Abstract
Objective
To describe the incidence and associated risk factors of New Onset Anisocoria (NOA) (new pupil size difference of at least 1mm) and its subtypes: NOA accompanied by abnormal (NOA-Abnormal) and normal (NOA-Normal) pupil reactivity in patients with acute neurological injuries.
Design
We tested the association of patients who experienced NOA subtypes with degree of midline shift using linear regression. We further explored differences between quantitative pupil characteristics associated with First-Time NOA (FT-NOA) and non-NOA at preceding observations using mixed effects logistic regression, adjusting for possible confounders.
Setting
All quantitative pupil observations were collected at two Neuro Intensive Care Units (ICU) by nursing staff as standard of care.
Patients
We conducted a retrospective two-center study of adult patients with intracranial pathology in the ICU with at least a 24 hour stay and 3 or more quantitative pupil measurements between 2016 and 2018.
Measurements and Main Results
We studied 221 patients (mean age 58, 41% women). Sixty-three percent experienced NOA. NOA-Abnormal occurring at any point during hospitalization was significantly associated with maximum midline shift (β=2.27 per mm, p=0.01). The occurrence of NOA-Normal was inversely associated with death (OR=0.34; 95% CI 0.16-0.71, p=0.01) in adjusted analyses. Subclinical continuous pupil size difference distinguished FT-NOA from non-NOA in up to four preceding pupil observations (or up to 8 hours prior). Minimum pupil reactivity between eyes also distinguished NOA-Abnormal from NOA-Normal prior to FT-NOA occurrence.
Conclusions
NOA occurs in over 60% of patients with neurologic emergencies. Pupil reactivity may be an important distinguishing characteristic of clinically relevant NOA phenotypes. NOA-Abnormal was associated with midline shift and NOA-Normal had an inverse relationship with death. Distinct quantitative pupil characteristics precede NOA occurrence and may allow for earlier prediction of neurologic decline. Further work is needed to determine whether quantitative pupillometry sensitively/specifically predicts clinically relevant anisocoria, enabling possible earlier treatments.
Keywords: Anisocoria, Neurocritical Care, Pupil Reactivity, Pupillometry, Neurologic Deterioration, Neurology
Introduction
In the appropriate context, New Onset Anisocoria (NOA), (pupil size asymmetry >1mm), is an important clinical hallmark of life-threatening intracranial injury. Anisocoria triggers emergent diagnostic work-up to rule out evolving intracranial pathology including occult aneurysms, cerebral infarction or hemorrhage, or impending herniation (1–6). Pupils are therefore closely monitored in high-risk patients. However, other etiologies including prior pupil trauma/dysfunction, medication, ambient lighting, or even normal healthy physiology (20–25% of the general population has pupil size differences of ≥ 0.4 mm) (7–9) warrant no emergent workup. Therefore, identifying anisocoric features associated with evolving intracranial injury is clinically relevant and can guide diagnostic and treatment decisions.
Traditional and subjective estimates of pupil size and reactivity vary widely between examiners (10, 11). The lack of quantitative pupil size and reactivity metrics has limited the characterization of potentially important anisocoria subtypes. Phenotypes including NOA accompanied by objective evidence of abnormal pupil reactivity (NOA-Abnormal) and NOA in the setting of normal pupil reactivity (NOA-Normal) could potentially distinguish emergent and non-emergent causes of anisocoria. Increased utilization of quantitative pupillometry through portable devices facilitates precise characterizations of anisocoria subtypes in patients with acute neurologic conditions.
Our objective was to describe the incidence of NOA in a heterogenous population of intensive care unit (ICU) patients using quantitative pupillometry data. We hypothesized that the occurrence of NOA-Abnormal would be significantly associated with midline shift, a marker of intracranial injury. Moreover, we also explored pupil reactivity characteristics prior to First-Time NOA (FT-NOA) that were associated with NOA occurrence. Understanding the clinical relevance of anisocoria and preceding predictors is potentially important for guiding emergency management decisions and earlier, appropriate resource utilization.
Materials and Methods
Population and Study Design
We prepared this report according to Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines (12). We conducted a two-center retrospective study of patients from the Brigham and Women’s and Massachusetts General Hospital Neurointensive Care Units admitted between 2016 and 2018 with intracranial pathology and at least three consecutive quantitative pupil observations during their hospitalization. We used convenience sampling of consecutively admitted patients within the data-collection period. To reduce potential bias, we excluded patients with non-intracranial injuries, history of glaucoma, cataracts, or retinal detachment, inaccessible brain imaging, or missing medication information (Supplementary Fig 1).
