Abstract
Background:
There is limited literature regarding potential disparities in non-melanoma skin cancer for skin of color patients.
Objective:
To examine disparities in non-melanoma skin cancer among Hispanic/Latino patients using Mohs micrographic surgery defect sizes, with a secondary aim of examining effect of insurance type.
Methods:
We conducted a multicenter retrospective study using data from three major institutions in Los Angeles County. A total of 3,486 Mohs micrographic surgeries of basal cell, squamous cell, and basosquamous cell carcinomas were analyzed.
Results:
Hispanic/Latino patients had 17% larger Mohs micrographic surgery defect sizes as compared to non-Hispanic white patients. More notably, when comparing defect sizes of squamous cell carcinomas to basal cell carcinomas, Hispanic/Latino patients had 80% larger defect sizes compared to non-Hispanic white patients who had 25% larger defect sizes. Compared to Medicare, patients with HMO and Medicaid/HMO had 22% and 52% larger defect sizes, respectively, whereas patients with PPO had 10% smaller defect sizes.
Limitations:
The data included was from a single county population.
Conclusion:
Disparities exist between skin of color and white patients regarding non-melanoma skin cancer. Patients and the medical community need to be cognizant that patients can develop skin cancer regardless of their race and ethnicity.
Keywords: skin cancer, non-melanoma skin cancer, racial disparities, ethnic disparities, skin of color, Hispanic, Latino, Mohs micrographic surgery
Capsule Summary
• Hispanic/Latino patients have significantly larger Mohs micrographic surgery defect sizes for non-melanoma skin cancer as compared to non-Hispanic white patients.
• It is imperative to educate primary care providers and patients regarding the risk of skin cancer regardless of race/ethnicity and promote sun-protective behaviors using media, schools, and other outreach efforts.
Introduction
Non-melanoma skin cancers (NMSCs), including basal cell carcinomas (BCCs) and squamous cell carcinomas (SCCs), are the most prevalent malignancy in the United States.1 Although the lifetime incidences of melanomas and NMSCs diagnosed in skin of color patients are relatively low - 5% of Hispanics, 4% of Asians, and 2% of Blacks, many studies have demonstrated increased morbidity and mortality associated particularly with melanoma in patients of color.2–5 Hispanics and Blacks have been shown to be less likely to report skin cancer signs and symptoms.6 Thus, it is conceivable that these patients would be more likely to present at later stages of diagnosis, portending poorer outcomes. Additionally, Hispanics and Blacks represent a significant proportion of uninsured individuals in the United States. Following the implementation of the Affordable Care Act in 2010, more of these individuals were able to attain health insurance, however, Sohn et al. demonstrated that minority groups have a greater propensity to lose their health insurance over the course of their lifetime.7–9 This disparity of greater loss of health coverage contributes to the overall heightened morbidity and mortality associated with a skin cancer diagnosis in this population. Furthermore, particularly among Hispanic and Black populations, there is a perception that a darker skin tone or lack of family history of skin cancer significantly lowers, if not eliminates, their personal risk of skin cancer.6 These perceptions can be harmful and raise serious concern for the skin health of patients of color, especially given their poorer prognoses and outcomes when diagnosed with melanoma or NMSC.10,11 In addition, disparities in skin cancer may also be influenced by differences in education level, demographic factors, and overall skin cancer risk perceptions.12
We aim to highlight specific disparities in NMSC by comparing Mohs micrographic surgery (MMS) defect sizes in Hispanic/Latino patients as compared to non-Hispanic white patients. Since MMS more precisely removes cancerous tissue while sparing non-cancerous tissue, MMS defect sizes serve as an approximation of carcinoma size and further reflect the severity of the cancer, given that more advanced lesions usually result in larger surgical defects to completely remove the malignancy. To date, the medical community lacks any literature detailing such differences between defect sizes following MMS.
Methods
We conducted an institutional review board-approved multicenter retrospective study using data from three major institutions in Los Angeles County, California, which is the most populous county in the United States. The institutions included Keck Medicine of USC, Los Angeles County + University of Southern California (LAC+USC) Medical Center, and the University of California Los Angeles (UCLA) Health System. Data on NMSC, comprised of BCC, SCC, and basosquamous carcinoma (BSCC), were obtained from the MMS case logs from January 2015 through December 2017 at Keck, June 2015 through December 2017 at LAC+USC, and January 2015 through November 2019 at UCLA. Data collected included age at time of MMS, gender, race/ethnicity, insurance type, date of MMS, type of NMSC, anatomic location, final surgical defect size (calculated as area, cm2), presence of perineural or lymphovascular invasion, recurrence, metastasis, death, and history of immunosuppression (solid organ transplant, hematologic disease, and/or other immunosuppression).
