Abstract
Multiple myeloma (MM) remains an incurable plasma cell malignancy. Although little is known about the etiology of MM, several metabolic risk factors such as obesity, diabetes, poor nutrition, many of which are modifiable, have been linked to the pathogenesis of numerous neoplasms including MM. In this article, we provide a detailed summary of what is known about the impact of obesity on the pathogenesis of MM, it’s influence on outcomes in MM patients, and discuss potential mechanisms through which obesity is postulated to influence MM risk and prognosis. Along with advancements in treatment modalities to improve survival in MM patients, focused efforts are needed to prevent or intercept MM at its earliest stages. The consolidated findings presented in this review highlight the need for clinical trials to assess if lifestyle modifications can reduce the incidence and improve outcomes of MM in high-risk populations. Data generated from such studies can help formulate evidence-based lifestyle recommendations for the prevention and control of MM.
Introduction
Multiple myeloma (MM) is the second most common hematologic malignancy characterized by neoplastic proliferation of plasma cell clones associated with overproduction of monoclonal immunoglobulins (1). It remains incurable despite many therapies approved in the last decade which improve survival.
In the US, the prevalence of obesity [body mass index (BMI) ≥30] and severe obesity (BMI ≥40) among adults (aged 20 years and over) has increased dramatically in recent decades. Indeed, from 1999–2000 through 2017–2018, the prevalence of obesity increased from 30.5% to 42.4% and that of severe obesity increased from 4.7% to 9.2% among adults (2). Similar rising trends in obesity (BMI ≥ age- and sex- specific 95th percentile of the 2000 Centers for Disease Control and Prevention growth charts) were noted in youth (aged 2–19 years) from 1999–2000 through 2015–2016 (3). The incidence of numerous obesity-related cancers, including MM, has increased in younger adults (25–49 years) from 1995–2014, with the largest annual percent change in the 30–34 years age group (2.21% increase) (4). These findings in the light of the rising prevalence of obesity across all age groups highlight the need for a closer review of the current literature evaluating the association between obesity and MM (5). In this review, we will discuss the available evidence for obesity’s role in the etiology and pathogenesis of plasma cell disorders as well as potential mechanisms.
Obesity and MGUS
The first study suggesting a link between obesity and monoclonal gammopathy of undetermined significance (MGUS) was published in 2010. In that study, the authors report screening 1 996 black and white women for MGUS and found that obese individuals are 1.8 times more likely to develop MGUS than those with a normal BMI (6). Complementary analyses using data from the National Health and Nutritional Examination Survey (NHANES) study (cross-sectional study involving 12 482 persons; 365 MGUS cases) and Age, Gene/Environment Susceptibility-Reykjavik Study (AGES-RS) (prospective cohort study with 5 725 participants; 575 MGUS cases) saw a trend towards increased risk for MGUS with increased BMI but effect estimates were not statistically significant (7, 8). Of note, the AGES-RS study looked at 11 different measures of obesity in their analysis, including weight (kg), baseline BMI (kg/m2), percent body fat (%), fat (kg), total body fat (cm2), visceral fat (cm2), subcutaneous fat (cm2), two versions of abdominal circumference (cm), self-reported lifetime maximum weight and midlife BMI, and found no significant association (8). Some limitations of this study, however, were a possibility of selection bias (mean age of cohort was 77 years implicating selection of a healthier population) and that Iceland has an exclusively white population and results may not be applicable to other races. Thus, based on current evidence from large population-based studies (Table 1), the association between obesity and risk of MGUS is controversial. Additional studies addressing the above limitations would be instrumental in further understanding the role of obesity as a risk factor for MGUS development.
Table 1:
Obesity and Monoclonal Gammopathy of Undetermined Significance
| Cohort/ Location | Study Design | N | Outcome | BMI (kg/m2) | Effect Estimate | 95% CI | Study |
|---|---|---|---|---|---|---|---|
| Obesity and the risk of MGUS | |||||||
| Southern Community Cohort Study (SCCS)/ Southeastern USA | Cohort | 1996 women; 60 MGUS | MGUS | >30 | OR 1.80 | 1.03–3.14 | Landgren et al Blood 2010 |
| National Health and Nutritional Examination Survey (NHANES), USA | Cross-sectional study | 12,482 persons; 365 MGUS | MGUS | Adjusted prevalence (%): | Standard Error: | Landgren et al Leukemia 2014 | |
| <25 | 1.99 | 0.27 | |||||
| 25–30 | 2.43 | 0.25 | |||||
| >30 | 2.72 | 0.32 | |||||
| Ptrend 0.20 | |||||||
| Age, Gene/ Environment Susceptibility-Reykjavik Study (AGES-RS)/ Reykjavik, Iceland | Prospective Cohort | 5725 persons, 575 MGUS | MGUS | Midlife ≥25kg/m2 | OR 1.15 | 0.90–1.45 | Thordardottir et al Blood Advances 2017 |
| LC-MGUS | OR 1.10 | 0.86–1.41 | |||||
| Obesity increases the risk of transformation of MGUS to MM | |||||||
| Age, Gene/ Environment Susceptibility-Reykjavik Study (AGES-RS)/ Reykjavik, Iceland | Prospective Cohort | 5725 persons, 575 MGUS→ 18 MM; 11 lymphoproliferative diseases (LPD) within 8 years | MGUS→ MM and other | HR 2.66 | 1.17–6.05 | Thordardottir et al Blood Advances 2017 | |
| US Veterans Health Administration database | Retrospective Cohort | 7878 MGUS (97% men)→ 329 MM | MGUS→ MM | 25–29.9 | HR 1.55 | 1.16–2.06 | Chang et al JNCI 2017 |
| ≥30 | HR 1.98 | 1.47–2.68 | |||||
Abbreviations - BMI: Body-Mass Index; 95%CI: 95% Confidence Interval; MGUS: Monoclonal Gammopathy of Undetermined Significance; LC-MGUS: Light Chain MGUS; MM: Multiple Myeloma; OR: Odds Ratio; HR: Hazard Ratio
Obesity increases the risk of transformation of MGUS to MM
Two large studies that assessed the impact of BMI on the risk of transformation of MGUS to MM found that overweight and obese individuals with MGUS were at a greater risk for progression to MM. Patients in the AGES-RS study (study characteristics outlined above) with midlife BMI ≥25 kg/m2 (mean age at time of “midlife” calculation 53.3 years for women and 52.1 years for men) had a 2.7 fold higher risk (95%CI 1.17–6.05) to develop MM and other lymphoproliferative diseases than normal weight individuals (8). These results were consistent with the US Veterans Health Administration Study (retrospective cohort of 7 878 MGUS cases followed prospectively for median duration of 68 months; 329 progressed to MM) that also showed an increased risk of progression to MM for overweight (Hazard Ratio, HR 1.55; 95%CI 1.16–2.06) and obese patients (HR 1.98; 95%CI 1.47–2.68) (9). BMI is a commonly used surrogate for obesity, but it does not provide detailed information regarding the distribution of body adiposity. A small cross sectional study of 40 MGUS and 32 MM patients found that MM patients had higher abdominal adipose tissue cross sectional areas and higher adipose tissue metabolic activity compared to patients with MGUS, thus suggesting that these adipose tissue parameters may play a role in the malignant transformation of MGUS to MM (10). This evidence (Table 1) suggests that obese patients are about twice as likely to transform from MGUS to MM.
Obesity and the risk of MM
Studies evaluating the association between obesity and risk of MM have been outlined in Table 2. A large prospective cohort study of hospitalized male U.S veterans (3 668 486 whites; 832 214 blacks) reported an increased risk of MM in obese white men (Relative Risk, RR 1.22; 95%CI 1.05–1.40) and obese black men (RR 1.26, 95%CI 1.02–1.56) as compared to non-obese white and black men respectively (11). The Iowa Women’s Health Study followed a prospective cohort of 37 083 post-menopausal women for 16 years and identified 95 cases of MM with increased incidence of MM in overweight and obese individuals as measured by various anthropometric parameters. The rate ratios (RR) for various parameters were – BMI ≥30 kg/m2; RR 1.5 (95%CI 0.92–2.6), weight ≥161 lbs; RR 1.9 (95%CI 1.1–3.4), waist circumference ≥36.26 inches; RR 2.0 (95%CI 1.1–3.5), as well as hip circumference ≥42.26 inches; RR 1.8 (95%CI 1.0–3.0) (12). Similarly, many other studies have demonstrated an increased incidence of MM with increasing BMI (13–16). For example, a meta-analysis of 10 prospective observational studies which collectively included 5 171 374 individuals evaluated the association between BMI and cancer risk found an excess risk for development of MM in both men (RR 1.11; 95%CI 1.05–1.18 per 5 kg/m2 increase in BMI) and women (RR 1.11; 95%CI 1.07–1.15 per 5 kg/m2 increase in BMI) (17). Analysis from the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial which enrolled 142 982 participants at 10 centers in the U.S in a randomized, controlled trial and identified 243 cases with plasma cell neoplasms, revealed increased MM risk with elevated BMI at baseline (HR 1.69; 95%CI 1.18–2.41 for BMI ≥30 kg/m2) and at age 20 years (HR 3.08; 95%CI 1.71–5.54 for BMI ≥30 kg/m2) (18). Findings of the National Institutes of Health-AARP Diet and Health Study, a large prospective cohort study in the U.S including 485 049 participants (489 MM cases) demonstrated that excess body weight, both in early and late adulthood was associated with increased MM risk (at cohort entry: HR 1.10, 95%CI 1.00–1.22; at age 50 years: HR 1.14, 95%CI 1.02–1.28; at age 35 years: HR 1.20, 95%CI 1.05–1.36 and at age 18 years: HR 1.13, 95%CI 0.98–1.32 per 5 kg/m2 increase in BMI) (19).
