Abstract
Aneurysms are malformations within the arterial vasculature brought on by the structural breakdown of the micro-architecture of the vessel wall, with aneurysms posing serious health risks in the event of their rupture. Blood flow within vessels is generally laminar with high, uni-directional wall shear stressors that modulate vascular endothelial cell functionality and regulate vascular smooth muscle cells. However, altered vascular geometry induced by bifurcations, significant curvature, stenosis, or clinical interventions can alter the flow, generating low stressor disturbed flow patterns. Disturbed flow is associated with altered cellular morphology, upregulated expression of proteins modulating inflammation, decreased regulation of vascular permeability, degraded extracellular matrix, and heightened cellular apoptosis. The understanding of the effects disturbed flow has on the cellular cascades which initiate aneurysms and promote their subsequent growth can further elucidate the nature of this complex pathology. This review summarizes the current knowledge about the disturbed flow and its relation to aneurysm pathology, the methods used to investigate these relations, as well as how such knowledge has impacted clinical treatment methodologies. This information can contribute to the understanding of the development, growth, and rupture of aneurysms and help develop novel research and aneurysmal treatment techniques.
Keywords: Disturbed Flow, Aneurysm, Vascular Cells, Inflammation
I. Introduction
Aneurysms are dilated or ballooned-out sections of the arterial wall due to their structural and mechanical weakening. Many aneurysms remain asymptomatic, posing a minimal threat to patient health, but their rupture causes severe morbidity or mortality. In the cerebral vasculature, aneurysms typically result from focal dilations of an artery, creating a bulbous outcrop (saccular aneurysm) with a well-defined ‘neck’ connecting the aneurysm to the parent artery. Such dilations are prevalent in ~3–5% of adults (Etminan & Rinkel, 2017) and their rupture is fatal in 30–40% of cases (Hackenberg, Hänggi, & Etminan, 2018), with survivors suffering severe neurological damage. Aneurysms are also prominent in the abdominal aorta (AAA), characterized by > 1.5-fold increase in the entire diameter of a vessel area (fusiform aneurysm), as opposed to a saccular aneurysm’s focal dilation of only one side of an artery. AAAs pose a serious health risk, with a 90% mortality rate upon their rupture (Boyd, Kuhn, Lozowy, & Kulbisky, 2016; Schmitz-Rixen et al., 2016). Surgical methods have been employed to protect an aneurysm from rupture yet may not be the ideal clinical option for patients for two reasons. First, surgical complications carry their own risk of morbidity or mortality (Lim et al., 2015), which can occur at a higher rate than that of aneurysm rupture. Currently, the clinical assessment of rupture risk is mainly based on aneurysm geometric characteristics. For example, abdominal aortic dilation typically ≥ 5cm in diameter is thought to pose a high risk of rupture and therefore, is often advised to be treated. Similarly, cerebral aneurysms > 7mm are also assumed to carry the greatest risk to patients(Korja, Lehto, & Juvela, 2014). However, not all large aneurysms rupture, and very small aneurysms may rupture at not-insignificant rates (Ikawa et al., 2019; Lee, Eom, Lee, Kim, & Kang, 2015). Secondly, surgical interventions can place a large economic burden upon patients (Modi et al., 2019; Monsivais et al., 2019; O’Brien-Irr et al., 2017), which go up significantly in the event of complications. Understanding the conditions that impact the initiation, development, and possible rupture of an aneurysm could help the assessment and clinical decision making when dealing with these potentially life-threatening arterial malformations.
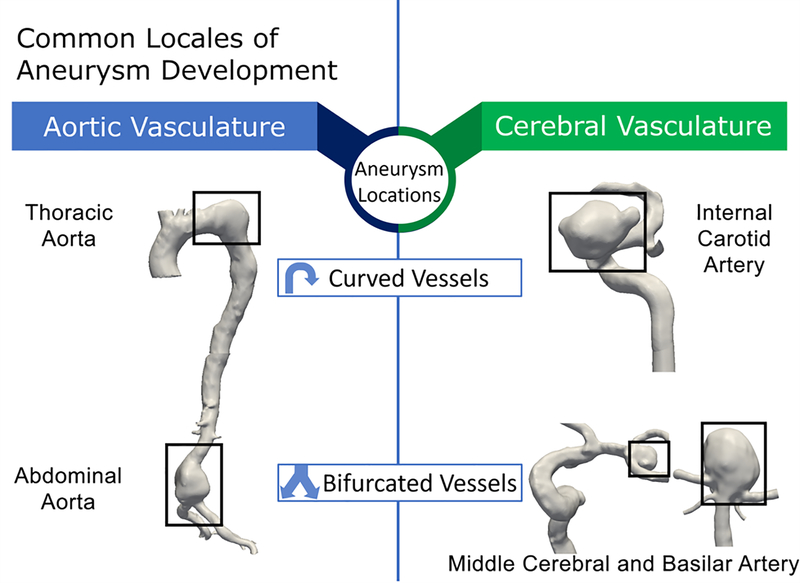
Although the full scope of mechanisms causing the development, expansion and possible rupture of aneurysms are unknown, flow disturbances in the vasculature play an important role. Aneurysms are often located at locales susceptible to disturbed flow (Bacigaluppi et al., 2014; D. A. Chistiakov, Orekhov, & Bobryshev, 2017; X. Liu, Sun, Fan, & Deng, 2015; Morbiducci et al., 2016; Roth et al., 2017), e.g. vessel bifurcations and significant vasculature curvatures (Figure 1). In straight sections of the vasculature, hemodynamic flow follows a laminar, semi-unidirectional pattern with tangential stressors on the vascular wall, referred to as wall shear stress (WSS), at rates that maintain vascular homeostasis and physiology(Baeyens, Bandyopadhyay, Coon, Yun, & Schwartz, 2016), such as ~15 dynes/cm2 in the internal carotid artery and ~4 dynes/cm2 in the supracelliac/infrarenal aorta(C. Cheng et al., 2007) (both areas of common aneurysm development). However, in some specific arterial areas, such as the apex of bifurcations and areas of high curvature, aberrant hemodynamic conditions often occur. Bifurcations in the cerebral arterial system are locales that can give rise to recirculating flow with oscillating stressors and heightened WSS rates at the bifurcation apex and low WSS adjacent to apices. In a study by Dolan et al., areas of high WSS coupled with positive WSS gradients act as a contributor towards pathological arterial remodeling and IA formation (Dolan, Kolega, & Meng, 2013), and this observation has been validated by further studies (Geers et al., 2017). Alternatively, areas of high vessel curvature in the cerebral internal carotid artery cause different detrimental hemodynamic conditions: high WSS along the outer edge of the curve, and oscillating flow with low WSS along the inner curvature. Abdominal aorta bifurcations generate differing flow patterns than their cerebral bifurcation counterparts: low, oscillatory flow with reduced WSS, high WSS gradients at the aortic wall, with the faster, more unidirectional flow in the central portion of the artery. Continued expansion of the aneurysmal wall can further exacerbate localized reductions in WSS and lead to larger areas of flow recirculation. The differences between flow conditions in the abdominal aorta versus cerebral arteries are due in part to adverse pressure gradients generated by the decreasing blood pressure from cardiac systole to diastole. Additionally, the deceleration of flow from the vascular aorta to the abdominal aortic bifurcation with the greatest flow alteration along the arterial wall, coupled with pressure gradients can lead to the reversal and recirculation of flow (Tanweer, Wilson, Metaxa, Riina, & Meng, 2014). Such pressure gradients are less prominent in the cerebral vasculature.
Figure 1:
Areas of high curvature and bifurcations in the aortic and cerebral vasculature are common locales of aneurysm growth (black hollow boxes). Areas represent aneurysm locations at the thoracic and abdominal portion of the aortic vasculature as well as the internal carotid, middle cerebral, and basilar arteries of the cerebral vasculature.
While the arterial system is sensitive to changing WSS, lengthy exposure to stressors outside physiological ranges may damage vascular cells and trigger pathological responses. The correlation of flow disturbances and aneurysms directs research toward 1) understanding disturbed flow’s role in aneurysm development (Achille, Tellides, Figueroa, & Humphrey, 2014; Can & Du, 2015), 2) analyzing disturbed flow to differentiate aneurysms likely to rupture from those to remain stable (Tsuji et al., 2017; Xiang et al., 2016), and 3) developing novel aneurysm treatment methodologies (X. Liu et al., 2015; Sindeev et al., 2018).
Vascular Structure.
Areas of likely aneurysm development, cerebral arteries, and the abdominal aorta are multi-layered systems with each layer helping to maintain arterial physiology: the inner-most tunica intima, middle tunica media, and outer-most tunica adventitia. The intima consists of an endothelial cell (EC) monolayer encapsulating the vessel’s lumenal space and is directly exposed to the hemodynamic environment. The EC layer regulates biomacromolecule permeability (Benn, Bredow, Casanova, Vukičević, & Knaus, 2016; Ghim et al., 2017; Mundi et al., 2017; Reglero-Real, Colom, Bodkin, & Nourshargh, 2016) and acts as hemodynamic mechanosensors transducing fluid force into biochemical signals (Cancel, Ebong, Mensah, Hirschberg, & Tarbell, 2016; Dorland & Huveneers, 2017). A representation of EC mechanotransductor pathways can be seen in Figure 2. Hemodynamic forces regulate ECs, with flow disruptions altering EC morphology, mechanotransductive signaling (Baratchi et al., 2017; J. D. Humphrey, Schwartz, Tellides, & Milewicz, 2015), protein expression (Baeyens et al., 2016; D. A. Chistiakov et al., 2017; Nakajima & Mochizuki, 2017), and the selective permeability of the vasculature (P. Liu et al., 2018).
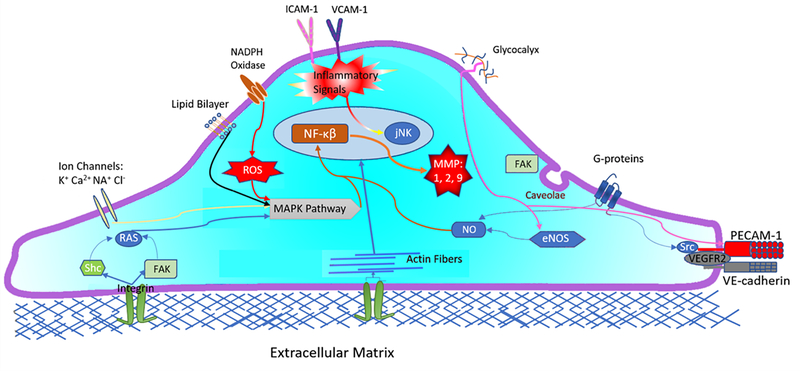
Figure 2:
Endothelial cell mechanoreceptors and their impact on select cellular signal pathways. Flow stressors impact ion channels (K+, Ca2+, Na+, Cl−), G-proteins, caveolae, nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, lipid bilayer, and glycocalyx. Signals are also transmitted from the cytoskeleton to activated integrins, platelet endothelial cell adhesion molecule-1 (PECAM-1), VE-cadherin and VEGFR2. Activated integrins phosphorylate non-receptor tyrosine kinases FAK and Shc which trigger Ras family GTPase. Ras triggers mitogen-activated protein kinases (MAPKs). MAPKs activation is also signaled from reactive oxygen species (ROS) production from NADPH oxidase and is modulated by K+ Cl− from ion channels. Cell-cell PECAM-1, VE-Cadherin, VEGRF2 complex and glycocalyx regulate eNOS and NO expression. NO and MAPK pathways phosphorize transcription factors such as nuclear factor-kappa β (NF-κB) and JNK. NF-κB is needed for metalloproteinase secretion (MMP-1,2,9) which increases cellular inflammation (VCAM-1 and ICAM-1 expression) and matrix degeneration. Increased cellular inflammation activates JNK expression, triggering cellular apoptosis.
The tunica media is made primarily of vascular smooth muscle cells (vSMCs) which regulate vessel contraction/dilation, modulating blood pressure in response to flow. VSMCs also produce a significant portion of extracellular matrix (ECM) molecules, such as elastin, collagen, fibrillin and proteoglycans which maintain arterial biomechanical and contractile integrity (Bogunovic et al., 2019; Michel, Jondeau, & Milewicz, 2018). Altered vSMCs reduce vessel contractility and integrity, features indicative of aneurysms (Jung, 2018; Penn, Witte, Komotar, & Sander Connolly, 2014). Moreover, prolonged pathophysiological conditions can trigger vSMC apoptosis (in high WSS) or heightened proliferation/migration (in low WSS) (Meng, Tutino, Xiang, & Siddiqui, 2014), further weakening the arterial wall and risking possible aneurysm rupture.
While similarities exist in the cellular changes giving rise to AAAs and IAs, differences occur between aneurysm types in terms of their triggers for initiation and expansion. As expressed in a study by Humphrey and Taylor, understating the contrast in the biology and hemodynamics of the differing aneurysm types can help improve the understanding of this vascular pathology (J. Humphrey & Taylor, 2008). A prominent factor of fusiform AAA initiation is intensified inflammation with the development of atherosclerotic plaque(s) that lead to an intraluminal thrombus (Kuivaniemi, Ryer, Elmore, & Tromp, 2015; Quintana & Taylor, 2019). The accumulation of inflammatory components within the artery is subsequently followed by an increased expression of oxidative stress (OS) and loss of vSMCs. These changes, coupled with an increase in collagen I, III, and a decrease in elastin, lead to AAA initiation. Further expansion of an AAA is driven by increases in atherosclerosis, enhanced expressions of proteases, and decreased amounts of collagen and elastin (Brangsch et al., 2017). In contrast, the initiation of saccular IAs is more often triggered by a loss of EC monolayer functionality, elastic lamina loss, and vSMC apoptosis, leading to a thinning of their arterial media (Meng et al., 2014). Whereas the expansion of a developed IA is noted by an increase in collagen I, a decrease in collagen III, IV, and elastin, and more inflammatory infiltrates, which exacerbate cellular apoptosis (Fennell, Kalani, Atwal, Martirosyan, & Spetzler, 2016).
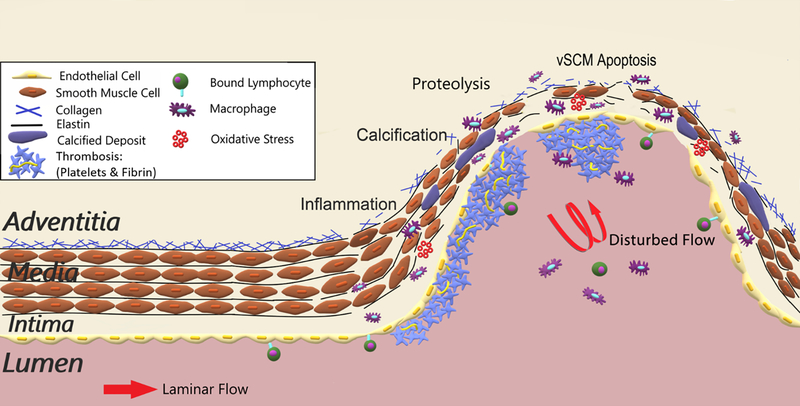
Figure 3 shows the general changes from healthy vessels to (AAA) aneurysms and their possible rupture: degraded EC monolayer, macrophage infiltration, protein breakdown(proteolysis) (Ramella et al., 2018; Sawyer et al., 2016), internal elastic lamina loss (Savastano, Bhambri, Andrew Wilkinson, & Pandey, 2018; Shunli Wang et al., 2018), and decreased collagen (Klaus et al., 2017; P. Liu et al., 2018).
Figure 3:
Diagram of healthy aortic artery to aneurysm pathology: inflammation, calcification, proteolysis, and apoptosis. Molecular triggers for vascular degeneration leading to aneurysms include lymphocyte adhesion, calcification, thombois, macrophage induction, and oxidative stress. These changes induce degradation of the endothelium, inflammation, proteolysis, thinning of the vascular media, breakdown of collagen and elastin, and cellular apoptosis. These changes are exacerbated by disturbed hemodynamic flow within aneurysms.
Early histological analysis of aneurysm tissues uncovered many of the cellular differences between healthy and aneurysmal tissues yet gave minimal insight into disturbed flow’s role in said changes. Several in vitro (Balaguru et al., 2016; Go et al., 2014; Xiang et al., 2016), in vivo (animal models) (Lysgaard Poulsen, Stubbe, & Lindholt, 2016; Y. Wang et al., 2015; Xiang et al., 2016), and in silico (J. Cebral et al., 2017; Seo, Eslami, Caplan, Tamargo, & Mittal, 2018; Tian, Gawlak, O’Donnell, Birukova, & Birukov, 2016) methodologies have been created to study disturbed flow’s impact on cellular structure, function, signal transduction, and protein expression. This paper overviews recent (2014–2020) understandings of disturbed flow’s role in aneurysm pathology, as well as established and novel experimental methods/technologies for investigating disturbed flow. Moreover, how the knowledge of disturbed flow has been utilized to develop new clinical aneurysm treatments is also discussed.
II. Disturbed Flow and Vascular Remodeling
EC Permeability
The permeation of macromolecules, such as macrophages and leukocytes, from inside the vessel lumen into the media layer is regulated by ECs (Cahill & Redmond, 2016b; Claesson-Welsh, 2015). The adventitial pressure caused by interstitial fluid is roughly 10 mmHg, with intraluminal arterial blood pressure at ~130/80 mmHg in humans. This outward pressure gradient across the arterial wall would make macromolecule transport uncontrolled without an internal regulatory layer. An altered permeability layer is associated with weakened arterial strength and aneurysms at higher rupture risk (Michel et al., 2018; Soldozy et al., 2019; Vakil et al., 2015).
Disturbed flow alters ECs’ permeability through the following mechanisms. First, ECs in laminar flow maintain an oblong geometry with cytoskeletal and focal adhesion complexes aligned along with flow directionality, forming parallel actin stress fibers across the cell to minimize mechanical tension (Gimbrone & García-Cardeña, 2016; Jufri, Mohamedali, Avolio, & Baker, 2015). Low WSS (< 5 dynes/cm2) disturbed flow may result in actin fibers aggregating at cell peripheries (Balaguru et al., 2016; Lay et al., 2019; Wong, LLanos, Boroda, Rosenberg, & Rabbany, 2016), changing ECs to a round or polygonal shape, which in turn increases the permeation of macromolecules into the vascular media (Gimbrone & García-Cardeña, 2016). Second, ECs are covered by proteoglycans and glycoproteins, collectively called glycocalyx, which maintains barrier functionality and fluids permeability (Uchimido, Schmidt, & Shapiro, 2019). ECs in laminar flow have a glycocalyx layer 24.4 + 5.95% thicker than those in disturbed flow (Scott Cooper, Emmott, McDonald, Campeau, & Leask, 2018; Harding, Mitra, Mensah, Herman, & Ebong, 2018; Mitra et al., 2018) with minimal glycocalyx coverage in aneurysms (IAs) and in pro-atherosclerotic regions (Bell & Baker, 2016; Diagbouga, Morel, Bijlenga, & Kwak, 2018). Third, degradation of vascular glycocalyx impacts the regulation of vascular endothelial growth factors (VEGFs) (Leblanc et al., 2019). Increased expression of VEGFs, a component of angiogenesis and the uncoupling of EC cell-cell junctions (T. Li et al., 2017; X. Li et al., 2017), triggers EC turnover which can cause ‘leaks’ in the EC monolayer. Fourth, higher expression of VEGF causes a decrease in EC adherence junction proteins such as vascular endothelial cadherin (VE-Cadherin). In disturbed flow, VE-Cadherin displays discontinuous cell-to-cell connections, resulting in ‘gaps’ in the endothelium (Barry, Wang, & Leckband, 2015; Reglero-Real et al., 2016). Disruptions to arterial permeability can also lead to the accumulation of lipoproteins and leukocytes into the vascular media, increasing vessel inflammation (Mundi et al., 2017). Additionally, when the endothelium is significantly degraded, the hemodynamic environment can directly impact vSMCs, changing their structure and functionality. vSMCs exposed to disturbed flow patterns often alter to their synthetic phenotype: a more rhomboid morphology with decreased contractile protein expression and increased elastolytic enzymes that degrade the ECM more than their elongated counterparts in laminar flow (Petsophonsakul et al., 2019). This change also elevates their growth rates and proliferation via phosphatidylinositol 3 kinase (PI3K)–protein kinase B and extracellular signal-regulated protein kinase 1/2 (ERK1/2) expression (Qiu et al., 2014), while reducing the ability of vSMCs to expand or contract in relation to changing hemodynamic conditions, altering the characteristics of the vascular wall and resulting in vascular remodeling, which is a known factor in aneurysm developmen and rupture.