Data Collection
We collected demographic, past medical, diagnostic, procedural, laboratory, neuroimaging (CT or MRI), Glasgow Coma Scale (GCS, a 12-point ordinal scale measuring eye opening, verbal, and motor responses to stimuli) (13), medication, and death at discharge information from electronic medical records. Trained team members reviewed radiology reports and raw images for evidence of mass effect (defined as local displacement of intracranial structures by a space-occupying lesion), midline shift, and uncal herniation. Midline shift was measured at the slice of maximum deviation at the level of the septum pellucidum (all measurements confirmed by CJO) (14). We recorded administration time and routes of medication classes with possible pupil influencing properties including vasopressors (15), osmotics (15), and anticholinergics (16)). (Supplementary Table 1). Study data were collected and managed using REDCap electronic data capture tools (17, 18).
Quantitative pupillometry data were recorded and collected using the NeurOptics NPi™−200 (NeurOptics Inc., Irvine CA, USA) pupillometer. Trained nursing staff conducted quantitative pupillometry as standard of care every two hours in participating neurocritical care units. In addition to recording pupil size and constriction velocity, the pupillometer calculates the Neurological Pupil Index (NPi), an algorithm that uses resting and constricted pupil size, percent change, constriction and dilation velocity, and latency (19, 20). NPi values range between 0 and 5. Values < 3 are considered “abnormal” and values ≥ 3 normal based on studies of normal populations. Its relation to constriction velocity has been externally examined in prior studies (20). For the purposes of this study, we defined patients with abnormal pupil reactivity as those with a recorded NPi < 3 in at least one eye. Our local Institutional Review Boards (H-37699, 2016P002718) approved the study. Our study was exempt from requiring consent, because it introduced no interventions and pupillometry is standard care.
Analysis
Primary:
Our primary outcome was maximum midline shift (mm) during ICU stay. Our secondary outcome was death at discharge. Our primary exposure was “NOA status.” We defined NOA as the new occurrence of pupil size asymmetry > 1mm (i.e., any anisocoric measurement without anisocoria in consecutive prior two measurements). We included anisocoric values in the first or second available observation as NOA, as we assumed these would also be new episodes. NOA-Abnormal was defined as NOA accompanied by abnormal pupil reactivity (NPi < 3), and NOA-Normal as NOA accompanied by normal pupil reactivity (NPi ≥ 3). NOA-Abnormal and Normal subgroups were mutually exclusive. The NOA-Abnormal subgroup consisted of patients with any instance of NOA-Abnormal in their ICU stay; NOA-Normal subgroup consisted of patients without any instances of NOA-Abnormal.
Primary Statistical Analysis:
We reported baseline characteristics of patients in three groups: NOA-Abnormal, NOA-Normal, and no NOA. Global p-values were obtained using ANOVA for continuous variables and Fisher’s Exact tests for categorical variables. To generate specific p-values to examine univariate significance for NOA-Abnormal and NOA-Normal, we used linear or logistic regression treating NOA status (NOA-Abnormal, NOA-Normal, no NOA) as a three factor exposure with no NOA as the reference. Time from admission to first anisocoric episode and number of anisocoric episodes for NOA-Abnormal and NOA-Normal subgroups were evaluated by t-test.
We used multivariable linear regression to test the association of NOA status with midline shift. We adjusted for hypothesized confounders including age and pupil-influencing medications during hospitalization, and average GCS over hospitalization. We used logistic regression to analyze our secondary outcome of death adjusting for age, medications, and midline shift. We used two-sided tests with a significance threshold alpha of 5% for the primary hypothesis. To address the potential influence of supratentorial versus infratentorial injuries, we performed stratified analyses of patients within those subgroups.
Exploratory:
We conducted further exploratory analyses to examine whether pupil characteristics at prior observations were associated with FT-NOA. We censored any observation occurring after the first occurrence of NOA. We constructed separate mixed effects linear regression models testing the association of FT-NOA with subclinical size difference (<1 mm) in four consecutively preceding measurements. Our NOA v. no NOA model included a random effect term for intra-patient correlation. We used linear regression to test the association of NOA-Abnormal versus NOA-Normal and minimum NPi in four consecutively preceding measurements in a subset of FT-NOA observations. We adjusted for GCS within an hour of pupil observation (Supplementary Table 2) and osmotic medications administered within two hours of a pupil observation (Supplementary Table 3). Due to the number of statistical tests and the risk of multiple comparisons, results for these analyses are exploratory and hypothesis-generating.
Missing Data:
Unpaired pupil observations were removed from analyses. Patients lacking any medication or GCS data for their hospitalization were excluded (Supplementary Fig 1). In our observational analyses, three additional study patients were removed because they did not have either GCS or medication data paired within one or two hours, respectively, of quantitative pupil measurements (Supplementary Table 5).
We used RStudio Version 1.1.463 (RStudio Team (2020). RStudio: Integrated Development for R. RStudio, PBC, Boston, MA URL http://www.rstudio.com/) for statistical analyses. Further details in Supplementary Methods.
