Abstract
Systemic insulin administration evokes sympathoexcitatory actions but the mechanisms underlying these observations are unknown. We reported that insulin sensitizes the response of thin-fibre primary afferents, as well as the dorsal root ganglion (DRG) that subserve them, to mechanical stimuli. However, little is known about the effects of insulin on primary neuronal responses to chemical stimuli. TRPV1, whose agonist is capsaicin (CAP), is widely expressed on chemically-sensitive metaboreceptors and/or nociceptors. The aim of this investigation was to determine the effects of insulin on CAP-activated currents in small DRG neurons and CAP-induced action potentials in thin-fibre muscle afferents of normal healthy rodents. Additionally, we investigated whether insulin potentiates sympathetic nerve activity (SNA) responses to CAP. In whole cell patch-clamp recordings from cultured mice DRG neurons in vitro, the fold change in CAP-activated current from pre- to post-application of insulin (n=13) was significantly (P<0.05) higher than those with a vehicle control (n=14). Similar results were observed in single-fibre recording experiments ex vivo as insulin potentiated CAP-induced action potentials compared to vehicle controls (n=9 per group, P<0.05). Furthermore, insulin receptor blockade with GSK1838705, significantly suppressed the insulin-induced augmentation in CAP-activated currents (n=13) as well as the response magnitude of CAP-induced action potentials (n=9). Likewise, the renal SNA response to CAP after intramuscular injection of insulin (n=8) was significantly (P<0.05) greater compared to vehicle (n=9). The findings suggest that insulin potentiates TRPV1 responsiveness to CAP at the DRG and muscle tissue levels, possibly contributing to the augmentation in sympathoexcitation during activities such as physical exercise.
Keywords: primary sensory neuron, transient receptor potential vanilloid 1, hyperinsulinemia, group IV muscle afferents, exercise pressor reflex, chemical sensitization
Graphical Abstract
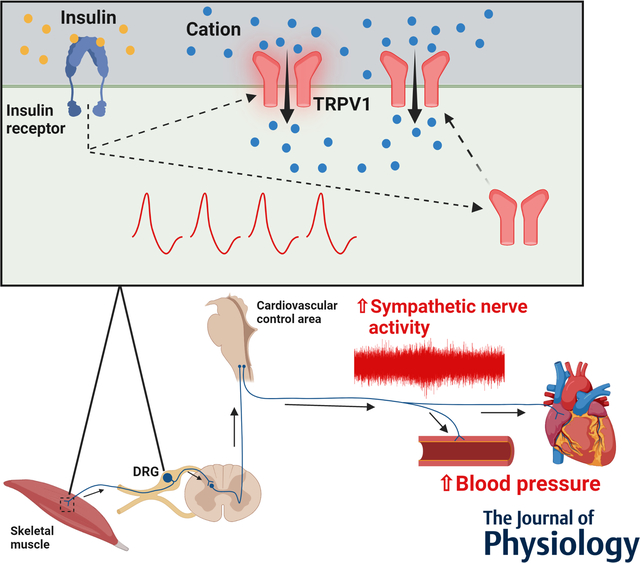
Insulin-induced enhancement of sympathetic nerve activity via sensitization of transient receptor potential vanilloid 1 (TRPV1) in small dorsal root ganglion (DRG) neurons and thin-fibre muscle afferents. Insulin increases neural discharge in response to capsaicin exposure, a TRPV1 agonist, in thin-fiber afferents at the level of the DRG in vitro and the skeletal muscle axon terminal ex vivo via sensitization of TRPV1. Further, in vivo studies demonstrate that intramuscular injection of insulin augments sympathetic nerve activity and blood pressure responses to intra-arterial injection of capsaicin. These findings suggest that insulin-induced sensitization of TRPV1 may contribute to the augmentation in sympathoexcitation during activities such as physical exercise. Design made in BioRender.
INTRODUCTION
It is well known that insulin, a major pancreatic hormone, not only plays a pivotal role in the regulation of glucose metabolism (Jensen & Richter, 2012), but also possesses the ability to influence brain function (Grote & Wright, 2016). For instance, insulin is involved in the regulation of the sympathetic nervous system within the brainstem (Morgan et al., 1993; Muntzel et al., 1994; Ward et al., 2011). Increasing evidence suggests that insulin centrally augments sympathetic nerve activity (Morgan et al., 1993; Muntzel et al., 1994; Ward et al., 2011; Mizuno et al., 2021). However, whether insulin contributes to peripherally (e.g. skeletal muscle reflexes) mediated sympathetic activation remains to be elucidated.
We previously demonstrated that insulin sensitizes the responsiveness of small dorsal root ganglion (DRG) neurons as well as thin-fibre muscle afferents to mechanical stimuli (Hotta et al., 2019a). This implies that insulin may evoke sympathoexcitation through a mechanically sensitive peripheral mechanism due to the fact that sensory information from primary afferents in the limbs evokes increases in sympathetic nerve activity. For example, in daily life, activation of mechanoreceptors in skeletal muscle during movement (Smith et al., 2006) and/or stimulation of nociceptors by noxious mechanical accidents on the skin (Burton et al., 2016), transmits afferent signals to the cardiovascular centers in the medulla oblongata that result in increased sympathetic outflow (Iwamoto et al., 1982; Person, 1989). However, little is known about the effect of insulin on DRG neurons or the thin fibre muscle afferents they subserve in response to chemical stimuli.
Transient receptor potential vanilloid 1 (TRPV1) (Julius et al., 1997) is widely expressed in small DRG neurons and thin-fibre afferents (Julius et al., 1997) that are nociceptors and/or metabolically sensitive skeletal muscle receptors. The purpose of this investigation was, therefore, to determine the effects of insulin on the capsaicin (CAP, a TRPV1 agonist)-activated whole-cell current response in small DRG neurons and CAP-induced action potentials in thin-fibre muscle afferents of normal healthy rodents. Furthermore, we investigated whether findings from in vivo and ex vivo experiments could be translated into in vivo conditions. Namely, we tested whether insulin has a substantial impact on CAP-evoked sympathetic nerve activity (SNA) in decerebrated rats.
MATERIALS AND METHODS
All studies were performed in accordance with the US Department of Health and Human Services NIH Guide for the Care and Use of Laboratory Animals. All experimental procedures were approved by the Animal Care Committee of Chubu University (#202120007, revision no. 21–038) and the Institutional Animal Care and Use Committee of the University of Texas Southwestern Medical Center (#2019-102849). All authors understood and conformed to the guidelines and ethical principles of the Journal of Physiology (Grundy, 2015).
The methods for making solutions, preparations, and recordings of single afferent fibre activity and whole cell current responses have been previously described (Kubo et al., 2012; Hotta et al., 2015, 2019b, 2019a).
Animals
Twenty-two male C57Bl/6J mice (3–6 weeks) whose body weight (BW) was approximately 12–24 g (Charles River, Yokohama, Japan) were used for whole-cell patch clamp recordings, and 100 male Sprague-Dawley rats(11 ± 3 weeks)whose BW were 352 ± 68 g (SLC, Shizuoka, Japan or Envigo, Indianapolis, IN) were used for single fibre recordings, immunohistochemistry, and in vivo experiments. Animals had free access to food and clean water. The animals were kept 1–4 per cage under a 12-hour light/dark cycle in an air-conditioned room (22–24°C) until required for experiments. The DRGs and muscle-nerve preparations were excised from mice and rats, respectively, that were euthanized with CO2.
Whole-cell patch clamp preparation
DRG culture
DRG neurons were prepared as previously reported (Kubo et al., 2012; Hotta et al., 2019a). Briefly, we collected DRGs from mice and digested DRGs with collagenase IV (1.0 mg/mL, Sigma, St Louis, MO, USA) for 30 min and trypsin-EDTA (0.05%, Sigma) for 20 min each at 37 °C. Then we terminated the enzyme reaction by using a trypsin inhibitor (0.08 mg/mL, Wako, Osaka, Japan) for 5 min at room temperature. We washed them with the Dulbecco’s modified Eagle’s medium (DMEM)/Ham’s F-12 (Wako) supplemented with nerve growth factor (0.1 μg/mL, NGF-7S, Sigma), fetal bovine serum (5%, Wako), Glutamax (1%, Invitrogen, Carlsbad, CA, USA), glucose (0.8%), penicillin-streptomycin (10 μL/mL, Sigma), and Dulbecco’s phosphate buffered saline (Sigma). We placed DRGs on glass coverslips coated with poly-L-lysine (0.1 mg/mL, Sigma) and laminin (4%, Invitrogen) after we suspended and dissociated them in the supplemented DMEM/Ham’s F-12 solution using a fire-polished Pasteur pipette. We maintained DRGs at 37 °C in a CO2 incubator replacing fresh supplemented DMEM at least every 2 days up to the day of current recording.
Whole-cell patch clamp recording and experimental procedure
We recorded inward currents and membrane voltages from cultured small DRG neurons (i.e., less than 30 μm in diameter) (Zhang et al., 2014) at room temperature as described previously (Kubo et al., 2012; Hotta et al., 2019a). The pipette solution contained (mM): NaCl 10, KCl 130, EGTA 1, MgCl2 1, HEPES 10, ATP 2 and GTP 0.2, adjusted to about pH 7.3 with 1 N KOH. We used HEPES-buffered solution containing (mM): NaCl 140, KCl 5, CaCl2 2, MgCl2 2, glucose 10, and HEPES 10, adjusted to approximately pH 7.4 with 1 N NaOH, as the extracellular bath solution.
The present study consisted of three application trials (vehicle control, insulin, insulin after pretreatment of insulin receptor antagonist), with only one trial applied to each neuron. In each trial, we first recorded CAP-activated current by exposing the recorded neuron to CAP (Sigma and Wako). Then we locally applied the following test solutions into the recording bath: 1) HEPES-buffered solution as vehicle control (control trial); or 2) 500 mU/mL of insulin (NovoRapid, Novo Nordisk, Tokyo, Japan) (insulin trial). We also pretreated the DRG neurons with 2–20 nM of an insulin receptor antagonist (GSK1838705, Tocris, Avonmouth, UK) 2–6 h before the insulin trial (GSK1838705 + insulin trial) to confirm the involvement of insulin receptors. The concentration of insulin used has been previously described (Rahmouni et al., 2004). Five min after the application of the test solutions, we recorded CAP-activated current to the same CAP stimulus again. The concentration of CAP we used was 1 μM and above (Tominaga et al., 1998). The concentration and exposure period of CAP were identical before and after the applications.
Currents and membrane voltages were recorded using Axopatch 200B (Axon Instruments, Inc., Foster City, CA, USA). Data were digitized and analyzed using Digidata 1550A, (Axon Instruments, Inc.) and Clampex software (Axon Instruments, Inc.). Neurons were defined as CAP sensitive if the response to CAP was greater than 50 pA (Xing et al., 2008). We calculated the total change transfer by using Clampfit software (Axon Instruments, Inc.) for comparison between three trials.