Vascular Inflammatory Response
While inflammation has been shown to play a key role in both AAA and IAs, AAA formation is often driven by heightened atherosclerosis and intraluminal thrombus formation, whereas inflammation-based degradation of an artery is considered as a greater factor in IA expansion and rupture (Piechota-Polanczyk et al., 2015; Quintana & Taylor, 2019; Starke, Chalouhi, Ding, & Hasan, 2015). Studies have demonstrated that chronic inflammatory responses cause ECM degradation in both IAs and AAAs (Andreata et al., 2018; Z. Cheng et al., 2018; Scott Cooper et al., 2018): 3-fold increase in CD45+ leucocytes in IAs vs healthy tissue (Strong, Amenta, Dumont, & Medel, 2015; Yan et al., 2016) and 15% of cells positively stained for CD68+ macrophages in AAAs versus 1% in healthy vessels (Fennell et al., 2016; Quan et al., 2018). Disturbed flow induces a 2-fold increase in expressed EC intracellular adhesion molecule-1 (ICAM-1) (J. Chen et al., 2015) and a 3-fold increase in EC vascular cell adhesion protein 1 (VCAM-1) (Reglero-Real et al., 2016) versus cells exposed to laminar flow. Expression of these molecules enhances lymphocyte, leukocyte, eosinophil, and basophil adhesion to EC cells (Bailey, Moreno, Haj, Simon, & Passerini, 2019; Chung, Kim, Lee, An, & Kwon, 2015). ECs then secrete cytokines, triggering inflammatory cell integrins to strengthen adherence, helping bound inflammatory cells penetrate the EC layer through cell-cell adhesion gaps (Rainger et al., 2015; Yeh et al., 2018). A second change is EC integrin’s response to disturbed flow, triggering 10 times relative increase in the expression of EC phosphodiesterases 4 (PDE4) which inhibits anti-inflammatory factor cyclic adenosine monophosphate (cAMP) (Yun et al., 2016) (AAA). This duality of inflammation and inhibited cAMP heightens atherosclerotic buildup of fats, cholesterol, and macrophages in the vessel media (IA) (Peshkova, Schaefer, & Koltsova, 2016), with pro-inflammatory M1 macrophages shown to be heightened within aneurysmal tissue (Dale et al., 2016). Inflammation also activates foam cells: specialized macrophages which ingest low-density lipoproteins, causing damaging atherosclerotic deposits on vessels (Dimitry A. Chistiakov, Melnichenko, Myasoedova, Grechko, & Orekhov, 2017; D. A. Chistiakov et al., 2017; Ollikainen et al., 2016). A third destructive change via disturbed flow is amplified transcription factor nuclear factor kappa-light-chain-enhancer activated B cells (NF-κβ) located in the animal cell cytosol. NF-κβ regulates pro-inflammatory genes tumor necrosis factor (TNF) cytokines, increasing leukocyte adhesion and transendothelial migration, interleukins (IL)-1β that activate T-lymphocytes cytokines for cellular proliferation, and the cellular migration and infiltration moderator IL-6 (Baeriswyl et al., 2019; T. Liu, Zhang, Joo, & Sun, 2017; Shimizu, Kushamae, Mizutani, & Aoki, 2019). NF-κβ also triggers proteolytic enzyme zinc-dependent endopeptidase matrix metalloperoxidases (MMPs): MMPs-1,2,9 degrade the AAA and IA ECM (Ramella et al., 2017; Signorelli et al., 2015; Turkmani, Edwards, & Chen, 2015), MMP-8,13 increase AAA inflammatory recruitment and transmigration (Travascio, 2017), and MMP-7,12 heighten inflammatory factor expression in IAs (W. Zhang, Qiao, Zhang, Luo, & Sun, 2017). Particularly MMP-2,9 increase expression in aneurysms: 2.4-fold MMP-2 in IAs and 449.2-fold increase in MMP-9 in AAA versus [their] healthy vasculature (Brangsch et al., 2017; Ciavarella et al., 2015; Rojas et al., 2018). Increased inflammation and deregulated anti-inflammatory responses expose vessels to lasting damage via ECM breakdown and weakened mechanical strength of arteries, promoting aneurysms (Z. Cheng et al., 2018).
Disturbed flow also impacts vascular health through the alteration of mast cells, specialized immune cells in connective tissues involved in allergic reaction and inflammation mediation. Mast cell degranulation is triggered in part by sensing damage-associated molecular patterns (DAMPs) from damaged and dying cells, which are heightened in disturbed flow (Gong, Liu, Jiang, & Zhou, 2020). Degranulated mast cells release histamines, proteolytic enzymes, heparin, and inflammatory mediators (Krystel-Whittemore, Dileepan, & Wood, 2016). Released histamine activates EC and vSMC H1 receptors, increasing vascular permeability (Ashina et al., 2015; Krystel-Whittemore et al., 2016; Signorelli et al., 2015) and contractility as to expel allergens from the body (Krystel-Whittemore et al., 2016) in both cerebral and aortic arteries. Mast cells degranulation also releases inflammatory cytokines: TNF-α (Peshkova et al., 2016; Sathyan et al., 2015) which regulate immune cells, interleukins IL-1β and IL-5 which stimulate B cell growth, chemoattractant cytokines IL-6, IL-8 and IL-12 which increase leukocyte cytotoxic T cells and natural killer cell production (Baratchi et al., 2017; Chatterjee, 2018; Green et al., 2018; Nishihara et al., 2017; Pan et al., 2017). Increased degranulation causes tight junction breakdown, inflammatory cell recruitment, and ECM remodeling (Brangsch et al., 2017; Ciavarella et al., 2015; Rojas et al., 2018).
The intensified intrusion of inflammatory factors into the vessel media lead to alterations and degradation of vSMCs. Inflammatory cytokines TNF-α and IL-1 trigger an increase in vSMC ICAM-1, IL-6, and MMPs. For example, the increase of ICAM-1 can improve the monocyte/macrophage and T-cell adhesion to cells, converting vSMCs from a contractile phenotype to their synthetic phenotype, and spuring atherosclerotic buildup/plaques (Novikova, Laktionov, & Karpenko, 2019).
Endothelial Cell Mechanotransduction
Disturbed flow alters EC expression of several cellular mechanotransduction complexes. First, disturbed flow alters platelet endothelial cell adhesion molecules (PECAM-1) (Moriguchi & Sumpio, 2015), increases EC growth factor (VEGF) and decreases VE-cadherin (Chatterjee, 2018). These altered molecules trigger cellular signals that increase EC immune cell adherence, leukocyte recruitment, and transendothelial migration. Second, disturbed flow impacts WSS sensitive integrin (Sun et al., 2016; Xanthis et al., 2019), transducers that activate Src homology domain 2-contaning kinase (Shc) (D. A. Chistiakov et al., 2017) and focal adhesion kinases (FAK) (Harada et al., 2017), which activate NF-κB (Baeriswyl et al., 2019). Activated NF-κB increases cellular response to cytokines and WSS through p38 mitogen-activated protein kinase (MAPK) (Moriguchi & Sumpio, 2015) and heightened cellular proliferation and apoptosis through Jun-amino-terminal kinase (JNK) (Nakajima & Mochizuki, 2017).
Additionally, while membrane-bound glycocalyx helps maintain EC permeability, it also plays a role in hemodynamic mechanotransduction (S. Cooper, McDonald, Burkat, & Leask, 2017; Harding et al., 2018; Mitra et al., 2018). Under physiologic laminar flow, glycocalyx triggers a 2-times relative increase in eNOS versus disturbed flow (Harding et al., 2018) and subsequent nitric oxide (NO) production (Cancel et al., 2016; Zeng, 2017). NO regulates vascular dilation/tone and limits vascular inflammation through the inhibition of NF-κB (Cha, Hwang, Kim, & Jun, 2018; McDonald, Cooper, Danielzak, & Leask, 2016) which inhibits the expression of ICAM-1. Disturbed flow degrades components of EC glycocalyx: sialic acid glycoproteins (Harding et al., 2018), long-chain polysaccharides heparan sulfate (McDonald et al., 2016), and hyaluronic acid (Mitra et al., 2018). Altered expression of these components hinders glycocalyx’s ability to function as a mechanotransductor, reduces expression of eNOS, increases ICAM-1, and lessens ECs function to regulate permeability (S. Li et al., 2015; W. Li & Wang, 2018; Mitra et al., 2018).
Alterations to EC mechanotranscuction also change intercellular signaling, which can stimulate vSCMs in the tunica media to undergo functional changes and/or increased proliferation. In a study by Zhu et al., atherogenic, disturbed shear stress was shown to increase the activation EC membrane-bound protein 3 (VAMP3) and synaptosomal associated protein 23 (SNAP23). Activation of these proteins cause the subsequent release of nonmembrane-bound microRNA miR-126–3p, an intercellular molecule that increases turnover of the vSMCs, leading to smooth muscle cell hyperplasia(Zhu et al., 2017).
Oxidative Stress on Vascular Cells
In arterial ECs, the maintenance of reactive oxygen species (ROS) and antioxidant defenses such as nitric oxide (NO) which inhibits platelet function and inflammatory cell adhesion (Karimi Galougahi, Ashley, & Ali, 2016; Ritchie, Drummond, Sobey, De Silva, & Kemp-Harper, 2017), must be balanced to maintain vascular health. A breakdown in said balance is referred to as Oxidative Stress (OS) and triggers pathophysiological cellular dysfunction via inflammation (Cahill & Redmond, 2016a), degraded adherence junctions (Chattopadhyay, Raghavan, & Rao, 2017), cellular proliferation, angiogenesis, and cellular apoptosis. Several ROS sources exist within the endothelium, forms of NADPH oxidase (NOX1 and NOXO1) (Siu, Gao, & Cai, 2016), mitochondrial oxidase, and iNOS (Burger, Turner, Munkonda, & Touyz, 2016; Siu et al., 2016; Tong et al., 2016). ECs exposed to disturbed flow alter the balance of ROS and OS defenses through multiple mechanisms. First, disturbed flow triggers a 1.5-times relative increased expression of NOX over laminar flow (D. A. Chistiakov et al., 2017; Yuan et al., 2018). Changes in NOX expression also cause a positive feedback loop within the vasculature: NOX activates ROS, which increases NOX, exacerbating vascular OS (Forrester, Kikuchi, Hernandes, Xu, & Griendling, 2018). Second, disturbed flow and NOX reduces the expression of atheroprotective endothelial nitric-oxide synthase (eNOS): 50% decrease versus laminar flow (Harding et al., 2018). eNOS aids in NO synthesis, with reductions weakening ROS/OS regulation (Balaguru et al., 2016). Third, in the presence of macrophages, NOX causes an increase in O2− and a 1.5-fold increase in H2O2 (Forrester et al., 2018; Hsieh, Liu, Huang, Tseng, & Wang, 2014; Siu et al., 2016). Amplified vSMC H2O2 exacerbates ECM osteopontin expression, an inflammatory mediator promoting vascular macrophage infiltration and lipid oxidation, generating atherosclerotic lesions which break down collagen and elastin, weakening the arterial media (Byon, Heath, & Chen, 2016). Increased O2− oxidates low-density lipoproteins which instigate vascular inflammation through monocyte and macrophage infiltration into the vessel wall (Q. Chen, Wang, Zhu, Xiao, & Zhang, 2018). Macrophages in the presence of OS also increase the proinflammatory cytokine TNFα which activates YAP/TAZ transcriptional factors for the regulation of VCAM-1 production (Choi, Kim, Kim, Seo, & Heo, 2018; Signorelli et al., 2018), increasing leukocyte adhesion and infiltration into the intima.
As aforementioned, the breakdown of the endothelium exposes the underlying vSMCs to direct hemodynamic tension, and OS which also has negative impacts on vSMCs’ functionality. Low shear stress has been shown to trigger ROS within vSMCs and enhance proprotein convertase subtilisin/kexin type 9 (PCSK9), an enzyme that regulates cholesterol via low-density lipoprotein receptor (LDLr) expression (Ding, Liu, et al., 2015). vSMC exposed to low-shear disturbed flow displays a bi-direction increase in PCSK9 and ROS, with each enhancing the expression of the other. This duality negatively impacts LDLr and leads to LDL deposition in the vasculature, increasing inflammation and/or exacerbating a possible atherosclerotic environment.
Cellular Apoptosis
When exposed to low (<2 dynes/cm2) WSS recirculating flow, the endoplasmic reticulum of arterial cells has reduced capability to correctly fold proteins, instead of releasing stress receptor-bound proteins to trigger apoptotic signaling (Chung et al., 2015). One such change is the 3-times increase in the relative expression of DNA damage-inducible transcript 3 protein/C/EBP homologous proteins (CHOP) in disturbed flow versus laminar flow. While the full extent of its impact on cellular cascades is unknown, increased expression of CHOP is critical of cellular apoptosis (Y. Li, Guo, Tang, Jiang, & Chen, 2014). The second group of proteins is apoptotic-signaling cysteine proteases (caspases) (Chung et al., 2015; Pan et al., 2017) with a 4-times relative expression increase of caspase3 (Serbanovic-Canic et al., 2017) and caspase12 (Pan et al., 2017) within recirculating flow versus cells in laminar flow. A third group are interleukins, with ECs in disturbed flow and treated with interleukin-1 receptor antagonist (IL-1RA), an inhibitor of pro-inflammatory IL-1β, exhibiting a 2-times decrease in the relative expression of CHOP and 2.75-times decrease in apoptosis compared with non-treated cells (Pan et al., 2017). Increased apoptosis has been noted via histological analysis of areas known to elicit disturbed hemodynamic flow: remodeled vessels (Emrich et al., 2015; Mahmoud et al., 2014; Zhao, Zhang, & Su, 2018) and aneurysms (Guo et al., 2016; L.-X. Jia et al., 2015).
Heterogeneity of Aneurysmal Wall Characteristics
The cellular changes associated with aneurysm initiation, expansion, and possible rupture are not uniform throughout affected arteries. Within the abdominal aortic wall, different degrees of ECM alteration (Jana, Hu, Shen, & Kassiri, 2019) and intraluminal thrombi (Leach, Kao, Zhu, Saloner, & Hope, 2019), have been noted and lead to non-uniform expansion of the vessel/AAA geometry. Varied expansion of the AAA also modifies hemodynamic dynamics within the afflicted artery (Salman, Ramazanli, Yavuz, & Yalcin, 2019), with areas of flow recirculation associated with greater platelet aggregation and thrombus deposition (Boyd et al., 2016). Change within the wall of IAs also varies in cellular alteration (Morel et al., 2018) and levels of cellular apoptosis (Juan Cebral et al., 2017), which play a role in the non-uniform expansion of an aneurysmal sac. In IAs, areas of higher flow vorticity and WSS are often associated with an increase in vascular inflammation, whereas areas of the aneurysmal wall exposed to lower WSS have a greater loss of vSMCs and increased wall degeneration (Juan Cebral et al., 2017). Studies have also shown the collagen content within the walls of aneurysms (IA and AAA) vary, with non-uniform collagen diameter, density, and orientation within the arterial lumen causing heterogenous mechanical strength of the aneurysm wall (Boyd et al., 2016; Juan Cebral et al., 2017; Morel et al., 2018; Rao et al., 2015; Robertson et al., 2015; Salman et al., 2019; Urabe et al., 2016). The varied expansion and mechanical strength of aneurysms, in turn, alter WSS extrema within a system: simulated aneurysms with assumed homogenous wall characteristics have altered wall stressors versus simulations incorporating measured (varied) wall thickness (Voß et al., 2016). Further understanding of how heterogenous mechanical strength of the aneurysmal wall impacts hemodynamics, or how hemodynamic conditions could trigger differing levels of vascular degradation, could improve the understanding aneurysm development and possible rupture.
Disturbed flow’s impact on arterial cells triggers a multitude of cellular cascades that cause pathologic vascular changes indicative of aneurysms. Increased inflammatory adhesion receptors, a breakdown in cell-cell adhesion proteins, and higher levels of oxidative stress can all lead to increased inflammation in the vasculature, cellular apoptosis, and weakening of the mechanical strength of the vessel wall. These changes result in a feedback loop phenomenon in which the maladaptive bulging of the vessel wall exacerbates disturbed flow patterns, leading to further cellular changes and possible rupture of aneurysms.
III. Methods for Studying Disturbed Flow on Vascular Cells
Methodologies developed to study disturbed flow’s impact on arterial cells have proven beneficial to understanding the complex cellular cascades indicative of aneurysms, as well as helping to improve clinical treatment options. Methodologies can be separated into 3 categories, in-vitro, in-vivo, and in-silico, each with their respective strengths in studying aneurysms.
In vitro systems
A variety of in vitro systems based on the principle of parallel-plate flow chambers (PPFC) have been employed to examine the cellular changes caused by disturbed flow. Specially designed flow chambers allow the study of desired flow patterns/shear stresses over controlled time periods and specific geometries, while providing a highly controlled and reproducible dynamic environment to the cells. PPFCs incorporate (at minimum) two plates spaced at a fixed geometry to create a flow channel, with channel height and width helping to control WSS generated in the system (Equation 1).
| (1) |
where Q is the flow rate, W and H are chamber width and height, μ (in Poise) as fluid dynamic viscosity, and τ is WSS (dyn/cm2). In PPFCs, a cellular monolayer is cultured onto a bio-inactive substrate such as glass or silicone and placed into a chamber to which flow is moved through the channel using a syringe or peristaltic pump. Characteristic values of flow waveforms and the generated wall shear stressors within PPFCs are often validated against measured flow data (ie. color Doppler ultrasound) from areas of flow interest within the vasculature (Y.-X. Wang et al., 2016). While PPFCs with rigid adherence to geometry (based on Equation (1)) were invaluable in the study of hemodynamics’ impact on arterial cells, they were incapable of determining how sudden stressor changes altered cells. Modified PPFCs have been developed with sudden geometric changes or varied chamber height across the flow path to generate both differing levels of WSS and varying WSS gradients within the same system (Sonmez, Cheng, Watkins, Roman, & Davidson, 2020). Such chambers make it possible to study how stressor variations impact arterial cells. The findings in PPFCs are often validated via comparison to histological/genetic/protein analysis of vascular cells in vivo. Such work has shown that similar changes exist between cells in PPFC and in vivo models for chemokine upregulation, neutrophil infiltration, increased activation of pro-inflammatory pathways (i.e NF-kβ) (Nowicki et al., 2014), growth factors and proliferation/migration (L. Jia et al., 2020), and nitric oxide production (Ding, et al., 2015).