Results
We collected quantitative pupillometry on 319 patients. Ninety-eight were excluded for history of pupil injury/surgery, no intracranial injury, mistimed or incomplete pupil measurements, inaccessible brain imaging, medications, or GCS measurements (Supplementary Fig 1). In our final cohort of 221 patients (9897 total observations), 90 were female, and median age was 60 years (25th – 75th percentile 50–68). Sixty-three patients were from Brigham and Women’s and 158 from Massachusetts General Hospital. Median follow-up time in the ICU was 97.5 hours (25th – 75th percentile 35.1–193.5). Common diagnoses included intraparenchymal hemorrhage (22%), ischemic stroke (22%), and brain tumor (16%) (Table 1, Supplementary Fig 2).
Table 1.
Baseline Characteristics of NOA-Status Patient Groups (N = 221 Patient Admissions)
| No NOA (N = 81) | NOA-Abnormal (N = 61) | NOA-Normal (N = 79) | Global p | NOA-Abnormal p | NOA-Normal p | |
|---|---|---|---|---|---|---|
|
| ||||||
| Demographics Mean [25%, 75%]/N (%) | ||||||
|
| ||||||
| Age | 60.8 [53.0, 70.0] | 57.0 [44.0, 67.0] | 55.6 [46.5, 66.0] | 0.09 | 0.18 | 0.03* |
| Female | 29 (35.8) | 27 (44.3) | 34 (43.3) | 0.54 | 0.31 | 0.35 |
| White Race | 60 (74.1) | 32 (52.4) | 61 (77.2) | 0.005* | 0.01* | 0.64 |
| Black Race | 7 (8.6) | 4 (6.6) | 5 (6.3) | 0.85 | 0.65 | 0.58 |
| Other Race a | 14 (17.3) | 25 (40.9) | 13 (16.5) | 0.001* | 0.002* | 0.89 |
| Hispanic/Latino Ethnicity | 3 (3.7) | 8 (13.1) | 9 (11.4) | 0.09 | 0.049* | 0.11 |
|
| ||||||
| Primary Diagnosis N (%) | ||||||
|
| ||||||
| Intraparenchymal Hemorrhage | 20 (2.5) | 11 (18.0) | 18 (22.8) | 0.63 | 0.34 | 0.78 |
| Stroke | 19 (23.5) | 10 (16.4) | 20 (25.3) | 0.43 | 0.30 | 0.78 |
| Tumor | 10 (12.3) | 10 (16.4) | 15 (19.0) | 0.51 | 0.49 | 0.25 |
| Traumatic Brain Injury | 8 (9.8) | 11 (18.0) | 9 (11.4) | 0.33 | 0.16 | 0.76 |
| Aneurysmal SAH | 9 (11.1) | 5 (8.2) | 8 (10.1) | 0.89 | 0.57 | 0.84 |
| CSVT | 0 | 4 (6.6) | 0 | 0.01* | 0.99 | 0.99 |
| Other b | 9 (11.1) | 6 (9.8) | 6 (7.6) | 0.77 | 0.81 | 0.45 |
|
| ||||||
| Injury Location N (%) | ||||||
|
| ||||||
| Supratentorial | 49 (60.5) | 49 (80.3) | 58 (73.4) | 0.03* | 0.01* | 0.08 |
| Infratentorial | 20 (24.6) | 6 (9.8) | 10 (12.7) | 0.04* | 0.03* | 0.055 |
| Global | 4 (4.9) | 3 (4.9) | 3 (3.8) | 0.99 | 0.99 | 0.73 |
|
| ||||||
| Medications N (%) | ||||||
|
| ||||||
| Pupil Influencing Medications | 75 (92.6) | 61 (100) | 77 (97.5) | 0.07 | 0.99 | 0.18 |
| Osmotic Medications | 35 (43.2) | 38 (62.3) | 41 (51.9) | 0.08 | 0.03* | 0.27 |
| Analgesic Medication | 52 (64.1) | 55 (90.1) | 68 (86.1) | <0.001* | 0.001* | 0.002* |
| Sedatives | 43 (53.1) | 49 (80.3) | 48 (60.8) | 0.002* | 0.001* | 0.33 |
| Vasopressors | 20 (24.7) | 29 (47.5) | 15 (18.9) | <0.001* | 0.01* | 0.38 |
| Antihypertensives | 56 (69.1) | 52 (85.2) | 62 (78.5) | 0.07 | 0.03* | 0.18 |
| Stimulants | 3 (3.7) | 6 (9.8) | 3 (3.8) | 0.22 | 0.15 | 0.98 |
| Anticholinergics | 15 (18.5) | 23 (37.7) | 25 (31.6) | 0.03* | 0.01* | 0.06 |
| Other Medications c | 29 (35.8) | 35 (57.4) | 23 (29.1) | 0.003* | 0.01* | 0.37 |
|
| ||||||
| Markers of Disease Severity Median [25%,75%]/ N (%) | ||||||
|
| ||||||
| Midline Shift (mm) | 0 [0, 4.0] | 4 [0, 8.0] | 2 [0, 8.0] | 0.02* | 0.01* | 0.13 |
| Average GCS Over Admission | 10 [6.0, 13.0] | 7 [5.0, 9.0] | 11 [8.0, 14.0] | <0.001* | 0.003* | 0.01* |
| Mass Effect | 55 (67.9) | 49 (80.3) | 58 (73.4) | 0.32 | 0.12 | 0.52 |
| Mechanical Ventilation | 60 (74.1) | 55 (90.2) | 57 (72.2) | 0.02* | 0.03* | 0.68 |
| Intracranial Pressure Monitor | 37 (45.7) | 32 (52.4) | 33 (41.8) | 0.48 | 0.42 | 0.62 |
| Emergent Surgery d | 23 (28.4) | 24 (39.3) | 23 (29.1) | 0.34 | 0.17 | 0.92 |
| Uncal Herniation | 22 (27.2) | 27 (44.3) | 21 (26.6) | 0.07 | 0.04* | 0.97 |
| Death | 32 (39.5) | 27 (44.3) | 15 (19.0) | 0.001* | 0.57 | 0.01* |
Univariate analysis of baseline characteristics and NOA status. Global p values were calculated using ANOVA or Fisher Exact Tests. NOA-Abnormal and NOA-Normal specific p values were calculated using logistic/linear regression models with NOA Status as a 3-level factor independent variable with No NOA as the reference level. Abbreviations: SAH = Subarachnoid Hemorrhage, CSVT = Cerebral Sinus Venous Thrombosis, ICP = Intracerebral Pressure.
