Abstract
Objective:
Socioeconomic factors may impact healthcare resource use and health-related quality of life (HRQL), but their association with post-critical illness outcomes is unknown. This study examines the associations between socioeconomic status, resource use, and HRQL in a cohort of children recovering from acute respiratory failure.
Design:
Secondary analysis of data from the RESTORE clinical trial.
Setting:
Thirty-one pediatric intensive care units.
Patients:
Children with acute respiratory failure enrolled whose parent/guardians consented for follow-up.
Measurements and Main Results:
Resource use included in-home care, number of healthcare providers, prescribed medications, home medical equipment, emergency department visits, and hospital readmission. Socioeconomic status was estimated by matching residential address to census tract-based median income. Health-related quality of life was measured using age-based parent-report instruments. Resource use interviews with matched census tract data (N=958) and HRQL questionnaires (N = 750/958) were assessed. Compared with high-income children, low-income children received care from fewer types of healthcare providers (β = −0.4; P = .004), used less newly prescribed medical equipment (OR = 0.4; P < .001) and had more emergency department visits (43% vs 33%; P = .04). In the youngest cohort (< 2 years of age), low income children had lower quality of life scores from physical ability (−8.6 points; P = .01) and bodily pain/discomfort (+8.2 points; P <.05). In addition, HRQL was lower in those who had more healthcare providers and prescribed medications. In older children, HRQL was lower if they had prescribed medications, emergency department visits, or hospital readmission.
Conclusions:
Children recovering from acute respiratory failure have ongoing healthcare resource use. Yet, lower income children use less in-home and outpatient services and use more hospital resources. Continued follow-up care, especially in lower income children, may help identify those in need of ongoing healthcare resources and those at-risk for decreased HRQL.
Keywords: pediatric critical care, socioeconomic factors, ventilators (mechanical), lung diseases (interstitial), censuses
Introduction
As a result of lower mortality rates, pediatric critical care providers are increasingly focused on post-discharge morbidity and, relatedly, improving the quality of life for survivors (1–4). Recovery after critical illness can profoundly impact the entire family, particularly if the child’s health has not returned to baseline and necessitates new healthcare needs after hospital discharge (5). While most pediatric intensive care (PICU) survivors return to their baseline health, a portion of these children develop new morbidities requiring healthcare resources that may diminished their health-related quality of life (HRQL) (3, 6, 7). Patient and family perceptions of health are critical to understanding the effectiveness of care and identifying who is at risk for altered HRQL (8–11).
Data support an inverse association between resource use and HRQL: lower quality of life is associated with increased resource use (12–14). The utilization of healthcare resources has financial implications for the entire family, regardless of socioeconomic status (SES), but differences in SES may affect the association between resource use and HRQL. SES typically relate to a family’s financial state and is generally positively associated with health outcomes—as SES increases, health outcomes improve (15–21). Resource use and its associations with SES and HRQL have not been sufficiently studied in pediatric critical care survivors (22–26). Here we quantify post-discharge resource use, examine the association of SES with resource use and HRQL, and examine the of resource use on HRQL in a cohort of children with acute respiratory failure, six months after PICU discharge. We hypothesized that post-critical illness, SES would be associated with a child’s resource use and HRQL.
Materials and Methods
Parents and/or guardians of 2138 subjects from the Randomized Evaluation of Sedation Titration for Respiratory Failure (RESTORE) study consented to follow-up, and 2002 subjects survived to hospital discharge (NCT00814099) (27, 28). A random sample of 1360 eligible subjects, stratified by site and age group, were selected for follow-up (29, 30). Each consenting family was contacted at 6 months (± 1 month) after the child’s hospital discharge to complete interviews assessing outcomes that included healthcare resource utilization and HRQL. A priori secondary outcomes—HRQL, functional status, and post-traumatic stress disorder—in this cohort were not significantly different between groups in the RESTORE trial (27). Our sample of analysis consists of subjects whose families completed follow-up interviews and whose residential address could be linked to a census tract. RESTORE was approved by the institutional review board at each participating site, and parental permission was obtained for all enrolled patients.
Data Collection
Baseline data were collected at RESTORE enrollment. Functional status was established at enrollment, hospital discharge, and six-month follow-up using the Pediatric Overall Performance Category (POPC) and Pediatric Cerebral Performance Category (PCPC) scales (31). Additional clinical variables included the PRISM III-12 score (32), severity of pediatric acute respiratory distress syndrome (PARDS) (33), number of dysfunctional organ systems (34), and duration of mechanical ventilation.
Because 63% of families who completed follow-up interviews declined to provide annual income (29), an approximation of SES was derived from census tract-level “median annual income by presence of own children under 18 years of age” from the year 2011 (midpoint of the RESTORE trial). We then categorized median income values into quartiles, following U.S. census-level research recommendations (19), and designated them as low income, low middle income, high middle income, and high income. Measurements of SES at the neighborhood level are feasible to collect and have been shown to be representative of individual data (35–39).
Follow-up
Parents and/or guardians were interviewed six months after PICU discharge and reported their education level and relationship status. Resource use variables were also self-reported and included care provided in the home by a healthcare professional or assistive personnel, the number and types of healthcare professionals providing on-going consultation and care, prescribed medications, homecare medical equipment, emergency department (ED) visits, and hospital readmission. HRQL was assessed using one of two measures: Infant Toddler Quality of Life Questionnaire-97 (ITQOL-97) (40) for children 2 years and younger and the Pediatric Quality of Life Inventory, Version 4.0 Generic Core Scales (PedsQL) (41) for children older than 2 years.
Statistical Analysis
To compare differences in clinical and resource use variables according to income quartile, the Cochrane-Armitage trend test was used for binary variables, the Pearson’s chi square test for nominal variables, and the Jonckheere-Terpstra test for ordinal and continuous variables (45). Adjusting for PICU as a cluster variable, linear and logistic regression was used to model the effects of independent variables on continuous HRQL and binary resource use variables using an exchangeable working assumption. Cumulative logit regression was used for ordinal resource use and HRQL variables using an independence working assumption. In all models, a three-degree of freedom test was used to assess overall significance for income quartile with outcome variables. Regression models adjusted for age category, having a preexisting condition, PARDS severity, worst MODS, duration of mechanical ventilation, and functional status. Comparisons between income quartiles were based on linear regression beta coefficients and on effect sizes d, defined as the difference in adjusted means divided by the standard deviation (SD) from normative samples (42, 43). Following Cohen (44), differences in HRQL between income quartiles were categorized as having small (0.2 ≤ d < 0.5), medium (0.5 ≤ d < 0.8), or large (d > 0.8) effects, and we view income quartiles as having a clinically important change in HRQL if their scores are more than half a SD apart (d ≥ 0.5). Backward stepwise regression was used to test if resource use was associated with HRQL, represented by the growth and development domain for the ITQOL and the total score for the PedsQL. Statistical significance was assessed at the 0.05 level. Statistical analyses were performed using SAS (version 9.4; SAS Institute).