To generate disturbed flow patterns, a ‘step-down’ section following the PPFC flow inlet results in well-defined swirling flow, followed by areas of flow reattachment, and resumed laminar flow (Gomez-Garcia et al., 2018; Tovar-Lopez et al., 2019). However, the total area exposed to disturbed flow in step-down PPFCs is minimal (~1mm monolayer length) and does not allow for easy modification of disturbed flow patterns, meaning studying varied disturbed flow characteristics is difficult to perform (Sonmez et al., 2020).
To overcome PPFC’s limitations, cone-plate viscometers utilize a bottom plate seeded with cells, with a rotatable top cone at fixed angle θ. Shear stress within the system can be calculated as:
| (2) |
with T as the % full-scale torque (dyne cm), r as the system radius, and ω as cone speed (rad/sec). Cone-plate viscometers can generate continuous, pulsatile laminar, or controlled disturbed flow patterns (Franzoni et al., 2016). Another system to expose cells to swirling flow is orbital shakers, maneuvering a plate seeded with cells in a circular motion to induce flow patterns(Warboys, Ghim, & Weinberg, 2019). Such systems helped uncover disturbed flow’s impact on cellular degeneration, endothelial senescence (Warboys et al., 2019), and barrier dysfunction (Z. H. Liu et al., 2017). yet typically only create uniform, temporally stable swirling flow patterns. Such uniformity and/or stability of flow disturbances is atypical of conditions in vivo. The inability of standard PPFCs to generate complex disturbed flow that better mimics flow within aneurysms limits the understanding of pattern characteristic’s impact on cellular changes.
Novel fabrication of flow chamber models which mimic aneurysm geometry helps overcome the limitations of many PPFCs, generating flow patterns more akin to those occurring in-vivo. One such chamber type focuses on the creation of flow channels with a simplistic bulbous portion at the bifurcation apex, mimicking a vessel bifurcation and a saccular IA respectfully (Nowicki et al., 2014). Another method casts polymeric organosilicon polydimethylsiloxane (PDMS) around a cylindrical object such as poly(methyl methacrylate) PMMA optical fibers with a drop of water-soluble sucrose wax added to the fiber to model a tubular structure with a simple saccular aneurysm (Mannino et al., 2015). Removing the fiber post-PDMS coating creates a hollow flow channel that can be seeded with ECs. Advancements in 3D printing have also been utilized to create “patient-specific” vessel models for studying aneurysms (Kaneko et al., 2018). A person’s 3D vascular structure is extracted from medical imaging data and fabricated using a 3D printer. The structure can then be coated in PDMS, and once cured, the printing material can be removed/dissolved and the now hollow PDMS structure can be seeded with ECs. Cells exposed to flow in said printed models mimic characteristics seen in-vivo: ECs elongated (0.2 minor/major axis) along with flow directionality and low inflammatory expression in straight areas while having an irregular shape (0.6 minor/major axis) and increased VCAM-1 expression in the aneurysmal sac(Kaneko et al., 2018). While such advancements in flow chambers better mimic flow conditions occurring in-vivo, flow patterns and WSS within complex models cannot be easily calculated as in PPFCs, instead of computational fluid dynamic (CFD) simulations must be utilized to understand the flow within these chambers: It is worth noting that such flow chambers, while novel designs can better represent the geometry of aneurysms, they are often constructed with rigid materials, or materials that may not accurately represent the material properties of aneurysmal tissue and their ability to expand or contract under varied flow conditions. Flow chambers also typically only allow the study of a monolayer of cells, limiting the ability to study how flow conditions trigger EC changes and the subsequent changes to vSMC within the same environment, thereby requiring higher degrees of system complexity to study the EC/vSMC interaction (van Engeland et al., 2018).
In-silico Systems: Computational Fluid Dynamic Simulation and Analysis
With advancements in computational power and modeling software, CFD in-silico tools have been shown to generate simulated flow within vessels that have non-significant differences to data measured in their in-vivo counterparts (Castro, Olivares, Putman, & Cebral, 2014; M. O. Khan, Steinman, & Valen-Sendstad, 2017). This has allowed the adaption of studying in-silico flow within complex “subject-specific” vasculatures (J. Cebral et al., 2017; Seo et al., 2018) to determine flow characteristics indicative of aneurysms (Arzani & Shadden, 2015; Boyd et al., 2016; Geers et al., 2017). Such work has shown disturbed flow as correlated with aneurysm growth and rupture (J. Cebral et al., 2017; Muhammad Owais Khan, Chnafa, Steinman, Mendez, & Nicoud, 2016; Varble, Xiang, Lin, Levy, & Meng, 2016), while histological data from patients undergoing intracranial aneurysm surgery have helped corroborate this connection to disturbances in simulated vascular flow to areas of cellular changes and aneurysm rupture (J. Cebral et al., 2017; Cebral et al., 2016). Theoretically, CFD models have strengths over current in-vivo measured flow, as clinically measured phase-contrast MRI has limited spatiotemporal resolution (1.0–3.5mm) and assumes flow uniformity within MRI resolution cells, reducing its accuracy in assessing flow in and around aneurysms (Itatani et al., 2017). In contrast, CFD models achieve markedly better resolutions versus MRI, allowing an enhanced depiction of hemodynamics in and around aneurysms.
While CFD-derived flow data improved the understanding of disturbed flow in relation to aneurysms, it uses assumptions concerning real cardiovascular flow: modeling blood as non-Newtonian and rigid vessel walls. Simulated wall rigidity simplifies CFD calculations and reduces simulation time yet causes known increases in simulated WSS (Yamaguchi et al., 2019), whereas vessels are compliant in relation to blood pressure and vessel physiology. While blood can be modeled as a Non-Newtonian fluid, adopting an appropriate blood model would alter flow velocity profiles within micro or stenotic vessels (Sriram, Intaglietta, & Tartakovsky, 2014). To ensure CFD assumptions do not lead to an improper assessment of disturbed flow, simulation results should be routinely cross-validated against phase-contrast MRI measurement, though neither can currently be treated as a gold standard under aneurysmal flow conditions. Additionally, studies often ensure in silico and in vitro flow systems agree with each other, in order to validate experimental outcomes. Simulated flow has shown to highly mimic the flow within in vitro systems (Hynes et al., 2020; Jain, Jiang, Strother, & Mardal, 2016; Y. X. Wang et al., 2016).
In-vivo
Longitudinal assessment of disturbed flow’s impact on vascular tissue in-vivo is fraught with difficulty: human histological samples are typically available only during aneurysm repair or post-rupture (Tavares Monteiro et al., 2014; Taylor et al., 2015; Welleweerd et al., 2015) making prolonged analysis of a subject unfeasible. Additionally, the majority of aneurysms remain asymptomatic, reducing the number of patients applicable for study (Thompson et al., 2015). To rectify this, techniques have been developed to create disturbed flow and aneurysms in animal models. One such method is the casting of a tapered conical device around a vessel, or the partial ligation of a vessel, allowing the study of cellular changes within varied flow conditions in a single animal (Dimitry A. Chistiakov et al., 2017; S. Cooper et al., 2017; Sheinberg et al., 2019). Conical devices cause continued narrowing of the vessel lumen, increasing WSS as the lumen narrows, and a region of swirling flow posterior to the cast (Bowden et al., 2016; Pfenniger et al., 2015). Ligation causes a sudden restriction of vessel diameter, generating an area of chronic hypertension, disturbed flow post ligation, and oscillating stressors prior due to a degree of flow reversal. Ligated or casted vessels are shown to have 1.07+0.31 increased loss of rabbit internal elastic lamina (Tutino et al., 2016), thinner media, and 1.5 fold increase NF- κβ (Go et al., 2014) versus normal arteries (Liaw, Fox, Siddiqui, Meng, & Kolega, 2014).
Aneurysm histological analyses show degraded ECM elastin, collagen, and fibronectin (Brangsch et al., 2019; Nosoudi et al., 2015; Platt & Shockey, 2016), so a second in-vivo method to create aneurysms was developed via chemically inducing such degradation. Elastase, a serine protease enzyme that cleaves elastin peptide bonds (Busch et al., 2016; Savastano et al., 2018) injected into a ligated vessel lumen and incubated for 20–30 minutes, degrades elastin, and inhibits Ca2+ inflow for vSMC contraction. Elastase-weakened vessels exposed to disturbed flow can result in aneurysmal growth (Brinjikji, Ding, Kallmes, & Kadirvel, 2016; Busch et al., 2016; Rowinska et al., 2014): 80% increased abdominal aorta diameter versus control (Shi Wang et al., 2018). It is worth nothing, arterial degradation stabilizes post-treatment, making elastase the primary culprit behind continual aneurysm growth unlikely, instead continued expansion being driven by disturbed flow (Bi et al., 2015). CaCl2 has also been used to trigger aneurysm development via the application of a calcium-chloride-soaked gauze to the arterial tunica adventitia. Absorbed calcium converts to calcium phosphate and precipitates into hydroxyapatite crystals in elastin (Androutsopoulou et al., 2018; John W Thompson et al., 2019). Calcium–elastin complexes decrease vessel elastin by 50% (Michineau et al., 2014), weakening vessel strength (Cao et al., 2017), and thinning the wall by 24% (Androutsopoulou et al., 2018). Chemically induced aneurysms show similar calcification, inflammation, OS, degraded elastin, vSMC apoptosis (Patelis et al., 2017; Sénémaud et al., 2017), and have geometric and resultant flow characteristics akin to natural IAs(Shunli Wang et al., 2018) making them ideal for testing novel treatments (Ley et al., 2017; Setozaki et al., 2017) and studying aneurysmal flow. To note, chemical degradation causes relatively uniform vessel alterations, yet changes in natural aneurysms are typically non-uniform (Samaniego, Roa, & Hasan, 2019) (as mentioned previously).
The third method for aneurysm creation is rodent genetic knockout models. Tissue inhibitor metalloproteinase (TIMP) hinders MMPs, increases lipid accumulation and ECM breakdown, and weakens the artery. TIMP−/− rodents having a 2.5-fold increase of atherosclerotic lesions and 33% decreased vessel thickness (X. Zhang, Ares, Taussky, Ducruet, & Grandhi, 2019), and develop larger aneurysms post-elastase incubation versus TIMP+/+ (Maguire, Pearce, Xiao, Oo, & Xiao, 2019b). Atherosclerosis-prone apolipoprotein E-deficient (apoE−/−) mice exploit the link between the aneurysm and high lipid concentration (Y. Wang et al., 2017), adventitia thickening, and vascular injury(Lo Sasso et al., 2016). ApoE−/− mice exposed to angiotensin II, a primary effector hormone for vasoconstriction, result in inflammation, EC dysfunction (Lo Sasso et al., 2016), and have increased aneurysm development: 77%(Ho et al., 2016) and 79% (Umebayashi et al., 2018) apoE−/− mice developing aneurysms compared with none in apoE+/+ mice. Aneurysms in apoE−/− mice have features akin to human AAA aneurysms: altered permeability, increased inflammation, and weakened mechanical properties (Sénémaud et al., 2017). eNOS−/− rodent models exploit arteries’ NO reliance to maintain the integrity by altering NO generating enzymes (Liaw, Dolan Fox, Siddiqui, Meng, & Kolega, 2014; John W. Thompson et al., 2019). eNOS−/− mice fed a high-fat diet (Pulathan, 2018) or exposed to angiotensin II (Phillips et al., 2018) have increased AAA development. Alternatively, iNOS−/− mice infused with angiotensin II showed minimal EC dysfunction or aneurysm depressed IA growth(Kossmann et al., 2014; Y. Wang et al., 2015).
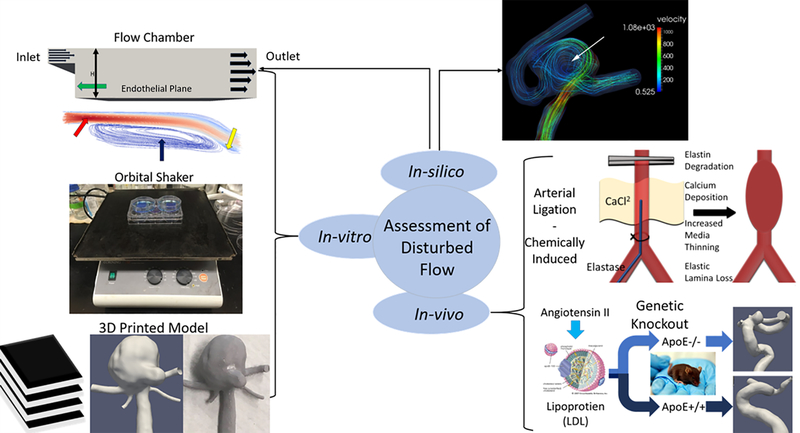
Methods used to study disturbed flow patterns and resultant vascular cell changes can be seen in Figure 4.
Figure 4:
Overview of methodologies for studying disturbed flow in the vasculature/aneurysms. In-vitro models such as step-down flow chambers (step-down, inlet, disturbed flow, flow re-attachment: green, red, blue, and yellow arrows respectively), orbital shaker, and 3D printed vascular models (medical imaging data, computational model, 3D printed) allow the study of cultured cells under controlled flow. In-vivo techniques such as arterial ligation, chemically induced aneurysms (elastase injection or CaCl2 exposure), and genetic knockout animals (lipoprotein image from https://www.britannica.com/science/lipoprotein#/media/1/342894/92254). In-silico simulated flow is often used alongside other methodologies to analyze flow characteristics within 3D in-vitro models or in-vivo developed aneurysms (white arrow).
IV: Applying Knowledge of Disturbed Flow Towards Clinical Outcomes
Surgical Interventions to Reduce Aneurysm Rupture
Surgical protection from rupture involves isolating the aneurysm from flow, yet methods vary depending on aneurysm type: saccular or fusiform. For saccular aneurysms, one option involves opening a section of the skull and implanting a metallic clip across an aneurysm’s ostium to seal it from the blood flow. Less invasive endovascular implantation of stents (a flexible low porosity membrane) diverts flow away from the aneurysm (Caroff et al., 2016), promotes stable aneurysm thrombi (Sarrami-Foroushani et al., 2019), and eventually helps regenerate an EC layer to seal the aneurysm. Endovascular insertion of platinum or titanium coils into the aneurysm sac limit also aims to limit aneurysmal flow, causing a 13–60% reduction in intra-aneurysmal velocity, varying by aneurysm location and coil length/stiffness (Fujimura et al., 2018). In broad-necked saccular aneurysms, neck >4mm or dome/neck ratio <1.5 (Piotin & Blanc, 2014), coils are often paired with stents to prevent coil dislocation (Nikoubashman, Pjontek, Brockmann, Tolba, & Wiesmann, 2015; Wu et al., 2019). Fusiform aneurysm treatments typically fall under two categories: open repair or endovascular repair. In open repair, a highly invasive incision is made in the body and the aneurysm surgically opened. A polytetrafluoroethylene or polyethylene terephthalate graft is then sewn into the vessel, isolating the weakened aneurysmal wall from the blood flow. Endovascular repair techniques insert a stent-graft into the femoral artery, which is guided to the area of the aneurysm, and opened and fastened in place to protect the aneurysm from the flow. While less invasive than open repair, endovascular repair may need additional surgery if the stent-graft placement requires future adjustment.
While stents limit aneurysm rupture risks, their design and implantation can cause unintended disruptions to flow within a treaded vessel (Ng et al., 2017; J. Wang et al., 2018). In-silico analysis of stents shows strut orientation can generate areas of low and oscillating WSS (Wei, Leo, Chen, & Li, 2019). CFD studies also show stent-strut geometry impacts the characteristics of generated disturbed flow, with rounded versus rectangular struts reducing the size of flow disturbances by a factor of two (Jiménez et al., 2014). With an ever-increasing amount of commercially available stents, understanding stent geometry, resultant hemodynamic patterns, and their impact on thrombi formation (Ngoepe, Frangi, Byrne, & Ventikos, 2018) is needed to help guide treatment options as to maximize patient outcomes (Paliwal et al., 2016; Wei et al., 2019).
Understanding the risks of disturbed flow has also guided the development of modified surgical techniques for arterial bypass, which diverts blood flow around diseased arteries to improve circulation (X. Liu, Sun, Fan, & Deng, 2014). A potential complication of an arterial bypass is Intimal Hyperplasia (thickening) occurring at grafting sites (Meirson et al., 2015; Stella, Vergara, Giovannacci, Quarteroni, & Prouse, 2019). CFD studies determined bypass grafts can generate areas of recirculating flow, triggering resultant Hyperplasia. Such work stresses the necessity of determining an optimal graft angle to limit unintended flow disturbances. As ideal graft angles are impacted by patient blood flow waveforms and arterial geometry, the application of in-silico tools to test potential graft placement/angles prior to surgical intervention could help to maximize clinical outcomes (Aliseda et al., 2017; Khruasingkeaw, Khunatorn, Rerkasem, & Srisuwan, 2016; Leong, Nackman, & Wei, 2018).
Pharmaceutical Intervention on Aneurysms
While surgical aneurysm treatments exist, pharmacological inhibition of cellular changes indicative of aneurysms is another avenue investigated for the clinical management of this pathology. Drugs to reduce cholesterol levels and alter lipid metabolism have been utilized to with reducing AAA rupture and lowering mortality post-AAA repair (Kokje, Hamming, & Lindeman, 2015; Salata et al., 2018), though conflicting outcomes have been seen in the use of such drugs to limit IA rupture (Can et al., 2018; Yoshimura et al., 2014). Pharmacological suppression of vascular inflammation in IAs and AAAs is targeted through a number of means: non-steroidal anti-inflammatory drugs (NSAIDs) (Y. F. Liu, Bai, & Qi, 2016; Ma et al., 2018; Nassiri et al., 2016), IL-1 inhibitors, and inhibiting mast cell degranulation (Aoki, 2015; Furukawa et al., 2020). Pharmaceutical control of NF-κB has been investigated to limit macrophage-induced vascular degradation: ~1.75-fold decrease in vessel diameter versus untreated vessels post elastase exposure (Fisher & Demel, 2019; Ren et al., 2016; Zhong et al., 2019). Other avenues include MMP inhibition (Maguire, Pearce, Xiao, Oo, & Xiao, 2019a), and antioxidants to reduce ROS/OS-induced cellular senescence and apoptosis (Lu et al., 2016; Yu et al., 2016; Yue, Chang, Xiao, Qi, & He, 2018). Yet inconsistent preclinical successes in the pharmacological suppression of aneurysm development/expansion suggest an improved understanding of cellular pathways and/or larger clinical trials are needed to improve such treatment options becoming a clinical staple (Lindeman & Matsumura, 2019).
