Abstract
Background:
In the United States, methadone for treatment of opioid use disorder is dispensed via highly-regulated accredited opioid treatment programs (OTP). During the COVID-19 pandemic, federal regulations were loosened, allowing for greater use of take-home methadone doses. We sought to understand how OTP leaders responded to these policy changes.
Methods:
We distributed a multistate electronic survey from September to November 2020 of OTP leadership to members of the American Association for the Treatment of Opioid Dependence (AATOD) who self-identified as leaders of OTPs. We asked study participants about how their OTP(s) implemented COVID-19-related policy changes into their clinical practice focusing on provision of take-home methadone doses, factors used to determine patient stability, and potential concerns about increased take-home doses. We used Chi-square test to compare survey responses between characterizations of the OTPs.
Results:
Of 170 survey respondents (17% response rate), the majority represented leadership of for-profit OTPs (69%) and were in a Southern state (54%). Routine allowances and practices related to take-home methadone doses varied across OTPs during the COVID-19 pandemic: 80 (47%) reported 14 days for newly enrolled patients (within past 90 days), 89 (52%) reported 14 days for “less stable” patients, and 112 (66%) reported 28 days for “stable” patients.
Conclusions:
We found that not all eligible OTP leaders adopted the practice of routinely allowing newly enrolled, “less stable,” and “stable” patients on methadone to have increased take-home doses up to the limit allowed by federal regulations during COVID-19. The pandemic provides an opportunity to critically re-evaluate long-established methadone and OTP regulations in preparation for future emergencies.
Keywords: Methadone, opioid-related disorders, COVID-19, survey, opioid treatment programs
Introduction
In response to the COVID-19 pandemic the Substance Abuse and Mental Health Services Administration (SAMHSA) and Drug Enforcement Administration (DEA) released guidance allowing for broad increases of take-home methadone doses.1 These are methadone doses that an opioid treatment program (OTP) can administer to patients being treated for opioid use disorder (OUD) for unobserved consumption on subsequent days. Specifically, states could request “blanket exceptions for all stable patients” to receive up to 28 days of take-home doses, and for “less stable” patients, up to 14 days of take-home methadone doses. While these changes sought to limit congregant settings and reduce the spread of COVID-19, they stood in contrast to the heavily regulated approach to methadone treatment where OTPs were required to follow specific federal and state regulations. It was unknown how OTPs would incorporate this guidance into practice.
Prior to the COVID-19 pandemic, some public health and addiction medicine experts called for expansion of methadone prescribing in primary care settings to improve access to care.2,3 Other countries allow for pharmacy-dispensed dosing4-6 which, in the U.S., would significantly increase access, particularly in rural communities.7-9 However, despite the worsening opioid overdose epidemic, the U.S. regulatory framework (42 CFR 8) for dispensing methadone at OTPs continued mostly unchanged.10,11 Additionally, states, localities, federal oversight agencies, payors, and accreditation bodies provide regulatory oversight which has created a patchwork of policies across the U.S.12
Concerns for diversion of take-home methadone and the risk of methadone-related overdose motivated these strict take-home methadone policies.13 Previously, all newly enrolled or unstable patients presented to the OTP daily to receive their methadone dose. The only exception is on anticipated closures (weekend days and holidays) when all patients receive take-home methadone. OTPs can request exceptions, for example if a patient has an acute illness, to the requirement to dose daily.14 Additional take-home methadone doses are allowed, but not required, after the program medical director considers federally-established criteria including time in treatment, absence of substance use, clinic attendance, home and relational stability, and others.15 Federal regulations permit up to six take-home doses (one-week supply) after nine months of continuous treatment engagement at the same OTP clinic, thirteen take-home doses (two-week supply) after one year, and 27 take-home doses (one-month supply) after two years.