Results
Of the 1360 subjects eligible and selected for RESTORE follow-up and matched to census tract data, 958/1360 (70%) of families completed healthcare resource interviews and 750/958 (78%) completed HRQL questionnaires (352 completed the ITQOL and 398 completed the PedsQL) (Figure 1). There were no significant differences in median income between subjects that were preliminarily eligible and those selected for follow-up (P = .23). However, of those eligible and selected, there were differences in the median income between the families that completed follow-up and those that did not ($58,482 vs $46,442; Kruskal-Wallis P < .001). Demographic, baseline, and hospital course characteristics for the study sample are summarized in Table 1. Preexisting conditions were present in one-third of the study population, the most common of which were asthma, seizure disorder, and neurologic/neuromuscular disorders. Most children were discharged to home (n = 872/958, 91%) and were at home at the time of follow-up (n = 932/958, 97%). There was no association among income quartile and preexisting conditions (P = .83), PRISM III-12 score (P = .51), PARDS severity (P = .16), or duration of mechanical ventilation (P = .32).
Figure 1. Flow Diagram for Subjects Included in Current Study.
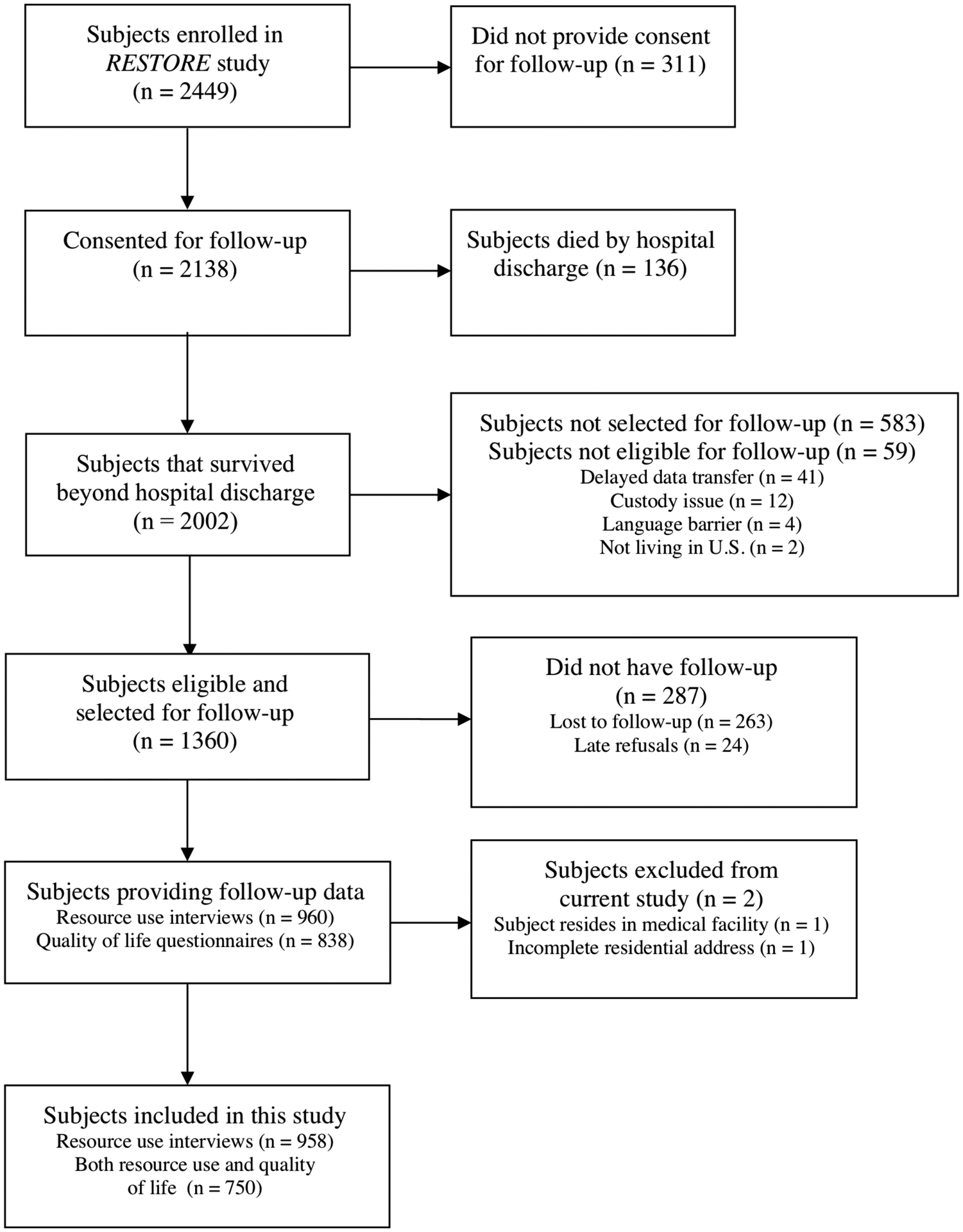
RESTORE = Randomized Evaluation of Sedation Titration for Respiratory Failure
Table 1.
Patient and Family Characteristics on Admission (n=958)
| Age at PICU admission, median (IQR), yr | 1.8 (0.4 – 7.9) |
| Age category, n (%) | |
| 2 wk to <1 yr | 357 (37) |
| 1 to <6 yr | 311 (33) |
| 6 to <18 yr | 290 (30) |
| Female, n (%) | 443 (46) |
| Race, n (%) | |
| White | 687 (72) |
| Black/African American | 171 (18) |
| Othera | 94 (10) |
| Hispanic or Latino, n (%) | 208 (22) |
| History of prematurity (< 36 wk post-menstrual age) | 138 (14) |
| Any preexisting condition, n (%) | 323 (34) |
| Asthma (prescribed bronchodilators or steroids) | 135 (14) |
| Seizure disorder (prescribed anticonvulsant medication) | 88 (9) |
| Neurologic/neuromuscular disorder | 83 (9) |
| Cancer | 51 (5) |
| Other | 47 (4) |
| Primary admitting diagnosis, n (%) | |
| Pneumonia or aspiration pneumonia | 412 (43) |
| Bronchiolitis | 248 (26) |
| Acute respiratory failure related to sepsis | 115 (12) |
| Asthma or reactive airway disease | 83 (9) |
| Other acute illnessesb | 77 (8) |
| Other chronic illnessesc | 23 (2) |
| Admission PRISM III-12 score, median (IQR) | 7 (3 – 12) |
| PARDS severity, n (%)d | |
| At risk/mild | 344 (36) |
| Moderate | 287 (30) |
| Severe | 327 (34) |
| Census tract-based household income, median, $ | 58,482 |
| Income quartile, $ | |
| Low Income | < 39,265 |
| Low Middle Income | 39,265 – 58,482 |
| High Middle Income | 58,483 – 87, 816 |
| High Income | > 87,816 |
Abbreviations: PICU, pediatric intensive care unit; IQR, interquartile range; PRISM III-12, Pediatric Risk of Mortality III score from first 12 hours in the PICU; PARDS, pediatric acute respiratory distress syndrome; GED, general education diploma.