Prediction of Aneurysm Development or Rupture
Aneurysm surgical complications can result in patient morbidity or mortality (Lim et al., 2015) and occur at higher rates than per-year rupture rates: 4–7% patient complication rate (W. Brinjikji et al., 2016; Kallmes et al., 2015) versus yearly rupture rate 0.76% (Murayama et al., 2016)-3.6% (Hishikawa et al., 2015) varied by patient age. It would benefit clinicians to discern aneurysms at high rupture risk from those unlikely to rupture to limit potentially risky and unnecessary treatments. Significant research has focused on identifying aneurysmal geometric and WSS characteristics indicative of aneurysm pathology (Detmer et al., 2018; H. Jiang et al., 2014; P. Jiang et al., 2018; Muluk, Muluk, Shum, & Finol, 2017; Qin et al., 2017; G.-x. Wang et al., 2019). Yet studies vary in characteristics used as well as resultant model strengths (Bijlenga et al., 2017; Qin et al., 2017; Valen-Sendstad & Steinman, 2014; Zhou, Zhu, Su, & Li, 2017). Also, WSS only identifies near-wall flow characteristics, overlooking bulk flow patterns in the aneurysmal sac. Disturbed flow analysis aims to improve both predictions of areas likely to develop aneurysms and rupture risk assessment by understanding the hemodynamic environment indicative of this pathology (Byrne, Mut, & Cebral, 2014; Fukazawa et al., 2015; Long et al., 2015; Kevin Sunderland & Jiang, 2019; K Sunderland et al., 2020; Varble, Trylesinski, Xiang, Snyder, & Meng, 2017).
Aneurysm Treatment through Regenerative Medicine
Confounding the cellular alterations induced by vortical flow via regenerative medicine has become an area of significant interest to combat aneurysms. Mesenchymal stem cells (MSCs) can be differentiated into a few cell types such as osteoblasts, adipocytes and chondrocytes as well as possessing anti-inflammatory and immunomudulatory properties (Yamawaki-Ogata, Hashizume, Fu, Usui, & Narita, 2014). The injection of rat MSCs into the vasculature of rats with an aneurysm and/or SAH signinficantly reduced apoptotic cells, lowered TUNNEL stained cells, and decreased inflammatory response (Ghonim et al., 2016). While the injection of MSCs into the aneurysmal wall holds promise for improving aneurysmal tissue, such direct injection methods come with an increased risk of damaging an already weakened vascular wall. As an alternative method, deverling cells with a perivascular scaffolds suppressed development and progression of AAA in rats (Parvizi, Petersen, van Spreuwel-Goossens, Kluijtmans, & Harmsen, 2018). While regenerative medicine-based approaches can potentially treat aneurysm, further studies are required to better understand how MSCs influence aneurysm pathology. Treatment of AAA using male or female rat derived MSCs have shown different effects: Mean elastase-induced aortic dilation of 121+5.2%, while male MSCs inhibited AAA growth to 87.8+6.9%, p=0.008) and female MSCs AAA growth to 75.2+8.3% p=0.0004) (Davis et al., 2015). Whereas proinflammatory TNF-α, interleukin 1β, and MCP-1 were decreased in elastase-exposed tissues treated with female MSCs. Comprehensive understanding of both the causes of aneurysm development and rupture, alongside the regenerative capabilities of human MSCs, could provide a beneficial therapeutic option for patients.
V: Future Perspectives and Concluding Remarks
While the investigations of disturbed flow’s impact on cellular changes indicative of aneurysms have improved the understanding of this complex pathology, much of these findings come from the analysis of relatively simplistic and temporally stable disturbed flow. Disturbed hemodynamic flow within the arterial system may vary significantly in terms of its spatial-temporal characteristics, especially in the aneurysmal sac. Currently, less is known to what extent varying disturbed flow characteristics, such as their stability over the cardiac cycle, may trigger differing levels of cellular changes, or if certain characteristics of disturbed flow are greater predictors of aneurysmal rupture. Advancements in CFD analytics toward quantifying disturbed flow characteristics, coupled alongside flow chambers designed to generate complex flow patterns could help overcome current informational gaps. Yet the complexity of flow within the aneurysmal sac may make identifying relevant characteristics difficult. Machine learning algorithms for pattern recognition and image analysis have already begun to be implemented in the identification of disturbed flow patterns within the vascular (Deng et al., 2018) and to reconstruct complex flow patterns from low-resolution data (Fukami, Fukagata, & Taira, 2019). Expansion of such techniques may help determine disturbed flow characteristics using their spatial flow information as opposed to condensing characteristics into a single numerical value like WSS-based indices. Further study on identifying flow characteristics that exacerbate/mitigate vascular changes could improve the understanding of aneurysm pathology and aid in developing novel treatment methodologies.
In much of the vasculature, laminar flow helps regulate cellular processes needed to maintain vessel health. The EC monolayer at the intercept of the vessel wall and fluid flow maintains control over vascular permeability, inflammatory processes, and the vessel’s response to changing hemodynamic conditions. Disturbances to laminar flow occur naturally within vessel bifurcations and areas of increased tortuosity, from pathophysiological vessel stenosis, and can result from surgical bypass grafting and stent deployment. Flow disturbances alter EC functionality, causing decreased regulation of vascular permeability, increased vascular inflammation, and higher EC death/turnover. Breakdown of EC functionality leads to alterations in the underlying media layer: degradation of vSMC and ECM reducing vascular expansion or contraction modulation and weakening the vascular wall. In this review, we have summarized the current understanding of disturbed flow as a trigger to altered vascular cells indicative of aneurysms (a summary can be seen in Table 1), the in-vitro, in-vivo, and in-silico research methodologies for studying disturbed flow, and the benefits such research has had on aneurysm treatment options.
Table 1:
Summary of Disturbed Flow’s Impact on Changes to Vascular Cells
| Functionality | Resultant Change due to Disturbed Flow | Model: Stimulus, Cell/Tissue | Reference |
|---|---|---|---|
| Cellular component | |||
|
| |||
| ECpermeability | |||
| VEGF | Increased: VEGF increase in 18% cyclic stress vs 5%(24 hrs) | In-vitro: Cyclic stretch of flexible cell plate (18%-5% elongation, 25 cycles/min), HPAEC | (Gawlak et al., 2016) |
| Increased: 3.3relative expression vs control | In-vivo: CaCl2 induction, rat aorta | (X. Li et al., 2017) | |
| VE-cadherin | Decreased / Disconnection between Cells | In-vivo: Arterial Ligation | (D. A. Chistiakov et al., 2017) |
| Decreased: 3.5 relative expression versus 10dyn/cm2 laminar flow | In-vitro: PPFC 1.8 dyn/cm2 pulsatile Flow, 4 hrs., HMEC | (Caolo et al., 2018) | |
| Atherosclerosis | |||
| PECAM-1 | Decreased: 4.0 relative expression versus 20 dyn/cm2 laminar flow | In-vitro: Orbital Shaker ±5 dyn/cm2 for 24 hrs, HUVEC | (D. A. Chistiakov et al., 2017) |
| Transduction | |||
| β1-Integrin | Decreased: 2.0 relative expression vs laminar flow |
In-vitro: Magnetic Tweezers 1 Hz bidirectional force (16 pN), 3 min, HUVEC & In-vitro: 1 Hz bidirectional flow, 15 dyn/cm2, HUVEC |
(Xanthis et al., 2019) |
| Integrin α5 | Increased: 2-fold vs laminar flow 12±4dyn/cm2 | In-vitro: PPFC 0.5±4dyn/cm2, HUVEC | (Sun et al., 2016) |
| Glycocalyx | Decreased: 44.4±10.3% mean fluorescent intensity, 13.8±8.4% thickness vs 12dyn/cm2 laminar flow |
In-vitro: PPFC with protuberance with recirculation zone 0–8 dynes/cm2 for 6 hrs, RFPEC & Artery Ligation, C57Bl/6 mice |
(Harding et al., 2018) |
| Decreased: Statistically significant vs inlet: Heparin Sulfate | In-vitro: Curved flow chamber with WSS 10 dyn/cm2, HAAEC | (Scott Cooper et al., 2018) | |
| Inflammatory Response | |||
| ICAM-1 | Increased: 3.0 Relative expression vs non-ligated | In-vivo: Arterial Ligation C57BL/6J Mice | (Y. Seo et al., 2019) |
| Increased: Statistically significant vs 15dyn/cm2 laminar flow | In-vitro: Cone-plate viscometer at ±5 dyn/cm2 at 1 Hz, HUVEC | (Go et al., 2014a) | |
| Increased: 1.5 relative expression vs 12dyn/cm2 laminar flow | In-vitro: PPFC ±0.5dyn/cm2 superimposed with 1 dyn/cm2 for 3 hrs, HAEC | (J. Chen et al., 2015) | |
| VCAM-1 | Increased: 2.0 increase MFI vs non-ligated | In-vivo: Arterial Ligation, 48 hrs. | (J. Chen et al., 2015) |
| Increased: 2.5–3 relative expression increase vs 12dyn/cm2 | In-vitro: PPFC 2hr 2dyn/cm2, 4hr stimulation by TNF-α, HAEC | (Bailey et al., 2019) | |
| Regulation of ECM | |||
| MMP2 | Increased: 1.25-fold increase vs 17dyn/cm2 laminar flow | In-vitro: PPFC with occlusion, 2hrs at 5dyn/cm2 disturbed flow | (Balaguru et al., 2016) |
| MMP-9 | Increased | In-vitro: Curved flow chamber | (Scott Cooper et al., 2018) |
| ROS / OS | |||
| NOX | Increased: 1.50 relative expression vs non-ligated artery | In-vivo: Carotid artery ligation, C57BL/6J mice (7 days) | (Yuan et al., 2018) |
| IL-1β | Increased: 10 relative expression increase vs laminar (4hrs) | In-vitro: Cone-plate viscometer, HAEC exposed for 0.5, 1, 2, 3, 4 hrs | (Pan et al., 2017) |
| IL-8 | Increased: 1.5 relative expression increase vs laminar control | In-vitro: Orbital shaker ±4dyn/cm2 at 0.5Hz, 2hrs, HUVEC | (Green et al., 2018) |
| eNOS | Decreased: 2.0 relative expression vs laminar flow | In-vitro: Step-down PPF 0–8 dyn/cm2 disturbed flow, 6 hrs, RFPEC | (Harding et al., 2018) |
| Decreased: 10 relative expression vs static culture | In-vitro: Cone-plate viscometer, 1Hz 0.5dyn/cm2, 24hrs, HUVEC | (Bibli et al., 2020) | |
| Apoptosis | |||
| Caspase 12 | Increase: 3.0 relative expression vs control, 4 hr. flow. | In-vitro: Cone-plate viscometer, HAEC exposed for 0.5, 1, 2, 3, 4 hrs | (Pan et al., 2017) |
| Caspase 3 | Increase:Vs 20dyne/cm2 laminar flow | In-vitro: Cone-plate viscometer, 48 hrs. | (Heo et al., 2015) |
| Increase: 4.0 relative expression vs 13dyn/cm2 periphery flow | In-vivo: Orbital shaker 4.8 dyn/cm2 For 72 hrs, HUVEC | (Serbanovic-Canic et al., 2017) | |
| Transcription Factors | |||
| NF-κβ | Increased: 1.5relative expression vs low WSS laminar flow | In-vivo: Arterial Cuff, 7 days-post Cuffing, ApoE−/− mice | (Baeriswyl et al., 2019) |
| Increased: 1.5 relative expression vs laminar flow | In-vitro: Cone-plate viscometer ±5 dyn/cm2 at 1Hz, HUVEC | (Go et al., 2014a) | |
| Nrf2 | Decreased: 5 relative expression vs 12±4dyn/cm2 | In-vitro: Orbital Shaker 0.5±4dyn/cm2 for 24 hrs, HUVEC | (Martin et al., 2014) |
| JNK | Increased: 4 relative expression vs uniform shear | In-vivo: Arterial ligation, ApoE−/− mice | (L. Wang et al., 2016) |
Studying disturbed flow’s role in arterial cell changes has led to an improved understanding of the pathological initiation, growth, and rupture of aneurysms. Uncovering the underlying changes to cellular morphology, inflammation, protein expression, and cellular apoptosis triggered by disturbed hemodynamic flow has helped improve the clinical management of aneurysms. Yet continued studies using novel research methodologies aim to improve the understanding of how complex disturbed flow impacts aneurysms. Further understanding of the spatial-temporal characteristics of disturbed flow and the specific changes they elicit within arteries could help drive the development of novel treatment options, improve the assessment of which aneurysms are at high rupture risk, and improve clinical outcomes for this deadly vascular pathology.
Funding Statement/ Acknowledgments
This work was supported by the National Institutes of Health (1R01HL146652-01A1 and 1R15HL145654-01 to FZ and 1R01EB029570-01A1 to JJ). It was also funded by American Heart Association (18PRE33990321) to KS.
Footnotes
Conflict of Interest
None of the authors of this work have any conflict of interests to declare at the time of submission.
Data Availability Statement
Data sharing is not applicable to this review article as no new data were created or analyzed in this work.
References
- Achille PD, Tellides G, Figueroa CA, & Humphrey JD (2014). A haemodynamic predictor of intraluminal thrombus formation in abdominal aortic aneurysms. Proceedings of the Royal Society A: Mathematical, Physical and Engineering Sciences, 470(2172), 20140163. doi: 10.1098/rspa.2014.0163 [DOI] [Google Scholar]
- Aliseda A, Chivukula VK, McGah P, Prisco AR, Beckman JA, Garcia GJM, … Mahr C. (2017). LVAD Outflow Graft Angle and Thrombosis Risk. ASAIO journal (American Society for Artificial Internal Organs : 1992), 63(1), 14–23. doi: 10.1097/MAT.0000000000000443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreata F, Syvannarath V, Clement M, Delbosc S, Guedj K, Fornasa G, … Caligiuri G. (2018). Macrophage CD31 Signaling in Dissecting Aortic Aneurysm. Journal of the American College of Cardiology, 72(1), 45–57. doi: 10.1016/j.jacc.2018.04.047 [DOI] [PubMed] [Google Scholar]
- Androutsopoulou V, Perrea DN, Kavantzas N, Angouras DC, Doulamis IP, & Tzani A (2018). Thoracic aortic aneurysm formation with the use of CaCl2: an experimental model in rabbits. Hellenic Journal οf Atherosclerosis, 9(1). [Google Scholar]
- Aoki T (2015). Inflammation mediates the pathogenesis of cerebral aneurysm and becomes therapeutic target. Neuroimmunol Neuroinflammation, 2, 86–92. [Google Scholar]
- Arzani A, & Shadden SC (2015). Characterizations and Correlations of Wall Shear Stress in Aneurysmal Flow. Journal of Biomechanical Engineering, 138(1), 014503–014503-014510. doi: 10.1115/1.4032056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashina K, Tsubosaka Y, Nakamura T, Omori K, Kobayashi K, Hori M, … Murata T. (2015). Histamine Induces Vascular Hyperpermeability by Increasing Blood Flow and Endothelial Barrier Disruption In Vivo. PLOS ONE, 10(7), e0132367. doi: 10.1371/journal.pone.0132367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacigaluppi S, Piccinelli M, Antiga L, Veneziani A, Passerini T, Rampini P, … Fontanella M. (2014). Factors affecting formation and rupture of intracranial saccular aneurysms. Neurosurgical Review, 37(1), 1–14. doi: 10.1007/s10143-013-0501-y [DOI] [PubMed] [Google Scholar]
- Baeriswyl DC, Prionisti I, Peach T, Tsolkas G, Chooi KY, Vardakis J, … Krams R. (2019). Disturbed flow induces a sustained, stochastic NF-κB activation which may support intracranial aneurysm growth in vivo. Scientific Reports, 9(1), 4738. doi: 10.1038/s41598-019-40959-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baeyens N, Bandyopadhyay C, Coon BG, Yun S, & Schwartz MA (2016). Endothelial fluid shear stress sensing in vascular health and disease. The Journal of Clinical Investigation, 126(3), 821–828. doi: 10.1172/JCI83083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey KA, Moreno E, Haj FG, Simon SI, & Passerini AG (2019). Mechanoregulation of p38 activity enhances endoplasmic reticulum stress–mediated inflammation by arterial endothelium. The FASEB Journal, 33(11), 12888–12899. doi: 10.1096/fj.201900236R [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaguru UM, Sundaresan L, Manivannan J, Majunathan R, Mani K, Swaminathan A, … Chatterjee S. (2016). Disturbed flow mediated modulation of shear forces on endothelial plane: A proposed model for studying endothelium around atherosclerotic plaques. Scientific Reports, 6, 27304. doi:10.1038/srep27304 https://www.nature.com/articles/srep27304#supplementary-information [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baratchi S, Khoshmanesh K, Woodman OL, Potocnik S, Peter K, & McIntyre P (2017). Molecular Sensors of Blood Flow in Endothelial Cells. Trends in Molecular Medicine, 23(9), 850–868. doi: 10.1016/j.molmed.2017.07.007 [DOI] [PubMed] [Google Scholar]
- Barry AK, Wang N, & Leckband DE (2015). Local VE-cadherin mechanotransduction triggers long-ranged remodeling of endothelial monolayers. Journal of Cell Science, 128(7), 1341. doi: 10.1242/jcs.159954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell J, & Baker S (2016). Biomarkers of glycocalyx injury are associated with delayed cerebral ischemia following aneurysmal subarachnoid hemorrhage: A case series supporting a new hypothesis. Neurocritical Care. [DOI] [PubMed] [Google Scholar]
- Benn A, Bredow C, Casanova I, Vukičević S, & Knaus P (2016). VE-cadherin facilitates BMP-induced endothelial cell permeability and signaling. Journal of Cell Science, 129(1), 206–218. doi: 10.1242/jcs.179960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi Y, Zhong H, Xu K, Qi X, Zhang Z, Wu G, & Han X (2015). Novel experimental model of enlarging abdominal aortic aneurysm in rabbits. Journal of Vascular Surgery, 62(4), 1054–1063. doi: 10.1016/j.jvs.2014.02.062 [DOI] [PubMed] [Google Scholar]
- Bijlenga P, Gondar R, Schilling S, Morel S, Hirsch S, Cuony J, … Schaller K. (2017). PHASES score for the management of intracranial aneurysm: a cross-sectional population-based retrospective study. Stroke, 48(8), 2105–2112. [DOI] [PubMed] [Google Scholar]
- Bogunovic N, Meekel JP, Micha D, Blankensteijn JD, Hordijk PL, & Yeung KK (2019). Impaired smooth muscle cell contractility as a novel concept of abdominal aortic aneurysm pathophysiology. Scientific Reports, 9(1), 6837. doi: 10.1038/s41598-019-43322-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowden N, Bryan M, Duckles H, Feng S, Hsiao S, Kim R, … Evans P. (2016). Experimental Approaches to Study Endothelial Responses to Shear Stress. Antioxidants & Redox Signaling, 25. doi: 10.1089/ars.2015.6553 [DOI] [PubMed] [Google Scholar]