Other Races: Asian (n=9), Native American (n=1), Unspecified (n=44).
Other Primary Diagnoses: Isolated IVH (n=1), Isolated hydrocephalus (n=2), Non-aneurysmal SAH (n=1), Epidural Hemorrhage (n=1), Status Epilepticus (n=13), Subdural Hematoma (n=6)
Other Medications: Budesonide (n=3), Buspirone (n=13), Cisatracurium (n=19), Dextran (n=11), Digoxin (n=2), Hydroxypropyl (n=1), Latanoprost (n=1), Montelukast (n=3), Naloxone (n=4), Polyvinyl Alcohol (n=17), Racepinephrine (n=1), Rocuronium (n=5), Tamsulosin (n=8), White petrolatum-mineral oil (n=49).
Emergent Surgeries: Craniotomy (n=37), Craniectomy (n=45), Subdural hematoma evacuation (n=1), Epidural hematoma evacuation (n=1).
NOA occurred at least once in 140 patients (63.3%) (Fig 1), and in a total of 534 out of 9897 observations (5.4%). Anisocoria (any pupil observation with a size difference >1mm) made up 980 (~10%) of observations. Sixty-one patients (27.6%) had at least one episode of NOA-Abnormal, and another 79 patients (35.7%) had at least one episode of NOA-Normal. The thirty-two patients who experienced both NOA-Abnormal and NOA-Normal during ICU stay were categorized as NOA-Abnormal for the primary analysis. Forty-three NOA measurements occurred in either the first or second recorded pupil measurement.
Figure 1: New Onset Anisocoria (NOA) in Patients with Intracranial Injury.
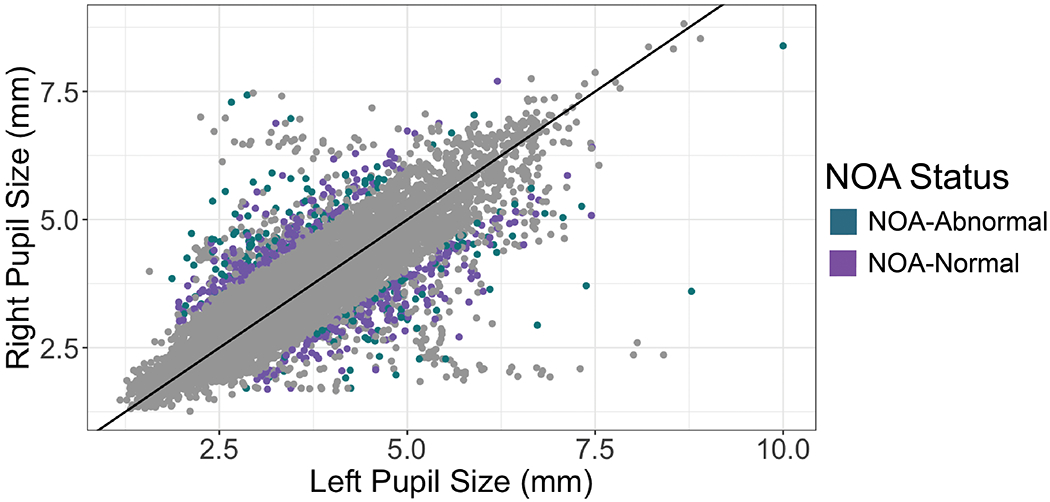
9897 total pupil observations from 221 patients with intracranial injury. New Onset Anisocoria (NOA), defined as a new episode of pupil size difference >1mm (i.e., was not preceded by anisocoria in two prior measurements) occurred in 63% of patients, and in 5.4% of total pupil observations. NOA with abnormal pupil reactivity (NOA-Abnormal) is highlighted in purple and occurred at least once in 61 patients (27%), and in 174 observations (1.8% of all observations, 32.5% of NOA observations). NOA with normal pupil reactivity (NOA-Normal) occurred in 360 observations (3.6% of all observations). Of note, data points in which pupil size difference is >1 mm but not designated as NOA-Abnormal or NOA-Normal are anisocoric measurements immediately preceded by another episode of anisocoria and are therefore not new.