Not all column percentages sum to 100% due to rounding.
Other includes Asian, Native Hawaiian or Other Pacific Islander, American Indian or Alaskan Native, and Multiracial.
Other acute diagnoses include acute respiratory failure related to multiple blood transfusions, laryngotracheobronchitis (croup/tracheitis), pertussis, pneumothorax, pulmonary edema, pulmonary hemorrhage, and thoracic trauma (pulmonary contusion or inhalation burns).
Other chronic diagnoses include acute chest syndrome/sickle cell disease, acute respiratory failure post-bone marrow transplant, chronic lung disease (cystic fibrosis or bronchopulmonary dysplasia), and pulmonary hypertension (not primary).
PARDS severity was defined using the 2015 Pediatric Acute Lung Injury Consensus Conference (PALICC).(33)
As presented in Table 2, resource use six months after PICU discharge was significantly different according to income quartile for the number of active healthcare providers (P < .001), prescription medications (P < .001), newly prescribed medical equipment (P = .003), and emergency department visits (P = .04). The majority of children (n = 606/958, 63%; P = .71) used medical equipment in the home, with more than half of those children (n = 353/606, 58%; P = .003) using newly prescribed medical equipment. Within six months after discharge, 41% (n = 386/958; P = .04) visited an emergency department and 34% (n = 328/958; P =.42) were readmitted to the hospital. Compared with children in the highest income quartile, more children in the lowest income quartile visited the ED (43% vs 33%). Children in the lowest income quartile had fewer different types of healthcare providers managing their care, specifically, occupational/physical therapy and gastroenterology services, used fewer medications, and were less likely to have additional medical equipment used in the home. The number of healthcare providers, medications prescribed, and new equipment post-PICU discharge increased as income quartile increased. Equipment listed in Supplemental Digital Content - Table S1.
Table 2.
Post-Intensive Care Resource Use According to Income Quartiles (n=958)
| Healthcare Resources | Low Income (n = 240) | Low Middle Income (n = 239) | High Middle Income (n = 239) | High Income (n = 240) | P a |
|---|---|---|---|---|---|
| .73 | |||||
| In home healthcare, n (%) | 68 (29) | 64 (27) | 62 (26) | 73 (31) | |
| Registered Nurse | 44 (18) | 41 (17) | 41 (17) | 59 (25) | |
| Nurse’s aid | 4 (2) | 5 (2) | 6 (3) | 3 (1) | |
| Licensed Practical Nurse | 3 (1) | 5 (2) | 4 (2) | 3 (1) | |
| Physical/Occupational Therapist | 26 (11) | 28 (12) | 25 (10) | 24 (10) | |
| Otherb | 11 (5) | 18 (8) | 17 (7) | 9 (4) | |
| Active healthcare providers, median (IQR) | 2 (1–3) | 2 (1–3) | 2 (1–4) | 2 (1–4) | <.001 |
| Healthcare providers, n (%) | |||||
| Pediatrician | 216 (90) | 216 (90) | 196 (82) | 195 (81) | |
| Pulmonologist | 55 (23) | 66 (28) | 73 (31) | 78 (33) | |
| Neurologist | 30 (13) | 35 (15) | 44 (18) | 42 (18) | |
| Cardiologist | 35 (15) | 22 (9) | 19 (8) | 31 (13) | |
| Gastroenterologist | 22 (9) | 21 (9) | 28 (12) | 38 (16) | |
| Occupational/physical therapist | 22 (9) | 21 (9) | 47 (20) | 39 (16) | |
| Medical equipment in home, n (%) | 143 (61) | 163 (69) | 142 (60) | 158 (66) | .71 |
| New equipment post-PICU dischargec | 74 (52) | 85 (52) | 88 (62) | 106 (67) | .003 |
| Prescribed medications, median (IQR) | 2 (1–4) | 2 (1–4) | 3 (1–5) | 3 (1–5) | <.001 |
| Emergency department visit, n (%) | 101 (43) | 104 (44) | 103 (43) | 78 (33) | .04 |
| Readmission, n (%) | 92 (38) | 66 (28) | 98 (41) | 72 (30) | .42 |
Abbreviations: IQR, interquartile range; PICU, pediatric intensive care unit.
P values for comparison between the income quartiles were calculated using the Cochrane-Armitage trend test for binary variables, the Pearson’s chi square test for nominal variables, and the Jonckheere-Terpstra test for ordinal and continuous variables.
Other includes counselor, neuropsychologist, speech therapy, respiratory therapy, vision therapy, and wound care.
Column percentages were calculated based on the number of subjects using medical equipment in the home for that income quartile.
In a multivariable model controlling for age group, preexisting conditions, PARDS severity, number of dysfunctional organs, duration of mechanical ventilation, and functional status at discharge, income quartile was significantly associated with number of healthcare providers, new medical equipment, ED visits, and hospital readmission (Table 3). Children in the lowest income quartile had fewer healthcare providers and were less likely to have newly prescribed medical equipment as compared with those in the highest income quartile. While emergency department visits were not associated with income quartile overall (P = .10), the odds of visiting the ED were approximately 50% higher for children in the lowest three income quartiles as compared with those in the highest income quartile. The odds of having a readmission were approximately 70% higher for children in the High Middle income quartile as compared with those in the High income quartile. Functional status at discharge was strongly predictive of resource use variables, with those with some degree of disability more than three times as likely to have in-home healthcare.
Table 3.