Muscle-nerve preparation
Single muscle group III and IV fibre recordings
Fifty rats (11 ± 3 weeks and BW: 358 ± 74 g) were used. These recordings were obtained using methods previously reported (Hotta et al., 2015, 2019b, 2019a; Ishizawa et al., 2020). We isolated the extensor digitorum longus (EDL) muscle along with the common peroneal nerve in a test chamber containing warmed modified Krebs-Henseleit solution (Krebs-buffer solution) superfused approximately at 34°C and pH 7.4. The solution contained (in mM): 110.9 NaCl, 4.7 KCl, 2.5 CaCl2, 1.2 MgSO4, 1.2 KH2PO4, 25.0 NaHCO3, and 20.0 glucose, and bubbled with a 5% CO2 + 95% O2 gas mixture. The common peroneal nerve was drawn into the recording chamber filled with paraffin oil. We repeatedly dissected the fibre using two sharpened watchmaker forceps and put one on a 0.1 mm φ gold recording electrode until we found a single group III or IV fibre.
We used fibre recordings in this experiment if the following three criteria were fulfilled (Taguchi et al., 2005): 1) the fibre responded to gentle probing by a smoothly rounded glass rod to the surface of EDL, 2) the increase in discharge rate in response to passive muscle stretching was intensity independent (intensity dependent discharge is a property typically observed in group I and II muscle spindle afferents that were not the focus of study), and 3) the conduction velocity of the fibre recorded was less than 2.0 m/s (characteristic of group IV fibres) and/or less than 15.0 m/s (characteristic of group III fibres) (Lawson & Waddell, 1991). We calculated conduction velocity from the distance and conduction latency between the stimulating electrodes on the receptive field and the recording electrode.
Changes in nerve activity voltage were continuously digitized using PowerLab 16/35 (ADInstruments, Sydney, NSW, Australia). Data were analyzed using Spike Histogram software (AD Instruments).
Experimental procedures and data analysis
After we identified a single group III or IV afferent fibre and its receptive field, we locally superfused 1 μM CAP (Sigma and Wako) for 30 s over the receptive field through a 3-mm-diameter metal tube as described previously (Taguchi et al., 2005; Hotta et al., 2015, 2019b; Ishizawa et al., 2020). We placed the opening of the tube as near to the receptive field as possible while still leaving space for the solution to flow to minimize dilution (Taguchi et al., 2005).
Then, we injected intramuscularly 5-μL of 1) Krebs-buffer solution as a vehicle control (control trial), 2) insulin (NovoRapid, Novo Nordisk) (0.5–5U/mL) (insulin trial) or 3) insulin (0.5–5U/mL) + GSK1838705 (40 nM) (insulin + GSK1838705 trial) near the receptive field using a microsyringe with a 30-gauge needle. The injection angle and depth were approximately 60–80° to the surface and 1 mm, respectively. Five, 10 and 20 min after the injection, we exposed the receptive field to the same CAP stimulus again.
Using methods previously described (Taguchi et al., 2005), we determined that the fibre responded to CAP when the change in the CAP-induced discharge rate fulfilled the following two criteria: 1) the net discharge rate exceeded 0.1 Hz during the 30 s stimulus period or post-stimulus period for 60 s, and 2) the instantaneous discharge rate of two consecutive discharges exceeded the mean plus 2 SD of the background activity before the stimulation. We defined the higher value in net increase of discharge rate at the period of 30-s CAP stimulus or at the period of 60-s post stimulus as the response magnitude before the injection. We also defined the maximal value of response magnitude at 5, 10, or 20 min after the injection as the representative value after the injection.
Fluorescent immunostaining
Three rats (19 weeks and BW: 518 ± 6 g) were transcardially perfused with saline followed by 4% paraformaldehyde for tissue fixation. The L4–6 DRGs were harvested and post-fixed overnight in 4% paraformaldehyde. The DRGs were stored in 10% and 20% sucrose, and afterwards sectioned at 10 μm using a cryostat. Triple immunostaining for TRPV1, insulin receptor, and the isolectin B4 (IB4) binding site in DRGs was performed by incubation with rabbit anti-TRPV1 [1:500, Cat#NB100–1617, RRID#AB_10002124, Novus Biological, Littleton, CO, USA] and mouse anti-Insulin Rβ [1:50, Cat#sc-57342, RRID#AB_784102, Santa Cruz Biotechnology, Santa Cruz, CA, USA] overnight at room temperature, after blocking with 10% normal goat serum / PBS plus 0.5% Triton X-100 for 1 h. The sections were rinsed in PBS and subsequently incubated with fluorescence-conjugated secondary antibodies (Alexa Fluor 405-conjugated goat anti-rabbit IgG [1:100, Cat#ab175652, RRID#AB_2687498, Abcam, Cambridge, MA, USA]; Cy3 goat anti-mouse IgG [1:50, Cat#115–165–146, RRID#AB_2338690, Jackson ImmunoResearch, West Grove, PA, USA]) and Alexa Fluor 488-conjugated IB4 [1:200, Cat# I21411, RRID#AB_2314662, Invitrogen] for 1 h at room temperature.
For quantitative analysis, 5 or 6 sections from each animal were arbitrarily selected (a total of 16 sections). The fluorescent images were observed and captured with a fluorescent microscope system (Axio Imager A2, ZEISS, Oberkochen, Germany). The number of positively stained small-diameter DRGs neurons (i.e. less than 30 μm in diameter) (Zhang et al., 2014) were counted and the percentage of insulin receptor- or IB4-positive neurons in TRPV1-positive neurons were calculated.
Recording of blood pressure and renal sympathetic activity in vivo
General surgical, and experimental procedures
Almost all surgical procedures were performed using methods previously described (Smith et al., 2001; Mizuno et al., 2016). Briefly, 47 rats (10 ± 1 weeks and BW: 335 ± 43 g). Rats were anesthetized with 1–4% isoflurane in 100% oxygen and subsequently mechanically ventilated after intubation. Sodium bicarbonate solution (8 mL 1 M NaHCO3 and 40 mL 5% dextrose in 152 mL Ringer solution) was continuously infused via the jugular vein at a rate of 3–5 ml/h/kg for stabilization of fluid balance and maintaining baseline arterial blood pressure (ABP). A left carotid arterial catheter connected to a pressure transducer (MLT0380/D, ADInstruments) was used for measuring ABP. Electrocardiograph (ECG) recordings were obtained using needle electrodes. To record renal SNA (RSNA), a branch of the left renal nerve was attached to bipolar electrodes (OT220–064a, Unique Medical, Osaka, Japan). For insulation and fixation, the nerve and electrodes were covered with silicone glue (Kwik-Sil, World Precision Instruments, Sarasota, FL, USA). The left common iliac artery was catheterized for CAP administration and the tip of the catheter advanced to the bifurcation of the abdominal aorta. For trapping injected drug in the hindlimb and avoiding exogenous insulin spill over into the systemic circulation, a reversible vascular occluder (FST, Foster City, CA, USA) was placed around the abdominal aorta and the inferior vena cava just above the aortic bifurcation. The right triceps surae muscles were exposed for intramuscular injection of insulin or saline used as a vehicle control. Finally, animals were placed on a stereotaxic head unit (David Kopf Instruments, Tujunga, CA, USA) and then, a precollicular decerebration procedure was performed for rendering the animals insentient. Isoflurane anesthesia was stopped immediately after the decerebration. To minimize brain edema, dexamethasone (0.2 mg) was given intravenously.
Following the minimum 1-h post decerebration recovery period, 0.1 mL of CAP (0.3 μg/100 μL) was administered into the arterial supply of the right leg 10 min after occluding the circulation of the hindlimb by the occluder. The occluder was released just after evaluating CAP-induced pressor responses. Blood was collected from the jugular and tail veins to assess blood glucose and plasma insulin levels. Then, a total of 30 μL of saline (vehicle control) or insulin (5 U/mL) was injected intramuscularly into six sites (5 μL/site) on the right triceps surae muscles using a microsyringe with a 30-gauge needle immediately after a 2nd occlusion of the hindlimb circulation. Ten min after the intramuscular injection of insulin or vehicle, CAP was injected as described above. Again, venous blood was drawn from the jugular and tail veins before releasing the occlusion.
After all of the experiments were conducted, hexamethonium bromide (60 mg/kg) was administered intravenously to validate that RSNA signals were recorded from postganglionic renal sympathetic fibres. We measured RSNA background noise over a 30-min period after the insentient decerebrated animal was humanely killed by intravenous injection of saturated potassium chloride (4 M, 2 ml/kg).
Data analysis
Data were analyzed as previously described (Mizuno et al., 2016). Briefly, RSNA, ABP and ECG signals were amplified, filtered, and continuously recorded on a computer via the PowerLab 16/35 (ADInstruments). Data analysis were performed using LabChart 8 application software (ADInstruments). RSNA was rectified, and mean arterial pressure (MAP) and heart rate (HR) were calculated from ABP and ECG recordings, respectively. 1-s averages of RSNA, MAP, and HR were used for each analysis. RSNA, MAP, and HR recorded for 30 s immediately before the onset of injection of CAP was used to determine baseline values. Baseline RSNA was designated as 100% and used to quantify RSNA responses to CAP stimulation. Subsequently, CAP-evoked changes in RSNA were expressed as a percentage of this baseline, and the relative changes in RSNA (ΔRSNA, %) from baseline were evaluated. The maximal MAP and HR responses to CAP were defined as the peak change from baseline (ΔMAP, mmHg, ΔHR, beats/min).
Measurements of blood glucose and plasma insulin
Blood glucose and plasma insulin levels were determined using a glucose meter (SARAYA, Osaka, Japan) and an ultrasensitive insulin ELISA kit (Morinaga Institute of Biological Science, Yokohama, Japan), respectively.
Statistical analysis
The Shapiro–Wilk test was first performed to confirm data normality. The unpaired t test or Mann-Whitney U test were used for pairwise comparisons as appropriate. In whole-cell patch clamp experiments, we compared the fold change in total charge transfer and response magnitude from before to after the application or injection between the three trials using one-way analysis of variance (ANOVA) with Bonferroni/Dunn multiple comparison test or the Kruskal-Wallis test with Mann‐Whitney U multiple comparison test with applied Bonferroni correction as appropriate. Regarding single fibre recording experiments, we used a Fisher’s exact test to compare the ratio of fibres responding to CAP. For analysis of ΔMAP, ΔHR, ΔRSNA, blood glucose, and plasma insulin levels obtained from in vivo experiments, data were analyzed by a two-way repeated measures ANOVA (trial-by-injection). If an interaction and/or a main effect was significant, then, Bonferroni/Dunn multiple comparison test was performed.
Statistical analyses were computed using Prism 5.0 (GraphPad Software, San Diego, CA, USA) and SPSS 25.0 for windows (IBM, Armonk, NY, USA) with the level of significance set at P < 0.05. All values are expressed as means ± SD.
RESULTS
Whole-cell patch clamp preparation
We used a total of 40 small DRG neurons which responded to CAP in the present study (φ = 21.4 ± 3.5 μm, range:15.3–29.2). The pipette-to-bath resistance was 6.5 ± 1.5 MΩ, and membrane potential was −52.1 ± 10.3 mV before exposure of CAP. Cell diameter and baseline membrane potential were not significantly different among the three trials (P = 0.186 and 0.218, respectively).