There is no precedent for federal regulators allowing for state-wide blanket exceptions to take-home methadone dosing. A rapid review of the literature evaluated impacts of large-scale events such as political and economic upheavals and natural disasters, but no prior research on respiratory pandemics existed to inform our research.16 As the COVID-19 pandemic progressed, evidence from Oregon demonstrated a 97% increase in take-home methadone doses after implementing the guidance;17 Connecticut OTPs increased take-home methadone doses without an increase in methadone-related overdoses.18 However, less is known about how OTP leaders, who ultimately determine if patients received take-home methadone doses, responded to the changes. Understanding how OTP leaders applied these changes could have broad OUD treatment policy implications in the post-COVID-19 era including determination of patient stability for take-home methadone, frequency and type of monitoring requirements (urine drug testing), and use of telemedicine. Variation of practice in certain states or regions could prompt policy clarification or standardization. Therefore, we conducted a multistate survey of OTP leaders to assess (1) how their OTPs changed take-home methadone provisions, (2) factors they considered in determining if patients were “stable” and “less stable,” and (3) potential concerns surrounding expanded access to take-home methadone doses.
Methods
We distributed an online survey to regional and state chapter members of the American Association for the Treatment of Opioid Dependence (AATOD)19 which include 28 states and the District of Columbia. AATOD national leadership sent email solicitations to the AATOD Board of Directors in September and November 2020 requesting they complete the survey and distribute an email to their chapter members with a link to the survey. The survey remained active throughout the 3-month period. Inclusion criteria were: (1) self-identified as an OTP leader who could describe their OTP’s response to the COVID-19 pandemic and (2) provided responses for the primary outcomes. Survey responses were collected with Research Electronic Data Capture (REDCap).20
Three primary outcomes related to OTP policy changes were considered. First, to assess the provision of take-home methadone doses, participants were asked if their OTP routinely allowed 14-day take-home methadone doses for new patients enrolled in the past 90 days and for “less stable” patients; or for 28-day take-home methadone doses for “stable” patients. These timeframes were based on SAMHSA guidance.21 A specific definition of “less-stable” or “stable” was not provided in the SAMHSA guidance or in the survey. Participants reported if their State Opioid Treatment Authority (SOTA) provided additional guidance restricting take-home methadone doses. Second, participants were asked their opinion of the top-3 patient-related factors that are the most important when determining patient stability (Figure 2). We used the criteria listed in federal guidelines that determine methadone take-home eligibility to create a list of factors.15 We added specific individual COVID-19 risks (i.e. patients with co-morbid medical condition and need to use public transportation) and the counselor’s general assessment of stability. Participants picked top-3 factors from the full list of 17 rather than rank-order all items to reduce survey burden and focus on the most important factors to determine patient stability. Finally, participants were asked about their level of concern for 6 potential program-related outcomes affected by the relaxed take-home methadone policies rated on a 5-point Likert scale (Figure 3). To our knowledge, there had not been previous studies evaluating the effect of increased take-home doses on OTPs. Therefore, we engaged with key-informants to identify potential program-related concerns. These informants were 10 addiction medicine clinicians and OTP leaders who were contacted by email, in-person discussion, and two group meetings. We identified the six most common concerns for inclusion based on their feedback.
Figure 2.
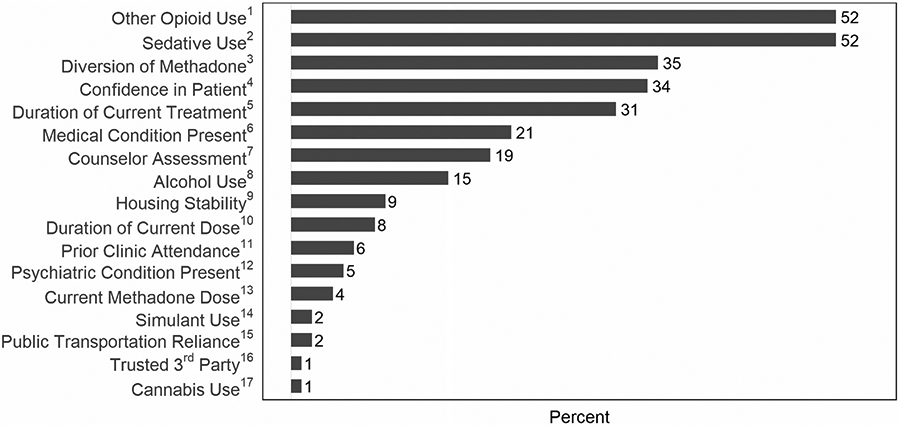
Participants’ top-3 patient factors to consider in determining patient stability. Participants were shown a list of factors (verbatim text is below) and asked to pick the top-3 they believed were most important when determining patient stability. The percentages represent how often each factor was chosen in the top-3 most important factors by participants (n = 170).