Data analysis was performed with linear mixed-effects regression models with a random intercept for site (UCLA vs non-UCLA). The outcome is log-transformed carcinoma area (cm2). Model I demonstrates the main effect of race/ethnicity, while Model II additionally adjusts for carcinoma type, insurance type, gender, and age (Table I).
Table I:
Patient demographics and differences in Mohs micrographic surgery (MMS) defect sizes. Model I contains the main effect of race/ethnicity, while Model II additionally adjusts for carcinoma type, insurance type, gender, and age.
| Model I | Model II | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Predictors | N(%) or M(SD) | % Change in MMS Defect | B | CI | p | % Change in MMS Defect Size Size | B | CI | p |
| (Intercept) | 0.31 | 0.11, 0.51 | 0.002 | 0.01 | −0.57, 0.60 | 0.968 | |||
| Race/Ethnicity | |||||||||
| Non-Hispanic White | 2649 (76.0%) | ref | - | - | ref | - | - | ||
| Hispanic/Latino | 307 (8.8%) | +17% | 0.16 | 0.04, 0.29 | 0.011 | +16% | 0.15 | 0.02, 0.29 | 0.024 |
| Asian | 59 (1.7%) | +12% | 0.11 | −0.16, 0.38 | 0.441 | +12% | 0.11 | −0.15, 0.37 | 0.39 |
| Other/Unknown/Mixed | 471 (13.5%) | +6% | 0.06 | −0.17, 0.04 | 0.226 | −5% | −0.05 | −0.15, 0.05 | 0.338 |
| Carcinoma Type | - | ref | - | - | |||||
| BCC | 1985 (56.9%) | - | ref | - | - | ||||
| BSCC | 17 (0.5%) | +101% | 0.70 | 0.21, 1.18 | 0.005 | ||||
| SCC | 1484 (42.6%) | +35% | 0.30 | 0.23, 0.37 | <0.001 | ||||
| Insurance | |||||||||
| Medicare | 1667 (47.8%) | - | ref | - | - | ||||
| HMO | 225 (6.5%) | +22% | 0.20 | 0.06, 0.34 | 0.006 | ||||
| Medi-Cal LA Care | 93 (2.7%) | +52% | 0.42 | 0.20, 0.64 | <0.001 | ||||
| Medi-Cal Restr. Ben. | 79 (2.3%) | +11% | 0.10 | −0.16, 0.35 | 0.45 | ||||
| Other Ins. | 339 (9.7%) | +5% | 0.05 | −0.07, 0.17 | 0.4 | ||||
| PPO | 1031 (29.6%) | −10% | −0.11 | −0.20, −0.02 | 0.016 | ||||
| Self-Pay | 53 (1.5%) | −1% | −0.01 | −0.28, 0.28 | 0.99 | ||||
| Gender | |||||||||
| Female | 1333 (38.2%) | - | ref | - | - | ||||
| Male | 2153 (61.8%) | +39% | 0.33 | 0.26, 0.40 | <0.001 | ||||
| Age* | 67.8 (14.2) | +4% | 0.04 | 0.02, 0.05 | <0.001 | ||||
Regression coefficient reflects a 5-year increase in age.
Results
A total of 3486 Mohs micrographic surgeries of BCCs, SCCs, and BSCCs were included (Table I). The mean age of patients was 67.8 years old. There were 2153 (61.8%) males and 1333 (38.2%) females. Among these patients, 2649 (76.0%) were non-Hispanic white, 307 (8.8%) were Hispanic/Latino, 59 (1.7%) were Asian, and 471 (13.5%) were Other/Unknown/Mixed. The “Other” races/ethnicities of Other/Unknown/Mixed category included 5 American Indian/Alaska Native, 3 Black/African American, 3 Native Hawaiian/Other Pacific Islander, and 1 Middle Eastern/North African. Among NMSC type, 1985 (56.9%) were BCC, 1484 (42.6%) were SCC, and 17 (0.5%) were BSCC. BCC and SCC distributions by race/ethnicity included 1485 BCC and 1154 SCC in non-Hispanic white, 218 BCC and 88 SCC in Hispanic/Latino, 32 BCC and 26 SCC in Asian, and 250 BCC and 216 SCC in Other/Unknown/Mixed (Figure 1). Insurance types included 1667 (47.8%) Medicare, 1031 (29.6%) preferred provider organization (PPO), 225 (6.5%) health maintenance organization (HMO), 93 (2.7%) Medi-Cal LA Care (Medicaid/HMO), 79 (2.3%) Medi-Cal (Medicaid) Restricted Benefit, 339 (9.7%) other insurance, and 53 (1.5%) self-pay (Table I).