Predictors of Resource Use Six Months After Pediatric Intensive Care Unity (PICU) Discharge (n = 958)
| Covariates | In home healthcare OR (95% CI) | Number of active healthcare providers β (95% CI) | New medical equipment OR (95% CI) | Number of prescribed medications β (95% CI) | ED visit OR (95% CI) | Readmission OR (95% CI) |
|---|---|---|---|---|---|---|
| Income quartiles (ref = High) | ||||||
| Low | 0.9 (0.6, 1.3) | −0.4* (−0.6, −0.1) | 0.4** (0.3, 0.7) | −0.2 (−0.5, 0.1) | 1.5* (1.1, 2.2) | 1.4 (0.8, 2.4) |
| Low Middle | 0.8 (0.5, 1.3) | −0.4* (−0.6, −0.1) | 0.5* (0.3, 0.8) | −0.2 (−0.6, 0.1) | 1.5* (1.1, 2.1) | 0.8 (0.6, 1.2) |
| High Middle | 0.8 (0.5, 1.2) | −0.03 (−0.3, 0.3) | 0.7 (0.4, 1.1) | 0.04 (−0.3, 0.4) | 1.5* (1.1, 2.1) | 1.7* (1.1, 2.4) |
| Age (ref = 2wk to <1yr) | ||||||
| 1yr to <3yr | 0.9 (0.5, 1.7) | 0.03 (−0.3, 0.3) | 0.4** (0.3, 0.7) | −0.2 (−0.6, 0.2) | 0.6* (0.4, 0.9) | 0.6* (0.4, 0.8) |
| 3yr to <6yr | 0.2** (0.1, 0.4) | −0.1 (−0.5, 0.3) | 0.4** (0.3, 0.6) | −0.1 (−0.6, 0.4) | 0.5* (0.3, 0.9) | 0.4** (0.2, 0.6) |
| 6yr to <18yr | 0.4** (0.2, 0.6) | −0.1 (−0.4, 0.1) | 0.7 (0.4, 1.2) | 0.4* (0.1, 0.8) | 0.5** (0.3, 0.7) | 0.4** (0.2, 0.6) |
| Preexisting condition | 1.9** (1.4, 2.6) | 0.1 (−0.1, 0.4) | 0.4 ** (0.3, 0.6) | 1.5* (1.2, 1.8) | 1.4 (0.98, 2.0) | 2.6** (1.7, 3.9) |
| PARDS on day 0/1a (ref = at risk/mild) | ||||||
| Moderate | 0.8 (0.5, 1.1) | 0.01 (−0.4, 0.4) | 0.8 (0.5, 1.1) | 0.1 (−0.2, 0.5) | 1.1 (0.8, 1.6) | 0.9 (0.6, 1.3) |
| Severe | 0.8 (0.5, 1.1) | −0.1 (−0.4, 0.2) | 0.6* (0.4, 0.9) | 0.1 (−0.3, 0.5) | 1.0 (0.8, 1.3) | 0.9 (0.7, 1.2) |
| Organ system dysfunctionb | 1.04 (0.9, 1.2) | 0.1* (0.01, 0.2) | 1.1 (0.99, 1.3) | 0.07 (−0.1, 0.2) | 1.1 (0.95, 1.3) | 1.2 (0.98, 1.4) |
| Duration of mechanical ventilation, per day | 1.05** (1.03, 1.1) | 0.03** (0.01, 0.05) | 1.04** (1.02, 1.1) | 0.03 (−0.001, 0.1) | 1.003 (0.99, 1.03) | 1.02* (0.99, 1.05) |
| Functional status at discharge (ref = age-appropriate) | ||||||
| Mild disability | 3.8** (2.4, 6.2) | 0.9** (0.5, 1.4) | 0.6 (0.4, 1.1) | 0.9** (0.4, 1.3) | 1.3 (0.9, 2.0) | 1.6* (1.1, 2.5) |
| Moderate disability | 4.5** (2.6, 7.7) | 1.7** (1.2, 2.3) | 0.7 (0.4, 1.0) | 1.1** (0.6, 1.7) | 2.6** (1.6, 4.2) | 2.0* (1.1, 3.5) |
| Severe disability or vegetative state | 7.6** (4.0, 14.2) | 1.5** (1.2, 1.8) | 1.1 (0.6, 1.8) | 2.2** (1.7, 2.7) | 1.3 (0.9, 2.0) | 2.7** (1.7, 4.2) |
Abbreviations: ED, emergency department; OR, odds ratio; CI, confidence interval; PARDS, pediatric acute respiratory distress syndrome.
P < .05
P < .001
PARDS severity was defined using the 2015 Pediatric Acute Lung Injury Consensus Conference (PALICC) criteria. (33)
Organ system dysfunction was measured continuously; every subject had respiratory dysfunction and dysfunction in up to five additional organ systems. (34)
As shown in Table 4, in a multivariable model controlling for the same covariates noted above, HRQL in children less than 2 years of age was associated with the low income quartile, relative to the high income quartile, for the ITQOL subscores measuring physical abilities (−8.6; 95% CI [−15.5, −1.8]; P <.05, medium effect size d = 0.55); bodily pain/discomfort (8.2; 95% CI [2.9, 13.5]; P <.05, medium effect size d = 0.51); and temperament and moods (−4.3; 95% CI[−7.1, −1.4]; P <.05, small effect size d = 0.36), though not with overall health. The physical abilities and bodily pain/discomfort subscores were more than a half a standard deviation different and so are considered clinically important. Functional status at discharge was highly predictive of most of the ITQOL domains, and those with severe disability scored 44.5 points lower on the physical disabilities domain than those with age-appropriate functional status. For the growth and development domain, as level of disability increased, scores decreased.
Table 4.
