Abstract
Personal care products (PCPs) refer to a wide variety of items commonly characterized as health or beauty products. PCPs contain a number of ingredients, often including a wide range of endocrine disrupting chemicals such as phthalates and parabens. The present study examines the association between self-reported PCP use and prenatal sex-steroids and thyroid hormones levels in women from Puerto Rico. We recruited pregnant women (n=1070) through the Puerto Rico PROTECT Cohort and collected blood, demographic and pregnancy-related data at recruitment and subsequent visits. PCP use in the 48-hours preceding the blood sample was collected through self-reported questionnaires. Nine hormones (corticotropin-releasing hormone [CRH], sex-hormone binding globulin [SHBG], estriol [E3], progesterone, testosterone, thyroid-stimulating hormone [TSH], total triiodothyronine [T3], total thyroxine [T4], and free thyroxine [fT4]) were measured in maternal serum samples at two points during pregnancy. Linear mixed models with random intercepts were used to examine associations between PCP use and serum hormone levels. Use of cosmetics significantly increased with age, household income and education level (p<0.01). Use of hair products, such as hair dyes and bleach, relaxers, and mousse, was associated with lower levels of all sex steroid hormones compared to non-use: SHBG (%Δ= −7.1, 95%CI: −12.4,−1.8), E3 (%Δ= −23.2, 95%CI: −32.2,−13.0), progesterone (%Δ= −21.5, 95%CI: −29.4,−12.9) and testosterone (%Δ= −21.5, 95%CI: −33.1,−7.8) adjusted for maternal age, education and pre-pregnancy body mass index. Our findings suggest that household income and education level influence PCP use among pregnant women in this study. Use of certain hair products was associated with lower concentrations of sex steroid hormones. Although there are limitations to questionnaire data, characterizing PCP use is inexpensive and may represent exposure from multiple classes of chemicals, including chemicals that may not specifically appear on product labels and/or have not been tested for endocrine disrupting potential, making it a useful complement to chemical biomarker data.
Keywords: personal care products, maternal hormones, pregnancy, endocrine disruptors, exposure measurement
Introduction
Personal care products (PCPs) refer to a wide variety of items that are commonly categorized as health or beauty products. In the U.S., widely used PCPs include cosmetics (such as facial makeup), shampoos, hair dyes and nail polish, while others meet the definition of over the counter drugs, including mouthwashes and skin protectants (e.g., lip balm) (FDA 2019, 2020). PCPs contain a number of ingredients ranging from preservatives to cleansing agents to fragrances. These ingredients can include a wide range of chemicals such as phthalates, bisphenol A (BPA), parabens, triclosan, dioxane, pigments and toxic metals (which we collectively refer to as “PCP-related chemicals”). Most PCPs are directly applied to the skin, allowing chemicals to cross the cutaneous barrier to reach the systemic circulation (Myers et al. 2015). Multiple epidemiological studies have shown that use of PCPs is associated with increased levels of phthalates diesters (Ashrap et al. 2018; Duty et al. 2005; Ferguson et al. 2017; Larsson et al. 2014; Parlett et al. 2013; Rodríguez-Carmona et al. 2020), phenols (Ferguson et al. 2017; Ko et al. 2016), and parabens (Den Hond et al. 2013; Larsson et al. 2014) in different populations. Many PCP-related chemicals are known endocrine disruptors that may interact with hormone systems, influencing synthesis, regulation, transport, metabolism, and interfere with hormone receptors (Gore et al. 2015; Lemini et al. 2003; Stoker et al. 2010). In fact, use of some PCPs has been associated with hormone-related adverse outcomes such as endometriosis and breast cancer (Gera et al. 2018; Llanos et al. 2017; Peinado et al. 2020; White et al. 2021).
Pregnancy is a period of particular vulnerability to the potential effects of endocrine-disrupting chemicals (EDCs) found in PCPs. Alterations in the delicate hormonal balance occurring during pregnancy can disrupt processes negatively affecting both mother and fetus. In fact, variation in maternal and placental hormone levels during pregnancy has been associated with pregnancy complications as well as adverse birth outcomes, including growth restriction, preterm birth and low birth weight (Gilles et al. 2018; Jelliffe-Pawlowski et al. 2010; Kumar et al. 2018; Mucci et al. 2003; Noyola-Martinez et al. 2019; Wadhwa et al. 2004). Furthermore, the prenatal hormonal milieu may contribute to children’s long-term health and disease risk, including future risk of neurodevelopmental impairment, polycystic ovary syndrome (PCOS), endometriosis, and prostate and breast cancer (Day et al. 2020; Filippou and Homburg 2017; Gore et al. 2015; Parazzini et al. 2017). Given the downstream health effects associated with the prenatal hormonal milieu, identifying factors contributing to variation in prenatal hormones is important.
Most PCP-related chemicals that are known or suspected endocrine disruptors can be measured in biological matrices including urine, serum, saliva, and breast milk. Although sensitive and relatively standardized analytical protocols exist today to measure these chemicals, it is not only expensive but requires extensive logistical and expert support. For studies assessing exposure, PCP use is typically self-reported and collected through questionnaires. These data are then matched to chemical concentrations in participants’ biospecimens to assess sources of exposure. Some studies suggest that questionnaire data may be a useful proxy to characterize exposure from PCP-related chemicals (Braun et al. 2014), while others suggest there is an inability to capture exposure variability for some chemicals using questionnaires (Buckley et al. 2012). Compared to direct assessment of chemical biomarkers, less is known about how PCP use (as assessed via self-report) relates to perinatal health. In fact, to date, no study has assessed PCP use in relation to prenatal hormone concentrations among pregnant women. In our previous work, we examined predictors of PCP-related chemicals, including phthalates, phenols, parabens, and triclocarban in pregnant women from Puerto Rico (Ashrap et al. 2018). We have also examined associations of these chemical analytes with prenatal hormone levels (Aker et al. 2016; Aker et al. 2019; Cathey et al. 2019). Here, we examined PCP use from self-reported questionnaire data as a proxy for PCP-related chemical exposures and assessed the association of PCP use with prenatal hormone levels. Compared to measuring individual chemicals in human biospecimens, self-reported questionnaires are inexpensive, have less logistical planning, and may represent exposure from multiple classes of chemicals or unidentified chemicals. Additionally, they provide information on frequency that can be used in risk assessment. The aims of this study were to 1) examine demographic factors associated with PCP use and serum concentrations of sex steroid and thyroid hormones and 2) examine the association between PCP use and serum sex steroid and thyroid hormones concentrations among pregnant women.
Methods
2.1. Study participants
Participants in this study were enrolled in the Puerto Rico PROTECT Cohort. Initiated in 2010, PROTECT is an ongoing prospective birth cohort designed to study environmental exposures in pregnant women and their children residing around the northern karst zone of Puerto Rico (Cantonwine et al. 2014; Meeker et al. 2013). Inclusion criteria for recruitment included women with: 1) singleton pregnancies, 2) ages 18–40 years, 3) resident of the northern karst zone, 4) no history of major obstetric or medical complication, 5) no use of oral contraceptives in the three months before pregnancy, and 6) no use of in vitro fertilization to achieve the current pregnancy. Women were recruited at approximately 14 ± 2 weeks and completed up to three prenatal study visits (18 ± 2 weeks [Visit 1], 22 ± 2 weeks [Visit 2] and 26 ± 2 weeks [Visit 3]). Participants underwent physical exams and completed a series of detailed questionnaires with items on demographics, occupation, lifestyle, and PCP use (Meeker et al. 2013). Blood samples were collected at Visit 1 and Visit 3. A total of 1070 women recruited between 2011–2017 with hormones measurements from at least one study visit were included in this study. The research protocol was approved by the Ethics and Research Committees of the University of Puerto Rico and participating clinics, the University of Michigan School of Public Health and Northeastern University. All study activities were fully explained to each participant before obtaining written consent for participation in the study.