1“Patient report or drug testing suggesting ongoing illicit opioid use.”
2“Patient report or drug testing suggesting ongoing benzodiazepine or sedative use.”
3“Concern for patient possibly diverting take-home methadone.”
4“Confidence that patient will take methadone take-home medication daily as directed.”
5“Length of time on current treatment episode (time enrolled at your OTP).”
6“Presence of other chronic medical conditions (i.e heart or lung disease) that make the patient at higher risk of morbidity/mortality from COVID-19.”
7“Counselor’s assessment of stability.”
8“Patient report or drug testing suggesting ongoing alcohol use.”
9“Housing assessment (i.e. stable housing, safe storage, or other substance use in household).”
10“Length of time on current methadone dose.”
11“Prior Group and/or clinic attendance.”
12“Presence of inadequately controlled/treated co-occurring psychiatric disorders.”
13“How high the patient’s dose of methadone is (total mg per day).”
14“Patient report or drug testing suggesting ongoing stimulant use (i.e cocaine or methamphetamine use).”
15“Patient must use public transportation to travel.”
16 “A trusted third party (or proxy) to manage doses.”
17“Patient report or drug testing suggesting ongoing cannabis/marijuana use.”
Figure 3.
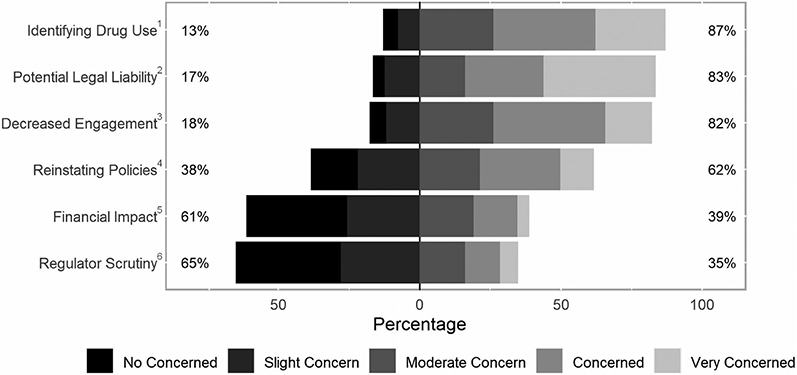
Level of concern of increased take-home doses. Participants were shown a list of potential outcomes (verbatim text below) and reported on a 5-point Likert scale their level of concern. Left column percentages represent the cumulative percentage reporting “No Concern” or “Slight concern.” Right column percentages represent the cumulative percentage reporting “Moderate Concern”, “Concerned” or “Very Concerned.”
1“Inability to identify when patient’s relapse/return to drug use”
2“Potential legal liabilities for diversion of, or overdose from, extra take-home doses of methadone”
3“Decreased patient engagement in counseling”
4“Difficulty around reinstating policies after the health emergency is over (i.e. taking back take-homes or reinforcing previous policies).”
5“Financial impact from decreased billable services.”
6“Potential for increased scrutiny from SOTA/DEA.”
Initial data analysis characterized the sample using descriptive statistics. Missing values were not imputed and are reported as missing. We compared outcomes between the type of OTP (for-profit, not-for-profit, or other) and OTP setting (urban, suburban, and rural). Likert response were dichotomized to “no to low concern” (1–2) versus “moderate to very concerned” (3–5). The association between take-home doses and level of concern was assessed using Chi-square test on complete cases. If the cell count was ≤5, we simulated the p-value using 2000 replicates.22 Analysis was completed using R with the “likert” package.23,24 The study was approved by the Johns Hopkins University Institutional Review Board.
Results
There were an estimated 1,015 potential participants with a total of 170 surveys (17% response rate) included in the analysis (Figure 1). The median number of individual OTPs managed was 1 (IQR 1–3) and the median number of patients served by each OTP was 415 (IQR 236–800). Most participants (71%) were administrative leaders, from for-profit OTPs (69%), and from the South (54%) (Table 1).
Figure 1.