Figure 1:
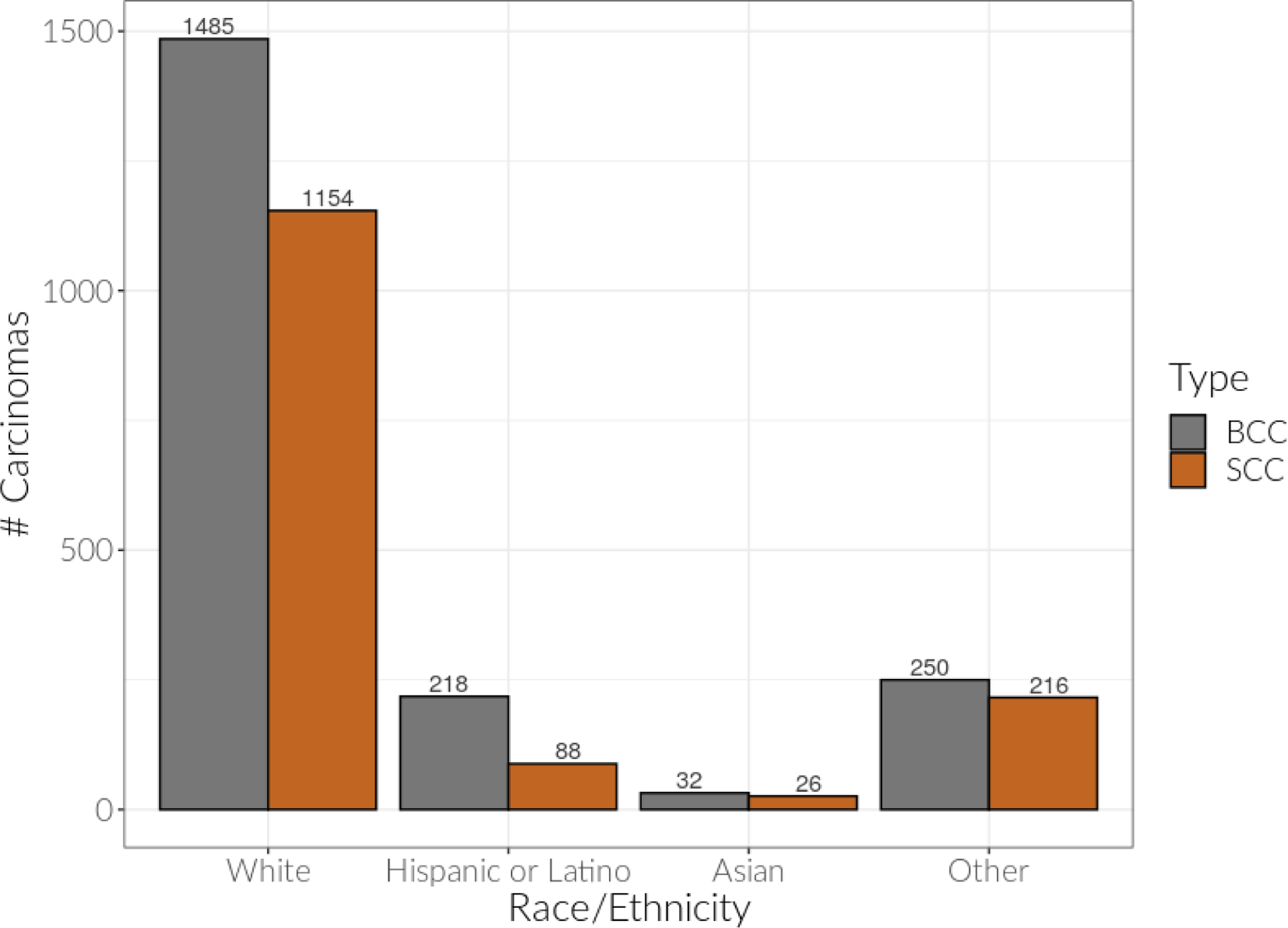
Basal cell carcinoma (BCC) and squamous cell carcinoma (SCC) distributions by race/ethnicity.