Figure 1 demonstrates representative CAP-activated inward currents. In 60.0% of all neurons recorded, the second CAP-activated current was reduced as compared with the first as shown in the upper and bottom panels of Fig. 1, a well known tachyphylactic phenomena. However, CAP-activated current increased after application of insulin in 53.8% of neurons (Fig. 1. middle panel, a 1.2-fold or greater increase was considered to be responsive). In GSK1838705 + insulin trials, total charge transfer after application was not increased but rather decreased (Fig. 1, bottom panel), similar to the control experiment (Fig. 1, upper panel). When we compared the effects of the different concentrations of GSK1838705 pretreatment (20 nM; n = 3 vs. 2 nM; n = 10), we did not observe any significant differences in the fold change from before to after insulin application (0.71 ± 0.16 vs. 0.64 ± 0.44, P = 0.674). As a result, we combined the data from the two concentration trials for analysis. Fold changes after the various applications are summarized in Fig. 2. As can be clearly seen, the insulin trial was significantly higher than control and GSK1838705 + insulin trials.
Figure 1. Sample recordings of capsaicin (CAP)-induced inward currents before and after the local application of HEPES-buffered solution (control), insulin, and insulin after pretreatment with an insulin receptor antagonist (GSK1838705 + insulin).
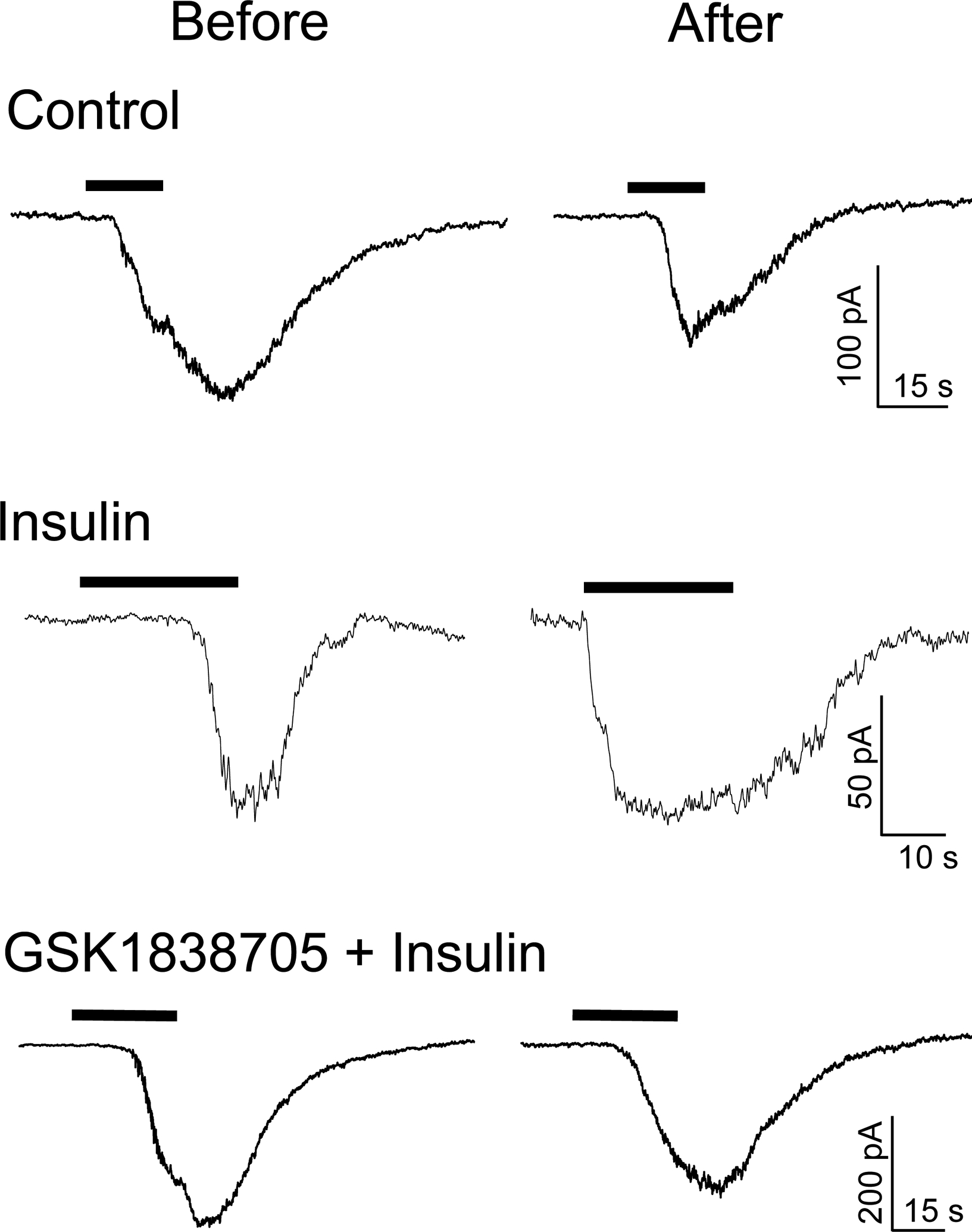
Black bars indicate the periods of exposure to 1 μM CAP. Each anteroposterior response was obtained from one small dorsal root ganglion neuron. In these experiments cell diameter for control, insulin, and GSK1838705 + insulin trials were 18.5, 18.3, 18.9 μm, respectively. Although control and GSK1838705 + insulin trials reduced the total charge transfers to 34% and 90% of those before application, respectively, the total charge transfer was increased by 296% after application in the insulin trial.
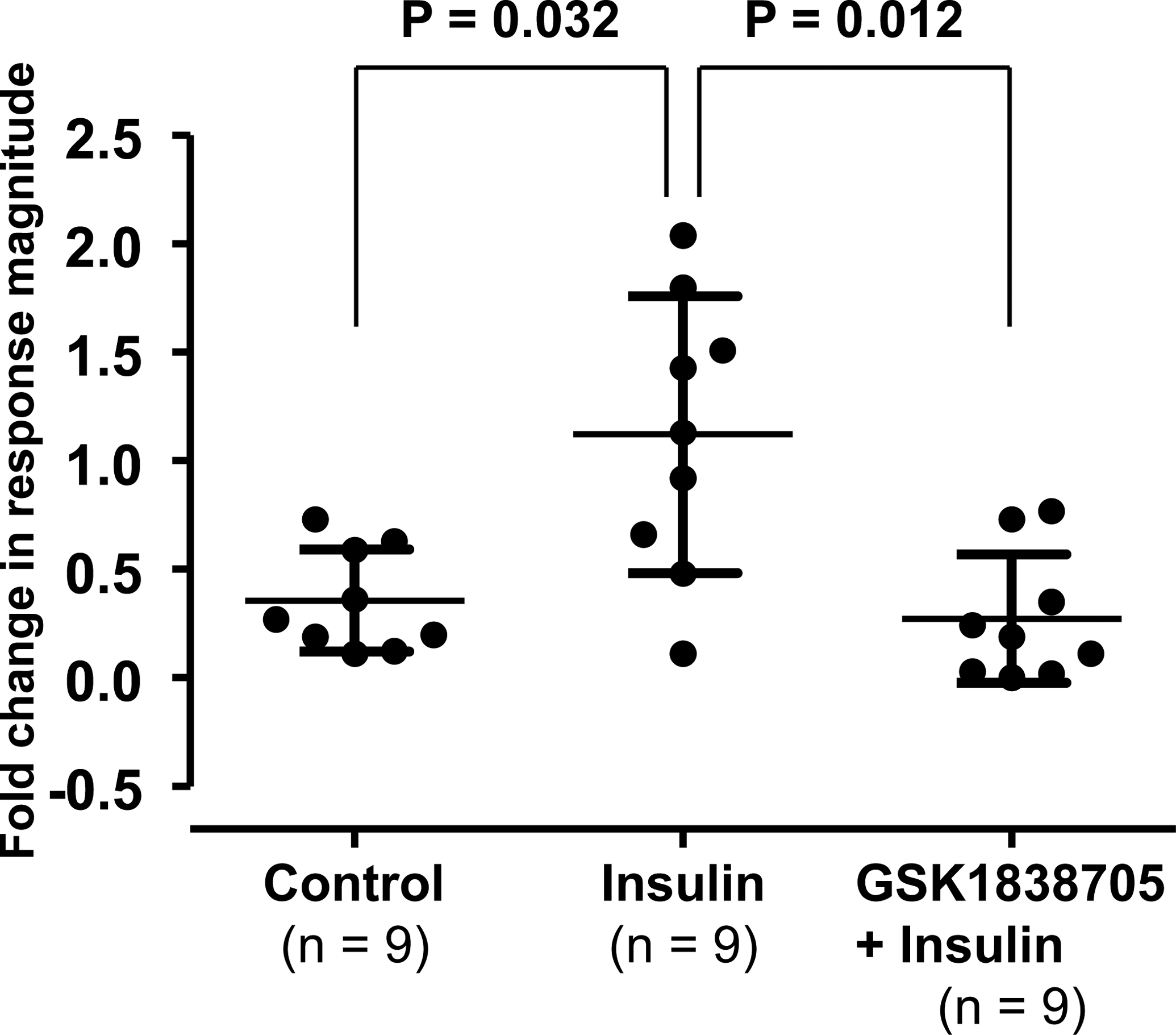
Figure 2. Fold change in total charge transfer in small dorsal root ganglion neurons from before to 5 min after the local application of HEPES-buffered solution (control), insulin, and insulin after pretreatment with an insulin receptor antagonist (GSK1838705 + insulin).