Table 2:
Obesity and Multiple Myeloma
| Cohort/ Location | Study Design | N | Outcome | BMI (kg/m2) | Effect Estimate | 95% CI | Study |
|---|---|---|---|---|---|---|---|
| Obesity and the risk of development of MM | |||||||
| Cancer registries from the Georgia Center for Cancer Statistics, the Metropolitan Detroit Cancer Surveillance System and the New Jersey Cancer registry, United States | Case-Control | White Race - 1086 Controls; 346 MM | MM | White: <18.5 | OR 0.6 | 0.2–2.3 | Brown et al Cancer Causes Control 2001 |
| 18.5–24.9 | OR 1.0 | ||||||
| 25–29.9 | OR 1.5 | 1.1–2.0 | |||||
| ≥30 | OR 1.9 | 1.2–3.1 | |||||
| Black Race - 903 Controls; 193 MM | MM | Black: <18.5 | OR 1.1 | 0.3–4.2 | |||
| 18.5–24.9 | OR 1.0 | ||||||
| 25–29.9 | OR 1.3 | 0.9–1.8 | |||||
| ≥30 | OR 1.5 | 0.9–2.4 | |||||
| Veterans affairs hospital across US | Prospective Cohort | 4.50 Million | MM | Obese | White men: RR 1.22 | 1.05–1.40 | Samanic et al Cancer Causes and Control 2004 |
| Black men: RR 1.26 | 1.02–1.56 | ||||||
| Iowa Women’s Health Study | Prospective Cohort | 37,083 participants; 95 MM cases | MM | 25–29.9 | Rate Ratio 1.3 | 0.78–2.0 | Blair et al Epidemiology 2005 |
| ≥30 | Rate Ratio 1.5 | 0.92–2.6 | |||||
| Sweden | Prospective Cohort | 362,552 male participants; 452 MM cases | MM | 25–29.9 | RR 0.96 | 0.79–1.16 | Samanic et al Cancer Causes Control 2006 |
| >30 | RR 0.58 | 0.37–0.93 | |||||
| Million Women Study, UK | Prospective Cohort | 1,222,630 women; 491 MM cases | MM | <22.5 | RR 0.8 | 0.64–1.0 | Reeves et al BMJ 2007 |
| 22.5–24.9 | RR 1.0 | 0.84–1.19 | |||||
| 25–27.4 | RR 1.11 | 0.92–1.32 | |||||
| 27.5–29.5 | RR 1.11 | 0.88–1.4 | |||||
| ≥30 | RR1.16 | 0.95–1.42 | |||||
| Norwegian Cohort, Norway | Prospective Cohort | 2,000,424 persons; 3,233 plasma cell neoplasms | MM | <18.5 | RR 0.69 | 0.38–1.25 | Engeland et al Am J Epidemiology 2007 |
| 18.5–24.9 | RR 1.0 | ||||||
| 25–29.9 | RR 1.14 | 1.06–1.22 | |||||
| ≥30 | RR 1.28 | 1.10–1.47 | |||||
| Participants from the Construction Industry’s Organization for Working Environment, Safety and Health; Sweden | Prospective Cohort | 336,381 male Swedish construction workers; 520 MM cases | MM | 18.5–25 | IRR 1.0 | Fernberg et al Cancer Research 2007 | |
| 25.1–30 | IRR 1.04 | 0.86–1.24 | |||||
| >30 | IRR 0.70 | 0.46–1.06 | |||||
| European Prospective Investigation into Cancer and Nutrition (EPIC) | Prospective Cohort | 371,983 participants; 139 MM cases | MM | Men: | Britton et al Haematologica 2008 | ||
| Weight (kg) <72.7 | RR 1.0 | ||||||
| 72.7–79.8 | RR 1.49 | 0.89–2.48 | |||||
| 79.9–87.7 | RR 1.49 | 0.88–2.53 | |||||
| ≥87.8 | RR 1.77 | 1.02–3.05 | |||||
| Ptrend 0.06 | |||||||
| BMI (kg/m2) <25 | RR 1.0 | ||||||
| 25–29.9 | RR 1.33 | 0.79–2.23 | |||||
| ≥30 | RR 1.17 | 0.80–1.72 | |||||
| Ptrend 0.26 | |||||||
| Women: | |||||||
| Weight (kg) <72.7 | RR 1.0 | ||||||
| 72.7–79.8 | RR 0.69 | 0.39–1.23 | |||||
| 79.9–87.7 | RR 1.19 | 0.71–1.99 | |||||
| ≥87.8 | RR 0.95 | 0.55–1.63 | |||||
| Ptrend 0.62 | |||||||
| BMI (kg/m2) <25 | RR 1.0 | ||||||
| 25–29.9 | RR 0.93 | 0.55–1.56 | |||||
| ≥30 | RR 1.06 | 0.72–1.58 | |||||
| Ptrend 0.89 | |||||||
| Swedish Twin Registry and Finnish Twin cohort | Prospective Cohort | 70,067 persons; 140 MM cases | MM | ≤18.49 | N/A | Söderberg et al Eur J Cancer 2009 | |
| 18.5–24.99 | RR 1.0 | ||||||
| 25–29.99 | RR 1.0 | 0.7–1.5 | |||||
| ≥30 | RR 2.1 | 1.1–3.7 | |||||
| The Netherlands Cohort Study on Diet and Cancer | Prospective Cohort | 120,852 participants; 4774 sub-cohort members; 279 MM cases | MM | BMI at baseline: 18.5–24.9 | HR 1.0 | Pylypchuk et al Am J Epidemiology 2009 | |
| 25–29.9 | HR 1.23 | 0.95–1.58 | |||||
| ≥30 | HR 1.13 | 0.68–1.88 | |||||
| per 4 kg/m2 increment | HR 1.13 | 0.97–1.31 | |||||
| BMI at Age 20 years: <20 | HR 1.0 | 0.69–1.45 | |||||
| 20-<21.5 | HR 1.0 | ||||||
| 21.5-<23 | HR 0.75 | 0.50–1.12 | |||||
| 23-<25 | HR 1.10 | 0.75–1.63 | |||||
| ≥25 | HR 1.16 | 0.69–1.95 | |||||
| per 4 kg/m2 increment | HR 1.06 | 0.87–1.30 | |||||
| The Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial | Prospective Cohort | 142, 982 participants; 243 MM | MM | BMI at age 20 years: | Troy et al Am J Epidemiology 2010 | ||
| <18.5 | HR 0.71 | 0.40–1.29 | |||||
| 18.5–24.9 | HR 1.0 | ||||||
| 25–29.9 | HR 1.33 | 0.94–1.88 | |||||
| ≥30 | HR 3.08 | 1.71–5.54 | |||||
| BMI at baseline: | |||||||
| <18.5 | N/A | ||||||
| 18.5–24.9 | HR 1.0 | ||||||
| 25–29.9 | HR 1.45 | 1.05–2.01 | |||||
| ≥30 | HR 1.69 | 1.18–2.41 | |||||
| The Japan Public Health Center-based Prospective Study, Japan | Prospective Cohort | 94,547 subjects; 88 MM cases | MM | <18.5 | HR 0.56 | 0.13–2.36 | Kanda et al Cancer, Epidemiology, Biomarkers & Prevention 2010 |
| 18.5–22.9 | HR 0.70 | 0.42–1.15 | |||||
| 23–24.9 | HR 1.0 | ||||||
| 25–29.9 | HR 0.79 | 0.45–1.38 | |||||
| ≥30 | HR 0.76 | 0.18–3.20 | |||||
| 1-unit increase | HR 1.01 | 0.95–1.09 | |||||
| California Teachers Study | Prospective Cohort | 121,216 women; 111 MM cases | MM | At cohort entry: <20 | RR 0.92 | 0.45–1.86 | Lu et al Epidemiology 2010 |
| 20–24.9 | RR 1.0 | ||||||
| 25–29.9 | RR 0.83 | 0.53–1.31 | |||||
| ≥30 | RR 0.86 | 0.48–1.55 | |||||
| Reported for age 18: <20 | RR 1.0 | ||||||
| 20–21.9 | RR 0.74 | 0.46–1.18 | |||||
| ≥22 | RR 0.97 | 0.61–1.53 | |||||
| National Institutes of Health – AARP Diet and Health Study, USA | Prospective Cohort | 485,049 participants; 489 MM cases | MM | BMI at baseline: | Hofmann et al Am J Epidemiology 2013 | ||
| <18.5 | HR 0.30 | 0.04–2.17 | |||||
| 18.5–22.49 | HR 1.0 | ||||||
| 22.5–24.9 | HR 1.02 | 0.73–1.43 | |||||
| 25–29.9 | HR 1.09 | 0.80–1.48 | |||||
| 30–34.9 | HR 1.26 | 0.89–1.78 | |||||
| ≥35 | HR 1.55 | 1.01–2.39 | |||||
| BMI at age 50 years: | |||||||
| <18.5 | HR 0.78 | 0.25–2.49 | |||||
| 18.5–22.49 | HR 1.0 | ||||||
| 22.5–24.9 | HR 1.14 | 0.85–1.52 | |||||
| 25–29.9 | HR 1.16 | 0.88–1.54 | |||||
| 30–34.9 | HR 1.23 | 0.84–1.80 | |||||
| ≥35 | HR 1.77 | 1.05–2.99 | |||||
| BMI at age 35 years: | |||||||
| <18.5 | HR 0.77 | 0.36–1.66 | |||||
| 18.5–22.49 | HR 1.0 | ||||||
| 22.5–24.9 | HR 1.42 | 1.12–1.79 | |||||
| 25–29.9 | HR 1.27 | 0.99–1.63 | |||||
| 30–34.9 | HR 1.41 | 0.89–2.22 | |||||
| ≥35 | HR 2.53 | 1.24–5.18 | |||||
| BMI at age 18 years: | |||||||
| <18.5 | HR 0.93 | 0.69–1.25 | |||||
| 18.5–22.49 | HR 1.0 | ||||||
| 22.5–24.9 | HR 1.12 | 0.88–1.44 | |||||
| >25 | HR 1.38 | 1.04–1.82 | |||||
| International Multiple Myeloma Consortium | Pooled Analysis of 8 case-control studies | 2,318 MM; 9,609 controls | MM | 25–29.9 | OR 1.1 | 1.0–1.3 | Birmann et al CEBP 2017 |
| 30–34.9 | OR 1.1 | 1.0–1.3 | |||||
| 35+ | OR 1.4 | 1.4–1.7 | |||||
| Nurses’ Health Study (NHS), Health Professionals Follow-up Study (HPFS), Women’s Health Study (WHS), USA | Prospective cohorts | 1,21,700 female nurses in NHS; 51,529 male health professionals in HPFS; 39,876 female health professionals; 575 MM | MM | Cumulative average BMI | HR 1.17 per 5 kg/m2 increase | 1.05–1.29 | Marinac et al BJC 2018 |
| Young adult BMI | HR 1.28 per 5 kg/m2 increase | 1.12–1.47 | |||||
| Change in BMI since young adulthood | HR 1.09 per 5 kg/m2 increase | 0.98–1.21 | |||||
| Cumulative average physical activity | HR 0.98 per 10 MET-hrs | 0.92–1.06 | |||||
| Cumulative average walking | HR 1.0 per 10 MET-hrs | 0.91–1.10 | |||||
| Nurses’ Health Study (NHS) and Health Professionals Follow-up Study (HPFS), USA | Prospective cohorts | 1,21,700 female nurses in NHS and 51,529 male health professionals in HPFS; 582 MM | Weight cycling and MM risk | Weight loss | HR 1.30 | 0.65–2.62 | Marinac et al JNCI Cancer Spectrum 2019 |
| Weight maintenance | HR 1 | ||||||
| Weight gain | HR 1.52 | 0.90–2.55 | |||||
| Light cycling | HR 1.54 | 0.95–2.49 | |||||
| Medium cycling | HR 1.25 | 0.76–2.05 | |||||
| Extreme cycling | HR 1.71 | 1.05–2.80 | |||||
| Body shape trajectories and MM Risk | Lean-stable | HR 1 | |||||
| Lean-increase | HR 1.17 | 0.89–1.53 | |||||
| Medium-stable | HR 1.27 | 0.97–1.66 | |||||
| Medium-increase | HR 1.62 | 1.22–2.14 | |||||
| Obesity increases MM mortality | |||||||
| Cancer Prevention Study II | Prospective Cohort | 900,053 participants; 708 MM deaths in M; 620 MM deaths in F | MM mortality | 25–29.9 | RR 1.18 (M) | 1.01–1.39 | Calle et al NEJM 2003 |
| RR 1.12 (F) | 0.93–1.34 | ||||||
| 30–34.9 | RR 1.44 (M) | 1.10–1.89 | |||||
| RR 1.47 (F) | 1.13–1.91 | ||||||
| 35–39.9 | RR 1.71(M) | 0.93–3.14 | |||||
| RR 1.44 (F) | 0.91–2.28 | ||||||
| National Cancer Institute Cohort Consortium | Pooled Analyses of 20 prospective studies | 1,564,218 participants; 1,388 MM deaths | MM mortality | BMI at study entry: 25<27.5 | HR 1.15 | 0.95–1.38 | Teras et al BJH 2014 |
| 27.5-<30 | HR 1.24 | 1.01–1.52 | |||||