2.2. Personal care products questionnaires
At each visit, a questionnaire was administered to collect information on self-reported product use in the 48-hours preceding the blood sample (i.e., recent use). The questionnaire included yes or no questions on use of PCPs, including the following product groups: perfumes/cologne/fragrance, soap (i.e., liquid and bar), lotions (i.e., hand and body lotions), cosmetics (e.g., “blush”, eye shadow, eyeliner, mascara), nail polish, shampoo and hair conditioners, hair spray (i.e., hair gel, hair spray), other hair products (i.e., hair bleach, relaxer, mousse, hair dyes), shaving cream and mouth wash.
2.3. Measurements of hormones
Serum samples were shipped to and analyzed at the Central Ligand Assay Satellite Services laboratory in the Department of Epidemiology at the University of Michigan, School of Public Health (see Supplemental Material for quality control and assurance details). Progesterone (Siemens, catalog no. 1586287) (ADVIA), sex hormone-binding globulin [SHBG] (Siemens, catalog no. 6520781) (ADVIA), testosterone (Siemens, catalog no. 5476206) (ADVIA), total triiodothyronine [T3] (Siemens, catalog no. 8427516) (ADVIA) total thyroxine [T4] (Siemens, catalog no. 9236439) (ADVIA), free T4 (fT4; Siemens, catalog no. 6490106) (ADVIA), and TSH (Siemens, catalog no. 8700387) (ADVIA) were measured using chemiluminescence immunoassays. Estriol [E3] (DiaMetra, catalog no. DKO019) (DiaMetra) and corticotropin-releasing hormone [CRH] (LifeSpan, catalog no. LS-F5352) (LifeSpan) were measured using enzyme immunoassays. The ratios of progesterone/E3 and T3/T4 were calculated and assessed as these ratios may be better indicators of adverse pregnancy outcomes than each of the individual hormone measurements (Dietrich et al. 2012; Romero et al. 1988). Overall, 1070 women had hormone measurements, from those 597 and 473 had one and two measurements, respectively.
2.4. Statistical analysis
Hormone concentrations below the limit of detection (LOD) were replaced by the LOD divided by the square root of two. Distributions of CRH, E3, progesterone, TSH, testosterone, and progesterone/E3 ratio were right skewed and natural log transformed for all analyses. Distributions of SHBG, fT4, T3, T4 and T3/T4 ratio were approximately normally-distributed and thus were not transformed. We also computed the geometric mean (GM) of hormone concentrations among women who did and did not recently use a PCP.
We first examined the correlations between sociodemographic variables and PCP recent use using Cramér V correlation coefficients. Cramér V is a measure of association between two categorical variables, giving a value between 0 and +1 (inclusive) but without direction (Cramer 1946). The coefficients were very similar to Spearman correlations, hence we added Spearman correlation signs (+/−). We then used generalized estimating equations (GEE) to test whether recent use of PCP differed by sociodemographic characteristics to explore potential predictors of recent use of PCP. GEE accounts for the repeated measurements of recent use of PCP by considering the inter-correlation between time points. To evaluate the association between recent use of PCP and hormone concentrations, we used linear mixed models (LMMs), with separate models for each hormone and random intercepts to account for correlation of repeated measurements. Results were presented as the mean percentage change (Δ%) in hormone concentrations and 95% confidence intervals (CIs) associated with use of a PCP group (yes/no). Crude models only included PCP variables as the exposure. Potential confounders were selected a priori from existing literature, available PROTECT data and analyses, and directed acyclic graphs. The covariates considered were maternal age, gravidity, gestational age at prenatal visit, infant sex, pre-pregnancy body mass index (BMI), marital status, insurance type, maternal education, employment status, smoking, exposure to secondhand smoke, alcohol consumption, time of sample collection and prenatal study visit. Each variable was evaluated in univariate analyses to determine those with significant impact in the estimates. Stepwise procedures assisted in the selection process. Finally, if a variable appreciably altered the beta coefficient of PCP exposure, it was included in the model. Final models were adjusted for maternal age (continuous), education (categorical), and pre-pregnancy BMI (categorical). Testosterone models were further adjusted for SHBG concentrations to account for SHBG bound-testosterone (Dunn et al. 1981).
To evaluate different windows of susceptibility, we additionally included interaction terms between recent use of PCP and each visit indicator into the LMMs to generate separate models for each visit. Using this method, the effect estimates of the covariates were still assessed using the LMM structure. These interaction terms were not significant, thus interaction terms were not included in final models. All statistical analyses were conducted using R version 3.6.2.
Results
A total of 1543 serum samples were analyzed from 1070 women (910 Visit 1 samples and 633 Visit 3 samples). Sociodemographic characteristics are shown in Table 1. Participants were 27 ± 5 years old on average and most (77%) were married or in a domestic partnership. Overall, participants had above a high school education (75%), were employed (60%), and had private health insurance (58%). More than 80% of women never smoked, while less than 2% reported smoking during pregnancy and 6% reported secondhand smoke exposure (>1h per day). The majority (90%) reported no alcohol consumption within the last few months.
Table 1.
Population demographics and pregnancy characteristics of 1070 women participating in the PROTECT study.