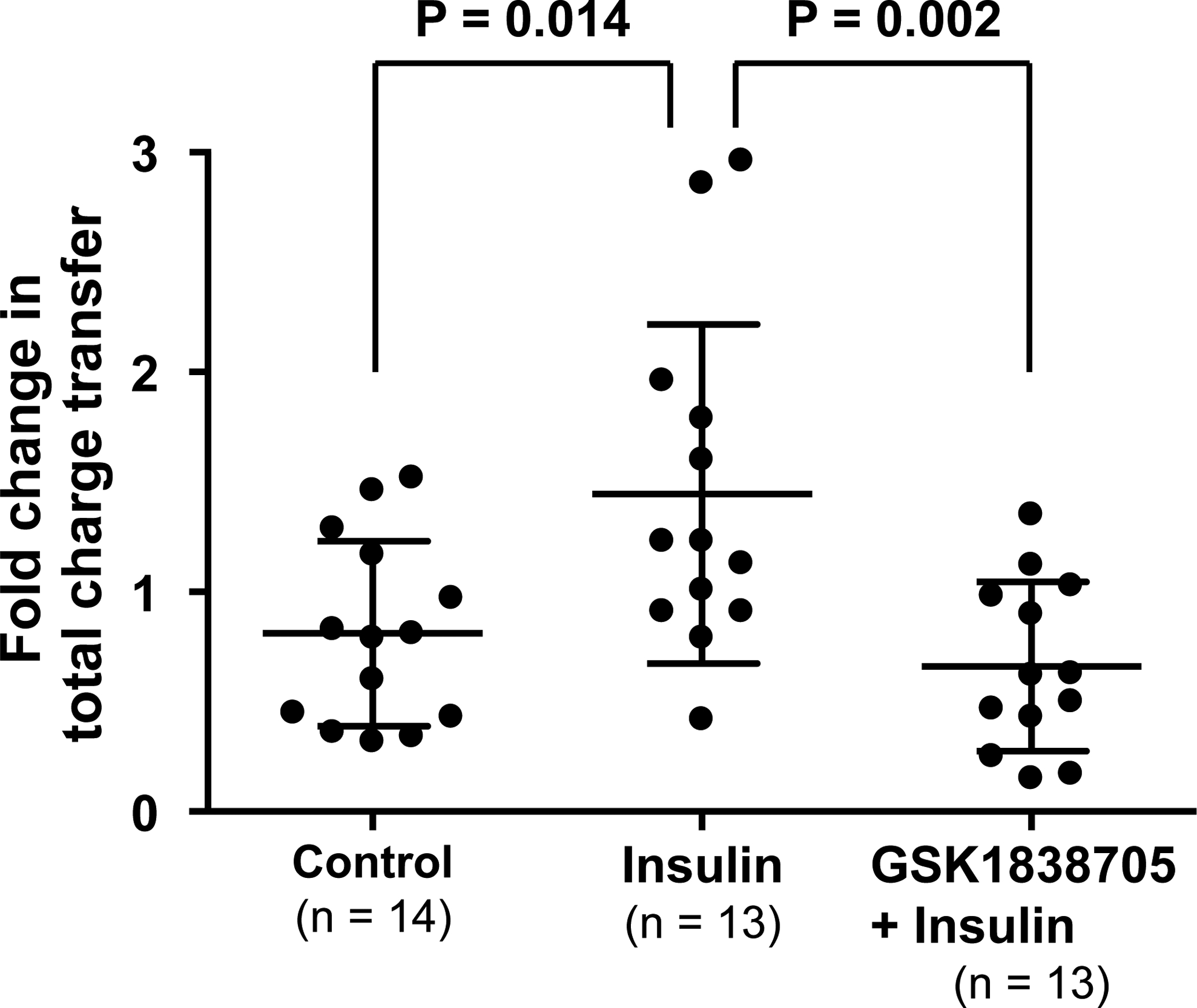
Means and SDs are shown. The data were analyzed by using a one-way ANOVA followed by Bonferroni/Dunn multiple comparison test.
Muscle-nerve preparation
We identified 64 group IV fibres. The conduction velocity and background activity were 0.79 ± 0.33 m/s and 0.20 ± 0.29 Hz, respectively. Twenty-seven of 64 fibres (42.2%) responded to CAP stimuli before injection of test solution. This result was in agreement with previous studies (Hotta et al., 2015, 2019b). Although 13 group III fibres (conduction velocity; 3.82 ± 2.76 m/s, background activity; 0.001 ± 0.002 Hz) were also identified, they were not found to be CAP-sensitive in the present study. Figure 3 shows the receptive fields of the group III and IV fibres recorded. Consistent with previous studies (Taguchi et al., 2005; Hotta et al., 2015, 2019b), most of the receptive fields were located around the musculotendinous junction.
Figure 3. Distribution of the receptive fields of group III and IV muscle afferent fibres.
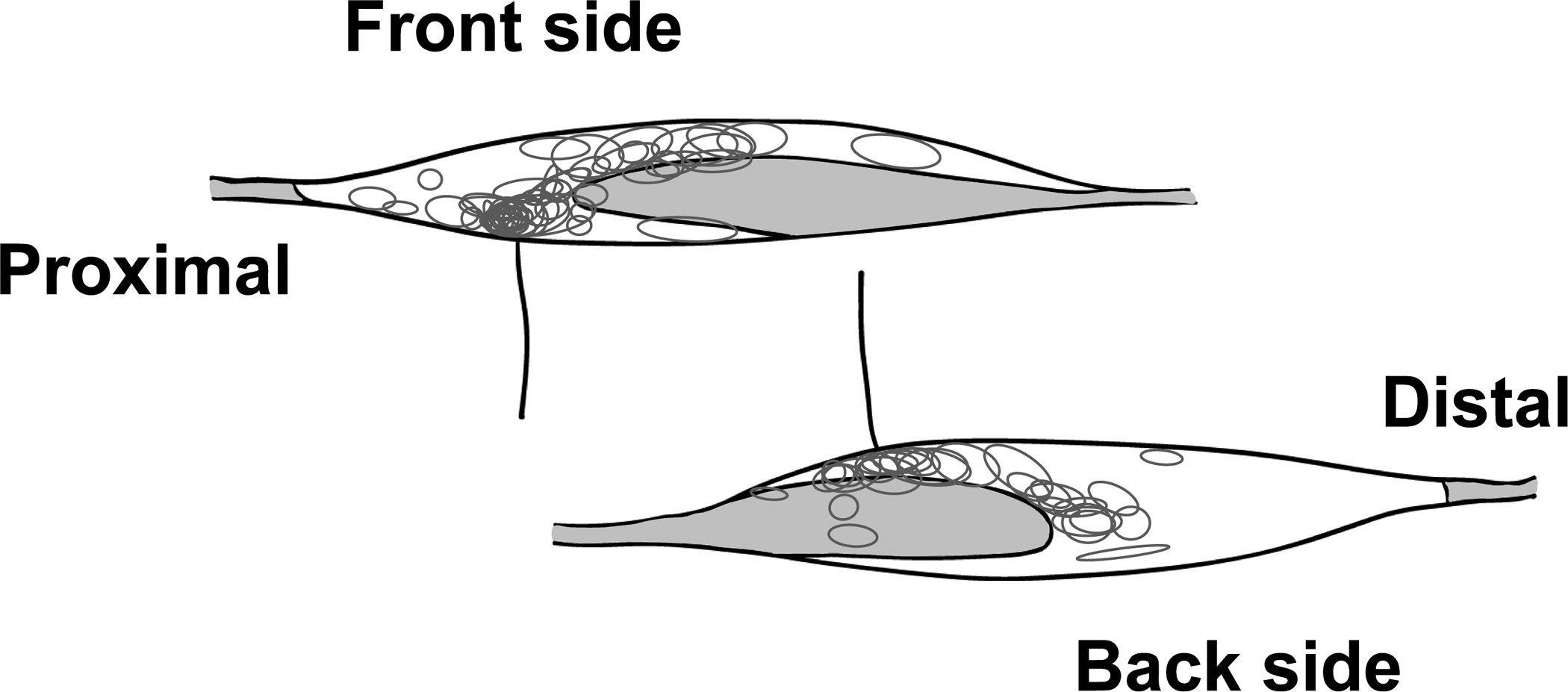
Each circle represents the receptive field of each thin-fibre muscle afferent observed. We defined the side facing the tibialis anterior as the front. The gray areas demonstrate the tendinous area.
Sample recordings of a CAP-sensitive fibres are shown in Fig. 4. Action potential discharges were observed during application of CAP and/or during the post-CAP stimulus period in CAP sensitive group IV fibres. Although in control and insulin + GSK1838705 trials, evoked discharges to a second exposure to CAP decreased (upper and bottom panels), the CAP-evoked discharge increased after the injection of insulin (middle panel). In insulin trials, two concentrations of insulin were utilized (0.5 U/mL; n = 5 vs. 5 U/mL; n = 4). We did not observe any significant differences in the fold change in response magnitude of the number of evoked discharges from before to after application between the concentrations (1.19 ± 0.85 vs.1.03 ± 0.33, P = 0.716). Hence, we combined the data from the two concentration trials for analysis. Figure 5 represents the fold change in response magnitude of the number of evoked discharges from pre- to post- injection of vehicle control, insulin and insulin + GSK1838705 in CAP-sensitive fibres. The magnitude of response to CAP before injection of each solution was not significantly different among the three trials (control, insulin, and GSK1838705 + insulin trials were 1.39 ± 1.34 Hz, 0.99 ± 0.94 Hz, 1.12 ± 0.95 Hz, respectively, P = 0.938). In 81.5% of all fibres measured, the response magnitude decreased by repetition of CAP exposure. When a 1.2-fold or greater increase was considered to be responsive, 44.4% of the fibres were affected by the injection of insulin. Importantly, the fold change in the response magnitude in the insulin trial was significantly higher than those of the control and GSK1838705 + insulin trials (Fig. 5).
Figure 4. Sample recordings of capsaicin (CAP)-evoked action potentials before and after the injection of Krebs-buffer solution (control), insulin, and the combination of an insulin receptor antagonist and insulin (GSK1838705 + insulin) in CAP-sensitive group IV muscle afferents.
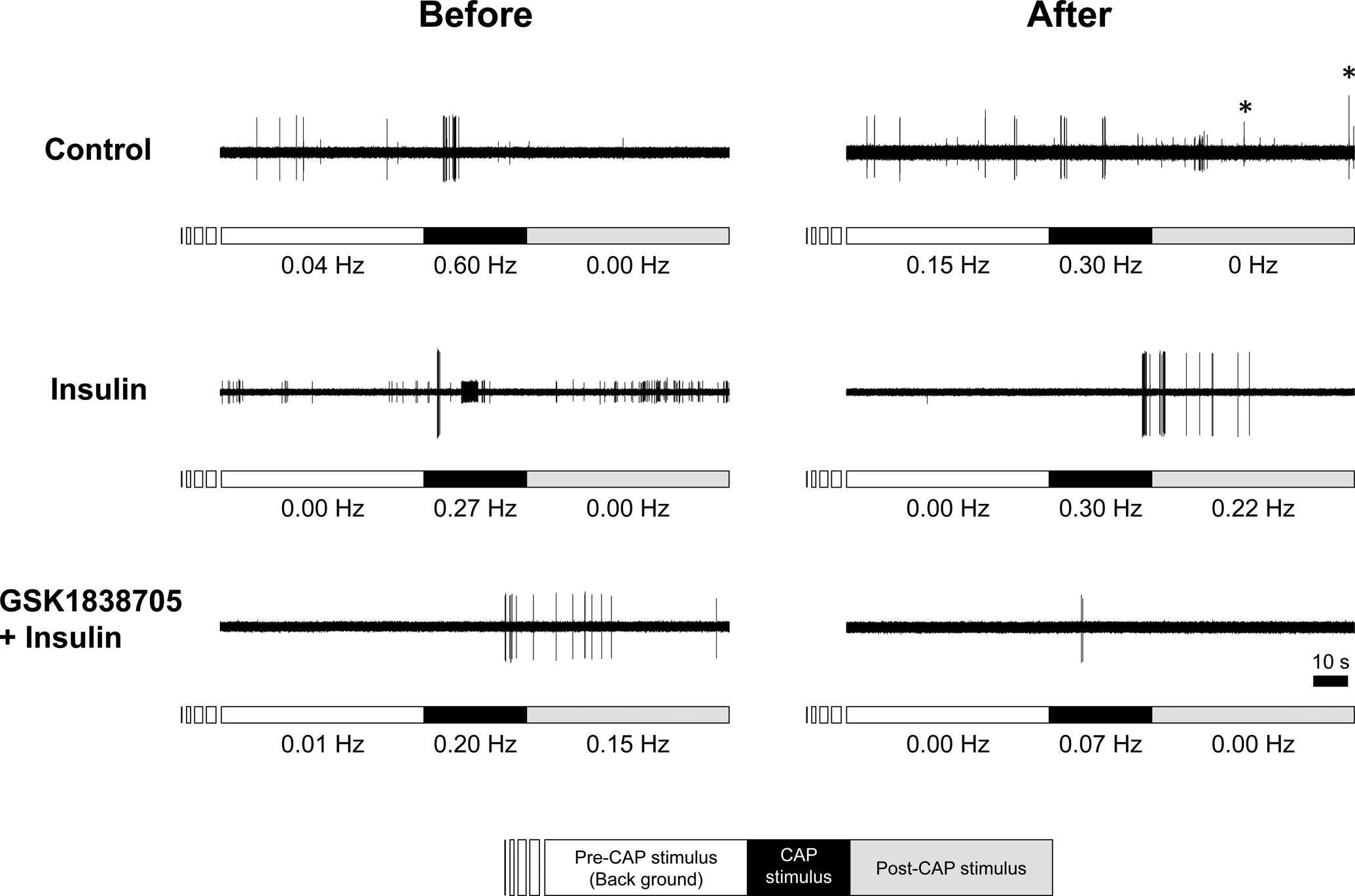
Bars just below the action potential recordings indicate the periods for measuring background activity (empty bar), period of exposure to 1 μM CAP for 30 s (black bar), and the post-stimulus period for 60 s (grey bar). The conduction velocity of these fibres for control, insulin, and GSK1838705 + insulin trials were 0.41, 0.88, and 0.84 m/s, respectively. The numbers shown below the bars were frequency of spike discharges. Voltage changes represented by asterisks identify untargeted action potentials. A number of small voltage changes observed in control and insulin trials also represent untargeted neural activity.