Among the three groups, there were significant differences in race, injury location, medications (analgesic, sedative, vasopressor, anticholinergic), average GCS over ICU stay, midline shift, mechanical ventilation, and death (Table 1). Mean anisocoric observations in the NOA-Abnormal group was 3 [25th percentile=2; 75th percentile=7] versus 2 [25th percentile=1, 75th percentile=3] observations throughout ICU stay in the NOA-Normal group (p<0.001). Median time to FT-NOA was 5.6 [25th percentile=0, 75th percentile=18.2] v. 17.3 [25th percentile=3.5, 75th percentile=37] hours in NOA-Abnormal and NOA-Normal groups respectively (p=0.02). The NOA-Abnormal subgroup had significant associations with supra and infratentorial location, various medication classes, average GCS, mechanical ventilation, and uncal herniation. NOA-Normal had a significant inverse relation with age (Table 1).
In our multivariable model adjusting for age, pupil-influencing medications, and average GCS, NOA-Abnormal was significantly associated with max midline shift (β=2.27 per mm, p=0.01, Standard Deviation (SD)=5.3). When split into subgroups based on injury location, NOA-Abnormal was no longer significant in the supratentorial subgroup (β=1.75 per mm, p=0.14, SD=5.6). NOA-Abnormal, however, did remain significantly associated with midline shift in the infratentorial subgroup (β=1.69 per mm, p=0.01, SD=1.5). Patients with primary infratentorial lesions and midline shift included three patients with brain tumors, one patient with aneurysmal subarachnoid hemorrhage and one patient with a spontaneous intraparenchymal hemorrhage (N=1), whose pathology all extended to the supratentorial region.
The secondary outcome of death for the whole cohort was significantly and inversely associated with NOA-Normal status (OR=0.34; 95% CI 0.16–0.71) after adjusting for confounders, but was not significantly associated with NOA-Abnormal (OR=1.09; 95% CI 0.52–2.23) (Table 2). There were no significant differences in results when patients were classified using only first time occurrence of NOA (FT-NOA) (Supplementary Table 4).
Table 2.
Multivariable Models of A. Max Midline Shift and B. Death (N=221 patients)
| A. Max Midline Shift during hospital admission | β | p |
|---|---|---|
|
| ||
| Total Cohort (221 total patients, 61 NOA-Abnormal, 79 NOA-Normal) | ||
|
| ||
| NOA-Abnormal | 2.27 | 0.01* |
| NOA-Normal | 1.41 | 0.10 |
| Age | 0.001 | 0.93 |
| Pupil Influencing Medications | 3.43 | 0.36 |
| GCS | −0.10 | 0.35 |
|
| ||
| Supratentorial Injury Subgroup (154 total patients, 49 NOA-Abnormal, 58 NOA-Normal) | ||
|
| ||
| NOA-Abnormal | 1.75 | 0.14 |
| NOA-Normal | 0.93 | 0.41 |
| Age | −0.01 | 0.66 |
| Pupil Influencing Medications | 4.40 | 0.44 |
| GCS | 0.005 | 0.97 |
|
| ||
| Infratentorial Injury Subgroup (36 total patients, 6 NOA-Abnormal, 10 NOA-Normal) | ||
|
| ||
| NOA- Abnormal | 1.69 | 0.01 |
| NOA-Normal | −0.02 | 0.97 |
| Age | 0.01 | 0.71 |
| Pupil Influencing Medications | NA | NA |
| GCS | −0.10 | 0.27 |
|
| ||
| B. Death by Discharge | OR [95% CI] | p |
|
| ||
| Total Cohort (221 total patients, 61 NOA-Abnormal, 79 NOA-Normal) | ||
|
| ||
| NOA-Abnormal | 1.09 [0.52, 2.23] | 0.81 |
| NOA-Normal | 0.34 [0.16, 0.71] | 0.01* |
| Age | 1.03 [1.01, 1.05] | 0.01* |
| Pupil Influencing Medications | 0.35 [0.11, 10.39] | 0.49 |
| Maximum Midline Shift (mm) | 1.09 [1.03, 1.16] | 0.003* |
|
| ||
| Supratentorial Injury Subgroup (154 total patients, 49 NOA-Abnormal, 58 NOA-Normal) | ||
|
| ||
| NOA-Abnormal | 0.91 [0.39, 2.12] | 0.83 |
| NOA-Normal | 0.31 [0.13, 0.73] | 0.01* |
| Age | 1.03 [1.01, 1.06] | 0.01* |
| Pupil Influencing Medications | 0.01 [0, 12.4] | 0.98 |
| Maximum Midline Shift (mm) | 1.07 [1.01, 1.15] | 0.02* |
|
| ||
| Infratentorial Injury Subgroup (36 total patients, 6 NOA-Abnormal, 10 NOA-Normal) | ||
|
| ||
| NOA-Abnormal | 1.03 [0.80, 10.07] | 0.97 |
| NOA-Normal | 0.20 [0.01, 1.59] | 0.19 |
| Age | 1.01 [0.95, 1.08] | 0.68 |
| Pupil Influencing Medications | NA | NA |
| Maximum Midline Shift (mm) | 1.02 [0.54, 1.77] | 0.48 |
βs are reported as effect per 1mm increase in midline shift. Stratified analysis of patients with supratentorial and infratentorial injury included. OR = Odds Ratio, CI = Confidence Interval. GCS = Glasgow Coma Scale. Midline Shift (MLS) mean and standard deviation (SD) per cohort: Total Cohort MLS (mean=4.0, SD=5.3), Supratentorial Subgroup MLS (mean=5.6, SD=5.6), Infratentorial Subgroup MLS (mean=0.5, SD=1.5). Pupil Influencing Medications includes any administration of medications listed in Supplementary Table 1 during admission.