| Variable | Mean (SD) |
| Maternal Age at Enrollment (years) | 26.9(5.5) |
| Mean Gestational Age at Delivery | 38.9(2.1) |
| Gravidity (# pregnancies) | 2(1) |
| N (%) | |
| Maternal Age (years) | |
| <25 | 394 (36.8%) |
| 25–30 | 393 (36.7%) |
| >30 | 280 (26.2%) |
| missing | 3 (0.3%) |
| Insurance Type | |
| Private | 623 (58.2%) |
| Public (Mi Salud) | 363 (33.9%) |
| missing | 84 (7.9%) |
| Maternal Education (years) | |
| ≤High school/GED | 228 (21.3%) |
| Some College or technical school | 349 (32.6%) |
| College degree | 328 (30.7%) |
| Masters degree or higher | 128 (12%) |
| missing | 37 (3.5%) |
| Income Status (US $) | |
| <$10,000 | 296 (27.7%) |
| ≥$10,000 to <$30,000 | 288 (26.9%) |
| ≥$30,000 to <$50,000 | 211 (19.7%) |
| ≥$50,000 | 118 (11%) |
| missing | 157 (14.7%) |
| Marital Status | |
| single | 210 (19.6%) |
| married or living together | 824 (77%) |
| missing | 36 (3.4%) |
| Gravidity (# pregnancies) | |
| 0 | 451 (42.1%) |
| 1 | 268 (25%) |
| >1 | 218 (20.4%) |
| missing | 33 (3.1%) |
| Prepregnancy BMI (kg m−2) | |
| ≤25 | 562 (52.5%) |
| >25 to ≤30 | 255 (23.8%) |
| >30 | 181 (16.9%) |
| missing | 72 (6.7%) |
| Employment Status | |
| employed | 643 (60.1%) |
| unemployed | 388 (36.3%) |
| missing | 39 (3.6%) |
| Smoking | |
| Never | 885 (82.7%) |
| Ever | 128 (12%) |
| Current | 17 (1.6%) |
| missing | 40 (3.7%) |
| Exposure to Second Hand Smoking | |
| None | 815 (76.2%) |
| Up to 1 hour | 44 (4.1%) |
| More than 1 hour | 70 (6.5%) |
| missing | 105 (9.8%) |
| Alcohol Consumption | |
| None | 539 (50.4%) |
| Before Pregnancy | 428 (40%) |
| Yes within the last few months | 66 (6.2%) |
| missing | 37 (3.5%) |
| Infant Sex | |
| Female | 539 (50.4%) |
| Male | 428 (40%) |
| missing | 66 (6.2%) |
Descriptive statistics for all maternal serum hormones have been previously described (Supplemental Table 1) (Aker et al. 2018; Cathey et al. 2019; Rivera-Núñez et al. 2021). Overall, all hormones except testosterone (n=5 below LOD) were detected in 100% of serum samples and concentrations of E3, SHBG, progesterone, and testosterone were all significantly higher at Visit 3 than at Visit 1 (p= 0.001).
Table 2 shows PCP use by sociodemographic characteristics. Age, insurance type, employment status and gravidity were the most frequent characteristics associated with the use of PCPs including liquid soap, hair conditioner, lotions, cosmetics and nail polish.
Table 2.
Proportion (%) of pregnant women using selected personal care product reported in the 48-h recall questionnaire in relation to population demographic and pregnancy characteristics
| Characteristic | Bar Soap | Liquid Soap | Cosmetic | Perfume | Lotion | Shaving Cream | Mouth Wash | Nail Polish | Shampoo | Condition | Hair Spray | Other Hair Products |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Maternal Age (years) | ||||||||||||
| <25 | 0.95 | 0.83 | 0.66 | 0.83 | 0.70 | 0.06 | 0.48 | 0.32 | 0.71 | 0.71 | 0.30 | 0.08 |
| 25–30 | 0.92 | 0.89 | 0.81 | 0.85 | 0.82 | 0.10 | 0.44 | 0.28 | 0.71 | 0.69 | 0.31 | 0.12 |
| >30 | 0.88 | 0.91 | 0.83 | 0.82 | 0.84 | 0.11 | 0.44 | 0.23 | 0.63 | 0.61 | 0.31 | 0.09 |
| p-value | 0.00 | 0.00 | 0.00 | 0.54 | 0.00 | 0.08 | 0.46 | 0.02 | 0.02 | 0.01 | 0.91 | 0.11 |
| Insurance Type | ||||||||||||
| Private | 0.90 | 0.89 | 0.82 | 0.83 | 0.83 | 0.11 | 0.46 | 0.25 | 0.67 | 0.65 | 0.27 | 0.10 |
| Public (Mi Salud) | 0.95 | 0.86 | 0.67 | 0.83 | 0.70 | 0.05 | 0.44 | 0.32 | 0.72 | 0.71 | 0.35 | 0.09 |
| p-value | 0.00 | 0.11 | 0.00 | 0.98 | 0.00 | 0.00 | 0.62 | 0.02 | 0.07 | 0.04 | 0.01 | 0.60 |
| Maternal Education (years) | ||||||||||||
| ≤High school/GED | 0.95 | 0.86 | 0.62 | 0.81 | 0.63 | 0.05 | 0.48 | 0.34 | 0.74 | 0.74 | 0.34 | 0.06 |
| Some College or technical school | 0.93 | 0.83 | 0.71 | 0.83 | 0.77 | 0.07 | 0.44 | 0.24 | 0.68 | 0.67 | 0.30 | 0.09 |
| College degree | 0.90 | 0.90 | 0.86 | 0.85 | 0.86 | 0.12 | 0.45 | 0.28 | 0.68 | 0.67 | 0.31 | 0.12 |
| Masters degree or higher | 0.89 | 0.95 | 0.87 | 0.86 | 0.86 | 0.13 | 0.46 | 0.28 | 0.64 | 0.59 | 0.24 | 0.08 |
| p-value | 0.13 | 0.00 | 0.00 | 0.52 | 0.00 | 0.00 | 0.77 | 0.05 | 0.15 | 0.03 | 0.30 | 0.07 |
| Income Status (US $) | ||||||||||||
| <$10,000 | 0.95 | 0.85 | 0.63 | 0.84 | 0.68 | 0.06 | 0.42 | 0.29 | 0.71 | 0.69 | 0.35 | 0.06 |
| ≥$10,000 to <$30,000 | 0.91 | 0.85 | 0.75 | 0.84 | 0.82 | 0.08 | 0.48 | 0.27 | 0.67 | 0.67 | 0.28 | 0.14 |
| ≥$30,000 to <$50,000 | 0.91 | 0.93 | 0.87 | 0.82 | 0.84 | 0.13 | 0.44 | 0.23 | 0.65 | 0.63 | 0.30 | 0.08 |
| ≥$50,000 | 0.91 | 0.91 | 0.90 | 0.87 | 0.85 | 0.09 | 0.47 | 0.25 | 0.69 | 0.67 | 0.22 | 0.09 |
| p value | 0.22 | 0.01 | 0.00 | 0.71 | 0.00 | 0.03 | 0.51 | 0.53 | 0.57 | 0.53 | 0.07 | 0.00 |
| Marital Status | ||||||||||||