Model I showed that Hispanic/Latino patients on average had MMS defect sizes that were 17% larger than non-Hispanic white patients (B=0.16, CI 0.04, 0.29, p=0.011) (Table I). Model II confirmed this after adjusting for key demographic variables of carcinoma type, insurance type, gender, and age; the effect estimate for race/ethnicity was consistent at 16% larger (B=0.15, CI 0.02, 0.29, p=0.024). Additionally, Model II demonstrated that in the overall data inclusive of all races/ethnicities, MMS defect sizes for BSCCs were on average 101% larger than BCCs (B=0.70, CI 0.21, 1.18, p=0.005) and MMS defect sizes for SCCs were on average 35% larger than BCCs (B=0.30, CI 0.23, 0.37, p<0.001). When Model I and Model II were further stratified by non-Hispanic white versus Hispanic/Latino patients, the discrepancy in size for SCC MMS defect sizes as compared to BCC MMS defect sizes was more pronounced for Hispanic/Latino patients at 80% larger (B=0.59, CI 0.26, 0.91, p<0.001) as compared to non- Hispanic white patients at 25% larger (B=0.22, CI 0.14, 0.30, p<0.001) (Table II). Males on average had 39% larger MMS defect sizes than females (B=0.33, CI 0.26, 0.40, p<0.001), and each 5-year increase in age was associated with a 4% increase in MMS defect size (B=0.04, CI 0.02, 0.05, p<0.001) (Table I). These results were also depicted in the descriptive box plots of the relationship between MMS defect size and the predictor variables of race/ethnicity, carcinoma type, age, and gender (Figure 2, supplemental figure: http://dx.doi.org/10.17632/p3zx9×37fk.1)) as well as bar graphs comparing SCC and BCC MMS defect sizes in non-Hispanic white and Hispanic/Latino patients (Figure 3).
Table II:
Differences in patient demographics and Mohs micrographic surgery (MMS) defect sizes in non-Hispanic white patients and Hispanic/Latino patients.
| Non-Hispanic White | Hispanic/Latino | |||||||
|---|---|---|---|---|---|---|---|---|
| Predictors | % Change in MMS Defect Size | B | CI | p | % Change in MMS Defect Size | B | CI | p |
| (Intercept) | −0.52 | −0.86, −0.18 | 0.002 | −0.57 | −1.67, 0.54 | 0.314 | ||
| Carcinoma (SCC vs. BCC) | +25% | 0.22 | 0.14, 0.30 | <0.001 | +80% | 0.59 | 0.26, 0.91 | <0.001 |
| Insurance (ref = Medicare) | ||||||||
| HMO | +5% | 0.05 | −0.11, 0.22 | 0.53 | +103% | 0.71 | 0.22, 1.19 | 0.004 |
| Medi-Cal LA Care | +92% | 0.65 | 0.36, 0.94 | <0.001 | −2% | −0.02 | −0.55, 0.51 | 0.936 |
| Medi-Cal Restr. Ben. | +5% | 0.05 | −0.60, 0.70 | 0.888 | +1% | 0.01 | −0.41, 0.44 | 0.946 |
| Other Ins. | +4% | 0.04 | −0.09, 0.18 | 0.526 | +2% | 0.02 | −0.50, 0.55 | 0.932 |
| PPO | −9% | −0.09 | −0.18, 0.01 | 0.08 | +3% | 0.03 | −0.57, 0.63 | 0.917 |
| Self-Pay | +2% | 0.02 | −0.39, 0.43 | 0.926 | −1% | −0.01 | −0.77, 0.74 | 0.976 |
| Age* | +4% | 0.04 | 0.02, 0.05 | <0.001 | +4% | 0.04 | −0.01, 0.09 | 0.152 |
| Gender (Male vs. Female) | +40% | 0.34 | 0.26, 0.42 | <0.001 | +25% | 0.22 | −0.08, 0.52 | 0.144 |
Regression coefficient reflects a 5-year increase in age.