| 30-<35 | HR 1.23 | 0.99–1.52 | |||||
| 35+ | HR 1.52 | 1.15–2.02 | |||||
| BMI (per 5kg/m2) | HR 1.09 | 1.03–1.16 | |||||
| Young adult BMI: 25-<27.5 | HR 1.11 | 0.87–1.40 | |||||
| 27.5-<30 | HR 1.49 | 1.03–2.16 | |||||
| 30+ | HR 1.82 | 1.22–2.73 | |||||
| BMI (per 5kg/m2) | HR 1.22 | 1.09–1.35 | |||||
| Japan Collaborative Cohort Study (JACC), Japan | Prospective Cohort | 109,698 subjects; 98 MM deaths | MM mortality | BMI (kg/m2): | Khan et al Asian Pac J Cancer Prev 2006 | ||
| <18.5 | HR 0.79 | 0.32–1.98 | |||||
| 18.5–25 | HR 1.0 | ||||||
| 25–30 | HR 0.72 | 0.39–1.33 | |||||
| ≥30 | HR 2.79 | 1.01–7.69 | |||||
| Walking/day: | |||||||
| ≥1 hr | HR 1.0 | ||||||
| 30 min–1 hr | HR 1.19 | 0.60–2.36 | |||||
| ≤30 min | HR 1.99 | 1.16–3.39 | |||||
| Million Women Study, UK | Prospective Cohort | 1,222,630 women; 284 MM deaths | MM mortality | <22.5 | RR 0.99 | 0.74–1.32 | Reeves et al BMJ 2007 |
| 22.5–24.9 | RR 1.0 | 0.78–1.28 | |||||
| 25–27.4 | RR 1.26 | 0.99–1.59 | |||||
| 27.5–29.5 | RR 1.13 | 0.82–1.55 | |||||
| ≥30 | RR 1.63 | 1.28–2.08 | |||||
Abbreviations - BMI: Body-Mass Index; 95%CI: 95% Confidence Interval; MM: Multiple Myeloma; OR: Odds Ratio; HR: Hazard Ratio; IRR: Incidence Rate Ratio; RR: Relative Risk
Consistent with these studies, a more recent pooled analysis of 8 case-control studies (2 318 MM; 9 609 controls) looked at the risk of MM in relation to BMI at 2 time points – usual adult BMI (at time of enrollment and study specific reference period) for all 8 studies and young adult BMI (ages 25–30 years) for 2 studies (20). They found that overweight or obese patients at time of study enrollment had a higher risk for MM (HR 1.1, 95%CI 1.0–1.3 for overweight and obese patients and HR 1.4, 95%CI 1.4–1.7 for patients with BMI 35+). Interestingly, patients with elevated BMI at both time points [study entry as well as at young adult age (ages 25–30 years)] had a significantly elevated MM risk compared with individuals with normal BMI on both measures (OR 1.7; 95%CI 1.1–2.6) (20) This study suggests that sustained BMI elevation during adulthood is associated with a higher risk than isolated elevated BMI in the recent years prior to diagnosis. Another pooled analysis in three large prospective cohorts, the Nurses’ Health Study (NHS), Health Professionals Follow-up Study (HPFS) and Women’s Health Study (WHS) with 575 MM cases demonstrated that MM risk increased by 17% per 5 kg/m2 increase in cumulative average BMI and by 28% per 5 kg/m2 increase in young adult BMI (21). Prospective data from the NHS and HPFS (582 MM cases) also revealed a statistically significant increased MM risk in weight cyclers compared with weight maintainers (HR 1.71; 95%CI 1.05–2.80) and in individuals with a medium-increase body shape trajectory compared with those with a lean-stable trajectory (HR 1.62; 95%CI 1.22–2.14) (22). On the contrary, analysis from a prospective cohort of Swedish men (362 552 participants, 452 MM cases) revealed a reduced MM risk in obese individuals with BMI >30 compared to individuals with BMIs in the normal range (RR 0.58; 95%CI 0.37–0.93) (23). The European Prospective Investigation into Cancer and Nutrition (EPIC) study (371 983 participants; 139 MM cases) showed statistically significant increased MM risk in heavier men (weight ≥ 87.8 kg) (RR 1.77; 95%CI 1.02–3.05) but no significant association was seen with higher BMI (BMI ≥28.7 kg/m2) (RR 1.52; 95%CI 0.92–2.51) (24). Similarly, several other studies showed no significant association between BMI and MM risk (25–28). However, the results of these studies need to be interpreted cautiously. Some of these studies reported a small number of incident MM cases possibly limiting their statistical power to detect a significant association. Overall, current evidence suggests that obesity, measured by various anthropometric parameters, increases the risk to develop MM in both men and women as well as white and black individuals. This risk is especially enhanced with sustained BMI elevation throughout adulthood. However, weight cycling has shown to have a detrimental effect on MM risk and attaining sustained weight loss poses many practical challenges. Additional studies are needed to develop a balanced approach to promote weight loss and mitigate the impact of obesity on MM risk.
Obesity increases MM mortality
Studies evaluating the association between obesity and MM mortality have been outlined in Table 2. The Cancer Prevention Study II (CPS-II), a prospective cohort of 9 00 053 participants, observed total 1 328 deaths due to MM and showed that overweight and obese individuals had a significantly higher mortality due to all cancers combined as well as for MM when compared to normal weight patients (29). Analysis from the Japan Collaborative Cohort Study (109 698 subjects; 98 MM deaths) revealed higher age and sex adjusted MM mortality risk with BMI ≥ 30kg/m2 (HR 2.79; 95%CI 1.01–7.69) and walking ≤ 30 minutes/day (HR 1.99; 95%CI 1.16–3.39) (30). Another UK based study showed similar association between BMI and MM mortality (13). Similarly, a pooled analysis of 20 prospective studies within the National Cancer Institute Cohort Consortium (1 564 218 participants; 1 388 MM deaths) showed that patients with a BMI above normal at study entry and in young adulthood had an increased risk of death due to MM (HR 1.09; 95%CI 1.03–1.16 and HR 1.22; 95%CI 1.09–1.35 respectively for every 5 kg/m2 increase in BMI) as well as an elevated waist circumference (HR 1.06; 95%CI 1.02–1.10 per 5 cm) (31). Data from the African American BMI-Mortality Pooling Project (7 prospective cohorts; 239 597 participants) evaluating BMI as a risk factor for increased MM mortality among African Americans reported a statistically significant (p=0.04) rising trend for MM death across BMI ranging from 18.5 to <60 kg/m2 with hazard ratios reaching 1.43 (men > women). It was hypothesized that elevated mortality could be due to increased incidence of MM and/or poorer survival (32). These studies support the association between obesity and elevated MM mortality.
The impact of obesity on outcomes in patients with MM Newly diagnosed MM
A study evaluating the association between BMI at the time of diagnosis of MM and overall survival in a cohort (n=2 968) of U.S. veterans found that patients who were underweight (BMI <18.5 kg/m2) had a higher all-cause mortality (HR 1.64; 95%CI 1.30–2.08) and those who were overweight (BMI 25–29.9 kg/m2) and obese (BMI ≥ 30 kg/m2) had a lower mortality (HR 0.82; 95%CI 0.75–0.91) and HR 0.75; 95%CI 0.67–0.84 respectively) when compared with the normal-weight group (BMI 18.5–24.9 kg/m2) (33). Additionally, patients with loss of ≥ 10% of baseline weight in the year leading up to MM diagnosis had significantly higher mortality (HR 1.52; 95%CI 1.34–1.72) compared with those who did not. However, after controlling for disease-related weight loss, age, race and co-morbidities being overweight or obese at the time of MM diagnosis was not significantly associated with decreased mortality (overweight: HR 0.98; 95%CI 0.86–1.11 and obese: HR 0.89; 95%CI 0.77–1.02) suggesting that disease-related weight loss was the primary predictor of outcomes (33).
Obesity and Post - Autologous Stem Cell Transplant (ASCT) MM
A single-center retrospective analysis of 142 MM patients undergoing ASCT revealed that increased BMI at transplant was associated with worse overall survival (OS) (HR 1.11; 95%CI 1.02–1.22) but not with progression free survival (PFS) (HR 1.02; 95%CI 0.97–1.08) in a multivariate analysis (34). A larger CIBMTR study analyzed the effect of BMI on outcomes of 1 087 MM patients who underwent autologous stem cell transplantation with high dose melphalan conditioning and found no overall effect of BMI on PFS, OS, progression, or non-relapse mortality (35).
Obesity and Relapsed MM
Retrospective analysis of 13 relapsed/refractory MM (RRMM) clinical trials (5 898 patients) demonstrated that overweight and obese patients had a trend towards slightly improved PFS (HR 0.90; 95%CI 0.82–0.99 and HR 0.88; 95%CI 0.79–0.97 respectively) and OS (HR 0.91; 95%CI 0.81–0.99 and HR 0.84; 95%CI 0.75–0.95 respectively) when compared to normal weight patients (36). Analysis from another prospective clinical trial (331 patients with RRMM) showed better median PFS (47.9 months vs 9.2 months) and OS (47.9 vs 20.5 months) in obese vs non-obese patients (37). These findings suggest that additional nutritional or muscle reserves often associated with higher BMIs (38) may confer a survival advantage in RRMM patients who have undergone multiple lines of therapy; however, additional studies assessing body composition parameters are needed before definitive conclusions can be drawn.