| single | 0.95 | 0.81 | 0.74 | 0.81 | 0.76 | 0.08 | 0.44 | 0.35 | 0.69 | 0.67 | 0.32 | 0.10 |
| married or living together | 0.91 | 0.89 | 0.77 | 0.84 | 0.79 | 0.09 | 0.46 | 0.26 | 0.69 | 0.67 | 0.30 | 0.09 |
| p-value | 0.02 | 0.00 | 0.52 | 0.23 | 0.30 | 0.66 | 0.68 | 0.01 | 0.99 | 0.90 | 0.63 | 0.83 |
| Employment Status | ||||||||||||
| employed | 0.91 | 0.90 | 0.82 | 0.86 | 0.84 | 0.10 | 0.46 | 0.25 | 0.66 | 0.65 | 0.29 | 0.11 |
| unemployed | 0.93 | 0.83 | 0.67 | 0.79 | 0.68 | 0.07 | 0.45 | 0.32 | 0.73 | 0.72 | 0.34 | 0.08 |
| p value | 0.12 | 0.00 | 0.00 | 0.00 | 0.00 | 0.12 | 0.85 | 0.01 | 0.02 | 0.01 | 0.07 | 0.07 |
| Gravidity (# pregnancies) | ||||||||||||
| 0 | 0.92 | 0.87 | 0.77 | 0.86 | 0.81 | 0.09 | 0.49 | 0.28 | 0.68 | 0.66 | 0.25 | 0.08 |
| 1 | 0.92 | 0.86 | 0.77 | 0.81 | 0.79 | 0.09 | 0.43 | 0.29 | 0.70 | 0.69 | 0.32 | 0.12 |
| >1 | 0.92 | 0.91 | 0.73 | 0.84 | 0.72 | 0.08 | 0.42 | 0.26 | 0.70 | 0.69 | 0.40 | 0.09 |
| p-value | 0.97 | 0.23 | 0.49 | 0.17 | 0.04 | 0.93 | 0.10 | 0.70 | 0.74 | 0.58 | 0.00 | 0.12 |
| Pre pregnancy BMI (kg m−2) | ||||||||||||
| ≤25 | 0.91 | 0.88 | 0.80 | 0.83 | 0.81 | 0.09 | 0.45 | 0.27 | 0.68 | 0.67 | 0.28 | 0.10 |
| >25 to ≤30 | 0.93 | 0.86 | 0.78 | 0.86 | 0.79 | 0.09 | 0.47 | 0.31 | 0.69 | 0.68 | 0.33 | 0.09 |
| >30 | 0.91 | 0.87 | 0.67 | 0.82 | 0.69 | 0.08 | 0.41 | 0.27 | 0.69 | 0.65 | 0.37 | 0.09 |
| p-value | 0.50 | 0.63 | 0.00 | 0.45 | 0.00 | 0.87 | 0.41 | 0.32 | 0.97 | 0.77 | 0.03 | 0.92 |
| Smoking | ||||||||||||
| Never | 0.92 | 0.87 | 0.76 | 0.83 | 0.78 | 0.09 | 0.46 | 0.26 | 0.69 | 0.67 | 0.29 | 0.09 |
| Ever | 0.92 | 0.88 | 0.76 | 0.88 | 0.81 | 0.08 | 0.42 | 0.45 | 0.74 | 0.74 | 0.41 | 0.15 |
| Current | 1.00 | 0.96 | 0.74 | 0.87 | 0.74 | 0.04 | 0.45 | 0.22 | 0.52 | 0.48 | 0.30 | 0.09 |
| p-value | 0.00 | 0.53 | 0.97 | 0.41 | 0.72 | 0.69 | 0.73 | 0.00 | 0.05 | 0.03 | 0.03 | 0.05 |
| Exposure to Second Hand Smoking | ||||||||||||
| None | 0.91 | 0.88 | 0.77 | 0.83 | 0.79 | 0.09 | 0.46 | 0.28 | 0.68 | 0.67 | 0.30 | 0.10 |
| Up to 1 hour | 0.95 | 0.85 | 0.85 | 0.85 | 0.79 | 0.05 | 0.48 | 0.31 | 0.74 | 0.74 | 0.34 | 0.10 |
| More than 1 hour | 0.97 | 0.81 | 0.73 | 0.91 | 0.75 | 0.07 | 0.42 | 0.33 | 0.76 | 0.74 | 0.37 | 0.12 |
| p-value | 0.21 | 0.24 | 0.31 | 0.17 | 0.73 | 0.57 | 0.71 | 0.56 | 0.32 | 0.33 | 0.47 | 0.71 |
| Alcohol Consumption | ||||||||||||
| None | 0.92 | 0.86 | 0.71 | 0.81 | 0.74 | 0.08 | 0.45 | 0.25 | 0.68 | 0.67 | 0.32 | 0.09 |
| Before Pregnancy | 0.92 | 0.89 | 0.83 | 0.87 | 0.84 | 0.08 | 0.47 | 0.31 | 0.69 | 0.67 | 0.28 | 0.10 |
| Yes within the last few months | 0.93 | 0.90 | 0.78 | 0.83 | 0.77 | 0.14 | 0.43 | 0.35 | 0.76 | 0.72 | 0.36 | 0.10 |
| p-value | 0.94 | 0.32 | 0.00 | 0.02 | 0.00 | 0.19 | 0.81 | 0.03 | 0.30 | 0.60 | 0.24 | 0.90 |
| Infant Sex | ||||||||||||
| Female | 0.93 | 0.87 | 0.77 | 0.86 | 0.77 | 0.09 | 0.46 | 0.30 | 0.68 | 0.66 | 0.29 | 0.11 |
| Male | 0.90 | 0.88 | 0.75 | 0.82 | 0.80 | 0.09 | 0.46 | 0.27 | 0.70 | 0.69 | 0.33 | 0.09 |
| p-value | 0.21 | 0.50 | 0.43 | 0.08 | 0.29 | 0.88 | 0.92 | 0.29 | 0.32 | 0.33 | 0.17 | 0.45 |
| Collection Year | ||||||||||||
| 2011–13 | 0.92 | 0.80 | 0.78 | 0.85 | 0.80 | 0.07 | 0.44 | 0.33 | 0.71 | 0.70 | 0.35 | 0.14 |
| 2014–17 | 0.92 | 0.91 | 0.77 | 0.83 | 0.79 | 0.08 | 0.50 | 0.29 | 0.69 | 0.67 | 0.28 | 0.08 |
| p-value (11–13v.14–17 | 0.71 | 0.00 | 0.60 | 0.38 | 0.94 | 0.59 | 0.07 | 0.17 | 0.55 | 0.42 | 0.04 | 0.00 |
| p-value (yr continuous) | 0.90 | 0.00 | 0.17 | 0.30 | 0.85 | 0.96 | 0.03 | 0.16 | 0.02 | 0.06 | 0.10 | 0.12 |
P-values from generalized estimating equations (GEE)
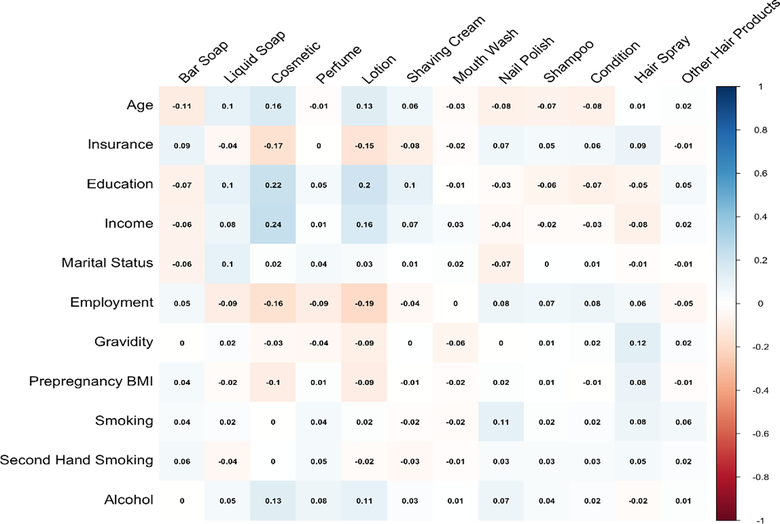
Additionally, use of certain PCPs increased with age, income or education. For example, younger women tended to more often report using hair shampoo and conditioner in the past 48 hours compared to older women (p<0.01), while use of cosmetics increased with maternal age, level of education and income (p<0.01). Although weak, these associations showed the highest positive correlations among all socioeconomic variables and recent use of PCP (Figure 1). Employment status was also associated with a higher use of cosmetics and lotions, with higher recent use among employed compared to unemployed women (p<0.01). Use of cosmetics and lotions was less frequent among women with higher pre-pregnancy BMI (p<0.01). Women with public health insurance reported more recent use of hair spray and nail polishers compared to those with private insurance (p<0.01). Overall, PCP use was similar between women recruited in 2011–2013 compared to those recruited in 2014–2017. More women used liquid soap in the 2014–2017 group compared to the 2011–2013 group (p<0.001). Additionally, less women used some hair care products in the 2014–2017 group compared to the 2011–2013 group.