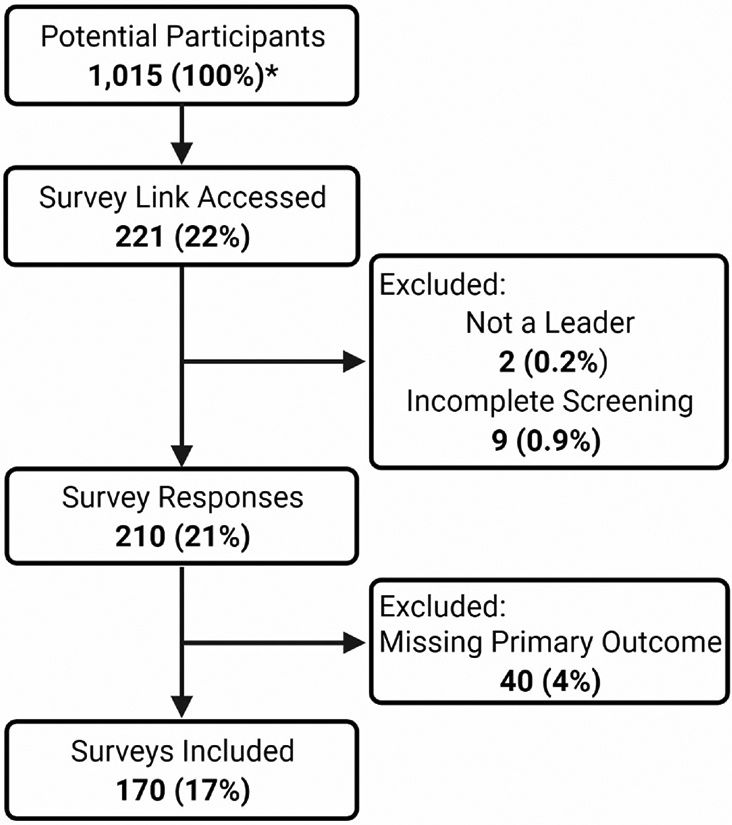
Flow diagram of study participants. *American Association for the Treatment of Opioid Dependence (AATOD) is comprised of 29 regional chapters with each chapter made up of opioid treatment program (OTP) members. OTP members potentially have multiple individuals in leadership positions who were included in the sampling frame. AATOD leadership estimated 1015 potential participants. The percentages are calculated using the number of potential participants as the denominator.
Table 1.
Participant Characteristics.
| Characteristic (n = 170) | Frequency (%) |
|---|---|
| Role | |
| Medical Director | 13 (8%) |
| Administrative leader | 121 (71%) |
| Nursing leader | 4 (2%) |
| Clinical leader | 9 (5%) |
| Other | 5 (11%) |
| Missing | 5 (3%) |
| Type of opioid treatment program | |
| Nonprofit | 38 (22%) |
| For-profit | 117 (69%) |
| Other | 10 (6%) |
| Missing | 5 (3%) |
| Opioid treatment program setting | |
| Urban/Large metropolitan area | 62 (37%) |
| Suburban/Medium metropolitan area | 78 (46%) |
| Rural or frontier | 25 (15%) |
| Missing | 5 (3%) |
| Insurance accepted | |
| Medicaid | 144 (85%) |
| Medicare | 141 (83%) |
| Private insurance | 104 (61%) |
| Self-pay/Sliding Scale | 113 (78%) |
| Government/VA | 78 (46%) |
| Census region | |
| Northeast | 10 (6%) |
| Midwest | 26 (15%) |
| South | 92 (54%) |
| West | 36 (21%) |
| Missing | 6 (4%) |
Provision of take-home doses
Of the 170 participants, 80 (47%) reported routinely allowing 14 days of take-home methadone doses for newly enrolled (past 90 days) patients, 89 (52%) reported routinely allowing for 14 days of take-home methadone doses for “less stable” patients, and 112 (66%) reported routinely allowing for 28 days of take-home methadone doses for “stable” patients.
Compared to SAHMSA guidance, 55 (32%) of participants reported more restrictive SOTA guidance for take-home methadone doses. Seven (4%) participants did not know about a difference in their SOTA policies. Respondents in states with more restrictive guidance versus same or unknown guidance reported a comparable proportion of routine take-home methadone doses for newly enrolled patients (53 vs. 46%; p = 0.54), “less stable” patients (44 vs. 56%; p = 0.17), and “stable” patients (64 vs. 67%; p = 0.83), respectively.
Factors determining patient stability and Take-Home methadone dose eligibility
The two most common factors for determining stability were ongoing non-prescribed opioid use (52%) and sedative use (52%). Figure 2 shows the proportion each factor was reported in the top-3.