- Boyd AJ, Kuhn DCS, Lozowy RJ, & Kulbisky GP (2016). Low wall shear stress predominates at sites of abdominal aortic aneurysm rupture. Journal of Vascular Surgery, 63(6), 1613–1619. doi: 10.1016/j.jvs.2015.01.040 [DOI] [PubMed] [Google Scholar]
- Brangsch J, Reimann C, Collettini F, Buchert R, Botnar RM, & Makowski MR (2017). Molecular imaging of abdominal aortic aneurysms. Trends in Molecular Medicine, 23(2), 150–164. [DOI] [PubMed] [Google Scholar]
- Brangsch J, Reimann C, Kaufmann JO, Adams LC, Onthank DC, Thöne-Reineke C, … Makowski MR. (2019). Concurrent Molecular Magnetic Resonance Imaging of Inflammatory Activity and Extracellular Matrix Degradation for the Prediction of Aneurysm Rupture. Circulation: Cardiovascular Imaging, 12(3), e008707. doi: 10.1161/CIRCIMAGING.118.008707 [DOI] [PubMed] [Google Scholar]
- Brinjikji W, Ding YH, Kallmes DF, & Kadirvel R (2016). From bench to bedside: utility of the rabbit elastase aneurysm model in preclinical studies of intracranial aneurysm treatment. Journal of NeuroInterventional Surgery, 8(5), 521–525. doi: 10.1136/neurintsurg-2015-011704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinjikji W, Lanzino G, Cloft HJ, Siddiqui AH, Boccardi E, Cekirge S, … Kallmes DF. (2016). Risk Factors for Ischemic Complications following Pipeline Embolization Device Treatment of Intracranial Aneurysms: Results from the IntrePED Study. American Journal of Neuroradiology, 37(9), 1673–1678. doi: 10.3174/ajnr.A4807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger D, Turner M, Munkonda MN, & Touyz RM (2016). Endothelial Microparticle-Derived Reactive Oxygen Species: Role in Endothelial Signaling and Vascular Function. Oxidative Medicine and Cellular Longevity, 2016, 10. doi: 10.1155/2016/5047954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch A, Holm A, Wagner N, Ergün S, Rosenfeld M, Otto C, … Lorenz U. (2016). Extra- and Intraluminal Elastase Induce Morphologically Distinct Abdominal Aortic Aneurysms in Mice and Thus Represent Specific Subtypes of Human Disease. Journal of Vascular Research, 53(1–2), 49–57. doi: 10.1159/000447263 [DOI] [PubMed] [Google Scholar]
- Byon CH, Heath JM, & Chen Y (2016). Redox signaling in cardiovascular pathophysiology: A focus on hydrogen peroxide and vascular smooth muscle cells. Redox biology, 9, 244–253. doi: 10.1016/j.redox.2016.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne G, Mut F, & Cebral J (2014). Quantifying the large-scale hemodynamics of intracranial aneurysms. American Journal of Neuroradiology, 35(2), 333–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill PA, & Redmond EM (2016a). Vascular endothelium – Gatekeeper of vessel health. Atherosclerosis, 248, 97–109. doi: 10.1016/j.atherosclerosis.2016.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill PA, & Redmond EM (2016b). Vascular endothelium – Gatekeeper of vessel health. Atherosclerosis, 248, 97–109. 10.1016/j.atherosclerosis.2016.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Can A, Castro VM, Dligach D, Finan S, Yu S, Gainer V, … Cai T. (2018). Lipid-lowering agents and high HDL (high-density lipoprotein) are inversely associated with intracranial aneurysm rupture. Stroke, 49(5), 1148–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Can A, & Du R (2015). Association of Hemodynamic Factors With Intracranial Aneurysm Formation and Rupture: Systematic Review and Meta-analysis. Neurosurgery, 78(4), 510–520. doi: 10.1227/neu.0000000000001083 [DOI] [PubMed] [Google Scholar]
- Cancel LM, Ebong EE, Mensah S, Hirschberg C, & Tarbell JM (2016). Endothelial glycocalyx, apoptosis and inflammation in an atherosclerotic mouse model. Atherosclerosis, 252, 136–146. doi: 10.1016/j.atherosclerosis.2016.07.930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Wu Q, Geng L, Chen X, Shen W, Wu F, & Chen Y (2017). Rapamycin inhibits CaCl2-induced thoracic aortic aneurysm formation in rats through mTOR-mediated suppression of proinflammatory mediators. Molecular medicine reports, 16(2), 1911–1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caroff J, Neki H, Mihalea C, D’Argento F, Abdel Khalek H, Ikka L, … Spelle L. (2016). Flow-Diverter Stents for the Treatment of Saccular Middle Cerebral Artery Bifurcation Aneurysms. American Journal of Neuroradiology, 37(2), 279–284. doi: 10.3174/ajnr.A4540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro MA, Olivares MCA, Putman CM, & Cebral JR (2014). Unsteady wall shear stress analysis from image-based computational fluid dynamic aneurysm models under Newtonian and Casson rheological models. Medical & Biological Engineering & Computing, 52(10), 827–839. doi: 10.1007/s11517-014-1189-z [DOI] [PubMed] [Google Scholar]
- Cebral J, Ollikainen E, Chung BJ, Mut F, Sippola V, Jahromi BR, … Robertson A. (2017). Flow conditions in the intracranial aneurysm lumen are associated with inflammation and degenerative changes of the aneurysm wall. American Journal of Neuroradiology, 38(1), 119–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cebral J, Ollikainen E, Chung BJ, Mut F, Sippola V, Jahromi BR, … Frösen J. (2017). Flow Conditions in the Intracranial Aneurysm Lumen Are Associated with Inflammation and Degenerative Changes of the Aneurysm Wall. American Journal of Neuroradiology, 38(1), 119–126. doi: 10.3174/ajnr.A4951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cebral JR, Duan X, Gade PS, Chung BJ, Mut F, Aziz K, & Robertson AM (2016). Regional Mapping of Flow and Wall Characteristics of Intracranial Aneurysms. Annals of Biomedical Engineering, 44(12), 3553–3567. doi: 10.1007/s10439-016-1682-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha S-H, Hwang Y, Kim K-N, & Jun H-S (2018). Palmitate induces nitric oxide production and inflammatory cytokine expression in zebrafish. Fish & Shellfish Immunology, 79, 163–167. doi: 10.1016/j.fsi.2018.05.025 [DOI] [PubMed] [Google Scholar]
- Chatterjee S (2018). Endothelial Mechanotransduction, Redox Signaling and the Regulation of Vascular Inflammatory Pathways. Frontiers in physiology, 9, 524–524. doi: 10.3389/fphys.2018.00524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay R, Raghavan S, & Rao GN (2017). Resolvin D1 via prevention of ROS-mediated SHP2 inactivation protects endothelial adherens junction integrity and barrier function. Redox biology, 12, 438–455. doi: 10.1016/j.redox.2017.02.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Green J, Yurdagul A, Albert P, McInnis MC, & Orr AW (2015). αvβ3 Integrins Mediate Flow-Induced NF-κB Activation, Proinflammatory Gene Expression, and Early Atherogenic Inflammation. The American Journal of Pathology, 185(9), 2575–2589. doi: 10.1016/j.ajpath.2015.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Wang Q, Zhu J, Xiao Q, & Zhang L (2018). Reactive oxygen species: key regulators in vascular health and diseases. British journal of pharmacology, 175(8), 1279–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng C, Helderman F, Tempel D, Segers D, Hierck B, Poelmann R, … Ursem NT. (2007). Large variations in absolute wall shear stress levels within one species and between species. Atherosclerosis, 195(2), 225–235. [DOI] [PubMed] [Google Scholar]
- Cheng Z, Zhou Y-Z, Wu Y, Wu Q-Y, Liao X-B, Fu X-M, & Zhou X-M (2018). Diverse roles of macrophage polarization in aortic aneurysm: destruction and repair. Journal of Translational Medicine, 16(1), 354–354. doi: 10.1186/s12967-018-1731-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chistiakov DA, Melnichenko AA, Myasoedova VA, Grechko AV, & Orekhov AN (2017). Mechanisms of foam cell formation in atherosclerosis. Journal of Molecular Medicine, 95(11), 1153–1165. doi: 10.1007/s00109-017-1575-8 [DOI] [PubMed] [Google Scholar]
- Chistiakov DA, Orekhov AN, & Bobryshev YV (2017). Effects of shear stress on endothelial cells: go with the flow. Acta Physiologica, 219(2), 382–408. doi: 10.1111/apha.12725 [DOI] [PubMed] [Google Scholar]
- Choi H-J, Kim N-E, Kim BM, Seo M, & Heo JH (2018). TNF-α-Induced YAP/TAZ Activity Mediates Leukocyte-Endothelial Adhesion by Regulating VCAM1 Expression in Endothelial Cells. International Journal of Molecular Sciences, 19(11), 3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung J, Kim KH, Lee SC, An SH, & Kwon K (2015). Ursodeoxycholic Acid (UDCA) Exerts Anti-Atherogenic Effects by Inhibiting Endoplasmic Reticulum (ER) Stress Induced by Disturbed Flow. Molecules and cells, 38(10), 851–858. doi: 10.14348/molcells.2015.0094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciavarella C, Alviano F, Gallitto E, Ricci F, Buzzi M, Velati C, … Pasquinelli G. (2015). Human Vascular Wall Mesenchymal Stromal Cells Contribute to Abdominal Aortic Aneurysm Pathogenesis Through an Impaired Immunomodulatory Activity and Increased Levels of Matrix Metalloproteinase-9. Circulation journal : official journal of the Japanese Circulation Society, 79. doi: 10.1253/circj.CJ-14-0857 [DOI] [PubMed] [Google Scholar]
- Claesson-Welsh L (2015). Vascular permeability--the essentials. Upsala journal of medical sciences, 120(3), 135–143. doi: 10.3109/03009734.2015.1064501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper S, Emmott A, McDonald KK, Campeau M-A, & Leask RL (2018). Increased MMP activity in curved geometries disrupts the endothelial cell glycocalyx creating a proinflammatory environment. PLOS ONE, 13(8), e0202526. doi: 10.1371/journal.pone.0202526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper S, McDonald K, Burkat D, & Leask RL (2017). Stenosis Hemodynamics Disrupt the Endothelial Cell Glycocalyx by MMP Activity Creating a Proinflammatory Environment. Annals of Biomedical Engineering, 45(9), 2234–2243. doi: 10.1007/s10439-017-1846-0 [DOI] [PubMed] [Google Scholar]
- Dale MA, Xiong W, Carson JS, Suh MK, Karpisek AD, Meisinger TM, … Baxter BT. (2016). Elastin-Derived Peptides Promote Abdominal Aortic Aneurysm Formation by Modulating M1/M2 Macrophage Polarization. The Journal of Immunology, 196(11), 4536–4543. doi: 10.4049/jimmunol.1502454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JP, Salmon M, Pope NH, Lu G, Su G, Sharma AK, … Upchurch GR Jr. (2015). Attenuation of aortic aneurysms with stem cells from different genders. journal of surgical research, 199(1), 249–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng L, Wang Y, Liu Y, Wang F, Li S, & Liu J (2018). A CNN-based vortex identification method. Journal of Visualization, 22. doi: 10.1007/s12650-018-0523-1 [DOI] [Google Scholar]
- Detmer FJ, Chung BJ, Mut F, Slawski M, Hamzei-Sichani F, Putman C, … Cebral JR. (2018). Development and internal validation of an aneurysm rupture probability model based on patient characteristics and aneurysm location, morphology, and hemodynamics. International journal of computer assisted radiology and surgery, 13(11), 1767–1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diagbouga MR, Morel S, Bijlenga P, & Kwak BR (2018). Role of hemodynamics in initiation/growth of intracranial aneurysms. European Journal of Clinical Investigation, 48(9), e12992. doi: 10.1111/eci.12992 [DOI] [PubMed] [Google Scholar]
- Ding Z,, S. L.,, X. W.,, X. D.,, Y. F.,, C. S., …, a. J. L. M. (2015). Hemodynamic Shear Stress via ROS Modulates PCSK9 Expression in Human Vascular Endothelial and Smooth Muscle Cells and Along the Mouse Aorta. Antioxidants & redox signaling, 22(9), 760–771. doi: 10.1089/ars.2014.6054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Z, Liu S, Wang X, Deng X, Fan Y, Sun C, … Mehta JL. (2015). Hemodynamic shear stress via ROS modulates PCSK9 expression in human vascular endothelial and smooth muscle cells and along the mouse aorta. Antioxidants & redox signaling, 22(9), 760–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan JM, Kolega J, & Meng H (2013). High wall shear stress and spatial gradients in vascular pathology: a review. Annals of Biomedical Engineering, 41(7), 1411–1427. doi: 10.1007/s10439-012-0695-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorland YL, & Huveneers S (2017). Cell–cell junctional mechanotransduction in endothelial remodeling. Cellular and Molecular Life Sciences, 74(2), 279–292. doi: 10.1007/s00018-016-2325-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emrich FC, Okamura H, Dalal AR, Penov K, Merk DR, Raaz U, … Fischbein MP. (2015). Enhanced Caspase Activity Contributes to Aortic Wall Remodeling and Early Aneurysm Development in a Murine Model of Marfan Syndrome. Arteriosclerosis, Thrombosis, and Vascular Biology, 35(1), 146–154. doi:: 10.1161/ATVBAHA.114.304364 [DOI] [PubMed] [Google Scholar]
- Etminan N, & Rinkel GJ (2017). Unruptured intracranial aneurysms: development, rupture and preventive management. Nature Reviews Neurology, 13, 126. doi: 10.1038/nrneurol.2017.14 [DOI] [PubMed] [Google Scholar]
- Fennell VS, Kalani MYS, Atwal G, Martirosyan NL, & Spetzler RF (2016). Biology of Saccular Cerebral Aneurysms: A Review of Current Understanding and Future Directions. Frontiers in Surgery, 3(43). doi: 10.3389/fsurg.2016.00043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher CL, & Demel SL (2019). Nonsteroidal Anti-Inflammatory Drugs: A Potential Pharmacological Treatment for Intracranial Aneurysm. Cerebrovascular Diseases Extra, 9(1), 31–45. doi: 10.1159/000499077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrester SJ, Kikuchi DS, Hernandes MS, Xu Q, & Griendling KK (2018). Reactive Oxygen Species in Metabolic and Inflammatory Signaling. Circulation Research, 122(6), 877–902. doi: 10.1161/CIRCRESAHA.117.311401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzoni M, Cattaneo I, Ene-Iordache B, Oldani A, Righettini P, & Remuzzi A (2016). Design of a cone-and-plate device for controlled realistic shear stress stimulation on endothelial cell monolayers. Cytotechnology, 68(5), 1885–1896. doi: 10.1007/s10616-015-9941-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimura S, Takao H, Suzuki T, Dahmani C, Ishibashi T, Mamori H, … Murayama Y. (2018). Hemodynamics and coil distribution with changing coil stiffness and length in intracranial aneurysms. Journal of NeuroInterventional Surgery, 10(8), 797–801. doi: 10.1136/neurintsurg-2017-013457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukami K, Fukagata K, & Taira K (2019). Super-resolution reconstruction of turbulent flows with machine learning. Journal of Fluid Mechanics, 870, 106–120. doi: 10.1017/jfm.2019.238 [DOI] [Google Scholar]
- Fukazawa K, Ishida F, Umeda Y, Miura Y, Shimosaka S, Matsushima S, … Suzuki H. (2015). Using Computational Fluid Dynamics Analysis to Characterize Local Hemodynamic Features of Middle Cerebral Artery Aneurysm Rupture Points. World Neurosurgery, 83(1), 80–86. doi: 10.1016/j.wneu.2013.02.012 [DOI] [PubMed] [Google Scholar]
- Furukawa H, Wada K, Tada Y, Kuwabara A, Sato H, Ai J, … Hashimoto T. (2020). Mast Cell Promotes the Development of Intracranial Aneurysm Rupture. Stroke, 51(11), 3332–3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geers AJ, Morales HG, Larrabide I, Butakoff C, Bijlenga P, & Frangi AF (2017). Wall shear stress at the initiation site of cerebral aneurysms. Biomechanics and Modeling in Mechanobiology, 16(1), 97–115. doi: 10.1007/s10237-016-0804-3 [DOI] [PubMed] [Google Scholar]
- Ghim M, Alpresa P, Yang S-W, Braakman ST, Gray SG, Sherwin SJ, … Weinberg PD. (2017). Visualization of three pathways for macromolecule transport across cultured endothelium and their modification by flow. American Journal of Physiology-Heart and Circulatory Physiology, 313(5), H959–H973. doi: 10.1152/ajpheart.00218.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghonim HT, Shah SS, Thompson JW, Ambekar S, Peterson EC, & Elhammady MS (2016). Stem cells as a potential adjunctive therapy in aneurysmal subarachnoid hemorrhage. Journal of vascular and interventional neurology, 8(5), 30. [PMC free article] [PubMed] [Google Scholar]
- Gimbrone MA, & García-Cardeña G (2016). Endothelial Cell Dysfunction and the Pathobiology of Atherosclerosis. Circulation Research, 118(4), 620–636. doi: 10.1161/CIRCRESAHA.115.306301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go Y-M, Son DJ, Park H, Orr M, Hao L, Takabe W, … Jones DP. (2014). Disturbed Flow Enhances Inflammatory Signaling and Atherogenesis by Increasing Thioredoxin-1 Level in Endothelial Cell Nuclei. PLOS ONE, 9(9), e108346. doi: 10.1371/journal.pone.0108346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Garcia MJ, Doiron AL, Steele RR, Labouta HI, Vafadar B, Shepherd RD, … Rinker KD. (2018). Nanoparticle localization in blood vessels: dependence on fluid shear stress, flow disturbances, and flow-induced changes in endothelial physiology. Nanoscale, 10(32), 15249–15261. [DOI] [PubMed] [Google Scholar]
- Gong T, Liu L, Jiang W, & Zhou R (2020). DAMP-sensing receptors in sterile inflammation and inflammatory diseases. Nature Reviews Immunology, 20(2), 95–112. doi: 10.1038/s41577-019-0215-7 [DOI] [PubMed] [Google Scholar]
- Green JP, Souilhol C, Xanthis I, Martinez-Campesino L, Bowden NP, Evans PC, & Wilson HL (2018). Atheroprone flow activates inflammation via endothelial ATP-dependent P2X7-p38 signalling. Cardiovascular Research, 114(2), 324–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo D, Wang Y-W, Ma J, Yan L, Li T-F, Han X-W, & Shui S-F (2016). Study on the role of Cathepsin B and JNK signaling pathway in the development of cerebral aneurysm. Asian Pacific Journal of Tropical Medicine, 9(5), 499–502. doi: 10.1016/j.apjtm.2016.03.020 [DOI] [PubMed] [Google Scholar]
- Hackenberg KAM, Hänggi D, & Etminan N (2018). Unruptured Intracranial Aneurysms. Stroke, 49(9), 2268–2275. doi: 10.1161/STROKEAHA.118.021030 [DOI] [PubMed] [Google Scholar]
- Harada T, Yoshimura K, Yamashita O, Ueda K, Morikage N, Sawada Y, & Hamano K (2017). Focal Adhesion Kinase Promotes the Progression of Aortic Aneurysm by Modulating Macrophage Behavior. Arteriosclerosis, Thrombosis, and Vascular Biology, 37(1), 156–165. doi: 10.1161/ATVBAHA.116.308542 [DOI] [PubMed] [Google Scholar]