Taken together, these findings suggest that the influence of BMI in plasma cell disorders maybe disease stage dependent. In normal individuals, an elevated BMI is associated with an increased risk of development of plasma cell disorders and increased MM mortality and in individuals with MGUS and SMM; it also appears to be associated with an increased risk of progression to MM. However, at time of MM diagnosis, transplant, or relapse, excess BMI appears to have no effect on survival, or is associated with improved survival with the latter implying that high BMI patients are able to tolerate therapies better or have less disease related weight loss or cachexia. Disease-related weight loss and body composition are important constructs to consider in future studies to clarify the association between obesity and clinical outcome in MM patients.
Mechanisms by which obesity leads to MM
Several mechanisms linking obesity and cancer risk have been described in the literature. The role of visceral adipocytes, bone marrow adipocytes, adipokines such has adiponectin, resistin and leptin, inflammatory cytokines, especially IL-6 and TNF-α, insulin and insulin-like growth factor I (IGF-I) and sex hormones is outlined below (Figure 1).
Figure 1:
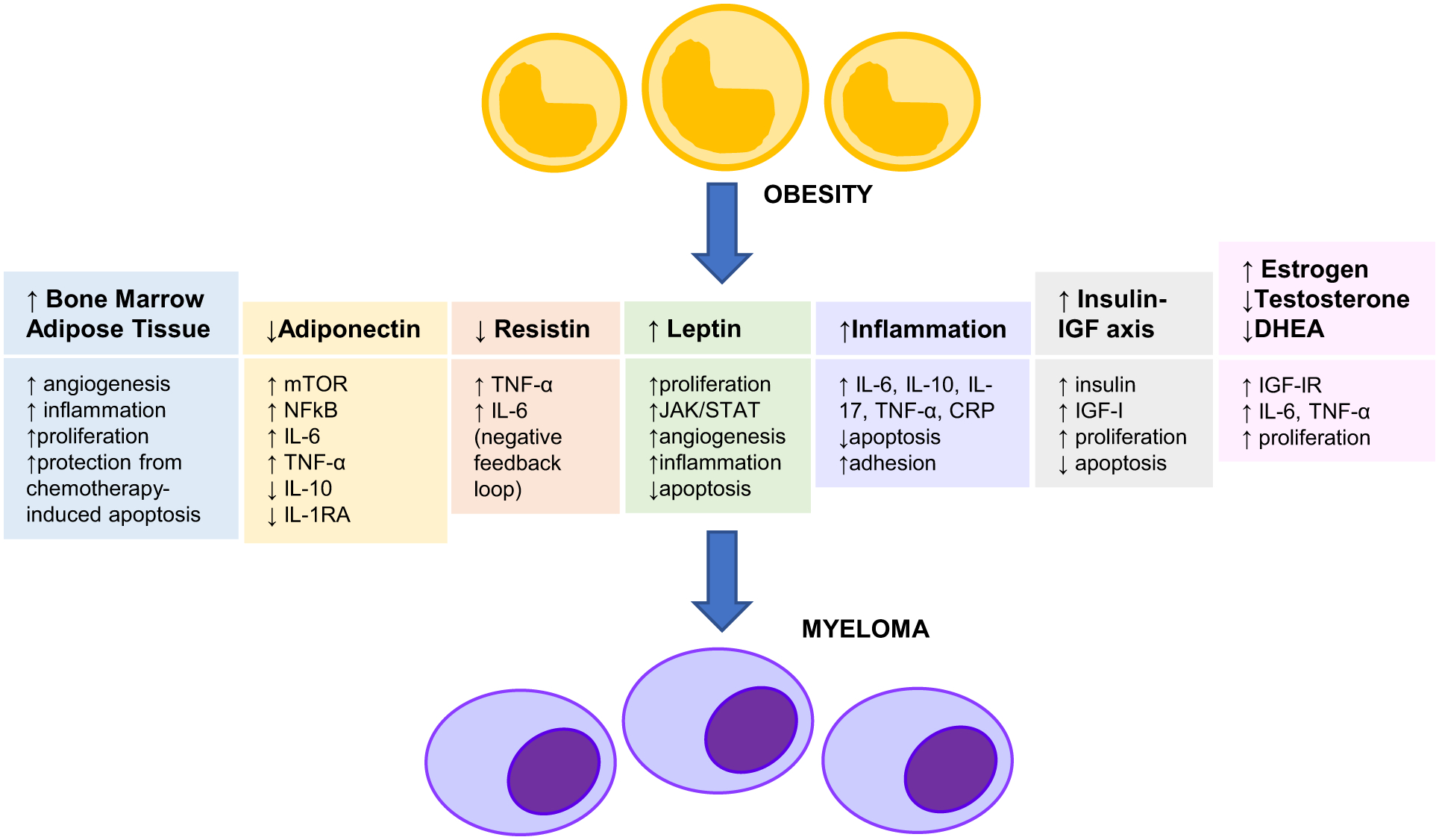
Role of Obesity in The Pathogenesis of Multiple Myeloma mTOR: Mechanistic/Mammalian Target of Rapamycin
JAK/STAT: Janus Kinase/Signal Transducer and Activator of Transcription
NFκB: Nuclear Transcription Factor κB IL-6: Interleukin-6
IL-10: Interleukin-10
IL-17: Interleukin-17
IL-1RA: Interleukin-1 Receptor Antagonist
TNF-α: Tumor Necrosis Factor-α
CRP: C-Reactive Protein
IGF-I: Insulin-like Growth Factor-I
IGF-IR: Insulin-like Growth Factor-I Receptor
Role of Visceral Adipocytes
Adipocytes are considered a major endocrine organ secreting adipokines and inflammatory factors (39). Adipocytes from overweight and obese persons display an altered cytokine profile when compared to normal weight persons due to increased production of inflammatory markers and leptin and decreased production of anti-inflammatory cytokines and adiponectin. The resultant enhanced inflammatory response has been postulated to increase genomic instability, disturb DNA repair, induce epigenetic changes and impact tumor progression (40, 41). Human MM cell lines were co-cultured with differentiated human adipocytes of normal, overweight, obese or super obese patients undergoing elective liposuction. This study demonstrated increased growth of MM cell lines exposed to all adipocyte conditioned media irrespective of BMI status, increased adhesion in MM cells exposed to obese/super obese adipocytes and increased tube formation in endothelial cells with overweight/obese/super obese adipocytes (42). Adipocytes have shown the ability to induce angiogenesis through secretion of angiogenic factors, adipokines (leptin) and cytokines (IL-6) and have thus emerged as potential targets for therapy (43). A recent study revealed that in obese MM patients, over-expression of acetyl-CoA synthetase 2 (ACSS2) induced by adipocyte-secreted cytokine angiotensin II, leads to stabilization of interferon regulatory factor 4 (IRF4) protein and upregulation of IRF4 transcriptional activity, thereby promoting myelomagenesis (44).
Role of Bone Marrow Adipocytes
Adipocytes are the major cellular component of the human bone marrow (BM) comprising up to 70% of the marrow volume and >10% of the total adipose mass in a healthy lean adult (45). While much of the emphasis on adiposity and MM focused on white adipose tissue (WAT), bone marrow adipose tissue (BMAT) is a newly recognized endocrine organ which is distinct from WAT. BMAT responds to growth hormones, insulin, thyroid hormone, release cytokines (IL-6, IL-1β, TNF-α, IGF-I) and adipokines (leptin, adiponectin) (46) and can influence neighboring cells in the bone microenvironment via autocrine, paracrine and endocrine signaling (47). BMAT has recently been found to contribute to oncogenesis and disease progression in MM by potentially influencing cell metabolism, angiogenesis, immune action and inflammation (48). Higher BMI correlates with higher level of BMAT, which in turn can provide a favorable microenvironment for MM cell growth (49, 50). BM adipocytes isolated from femoral biopsies of MM patients were shown to support MM growth in vitro and possibly protect MM cells from chemotherapy-induced apoptosis (51, 52). Increased BM angiogenesis has been associated with poor clinical outcomes in MM patients (53).
Role of Adiponectin
Adiponectin is a polypeptide hormone with anti-proliferative, anti-inflammatory and insulin sensitizing properties (54). Obese individuals have lower circulating levels of adiponectin as compared to normal weight individuals even though adiponectin is known to be mainly secreted by visceral adipose tissue (55) and decreased circulating adiponectin levels have been proposed to contribute to myelomagenesis in obese individuals. Individuals with higher adiponectin levels in a case-control study (73MM cases and 73 gender and age matched controls) had lower risk of MM (Q2: OR 0.44, Q3: OR 0.25 and Q4: OR 0.06 across quartiles of adiponectin) (56). Similarly, another study showed an inverse association between circulating levels of total and high molecular weight (HMW) adiponectin and subsequent MM risk in a prospective study of 174 MM patients and 348 controls within the PLCO Cancer Screening Trial (highest vs lowest quartile: OR 0.49 Ptrend 0.03 for total adiponectin and OR 0.44 Ptrend 0.01 for HMW adiponectin) (57). Another pooled investigation of 624 MM cases and 1 246 individually matched controls from seven cohorts participating in the MM Cohort Consortium revealed that higher total adiponectin levels were associated with reduced MM risk (highest vs lowest quartile: OR 0.64 Ptrend 0.001), particularly among overweight (OR 0.41 Ptrend <0.001) and obese individuals (OR 0.41 Ptrend 0.039) (58). They also measured circulating adiponectin levels in 213 patients (84 MGUS, 104 SMM and 25 MM) and found that adiponectin levels were significantly lower among patients with smoldering MM (SMM)/MM when compared to those with MGUS (16% lower for SMM and 20% lower for MM) (59). These findings suggest that higher circulating adiponectin levels in the pre-diagnostic stage may be protective against MM development. L-4F, an apolipoprotein peptide mimetic when used to augment serum concentration of HMW adiponectin in obese mice, improved bone mass by stimulating osteoblasts (60). Another study in MM bearing mice showed similar effects of L-4F on MM bone disease along with reduction in tumor burden and improved survival (61). These studies (Table 3) suggested that decreased host-derived adiponectin promotes myeloma growth and osteolysis and increasing adiponectin levels have a potential benefit in reducing the risk of MM and associated bone disease.