Figure 1.
Heat map of Cramér V coefficients between frequency of personal care product use and sociodemographic characteristics.
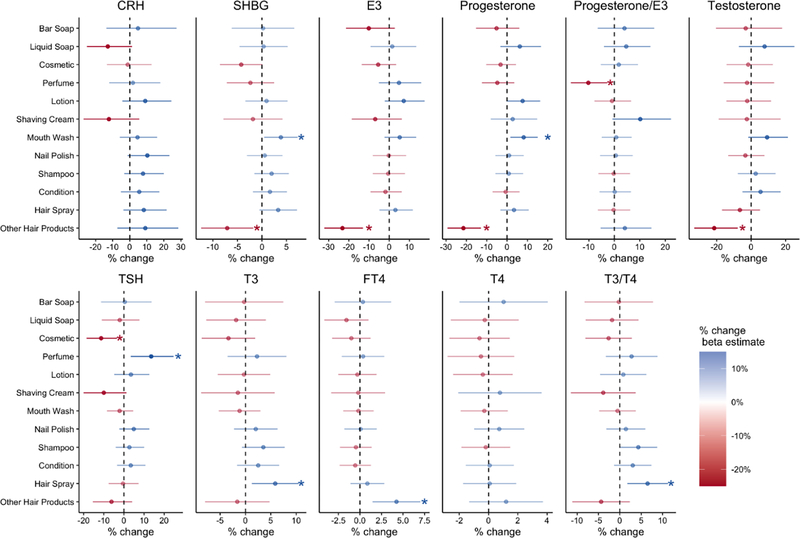
Figure 2 shows changes in prenatal hormone concentrations by use of PCPs. PCPs in the creams and lotions categories were associated with increased sex steroid hormone levels. For example, use of mouthwash was associated with higher levels of SHBG (%Δ= 3.8, 95%CI: 0.4, 7.3) and progesterone (%Δ= 8.1, 95%CI: 1.6, 15.1) levels compared to no use of mouthwash. By contrast, women using other hair products (i.e., hair bleach, relaxer, mousse, hair dyes) showed decreases in all sex steroid hormones levels including significant changes in SHBG (%Δ= −7.1, 95%CI: −12.4, −1.8), E3 (%Δ= −23.2, 95%CI: −32.2, −13.0), progesterone (%Δ= −21.5, 95%CI: −29.4, −12.9) and testosterone (%Δ= −21.5, 95%CI: −33.1, −7.8) compared to women reporting not using other hair products. Although not statistically significant, recent use of cosmetics was associated with decreases in all sex steroid hormones as well.
Figure 2. The percent difference in serum hormone concentration (95% confidence interval) in relation to self-reported use of personal care products.
CRH: corticotropin-releasing hormone, SHBG: sex hormone-binding globulin, E3: estriol, TSH:, thyroid-stimulating hormone, T3: total triiodothyronine, FT4: free total thyroxine, T4: total thyroxine
Prenatal thyroid hormones levels showed fewer associations with use of PCPs. Overall, use of cosmetics was associated with small, non-statistically significant decreases in all thyroid hormones (TSH, T3, FT4, and T4). Use of perfume was associated with changes in TSH (%Δ= 13.5, 95%CI: 3.4, 24.7), use of hair spray was associated with changes in T3 (%Δ= 5.9, 95%CI: 1.2, 10.5), and use of other hair products was associated with changes in FT4 levels ((%Δ= 4.2, 95%CI: 1.5, 7.0).
Discussion
In this study we examined associations between prenatal PCP use and maternal serum hormones measured at two time points during pregnancy. We identified socioeconomic variables such as income, education, employment status and type of insurance as factors influencing PCP use among pregnant women in Puerto Rico. In contrast to other studies reporting PCP use during pregnancy, use of PCPs did not change between study visits. Finally, use of hair products such as hair dyes and bleach, relaxers and mousse was associated with lower levels of sex steroid hormones.
To our knowledge, this is the first study examining prenatal PCP use in relation to maternal serum hormone concentrations. However, the use of PCPs during pregnancy has been described in previous studies examining frequency and changes in use. In three different U.S. cities, PCP frequently used by pregnant women included makeup (specifically eye makeup, lipstick), and hand creams, and/or lotions (Buckley et al. 2012). In Taiwan, commonly used products among pregnant women included skin toners and essential oils (Hsieh et al. 2019). In France, pregnant women reported using toothpaste, face cream, deodorant and make up remover most frequently among other PCPs (Nakiwala et al. 2020). Similar to previous reports about life habit changes during pregnancy, such as diet and water intake (Barrett et al. 2014; Forssén et al. 2009), studies have shown that women also modify their use of PCPs including cosmetic, lotions and hair styling products during pregnancy (Katsikantami et al. 2020; Marie et al. 2016). In the Plastic and Personal-care Product Use in Pregnancy (P4) study, among a Canadian cohort of 80 pregnant and postpartum women, general hygiene (e.g., soap) products were the most commonly and consistently used over time, while use of cosmetics declined through pregnancy and post-delivery (Lang et al. 2016). In contrast, we observed minimal changes in PCP use between the two study visits (approximately 8 weeks apart) in the PROTECT cohort. Our questionnaire did not assess duration and magnitude of use, which may have impacted our ability to capture variability in use across pregnancy. Additionally, our findings may suggest cultural differences in the use of PCPs. A strong culture of beauty impacts Latina women (Nielsen 2015), which may influence consistent use of cosmetics through pregnancy.
Use of PCPs in relation to socioeconomic variables among pregnant women has been reported less frequently. The P4 study showed a strong influence of household income on frequency of PCP application, where participants reporting a household income >$100,000 applied PCPs more often than participants with lower household income. Our analysis showed a trend suggesting an association between higher income and education with higher frequency of cosmetics use. Additionally, employed women reported using more cosmetics than unemployed women. Studies in non-pregnant populations have also reported associations between frequency of use and socioeconomic markers, such as household income and education (Park et al. 2018; Shaaban and Alhajri 2020). Higher income and education might make cosmetics more accessible for women in the upper income and education categories. These data are important to identify populations at increased risk of chemical exposures associated with PCPs.