Level of concern for increased take-home methadone doses
Participant’s level of concern was generally higher for difficulty identifying when a patient had returned to drug use, potential legal liabilities related to overdose from or diversion of take-home methadone doses, decreased engagement in treatment at the OTP, and reinstating policies after the public health emergency is over. The distribution of responses for each concern is presented in Figure 3.
Comparison by type of OTP and OTP setting on outcomes
We found no difference in the provision of take-home methadone doses for newly enrolled, “less-stable,” or “stable” patients across type of OTP or OTP setting (Table 2). For most ratings on the level of concern there was no difference by type of OTP or OTP setting with the exception for the financial impact of decrease in billable services. Of participants from nonprofit clinics, 58% reported they were moderate-to-very concerned about the financial impact related to these policy changes compared to 33% from for-profit and 33% from other types of clinics (p = 0.02). A post-hoc analysis comparing participants from nonprofit and for-profit suggested these two groups differed significantly on the proportion reporting no/low concern and moderate-to-very concerned (p = 0.01).
Table 2.
Comparison of the provision of take-home doses and level of concern for increased take-home doses by type of opioid treatment program and settinga.
| Type of opioid treatment program | Opioid treatment program setting | |||||
|---|---|---|---|---|---|---|
| Provision of take-home doses | ||||||
| Nonprofit | For-profit | Other | Urban | Suburban | Rural | |
| New patient | ||||||
| Yes | 19 (50%) | 57 (49%) | 3 (33%) | 29 (47%) | 35 (45%) | 15 (60%) |
| No | 19 (50%) | 60 (51%) | 6 (67%) | 33 (53%) | 42 (55%) | 10 (40%) |
| Less stable | ||||||
| Yes | 20 (53%) | 62 (53%) | 5 (50%) | 33 (53%) | 40 (51%) | 14 (56%) |
| No | 18 (47%) | 55 (47%) | 5 (50%) | 29 (47%) | 38 (49%) | 11 (44%) |
| Stable | ||||||
| Yes | 25 (66%) | 77 (66%) | 7 (70%) | 42 (68%) | 51 (65%) | 16 (64%) |
| No | 13 (34%) | 40 (34%) | 3 (30%) | 20 (32%) | 27 (35%) | 9 (36%) |
| Level of concern for increased take-home dosesb | ||||||
| Difficult identifying substance use | ||||||
| No-low | 6 (16%) | 14 (12%) | 1 (10%) | 11 (18%) | 8 (10%) | 2 (8%) |
| Moderate-very | 32 (84%) | 102 (88%) | 9 (90%) | 51 (82%) | 69 (90%) | 23 (92%) |
| Potential legal liability | ||||||
| No-low | 10 (26%) | 16 (14%) | 0 (0%) | 11 (18%) | 12 (16%) | 3 (12%) |
| Moderate-very | 28 (74%) | 101 (86%) | 9 (100%) | 51 (82%) | 65 (84%) | 22 (88%) |
| Decreased engagement | ||||||
| No-low | 4 (11%) | 23 (20%) | 2 (22%) | 12 (20%) | 13 (17%) | 4 (16%) |
| Moderate-very | 34 (89%) | 94 (80%) | 7 (78%) | 49 (80%) | 65 (83%) | 21 (84%) |
| Difficulty reinstating policies | ||||||
| No-low | 20 (53%) | 40 (34%) | 3 (33%) | 24 (39%) | 28 (36%) | 11 (44%) |
| Moderate-very | 18 (47%) | 77 (66%) | 6 (67%) | 37 (61%) | 50 (64%) | 14 (56%) |
| Financial impactc | ||||||
| No-low | 16 (42%) | 78 (67%) | 6 (67%) | 39 (64%) | 46 (60%) | 15 (60%) |
| Moderate-very | 22 (58%) | 38 (33%) | 3 (33%) | 22 (36%) | 31 (40%) | 10 (40%) |
| Increased state or federal scrutiny | ||||||
| No-low | 30 (79%) | 73 (62%) | 4 (44%) | 45 (74%) | 48 (62%) | 14 (56%) |
| Moderate-very | 8 (21%) | 44 (38%) | 5 (56%) | 16 (26%) | 30 (38%) | 11 (44%) |
We used a Chi-Square test on complete cases, n = 164; Six participants (4%) out of 170 did not provide the type of opioid treatment program or setting and were not included in the analysis.