Table 3:
Obesity and Adipokines
| Cohort/ Location | Study Design | N | Outcome | BMI (kg/m2) | Effect Estimate | 95% CI | Study |
|---|---|---|---|---|---|---|---|
| Obesity and adiponectin | |||||||
| Veterans’ Administration General Hospital of Athens, Greece (NIMTS) | Case-Control study | 73 MM and 73 gender and age matched controls | Adiponectin level and MM risk | (Quartiles of adiponectin)Q1: OR 1.0 | Dalamaga et al Cancer Causes Control 2009 | ||
| Q2: OR 0.44 | 0.18–1.07 | ||||||
| Q3: 0.25 | 0.09–0.68 | ||||||
| Q4 : 0.06 | 0.01–0.25 | ||||||
| Prostate, Lung, Colorectal and Ovarian (PLCO)Cancer Screening Trial | Prospective study | 174 MM patients and 348 controls | Total Adiponectin levels and MM risk | (highest vs lowest quartile) OR 0.49 | 0.26–0.93 | Hofmann et al Blood 2012 | |
| HMW Adiponectin level and MM risk | OR 0.44 | 0.23–0.85 | |||||
| MM Cohort Consortium | Prospective study | 624 MM cases and 1246 individually matched controls | Adiponectin levels and MM risk | Overall | OR 0.64 | 0.47–0.85 | Hofmann et al Cancer Research 2016 |
| <25 | OR 1.2 | 0.73–2.0 | |||||
| 25–29.9 | OR 0.41 | 0.26–0.65 | |||||
| ≥30 | OR 0.41 | 0.17–0.98 | |||||
| NCT01109407 and NCT01402284 clinical studies | Observational study | 213 patients (84 MGUS, 104 SMM, 25 MM) | Adiponectin levels compared to MGUS | - | SMM: 16% lower | −31% to 2% | Hofmann et al Obesity 2017 |
| MM: 20% lower | −40% to 7% | ||||||
| Obesity and resistin | |||||||
| Veterans’ Administration General Hospital of Athens, Greece, (NIMTS) | Case-Control study | 73 MM and 73 gender and age matched controls | Resistin levels and MM risk | (Quartiles of resistin) Q1: OR 1.0 | Dalamaga et al Cancer Causes Control 2009 | ||
| Q2: OR 0.30 | 0.11–0.80 | ||||||
| Q3: OR 0.13 | 0.04–0.39 | ||||||
| Q4: 0.03 | 0.01–0.18 | ||||||
| MM Cohort Consortium | Prospective study | 178 MM cases and 358 individually matched controls | Resistin levels and MM risk | (Quartiles of resistin) Q1: OR 1 | Santo et al BJC 2017 | ||
| Q2: OR 0.77 | 0.43–1.38 | ||||||
| Q3: OR 0.44 | 0.24–0.83 | ||||||
| Q4: OR 0.54 | 0.29–0.995 | ||||||
| Obesity and leptin | |||||||
| Veterans’ Administration General Hospital of Athens, Greece, (NIMTS) | Case-Control study | 73 MM and 73 gender and age matched controls | Leptin levels and MM risk | (Quartiles of leptin) Q1: OR 1.0 | Dalamaga et al Cancer Causes Control 2009 | ||
| Q2: OR 1.65 | 0.59–4.44 | ||||||
| Q3: OR 0.99 | 0.35–2.86 | ||||||
| Q4: 2.09 | 0.79–5.52 | ||||||
Abbreviations - BMI: Body-Mass Index; 95%CI: 95% Confidence Interval; MGUS: Monoclonal Gammopathy of Undetermined Significance; MM: Multiple Myeloma; OR: Odds Ratio
One of the proposed biological mechanisms to explain this inverse association between adiponectin levels and MM risk is that adiponectin activates adiponectin receptors expressed by normal and MM cells (62) which in turn activate AMP kinase leading to mechanistic/mammalian target of rapamycin (mTOR) inhibition and downregulation of oxidative phosphorylation (63). The anti-inflammatory effects of adiponectin may be explained by inhibition of nuclear transcription factor κB (NF-κβ) and pro-inflammatory cytokine secretion (IL-6 or TNF-α) and induced expression of anti-inflammatory cytokines (IL-10 or IL-1 receptor antagonist) by adipocytes (64, 65). Experimental studies conducted in vitro and in animal models have also demonstrated adiponectin induced MM cell apoptosis (62).
Role of Resistin
Resistin is an adipokine that was initially shown to induce insulin resistance in rodents (66) and data linking obesity, resistin levels and MM risk in humans is controversial (Table 3). Some studies have found elevated resistin levels correlate with body fat mass (67) while others have found no association (68, 69). Human resistin is thought to be expressed at higher levels in circulating blood monocytes (70) and increases nuclear factor κB-related monocytic secretion of pro-inflammatory cytokines such as IL-6 (71, 72). Resistin is considered an inflammatory factor associated with other inflammatory markers (56). A prospective case-control study (178 MM cases and 358 individually matched controls from three cohorts participating in the MM cohort consortium) demonstrated a statistically significant inverse association between resistin levels and MM risk (OR 0.44 and OR 0.54 for third and fourth quartiles respectively vs the lowest quartile; Ptrend 0.03) among men (73). Lower resistin levels have been associated with increased MM risk. Thus, this has been postulated to reflect a compensatory negative feedback effect on the resistin pathway following production of IL-6, TNF-α and/or other cytokines that have known effects on proliferation and survival of MM cells (56).
Inflammatory cytokines
Obesity is a state of chronic inflammation associated with high levels of several inflammatory cytokines produced primarily by the adipose tissue - IL-6, C-reactive protein (CRP), and TNF-α (74). IL-6 levels have correlated with BM plasmacytosis, serum lactate dehydrogenase, beta 2 microglobulin and calcium in MM (75) as well as shorter survival (76–78). IL-6 also inhibits apoptosis in myeloma cells and promotes survival (79), and activates the Janus Kinase/Signal Transducer and Activator of Transcription (JAK/STAT3), phosphatidylinositol 3-kinase/protein kinase B (PI3K/AKT), mitogen-activated protein kinase/extracellular signal-regulated kinase (MAPK/ERK) pathways which are known to increase production of anti-apoptotic proteins like myeloid cell leukemia-1 (Mcl-1), B-cell lymphoma – extra-large (Bcl-xL) and c-Myc in MM cells promoting survival and resistance to chemotherapy (80). In addition, IL-6 is also postulated to cause drug resistance through epigenetic modulation proteins, and it enhances DNA methyltransferase-1 thereby promoting methylation and deactivation of p53, facilitating MM cells to avoid apoptosis (80). IL-6 is thought to be secreted by plasma cells by an autocrine mechanism and through interactions between adhesion molecules on MM plasma cells and their respective receptors on BM stromal cells via a paracrine mechanism (81).
CRP is a polypeptide protein secreted by hepatocytes in response to IL-6 and has been used as an indicator of IL-6 production. A phase II trial of IL-1 receptor antagonist (IL-1Ra) and low-dose dexamethasone for patients with SMM or indolent MM who were at risk of progression to active MM demonstrated statistically significant improvement in PFS and OS in those with reduction in high sensitivity CRP (hs-CRP) (defined as ≥40% decrease in hs-CRP at 6 months when compared to baseline value) versus those without a reduction in CRP (median PFS: 104 months vs 11 months; median OS: not reached vs 7.9 years) (82). In that study, the authors hypothesized that IL-1Ra would inhibit IL-1 mediated production of IL-6 and MM cell growth and reduction in hs-CRP indicated successful targeting of IL-1/IL-6 pathway. In another study, elevated pre-transplant CRP (> upper limit of normal i.e. 8mg/L) was found to have an independent negative prognostic impact on post-transplant survival on multivariate analysis (HR 2.0; 95%CI 1.0–3.8) in MM patients undergoing delayed ASCT (83). In addition, there is evidence to suggest that CRP binds activating FCγ receptors, activates PI3K/AKT, MAPK/ERK and NF-κβ pathways, and inhibits caspase cascade activation induced by chemotherapy drugs and synergizes with IL-6 to protect MM cells from chemotherapy induced apoptosis (84).
TNF-α is a potent inflammatory mediator. TNF-α mRNA and protein are expressed by MM plasma cells (85). TNF-α induces expression of adhesion molecules on MM cell lines and on BM stromal cells leading to increased binding of MM cells to BM stromal cells and increased IL-6 secretion (86).
Role of Leptin
Leptin is a protein coded by the ‘obese’ gene, is mainly secreted by white adipocytes, is an important regulator of caloric intake and metabolic functions and correlates positively with body fat mass (87, 88). Leptin receptor is thought to be structurally and functionally similar to IL-6 receptors (87) and to some hematopoietic growth factor receptors (56). Several variants of leptin receptors with cytoplasmic domains of different lengths have been detected in different tissues. Pre-clinical studies have demonstrated that full-length leptin receptors can mediate activation of the JAK/STAT pathway and stimulate transcription through IL-6 responsive gene elements with a specificity like IL-6 receptors (89). There is evidence to support that leptin induces IL-6 expression in a concentration and time dependent manner by activation of leptin receptor which in turn activates insulin receptor substrate-1 (IRS-1), PI3K/AKT, activator protein 1 (AP-1) pathways (90, 91). Leptin is also thought to have proliferative and anti-apoptotic effects in T-lymphocytes, leukemic cells, hematopoietic progenitor cells, influences angiogenesis and stimulates cytokine secretion from T-lymphocytes and monocytes (87, 88). Newly diagnosed MM patients had higher leptin levels compared with healthy controls (56, 92), (Table 3). Furthermore, leptin increased expression of genes implicated in cell growth, viability and intracellular signaling and stimulated proliferation in myeloma cell lines suggesting leptin maybe a potential therapeutic target (92, 93).
Insulin and IGF axis
Obesity and diabetes mellitus (DM) are known hyperinsulinemic states and hyperinsulinemia has been linked to increased cancer risk (20, 94). IGF-I levels are less consistently elevated in obese/diabetic patients than insulin levels thereby suggesting that insulin is mainly implicated in the increased cancer risk (95). Insulin is a potent growth and survival factor for human MM cell signaling through hybrid receptors (96). MM cell lines express IGF-I, IGF-II and insulin receptors and respond to IGFs by evading apoptosis via distinct intracellular apoptotic pathways (96, 97). Insulin and IGFs protect MM cells from dexamethasone induced apoptosis and thus may play a role in maintenance of the malignant clone (97). However, the NHANES study analyzed associations between various obesity-related metabolic biomarkers in relation to MGUS prevalence, including C-peptide, insulin and glucose, but did not find significant associations (7). Metformin, an oral hypoglycemic agent commonly used in DM, lowers circulating levels of glucose and insulin by promoting uptake of glucose in the tissues and decreasing hepatic gluconeogenesis. Metformin use for ≥4 years has been associated with 53% lower risk of transformation of MGUS to MM in a cohort of diabetic U.S. veterans (98) possibly by interruption of the insulin and IGF receptor signaling pathways.