Figure 2:
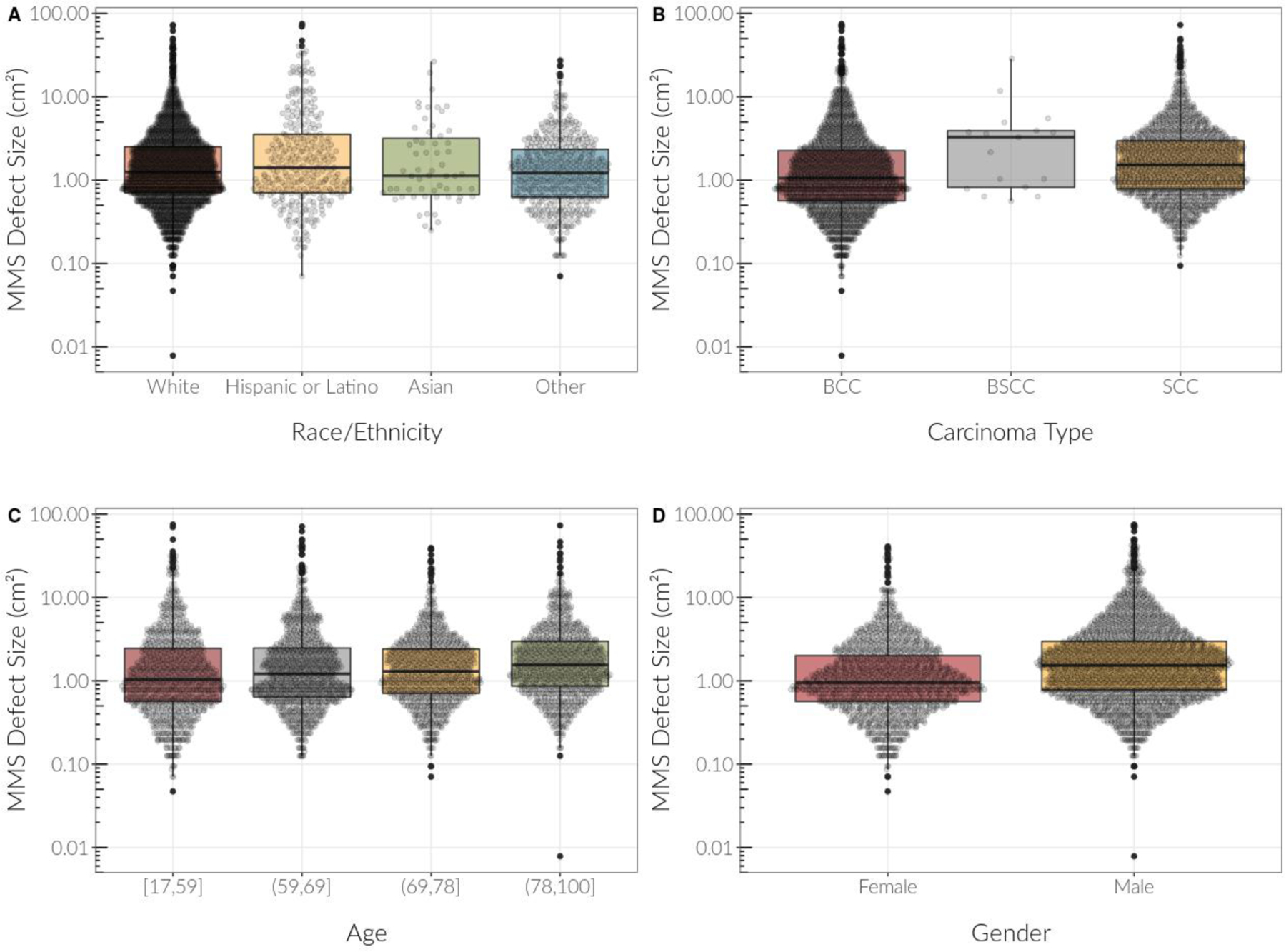
Descriptive boxplots of the relationship between Mohs micrographic surgery (MMS) defect sizes and other predictor variables including race/ethnicity, carcinoma type, age, and gender. Medians are reflected as horizontal lines, whereas the bars represent 1.5 interquartile ranges. MMS defect size is plotted using log scale.