Specific prompts for each concern are in Figure 3.
p = 0.02 For the comparison of level of concern by type of opioid treatment program.
The frequency of the top-3 factors used to determine stability was the same across type of OTP and OTP setting and matched the overall sample (Figure 2).
Discussion
In this survey of OTP leaders’ practices in response to changes in take-home methadone guidance during the COVID-19 pandemic, approximately half of respondents reported routinely dispensing 14-day take-home methadone doses to newly enrolled and “less stable” patients and approximately two-thirds reported routinely dispensing 28-day take-home methadone doses to “stable” patients. Nearly one-third of respondents reported their SOTA provided “more restrictive” take-home methadone guidance than what SAMHSA allowed suggesting state-level policies may have hindered take-home methadone dosing, consistent with known variability in state-level OTP policies.12
Ongoing opioid use or sedative use were top factors in determining patient stability. This is understandable given the detection of ongoing opioid or sedative use is easily accessible with toxicology testing and provides objective data to OTP leaders. Also the use of these substances is a risk for overdose-related mortality.25 Notably, the concern for possible methadone diversion was also common and potentially the easiest to address. Newer strategies including electronic pillboxes or smartphone app technologies allow for controlled and/or direct observation of take-home methadone dispensing and consumption.26-28 Routine implementation of this technology to mitigate diversion may overcome this known barrier to take-home dosing of methadone.13
While the goal was to reduce COVID-19 transmission through increased take-home methadone doses, OTP leaders were concerned about how these changes could negatively impact their programs. OTPs provide support and accountability for people with OUD and by increasing take-home methadone it was more difficult to complete these core functions. Also, OTP leaders are acclimated with the strict requirements of methadone take-home doses and the sudden change in this highly regulated environment potentially explains why participants were concerned about the legal liabilities associated with take-home methadone. Fortunately, many of these concerns could be addressed through technology to limit diversion and with research that clarifies the actual incidence of adverse outcomes such as methadone-related overdose.
OTPs require income generated from care delivery that could be reduced through the significant increase of take-home methadone. Overall, most OTP leaders were less concerned about the financial impact and there was a small but statistically difference between nonprofit and for-profit OTPs. The ability of an OTP to change practice to deliver telemedicine/virtual services might have mitigated funding concerns overall and explain the differences between for-profit and nonprofit OTPs.29 By continuing to allow for telemedicine and reimbursing at a sufficient rate, OTPs could stabilize or increase deliverable services, improve their financial stability, and increase patient access.30
Limitations of our study include the convenient sampling method of AATOD members which potentially limit generalizability; only states with AATOD members could be included and states that experienced significant initial COVID-19 surges were not represented. While we were able to estimate the total number of potential participants, this sampling method did not allow us to determine if there were systematic differences between responders and non-responders. In addition, this study determined the opinions of program leaders regarding important factors for determining patient stability for take-home eligibility. Actual patient-level decisions could have been determined on differently held opinions. A study of all Connecticut-based OTP clinics found variability in how leadership used the federal criteria to determine eligibility for take-home dosing during the COVID-19 pandemic.18 The relaxation of federal rules allowed for OTP medical providers to have more flexibility when determining patient stability,31 and characterizing how this was implemented in practice could shape future OTP policy reform. Finally, our survey questions were reviewed by addiction experts and pilot tested but were not formally validated.
The pandemic has created the circumstance to revisit numerous policies around addiction treatment,32 particularly in reconsidering what patient-centered methadone treatment should look like moving forward post-pandemic.33,34 While some patients may require the pre-pandemic high level of supervision of OTPs, many may not. Pharmacy-based methadone dosing, technologies including time-release lockboxes and smartphone apps, and reimbursing tele-health services could be a way to mitigate OTP leaders’ and regulators’ concerns while providing more patient-centered care and addressing the structural racism inherent in OTP federal policies.7,11 Our study provides evidence to help guide future public health and policy changes and highlights potential research opportunities to improve the care for people with OUD.