Predictors of Health-Related Quality of Life (ITQOL) in Young Children Six Months After PICU Discharge (n = 352)
| Covariates | Overall health OR (95% CI) | Physical abilities β (95% CI) | Growth and development β (95% CI) | Pain and discomfort β (95% CI) | Temperament and moods β (95% CI) | General health β (95% CI) |
|---|---|---|---|---|---|---|
| Income quartiles (ref = High) | ||||||
| Low | 0.6 (0.3, 1.0) | −8.6* (−15.5, −1.8) | −0.8 (−5.1, 3.5) | 8.2* (2.9, 13.5) | −4.3* (−7.1, −1.4) | 1.1 (−3.2, 5.4) |
| Low Middle | 0.7 (0.4, 1.1) | 3.8 (−3.1, 10.8) | 1.7 (−2.0, 5.5) | 0.7 (−3.3, 4.7) | −0.98 (−3.8, 1.8) | −0.8 (−6.4, 4.8) |
| High Middle | 0.6 (0.3, 1.1) | 2.7 (−4.5, 9.9) | −2.6 (−6.4, 1.1) | 0.02 (−5.4, 5.5) | −1.6 (−5.1, 1.8) | −3.4 (−8.1, 1.2) |
| Age at follow-up (ref = <1yr) | ||||||
| 1yr to <2yr | 0.9 (0.6, 1.1) | −0.8 (−7.1, 5.6) | −1.8 (−5.9, 2.3) | 2.3 (−1.6, 6.2) | 0.3 (−2.6, 3.2) | −2.0 (−5.3, 1.3) |
| 2yr to <6yr | 0.4 (0.2, 1.0) | −7.9 (−29.4, 13.6) | −4.0 (−14.8, 6.8) | −3.8 (−16.1, 8.5) | −2.5 (−8.2, 3.1) | −4.0 (−12.0, 4.0) |
| Preexisting condition | 0.5* (0.3, 0.9) | −6.6 (−18.9, 5.7) | −4.9 (−11.4, 1.6) | 3.1 (−3.5, 9.7) | −0.9 (−5.0, 3.1) | −5.5 (−11.1, 0.1) |
| PARDS on day 0/1a (ref = at risk/mild) | ||||||
| Moderate | 0.7 (0.4, 1.2) | 1.6 (−4.3, 7.4) | −2.2 (−6.6, 2.3) | 0.02 (−5.5, 5.5) | −1.8 (−4.6, 1.0) | −3.0 (−7.8, 1.6) |
| Severe | 0.9 (0.6, 1.5) | −0.5 (−9.6, 8.6) | −1.6 (−7.5, 4.3) | −1.9 (−6.0, 2.3) | −3.5* (−6.2, −0.8) | −3.3 (−6.6, −0.05) |
| Organ system dysfunctionb | 1.03 (0.8, 1.3) | 1.8 (−0.7, 4.3) | 2.1* (0.2, 3.9) | 1.1 (−0.6, 2.9) | 0.4 (−0.7, 1.5) | −0.007 (−1.5, 1.4) |
| Duration of mechanical ventilation, per day | 0.97 (0.9, 1.03) | −0.4 (−0.97, 0.2) | −0.2 (−0.6, 0.3) | 0.1 (−0.5, 0.6) | 0.04 (−0.2, 0.3) | −0.3 (−0.6, 0.05) |
| Functional status at discharge (ref = age-appropriate) | ||||||
| Mild disability | 0.3 (0.1, 1.2) | −16.4 (−33.7, 0.9) | −13.5* (−21.8, −5.2) | −4.0 (−14.3, 6.3) | −0.3 (−6.7, 6.1) | −6.0 (−16.9, 4.9) |
| Moderate disability | 0.2** (0.1, 0.5) | −18.9** (−27.8, −10.0) | −22.6** (−33.1, −12.2) | 1.6 (−5.7, 8.8) | −2.2 (−7.9, 3.6) | −13.2** (−20.7, −5.8) |
| Severe disability or vegetative state | 0.2** (0.1, 0.5) | −44.5** (−66.8, −22.1) | −26.8* (−42.8, −10.8) | −9.9 (−24.1, 4.4) | −10.0* (−19.3, −0.7) | −12.3* (−21.5, −3.1) |
Abbreviations: PICU, pediatric intensive care unit; OR, odds ratio; CI, confidence interval; PARDS, pediatric acute respiratory distress syndrome. Higher scores indicate better health-related quality of life.
P < .05
P < .001
PARDS severity was defined using the 2015 Pediatric Acute Lung Injury Consensus Conference (PALICC) criteria. (33)
Organ system dysfunction was measured continuously; every subject had respiratory dysfunction and dysfunction in up to five additional organ systems. (34)
In older children whose parents and/or guardians completed the PedsQL, income quartile was not associated with the total score. The emotional functioning subscore was associated with high middle income (−6.0; 95% CI [−11.9, −0.2]; P < .05, small effect size d = 0.35) and the school functioning subscore was associated with low middle Income (−8.6; 95% CI [−15.6, −1.6]; P < .05, small effect size d = 0.43) versus High Income (Supplemental Digital Content – Table S2). Age was associated with PedsQL scores; the youngest age category having higher scores than the other three age categories. In total score as well as all subscores, children in the 8 to <13 year old age category had the lowest scores of all age categories. In terms of functional status, children with moderate disability had the worst PedsQL scores in all domains compared with all other levels of functional status.
In a backward stepwise regression model controlling for income quartile and the same covariates noted above, two of the six resource use variables were associated with lower scores on the ITQOL’s growth and development domain: scores were 2.0 points lower for each additional healthcare provider (P < .001) and 2.6 points lower for each prescribed medication (P = .004). In another backward stepwise regression model controlling for the same covariates, three of the six resource use variables were associated with lower PedsQL total scores: 1.6 points lower for each prescribed medication (P = .04), 4.4 points lower if the child had visited the ED (P = .05), and 5.8 points lower if the child was readmitted (P = .02).
Discussion
Among children recovering from acute respiratory failure, there are differences in post-PICU resource use and quality of life according to income level. Six months after PICU discharge, lower income children received care from fewer different healthcare providers and used less newly prescribed medical equipment, despite ongoing healthcare needs. Quality of life in children under two years of age was affected by their physical inabilities, bodily pain/discomfort, and to a lesser extent, their temperament and mood with respect to income quartiles whereas no clinically important differences were found in older children. Despite younger children in low income quartiles experiencing decreased physical abilities and increased bodily pain/discomfort, they had decreased use of occupational and physical therapy. Overall, these findings indicate that income-based differences in resource use in all age groups and diminished HRQL in young children exist post-critical illness, which may be attributed to disparate allocation of resources.
A portion of children in each income quartile did not see a pediatrician in the six months after PICU discharge (10% lowest quartile; 19% highest quartile). More children in the lowest income quartile visited the ED and were readmitted to the hospital but had fewer healthcare providers and less newly prescribed medical equipment. One study that followed healthcare utilization for two years after PICU discharge found that one-fifth of the patients were referred to a specialist alone and one half were readmitted to the hospital, the majority of these to the PICU (46). It is possible that better care coordination post-PICU discharge could help identify patients at risk for readmission or preventable health problems? Even if resource utilization within the first six months after discharge is minimal, post-PICU sequelae may take time to emerge, particularly for children who are concurrently developing (47,48).