Multiple studies have also shown that women using PCPs have higher levels of PCP-related chemicals (Braun et al. 2014; Hsieh et al. 2019). For example, pregnant women using more than one “leave-on” product (e.g., body lotion, perfume) had higher levels of certain phthalates (e.g., mono-ethyl phthalate) (Hsieh et al. 2019). In addition to higher levels of phthalates, another study reported that women using lotions, cosmetics and perfumes had the highest levels of urinary butyl-paraben (Braun et al. 2014). We have discussed PCP-related chemical biomarkers in previous PROTECT reports. For example, we showed that urinary phthalates, phenols and parabens were widely detectable in this population and tended to be found at higher concentrations as compared to women of reproductive age in the general U.S. population (Ashrap et al. 2018; Ferguson et al. 2019). In that prior work, we also observed positive associations between PCP-related chemicals and use of soaps, sunscreens, lotions and cosmetics. Most notably, triclosan and triclocarban concentrations were higher among women reporting use of liquid soap and bar soap. In a separate report, we observed associations between urinary triclosan and prenatal E3 and SHBG levels (Aker et al. 2019). Here we did not observe significant associations between use of liquid or bar soap and any of the maternal hormones examined. Our self-reported questionnaires likely represent exposures to multiple classes of chemicals while typically, a single exposure biomarker captures one chemical from multiple routes of exposure. While using biomarkers is generally more accurate for assessment of a single chemical analyte, associations involving chemical mixtures are more difficult and expensive to assess, particularly when multiple chemical types are present. In an effort to examine the impact of more than one PCPs in in each of our models, we conducted multiple adjustment analysis in those statistically significant (Supplemental Table 2). However, we did not see any difference between models. Importantly, self-reported PCP use as a way to measure exposure would also capture chemicals in products that may not specifically appear on product labels (e.g., “fragrance”) and/or have not been tested for endocrine disrupting potential. An additional limitation is that although self-reported questionnaires are appropriate to collect personal habits such as PCP use, participants may not accurately remember exposure. It is also possible that participants may not have had their typical PCP use routine during the relevant 48-hr timeframe that was captured in the questionnaire. Nevertheless, self-reported questionnaires are useful to generate valuable use data to conduct risk assessment.
We reported consistent inverse associations between the use of “other” hair products category (which included hair dyes, bleach, mousse and relaxers) and sex steroid hormones. One study found that self-reported pre-pregnancy use of hair dyes was associated with increased risk of low birthweight (Jiang et al. 2018). Because of their potential for hormone disruption and carcinogenicity, use of hair products have been more extensively studied in relation to risk of breast cancer in African American and White women (Gera et al. 2018). For example, the Sister Study, a study of women ages 35–74 years who had a sister with breast cancer (n=46,709), observed that Black and White women reporting use of chemical strengtheners and permanent hair dyes had an increased risk of breast cancer compared to women reporting no use (Eberle et al. 2020). The Women’s Circle of Health Study (n= 4,285 African American and White women) reported that use of dark hair dye shades and chemical relaxers was associated with increased risk of breast cancer, and ER+ disease, specifically, in both African American and White women (Llanos et al. 2017). Hair care products contain a variety of PCP-related chemical additives that can hold fragrance (e.g., diethyl phthalate), extend shelf life (e.g., parabens), and prevent bacterial growth (e.g., triclosan) (FDA 2021; Helm et al. 2018). For example, Helm et al., identified 45 of 66 target chemicals in hair products used by Black women including cyclosiloxane, fragrance chemicals and diethyl phthalate (DEP) at the highest levels. We previously reported associations between the use of the “other” hair products and urinary parabens (Ashrap et al. 2018). We further observed that paraben concentrations were inversely associated with estradiol and SHBG (Aker et al. 2016). Parabens are commonly used as preservatives to extend shelf life not only in hair care products but also in makeup, moisturizers, and shaving products among others. Parabens and other EDCs bind to hormone receptors potentially enhancing, dampening or blocking hormonal action (Vo et al. 2010). EDCs can also alter the number of hormone receptors influencing the levels of circulating hormones (Martinez-Arguelles et al. 2014). Alterations in hormone levels can have vast consequences beyond health at birth including changes in infant and child growth, pubertal trajectories and may influence development of hormone-sensitive cancers such as breast, uterine and ovarian cancer (Adair 2001; Bukowski et al. 2012; Richards et al. 2002).
Our study is the first to assess associations between PCP use and maternal serum hormones in a pregnant population. The PROTECT study design allowed us to examine different windows of exposure as well as multiple relevant hormone measures. In spite of the longitudinal design, our study had some limitations. Recall bias is an inherent limitation of self-reported questionnaire data and might have decreased our ability to detect associations. Additionally, we had no data on duration of use and were unable to identify specific PCP brands to provide additional insights to our results. Although we did not observe changes in PCP use between visits in this study, we do not know if women made changes before enrolling in the study (i.e., during the preconception period or immediately after learning about their pregnancies). Also, circulating hormone levels represent maternal, fetal, and placental contributions and it is not clear which of these sources may be most impacted by PCP-related chemicals.
Conclusion
Our findings suggest that pregnant women in Puerto Rico use PCPs during pregnancy with minimum changes between trimesters. Socioeconomic factors including household income and education level influence PCP use. These findings are important for identifying populations at risk of potentially harmful PCP-related chemical exposures. Our results also suggest that chemicals some hair products may alter sex steroid hormones during pregnancy. Disruptions in prenatal sex steroid hormones may contribute to adverse maternal and fetal outcomes. As such, additional research should address the public health impact of exposure to chemicals in hair products in pregnant populations. Primary physicians and obstetricians should inform and offer guidance to reproductive age women about the potential health impact of EDCs such as those found in hair products. Finally, self-reported questionnaires like the ones we used in this study capture exposures from multiple classes of chemicals including chemicals that may not specifically appear on product labels and/or have not been tested for endocrine disrupting potential making it a useful complement to chemical biomarker data.
Supplementary Material
Acknowledgments
We are extremely grateful to all PROTECT study participants and their families. The authors also thank the nurses and research staff who participated in cohort recruitment and follow up, as well as the Federally Qualified Health Centers (FQHC) in Puerto Rico that facilitated participant recruitment, including Morovis Community Health Center, Prymed in Ciales, Camuy Health Services, Inc. and the Delta OBGyn Group in Manatí, as well as the Manatí Medical Center and the Metro Pavía Hospital in Arecibo.
Funding
This work was supported by the Superfund Research Program of the National Institute of Environmental Health Sciences (NIEHS), National Institutes of Health (NIH; grant number P42ES017198). Additional support was provided from NIEHS (grant number P30ES017885, P30ES005022, R01ES032203) and the Environmental influences on Child Health Outcomes (ECHO) program (grant number UH3OD023251). ECHO is a nationwide research program supported by the NIH, Office of the Director to enhance child health. First author also received support from NIEHS (grant number 3R01ES029275-02S1).
The research protocol was approved by the Ethics and Research Committees of the University of Puerto Rico and participating clinics, the University of Michigan School of Public Health and Northeastern University. All study activities were fully explained to each participant before obtaining written consent for participation in the study.
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adair LS. 2001. Size at birth predicts age at menarche. Pediatrics 107:E59. [DOI] [PubMed] [Google Scholar]
- ADVIA. Siemens cat# 01586287, rrid:Ab_2783800. Available: https://scicrunch.org/resolver/AB_2783800 2020].
- ADVIA. Siemens cat# 08700387, rrid:Ab_2783806. Available: https://scicrunch.org/resolver/AB_2783806.
- ADVIA. Siemens cat# 06490106, rrid:Ab_2783805. Available: https://scicrunch.org/resolver/AB_2783805
- ADVIA. Siemens cat# 09236439, rrid:Ab_2783803. Available: https://scicrunch.org/resolver/AB_2783803.