- Harding IC, Mitra R, Mensah SA, Herman IM, & Ebong EE (2018). Pro-atherosclerotic disturbed flow disrupts caveolin-1 expression, localization, and function via glycocalyx degradation. Journal of Translational Medicine, 16(1), 364. doi: 10.1186/s12967-018-1721-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hishikawa T, Date I, Tokunaga K, Tominari S, Nozaki K, Shiokawa Y, … Morita A. (2015). Risk of rupture of unruptured cerebral aneurysms in elderly patients. Neurology, 85(21), 1879–1885. doi: 10.1212/wnl.0000000000002149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho Y-C, Wu M-L, Gung P-Y, Chen C-H, Kuo C-C, & Yet S-F (2016). Heme oxygenase-1 deficiency exacerbates angiotensin II-induced aortic aneurysm in mice. Oncotarget, 7(42), 67760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh H-J, Liu C-A, Huang B, Tseng AH, & Wang DL (2014). Shear-induced endothelial mechanotransduction: the interplay between reactive oxygen species (ROS) and nitric oxide (NO) and the pathophysiological implications. Journal of Biomedical Science, 21(1), 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey J, & Taylor C (2008). Intracranial and abdominal aortic aneurysms: similarities, differences, and need for a new class of computational models. Annu. Rev. Biomed. Eng, 10, 221–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey JD, Schwartz MA, Tellides G, & Milewicz DM (2015). Role of Mechanotransduction in Vascular Biology. Circulation Research, 116(8), 1448–1461. doi: 10.1161/CIRCRESAHA.114.304936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes W, Pepona M, Robertson C, Alvarado J, Dubbin K, Triplett M, … Moya M. (2020). Examining metastatic behavior within 3D bioprinted vasculature for the validation of a 3D computational flow model. Science advances, 6(35), eabb3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikawa F, Morita A, Tominari S, Nakayama T, Shiokawa Y, Date I, … Kayama T. (2019). Rupture risk of small unruptured cerebral aneurysms. J Neurosurg, 1. [DOI] [PubMed] [Google Scholar]
- Itatani K, Miyazaki S, Furusawa T, Numata S, Yamazaki S, Morimoto K, … Yaku H. (2017). New imaging tools in cardiovascular medicine: computational fluid dynamics and 4D flow MRI. General Thoracic and Cardiovascular Surgery, 65(11), 611–621. doi: 10.1007/s11748-017-0834-5 [DOI] [PubMed] [Google Scholar]
- Jain K, Jiang J, Strother C, & Mardal K-A (2016). Transitional hemodynamics in intracranial aneurysms — Comparative velocity investigations with high resolution lattice Boltzmann simulations, normal resolution ANSYS simulations, and MR imaging. Medical Physics, 43(11), 6186–6198. doi: 10.1118/1.4964793 [DOI] [PubMed] [Google Scholar]
- Jana S, Hu M, Shen M, & Kassiri Z (2019). Extracellular matrix, regional heterogeneity of the aorta, and aortic aneurysm. Experimental & Molecular Medicine, 51(12), 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia L-X, Zhang W-M, Zhang H-J, Li T-T, Wang Y-L, Qin Y-W, … Du J. (2015). Mechanical stretch-induced endoplasmic reticulum stress, apoptosis and inflammation contribute to thoracic aortic aneurysm and dissection. The Journal of Pathology, 236(3), 373–383. doi: 10.1002/path.4534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia L, Wang L, Wei F, Li C, Wang Z, Yu H, … Jiang A. (2020). Effects of Caveolin-1-ERK1/2 pathway on endothelial cells and smooth muscle cells under shear stress. Experimental Biology and Medicine, 245(1), 21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Shen J, Weng Y-X, Pan J-W, Yu J-B, Wan Z-A, & Zhan R (2014). Morphology parameters for mirror posterior communicating artery aneurysm rupture risk assessment. Neurologia medico-chirurgica, oa. 2014–0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang P, Liu Q, Wu J, Chen X, Li M, Li Z, … Cao Y. (2018). A novel scoring system for rupture risk stratification of intracranial aneurysms: a hemodynamic and morphological study. Frontiers in neuroscience, 12, 596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez JM, Prasad V, Yu MD, Kampmeyer CP, Kaakour A-H, Wang P-J, … Davies PF. (2014). Macro- and microscale variables regulate stent haemodynamics, fibrin deposition and thrombomodulin expression. Journal of The Royal Society Interface, 11(94), 20131079. doi: 10.1098/rsif.2013.1079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jufri NF, Mohamedali A, Avolio A, & Baker MS (2015). Mechanical stretch: physiological and pathological implications for human vascular endothelial cells. Vascular Cell, 7(1), 8. doi: 10.1186/s13221-015-0033-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung K-H (2018). New Pathophysiological Considerations on Cerebral Aneurysms. Neurointervention, 13(2), 73–83. doi: 10.5469/neuroint.2018.01011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallmes DF, Hanel R, Lopes D, Boccardi E, Bonafé A, Cekirge S, … Lylyk P. (2015). International Retrospective Study of the Pipeline Embolization Device: A Multicenter Aneurysm Treatment Study. American Journal of Neuroradiology, 36(1), 108–115. doi: 10.3174/ajnr.A4111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko N, Mashiko T, Namba K, Tateshima S, Watanabe E, & Kawai K (2018). A patient-specific intracranial aneurysm model with endothelial lining: a novel in vitro approach to bridge the gap between biology and flow dynamics. Journal of NeuroInterventional Surgery, 10(3), 306–309. doi: 10.1136/neurintsurg-2017-013087 [DOI] [PubMed] [Google Scholar]
- Karimi Galougahi K, Ashley EA, & Ali ZA (2016). Redox regulation of vascular remodeling. Cellular and Molecular Life Sciences, 73(2), 349–363. doi: 10.1007/s00018-015-2068-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan MO, Chnafa C, Steinman DA, Mendez S, & Nicoud F (2016). Large Eddy Simulation of” turbulent-like” flow in intracranial aneurysms. Paper presented at the APS Meeting Abstracts. [Google Scholar]
- Khan MO, Steinman DA, & Valen-Sendstad K (2017). Non-Newtonian versus numerical rheology: Practical impact of shear-thinning on the prediction of stable and unstable flows in intracranial aneurysms. International Journal for Numerical Methods in Biomedical Engineering, 33(7), e2836. doi: 10.1002/cnm.2836 [DOI] [PubMed] [Google Scholar]
- Khruasingkeaw S, Khunatorn Y, Rerkasem K, & Srisuwan T (2016). Wall shear stress distribution in arteriovenous graft anastomosis using computational fluid dynamics. International Journal of Pharma Medicine and Biological Sciences, 5(1), 71. [Google Scholar]
- Klaus V, Tanios-Schmies F, Reeps C, Trenner M, Matevossian E, Eckstein HH, & Pelisek J (2017). Association of Matrix Metalloproteinase Levels with Collagen Degradation in the Context of Abdominal Aortic Aneurysm. European Journal of Vascular and Endovascular Surgery, 53(4), 549–558. doi: 10.1016/j.ejvs.2016.12.030 [DOI] [PubMed] [Google Scholar]
- Kokje VBC, Hamming JF, & Lindeman JHN (2015). Editor’s Choice – Pharmaceutical Management of Small Abdominal Aortic Aneurysms: A Systematic Review of the Clinical Evidence. European Journal of Vascular and Endovascular Surgery, 50(6), 702–713. doi: 10.1016/j.ejvs.2015.08.010 [DOI] [PubMed] [Google Scholar]
- Korja M, Lehto H, & Juvela S (2014). Lifelong Rupture Risk of Intracranial Aneurysms Depends on Risk Factors. Stroke, 45(7), 1958–1963. doi: 10.1161/STROKEAHA.114.005318 [DOI] [PubMed] [Google Scholar]
- Kossmann S, Hu H, Steven S, Schönfelder T, Fraccarollo D, Mikhed Y, … Karbach SH. (2014). Inflammatory monocytes determine endothelial nitric-oxide synthase uncoupling and nitro-oxidative stress induced by angiotensin II. Journal of Biological Chemistry, 289(40), 27540–27550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystel-Whittemore M, Dileepan KN, & Wood JG (2016). Mast Cell: A Multi-Functional Master Cell. Frontiers in Immunology, 6(620). doi: 10.3389/fimmu.2015.00620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuivaniemi H, Ryer EJ, Elmore JR, & Tromp G (2015). Understanding the pathogenesis of abdominal aortic aneurysms. Expert review of cardiovascular therapy, 13(9), 975–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lay AJ, Coleman PR, Formaz-Preston A, Ting KK, Roediger B, Weninger W, … Gamble JR. (2019). ARHGAP18: A Flow‐Responsive Gene That Regulates Endothelial Cell Alignment and Protects Against Atherosclerosis. Journal of the American Heart Association, 8(2), e010057. doi: 10.1161/JAHA.118.010057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach JR, Kao E, Zhu C, Saloner D, & Hope MD (2019). On the Relative Impact of Intraluminal Thrombus Heterogeneity on Abdominal Aortic Aneurysm Mechanics. Journal of Biomechanical Engineering, 141(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblanc ME, Saez-Torres KL, Cano I, Hu Z, Saint-Geniez M, Ng Y-S, & D’Amore PA (2019). Glycocalyx regulation of vascular endothelial growth factor receptor 2 activity. The FASEB Journal, 33(8), 9362–9373. doi: 10.1096/fj.201900011R [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee G-J, Eom K-S, Lee C, Kim D-W, & Kang S-D (2015). Rupture of Very Small Intracranial Aneurysms: Incidence and Clinical Characteristics. Journal of cerebrovascular and endovascular neurosurgery, 17(3), 217–222. doi: 10.7461/jcen.2015.17.3.217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong CM, Nackman GB, & Wei T (2018). Flow patterns through vascular graft models with and without cuffs. PLOS ONE, 13(2), e0193304–e0193304. doi: 10.1371/journal.pone.0193304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley D, Mühl-Benninghaus R, Yilmaz U, Körner H, Cattaneo GFM, Mailänder W, … Simgen A. (2017). The Derivo Embolization Device, a Second-Generation Flow Diverter for the Treatment of Intracranial Aneurysms, Evaluated in an Elastase-Induced Aneurysm Model. Clinical Neuroradiology, 27(3), 335–343. doi: 10.1007/s00062-015-0493-9 [DOI] [PubMed] [Google Scholar]
- Li S, Xu J, Yao W, Li H, Liu Q, Xiao F, … Ruan W. (2015). Sevoflurane pretreatment attenuates TNF-α-induced human endothelial cell dysfunction through activating eNOS/NO pathway. Biochemical and Biophysical Research Communications, 460(3), 879–886. doi: 10.1016/j.bbrc.2015.03.126 [DOI] [PubMed] [Google Scholar]
- Li T, Wang H, Li X, Ge H, Sun H, & Ma D (2017). Predictive significance of VEGFA variations in intracranial aneurysm. Int J Clin Exp Med, 10(9), 13802–13807. [Google Scholar]
- Li W, & Wang W (2018). Structural alteration of the endothelial glycocalyx: contribution of the actin cytoskeleton. Biomechanics and Modeling in Mechanobiology, 17(1), 147–158. doi: 10.1007/s10237-017-0950-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Fang Q, Tian X, Wang X, Ao Q, Hou W, … Bai S. (2017). Curcumin attenuates the development of thoracic aortic aneurysm by inhibiting VEGF expression and inflammation. Molecular medicine reports, 16(4), 4455–4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Guo Y, Tang J, Jiang J, & Chen Z (2014). New insights into the roles of CHOP-induced apoptosis in ER stress. Acta Biochimica et Biophysica Sinica, 46(8), 629–640. doi: 10.1093/abbs/gmu048 [DOI] [PubMed] [Google Scholar]
- Liaw N, Dolan Fox JM, Siddiqui AH, Meng H, & Kolega J (2014). Endothelial Nitric Oxide Synthase and Superoxide Mediate Hemodynamic Initiation of Intracranial Aneurysms. PLOS ONE, 9(7), e101721. doi: 10.1371/journal.pone.0101721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liaw N, Fox JMD, Siddiqui AH, Meng H, & Kolega J (2014). Endothelial nitric oxide synthase and superoxide mediate hemodynamic initiation of intracranial aneurysms. PLOS ONE, 9(7), e101721–e101721. doi: 10.1371/journal.pone.0101721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim S, Halandras PM, Park T, Lee Y, Crisostomo P, Hershberger R, … Cho JS. (2015). Outcomes of endovascular abdominal aortic aneurysm repair in high-risk patients. Journal of Vascular Surgery, 61(4), 862–868. doi: 10.1016/j.jvs.2014.11.081 [DOI] [PubMed] [Google Scholar]
- Lindeman JH, & Matsumura JS (2019). Pharmacologic Management of Aneurysms. Circulation Research, 124(4), 631–646. doi: 10.1161/CIRCRESAHA.118.312439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Song Y, Zhou Y, Liu Y, Qiu T, An Q, … Zhu W. (2018). Cyclic Mechanical Stretch Induced Smooth Muscle Cell Changes in Cerebral Aneurysm Progress by Reducing Collagen Type IV and Collagen Type VI Levels. Cellular Physiology and Biochemistry, 45(3), 1051–1060. doi: 10.1159/000487347 [DOI] [PubMed] [Google Scholar]
- Liu T, Zhang L, Joo D, & Sun S-C (2017). NF-κB signaling in inflammation. Signal transduction and targeted therapy, 2, 17023. doi: 10.1038/sigtrans.2017.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Sun A, Fan Y, & Deng X (2014). Physiological Significance of Helical Flow in the Arterial System and its Potential Clinical Applications. Annals of Biomedical Engineering, 43. doi: 10.1007/s10439-014-1097-2 [DOI] [PubMed] [Google Scholar]
- Liu X, Sun A, Fan Y, & Deng X (2015). Physiological Significance of Helical Flow in the Arterial System and its Potential Clinical Applications. Annals of Biomedical Engineering, 43(1), 3–15. doi: 10.1007/s10439-014-1097-2 [DOI] [PubMed] [Google Scholar]
- Liu YF, Bai YQ, & Qi M (2016). Daidzein attenuates abdominal aortic aneurysm through NF-κB, p38MAPK and TGF-β1 pathways. Molecular medicine reports, 14(1), 955–962. [DOI] [PubMed] [Google Scholar]
- Liu ZH, Li C, Chen JW, Shen Y, Gao J, Shen WF, … Lu L. (2017). C1q/TNF-related protein 1 promotes endothelial barrier dysfunction under disturbed flow. Biochemical and Biophysical Research Communications, 490(2), 580–586. doi: 10.1016/j.bbrc.2017.06.081 [DOI] [PubMed] [Google Scholar]
- Lo Sasso G, Schlage WK, Boué S, Veljkovic E, Peitsch MC, & Hoeng J (2016). The Apoe(−/−) mouse model: a suitable model to study cardiovascular and respiratory diseases in the context of cigarette smoke exposure and harm reduction. Journal of Translational Medicine, 14(1), 146–146. doi: 10.1186/s12967-016-0901-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long Y, Zhong J, Yu H, Yan H, Zhuo Z, Meng Q, … Li H. (2015). A scaling aneurysm model-based approach to assessing the role of flow pattern and energy loss in aneurysm rupture prediction. Journal of Translational Medicine, 13(1), 311. doi: 10.1186/s12967-015-0673-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu W-W, Jia L-X, Ni X-Q, Zhao L, Chang J-R, Zhang J-S, … Qi Y-F. (2016). Intermedin1−53 Attenuates Abdominal Aortic Aneurysm by Inhibiting Oxidative Stress. Arteriosclerosis, Thrombosis, and Vascular Biology, 36(11), 2176–2190. doi: 10.1161/ATVBAHA.116.307825 [DOI] [PubMed] [Google Scholar]
- Lysgaard Poulsen J, Stubbe J, & Lindholt JS (2016). Animal Models Used to Explore Abdominal Aortic Aneurysms: A Systematic Review. European Journal of Vascular and Endovascular Surgery, 52(4), 487–499. doi: 10.1016/j.ejvs.2016.07.004 [DOI] [PubMed] [Google Scholar]
- Ma X, Yao H, Yang Y, Jin L, Wang Y, Wu L, … Cheng K. (2018). miR-195 suppresses abdominal aortic aneurysm through the TNF-α/NF-κB and VEGF/PI3K/Akt pathway. International journal of molecular medicine, 41(4), 2350–2358. [DOI] [PubMed] [Google Scholar]
- Maguire EM, Pearce SWA, Xiao R, Oo AY, & Xiao Q (2019a). Matrix Metalloproteinase in Abdominal Aortic Aneurysm and Aortic Dissection. Pharmaceuticals (Basel, Switzerland), 12(3), 118. doi: 10.3390/ph12030118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire EM, Pearce SWA, Xiao R, Oo AY, & Xiao Q (2019b). Matrix Metalloproteinase in Abdominal Aortic Aneurysm and Aortic Dissection. Pharmaceuticals, 12(3), 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoud M, Kim R, de Luca A, Gauci I, Hsiao S, & Evans P (2014). 189 Disturbed Flow Promotes Endothelial Cell Injury Via the Induction of Developmental Genes. Heart, 100(Suppl 3), A105–A105. doi: 10.1136/heartjnl-2014-306118.189 [DOI] [Google Scholar]
- Mannino RG, Myers DR, Ahn B, Wang Y, Margo R, Gole H, … Lam WA. (2015). “Do-it-yourself in vitro vasculature that recapitulates in vivo geometries for investigating endothelial-blood cell interactions”. Scientific Reports, 5, 12401–12401. doi: 10.1038/srep12401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald KK, Cooper S, Danielzak L, & Leask RL (2016). Glycocalyx Degradation Induces a Proinflammatory Phenotype and Increased Leukocyte Adhesion in Cultured Endothelial Cells under Flow. PLOS ONE, 11(12), e0167576. doi: 10.1371/journal.pone.0167576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meirson T, Orion E, Di Mario C, Webb C, Patel N, Channon KM, … Taggart DP. (2015). Flow patterns in externally stented saphenous vein grafts and development of intimal hyperplasia. The Journal of Thoracic and Cardiovascular Surgery, 150(4), 871–879. doi: 10.1016/j.jtcvs.2015.04.061 [DOI] [PubMed] [Google Scholar]
- Meng H, Tutino V, Xiang J, & Siddiqui A (2014). High WSS or low WSS? Complex interactions of hemodynamics with intracranial aneurysm initiation, growth, and rupture: toward a unifying hypothesis. American Journal of Neuroradiology, 35(7), 1254–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel J-B, Jondeau G, & Milewicz DM (2018). From genetics to response to injury: vascular smooth muscle cells in aneurysms and dissections of the ascending aorta. Cardiovascular Research, 114(4), 578–589. doi: 10.1093/cvr/cvy006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michineau S, Franck G, Wagner-Ballon O, Dai J, Allaire E, & Gervais M (2014). Chemokine (CXC motif) receptor 4 blockade by AMD3100 inhibits experimental abdominal aortic aneurysm expansion through anti-inflammatory effects. Arteriosclerosis, Thrombosis, and Vascular Biology, 34(8), 1747–1755. [DOI] [PubMed] [Google Scholar]