Acknowledgments
We wish to thank Mark Parrino, President of the American Association for the Treatment of Opioid Dependence for facilitating survey distribution. We would like to acknowledge Research in Addiction Medicine Scholars (RAMS) program fellows and the faculty and fellows of the Division of General Internal Medicine at Oregon Health & Science University School of Medicine and Johns Hopkins University School of Medicine for their feedback on the survey development. Figure 1 created with BioRender.com. The funding organizations had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Funding
XAL was supported by the Samuel H. Wise General Internal Medicine Fellowship and the Research in Addiction Medicine Scholars Program [R25DA033211]. JDP was supported by the Research in Addiction Medicine Scholars Program [R25DA033211] and the National Heart, Lung, and Blood Institute [T32 HP10025]. PTK was supported by the National Institutes of Health awards UH3DA044831 and UG1DA015815. GC was supported by the National Institute on Alcohol Abuse and Alcoholism [K24 AA027483].
Footnotes
Disclosure statement
KBS serves on the Boards of Directors of the American Association for the Treatment of Opioid Dependence, and the Maryland Association for the Treatment of Opioid Dependence. He also serves on the Center for Substance Abuse Treatment National Advisory Council of the U.S. Department of Health and Human Services, Substance Abuse and Mental Health Administration. The other authors – XAL, JDP, PTK, and GC – declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. No potential conflict of interest was reported by the author(s).
References
- [1].Substance Abuse and Mental Health Services Administration. Opioid treatment program (OTP) guidance. Substance Abuse and Mental Health Services Administration; 2020. https://www.samhsa.gov/sites/default/files/otp-guidance-20200316.pdf. [Google Scholar]
- [2].Kleinman RA, Morris NP. Federal barriers to addressing the opioid epidemic. J Gen Intern Med. 2020;35(4):1304–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Samet JH, Botticelli M, Bharel M. Methadone in primary care – one small step for congress, one giant leap for addiction treatment. N Engl J Med. 2018;379(1):7–8. [DOI] [PubMed] [Google Scholar]
- [4].Joudrey PJ, Edelman EJ, Wang EA. Methadone for opioid use disorder – decades of effectiveness but still miles away in the US. JAMA Psychiatry. 2020;77(11):1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Priest KC, Gorfinkel L, Klimas J, Jones AA, Fairbairn N, McCarty D. Comparing Canadian and United States opioid agonist therapy policies. Int J Drug Policy. 2019;74:257–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Calcaterra SL, Bach P, Chadi A, et al. Methadone matters: what the United States can learn from the global effort to treat opioid addiction. J Gen Intern Med. 2019;34(6):1039–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Joudrey PJ, Chadi N, Roy P, et al. Pharmacy-based methadone dispensing and drive time to methadone treatment in five states within the United States: a cross-sectional study. Drug Alcohol Depend. 2020; 211:107968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Johnson Q, Mund B, Joudrey PJ. Improving rural access to opioid treatment programs. J Law Med Ethics. 2018;46(2):437–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kleinman RA. Comparison of driving times to opioid treatment programs and pharmacies in the US. JAMA Psychiatry. 2020; 77(11):1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Centers for Disease Control and Prevention. Overdose deaths accelerating during COVID-19. Health Alert Network Advisory. https://www.cdc.gov/media/releases/2020/p1218-overdose-deaths-covid-19.html. Published December 2020. Accessed February 10, 2021. [Google Scholar]
- [11].Peterkin A, Davis CS, Weinstein Z. Permanent methadone treatment reform needed to combat the opioid crisis and structural racism. Journal of Addiction Medicine. 2021;. doi: 10.1097/ADM.0000000000000841 [DOI] [PubMed] [Google Scholar]
- [12].Jackson JR, Harle CA, Silverman RD, Simon K, Menachemi N. Characterizing variability in state-level regulations governing opioid treatment programs. Journal of Substance Abuse Treatment. 2020;115:108008. [DOI] [PubMed] [Google Scholar]
- [13].U.S. Government Accountability Office. Methadone-Associated Overdose Deaths: Factors Contributing to Increased Deaths and Efforts to Prevent Them; 2009. GAO-09-341. www.gao.gov/assets/gao-09-341.pdf. Accessed May 28, 2021. [Google Scholar]
- [14].Peles E, Schreibe S, Sason A, Adelson M. Earning “Take-home” privileges and long-term outcome in a methadone maintenance treatment program. Journal of Addiction Medicine. 2011;5(2):92–98. [DOI] [PubMed] [Google Scholar]
- [15].Substance Abuse and Mental Health Services Administration (SAMHSA). Federal Guidelines for Opioid Treatment Programs. Rockville, MD: U.S. Department of Health and Human Services; 2015. [Google Scholar]
- [16].Zolopa C, Hoj S, Bruneau J, et al. A rapid review of the impacts of “big events” on risks, harms, and service delivery among people who use drugs: implications for responding to COVID-19. Int J Drug Policy. 2021;92:103127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].McIlveen JW, Hoffman K, Priest KC, Choi D, Korthuis PT, McCarty D. Reduction in Oregon’s medication dosing visits after the SARS-CoV-2 relaxation of restrictions on take-home medication. Journal of Addiction Medicine. 2021. doi: 10.1097/adm.0000000000000812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Brothers S, Viera A, Heimer R. Changes in methadone program practices and fatal methadone overdose rates in Connecticut during COVID-19. J Subst Abuse Treat. 2021;131:108449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].American Association for the Treatment of Opioid Dependence, Inc. (AATOD). Our board of directors. http://www.aatod.org/about-us/our-board-of-directors/. Accessed June 4, 2021.