Sex Hormones
Rising BMI is associated with increased levels of estrone, estradiol and free estradiol in postmenopausal women via increased aromatase activity (99). There is considerable biologic interplay between sex hormone signaling and obesity related pathways associated with increased MM risk, for example, expressions of estrogen receptor (ER) and IGF-I receptor (IGF-IR) have been shown to correlate with each other (100), estrogen increases IGF-IR levels (101) and IGF-I in turn enhances ER responsiveness to estrogen (102). Estradiol administration can also stimulate secretion of cytokines including TNF-α (103). Obesity is associated with decreased circulating total and free testosterone levels in men possibly due to lower sex hormone binding globulins (SHBG) and genuine hypothalamic-pituitary-testicular axis suppression respectively (104). Low testosterone levels are found in most MM patients as well (105). Several cross-sectional studies have found a statistically significant negative correlation between plasma free dehydroepiandrosterone (DHEA) levels and measures of total adiposity (BMI, body fat mass and %fat) in both men and women (106). There is evidence to suggest that DHEA directly inhibited MM proliferation and IL-6 production by BM stromal cells (107). We thereby, postulate a possible association between lower circulating DHEA levels in obese individuals and increased MM risk.
Conclusion and Future Directions:
In conclusion, this review outlines the current associations between obesity and MM. Obesity has been shown to increase the risk of MM and the malignant transformation of MGUS to MM. Similarly, not surprisingly, obesity is also associated with increased MM mortality. Obesity, associated with increased visceral and bone marrow adipose tissue, is thought to contribute to myelomagenesis by increased levels of leptin, inflammatory cytokines like IL-6 and TNF-α, increased insulin and IGF levels as well as decreased circulating levels of adiponectin. The effect of obesity on outcomes in MM patients is not completely understood but may be clarified with additional studies investigating the influence of weight and body composition throughout the disease trajectory.
Obesity is a particularly interesting risk factor for MM because it is the only known risk factor that has the potential to be prevented or modified. However, prospective trials with lifestyle interventions promoting weight loss or improved body composition are warranted to clarify the degree to which altering these exposures impacts disease pathogenesis and outcomes. Studies to evaluate the feasibility of sustained weight loss and its impact on quality of life would also be essential in the light of possible detrimental effects of weight cycling on MM risk. In addition, broader pre-clinical studies to further characterize the complex molecular pathways by which obesity influences myelomagenesis can possibly provide potential exciting targets for treatment.
Acknowledgements
This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748 and supported by the American Society of Hematology Clinical Research Training Institute Award, TREC Training Workshop R25CA203650, International Myeloma Society and Paula and Rodger Riney Foundation CDA, Parker Institute of Cancer Immunotherapy CDA and NCI K12 CDA (U.A.S). This study was funded in part by the National Institutes of Health (F32 CA220859, K22 CA251648) and the American Cancer Society (PF-17-231-01-CCE). This research was also supported by a Stand Up To Cancer Dream Team Research Grant (Grant Number: SU2C-AACR-DT28-18). Stand Up To Cancer is a program of the Entertainment Industry Foundation. Research grants are administered by the American Association for Cancer Research, the scientific partner of Stand Up To Cancer. Opinions, interpretations, conclusions and recommendations are those of the author(s) and are not necessarily endorsed by Stand Up To Cancer, the Entertainment Industry Foundation, or the American Association for Cancer Research (C.R.M.).
Competing Interests:
U.A.S. has received research funding from Celgene/Bristol Myers Squibb, Janssen to her institution and honorariums for continuing medical education activity from the Physicians Education Resource, all outside of the submitted work. R.P., S.M.T. and C.R.M declare no competing financial interests.
Contributor Information
Richa Parikh, University of Arkansas for Medical Sciences, Myeloma Center, Little Rock, AR, USA..
Syed Maaz Tariq, Jinnah Sindh Medical University, Karachi City, Sindh, Pakistan..
Catherine R. Marinac, Division of Population Sciences, Dana-Farber Cancer Institute, Harvard Medical School, Boston, MA, USA..
Urvi A. Shah, Myeloma Service, Department of Medicine, Memorial Sloan Kettering Cancer Center, 1275 York Avenue, New York City, NY 10065, USA..
References:
- 1.Kumar SK, Callander NS, Alsina M, Atanackovic D, Biermann JS, Castillo J, et al. NCCN Guidelines Insights: Multiple Myeloma, Version 3.2018. J Natl Compr Canc Netw. 2018;16(1):11–20. [DOI] [PubMed] [Google Scholar]
- 2.Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of Obesity and Severe Obesity Among Adults: United States, 2017–2018. NCHS data brief. 2020(360):1–8. [PubMed] [Google Scholar]
- 3.Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of Obesity Among Adults and Youth: United States, 2015–2016. NCHS data brief. 2017(288):1–8. [PubMed] [Google Scholar]
- 4.Sung H, Siegel RL, Rosenberg PS, Jemal A. Emerging cancer trends among young adults in the USA: analysis of a population-based cancer registry. The Lancet Public health. 2019;4(3):e137–e47. [DOI] [PubMed] [Google Scholar]
- 5.Marinac CR, Birmann BM. Rising cancer incidence in younger adults: is obesity to blame? The Lancet Public health. 2019;4(3):e119–e20. [DOI] [PubMed] [Google Scholar]
- 6.Landgren O, Rajkumar SV, Pfeiffer RM, Kyle RA, Katzmann JA, Dispenzieri A, et al. Obesity is associated with an increased risk of monoclonal gammopathy of undetermined significance among black and white women. Blood, The Journal of the American Society of Hematology. 2010;116(7):1056–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Landgren O, Graubard BI, Katzmann JA, Kyle RA, Ahmadizadeh I, Clark R, et al. Racial disparities in the prevalence of monoclonal gammopathies: a population-based study of 12,482 persons from the National Health and Nutritional Examination Survey. Leukemia. 2014;28(7):1537–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thordardottir M, Lindqvist EK, Lund SH, Costello R, Burton D, Korde N, et al. Obesity and risk of monoclonal gammopathy of undetermined significance and progression to multiple myeloma: a population-based study. Blood Adv. 2017;1(24):2186–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang S-H, Luo S, Thomas TS, O’Brian KK, Colditz GA, Carlsson NP, et al. Obesity and the transformation of monoclonal gammopathy of undetermined significance to multiple myeloma: a population-based cohort study. JNCI: Journal of the National Cancer Institute. 2017;109(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Veld J, O’Donnell EK, Reagan MR, Yee AJ, Torriani M, Rosen CJ, et al. Abdominal adipose tissue in MGUS and multiple myeloma. Skeletal radiology. 2016;45(9):1277–83. [DOI] [PubMed] [Google Scholar]
- 11.Samanic C, Gridley G, Chow WH, Lubin J, Hoover RN, Fraumeni JF, Jr. Obesity and cancer risk among white and black United States veterans. Cancer causes & control : CCC. 2004;15(1):35–43. [DOI] [PubMed] [Google Scholar]
- 12.Blair CK, Cerhan JR, Folsom AR, Ross JA. Anthropometric characteristics and risk of multiple myeloma. Epidemiology. 2005:691–4. [DOI] [PubMed] [Google Scholar]
- 13.Reeves GK, Pirie K, Beral V, Green J, Spencer E, Bull D. Cancer incidence and mortality in relation to body mass index in the Million Women Study: cohort study. BMJ (Clinical research ed). 2007;335(7630):1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Engeland A, Tretli S, Hansen S, Bjørge T. Height and body mass index and risk of lymphohematopoietic malignancies in two million Norwegian men and women. American journal of epidemiology. 2007;165(1):44–52. [DOI] [PubMed] [Google Scholar]
- 15.Söderberg KC, Kaprio J, Verkasalo PK, Pukkala E, Koskenvuo M, Lundqvist E, et al. Overweight, obesity and risk of haematological malignancies: a cohort study of Swedish and Finnish twins. European journal of cancer (Oxford, England : 1990). 2009;45(7):1232–8. [DOI] [PubMed] [Google Scholar]
- 16.Brown LM, Gridley G, Pottern LM, Baris D, Swanso CA, Silverman DT, et al. Diet and nutrition as risk factors for multiple myeloma among blacks and whites in the United States. Cancer Causes Control. 2001;12(2):117–25. [DOI] [PubMed] [Google Scholar]
- 17.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet (London, England). 2008;371(9612):569–78. [DOI] [PubMed] [Google Scholar]
- 18.Troy JD, Hartge P, Weissfeld JL, Oken MM, Colditz GA, Mechanic LE, et al. Associations between anthropometry, cigarette smoking, alcohol consumption, and non-Hodgkin lymphoma in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. American journal of epidemiology. 2010;171(12):1270–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hofmann JN, Moore SC, Lim U, Park Y, Baris D, Hollenbeck AR, et al. Body mass index and physical activity at different ages and risk of multiple myeloma in the NIH-AARP diet and health study. American journal of epidemiology. 2013;177(8):776–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Birmann BM, Andreotti G, De Roos AJ, Camp NJ, Chiu BCH, Spinelli JJ, et al. Young Adult and Usual Adult Body Mass Index and Multiple Myeloma Risk: A Pooled Analysis in the International Multiple Myeloma Consortium (IMMC). Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2017;26(6):876–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marinac CR, Birmann BM, Lee IM, Rosner BA, Townsend MK, Giovannucci E, et al. Body mass index throughout adulthood, physical activity, and risk of multiple myeloma: a prospective analysis in three large cohorts. British journal of cancer. 2018;118(7):1013–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marinac CR, Suppan CA, Giovannucci E, Song M, Kværner AS, Townsend MK, et al. Elucidating Under-Studied Aspects of the Link Between Obesity and Multiple Myeloma: Weight Pattern, Body Shape Trajectory, and Body Fat Distribution. JNCI cancer spectrum. 2019;3(3):pkz044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Samanic C, Chow WH, Gridley G, Jarvholm B, Fraumeni JF Jr. Relation of body mass index to cancer risk in 362,552 Swedish men. Cancer causes & control : CCC. 2006;17(7):901–9. [DOI] [PubMed] [Google Scholar]