- ADVIA. Siemens cat# 05476206, rrid:Ab_2783804 Available: https://scicrunch.org/resolver/AB_2783804
- ADVIA. Siemens cat# 05476206, rrid:Ab_2783804. Available: https://scicrunch.org/resolver/AB_2783804.
- ADVIA. Siemens cat# 06520781, rrid:Ab_2783801. Available: https://scicrunch.org/resolver/AB_2783801
- Aker AM, Watkins DJ, Johns LE, Ferguson KK, Soldin OP, Anzalota Del Toro LV, et al. 2016. Phenols and parabens in relation to reproductive and thyroid hormones in pregnant women. Environ Res 151:30–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aker AM, Johns L, McElrath TF, Cantonwine DE, Mukherjee B, Meeker JD. 2018. Associations between maternal phenol and paraben urinary biomarkers and maternal hormones during pregnancy: A repeated measures study. Environ Int 113:341–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aker AM, Ferguson KK, Rosario ZY, Mukherjee B, Alshawabkeh AN, Calafat AM, et al. 2019. A repeated measures study of phenol, paraben and triclocarban urinary biomarkers and circulating maternal hormones during gestation in the puerto rico protect cohort. Environ Health 18:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashrap P, Watkins DJ, Calafat AM, Ye X, Rosario Z, Brown P, et al. 2018. Elevated concentrations of urinary triclocarban, phenol and paraben among pregnant women in northern puerto rico: Predictors and trends. Environ Int 121:990–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Just AC, Williams PL, Smith KW, Calafat AM, Hauser R. 2014. Personal care product use and urinary phthalate metabolite and paraben concentrations during pregnancy among women from a fertility clinic. J Expo Sci Environ Epidemiol 24:459–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley JP, Palmieri RT, Matuszewski JM, Herring AH, Baird DD, Hartmann KE, et al. 2012. Consumer product exposures associated with urinary phthalate levels in pregnant women. J Expo Sci Environ Epidemiol 22:468–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukowski R, Chlebowski RT, Thune I, Furberg AS, Hankins GD, Malone FD, et al. 2012. Birth weight, breast cancer and the potential mediating hormonal environment. PLoS One 7:e40199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantonwine DE, Cordero JF, Rivera-Gonzalez LO, Anzalota Del Toro LV, Ferguson KK, Mukherjee B, et al. 2014. Urinary phthalate metabolite concentrations among pregnant women in northern puerto rico: Distribution, temporal variability, and predictors. Environ Int 62:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cathey AL, Watkins D, Rosario ZY, Vélez C, Alshawabkeh AN, Cordero JF, et al. 2019. Associations of phthalates and phthalate replacements with crh and other hormones among pregnant women in puerto rico. J Endocr Soc 3:1127–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer H 1946. Mathematical methods of statistics:Princeton University Press. [Google Scholar]
- Day DB, Collett BR, Barrett ES, Bush NR, Swan SH, Wang C, et al. 2020. Prenatal sex hormones and behavioral outcomes in children. Psychoneuroendocrinology 113:104547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Den Hond E, Paulussen M, Geens T, Bruckers L, Baeyens W, David F, et al. 2013. Biomarkers of human exposure to personal care products: Results from the flemish environment and health study (flehs 2007–2011). Sci Total Environ 463–464:102–110. [DOI] [PubMed] [Google Scholar]
- DiaMetra. Diametra cat# dko019, rrid:Ab_2783799. Available: https://scicrunch.org/resolver/AB_2783799
- Dietrich JW, Landgrafe G, Fotiadou EH. 2012. Tsh and thyrotropic agonists: Key actors in thyroid homeostasis. J Thyroid Res 2012:351864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn JF, Nisula BC, Rodbard D. 1981. Transport of steroid hormones: Binding of 21 endogenous steroids to both testosterone-binding globulin and corticosteroid-binding globulin in human plasma. J Clin Endocrinol Metab 53:58–68. [DOI] [PubMed] [Google Scholar]
- Duty SM, Ackerman RM, Calafat AM, Hauser R. 2005. Personal care product use predicts urinary concentrations of some phthalate monoesters. Environ Health Perspect 113:1530–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberle CE, Sandler DP, Taylor KW, White AJ. 2020. Hair dye and chemical straightener use and breast cancer risk in a large us population of black and white women. Int J Cancer 147:383–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FDA. 2019. Fda fact sheet: Sunscreen drug products for over-the-counter-human use.Food and Drug Administration. [Google Scholar]
- FDA. 2020. Title 21--food and drugs chapter i--food and drug administration department of health and human service subchapter d - drugs for human use.Food and Drug Administration. [Google Scholar]
- FDA FaDA. 2021. Cosmetic products & ingredients.
- Ferguson KK, Colacino JA, Lewis RC, Meeker JD. 2017. Personal care product use among adults in nhanes: Associations between urinary phthalate metabolites and phenols and use of mouthwash and sunscreen. J Expo Sci Environ Epidemiol 27:326–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson KK, Rosen EM, Rosario Z, Feric Z, Calafat AM, McElrath TF, et al. 2019. Environmental phthalate exposure and preterm birth in the protect birth cohort. Environ Int 132:105099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippou P, Homburg R. 2017. Is foetal hyperexposure to androgens a cause of pcos? Hum Reprod Update 23:421–432. [DOI] [PubMed] [Google Scholar]
- Gera R, Mokbel R, Igor I, Mokbel K. 2018. Does the use of hair dyes increase the risk of developing breast cancer? A meta-analysis and review of the literature. Anticancer Res 38:707–716. [DOI] [PubMed] [Google Scholar]
- Gilles M, Otto H, Wolf IAC, Scharnholz B, Peus V, Schredl M, et al. 2018. Maternal hypothalamus-pituitary-adrenal (hpa) system activity and stress during pregnancy: Effects on gestational age and infant’s anthropometric measures at birth. Psychoneuroendocrinology 94:152–161. [DOI] [PubMed] [Google Scholar]
- Gore AC, Chappell VA, Fenton SE, Flaws JA, Nadal A, Prins GS, et al. 2015. Edc-2: The endocrine society’s second scientific statement on endocrine-disrupting chemicals. Endocr Rev 36:E1–e150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helm JS, Nishioka M, Brody JG, Rudel RA, Dodson RE. 2018. Measurement of endocrine disrupting and asthma-associated chemicals in hair products used by black women. Environ Res 165:448–458. [DOI] [PubMed] [Google Scholar]
- Hsieh CJ, Chang YH, Hu A, Chen ML, Sun CW, Situmorang RF, et al. 2019. Personal care products use and phthalate exposure levels among pregnant women. Sci Total Environ 648:135–143. [DOI] [PubMed] [Google Scholar]
- Jelliffe-Pawlowski LL, Baer RJ, Currier RJ. 2010. Second trimester serum predictors of preterm birth in a population-based sample of low-risk pregnancies. Prenat Diagn 30:727–733. [DOI] [PubMed] [Google Scholar]
- Jiang C, Hou Q, Huang Y, Ye J, Qin X, Zhang Y, et al. 2018. The effect of pre-pregnancy hair dye exposure on infant birth weight: A nested case-control study. BMC Pregnancy Childbirth 18:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsikantami I, Tzatzarakis MN, Karzi V, Stavroulaki A, Xezonaki P, Vakonaki E, et al. 2020. Biomonitoring of bisphenols a and s and phthalate metabolites in hair from pregnant women in crete. Sci Total Environ 712:135651. [DOI] [PubMed] [Google Scholar]
- Ko A, Kang HS, Park JH, Kwon JE, Moon GI, Hwang MS, et al. 2016. The association between urinary benzophenone concentrations and personal care product use in korean adults. Arch Environ Contam Toxicol 70:640–646. [DOI] [PubMed] [Google Scholar]
- Kumar S, Gordon GH, Abbott DH, Mishra JS. 2018. Androgens in maternal vascular and placental function: Implications for preeclampsia pathogenesis. Reproduction 156:R155–r167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang C, Fisher M, Neisa A, MacKinnon L, Kuchta S, MacPherson S, et al. 2016. Personal care product use in pregnancy and the postpartum period: Implications for exposure assessment. Int J Environ Res Public Health 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson K, Ljung Björklund K, Palm B, Wennberg M, Kaj L, Lindh CH, et al. 2014. Exposure determinants of phthalates, parabens, bisphenol a and triclosan in swedish mothers and their children. Environ Int 73:323–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemini C, Jaimez R, Avila ME, Franco Y, Larrea F, Lemus AE. 2003. In vivo and in vitro estrogen bioactivities of alkyl parabens. Toxicol Ind Health 19:69–79. [DOI] [PubMed] [Google Scholar]
- LifeSpan. Lifespan cat# ls-f5352, rrid:Ab_2783798. Available: https://scicrunch.org/resolver/AB_2783798.