- [20].Harris PA, Taylor R, Minor BL, REDCap Consortium, et al. The REDCap consortium: Building an international community of software platform partners. J Biomed Inform. 2019;95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Substance Abuse and Mental Health Services Administration. Opioid Treatment Program (OTP) Guidance; 2020. https://www.samhsa.gov/sites/default/files/otp-guidance-20200316.pdf. Updated March 29, 2020. Accessed May 28, 2020.
- [22].Hope ACA. A simplified Monte Carlo Significance Test. J R Stat Soc. 1968;30(3):582–598. [Google Scholar]
- [23].R Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2020. [Google Scholar]
- [24].Bryer J, Speerschneider K. Likert: analysis and visualization likert items. R package version 1.3.5; 2016. [Google Scholar]
- [25].Xu KY, Hartz SM, Borodovsky JT, Bierut LJ, Grucza RA. Association between benzodiazepine use with or without opioid use and all-cause mortality in the United States, 1999–2015. JAMA Netw Open. 2020;3(12):e2028557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Dunn KE, Brooner RK, Stoller KB. Technology-assisted methadone take-home dosing for dispensing methadone to persons with opioid use disorder during the Covid-19 pandemic. J Subst Abuse Treat. 2021; 121:108197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kidorf M, Brooner RK, Dunn KE, Peirce JM. Use of an electronic pillbox to increase number of methadone take-home doses during the COVID-19 pandemic. J Subst Abuse Treat. 2021;126:108328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Godersky ME, Klein JW, Merrill JO, et al. Acceptability and feasibility of a mobile health application for video directly observed therapy of buprenorphine for opioid use disorders in an office-based setting. J Addict Med. 2020;14(4):319–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Alegría M, Frank RG, Hansen HB, Sharfstein JM, Shim RS, Tierney M. Transforming mental health and addiction services. Health Aff. 2021;40(2):226–234. [DOI] [PubMed] [Google Scholar]
- [30].Hunter SB, Dopp AR, Ober AJ, Uscher-Pines L Uscher-Pines L Clinician perspectives on methadone service delivery and the use of telemedicine during the COVID-19 pandemic: a qualitative study. J Subst Abuse Treat. 2021;124:108288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Hatch-Maillette MA, Peavy KM, Tsui JI, Banta-Green CJ, Woolworth S, Grekin P. Rethinking patient stability for methadone in opioid treatment programs during a global pandemic: provider perspectives. J Subst Abuse Treat. 2021;124(November 2020):108223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Bao Y, Williams AR, Schackman BR. COVID-19 could change the way we respond to the opioid crisis-for the better. Psychiatr Serv. 2020;71(12):1214–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Greenblatt AD, Magidson JF, Belcher AM, Gandhi D, Weintraub E. Overdue for an overhaul: how opioid treatment programs can learn from COVID-19. Mayo Clin Proc. 2020;95(10):2076–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Joseph G, Torres-Lockhart K, Stein MR, Mund PA, Nahvi S. Reimagining patient-centered care in opioid treatment programs: Lessons from the Bronx during COVID-19. J Subst Abuse Treat. 2021;122(November):108219. [DOI] [PMC free article] [PubMed] [Google Scholar]