Figure 5. Fold change in response magnitude to capsaicin (CAP) before to after the intramuscular injection of Krebs-buffer solution (control), insulin, and the mixture of an insulin receptor antagonist and insulin (GSK1838705 + insulin) in CAP-sensitive group IV muscle afferents.
Means and SDs are shown. The data were analyzed by using the Kruskal-Wallis test with Mann‐Whitney U multiple comparison test with applied Bonferroni correction.
We observed that some CAP-insensitive group IV fibres responded to CAP after the injection of insulin as shown in Figure 6. Because no significant difference in the proportion of responsiveness to CAP between two insulin concentration trials was detected (71.4%, n = 7 in 0.5 U/mL trial vs. 37.5%, n = 8 in 5 U/mL trial, P = 0.315), we combined the data from the two concentration trials and treated as one insulin trial. Table 1 shows the proportion of CAP-insensitive group IV fibres which responded to CAP after the injection of either the vehicle control or insulin. Importantly, the proportion of fibres responsive to CAP was significantly different between two injection trials. This finding suggests that insulin converted CAP-insensitive group IV fibres to CAP-sensitive neurons. None of the 7 CAP-insensitive group IV fibres responded to CAP after injection of insulin + GSK1838705. We further examined the effects of injection of insulin on group III fibres; however, neither injection of insulin (n = 5), vehicle control (n = 5) nor insulin + GSK1838705 (n = 3) changed the responsiveness to CAP.
Figure 6. Typical examples where the injection of Krebs-buffer solution (control) or insulin induces capsaicin (CAP) sensitivity in fibres that were initially CAP- insensitive.
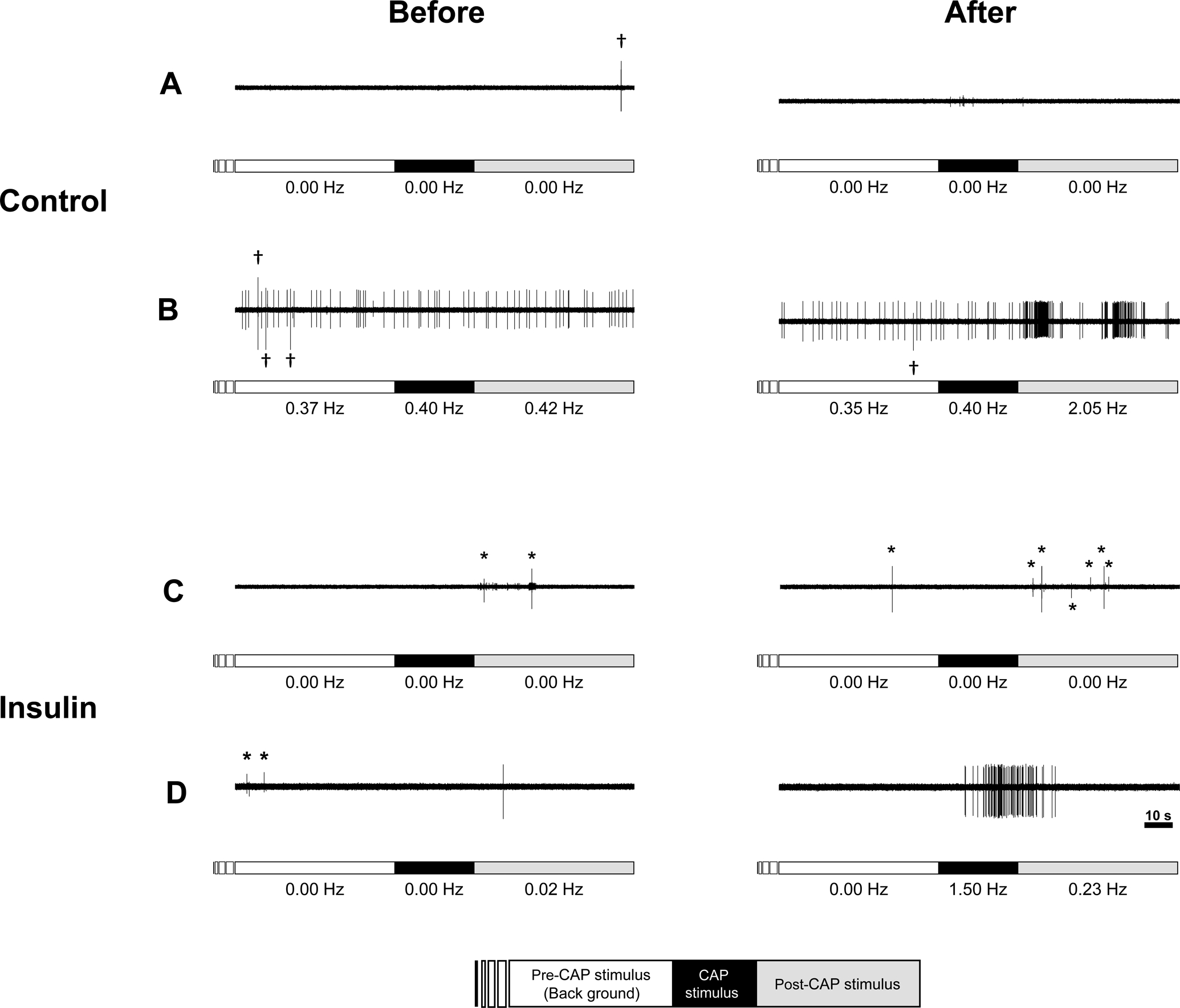
Bars just below the action potential recordings indicate the periods for measuring background activity (empty bar), period of exposure to 1 μM CAP for 30 s (black bar), and the post-stimulus period for 60 s (grey bar). Voltage changes represented by asterisks identify untargeted action potentials. Daggers show artifacts. The conduction velocity of these fibres for control (A and B) and insulin (C and D) were 0.67, 0.46, 0.89 and 1.62 m/s, respectively. While vehicle control (A) or insulin (C) did not change CAP sensitivity, CAP-insensitive group IV fibres which responded to CAP after the injection of either the vehicle control (B) or insulin (D).
Table 1.
Proportion of responsiveness to capsaicin (CAP) after the injection of Krebs-buffer solution (control) and insulin in CAP-insensitive group IV muscle afferents.
| Response to CAP stimulus | P value | ||
|---|---|---|---|
| Yes | No | ||
| Control (n = 15) | 1 (6.7%) | 14 (93.3%) | 0.014 |
| Insulin (n = 15) | 8 (53.3%) | 7 (46.7%) | |
Values are numbers of fibres (percentages). The P value was derived from a Fisher’s exact test.
Co-localization of TRPV1, insulin receptor and IB4 binding in L4-L6 DRGs neurons.
We randomly collected 16 sections of DRGs from 3 rats. Sample photos are shown in Figure 7A. Figure 7B and C shows that a total 28.7% of TRPV1-positive neurons examined expressed insulin receptors. Of these, 21.8% and 6.9% were IB4-positive and -negative neurons, respectively. 71.3% of TRPV1-positive neurons examined did not have any insulin receptors regardless of IB4 positivity or not.
Figure 7. Co-localization of transient receptor potential vanilloid 1 (TRPV1), insulin receptor (IR), and isolectin B4 (IB4) binding in L4-L6 dorsal root ganglion (DRG) neurons in rats.