- Mitra R, Qiao J, Madhavan S, O’Neil GL, Ritchie B, Kulkarni P, … Ebong EE. (2018). The comparative effects of high fat diet or disturbed blood flow on glycocalyx integrity and vascular inflammation. Translational Medicine Communications, 3(1), 10. doi: 10.1186/s41231-018-0029-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modi S, Shah K, Schultz L, Tahir R, Affan M, & Varelas P (2019). Cost of hospitalization for aneurysmal subarachnoid hemorrhage in the United States. Clinical Neurology and Neurosurgery, 182, 167–170. doi: 10.1016/j.clineuro.2019.05.018 [DOI] [PubMed] [Google Scholar]
- Monsivais D, Morales M, Day A, Kim D, Hoh B, & Blackburn S (2019). Cost Analysis of Endovascular Coiling and Surgical Clipping for the Treatment of Ruptured Intracranial Aneurysms. World Neurosurgery, 124, e125–e130. doi: 10.1016/j.wneu.2018.12.028 [DOI] [PubMed] [Google Scholar]
- Morbiducci U, Kok AM, Kwak BR, Stone PH, Steinman DA, & Wentzel JJ (2016). Atherosclerosis at arterial bifurcations: evidence for the role of haemodynamics and geometry. Thrombosis and haemostasis, 115(3), 484–492. doi: 10.1160/th15-07-0597 [DOI] [PubMed] [Google Scholar]
- Morel S, Diagbouga MR, Dupuy N, Sutter E, Braunersreuther V, Pelli G, … Isidor N. (2018). Correlating clinical risk factors and histological features in ruptured and unruptured human intracranial aneurysms: the Swiss AneuX Study. Journal of Neuropathology & Experimental Neurology, 77(7), 555–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriguchi T, & Sumpio BE (2015). PECAM-1 phosphorylation and tissue factor expression in HUVECs exposed to uniform and disturbed pulsatile flow and chemical stimuli. Journal of Vascular Surgery, 61(2), 481–488. doi: 10.1016/j.jvs.2013.09.059 [DOI] [PubMed] [Google Scholar]
- Muluk SL, Muluk PD, Shum J, & Finol EA (2017). On the use of geometric modeling to predict aortic aneurysm rupture. Annals of vascular surgery, 44, 190–196. [DOI] [PubMed] [Google Scholar]
- Mundi S, Massaro M, Scoditti E, Carluccio MA, van Hinsbergh VWM, Iruela-Arispe ML, & De Caterina R (2017). Endothelial permeability, LDL deposition, and cardiovascular risk factors—a review. Cardiovascular Research, 114(1), 35–52. doi: 10.1093/cvr/cvx226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murayama Y, Takao H, Ishibashi T, Saguchi T, Ebara M, Yuki I, … Molyneux AJ. (2016). Risk analysis of unruptured intracranial aneurysms: prospective 10-year cohort study. Stroke, 47(2), 365–371. [DOI] [PubMed] [Google Scholar]
- Nakajima H, & Mochizuki N (2017). Flow pattern-dependent endothelial cell responses through transcriptional regulation. Cell cycle (Georgetown, Tex.), 16(20), 1893–1901. doi: 10.1080/15384101.2017.1364324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassiri F, Ibrahim GM, Badhiwala JH, Witiw CD, Mansouri A, Alotaibi NM, & Macdonald RL (2016). A Propensity Score-Matched Study of the Use of Non-steroidal Anti-inflammatory Agents Following Aneurysmal Subarachnoid Hemorrhage. Neurocritical Care, 25(3), 351–358. doi: 10.1007/s12028-016-0266-6 [DOI] [PubMed] [Google Scholar]
- Ng J, Bourantas CV, Torii R, Ang HY, Tenekecioglu E, Serruys PW, & Foin N (2017). Local Hemodynamic Forces After Stenting. Arteriosclerosis, Thrombosis, and Vascular Biology, 37(12), 2231–2242. doi: 10.1161/ATVBAHA.117.309728 [DOI] [PubMed] [Google Scholar]
- Ngoepe M, Frangi A, Byrne J, & Ventikos Y (2018). Thrombosis in Cerebral Aneurysms and the Computational Modeling Thereof: A Review. Frontiers in physiology, 9, 306. doi: 10.3389/fphys.2018.00306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikoubashman O, Pjontek R, Brockmann M-A, Tolba R, & Wiesmann M (2015). Retrieval of Migrated Coils with Stent Retrievers: An Animal Study. American Journal of Neuroradiology, 36(6), 1162–1166. doi: 10.3174/ajnr.A4240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishihara M, Aoki H, Ohno S, Furusho A, Hirakata S, Nishida N, … Fukumoto Y. (2017). The role of IL-6 in pathogenesis of abdominal aortic aneurysm in mice. PLOS ONE, 12(10), e0185923–e0185923. doi: 10.1371/journal.pone.0185923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosoudi N, Nahar-Gohad P, Sinha A, Chowdhury A, Gerard P, Carsten CG, … Vyavahare NR. (2015). Prevention of Abdominal Aortic Aneurysm Progression by Targeted Inhibition of Matrix Metalloproteinase Activity With Batimastat-Loaded Nanoparticles. Circulation Research, 117(11), e80–e89. doi: 10.1161/CIRCRESAHA.115.307207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novikova OA, Laktionov PP, & Karpenko AA (2019). The roles of mechanotransduction, vascular wall cells, and blood cells in atheroma induction. Vascular, 27(1), 98–109. [DOI] [PubMed] [Google Scholar]
- Nowicki KW, Hosaka K, He Y, McFetridge PS, Scott EW, & Hoh BL (2014). Novel high-throughput in vitro model for identifying hemodynamic-induced inflammatory mediators of cerebral aneurysm formation. Hypertension, 64(6), 1306–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien-Irr MS, Harris LM, Dosluoglu HH, Cherr GS, Rivero M, Noor S, … Dryjski ML. (2017). Factors that affect cost and clinical outcome of endovascular aortic repair for abdominal aortic aneurysm. Journal of Vascular Surgery, 65(4), 997–1005. doi: 10.1016/j.jvs.2016.08.090 [DOI] [PubMed] [Google Scholar]
- Ollikainen E, Tulamo R, Lehti S, Lee-Rueckert M, Hernesniemi J, Niemelä M, … Frösen J. (2016). Smooth Muscle Cell Foam Cell Formation, Apolipoproteins, and ABCA1 in Intracranial Aneurysms: Implications for Lipid Accumulation as a Promoter of Aneurysm Wall Rupture. Journal of neuropathology and experimental neurology, 75(7), 689–699. doi: 10.1093/jnen/nlw041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paliwal N, Yu H, Xu J, Xiang J, Siddiqui A, Yang X, … Meng H. (2016). Virtual Stenting Workflow with Vessel-Specific Initialization and Adaptive Expansion for Neurovascular Stents and Flow Diverters. Computer Methods in Biomechanics and Biomedical Engineering, 19, 1–9. doi: 10.1080/10255842.2016.1149573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan L, Hong Z, Yu L, Gao Y, Zhang R, Feng H, … Wang G. (2017). Shear stress induces human aortic endothelial cell apoptosis via interleukin-1 receptor-associated kinase 2-induced endoplasmic reticulum stress. Molecular medicine reports, 16(5), 7205–7212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvizi M, Petersen A, van Spreuwel-Goossens C, Kluijtmans S, & Harmsen M (2018). Perivascular scaffolds loaded with adipose tissue-derived stromal cells attenuate development and progression of abdominal aortic aneurysm in rats. Journal of Biomedical Materials Research Part A, 106(9), 2494–2506. [DOI] [PubMed] [Google Scholar]
- Patelis N, Moris D, Schizas D, Damaskos C, Perrea D, Bakoyiannis C, … Georgopoulos S. (2017). Animal models in the research of abdominal aortic aneurysms development. Physiological research, 66(6), 899–915. [DOI] [PubMed] [Google Scholar]
- Penn DL, Witte SR, Komotar RJ, & Sander Connolly E (2014). The role of vascular remodeling and inflammation in the pathogenesis of intracranial aneurysms. Journal of Clinical Neuroscience, 21(1), 28–32. doi: 10.1016/j.jocn.2013.07.004 [DOI] [PubMed] [Google Scholar]
- Peshkova IO, Schaefer G, & Koltsova EK (2016). Atherosclerosis and aortic aneurysm–is inflammation a common denominator? The FEBS journal, 283(9), 1636–1652. [DOI] [PubMed] [Google Scholar]
- Petsophonsakul P, Furmanik M, Forsythe R, Dweck M, Schurink GW, Natour E, … Schurgers L. (2019). Role of Vascular Smooth Muscle Cell Phenotypic Switching and Calcification in Aortic Aneurysm Formation. Arteriosclerosis, Thrombosis, and Vascular Biology, 39(7), 1351–1368. doi: 10.1161/ATVBAHA.119.312787 [DOI] [PubMed] [Google Scholar]
- Pfenniger A, Meens MJ, Pedrigi RM, Foglia B, Sutter E, Pelli G, … Kwak BR. (2015). Shear stress-induced atherosclerotic plaque composition in ApoE−/− mice is modulated by connexin37. Atherosclerosis, 243(1), 1–10. doi: 10.1016/j.atherosclerosis.2015.08.029 [DOI] [PubMed] [Google Scholar]
- Phillips EH, Chang MS, Gorman S, Qureshi HJ, Ejendal KFK, Kinzer-Ursem TL, … Goergen CJ. (2018). Angiotensin II Infusion Does Not Cause Abdominal Aortic Aneurysms in Apolipoprotein E-Deficient Rats. Journal of Vascular Research, 55(1), 1–12. doi: 10.1159/000484086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piechota-Polanczyk A, Jozkowicz A, Nowak W, Eilenberg W, Neumayer C, Malinski T, … Brostjan C. (2015). The Abdominal Aortic Aneurysm and Intraluminal Thrombus: Current Concepts of Development and Treatment. Frontiers in cardiovascular medicine, 2, 19–19. doi: 10.3389/fcvm.2015.00019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piotin M, & Blanc R (2014). Balloons and stents in the endovascular treatment of cerebral aneurysms: vascular anatomy remodeled. Frontiers in neurology, 5, 41–41. doi: 10.3389/fneur.2014.00041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt MO, & Shockey WA (2016). Endothelial cells and cathepsins: Biochemical and biomechanical regulation. Biochimie, 122, 314–323. doi: 10.1016/j.biochi.2015.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulathan Z (2018). Experimental Models in Abdominal Aortic Aneurysm Abdominal Aortic Aneurysm-From Basic Research to Clinical Practice: IntechOpen. [Google Scholar]
- Qin H, Yang Q, Zhuang Q, Long J, Yang F, & Zhang H (2017). Morphological and hemodynamic parameters for middle cerebral artery bifurcation aneurysm rupture risk assessment. Journal of Korean Neurosurgical Society, 60(5), 504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J, Zheng Y, Hu J, Liao D, Gregersen H, Deng X, … Wang G. (2014). Biomechanical regulation of vascular smooth muscle cell functions: from in vitro to in vivo understanding. Journal of The Royal Society Interface, 11(90), 20130852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan K, Li S, Wang D, Shi Y, Yang Z, Song J, … Zhu W. (2018). Berberine Attenuates Macrophages Infiltration in Intracranial Aneurysms Potentially Through FAK/Grp78/UPR Axis. Frontiers in Pharmacology, 9(565). doi: 10.3389/fphar.2018.00565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana RA, & Taylor WR (2019). Cellular mechanisms of aortic aneurysm formation. Circulation Research, 124(4), 607–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainger G, Chimen M, Harrison M, Yates C, Harrison P, Watson S, … Nash G. (2015). The role of platelets in the recruitment of leukocytes during vascular disease. Platelets, 26, 1–14. doi: 10.3109/09537104.2015.1064881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramella M, Bernardi P, Fusaro L, Manfredi M, Casella F, Porta CM, … Settembrini AM. (2018). Relevance of inflammation and matrix remodeling in abdominal aortic aneurysm (AAA) and popliteal artery aneurysm (PAA) progression. American journal of translational research, 10(10), 3265. [PMC free article] [PubMed] [Google Scholar]
- Ramella M, Boccafoschi F, Bellofatto K, Follenzi A, Fusaro L, Boldorini R, … Cannas M. (2017). Endothelial MMP-9 drives the inflammatory response in abdominal aortic aneurysm (AAA). American journal of translational research, 9(12), 5485–5495. [PMC free article] [PubMed] [Google Scholar]
- Rao J, Brown BN, Weinbaum JS, Ofstun EL, Makaroun MS, Humphrey JD, & Vorp DA (2015). Distinct macrophage phenotype and collagen organization within the intraluminal thrombus of abdominal aortic aneurysm. Journal of Vascular Surgery, 62(3), 585–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reglero-Real N, Colom B, Bodkin JV, & Nourshargh S (2016). Endothelial Cell Junctional Adhesion Molecules. Arteriosclerosis, Thrombosis, and Vascular Biology, 36(10), 2048–2057. doi: 10.1161/ATVBAHA.116.307610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren J, Liu Z, Wang Q, Giles J, Greenberg J, Sheibani N, … Liu B. (2016). Andrographolide Ameliorates Abdominal Aortic Aneurysm Progression by Inhibiting Inflammatory Cell Infiltration through Downregulation of Cytokine and Integrin Expression. Journal of Pharmacology and Experimental Therapeutics, 356(1), 137–147. doi: 10.1124/jpet.115.227934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie RH, Drummond GR, Sobey CG, De Silva TM, & Kemp-Harper BK (2017). The opposing roles of NO and oxidative stress in cardiovascular disease. Pharmacological Research, 116, 57–69. doi: 10.1016/j.phrs.2016.12.017 [DOI] [PubMed] [Google Scholar]
- Robertson AM, Duan X, Aziz KM, Hill MR, Watkins SC, & Cebral JR (2015). Diversity in the Strength and Structure of Unruptured Cerebral Aneurysms. Annals of Biomedical Engineering, 43(7), 1502–1515. doi: 10.1007/s10439-015-1252-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas HA, Fernandes K. S. d. S., Ottone MR, Magalhães K. C. S. F. d., Albuquerque L. A. F. d., Pereira JLB, … Simões RT (2018). Levels of MMP-9 in patients with intracranial aneurysm: Relation with risk factors, size and clinical presentation. Clinical Biochemistry, 55, 63–68. doi: 10.1016/j.clinbiochem.2018.03.005 [DOI] [PubMed] [Google Scholar]
- Roth W, Morgello S, Goldman J, Mohr JP, Elkind MSV, Marshall RS, & Gutierrez J (2017). Histopathological Differences Between the Anterior and Posterior Brain Arteries as a Function of Aging. Stroke, 48(3), 638–644. doi: 10.1161/STROKEAHA.116.015630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowinska Z, Gorressen S, Merx MW, Koeppel TA, Liehn EA, & Zernecke A (2014). Establishment of a New Murine Elastase-Induced Aneurysm Model Combined with Transplantation. PLOS ONE, 9(7), e102648. doi: 10.1371/journal.pone.0102648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salata K, Syed M, Hussain MA, de Mestral C, Greco E, Mamdani M, … Verma S. (2018). Statins reduce abdominal aortic aneurysm growth, rupture, and perioperative mortality: a systematic review and meta-analysis. Journal of the American Heart Association, 7(19), e008657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salman HE, Ramazanli B, Yavuz MM, & Yalcin HC (2019). Biomechanical investigation of disturbed hemodynamics-induced tissue degeneration in abdominal aortic aneurysms using computational and experimental techniques. Frontiers in Bioengineering and Biotechnology, 7, 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaniego EA, Roa JA, & Hasan D (2019). Vessel wall imaging in intracranial aneurysms. Journal of NeuroInterventional Surgery, 11(11), 1105–1112. doi: 10.1136/neurintsurg-2019-014938 [DOI] [PubMed] [Google Scholar]
- Sarrami-Foroushani A, Lassila T, Hejazi SM, Nagaraja S, Bacon A, & Frangi AF (2019). A computational model for prediction of clot platelet content in flow-diverted intracranial aneurysms. Journal of Biomechanics, 91, 7–13. doi: 10.1016/j.jbiomech.2019.04.045 [DOI] [PubMed] [Google Scholar]
- Sathyan S, Koshy LV, Srinivas L, Easwer HV, Premkumar S, Nair S, … Banerjee M. (2015). Pathogenesis of intracranial aneurysm is mediated by proinflammatory cytokine TNFA and IFNG and through stochastic regulation of IL10 and TGFB1 by comorbid factors. Journal of Neuroinflammation, 12(1), 135. doi: 10.1186/s12974-015-0354-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savastano LE, Bhambri A, Andrew Wilkinson D, & Pandey AS (2018). Chapter 2 - Biology of Cerebral Aneurysm Formation, Growth, and Rupture. In Ringer AJ(Ed.), Intracranial Aneurysms (pp. 17–32): Academic Press. [Google Scholar]
- Sawyer DM, Pace LA, Pascale CL, Kutchin AC, O’Neill BE, Starke RM, & Dumont AS (2016). Lymphocytes influence intracranial aneurysm formation and rupture: role of extracellular matrix remodeling and phenotypic modulation of vascular smooth muscle cells. Journal of Neuroinflammation, 13(1), 185. doi: 10.1186/s12974-016-0654-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz-Rixen T, Keese M, Hakimi M, Peters A, Böckler D, Nelson K, & Grundmann RT (2016). Ruptured abdominal aortic aneurysm—epidemiology, predisposing factors, and biology. Langenbeck’s Archives of Surgery, 401(3), 275–288. doi: 10.1007/s00423-016-1401-8 [DOI] [PubMed] [Google Scholar]
- Sénémaud J, Caligiuri G, Etienne H, Delbosc S, Michel J-B, & Coscas R (2017). Translational Relevance and Recent Advances of Animal Models of Abdominal Aortic Aneurysm. Arteriosclerosis, Thrombosis, and Vascular Biology, 37(3), 401–410. doi: 10.1161/ATVBAHA.116.308534 [DOI] [PubMed] [Google Scholar]
- Seo J-H, Eslami P, Caplan J, Tamargo RJ, & Mittal R (2018). A Highly Automated Computational Method for Modeling of Intracranial Aneurysm Hemodynamics. Frontiers in physiology, 9, 681–681. doi: 10.3389/fphys.2018.00681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serbanovic-Canic J, de Luca A, Warboys C, Ferreira PF, Luong LA, Hsiao S, … Souilhol C. (2017). Zebrafish model for functional screening of flow-responsive genes. Arteriosclerosis, Thrombosis, and Vascular Biology, 37(1), 130–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setozaki S, Minakata K, Masumoto H, Hirao S, Yamazaki K, Kuwahara K, … Sakata R. (2017). Prevention of abdominal aortic aneurysm progression by oral administration of green tea polyphenol in a rat model. Journal of Vascular Surgery, 65(6), 1803–1812.e1802. doi: 10.1016/j.jvs.2016.06.003 [DOI] [PubMed] [Google Scholar]
- Sheinberg DL, McCarthy DJ, Elwardany O, Bryant J-P, Luther E, Chen SH, … Starke RM. (2019). Endothelial dysfunction in cerebral aneurysms. 47(1), E3. doi: 10.3171/2019.4.focus19221 [DOI] [PubMed] [Google Scholar]
- Shimizu K, Kushamae M, Mizutani T, & Aoki T (2019). Intracranial Aneurysm as a Macrophage-mediated Inflammatory Disease. Neurologia medico-chirurgica, 59(4), 126–132. doi: 10.2176/nmc.st.2018-0326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Signorelli F, Gory B, Riva R, Labeyrie P-E, Pelissou-Guyotat I, & Turjman F (2015). Hemodynamics, inflammation, vascular remodeling, and the development and rupture of intracranial aneurysms: a review. Neuroimmunology and Neuroinflammation, 2(2), 59. [Google Scholar]