- 24.Britton JA, Khan AE, Rohrmann S, Becker N, Linseisen J, Nieters A, et al. Anthropometric characteristics and non-Hodgkin’s lymphoma and multiple myeloma risk in the European Prospective Investigation into Cancer and Nutrition (EPIC). Haematologica. 2008;93(11):1666–77. [DOI] [PubMed] [Google Scholar]
- 25.Fernberg P, Odenbro A, Bellocco R, Boffetta P, Pawitan Y, Zendehdel K, et al. Tobacco use, body mass index, and the risk of leukemia and multiple myeloma: a nationwide cohort study in Sweden. Cancer research. 2007;67(12):5983–6. [DOI] [PubMed] [Google Scholar]
- 26.Kanda J, Matsuo K, Inoue M, Iwasaki M, Sawada N, Shimazu T, et al. Association of anthropometric characteristics with the risk of malignant lymphoma and plasma cell myeloma in a Japanese population: a population-based cohort study. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2010;19(6):1623–31. [DOI] [PubMed] [Google Scholar]
- 27.Lu Y, Sullivan-Halley J, Henderson KD, Ma H, Horn-Ross PL, Reynolds P, et al. Anthropometric characteristics and multiple myeloma risk. Epidemiology. 2010;21(2):272–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pylypchuk RD, Schouten LJ, Goldbohm RA, Schouten HC, van den Brandt PA. Body mass index, height, and risk of lymphatic malignancies: a prospective cohort study. American journal of epidemiology. 2009;170(3):297–307. [DOI] [PubMed] [Google Scholar]
- 29.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of US adults. New England Journal of Medicine. 2003;348(17):1625–38. [DOI] [PubMed] [Google Scholar]
- 30.Khan MM, Mori M, Sakauchi F, Matsuo K, Ozasa K, Tamakoshi A. Risk factors for multiple myeloma: evidence from the Japan Collaborative Cohort (JACC) study. Asian Pac J Cancer Prev. 2006;7(4):575–81. [PubMed] [Google Scholar]
- 31.Teras LR, Kitahara CM, Birmann BM, Hartge PA, Wang SS, Robien K, et al. Body size and multiple myeloma mortality: a pooled analysis of 20 prospective studies. Br J Haematol. 2014;166(5):667–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sonderman JS, Bethea TN, Kitahara CM, Patel AV, Harvey C, Knutsen SF, et al. Multiple Myeloma Mortality in Relation to Obesity Among African Americans. J Natl Cancer Inst. 2016;108(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beason TS, Chang SH, Sanfilippo KM, Luo S, Colditz GA, Vij R, et al. Influence of body mass index on survival in veterans with multiple myeloma. Oncologist. 2013;18(10):1074–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Williams A, Baruah D, Patel J, Szabo A, Chhabra S, Dhakal B, et al. Prevalence and significance of sarcopenia in multiple myeloma patients undergoing autologous hematopoietic cell transplantation. Bone marrow transplantation. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vogl DT, Wang T, Pérez WS, Stadtmauer EA, Heitjan DF, Lazarus HM, et al. Effect of obesity on outcomes after autologous hematopoietic stem cell transplantation for multiple myeloma. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2011;17(12):1765–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ershler R, Fernandes LL, Kanapuru B, Gwise T, Kluetz PG, Theoret MR, et al. FDA analysis: Impact of BMI on efficacy outcomes in multiple myeloma trials. Journal of Clinical Oncology. 2020;38(15_suppl):8543-. [Google Scholar]
- 37.Roy V, Swaika A, Kumar S, Mikhael J, Chanan-Khan A, Lacy M, et al. Influence of Obesity on Outcomes of Patients with Relapsed Refractory Multiple Myeloma. Clinical Lymphoma, Myeloma and Leukemia. 2017;17(1):e139–e40. [Google Scholar]
- 38.Caan BJ, Cespedes Feliciano EM, Kroenke CH. The Importance of Body Composition in Explaining the Overweight Paradox in Cancer-Counterpoint. Cancer research. 2018;78(8):1906–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nieman KM, Romero IL, Van Houten B, Lengyel E. Adipose tissue and adipocytes support tumorigenesis and metastasis. Biochimica et biophysica acta. 2013;1831(10):1533–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30(7):1073–81. [DOI] [PubMed] [Google Scholar]
- 41.Kidane D, Chae WJ, Czochor J, Eckert KA, Glazer PM, Bothwell AL, et al. Interplay between DNA repair and inflammation, and the link to cancer. Critical reviews in biochemistry and molecular biology. 2014;49(2):116–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bullwinkle EM, Parker MD, Bonan NF, Falkenberg LG, Davison SP, DeCicco-Skinner KL. Adipocytes contribute to the growth and progression of multiple myeloma: Unraveling obesity related differences in adipocyte signaling. Cancer letters. 2016;380(1):114–21. [DOI] [PubMed] [Google Scholar]
- 43.Hefetz-Sela S, Scherer PE. Adipocytes: impact on tumor growth and potential sites for therapeutic intervention. Pharmacology & therapeutics. 2013;138(2):197–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li Z, Liu H, He J, Wang Z, Yin Z, You G, et al. Acetyl-CoA Synthetase 2: A Critical Linkage in Obesity-Induced Tumorigenesis in Myeloma. Cell metabolism. 2021;33(1):78–93.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cawthorn WP, Scheller EL, Parlee SD, Pham HA, Learman BS, Redshaw CM, et al. Expansion of Bone Marrow Adipose Tissue During Caloric Restriction Is Associated With Increased Circulating Glucocorticoids and Not With Hypoleptinemia. Endocrinology. 2016;157(2):508–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Caers J, Deleu S, Belaid Z, De Raeve H, Van Valckenborgh E, De Bruyne E, et al. Neighboring adipocytes participate in the bone marrow microenvironment of multiple myeloma cells. Leukemia. 2007;21(7):1580–4. [DOI] [PubMed] [Google Scholar]
- 47.Rosen CJ, Ackert-Bicknell C, Rodriguez JP, Pino AM. Marrow fat and the bone microenvironment: developmental, functional, and pathological implications. Critical reviews in eukaryotic gene expression. 2009;19(2):109–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Falank C, Fairfield H, Reagan MR. Signaling Interplay between Bone Marrow Adipose Tissue and Multiple Myeloma cells. Frontiers in endocrinology. 2016;7:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pan SY, Johnson KC, Ugnat AM, Wen SW, Mao Y. Association of obesity and cancer risk in Canada. American journal of epidemiology. 2004;159(3):259–68. [DOI] [PubMed] [Google Scholar]
- 50.Wallin A, Larsson SC. Body mass index and risk of multiple myeloma: a meta-analysis of prospective studies. European journal of cancer (Oxford, England : 1990). 2011;47(11):1606–15. [DOI] [PubMed] [Google Scholar]
- 51.Caers J, Deleu S, Belaid Z, De Raeve H, Van Valckenborgh E, De Bruyne E, et al. Neighboring adipocytes participate in the bone marrow microenvironment of multiple myeloma cells. Leukemia. 2007;21(7):1580–4. [DOI] [PubMed] [Google Scholar]
- 52.Liu Z, Xu J, He J, Liu H, Lin P, Wan X, et al. Mature adipocytes in bone marrow protect myeloma cells against chemotherapy through autophagy activation. Oncotarget. 2015;6(33):34329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rajkumar SV, Leong T, Roche PC, Fonseca R, Dispenzieri A, Lacy MQ, et al. Prognostic value of bone marrow angiogenesis in multiple myeloma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2000;6(8):3111–6. [PubMed] [Google Scholar]
- 54.Roberts DL, Dive C, Renehan AG. Biological mechanisms linking obesity and cancer risk: new perspectives. Annual review of medicine. 2010;61:301–16. [DOI] [PubMed] [Google Scholar]
- 55.Greenberg AS, Obin MS. Obesity and the role of adipose tissue in inflammation and metabolism. The American journal of clinical nutrition. 2006;83(2):461s–5s. [DOI] [PubMed] [Google Scholar]
- 56.Dalamaga M, Karmaniolas K, Panagiotou A, Hsi A, Chamberland J, Dimas C, et al. Low circulating adiponectin and resistin, but not leptin, levels are associated with multiple myeloma risk: a case-control study. Cancer causes & control : CCC. 2009;20(2):193–9. [DOI] [PubMed] [Google Scholar]
- 57.Hofmann JN, Liao LM, Pollak MN, Wang Y, Pfeiffer RM, Baris D, et al. A prospective study of circulating adipokine levels and risk of multiple myeloma. Blood. 2012;120(22):4418–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hofmann JN, Birmann BM, Teras LR, Pfeiffer RM, Wang Y, Albanes D, et al. Low Levels of Circulating Adiponectin Are Associated with Multiple Myeloma Risk in Overweight and Obese Individuals. Cancer research. 2016;76(7):1935–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hofmann JN, Mailankody S, Korde N, Wang Y, Tageja N, Costello R, et al. Circulating Adiponectin Levels Differ Between Patients with Multiple Myeloma and its Precursor Disease. Obesity (Silver Spring, Md). 2017;25(8):1317–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Peterson SJ, Drummond G, Kim DH, Li M, Kruger AL, Ikehara S, et al. L-4F treatment reduces adiposity, increases adiponectin levels, and improves insulin sensitivity in obese mice. Journal of lipid research. 2008;49(8):1658–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fowler JA, Lwin ST, Drake MT, Edwards JR, Kyle RA, Mundy GR, et al. Host-derived adiponectin is tumor-suppressive and a novel therapeutic target for multiple myeloma and the associated bone disease. Blood. 2011;118(22):5872–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Medina EA, Oberheu K, Polusani SR, Ortega V, Velagaleti GV, Oyajobi BO. PKA/AMPK signaling in relation to adiponectin’s antiproliferative effect on multiple myeloma cells. Leukemia. 2014;28(10):2080–9. [DOI] [PubMed] [Google Scholar]
- 63.Tanabe H, Fujii Y, Okada-Iwabu M, Iwabu M, Nakamura Y, Hosaka T, et al. Crystal structures of the human adiponectin receptors. Nature. 2015;520(7547):312–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ajuwon KM, Spurlock ME. Adiponectin inhibits LPS-induced NF-kappaB activation and IL-6 production and increases PPARgamma2 expression in adipocytes. American journal of physiology Regulatory, integrative and comparative physiology. 2005;288(5):R1220–5. [DOI] [PubMed] [Google Scholar]