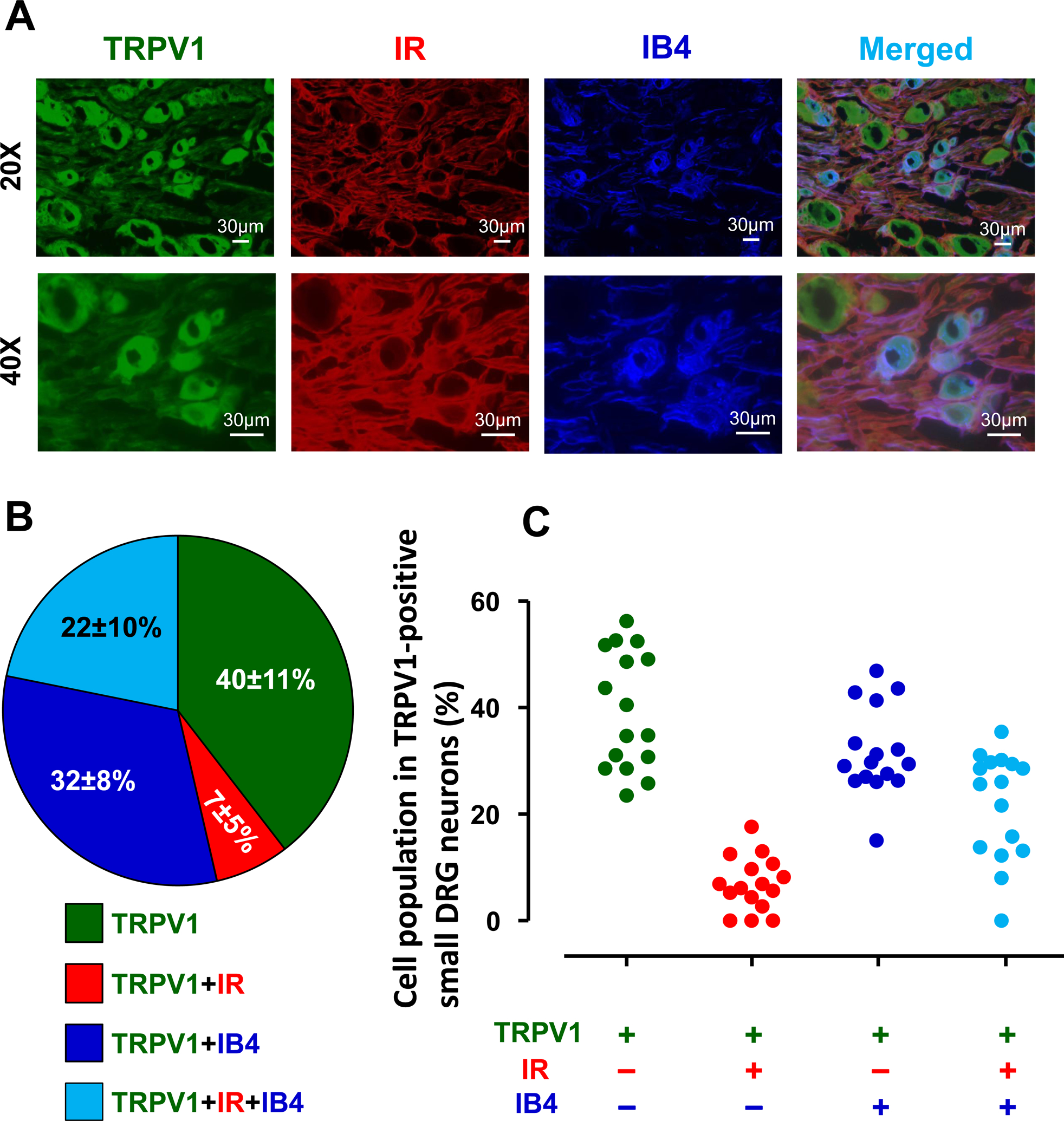
Representative images showing the expression of TRPV1, IR, and IB4-positive neurons in L4-L6 DRGs under 20X and 40X magnification (A). White bar = 30 μm. The percentages of IR and/or IB4-positive neruons in TRPV1-positive small DRG neurons (B). Means and SDs are shown. Individual data (C) obtained from distinct sections (n = 16) are also shown.
Sympathoexcitatory reflex in vivo
Figure 8A and B demonstrate the changes in blood glucose and plasma insulin levels, respectively, in the occluded circulation (samples were collected from the tail vein) and systemic circulation (samples were collected from the jugular vein) before and after the injection of insulin or vehicle. Blood glucose obtained from the tail vein was not significantly changed from before to after injection regardless of trial (vehicle, n=23; or insulin, n=27; Fig. 8A). However, plasma insulin in the tail vein was significantly increased by the intramuscular injection of insulin, but not vehicle (Fig. 8B). In addition, we succeeded in collecting 10 data sets of blood samples from the jugular vein (n = 5 in each trial). Importantly, neither the injection of insulin nor vehicle significantly altered blood glucose (Fig. 8A) or plasma insulin levels (Fig. 8B).
Figure 8. Blood glucose (A) and plasma insulin (B) obtained from the tail and jugular veins, and arterial blood pressure (ABP) and renal sympathetic nerve activity (RSNA) responses to capsaicin (CAP) stimulation (C and D) before and after the injection of saline (control) or insulin.
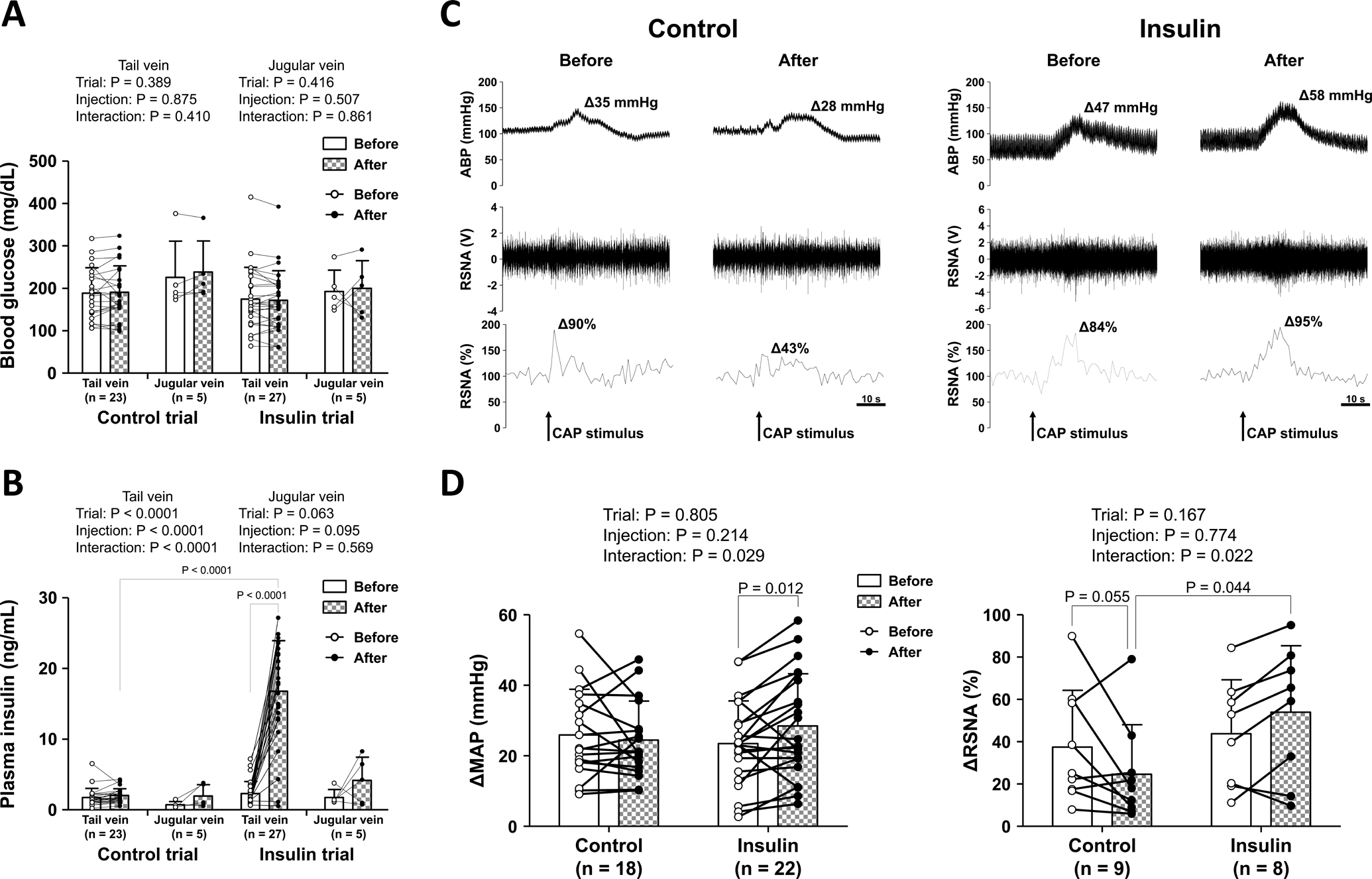
The open (before) and closed (after) circles represent individual data (A, B, and D). Means and SDs are also shown. The data were analyzed by a two-way repeated measures ANOVA (trial-by-injection) followed by Bonferroni/Dunn multiple comparison test (A, B, and D). Representative tracings of individual ABP (upper panel) and RSNA (raw data in middle panel, and relative rectified data in lower panel) responses to CAP before and after intramuscular injection of vehicle or insulin (C).
Figure 8C shows representative recordings of the ABP and RSNA responses to CAP before and after intramuscular injection of vehicle (left) or insulin (right). In the present study, we succeeded in measuring RSNA in 17 and MAP in 40 of 47 rats. Baseline MAP and RSNA (i.e. the signal to noise ratio of RSNA in the 30 s before injection of CAP) were not significantly different than the baseline values after intramuscular injection of vehicle or insulin (baseline MAP: 89.2 ± 29.6 mmHg in control trial [n = 18] vs. 92.1 ± 29.0 mmHg in insulin trial [n = 22], P = 0.882, signal to noise ratio of RSNA: 6.75 ± 5.68 in control trial [n = 9] vs. 3.44 ± 1.99 in insulin trial [n = 8], P = 0.132). However, baseline HR was significantly greater in the control trial than the insulin trial (500 ± 35 bpm in control trial [n = 18] vs. 468 ± 49 bpm in insulin trial [n = 22], P = 0.042). Notably, although the ΔMAP in response to CAP was not significantly changed before and after intramuscular injection of the vehicle, the injection of insulin significantly increased the CAP-induced ΔMAP (Fig. 8D, left). Furthermore, the CAP-induced ΔRSNA after intramuscular injection of insulin was significantly greater than that after injection of the vehicle (Fig. 8D, right). The ΔHR in response to CAP was not significantly different between trials and did not significantly change before and after intramuscular injection of vehicle or insulin (before: Δ1.9 ± 3.0 bpm, after: Δ1.4 ± 2.5 bpm in control trial vs. before: Δ3.3 ± 5.7 bpm, after: Δ4.8 ± 5.4 bpm in insulin trial, trial effect: P =0.070, injection effect: P = 0.434, interaction: P = 0.112).
DISCUSSION
The major novel findings from this investigation are as follows: 1) insulin significantly augmented the response to CAP in small DRG neurons derived from young mice in vitro, 2) insulin significantly sensitized group IV muscle afferents to CAP ex vivo; 3) the insulin receptor antagonist, GSK1838705, suppressed this sensitization at both the DRG and muscle tissue levels, suggesting a sensitizing mechanism in somatosensory primary neurons involving an insulin/insulin receptor signaling pathway; 4) insulin maintains the ability to convert some group IV muscle afferents originally CAP-insensitive to CAP-sensitive fibres; 5) co-localization with insulin receptors was observed in about 29% of the TRPV1-positive small DRG neurons examined; and 6) insulin significantly increased RSNA responses to CAP, resulting in a significant augmentation of the MAP response.
Possible mechanism of sensitization
From the present experiments, it was evident that sensitization occurred via an insulin/insulin receptor pathway. As evidence, in control trials or after pharmacologically antagonizing insulin receptors, the response to CAP was decreased by repetitive exposures in both DRG and muscle-nerve preparations. This could be due to CAP-induced desensitization of TRPV1 (Tominaga et al., 1998). Conversely, approximately 54% of DRG neurons and 44% of group IV fibres were sensitized to CAP by insulin. This suggests that the desensitizing effects of CAP can be reversed in the presence of insulin. It is known that 16–30% of TRPV1 and insulin receptors colocalize on primary neurons (Baiou et al., 2007; Lázár et al., 2018) and both tend to be expressed in relatively small diameter primary fibres (Baiou et al., 2007). Consistent with previous studies, we observed an approximate 29 % co-localization rate between TRPV1 and insulin receptors in small DRG neurons. As such, it is reasonable to suggest that an insulin/insulin receptor pathway modulates TRPV1 sensitivity and/or expression accounting for the augmentations in responsiveness noted in the current investigation.