Exploratory Analysis:
Our exploratory cohort included 218 patients and 3625 observations after censoring observations after FT-NOA and excluding patients without pupil observations within one or two hours of GCS or medication documentation. We found that subclinical continuous pupil size difference (mm) was significantly associated with future FT-NOA versus non-anisocoric measurements up to four observations (or approximately 8 hours) prior. We found that minimum NPi between eyes was significantly different between FT-NOA phenotypes up to four observations prior to FT-NOA (Table 3, Fig 2, Supplementary Fig 3).
Table 3.
Quantitative Pupil Characteristics Prior to First-Time NOA (218 patients, 3625 total observations)
| A. Size difference (mm) prior to pupil observations | NOA (M=138) vs. No NOA (M=3487) | |
|---|---|---|
|
| ||
| Diff Size | OR [95% CI] | p |
|
| ||
| One observation prior (N=191, M=3282) | 10.34 [5.08, 20.80] | <0.001* |
| Two observations prior (N=167 M=2898) | 6.99 [3.09, 15.45] | <0.001* |
| Three observations prior (N=146, M=2314) | 6.04 [2.40, 14.77] | <0.001* |
| Four observations prior (N=123, M=1773) | 13.27 [4.65, 36.95] | <0.001* |
|
| ||
| B. Minimum NPi prior to FT-NOA pupil observations | NOA-Abnormal (M=44) vs. NOA-Normal (M=94) | |
|
| ||
| Min NPi | OR [95% CI] | p |
|
| ||
| One observation prior (N=107, M=107) | 0.25 [0.12, 0.45] | <0.001* |
| Two observations prior (N=88, M=88) | 0.26 [0.11, 0.47] | <0.001* |
| Three observations prior (N=69, M=69) | 0.21 [0.07, 0.44] | <0.001* |
| Four observations prior (N=52, M=52) | 0.24 [0.07, 0.53] | 0.004* |
Panel A reports univariate mixed effects logistic regression models to account for inter-subject variability testing first-time NOA and size difference up to four observations prior. Panel B reports univariate logistic regression for first-time NOA phenotype and minimum NPi. All models adjust for GCS and osmotic medications prior to observation. Abbreviations: OR = Odds Ratio, CI = Confidence Interval, NOA = New Onset Anisocoria, FT-NOA = First-Time NOA, N=patients, M=observations. Observational sample sizes are included in Supplementary Methods.
Figure 2: Pupil Size Difference (mm) Prior to First-Time New Onset Anisocoria (FT-NOA).
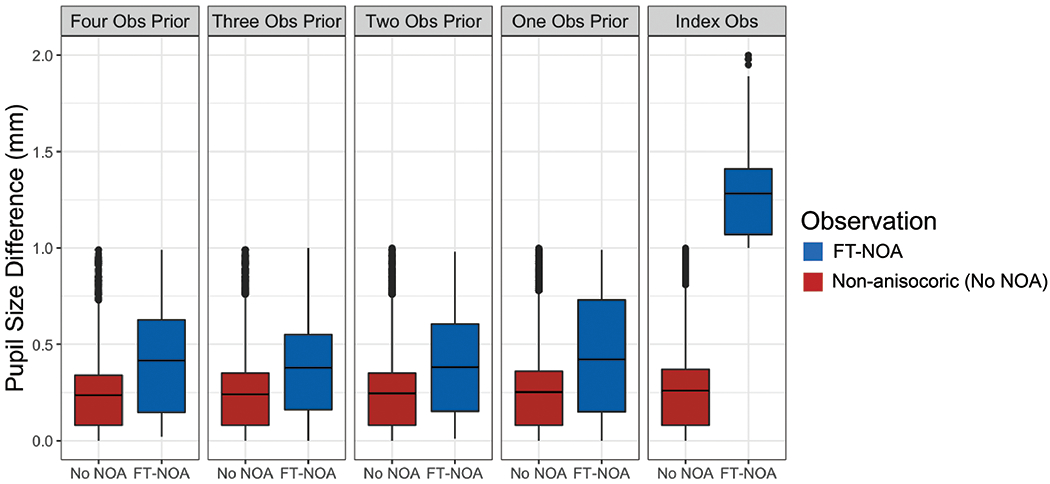
Fig 2: Subclinical pupil size difference (mm) is significantly larger up to four observations prior to first-time NOA occurrence than in observations prior to non-anisocoric observations (Obs).
