activity. Cancer Res. 66, 10425–10433 (2006). [DOI] [PubMed] [Google Scholar]
- 135.Chen X et al. Metal-based proteasomal deubiquitinase inhibitors as potential anticancer agents. Cancer Metastasis Rev. 36, 655–668 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Skrott Z et al. Alcohol-abuse drug disulfiram targets cancer via p97 segregase adaptor NPL4. Nature 552, 194–199 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yang H et al. Repurposing old drugs as new inhibitors of the ubiquitin-proteasome pathway for cancer treatment. Semin. Cancer Biol 68, 105–122 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Liu P et al. Disulfiram targets cancer stem-like cells and reverses resistance and cross-resistance in acquired paclitaxel-resistant triple-negative breast cancer cells. Br. J. Cancer 109, 1876–1885 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wang Y et al. Blocking the formation of radiation-induced breast cancer stem cells. Oncotarget. 5, 3743–3755 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wu L et al. Disulfiram and BKM120 in combination with chemotherapy impede tumor progression and delay tumor recurrence in tumor initiating cell-rich TNBC. Sci. Rep 9, 236 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Gehrmann M Drug evaluation: STA-4783–enhancing taxane efficacy by induction of Hsp70. Curr. Opin. Investig. Drugs 7, 574–580 (2006). [PubMed] [Google Scholar]
- 142.Yadav AA, Patel D, Wu X & Hasinoff BB Molecular mechanisms of the biological activity of the anticancer drug elesclomol and its complexes with Cu(II), Ni(II) and Pt(II). J. Inorg. Biochem 126, 1–6 (2013). [DOI] [PubMed] [Google Scholar]
- 143.Nagai M et al. The oncology drug elesclomol selectively transports copper to the mitochondria to induce oxidative stress in cancer cells. Free. Radic. Biol. Med 52, 2142–2150 (2012). [DOI] [PubMed] [Google Scholar]
- 144.Berkenblit A et al. Phase I clinical trial of STA-4783 in combination with paclitaxel in patients with refractory solid tumors. Clin. Cancer Res 13, 584–590 (2007). [DOI] [PubMed] [Google Scholar]
- 145.Chakravarty R, Chakraborty S & Dash A 64Cu2+ ions as PET Probe: an emerging paradigm in molecular imaging of cancer. Mol. Pharm 13, 3601–3612 (2016). [DOI] [PubMed] [Google Scholar]
- 146.Qin C et al. Theranostics of malignant melanoma with 64CuCl2. J. Nucl. Med 55, 812–817 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Peng F, Lu X, Janisse J, Muzik O & Shields AF PET of human prostate cancer xenografts in mice with increased uptake of 64CuCl2. J. Nucl. Med 47, 1649–1652 (2006). [PubMed] [Google Scholar]
- 148.Kim KI et al. Detection of increased 64Cu uptake by human copper transporter 1 gene overexpression using PET with 64CuCl2 in human breast cancer xenograft model. J. Nucl. Med 55, 1692–1698 (2014). [DOI] [PubMed] [Google Scholar]
- 149.Jorgensen JT, Persson M, Madsen J & Kjaer A High tumor uptake of 64Cu: implications for molecular imaging of tumor characteristics with copper-based PET tracers. Nucl. Med. Biol 40, 345–350 (2013). [DOI] [PubMed] [Google Scholar]
- 150.Ferrari C et al. Copper-64 dichloride as theranostic agent for glioblastoma multiforme: a preclinical study. Biomed. Res. Int 2015, 129764 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Cai H et al. Reduced 64Cu uptake and tumor growth inhibition by knockdown of human copper transporter 1 in xenograft mouse model of prostate cancer. J. Nucl. Med 55, 622–628 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Capasso E et al. Role of 64CuCl2 PET/CT in staging of prostate cancer. Ann. Nucl. Med 29, 482–8 (2015). [DOI] [PubMed] [Google Scholar]
- 153.Piccardo A et al. 64CuCl2 PET/CT in prostate cancer relapse. J. Nucl. Med 59, 444–451 (2018). [DOI] [PubMed] [Google Scholar]
- 154.Panichelli P et al. Imaging of brain tumors with copper-64 chloride: early experience and results. Cancer Biother Radiopharm. 31, 159–167 (2016). [DOI] [PubMed] [Google Scholar]
- 155.Santini C et al. Advances in copper complexes as anticancer agents. Chem. Rev 114, 815–862 (2014). [DOI] [PubMed] [Google Scholar]
- 156.De Luca A, Barile A, Arciello M & Rossi L Copper homeostasis as target of both consolidated and innovative strategies of anti-tumor therapy J. Trace Elem. Med. Biol 55, 204–13 (2019). [DOI] [PubMed] [Google Scholar]
- 157.Kellett A, Molphy Z, McKee V, Slator C Recent advances in anticancer copper compounds. in Metal-based Anticancer Agents. p. 91–119 (Royal Society of Chemistry, 2019). [Google Scholar]
- 158.Gandin V et al. In vitro and in vivo anticancer activity of copper(I) complexes with homoscorpionate tridentate tris(pyrazolyl)borate and auxiliary monodentate phosphine ligands. J. Med. Chem 57, 4745–4760 (2014). [DOI] [PubMed] [Google Scholar]
- 159.Marzano C et al. [CU(thp)4]n[X]-n Compounds for the Treatment of a Broad Range of Human Solid Tumors, Including Refractory Tumors. US Patent 9114149, Abstract (2015). Inventors; Universita degli Studi di Camerino, Universita degli Studi di Padova, assignee.