Figure 3:
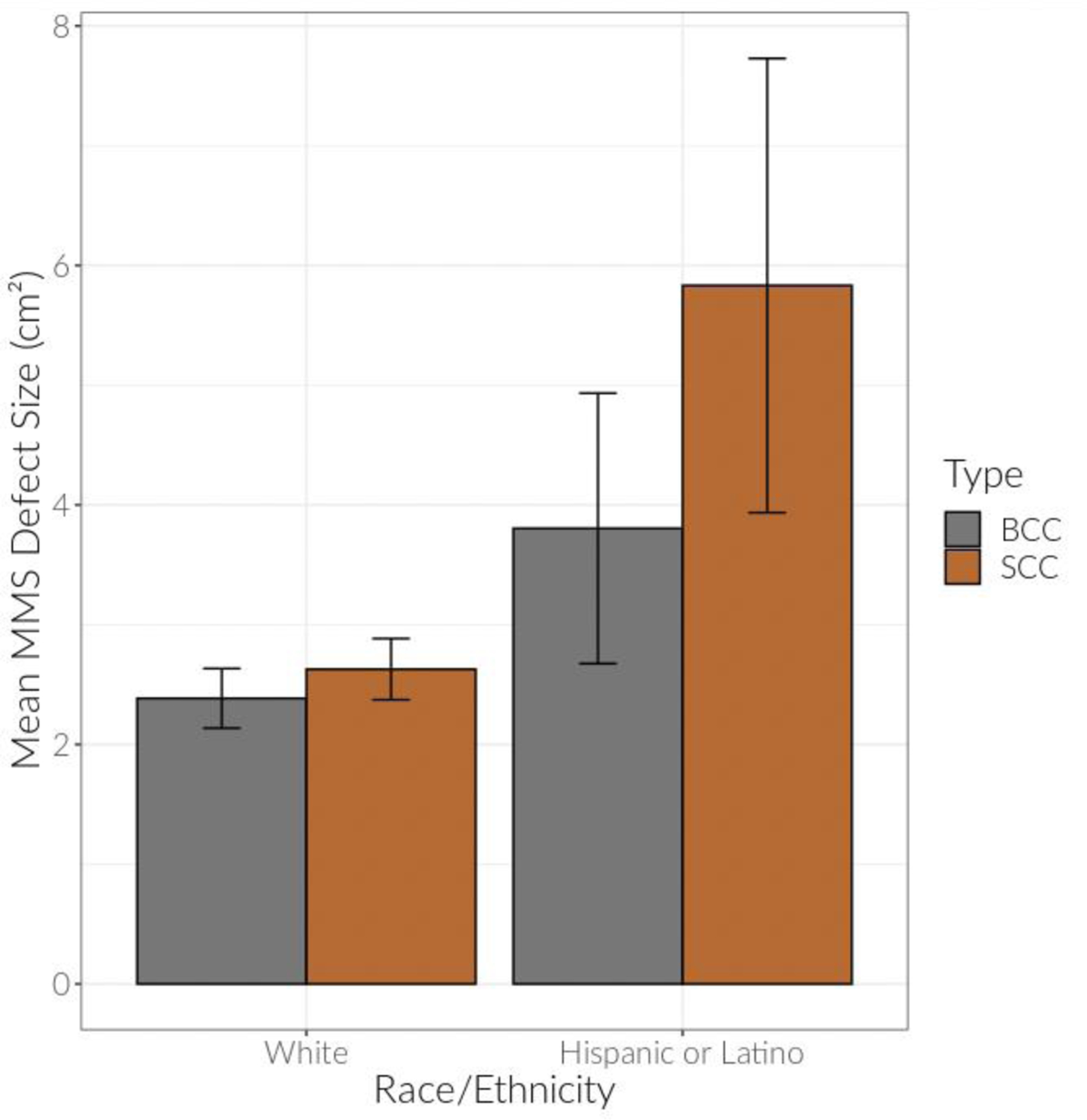
Mean Mohs micrographic surgery (MMS) defect sizes of basal cell carcinomas and squamous cell carcinomas in non-Hispanic white versus Hispanic/Latino patients. Error bars represent raw 95% confidence intervals.
Compared to patients with Medicare, patients with HMO had 22% larger MMS defect sizes (B=0.20, CI 0.06, 0.34, p=0.006) and those with Medi-Cal LA Care (Medicaid/HMO) had 52% larger MMS defect sizes (B=0.42, CI 0.20, 0.64, p<0.001). In contrast, patients with PPO insurances had 10% smaller MMS defect sizes as compared to patients with Medicare (B= −0.11, CI −0.20, −0.02, p=0.016). This statistical analysis was age-adjusted (Table I). With regards to race/ethnicity and insurance type, the majority of non-Hispanic white patients had Medicare (49.7%), followed by PPO (31.6%), other insurance (9.4%), HMO (6.2%), Medi-Cal LA Care (1.8%), self-pay (0.9%), and Medi-Cal Restricted Benefit (0.3%). In comparison, Hispanic/Latino patients had insurances of Medicare (33.9%), Medi-Cal Restricted Benefit (21.2%), HMO (12.4%), Medi-Cal LA Care (10.4%), other insurance (9.8%), PPO (8.1%), and self-pay (4.2%).