- 65.Dalamaga M, Christodoulatos GS. Adiponectin as a biomarker linking obesity and adiposopathy to hematologic malignancies. Hormone molecular biology and clinical investigation. 2015;23(1):5–20. [DOI] [PubMed] [Google Scholar]
- 66.Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, et al. The hormone resistin links obesity to diabetes. Nature. 2001;409(6818):307–12. [DOI] [PubMed] [Google Scholar]
- 67.Yannakoulia M, Yiannakouris N, Blüher S, Matalas AL, Klimis-Zacas D, Mantzoros CS. Body fat mass and macronutrient intake in relation to circulating soluble leptin receptor, free leptin index, adiponectin, and resistin concentrations in healthy humans. J Clin Endocrinol Metab. 2003;88(4):1730–6. [DOI] [PubMed] [Google Scholar]
- 68.Lee JH, Chan JL, Yiannakouris N, Kontogianni M, Estrada E, Seip R, et al. Circulating resistin levels are not associated with obesity or insulin resistance in humans and are not regulated by fasting or leptin administration: cross-sectional and interventional studies in normal, insulin-resistant, and diabetic subjects. J Clin Endocrinol Metab. 2003;88(10):4848–56. [DOI] [PubMed] [Google Scholar]
- 69.Huang X, Yang Z. Resistin’s, obesity and insulin resistance: the continuing disconnect between rodents and humans. J Endocrinol Invest. 2016;39(6):607–15. [DOI] [PubMed] [Google Scholar]
- 70.Savage DB, Sewter CP, Klenk ES, Segal DG, Vidal-Puig A, Considine RV, et al. Resistin / Fizz3 expression in relation to obesity and peroxisome proliferator-activated receptor-gamma action in humans. Diabetes. 2001;50(10):2199–202. [DOI] [PubMed] [Google Scholar]
- 71.Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nature reviews Immunology. 2006;6(10):772–83. [DOI] [PubMed] [Google Scholar]
- 72.Lee S, Lee HC, Kwon YW, Lee SE, Cho Y, Kim J, et al. Adenylyl cyclase-associated protein 1 is a receptor for human resistin and mediates inflammatory actions of human monocytes. Cell metabolism. 2014;19(3):484–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Santo L, Teras LR, Giles GG, Weinstein SJ, Albanes D, Wang Y, et al. Circulating resistin levels and risk of multiple myeloma in three prospective cohorts. British journal of cancer. 2017;117(8):1241–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hosgood HD, Gunter MJ, Murphy N, Rohan TE, Strickler HD. The relation of obesity-related hormonal and cytokine levels with multiple myeloma and non-hodgkin lymphoma. Frontiers in oncology. 2018;8:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Solary E, Guiguet M, Zeller V, Casasnovas RO, Caillot D, Chavanet P, et al. Radioimmunoassay for the measurement of serum IL-6 and its correlation with tumour cell mass parameters in multiple myeloma. Am J Hematol. 1992;39(3):163–71. [DOI] [PubMed] [Google Scholar]
- 76.Lauta VM. A review of the cytokine network in multiple myeloma: diagnostic, prognostic, and therapeutic implications. Cancer. 2003;97(10):2440–52. [DOI] [PubMed] [Google Scholar]
- 77.Kyrtsonis MC, Dedoussis G, Zervas C, Perifanis V, Baxevanis C, Stamatelou M, et al. Soluble interleukin-6 receptor (sIL-6R), a new prognostic factor in multiple myeloma. British journal of haematology. 1996;93(2):398–400. [DOI] [PubMed] [Google Scholar]
- 78.Stasi R, Brunetti M, Parma A, Di Giulio C, Terzoli E, Pagano A. The prognostic value of soluble interleukin-6 receptor in patients with multiple myeloma. Cancer. 1998;82(10):1860–6. [PubMed] [Google Scholar]
- 79.Gadó K, Domján G, Hegyesi H, Falus A. Role of interleukin-6 in the pathogenesis of multiple myeloma. Cell biology international. 2000;24(4):195–209. [DOI] [PubMed] [Google Scholar]
- 80.Harmer D, Falank C, Reagan MR. Interleukin-6 Interweaves the Bone Marrow Microenvironment, Bone Loss, and Multiple Myeloma. Frontiers in endocrinology. 2018;9:788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Teoh G, Anderson KC. Interaction of tumor and host cells with adhesion and extracellular matrix molecules in the development of multiple myeloma. Hematology/oncology clinics of North America. 1997;11(1):27–42. [DOI] [PubMed] [Google Scholar]
- 82.Lust JA, Lacy MQ, Zeldenrust SR, Witzig TE, Moon-Tasson LL, Dinarello CA, et al. Reduction in C-reactive protein indicates successful targeting of the IL-1/IL-6 axis resulting in improved survival in early stage multiple myeloma. Am J Hematol. 2016;91(6):571–4. [DOI] [PubMed] [Google Scholar]
- 83.Chakraborty R, Muchtar E, Kumar SK, Buadi FK, Dingli D, Dispenzieri A, et al. Elevated pre-transplant C-reactive protein identifies a high-risk subgroup in multiple myeloma patients undergoing delayed autologous stem cell transplantation. Bone marrow transplantation. 2018;53(2):155–61. [DOI] [PubMed] [Google Scholar]
- 84.Yang J, Wezeman M, Zhang X, Lin P, Wang M, Qian J, et al. Human C-reactive protein binds activating Fcgamma receptors and protects myeloma tumor cells from apoptosis. Cancer Cell. 2007;12(3):252–65. [DOI] [PubMed] [Google Scholar]
- 85.Garrett IR, Durie BG, Nedwin GE, Gillespie A, Bringman T, Sabatini M, et al. Production of lymphotoxin, a bone-resorbing cytokine, by cultured human myeloma cells. The New England journal of medicine. 1987;317(9):526–32. [DOI] [PubMed] [Google Scholar]
- 86.Hideshima T, Chauhan D, Schlossman R, Richardson P, Anderson KC. The role of tumor necrosis factor alpha in the pathophysiology of human multiple myeloma: therapeutic applications. Oncogene. 2001;20(33):4519–27. [DOI] [PubMed] [Google Scholar]
- 87.Fantuzzi G, Faggioni R. Leptin in the regulation of immunity, inflammation, and hematopoiesis. Journal of leukocyte biology. 2000;68(4):437–46. [PubMed] [Google Scholar]
- 88.Brennan AM, Mantzoros CS. Drug Insight: the role of leptin in human physiology and pathophysiology--emerging clinical applications. Nature clinical practice Endocrinology & metabolism. 2006;2(6):318–27. [DOI] [PubMed] [Google Scholar]
- 89.Baumann H, Morella KK, White DW, Dembski M, Bailon PS, Kim H, et al. The full-length leptin receptor has signaling capabilities of interleukin 6-type cytokine receptors. Proc Natl Acad Sci U S A. 1996;93(16):8374–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yang WH, Liu SC, Tsai CH, Fong YC, Wang SJ, Chang YS, et al. Leptin induces IL-6 expression through OBRl receptor signaling pathway in human synovial fibroblasts. PloS one. 2013;8(9):e75551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tang CH, Lu DY, Yang RS, Tsai HY, Kao MC, Fu WM, et al. Leptin-induced IL-6 production is mediated by leptin receptor, insulin receptor substrate-1, phosphatidylinositol 3-kinase, Akt, NF-kappaB, and p300 pathway in microglia. J Immunol. 2007;179(2):1292–302. [DOI] [PubMed] [Google Scholar]
- 92.Reseland JE, Reppe S, Olstad OK, Hjorth-Hansen H, Brenne AT, Syversen U, et al. Abnormal adipokine levels and leptin-induced changes in gene expression profiles in multiple myeloma. European journal of haematology. 2009;83(5):460–70. [DOI] [PubMed] [Google Scholar]
- 93.Yu W, Cao DD, Li QB, Mei HL, Hu Y, Guo T. Adipocytes secreted leptin is a pro-tumor factor for survival of multiple myeloma under chemotherapy. Oncotarget. 2016;7(52):86075–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Colditz GA, Peterson LL. Obesity and Cancer: Evidence, Impact, and Future Directions. Clin Chem. 2018;64(1):154–62. [DOI] [PubMed] [Google Scholar]
- 95.Strickler HD, Wylie-Rosett J, Rohan T, Hoover DR, Smoller S, Burk RD, et al. The relation of type 2 diabetes and cancer. Diabetes technology & therapeutics. 2001;3(2):263–74. [DOI] [PubMed] [Google Scholar]
- 96.Sprynski AC, Hose D, Kassambara A, Vincent L, Jourdan M, Rossi JF, et al. Insulin is a potent myeloma cell growth factor through insulin/IGF-1 hybrid receptor activation. Leukemia. 2010;24(11):1940–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Xu F, Gardner A, Tu Y, Michl P, Prager D, Lichtenstein A. Multiple myeloma cells are protected against dexamethasone-induced apoptosis by insulin-like growth factors. British journal of haematology. 1997;97(2):429–40. [DOI] [PubMed] [Google Scholar]
- 98.Chang SH, Luo S, O’Brian KK, Thomas TS, Colditz GA, Carlsson NP, et al. Association between metformin use and progression of monoclonal gammopathy of undetermined significance to multiple myeloma in US veterans with diabetes mellitus: a population-based retrospective cohort study. The Lancet Haematology. 2015;2(1):e30–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.McTiernan A, Wu L, Chen C, Chlebowski R, Mossavar-Rahmani Y, Modugno F, et al. Relation of BMI and physical activity to sex hormones in postmenopausal women. Obesity (Silver Spring, Md). 2006;14(9):1662–77. [DOI] [PubMed] [Google Scholar]
- 100.Peyrat JP, Bonneterre J, Beuscart R, Djiane J, Demaille A. Insulin-like growth factor 1 receptors in human breast cancer and their relation to estradiol and progesterone receptors. Cancer research. 1988;48(22):6429–33. [PubMed] [Google Scholar]
- 101.Yu H, Shu XO, Li BD, Dai Q, Gao YT, Jin F, et al. Joint effect of insulin-like growth factors and sex steroids on breast cancer risk. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2003;12(10):1067–73. [PubMed] [Google Scholar]
- 102.Ellis MJ, Jenkins S, Hanfelt J, Redington ME, Taylor M, Leek R, et al. Insulin-like growth factors in human breast cancer. Breast Cancer Res Treat. 1998;52(1–3):175–84. [DOI] [PubMed] [Google Scholar]
- 103.Hosgood HD, Gunter MJ, Murphy N, Rohan TE, Strickler HD. The Relation of Obesity-Related Hormonal and Cytokine Levels With Multiple Myeloma and Non-Hodgkin Lymphoma. Front Oncol. 2018;8:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Grossmann M. Hypogonadism and male obesity: Focus on unresolved questions. Clin Endocrinol (Oxf). 2018;89(1):11–21. [DOI] [PubMed] [Google Scholar]
- 105.John S, Sharma N, Sborov DW, Williams N, Jones D, Benson DM, et al. Most multiple myeloma patients have low testosterone. Leuk Lymphoma. 2019;60(3):836–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tchernof A, Labrie F. Dehydroepiandrosterone, obesity and cardiovascular disease risk: a review of human studies. Eur J Endocrinol. 2004;151(1):1–14. [DOI] [PubMed] [Google Scholar]
- 107.Liu S, Ishikawa H, Li FJ, Ma Z, Otsuyama K, Asaoku H, et al. Dehydroepiandrosterone can inhibit the proliferation of myeloma cells and the interleukin-6 production of bone marrow mononuclear cells from patients with myeloma. Cancer research. 2005;65(6):2269–76. [DOI] [PubMed] [Google Scholar]