- Llanos AAM, Rabkin A, Bandera EV, Zirpoli G, Gonzalez BD, Xing CY, et al. 2017. Hair product use and breast cancer risk among african american and white women. Carcinogenesis 38:883–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marie C, Cabut S, Vendittelli F, Sauvant-Rochat MP. 2016. Changes in cosmetics use during pregnancy and risk perception by women. Int J Environ Res Public Health 13:383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Arguelles DB, Campioli E, Lienhart C, Fan J, Culty M, Zirkin BR, et al. 2014. In utero exposure to the endocrine disruptor di-(2-ethylhexyl) phthalate induces long-term changes in gene expression in the adult male adrenal gland. Endocrinology 155:1667–1678. [DOI] [PubMed] [Google Scholar]
- Meeker JD, Cantonwine DE, Rivera-González LO, Ferguson KK, Mukherjee B, Calafat AM, et al. 2013. Distribution, variability, and predictors of urinary concentrations of phenols and parabens among pregnant women in puerto rico. Environ Sci Technol 47:3439–3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucci LA, Lagiou P, Tamimi RM, Hsieh CC, Adami HO, Trichopoulos D. 2003. Pregnancy estriol, estradiol, progesterone and prolactin in relation to birth weight and other birth size variables (united states). Cancer Causes Control 14:311–318. [DOI] [PubMed] [Google Scholar]
- Myers SL, Yang CZ, Bittner GD, Witt KL, Tice RR, Baird DD. 2015. Estrogenic and anti-estrogenic activity of off-the-shelf hair and skin care products. J Expo Sci Environ Epidemiol 25:271–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakiwala D, Vernet C, Lyon-Caen S, Lavorel A, Rolland M, Cracowski C, et al. 2020. Use of personal care products during pregnancy in relation to urinary concentrations of select phenols: A longitudinal analysis from the sepages feasibility study. Int J Hyg Environ Health 227:113518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen. 2015. Hispanic consumers are the ‘foundation’ for beauty category sales.
- Noyola-Martinez N, Halhali A, Barrera D. 2019. Steroid hormones and pregnancy. Gynecol Endocrinol 35:376–384. [DOI] [PubMed] [Google Scholar]
- Parazzini F, Esposito G, Tozzi L, Noli S, Bianchi S. 2017. Epidemiology of endometriosis and its comorbidities. Eur J Obstet Gynecol Reprod Biol 209:3–7. [DOI] [PubMed] [Google Scholar]
- Park GH, Nam C, Hong S, Park B, Kim H, Lee T, et al. 2018. Socioeconomic factors influencing cosmetic usage patterns. J Expo Sci Environ Epidemiol 28:242–250. [DOI] [PubMed] [Google Scholar]
- Parlett LE, Calafat AM, Swan SH. 2013. Women’s exposure to phthalates in relation to use of personal care products. J Expo Sci Environ Epidemiol 23:197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peinado FM, Ocón-Hernández O, Iribarne-Durán LM, Vela-Soria F, Ubiña A, Padilla C, et al. 2020. Cosmetic and personal care product use, urinary levels of parabens and benzophenones, and risk of endometriosis: Results from the endea study. Environ Res:110342. [DOI] [PubMed] [Google Scholar]
- Richards M, Hardy R, Kuh D, Wadsworth ME. 2002. Birthweight, postnatal growth and cognitive function in a national uk birth cohort. Int J Epidemiol 31:342–348. [PubMed] [Google Scholar]
- Rivera-Núñez Z, Ashrap P, Barrett ES, Watkins DJ, Cathey AL, Vélez-Vega CM, et al. 2021. Association of biomarkers of exposure to metals and metalloids with maternal hormones in pregnant women from puerto rico. Environ Int 147:106310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Carmona Y, Ashrap P, Calafat AM, Ye X, Rosario Z, Bedrosian LD, et al. 2020. Determinants and characterization of exposure to phthalates, dehtp and dinch among pregnant women in the protect birth cohort in puerto rico. J Expo Sci Environ Epidemiol 30:56–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero R, Scoccia B, Mazor M, Wu YK, Benveniste R. 1988. Evidence for a local change in the progesterone/estrogen ratio in human parturition at term. Am J Obstet Gynecol 159:657–660. [DOI] [PubMed] [Google Scholar]
- Shaaban H, Alhajri W. 2020. Usage patterns of cosmetic and personal care products among female population in saudi arabia: Important factors for exposure and risk assessment. J Environ Public Health 2020:8434508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoker TE, Gibson EK, Zorrilla LM. 2010. Triclosan exposure modulates estrogen-dependent responses in the female wistar rat. Toxicol Sci 117:45–53. [DOI] [PubMed] [Google Scholar]
- Vo TT, Yoo YM, Choi KC, Jeung EB. 2010. Potential estrogenic effect(s) of parabens at the prepubertal stage of a postnatal female rat model. Reprod Toxicol 29:306–316. [DOI] [PubMed] [Google Scholar]
- Wadhwa PD, Garite TJ, Porto M, Glynn L, Chicz-DeMet A, Dunkel-Schetter C, et al. 2004. Placental corticotropin-releasing hormone (crh), spontaneous preterm birth, and fetal growth restriction: A prospective investigation. Am J Obstet Gynecol 191:1063–1069. [DOI] [PubMed] [Google Scholar]
- White AJ, Gregoire AM, Taylor KW, Eberle C, Gaston S, O’Brien KM, et al. 2021. Adolescent use of hair dyes, straighteners and perms in relation to breast cancer risk. Int J Cancer 148:2255–2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.