- 160.Trejo-Solis C et al. Copper compound induces autophagy and apoptosis of glioma cells by reactive oxygen species and JNK activation. BMC Cancer 12, 156 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Serment-Guerrero J et al. Genotoxicity of the copper antineoplastic coordination complexes casiopeinas. Toxicol. In Vitro 25, 1376–1384 (2011). [DOI] [PubMed] [Google Scholar]
- 162.Hirayama T, Van de Bittner GC, Gray LW, Lutsenko S & Chang CJ Near-infrared fluorescent sensor for in vivo copper imaging in a murine Wilson disease model. Proc. Natl Acad. Sci. USA 109, 2228–2233 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Heffern MC et al. In vivo bioluminescence imaging reveals copper deficiency in a murine model of nonalcoholic fatty liver disease. Proc. Natl Acad. Sci. USA 113, 14219–14224 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Cui L et al. Mitochondrial copper depletion suppresses triple-negative breast cancer in mice. Nat. Biotechnol 39, 357–367 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Iovan DA, Jia S & Chang CJ Inorganic chemistry approaches to activity-based sensing: from metal sensors to bioorthogonal metal chemistry. Inorg. Chem 58, 13546–13560 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ramos-Torres KM, Kolemen S & Chang CJ Thioether coordination chemistry for molecular imaging of copper in biological systems. Isr. J. Chem 56, 724–737 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Morgan MT et al. Stabilization of aliphatic phosphines by auxiliary phosphine sulfides offers zeptomolar affinity and unprecedented selectivity for probing biological Cu(I). Angew. Chem. Int. Ed 57, 9711–9715 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Wang J et al. Inhibition of human copper trafficking by a small molecule significantly attenuates cancer cell proliferation. Nat. Chem 7, 968–979 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Karginova O et al. Inhibition of copper transport induces apoptosis in triple-negative breast cancer cells and suppresses tumor angiogenesis. Mol. Cancer Ther 18, 873–885 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Haddad MR et al. Cerebrospinal fluid-directed rAAV9-rsATP7A plus subcutaneous copper histidinate advance survival and outcomes in a Menkes disease mouse model. Mol. Ther. Methods Clin. Dev 10, 165–178 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Su TA et al. A modular ionophore platform for liver-directed copper supplementation in cells and animals. J. Am. Chem. Soc 140, 13764–13774 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Pinho JO et al. Therapeutic potential of a copper complex loaded in pH-sensitive long circulating liposomes for colon cancer management. Int. J. Pharm 599, 120463 (2021). [DOI] [PubMed] [Google Scholar]
- 173.Pinho JO et al. Copper complex nanoformulations featuring highly promising therapeutic potential in murine melanoma models. Nanomedicine 14, 835–850. (2019). [DOI] [PubMed] [Google Scholar]
- 174.Hunsaker EW & Franz KJ Emerging opportunities to manipulate metal trafficking for therapeutic benefit. Inorg. Chem 58, 13528–13545 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Walshe JM Penicillamine, a new oral therapy for Wilson’s disease. Am. J. Med 21, 487–495 (1956). [DOI] [PubMed] [Google Scholar]
- 176.Brewer GJ et al. Treatment of Wilson’s disease with ammonium tetrathiomolybdate. I. Initial therapy in 17 neurologically affected patients. Arch. Neurol 51, 545–554 (1994). [DOI] [PubMed] [Google Scholar]
- 177.NCRR, University of Michigan. Study of Tetrathiomolybdate in Patients With Wilson Disease https://ClinicalTrials.gov/show/NCT00004339 (1994).
- 178.Bell RG & Smith HW Preliminary report on clinical trials of Antabuse. Can. Med. Assoc. J 60, 286–288 (1949). [PMC free article] [PubMed] [Google Scholar]
- 179.Limited CMDP. Treatment Continuation Study for Patients With ALS/MND Who Completed Study CMD-2019–001 https://ClinicalTrials.gov/show/NCT04313166 (2020).
- 180.Torres JB et al. PET Imaging of copper trafficking in a mouse model of Alzheimer disease. J. Nucl. Med 57, 109–114 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Mao X & Schimmer AD The toxicology of clioquinol. Toxicol. Lett 182, 1–6 (2008). [DOI] [PubMed] [Google Scholar]
- 182.Ltd CP. 64Cu-SAR-bisPSMA and 67Cu-SAR-bisPSMA for Identification and Treatment of PSMA-expressing Metastatic Castrate Resistant Prostate Cancer https://ClinicalTrials.gov/show/NCT04868604 (2021).