We report a disproportionate use of post-PICU resources, specifically in regard to active healthcare providers. Despite decreased physical abilities and increased bodily pain/discomfort in low income children less than 2 years old, only 9% of low and low middle income children used occupational/physical therapy services post-PICU. This is nearly half of what was reported to be used in the higher income quartiles. This discrepancy in services may indicate decreased access and/or disproportionate prescribing practices to occupational/physical therapy services in this vulnerable cohort. This finding is supported by research exploring social disparities, including income, in early intervention service use (49). Together, these data indicate the need for increased vigilance regarding early intervention services post-PICU, especially occupational and physical therapy to address physical function needs.
Measures of HRQL can reveal critical developments in a patient’s health not detected by clinical variables or physiological endpoints. Health in general, as well as growth and development, for young children did not differ by income quartile, but physical abilities were rated significantly lower for low income children. Regardless of income level, studies have shown that PICU survivors can be discharged with or subsequently develop impairments in physical functioning that may relate to illness course, treatments, or both (48, 50). Controlling for illness course variables, a large study conducted in French ICUs found that only the physical functioning items on the HRQL instrument were lower for low SES adults (51). Furthermore, there were no differences in mortality rates or length of stay in the same study. Because the low-income group in our study also had fewer healthcare providers and less new equipment in the home, it is possible that a SES difference in healthcare access is contributing to diminished parent-reported physical abilities.
We demonstrated that in all age groups that higher HRQL scores were associated with children having fewer prescribed medications, suggesting that these medications indicate ongoing health issues that substantially affect a child’s life. Interestingly, having in-home healthcare and newly prescribed medical equipment were not associated with HRQL after adjustment for other factors. For younger children, having more healthcare providers was associated with lower scores on the growth and development domain, but this was not the case for older children. It is possible that older children could have had ongoing health issues for longer periods of time, and families have become accustomed to actively seeing healthcare providers to maintain their children’s health or that a child’s age affects the family’s perceived burden of having more healthcare providers. Overall, increased awareness and anticipatory guidance at PICU discharge regarding these associations and a child’s quality of life is important among providers.
Strengths and Limitations
While the census-based methods described in this study were executed with accuracy, they may not reflect the actual income of each family. However, neighborhood-level income data can provide a reasonable approximation to individual-level income data (35–39). Our data reflected several trends that provide support for the use of this census-tract derived income method: children in the lowest income quartile were more likely to be Black or Hispanic and have a history of asthma; children in the highest income quartile were more likely to be White, with lower rates of asthma. Fewer low-income families who consented to follow-up actually completed it, potentially because of a change residential location due to housing instability. These demographic and health-related trends are well documented in U.S. health disparity literature, though less follow-up of lower income subjects is also a limitation of this study (52, 53). It is possible that an alternative measure of SES, such as health insurance status, may have impacted resource use and HRQL due to its association with healthcare access and lack of consistent primary care. While HRQL was assessed six months after PICU discharge, we cannot be certain that the HRQL outcomes are directly caused by critical illness or the PICU care and treatment. No baseline HRQL measurements were available to evaluate potential changes in HRQL and subsequent associations with SES.
Conclusion
Six months after PICU discharge, many children recovering from acute respiratory failure have ongoing healthcare resource use. After controlling for illness and functional status characteristics, children in the lowest income quartile had fewer healthcare providers managing their care, fewer medications prescribed post-PICU, and were less likely to have newly prescribed homecare medical equipment. Despite fewer healthcare providers and prescribed medications, children less than 2 years of age in the lowest income quartile experienced decreased HRQL in the domains of physical abilities, bodily pain/discomfort, and temperament and mood. PICU survivors require ongoing vigilance to identify emerging health concerns. Follow-up care could help identify children in need of healthcare resources and those at risk for decreased health-related quality of life.
Supplementary Material
Acknowledgements
The authors gratefully acknowledge the work of the RESTORE study investigators, who include: Martha A.Q. Curley (Principal Investigator; School of Nursing and the Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA; Critical Care and Cardiovascular Program, Boston Children’s Hospital, Boston, MA); David Wypij, (Principal Investigator - Data Coordinating Center; Department of Biostatistics, Harvard T.H. Chan School of Public Health; Department of Pediatrics, Harvard Medical School; Department of Cardiology, Boston Children’s Hospital, Boston, MA); Geoffrey L. Allen (Children’s Mercy Hospital, Kansas City, MO); Derek C. Angus (Clinical Research, Investigation, and Systems Modeling of Acute Illness Center, Pittsburgh, PA); Lisa A. Asaro (Department of Cardiology, Boston Children’s Hospital, Boston, MA); Judy A. Ascenzi (The Johns Hopkins Hospital, Baltimore, MD); Scot T. Bateman (University of Massachusetts Memorial Children’s Medical Center, Worcester, MA); Santiago Borasino (Children’s Hospital of Alabama, Birmingham, AL); Cindy Darnell Bowens (Children’s Medical Center of Dallas, Dallas, TX); G. Kris Bysani (Medical City Children’s Hospital, Dallas, TX); Ira M. Cheifetz (Duke Children’s Hospital, Durham, NC); Allison S. Cowl (Connecticut Children’s Medical Center, Hartford, CT); Brenda L. Dodson (Department of Pharmacy, Boston Children’s Hospital, Boston, MA); E. Vincent S. Faustino (Yale-New Haven Children’s Hospital, New Haven, CT); Lori D. Fineman (University of California San Francisco Benioff Children’s Hospital at San Francisco, San Francisco, CA); Heidi R. Flori (University of California at San Francisco Benioff Children’s Hospital at Oakland, Oakland, CA); Linda S. Franck (University of California at San Francisco School of Nursing, San Francisco, CA); Rainer G. Gedeit (Department of Pediatrics, Medical College of Wisconsin, Milwaukee, WI); Mary Jo C. Grant (Primary Children’s Hospital, Salt Lake City, UT); Andrea L. Harabin (National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, MD); Catherine Haskins-Kiefer (Florida Hospital for Children, Orlando, FL); James H. Hertzog (Nemours/Alfred I. duPont Hospital for Children, Wilmington, DE); Larissa Hutchins (The Children’s Hospital of Philadelphia, Philadelphia, PA); Aileen L. Kirby (Oregon Health & Science University Doernbecher Children’s Hospital, Portland, OR); Ruth M. Lebet (School of Nursing, University of Pennsylvania, Philadelphia, PA); Michael A. Matthay (University of California at San Francisco School of Medicine, San Francisco, CA); Gwenn E. McLaughlin (Holtz Children’s Hospital, Jackson Health System, Miami, FL); JoAnne E. Natale (University of California Davis Children’s Hospital, Sacramento, CA); Phineas P. Oren (St. Louis Children’s Hospital, St. Louis, MO); Nagendra Polavarapu (Advocate Children’s Hospital-Oak Lawn, Oak Lawn, IL); James B. Schneider (Cohen Children’s Medical Center of New York, Hyde Park, NY); Adam J. Schwarz (Children’s Hospital of Orange County, Orange, CA); Thomas P. Shanley (C. S. Mott Children’s Hospital at the University of Michigan, Ann Arbor, MI); Shari Simone (University of Maryland Medical Center, Baltimore, MD); Lewis P. Singer (The Children’s Hospital at Montefiore, Bronx, NY); Lauren R. Sorce (Ann & Robert H. Lurie Children’s Hospital of Chicago, Chicago, IL); Edward J. Truemper (Children’s Hospital and Medical Center, Omaha, NE); Michele A. Vander Heyden (Children’s Hospital at Dartmouth, Dartmouth, NH); R. Scott Watson (Center for Child Health, Behavior, and Development, Seattle Children’s Research Institute, Seattle, WA); Claire R. Wells (University of Arizona Medical Center, Tucson, AZ).