Discussion
To our knowledge, this is the first study to describe the incidence and risk factors of quantitative NOA in a heterogenous population of patients with intracranial pathology requiring ICU care. Our use of quantitative pupillometry enables objective delineation of clinical anisocoria phenotypes that may help distinguish between severe intracranial processes and benign etiologies of pupillary asymmetry (21).
We observed that NOA-Abnormal was significantly associated with midline shift after adjusting for confounders. NOA-Abnormal was also associated with other markers of disease severity, notably mechanical ventilation and uncal herniation. Conversely, NOA-Normal appeared to have an inverse association with death. In our exploratory analysis, subclinical pupil size difference was significantly associated with FT-NOA up to four measurements (median ~8 hours) prior to clinical observation of FT-NOA. We also observed significant differences in minimum NPi between NOA phenotypes.
Our finding that NOA-Abnormal was significantly associated with midline shift suggests that NOA with concomitant abnormal pupil reactivity portends greater likelihood of evolving intracranial injury and compression of midline structures. We found that NOA-Abnormal was significantly associated with death in our infratentorial subgroup, but not for our total population. The lack of significance of death and NOA-Abnormal may signify that supratentorial midline shift is not invariably associated with death.
On the contrary, NOA-Normal was not significantly associated with increasing midline shift. Moreover, NOA-Normal was less likely to be associated with death and was more likely to occur in younger patients. These findings further suggest fundamental differences between patients with NOA-Abnormal and NOA-Normal subtypes. While our study was not designed to definitively establish the etiology of NOA-Normal, it is possible that this finding occurs in the setting of analgesic administration, undiagnosed pupil dysfunction, or pre-existing subclinical (<1mm) physiologic anisocoria. We do not conclude that NOA with normal reactivity is a “good prognostic indicator,” but suspect that other associated features, like its association with younger age or possibly the mechanism of injury, might be reasons for this association. It is important to note that NOA-Normal was not ubiquitously non-emergent. In our analysis of 38 images within 2 hours of NOA occurrence, 4 scans showing new/worsening mass effect were preceded by NOA-Normal (Supplementary Table 6). We conclude that while NOA-Abnormal subtypes warrant higher suspicion for evolution of intracranial injury, the finding of NOA-Normal should not be dismissed.
In our exploratory analysis, the finding that subclinical difference in size was greater in up to four measurements prior to FT-NOA compared with measurements prior to non-anisocoria suggests that increasing pupil size difference, even if not “clinical” (i.e., greater than 1 mm difference), may be a prelude to NOA and/or evolving intracranial pathology. Our observation that minimum NPi was lower up to four measurements prior to FT-NOA-Abnormal compared with measurements leading up to FT-NOA-Normal suggests that pupil characteristics before FT-NOA may distinguish between future NOA phenotypes.
Pupil size and features are dynamic and sensitive to myriad influences including medication, ambient light, advancing age, repetitive testing, cognitive load, and pain (22–24). However, because pupil features like reactivity are sensitive to both pathology affecting pupil pathways and resilient in the setting of global neurologic injury, they are important clinical markers of evolving injury and recovery potential in brain-injured patients (25, 26).
Pupillary responses to intracranial pathology have been studied since the 19th century (1, 27, 28). Studies have reported qualitative anisocoria in the setting of large hemorrhagic and ischemic strokes (3, 29). However, because anisocoria can be due to more mundane etiologies including medication (particularly anticholinergics) (16), and diabetes mellitus (16, 21), some cases of newly recognized anisocoria lead to unnecessary emergent diagnostic testing and resource utilization. Therefore, a better understanding of when and which kinds of anisocoria signify worsening intracranial pathology is clinically relevant.
Several studies have sought to elucidate the clinical utility of quantitative pupillometry in the setting of emergent neurologic care (30, 31). In 2018, McNett and colleagues found that intracranial pressure was associated with constriction velocity and NPi (32). El Ahmadieh reported that NPi at assessment in 36 patients with traumatic brain injury was significantly lower in patients who needed neurosurgical interventions versus those who did not (33).