Small DRG neurons can be divided into two populations: (1) IB4-positive, non-peptidergic neurons, and (2) IB4-negative, peptidergic neurons (Priestley et al., 2002). In the present investigation, the co-localization of TRPV1 and insulin receptors was observed both in IB4-positive and -negative neuronal populations. Hence, both populations of DRG neurons could be involved in the insulin/insulin receptor-dependent sensitization of TRPV1 to CAP.
Sensitization of TRPV1 can occur by three mechanisms; 1) protein phosphorylation of the channel, 2) translocation of the channel to the cell surface, and 3) up-regulation of TRPV1 transcription and translation (Sousa-Valente et al., 2014). As to the third mechanism, indeed long-term application of insulin has been reported to increase total TRPV1 protein level in the human neuroblastoma cell line SH-SY5Y transfected with TRPV1 (Lilja et al., 2007). However, it is unlikely that this mechanism operated in the present acute investigation.
The insulin receptor itself is a tyrosine kinase that activates phosphoinositide-3-kinase (PI3K)/Akt pathways and protein kinase C (PKC). These are Ser/Thr kinases, and the Ser/Thr-phosphorylation of TRPV1 induced by PKC has been demonstrated to potentiate channel activity (Numazaki et al., 2003; Zhang et al., 2005; Wang et al., 2015). Tyr-phosphorylation is induced via PI3K/PKC pathway (Zhang et al., 2005). Tyr-phosphorylation of TRPV1 enhances its recruitment to the cell surface (Zhang et al., 2005). In fact, evidence from embryonic rat DRG neurons and HEK 293 cells heterologously expressing TRPV1 demonstrated that insulin was involved in protein trafficking influencing the translocation of TRPV1 from the cytosol to the plasma membrane (Van Buren et al., 2005). Therefore, it is reasonable to suggest that these mechanisms could have been involved with the insulin-induced augmentation in responsiveness to CAP in the current study. Although speculative, activation of a TRPV1 translocation mechanism could account for converting 53% of group IV fibres that were initially CAP-insensitive to being sensitive to CAP after the injection of insulin.
The present study used a supraphysiological concentration of insulin (Sjöstrand et al., 1999). This strategy was based on our previous investigation using a high dose of insulin (500 mU/mL) to induce mechanical sensitization in small DRG neurons in vitro and thin-fibre muscle afferents ex vivo (Hotta et al., 2019a). Further investigations are warranted to clarify whether the insulin-induced sensitization of TRPV1 is observed in a dose dependent manner and within the physiological range of insulin concentrations.
Implications
The findings of the present study suggest that insulin regulates or modifies the sensitivity of chemically-sensitive somatosensory thin fibres. Activation of these peripheral fibres during the performance of exercise or other daily activities is known to mediate increases in sympathetic outflow. Importantly, intramuscular injection of insulin into the hindlimb significantly augmented the RSNA response to CAP in the decerebrate rats of the present study (Fig. 8C and D). As such, sensitization of these fibres may account for the noted sympathoexcitatory actions of insulin during physical exercise in daily life.
We previously demonstrated that small DRG neurons and thin-fibre muscle afferents were sensitized to mechanical stimuli by insulin (Hotta et al., 2019a). Although there is less evidence that TRPV1 plays a role in mechano sensitivity under normal conditions (Nishihara et al., 2011), it has been established that TRPV1 is associated with the development of mechanical hypersensitivity under some conditions (Fujii et al., 2008; Walder et al., 2012; Ota et al., 2013). Thus, it is possible that TRPV1 is responsible for the insulin-induced potentiation in mechano-sensitivity we previously reported (Majhi & Pourteymour, 2020). In the wake of the current findings, this is of considerable interest as increased sensitivity to both mechanical and chemical stimuli in the periphery may be mediated by similar mechanisms. Further research aimed at determining whether insulin-induced mechanical sensitization is abrogated by TRPV1 blockade and/or abolished by reducing TRPV1 expression is warranted.
The present study intimating insulin/insulin receptor-mediated TRPV1 sensitization has the following potential clinical implications First, administration of insulin to diabetic patients could induce severe neuropathic pain (Leow & Wyckoff, 2005). This is known as insulin neuritis or treatment-induced diabetic neuropathy (Hwang & Davies, 2016). Although the mechanisms have yet to be elucidated (Nicodemus et al., 2017), sensitization of TRPV1 is a clear candidate for this nociceptive channel overactivity (Majhi & Pourteymour, 2020). Second, TRPV1, located on thin-fibre muscle afferents, has been suggested to be a key receptor contributing to muscle metaboreflex-induced increases in blood pressure (Smith et al., 2010; Mizuno et al., 2011; Li & Garry, 2020). Recent animal and human studies have shown that the chemically sensitive muscle metaboreflex is exaggerated in type 2 diabetes mellitus (Holwerda et al., 2016; Ishizawa et al., 2021), which may increase the risk for adverse cardiovascular events and stroke during or immediately after a bout of exercise (Hoberg et al., 1990; Mittleman et al., 1993). Because hyperinsulinemia is a common characteristic of individuals in an insulin resistant state, findings from the current investigation might explain the mechanism underlying the exaggerated skeletal muscle metaboreflex previously demonstrated in type 2 diabetes. Of relevance to both clinical implications noted, the testing of pharmacological approaches to manipulate TRPV1 activity in humans has begun and may prove to mitigate the negative effects of insulin-induced TRPV1 sensitization after the pathogenesis of disease (Weyer-Menkhoff & Lötsch, 2018).
Conclusion
The results of this investigation demonstrate that insulin potentiates CAP-induced neural responses at the DRG and skeletal muscle tissue levels. Likewise, insulin was shown to augment the sympathetic nerve activity response to CAP in vivo. These findings support the hypothesis that insulin sensitizes peripheral sensory afferent pathways, possibly contributing to increased sympathoexcitation during activities such as physical exercise.
Supplementary Material
KEYPOINT.
Evidence suggests insulin centrally activates the sympathetic nervous system, and a chemical stimulus to tissues activates the sympathetic nervous system via thin fibre muscle afferents.
Insulin is reported to modulate putative chemical sensitive channels in the dorsal root ganglion neurons of these afferents.
In the present study, we demonstrate that insulin potentiates the responsiveness of thin fibre afferents to capsaicin at muscle tissue levels as well as at the level of dorsal root ganglion neurons. In addition, we demonstrate that insulin augments the sympathetic nerve activity response to capsaicin in vivo.
These data suggests that sympathoexcitation is peripherally mediated via insulin-induced chemical sensitization.
The present study proposes a possible physiological role of insulin in the regulation of chemical sensitivity in somatosensory thin fibre muscle afferents.
TRANSLATIONAL PERSPECTIVE.
We tested the hypothesis that insulin contributes to sympathetic activation via sensitization of transient receptor potential vanilloid 1 (TRPV1) located in peripheral tissues. The present study demonstrated that insulin potentiates the responsiveness of thin fibre afferents to capsaicin, a TRPV1 agonist, at the levels of the dorsal root ganglion in vitro and the skeletal muscle axon terminal ex vivo. Further, in vivo studies showed that intramuscular injection of insulin augments sympathetic and pressor responses to intra-arterial injection of capsaicin. These findings imply that insulin-induced sensitization of TRPV1, known to be expressed in noci-sensitive and metabolically-sensitive afferent neurons, enhances the cardiovascular response to pain stimuli and/or chemical stimuli, the latter commonly generated during physical activity. Dissection of the mechanisms underlying insulin-induced TRPV1 sensitization may lead to the development of novel therapeutic strategies to ameliorate insulin neuritis or treatment-induced diabetic neuropathy evoked by administration of insulin to diabetic patients. Additionally, insulin-induced alterations may contribute importantly to the abnormal circulatory response to exercise in patients with type 2 diabetes. To address this possibility, further investigation is planned to elucidate whether postprandial insulin secretion could potentially alter cardiovascular responses to exercise.
ACKNOWLEDGEMENTS
We thank Chiaki Kihara, Kimie Kato and Martha Romero for their expert technical assistance. This work is dedicated to the memory of our co-author, friend, colleague and mentor Dr. Jere H. Mitchell, a giant in the field of neural cardiovascular control, whose guidance and brilliance led to the completion of this work and many others over his illustrious 60 year career.
GRANTS
This work was supported in part by JSPS KAKENHI JP17K01769 (to NH), the Grant Short-term Research Project from the Research Institute of Life and Health Sciences of Chubu University (to NH), the Health Science Center Foundation (to NH), the Nakatomi Foundation (to NH), the Lawson & Rogers Lacy Research Fund in Cardiovascular Disease (to JHM), and the National Heart, Lung, and Blood Institute (R01HL-151632) (to MM).
Abbreviations
- DRG
dorsal root ganglion
- TRPV1
transient receptor potential vanilloid 1
- CAP
capsaicin
- SNA
sympathetic nerve activity
- EDL
extensor digitorum longus
- IB4
isolectin B4
- ABP
arterial blood pressure
- ECG
electrocardiograph
- RSNA
renal sympathetic nerve activity
- MAP
mean arterial pressure
- HR
heart rate
- PI3K
phosphoinositide-3-kinase
- PKC
protein kinase C
- IR
insulin receptor
Biography

Amane Hori is a Ph.D. student at the graduate school of the Chubu University in Japan, where he has a research fellowship for young scientists at Japan Society for the Promotion of Science. He received his undergraduate degree and MS from Chubu University. His research focuses on neural control of the cardiovascular responses to exercise. He now examines the effects of diabetes and mental/physical stress on cardiovascular control during exercise.
Footnotes
COMPETING INTERESTS
The authors declare that they have no competing interests.
DATA AVAILABILITY STATEMENT
The data that support the present findings are available from the corresponding author on reasonable request.