Copyright Form Disclosure:
Dr. Kachmar received funding from the Rita and Alex Hillman Foundation. Drs. Watson, Wypij, Perry, and Curley received support for article research from the National Institutes of Health (NIH). Dr. Wypij’s institution received funding from the National Institute of Child Health and Human Development (NICHD)/NIH. Dr. Perry’s institution received funding from the Eunice K. Shriver NICHD/NIH and the Alex and Rita Hillman Scholars Program. Dr. Curley received funding from the NICHD/NIH and the National Heart, Lung, and Blood Institute.
Conflicts of Interest and Sources of Funding:
No conflicts of interest. AGK was supported by the Rita & Alex Hillman Foundation. The Randomized Evaluation of Sedation Titration for Respiratory Failure study was supported by grants from the National Heart, Lung, and Blood Institute and the National Institute of Nursing Research, National Institutes of Health (U01HL086622 to Dr. Curley and U01 HL086649 to Dr. Wypij).
References
- 1.Society of Critical Care Medicine. Critical Care Statistics. [cited January 31, 2020]. Available from: http://www.sccm.org/Communications/Pages/CriticalCareStats.aspx.
- 2.Namachivayam P, Shann F, Shekerdemian L, et al. Three decades of pediatric intensive care: Who was admitted, what happened in intensive care, and what happened afterward. Pediatr Crit Care Med 2010; 11: 549–555. [DOI] [PubMed] [Google Scholar]
- 3.Pollack MM, Holubkov R, Funai T, et al. Pediatric intensive care outcomes: development of new morbidities during pediatric critical care. Pediatr Crit Care Med 2014; 15: 821–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pollack MM, Holubkov R, Funai T, et al. Simultaneous prediction of new morbidity, mortality, and survival without new morbidity from pediatric intensive care: a new paradigm for outcomes assessment. Crit Care Med 2015; 43: 1699–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shudy M, de Almeida ML, Ly S, et al. Impact of pediatric critical illness and injury on families: a systematic literature review. Pediatrics 2006; 118 Suppl 3: S203–218. [DOI] [PubMed] [Google Scholar]
- 6.Pinto NP, Rhinesmith EW, Kim TY, et al. Long-term function after pediatric critical illness: results from the survivor outcomes study. Pediatr Crit Care Med 2017; 18: e122–e130. [DOI] [PubMed] [Google Scholar]
- 7.Pollack MM, Banks R, Holubkov R, et al. Morbidity and mortality in critically ill children: I. pathophysiologies and potential therapeutic solutions. Critical Care Medicine 2020; 48: 790–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sachdeva R Functional outcomes in pediatric models. Current Opinion Crit Care 1997; 3: 179–182. [Google Scholar]
- 9.Taylor A, Butt W. The evaluation of outcome following paediatric intensive care: the. The evaluation of outcome following paediatric intensive care: the major issues identified 2000; 11: 239–244. [Google Scholar]
- 10.Sturms LM, van der Sluis CK, Groothoff JW, et al. The health-related quality of life of pediatric traffic victims. J Trauma 2002; 52: 88–94. [DOI] [PubMed] [Google Scholar]
- 11.Morrison AL, Gillis J, O’Connell AJ, et al. Quality of life of survivors of pediatric intensive care. Pediatr Crit Care Med 2002; 3: 1–5. [DOI] [PubMed] [Google Scholar]
- 12.Varni JW, Bendo CB, Nurko S, et al. Health-related quality of life in pediatric patients with functional and organic gastrointestinal diseases. J Pediatr 2015; 166: 85–90. [DOI] [PubMed] [Google Scholar]
- 13.Rodday A, Graham R, Weidner R, et al. Predicting health care ration for children with respiratory insufficiency using parent-proxy ratings of children’s health-related quality of life. J Pediatr Health Care 2017; 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seid M, Varni JW, Segall D, et al. Health-related quality of life as a predictor of pediatric healthcare costs: a two-year prospective cohort analysis. Health Qual Life Outcomes 2004; 2: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adler NE, Boyce T, Chesney MA, et al. Socioeconomic status and health. The challenge of the gradient. Am Psychol 1994; 49: 15–24. [DOI] [PubMed] [Google Scholar]
- 16.Anderson NB, Armstead CA. Toward understanding the association of socioeconomic status and health: a new challenge for the biopsychosocial approach. Psychosom Med 1995; 57: 213–225. [DOI] [PubMed] [Google Scholar]
- 17.Bunker J, Gomby D, Kehrer B. Pathways to Health: The Role of Social Factors. Menlo Park, CA: The Henry J. Kaiser Family Foundation; 1989. [Google Scholar]
- 18.Chen E Why socioeconomic status affects the health of children: a psychosocial perspective. Curr Dir Psychol Sci 2004; 12: 112–115. [Google Scholar]
- 19.Gornick ME. Measuring the effects of socioeconomic status on health care. In: Swift EK, editor. Guidance for the National Healthcare Disparities Report. Washington, DC: National Academies Press; 2002. [PubMed] [Google Scholar]
- 20.Nepomnyaschy L Socioeconomic gradients in infant health across race and ethnicity. Matern Child Health J 2009; 13: 720–731. [DOI] [PubMed] [Google Scholar]
- 21.Larson K, Halfon N. Family income gradients in the health and health care access of US children. Matern Child Health J 2010; 14: 332–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones S, Rantell K, Stevens K, et al. Outcome at 6 months after admission for pediatric intensive care: a report of a national study of pediatric intensive care units in the United kingdom. Pediatrics 2006; 118: 2101–2108. [DOI] [PubMed] [Google Scholar]
- 23.Simon AE, Chan KS, Forrest CB. Assessment of children’s health-related quality of life in the United States with a multidimensional index. Pediatrics 2008; 121: e118–126. [DOI] [PubMed] [Google Scholar]