- Signorelli F, Sela S, Gesualdo L, Chevrel S, Tollet F, Pailler-Mattei C, … Schul DB. (2018). Hemodynamic Stress, Inflammation, and Intracranial Aneurysm Development and Rupture: A Systematic Review. World Neurosurgery, 115, 234–244. doi: 10.1016/j.wneu.2018.04.143 [DOI] [PubMed] [Google Scholar]
- Sindeev S, Arnold PG, Frolov S, Prothmann S, Liepsch D, Balasso A, … Kirschke JS. (2018). Phase-contrast MRI versus numerical simulation to quantify hemodynamical changes in cerebral aneurysms after flow diverter treatment. PLOS ONE, 13(1), e0190696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu KL, Gao L, & Cai H (2016). Differential roles of protein complexes NOX1-NOXO1 and NOX2-p47phox in mediating endothelial redox responses to oscillatory and unidirectional laminar shear stress. Journal of Biological Chemistry, 291(16), 8653–8662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soldozy S, Norat P, Elsarrag M, Chatrath A, Costello JS, Sokolowski JD, … Park MS. (2019). The biophysical role of hemodynamics in the pathogenesis of cerebral aneurysm formation and rupture. 47(1), E11. doi: 10.3171/2019.4.focus19232 [DOI] [PubMed] [Google Scholar]
- Sonmez UM, Cheng Y-W, Watkins SC, Roman BL, & Davidson LA (2020). Endothelial cell polarization and orientation to flow in a novel microfluidic multimodal shear stress generator. Lab on a Chip, 20(23), 4373–4390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sriram K, Intaglietta M, & Tartakovsky DM (2014). Non-Newtonian flow of blood in arterioles: consequences for wall shear stress measurements. Microcirculation (New York, N.Y. : 1994), 21(7), 628–639. doi: 10.1111/micc.12141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starke RM, Chalouhi N, Ding D, & Hasan DM (2015). Potential role of aspirin in the prevention of aneurysmal subarachnoid hemorrhage. Cerebrovascular Diseases, 39(5–6), 332–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stella S, Vergara C, Giovannacci L, Quarteroni A, & Prouse G (2019). Assessing the Disturbed Flow and the Transition to Turbulence in the Arteriovenous Fistula. Journal of Biomechanical Engineering, 141(10). doi: 10.1115/1.4043448 [DOI] [PubMed] [Google Scholar]
- Strong MJ, Amenta PS, Dumont AS, & Medel R (2015). The role of leukocytes in the formation and rupture of intracranial aneurysms. Neuroimmunol Neuroinflammation, 2, 8. [Google Scholar]
- Sun X, Fu Y, Gu M, Zhang L, Li D, Li H, … Zhu Y. (2016). Activation of integrin α5 mediated by flow requires its translocation to membrane lipid rafts in vascular endothelial cells. Proceedings of the National Academy of Sciences, 113(3), 769–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunderland K, & Jiang J (2019). Multivariate analysis of hemodynamic parameters on intracranial aneurysm initiation of the internal carotid artery. Medical engineering & physics, 74, 129–136. [DOI] [PubMed] [Google Scholar]
- Sunderland K, Wang M, Pandey A, Gemmete J, Huang Q, Goudge A, & Jiang J (2020). Quantitative analysis of flow vortices: differentiation of unruptured and ruptured medium-sized middle cerebral artery aneurysms. Acta Neurochirurgica, 1–11. [DOI] [PubMed] [Google Scholar]
- Tanweer O, Wilson TA, Metaxa E, Riina HA, & Meng H (2014). A comparative review of the hemodynamics and pathogenesis of cerebral and abdominal aortic aneurysms: lessons to learn from each other. Journal of cerebrovascular and endovascular neurosurgery, 16(4), 335–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavares Monteiro JA, da Silva ES, Raghavan ML, Puech-Leão P, de Lourdes Higuchi M, & Otoch JP (2014). Histologic, histochemical, and biomechanical properties of fragments isolated from the anterior wall of abdominal aortic aneurysms. Journal of Vascular Surgery, 59(5), 1393–1401.e1392. doi: 10.1016/j.jvs.2013.04.064 [DOI] [PubMed] [Google Scholar]
- Taylor BE, Appelboom G, Zilinyi R, Goodman A, Chapel D, LoPresti M, & Connolly ES Jr (2015). Role of the complement cascade in cerebral aneurysm formation, growth, and rupture. Neuroimmunology and Neuroinflammation, 2(2), 93. [Google Scholar]
- Thompson BG, Brown RD, Amin-Hanjani S, Broderick JP, Cockroft KM, Connolly ES, … Torner J. (2015). Guidelines for the Management of Patients With Unruptured Intracranial Aneurysms. Stroke, 46(8), 2368–2400. doi: 10.1161/STR.0000000000000070 [DOI] [PubMed] [Google Scholar]
- Thompson JW, Elwardany O, McCarthy DJ, Sheinberg DL, Alvarez CM, Nada A, … Starke RM. (2019). In vivo cerebral aneurysm models. 47(1), E20. doi: 10.3171/2019.4.focus19219 [DOI] [PubMed] [Google Scholar]
- Thompson JW, Elwardany O, McCarthy DJ, Sheinberg DL, Alvarez CM, Nada A, … Starke RM. (2019). In vivo cerebral aneurysm models. Neurosurgical focus, 47(1), E20. [DOI] [PubMed] [Google Scholar]
- Tian Y, Gawlak G, O’Donnell JJ, Birukova AA, & Birukov KG (2016). Activation of vascular endothelial growth factor (VEGF) receptor 2 mediates endothelial permeability caused by cyclic stretch. Journal of Biological Chemistry, 291(19), 10032–10045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong X, Khandelwal AR, Wu X, Xu Z, Yu W, Chen C, … Zeng C. (2016). Pro-atherogenic role of smooth muscle Nox4-based NADPH oxidase. Journal of Molecular and Cellular Cardiology, 92, 30–40. doi: 10.1016/j.yjmcc.2016.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovar-Lopez F, Thurgood P, Gilliam C, Nguyen N, Pirogova E, Khoshmanesh K, & Baratchi S (2019). A Microfluidic System for Studying the Effects of Disturbed Flow on Endothelial Cells. Frontiers in Bioengineering and Biotechnology, 7(81). doi: 10.3389/fbioe.2019.00081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travascio F (2017). The Role of Matrix Metalloproteinase in Human Body Pathologies: BoD–Books on Demand.
- Tsuji M, Ishikawa T, Ishida F, Furukawa K, Miura Y, Shiba M, … Suzuki H. (2017). Stagnation and complex flow in ruptured cerebral aneurysms: a possible association with hemostatic pattern. 126(5), 1566. doi: 10.3171/2016.3.Jns152264 [DOI] [PubMed] [Google Scholar]
- Turkmani AH, Edwards NJ, & Chen PR (2015). The role of inflammation in cerebral aneurysms. Neuroimmunology and Neuroinflammation, 2(2), 102. [Google Scholar]
- Tutino VM, Liaw N, Spernyak JA, Ionita CN, Siddiqui AH, Kolega J, & Meng H (2016). Assessment of Vascular Geometry for Bilateral Carotid Artery Ligation to Induce Early Basilar Terminus Aneurysmal Remodeling in Rats. Current neurovascular research, 13(1), 82–92. doi: 10.2174/1567202612666151027143149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchimido R, Schmidt EP, & Shapiro NI (2019). The glycocalyx: a novel diagnostic and therapeutic target in sepsis. Critical Care, 23(1), 16. doi: 10.1186/s13054-018-2292-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umebayashi R, Uchida HA, Kakio Y, Subramanian V, Daugherty A, & Wada J (2018). Cilostazol Attenuates Angiotensin II–Induced Abdominal Aortic Aneurysms but Not Atherosclerosis in Apolipoprotein E–Deficient Mice. Arteriosclerosis, Thrombosis, and Vascular Biology, 38(4), 903–912. doi: 10.1161/ATVBAHA.117.309707 [DOI] [PubMed] [Google Scholar]
- Urabe G, Hoshina K, Shimanuki T, Nishimori Y, Miyata T, & Deguchi J (2016). Structural analysis of adventitial collagen to feature aging and aneurysm formation in human aorta. Journal of Vascular Surgery, 63(5), 1341–1350. [DOI] [PubMed] [Google Scholar]
- Vakil P, Ansari SA, Cantrell CG, Eddleman CS, Dehkordi FH, Vranic J, … Carroll TJ. (2015). Quantifying Intracranial Aneurysm Wall Permeability for Risk Assessment Using Dynamic Contrast-Enhanced MRI: A Pilot Study. American Journal of Neuroradiology, 36(5), 953–959. doi: 10.3174/ajnr.A4225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valen-Sendstad K, & Steinman D (2014). Mind the gap: impact of computational fluid dynamics solution strategy on prediction of intracranial aneurysm hemodynamics and rupture status indicators. American Journal of Neuroradiology, 35(3), 536–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Engeland NC, Pollet AM, den Toonder JM, Bouten CV, Stassen OM, & Sahlgren CM (2018). A biomimetic microfluidic model to study signalling between endothelial and vascular smooth muscle cells under hemodynamic conditions. Lab on a Chip, 18(11), 1607–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varble N, Trylesinski G, Xiang J, Snyder K, & Meng H (2017). Identification of vortex structures in a cohort of 204 intracranial aneurysms. Journal of The Royal Society Interface, 14(130), 20170021. doi: 10.1098/rsif.2017.0021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varble N, Xiang J, Lin N, Levy E, & Meng H (2016). Flow instability detected by high-resolution computational fluid dynamics in fifty-six middle cerebral artery aneurysms. Journal of Biomechanical Engineering, 138(6), 061009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voß S, Glaßer S, Hoffmann T, Beuing O, Weigand S, Jachau K, … Berg P. (2016). Fluid-structure simulations of a ruptured intracranial aneurysm: constant versus patient-specific wall thickness. Computational and mathematical methods in medicine, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G. x., Wang S, Liu L. l., Gong M. f., Zhang D, Yang C. y., & Wen L (2019). A simple scoring model for prediction of rupture risk of anterior communicating artery aneurysms. Frontiers in neurology, 10, 520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Jin X, Huang Y, Ran X, Luo D, Yang D, … Wang G. (2018). Endovascular stent-induced alterations in host artery mechanical environments and their roles in stent restenosis and late thrombosis. Regenerative Biomaterials, 5(3), 177–187. doi: 10.1093/rb/rby006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Dai D, Kolumam Parameswaran P, Kadirvel R, Ding Y-H, Robertson AM, & Kallmes DF (2018). Rabbit aneurysm models mimic histologic wall types identified in human intracranial aneurysms. Journal of NeuroInterventional Surgery, 10(4), 411–415. doi: 10.1136/neurintsurg-2017-013264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Dong H, Liu C, Xu G, Hu X, Fan Y, & Chen L (2018). Early growth response factor-1 DNA enzyme 1 inhibits the formation of abdominal aortic aneurysm in rats. Experimental and therapeutic medicine, 16(1), 141–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y-X, Xiang C, Liu B, Zhu Y, Luan Y, Liu S-T, & Qin K-R (2016). A multi-component parallel-plate flow chamber system for studying the effect of exercise-induced wall shear stress on endothelial cells. Biomedical engineering online, 15(2), 659–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Dinh TN, Nield A, Krishna SM, Denton K, & Golledge J (2017). Renal Denervation Promotes Atherosclerosis in Hypertensive Apolipoprotein E-Deficient Mice Infused with Angiotensin II. Frontiers in physiology, 8(215). doi: 10.3389/fphys.2017.00215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Emeto TI, Lee J, Marshman L, Moran C, Seto S. w., & Golledge J (2015). Mouse Models of Intracranial Aneurysm. Brain Pathology, 25(3), 237–247. doi: 10.1111/bpa.12175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warboys CM, Ghim M, & Weinberg PD (2019). Understanding mechanobiology in cultured endothelium: A review of the orbital shaker method. Atherosclerosis, 285, 170–177. doi: 10.1016/j.atherosclerosis.2019.04.210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei L, Leo HL, Chen Q, & Li Z (2019). Structural and Hemodynamic Analyses of Different Stent Structures in Curved and Stenotic Coronary Artery. Frontiers in Bioengineering and Biotechnology, 7(366). doi: 10.3389/fbioe.2019.00366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welleweerd JC, Nelissen BGL, Koole D, de Vries J-PPM, Moll FL, Pasterkamp G, … de Borst GJ. (2015). Histological analysis of extracranial carotid artery aneurysms. PLOS ONE, 10(1), e0117915–e0117915. doi: 10.1371/journal.pone.0117915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong AK, LLanos P, Boroda N, Rosenberg SR, & Rabbany SY (2016). A Parallel-Plate Flow Chamber for Mechanical Characterization of Endothelial Cells Exposed to Laminar Shear Stress. Cellular and Molecular Bioengineering, 9(1), 127–138. doi: 10.1007/s12195-015-0424-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Tong X, Liu Q, Cao Y, Zhao Y, & Wang S (2019). Microsurgical ligation for incompletely coiled or recurrent intracranial aneurysms: a 17-year single-center experience. Chinese Neurosurgical Journal, 5(1), 7. doi: 10.1186/s41016-019-0153-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xanthis I, Souilhol C, Serbanovic-Canic J, Roddie H, Kalli AC, Fragiadaki M, … Evans PC. (2019). β1 integrin is a sensor of blood flow direction. Journal of Cell Science, 132(11), jcs229542. doi: 10.1242/jcs.229542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang J, Yu J, Snyder KV, Levy EI, Siddiqui AH, & Meng H (2016). Hemodynamic-morphological discriminant models for intracranial aneurysm rupture remain stable with increasing sample size. Journal of NeuroInterventional Surgery, 8(1), 104–110. doi: 10.1136/neurintsurg-2014-011477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi R, Kotani T, Tanaka G, Tupin S, Osman K, Shafii NS, … Ohta M. (2019). Effects of Elasticity on Wall Shear Stress in Patient-Specific Aneurysm of Cerebral Artery. Journal of Flow Control, Measurement & Visualization, Vol.07No.02, 14. 10.4236/jfcmv.2019.72006 [DOI] [Google Scholar]
- Yamawaki-Ogata A, Hashizume R, Fu X-M, Usui A, & Narita Y (2014). Mesenchymal stem cells for treatment of aortic aneurysms. World journal of stem cells, 6(3), 278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y-W, Fan J, Bai S-L, Hou W-J, Li X, & Tong H (2016). Zinc Prevents Abdominal Aortic Aneurysm Formation by Induction of A20-Mediated Suppression of NF-κB Pathway. PLOS ONE, 11(2), e0148536. doi: 10.1371/journal.pone.0148536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh Y-T, Serrano R, François J, Chiu J-J, Li Y-SJ, del Álamo JC, … Lasheras JC. (2018). Three-dimensional forces exerted by leukocytes and vascular endothelial cells dynamically facilitate diapedesis. Proceedings of the National Academy of Sciences, 115(1), 133–138. doi: 10.1073/pnas.1717489115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura Y, Murakami Y, Saitoh M, Yokoi T, Aoki T, Miura K, … Group SR. (2014). Statin use and risk of cerebral aneurysm rupture: a hospital-based case–control study in Japan. Journal of Stroke and Cerebrovascular Diseases, 23(2), 343–348. [DOI] [PubMed] [Google Scholar]
- Yu Z, Morimoto K, Yu J, Bao W, Okita Y, & Okada K (2016). Endogenous superoxide dismutase activation by oral administration of riboflavin reduces abdominal aortic aneurysm formation in rats. Journal of Vascular Surgery, 64(3), 737–745. doi: 10.1016/j.jvs.2015.03.045 [DOI] [PubMed] [Google Scholar]
- Yuan S, Yurdagul A, Peretik JM, Alfaidi M, Yafeai ZA, Pardue S, … Orr AW. (2018). Cystathionine γ-Lyase Modulates Flow-Dependent Vascular Remodeling. Arteriosclerosis, Thrombosis, and Vascular Biology, 38(9), 2126–2136. doi: 10.1161/ATVBAHA.118.311402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue J, Chang S, Xiao Z, Qi Y, & He J (2018). The protective effect of puerarin on angiotensin II-induced aortic aneurysm formation by the inhibition of NADPH oxidase activation and oxidative stress-triggered AP-1 signaling pathways. Oncology letters, 16(3), 3327–3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun S, Budatha M, Dahlman JE, Coon BG, Cameron RT, Langer R, … Schwartz MA. (2016). Interaction between integrin α 5 and PDE4D regulates endothelial inflammatory signalling. Nature cell biology, 18(10), 1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Y (2017). Endothelial glycocalyx as a critical signalling platform integrating the extracellular haemodynamic forces and chemical signalling. Journal of Cellular and Molecular Medicine, 21(8), 1457–1462. doi: 10.1111/jcmm.13081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Qiao C, Zhang X, Luo H, & Sun X (2017). The expression of MMP-7 in serum and aneurysm tissues of patients with abdominal aortic aneurysm associated with hypertension and the clinical efficacy of endovascular exclusion. Eur Rev Med Pharmacol Sci, 21(20), 4623–4631. [PubMed] [Google Scholar]
- Zhang X, Ares WJ, Taussky P, Ducruet AF, & Grandhi R (2019). Role of matrix metalloproteinases in the pathogenesis of intracranial aneurysms. Neurosurgical focus, 47(1), E4. [DOI] [PubMed] [Google Scholar]
- Zhao W, Zhang H, & Su JY (2018). MicroRNA-29a contributes to intracranial aneurysm by regulating the mitochondrial apoptotic pathway. Molecular medicine reports, 18(3), 2945–2954. [DOI] [PubMed] [Google Scholar]
- Zhong L, He X, Si X, Wang H, Li B, Hu Y, … Bin J. (2019). SM22α (Smooth Muscle 22α) Prevents Aortic Aneurysm Formation by Inhibiting Smooth Muscle Cell Phenotypic Switching Through Suppressing Reactive Oxygen Species/NF-κB (Nuclear Factor-κB). Arteriosclerosis, Thrombosis, and Vascular Biology, 39(1), e10–e25. doi: 10.1161/ATVBAHA.118.311917 [DOI] [PubMed] [Google Scholar]
- Zhou G, Zhu Y, Yanling, Su M, & Li M. (2017). Association of wall shear stress with intracranial aneurysm rupture: systematic review and meta-analysis. Scientific Reports, 7(1), 5331. doi: 10.1038/s41598-017-05886-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J-J, Liu Y-F, Zhang Y-P, Zhao C-R, Yao W-J, Li Y-S, … Wang X-F. (2017). VAMP3 and SNAP23 mediate the disturbed flow-induced endothelial microRNA secretion and smooth muscle hyperplasia. Proceedings of the National Academy of Sciences, 114(31), 8271–8276. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this review article as no new data were created or analyzed in this work.