REFERENCES
- Baiou D, Santha P, Avelino A, Charrua A, Bacskai T, Matesz K, Cruz F & Nagy I (2007). Neurochemical characterization of insulin receptor-expressing primary sensory neurons in wild-type and vanilloid type 1 transient receptor potential receptor knockout mice. J Comp Neurol 503, 334–347. [DOI] [PubMed] [Google Scholar]
- Van Buren JJ, Bhat S, Rotello R, Pauza ME & Premkumar LS (2005). Sensitization and translocation of TRPV1 by insulin and IGF-I. Mol Pain 1, doi: 10.1186/1744-8069-1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton AR, Fazalbhoy A & Macefield VG (2016). Sympathetic responses to noxious stimulation of muscle and skin. Front Neurol 7, 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii Y, Ozaki N, Taguchi T, Mizumura K, Furukawa K & Sugiura Y (2008). TRP channels and ASICs mediate mechanical hyperalgesia in models of inflammatory muscle pain and delayed onset muscle soreness. Pain 140, 292–304. [DOI] [PubMed] [Google Scholar]
- Grote CW & Wright DE (2016). A Role for Insulin in Diabetic Neuropathy. Front Neurosci 10, 581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy D (2015). Principles and standards for reporting animal experiments in The Journal of Physiology and Experimental Physiology. J Physiol 593, 2547–2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoberg E, Schuler G, Kunze B, Obermoser A-L, Hauer K, Mautner H-P, Schlierf G & Kübler W (1990). Silent myocardial ischemia as a potential link between lack of premonitoring symptoms and increased risk of cardiac arrest during physical stress. Am J Cardiol 65, 583–589. [DOI] [PubMed] [Google Scholar]
- Holwerda SW, Restaino RM, Manrique C, Lastra G, Fisher JP & Fadel PJ (2016). Augmented pressor and sympathetic responses to skeletal muscle metaboreflex activation in type 2 diabetes patients. Am J Physiol Heart Circ Physiol 310, H300–H309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotta N, Katanosaka K, Mizumura K, Iwamoto GA, Ishizawa R, Kim HK, Vongpatanasin W, Mitchell JH, Smith SA & Mizuno M (2019a). Insulin potentiates the response to mechanical stimuli in small dorsal root ganglion neurons and thin fibre muscle afferents in vitro. J Physiol 597, 5049–5062. [DOI] [PubMed] [Google Scholar]
- Hotta N, Kubo A & Mizumura K (2015). Effect of protons on the mechanical response of rat muscle nociceptive fibres and neurons in vitro. Neurosci Res 92, 46–52. [DOI] [PubMed] [Google Scholar]
- Hotta N, Kubo A & Mizumura K (2019b). Chondroitin sulfate attenuates acid-induced augmentation of the mechanical response in rat thin-fibre muscle afferents in vitro. J Appl Physiol 126, 1160–1170. [DOI] [PubMed] [Google Scholar]
- Hwang YT & Davies G (2016). ‘Insulin neuritis’ to ‘treatment-induced neuropathy of diabetes’: New name, same mystery. Pract Neurol 16, 53–55. [DOI] [PubMed] [Google Scholar]
- Ishizawa R, Kim HK, Hotta N, Iwamoto GA, Mitchell JH, Smith SA, Vongpatanasin W & Mizuno M (2021). TRPV1 (Transient Receptor Potential Vanilloid 1) Sensitization of Skeletal Muscle Afferents in Type 2 Diabetic Rats With Hyperglycemia. Hypertension 77, 1360–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizawa R, Kim HK, Hotta N, Iwamoto GA, Vongpatanasin W, Mitchell JH, Smith SA & Mizuno M (2020). Skeletal muscle reflex–induced sympathetic dysregulation and sensitization of muscle afferents in type 1 diabetic rats. Hypertension 75, 1072–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto GA, Kaufmann MP, Botterman BR & Mitchell JH (1982). Effects of lateral reticular nucleus lesions on the exercise pressor reflex in cats. Circ Res 51, 400–403. [DOI] [PubMed] [Google Scholar]
- Jensen TE & Richter EA (2012). Regulation of glucose and glycogen metabolism during and after exercise. J Physiol 590, 1069–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julius D, Caterina MJ, Schumacher MA, Tominaga M, Rosen TA & Levine JD (1997). The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389, 816–824. [DOI] [PubMed] [Google Scholar]
- Kubo A, Katanosaka K & Mizumura K (2012). Extracellular matrix proteoglycan plays a pivotal role in sensitization by low pH of mechanosensitive currents in nociceptive sensory neurones. J Physiol 590, 2995–3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson SN & Waddell PJ (1991). Soma neurofilament immunoreactivity is related to cell size and fibre conduction velocity in rat primary sensory neurons. J Physiol 435, 41–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lázár BA, Jancsó G, Nagy I, Horváth V & Sántha P (2018). The insulin receptor is differentially expressed in somatic and visceral primary sensory neurons. Cell Tissue Res; DOI: 10.1007/s00441-018-2868-0. [DOI] [PubMed] [Google Scholar]
- Leow MKS & Wyckoff J (2005). Under-recognised paradox of neuropathy from rapid glycaemic control. Postgrad Med J 81, 103–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q & Garry MG (2020). A murine model of the exercise pressor reflex. J Physiol 598, 3155–3171. [DOI] [PubMed] [Google Scholar]
- Lilja J, Laulund F & Forsby A (2007). Insulin and insulin-like growth factor type-I up-regulate the vanilloid receptor-1 (TRPV1) in stably TRPV1-expressing SH-SY5Y neuroblastoma cells. J Neurosci Res 85, 1413–1419. [DOI] [PubMed] [Google Scholar]
- Majhi RK & Pourteymour S (2020). Insulin sensitizes mechanosensitive ion channels, which aggravates pain. J Physiol 598, 19–21. [DOI] [PubMed] [Google Scholar]
- Mittleman MA, Maclure M, Tofler GH, Sherwood JB, Goldberg RJ & Muller JE (1993). Triggering of acute myocardial infarction by heavy physical exertion. Protection against triggering by regular exertion. Determinants of Myocardial Infarction Onset Study Investigators. N Engl J Med 329, 1677–1683. [DOI] [PubMed] [Google Scholar]
- Mizuno M, Hotta N, Ishizawa R, Kim HK, Iwamoto GA, Vongpatanasin W, Mitchell JH & Smith SA (2021). The Impact of Insulin resistance in Cardiovascular Control during Exercise in Diabetes. Exerc Sport Sci Rev 49, 157–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno M, Mitchell JH, Crawford S, Huang C-L, Maalouf N, Hu M-C, Moe OW, Smith SA & Vongpatanasin W (2016). High dietary phosphate intake induces hypertension and augments exercise pressor reflex function in rats. Am J Physiol - Regul Integr Comp Physiol 311, R39–R48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno M, Murphy MN, Mitchell JH & Smith SA (2011). Antagonism of the TRPv1 receptor partially corrects muscle metaboreflex overactivity in spontaneously hypertensive rats. J Physiol 589, 6191–6204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan DA, Balon TW, Ginsberg BH & Mark AL (1993). Nonuniform regional sympathetic nerve responses to hyperinsulinemia in rats. Am J Physiol 264, R423–R427. [DOI] [PubMed] [Google Scholar]
- Muntzel MS, Morgan DA, Mark AL & Johnson AK (1994). Intracerebroventricular insulin produces nonuniform regional increases in sympathetic nerve activity. Am J Physiol 267, R1350–R1355. [DOI] [PubMed] [Google Scholar]
- Nicodemus JM, Enriquez C, Marquez A, Anaya CJ & Jolivalt CG (2017). Murine model and mechanisms of treatment-induced painful diabetic neuropathy. Neuroscience 354, 136–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishihara E, Hiyama TY & Noda M (2011). Osmosensitivity of transient receptor potential vanilloid 1 is synergistically enhanced by distinct activating stimuli such as temperature and protons. PLoS One 6, e22246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numazaki M, Tominaga T, Takeuchi K, Murayama N, Toyooka H & Tominaga M (2003). Structural determinant of TRPV1 desensitization interacts with calmodulin. Proc Natl Acad Sci U S A 100, 8002–8006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ota H, Katanosaka K, Murase S, Kashio M, Tominaga M & Mizumura K (2013). TRPV1 and TRPV4 Play Pivotal Roles in Delayed Onset Muscle Soreness. PLoS One; DOI: 10.1371/journal.pone.0065751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Person RJ (1989). Somatic and vagal afferent convergence on solitary tract neurons in cat: electrophysiological characteristics. Neuroscience 30, 283–295. [DOI] [PubMed] [Google Scholar]
- Priestley JV, Michael GJ, Averill S, Liu M & Willmott N (2002). Regulation of nociceptive neurons by nerve growth factor and glial cell line derived neurotrophic factor. Can J Physiol Pharmacol 80, 495–505. [DOI] [PubMed] [Google Scholar]
- Rahmouni K, Morgan DA, Morgan GM, Liu X, Sigmund CD, Mark AL & Haynes WG (2004). Hypothalamic PI3K and MAPK differentially mediate regional sympathetic activation to insulin. J Clin Invest 114, 652–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjöstrand M, Holmäng A & Lönnroth P (1999). Measurement of interstitial insulin in human muscle. Am J Physiol Endocrinol Metab 276, E151–E154 [DOI] [PubMed] [Google Scholar]
- Smith SA, Leal AK, Williams MA, Murphy MN, Mitchell JH & Garry MG (2010). The TRPv1 receptor is a mediator of the exercise pressor reflex in rats. J Physiol 588, 1179–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SA, Mitchell JH & Garry MG (2001). Electrically induced static exercise elicits a pressor response in the decerebrate rat. J Physiol 537, 961–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SA, Mitchell JH & Garry MG (2006). The mammalian exercise pressor reflex in health and disease. Exp Physiol 91, 89–102. [DOI] [PubMed] [Google Scholar]
- Sousa-Valente J, Andreou AP, Urban L & Nagy I (2014). Transient receptor potential ion channels in primary sensory neurons as targets for novel analgesics. Br J Pharmacol 171, 2508–2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi T, Sato J & Mizumura K (2005). Augmented mechanical response of muscle thin-fibre sensory receptors recorded from rat muscle-nerve preparations in vitro after eccentric contraction. J Neurophysiol 94, 2822–2831. [DOI] [PubMed] [Google Scholar]
- Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI & Julius D (1998). The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron 21, 531–543. [DOI] [PubMed] [Google Scholar]
- Walder RY, Radhakrishnan R, Loo L, Rasmussen LA, Mohapatra DP, Wilson SP & Sluka KA (2012). TRPV1 is important for mechanical and heat sensitivity in uninjured animals and development of heat hypersensitivity after muscle inflammation. Pain 153, 1664–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Joseph J, Ro JY & Chung MK (2015). Modality-specific mechanisms of protein kinase C-induced hypersensitivity of TRPV1: S800 is a polymodal sensitization site. Pain 156, 931–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward KR, Bardgett JF, Wolfgang L & Stocker SD (2011). Sympathetic response to insulin is mediated by melanocortin 3/4 receptors in the hypothalamic paraventricular nucleus. Hypertension 57, 435–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weyer-Menkhoff I & Lötsch J (2018). Human pharmacological approaches to TRP-ion-channel-based analgesic drug development. Drug Discov Today 23, 2003–2012. [DOI] [PubMed] [Google Scholar]
- Xing J, Sinoway L & Li J (2008). Differential responses of sensory neurones innervating glycolytic and oxidative muscle to protons and capsaicin. J Physiol 586, 3245–3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Huang J & McNaughton PA (2005). NGF rapidly increases membrane expression of TRPV1 heat-gated ion channels. EMBO J 24, 4211–4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Qin W, Qian Z, Liu X, Wang H, Gong S, Sun YG, Snutch TP, Jiang X & Tao J (2014). Peripheral pain is enhanced by insulin-like growth factor 1 through a G protein-mediated stimulation of T-type calcium channels. Sci Signal; DOI: 10.1126/scisignal.2005283. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the present findings are available from the corresponding author on reasonable request.