- 24.Ward SL, Turpin A, Spicer AC, et al. Long-term pulmonary function and quality of life in children after Acute respiratory Distress Syndrome: a feasibility investigation. Pediatr Crit Care Med 2017; 18: e48–e55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martin-Herz SP, Zatzick DF, McMahon RJ. Health-related quality of life in children and adolescents following traumatic injury: a review. Clin Child Fam Psychol Rev 2012; 15: 192–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cassedy A, Drotar D, Ittenbach R, et al. The impact of socio-economic status on health related quality of life for children and adolescents with heart disease. Health Qual Life Outcomes 2013; 11: 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Curley MAQ, Wypij D, Watson RS, et al. Protocolized sedation vs usual care in pediatric patients mechanically ventilated for acute respiratory failure: a randomized clinical trial. JAMA 2015; 313: 379–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Curley MAQ, Gedeit RG, Dodson BL, et al. Methods in the design and implementation of the Randomized Evaluation of Sedation Titration for Respiratory Failure (RESTORE) clinical trial. Trials 2018; 19: 687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Watson RS, Asaro LA, Hertzog JH, et al. Long-Term Outcomes after Protocolized Sedation versus Usual Care in Ventilated Pediatric Patients. Am J Respir Crit Care Med 2018; 197: 1457–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Watson RS, Asaro LA, Hutchins L, et al. Risk factors for functional decline and impaired quality of life after pediatric respiratory failure. Am J Respir Crit Care Med 2019; 200: 900–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fiser DH. Assessing the outcome of pediatric intensive care. The Journal of Pediatrics 1992; 121: 68–74. [DOI] [PubMed] [Google Scholar]
- 32.Pollack MM, Patel KM, Ruttimann UE. PRISM III: an updated Pediatric Risk of Mortality score. Crit Care Med 1996; 24: 743–752. [DOI] [PubMed] [Google Scholar]
- 33.Pediatric Acute Lung Injury Consensus Confrence Group (PALICC). Pediatric acute respiratory distress syndrome: consensus recommendations from the Pediatric Acute Lung Injury Consensus Conference. Pediatr Crit Care Med 2015; 16: 428–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weiss SL, Asaro LA, Flori HR, et al. Multiple organ dysfunction in children mechanically ventilated for acute respiratory failure. Pediatr Crit Care Med 2017; 18: 319–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Braveman P, Cubbin C, Egerter S, et al. Socioeconomic status in health research: one size does not fit all. JAMA 2005; 294: 2879 – 2888. [DOI] [PubMed] [Google Scholar]
- 36.Yen IH, Syme SL. The social environment and health: a discussion of the epidemiologic literature. Annu Rev Public Health 1999; 20: 287–308. [DOI] [PubMed] [Google Scholar]
- 37.Robert S Socioeconomic position and health: the independent contribution of community socioeconomic context. Annu Rev Sociol 1999; 25: 489–516. [Google Scholar]
- 38.Kachmar AG, Connolly CA, Wolf S, et al. Socioeconomic status in pediatric health research: A scoping review. J Pediatr 2019; 213: 163–170. [DOI] [PubMed] [Google Scholar]
- 39.Sampson R, Morenoff J, Gannon-Rowley T. Assessing “neighborhood effects”: social processes and new directions in research. Annu Rev Sociol 2002; 28: 443–478. [Google Scholar]
- 40.Raat H, Landgraf JM, Oostenbrink R, et al. Reliability and validity of the Infant and Toddler Quality of Life Questionnaire (ITQOL) in a general population and respiratory disease sample. Qual Life Res 2007; 16: 445–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Varni JW, Seid M, Kurtin PS. PedsQL 4.0: reliability and validity of the Pediatric Quality of Life Inventory version 4.0 generic core scales in healthy and patient populations. Med Care 2001; 39: 800–812. [DOI] [PubMed] [Google Scholar]
- 42.Varni JW, Burwinkle TM, Seid M, Skarr D. The PedsQL 4.0 as a pediatric population health measure: feasibility, reliability, and validity. Ambul Pediatr 2003;3:349–341. [DOI] [PubMed] [Google Scholar]
- 43.HealthActCHQ Inc. U.S. based norms: The infant toddler quality of life questionnaire (ITQOL-97). Boston, MA: HealthActCHQ, 2017. [Google Scholar]
- 44.Cohen J Statistical power analysis for the behavioral sciences, 2nd edition. New York, NY: Routledge, 1988. [Google Scholar]
- 45.Bewick J, Cheek L, Ball J. Statistics review 10: Further nonparametic methods. Pediatr Crit Care Med 2004; 8: 196–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yagiela LM, Barbaro RP, Quasney MW, et al. Outcomes and patterns of healthcare utilization after hospitalization for pediatric critical illness due to respiratory failure. Pediatr Crit Care Med 2019; 20: 120–127. [DOI] [PubMed] [Google Scholar]
- 47.Needham DM, Davidson J, Cohen H, et al. Improving long-term outcomes after discharge from intensive care unit: report from a stakeholders’ conference. Crit Care Med 2012; 40: 502–509. [DOI] [PubMed] [Google Scholar]
- 48.Manning JC, Pinto NP, Rennick JE, et al. Conceptualizing Post- Intensive Care Syndrome in children-The PICS-p framework. Pediatr Crit Care Med 2018. [DOI] [PubMed] [Google Scholar]
- 49.Khetani MA, Richardson Z, McManus BM. Social disparities in early intervention service use and provider-reported outcomes. J Dev Behav Pediatr 2017; 38(7): 501–509. doi: 10.1097/DBP.0000000000000474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van Zellem L, Utens EM, de Wildt SN, et al. Analgesia-sedation in PICU and neurological outcome: a secondary analysis of long-term neuropsychological follow-up in meningococcal septic shock survivors. Pediatr Crit Care Med 2014; 15: 189–196. [DOI] [PubMed] [Google Scholar]
- 51.Bastian K, Hollinger A, Mebazaa A, et al. Association of social deprivation with 1-year outcome of ICU survivors: results from the FROG-ICU study. Intensive Care Med 2018; 44: 2025–2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gem J The urban environment and childhood asthma study. J Allergy Clin Immunol 2010; 125: 545–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Flores G, Committee on Pediatric Research. Technical report--racial and ethnic disparities in the health and health care of children. Pediatrics 2010; 125: e979–e1020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