Discussion
Although skin of color patients are at risk of developing NMSC, the majority of skin cancer studies have focused on Caucasian skin.5,13 Skin cancers in skin of color patients often present with elevated morbidity and mortality, perhaps due to a lack of awareness, diagnosis at a more advanced stage, or socioeconomic factors such as barriers accessing care.5
In our multicenter retrospective study of MMS patients with NMSC at three major academic centers in Los Angeles County, California, we used MMS defect size as an approximation of carcinoma size and found that Hispanic/Latino patients have statistically significant larger MMS defect sizes than non-Hispanic white patients for NMSCs overall, even after adjusting for variables including carcinoma type, insurance type, gender, and age. This statistical significance persisted with SCCs, which have potential to be more aggressive than BCCs. Asian and Other/Unknown/Mixed races were not significant determinants of MMS defect size. Furthermore, patients with HMO and Medi-Cal LA Care (Medicaid/HMO) insurances had statistically significant larger MMS defect sizes as compared to Medicare patients, whereas patients with PPO insurance had the smallest MMS defect sizes. These findings of larger MMS defect sizes are critical as they are a further indication of health disparities based on race/ethnicity and type of insurance. Furthermore, larger MMS defect sizes reflect more advanced disease and may also be associated with higher morbidity.
Findings of larger MMS defect sizes in Hispanic/Latino patients are likely multifactorial. Hispanic/Latino patients may not be aware of their risk for developing skin cancer and thus may have decreased use of photoprotection or sun-avoidant behaviors, reduced recognition of potential skin cancers, and subsequent delay in seeking care. A study by Buster et al. found that Hispanics and Blacks more often believed that they could not lower their risk of skin cancer, skin cancer diagnosis is preceded by pain or other symptoms, and there are too many skin cancer prevention recommendations to follow.12 In another study by Taylor, Hispanics and Blacks reported low sunscreen use due to misconceptions that it is unnecessary to use sun protection.14 When sunscreen was used, it was often applied insufficiently and not reapplied.14 A study by Sanclemente et al. of Columbian high school students found that most students did not practice sun protection despite having moderate to very high sun exposure and light skin phototypes (majority were Fitzpatrick skin types I-III).15 Another factor possibly contributing to the larger surgery defect sizes in Hispanic/Latino patients may be a delay in diagnosis, as clinicians may either not be aware of the skin cancer risk in skin of color patients or have difficulty recognizing NMSC in skin of color.13
Our study also found that when compared to patients with Medicare, larger MMS defect sizes were seen in patients with HMO and Medi-Cal LA Care (Medicaid/HMO) insurances and smaller defect sizes were seen in patients with PPO insurances, even when controlled for age. Most non-Hispanic white patients in our study had Medicare and PPO insurances, whereas the majority of Hispanic/Latino patients had Medicare, Medi-Cal Restricted Benefit, HMO, and Medi-Cal LA Care. These findings show how race/ethnicity may relate to type of insurance coverage in their overall effects on health disparities. Disparities in health insurance are multifactorial and stem in part from both socioeconomic status and race.16 In dermatology, underinsured patients have worse outcomes, may receive delayed diagnoses, and experience delays in interventions and treatments.17,18 Regarding melanoma, type of insurance (e.g. public versus private) is statistically significantly associated with the stage of melanoma diagnosis, with uninsured and Medicaid patients being at greater risk for late stage disease at diagnosis.16,19 Furthermore, privately insured patients may have a higher acceptance rate as new patients and experience shorter wait times to see a dermatologist.20
Recommendations based on our findings include the following: a more aggressive effort to promote sun-protective behaviors through media campaigns, community outreach targeting religious and cultural organizations, and increased education in schools and physician offices (particularly primary care physicians) regarding the risk of developing skin cancer regardless of race and ethnicity. Furthermore, additional focus should be placed on recognition of skin cancer in skin of color patients for dermatologists and primary care physicians during their residency training and in continuing medical education.
Limitations of this study include limited data for non-Hispanic Black and Asian patients due to a small sample size. There is potential heterogeneity between the specific racial/ethnic categories and the Other/Unknown/Mixed categories, which may have limited the ability to detect an effect and can be more closely examined in future studies. Additionally, there were slightly different time periods for data collection at each of the institutions to allow for similar sample sizes between USC (Keck and LAC+USC) and UCLA.
Funding sources:
This publication was supported by grants UL1TR001855 and UL1TR000130 from the National Center for Advancing Translational Science (NCATS) of the U.S. National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviation and Acronym List
- NMSCs
Non-melanoma skin cancers
- BCCs
Basal cell carcinomas
- SCCs
Squamous cell carcinomas
- BSCCs
Basosquamous carcinomas
- MMS
Mohs micrographic surgery
- HMO
Health maintenance organization
- PPO
Preferred provider organization
- LAC+USC
Los Angeles County + University of Southern California
- UCLA
University of California Los Angeles
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest:
Jenny C. Hu, MD, MPH is a consultant and on an advisory board for Regeneron Pharmaceuticals, Inc.
Gino K. In, MD, MPH is on advisory boards for Regeneron Pharmaceuticals, Inc., Castle Biosciences, Novartis, Bristol Meyers Squibb, and Array Medical
IRB approval: University of Southern California IRB #HS-19–00176; University of California, Los Angeles IRB #19–000613
Mendeley Link for Supplemental Figure: https://data.mendeley.com/datasets/p3zx9x37fk/1
References
- 1.Rogers HW, Weinstock MA, Feldman SR, Coldiron BM. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the U.S. population, 2012. JAMA Dermatol 2015;151(10):1081–1086. [DOI] [PubMed] [Google Scholar]
- 2.Gloster HM, Neal K. Skin cancer in skin of color. J Am Acad Dermatol 2006;55(5):741–60; quiz 761. [DOI] [PubMed] [Google Scholar]
- 3.Hu S, Parmet Y, Allen G, et al. Disparity in melanoma: A trend analysis of melanoma incidence and stage at diagnosis among whites, hispanics, and blacks in florida. Arch Dermatol 2009;145(12):1369–1374. [DOI] [PubMed] [Google Scholar]
- 4.Cormier JN, Xing Y, Ding M, et al. Ethnic differences among patients with cutaneous melanoma. Arch Intern Med 2006;166(17):1907–1914. [DOI] [PubMed] [Google Scholar]
- 5.Agbai ON, Buster K, Sanchez M, et al. Skin cancer and photoprotection in people of color: A review and recommendations for physicians and the public. J Am Acad Dermatol 2014;70(4):748–762. [DOI] [PubMed] [Google Scholar]
- 6.Buchanan Lunsford N, Berktold J, Holman DM, Stein K, Prempeh A, Yerkes A. Skin cancer knowledge, awareness, beliefs and preventive behaviors among black and hispanic men and women. Prev Med Rep 2018;12:203–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sohn H. Racial and ethnic disparities in health insurance coverage: Dynamics of gaining and losing coverage over the life-course. Popul Res Policy Rev 2017;36(2):181–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buchmueller TC, Levinson ZM, Levy HG, Wolfe BL. Effect of the affordable care act on racial and ethnic disparities in health insurance coverage. Am J Public Health 2016;106(8):1416–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen J, Vargas-Bustamante A, Mortensen K, Ortega AN. Racial and ethnic disparities in health care access and utilization under the affordable care act. Med Care 2016;54(2):140–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu XC, Eide MJ, King J, et al. Racial and ethnic variations in incidence and survival of cutaneous melanoma in the united states, 1999–2006. J Am Acad Dermatol 2011;65(5 Suppl 1):S26–37. [DOI] [PubMed] [Google Scholar]
- 11.Kaufman BP, Alexis AF. Skin cancer mortality in patients with skin of color. Cutis 2017;99(5):307–308. [PubMed] [Google Scholar]
- 12.Buster KJ, You Z, Fouad M, Elmets C. Skin cancer risk perceptions: A comparison across ethnicity, age, education, gender, and income. J Am Acad Dermatol 2012;66(5):771–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Higgins S, Nazemi A, Chow M, Wysong A. Review of nonmelanoma skin cancer in african americans, hispanics, and asians. Dermatol Surg 2018;44(7):903–910. [DOI] [PubMed] [Google Scholar]
- 14.Taylor SC. Enhancing the care and treatment of skin of color, part 2: Understanding skin physiology. Cutis 2005;76(5):302–306. [PubMed] [Google Scholar]
- 15.Sanclemente G, Zapata JF, García JJ, Gaviria A, Gómez LF, Barrera M. Lack of correlation between minimal erythema dose and skin phototype in a colombian scholar population. Skin Res Technol 2008;14(4):403–409. [DOI] [PubMed] [Google Scholar]
- 16.Sauaia A, Dellavalle RP. Health care inequities: An introduction for dermatology providers. Dermatol Clin 2009;27(2):103–7, v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shwe S, Kassira S, Kim DJ, Elsensohn A, Lee P. How to navigate dermatology care for the uninsured. J Am Acad Dermatol 2019;80(6):1809–1813. [DOI] [PubMed] [Google Scholar]
- 18.Buster KJ, Stevens EI, Elmets CA. Dermatologic health disparities. Dermatol Clin 2012;30(1):53–9, viii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roetzheim RG, Pal N, Tennant C, et al. Effects of health insurance and race on early detection of cancer. J Natl Cancer Inst 1999;91(16):1409–1415. [DOI] [PubMed] [Google Scholar]
- 20.Alghothani L, Jacks SK, Vander Horst A, Zirwas MJ. Disparities in access to dermatologic care according to insurance type. Arch Dermatol 2012;148(8):956–957. [DOI] [PubMed] [Google Scholar]