Our work is consistent with previous literature describing qualitative pupil asymmetry and/or decreased reactivity in the setting of expanding lesions (2), strokes with edema (3), and acute intraparenchymal and subdural hemorrhage with rapid compression (34, 35). It is also consistent with recent research that found a negative association between NPi and midline shift (36). We build on prior knowledge by describing the incidence and characteristics of distinct clinical anisocoria subtypes in a critically ill population. Our observation that NOA occurred in more than 60% of patients suggests that anisocoria is commonplace among critically ill patients with intracranial conditions, and that distinguishing benign from emergent NOA is important to clinical management decisions.
We acknowledge several study limitations. Due to the lack of prior foundational work, we conducted multiple tests of association, increasing the potential for false positive findings. We attempted to mitigate this by selecting one primary hypothesis. The remainder of our analyses are hypothesis generating for more definitive study. Our relatively small sample size may also have increased the potential for false negative findings. Because our study was observational, we cannot exclude residual confounding, or establish causal relations. The relation of NOA and its phenotypes may vary among the heterogenous neurologic diagnoses we studied. We lacked statistical power to study variation by diagnosis, or to confirm the significant association of our secondary outcome, NOA-Normal and decreased mortality. We did not have information on cognitive load, pain, or ambient light levels, which have been reported to affect pupil characteristics including reactivity (37). Due to variations in timing of GCS scores, medications, and pupil measurements, we relied on pairing measurements closest in time. Our inability to find a significant association between diabetes mellitus and NOA (21) may have been due to limited data regarding duration/severity, or exclusion of patients who may have also had diabetes. We also did not collect information on withdrawal of life-sustaining therapies. It is also worth noting that some studies use different cut-offs to define anisocoria (>0.4 mm, 0.6 mm, 1 mm) (38, 39) (40). However, given that we observed that 63% of patients experienced anisocoria using a cutoff of 1 mm in our critically ill population, we submit our definition was appropriate.
Despite limitations, our study had several strengths. We had access to nearly 10,000 unique pupil observations recorded in a heterogenous patient cohort across two intensive care settings. We performed fundamental groundwork analyses to determine the appropriate observation-level confounders including GCS and osmotic medication administration. Our work enabled us to rigorously examine both patient- and pupil observation-level risk factors associated with NOA, and to adjust for important patient and temporal covariates including demographics, arousal state, and medications. Importantly, our quantitative methodology enables validation and generalization beyond our institution.
Conclusion
Our study demonstrated that NOA occurs in over 60% of patients with acute intracranial pathology. NOA-Abnormal is associated with multiple markers of disease severity including midline shift, in contrast to NOA-Normal, which was inversely associated with death. Moreover, we found that subclinical pupil size difference was significantly associated future NOA. Minimum NPi significantly differed between NOA-subtypes, suggesting that certain quantitative pupil profiles can predict and distinguish between potentially relevant anisocoria phenotypes. Further studies are needed to determine the sensitivity and specificity of quantitative pupil metrics in predicting NOA to best determine how pupillometry can support and improve clinical decision-making in neurologic emergencies.
Supplementary Material
Supplementary Table 1: Pupil Influencing Medication Categories
Supplementary Table 2: Observational Level NOA and Glasgow Coma Scale
Supplementary Table 3: Observational Level NOA and Medication Categories
Supplementary Table 4: Multivariable Models of Max Midline Shift and Death using First-Time NOA
Supplementary Table 5: Missing Data Variables of Interest
Supplementary Table 6: Radiographic Findings and New Onset Anisocoria
Supplementary Figure 1: Inclusion/Exclusion Criteria
Supplementary Figure 2: Diagnostic Breakdown of New Onset Anisocoria (NOA)
Supplementary Fig 2: Breakdown of NOA-status (patients who experienced at least one episode of NOA-Abnormal, NOA-Normal, or non-anisocoric episodes during ICU stay) among the six most frequent diagnoses.
Supplementary Figure 3: NPi Prior to First-Time New Onset Anisocoria (FT-NOA)
Supplemental Fig 3: Minimum NPi between eyes is significantly lower in up to four observations prior to FT NOA-Abnormal observations than in FT NOA-Normal observations.*Abbreviations: NOA-N = First-Time NOA-Normal, NOA-Ab = First-Time NOA-Abnormal
Significance of Work.
New onset anisocoria occurs frequently in the Neuro ICU. Anisocoria accompanied by abnormal pupil reactivity is significantly associated with increased midline shift, while anisocoria without abnormal pupil reactivity was more likely a non-emergent finding.
Financial Support
CJO receives support from NIH/NINDS K23NS116033 and the Peter Paul Young Career Development Foundation at Boston University.
EJB is supported in part by 2R01 HL092577; 1R01 HL141434 01A1; 2U54HL120163; 1R01AG066010; 1R01AG066914; American Heart Association, AHA_18SFRN34110082.
DMG receives support from R01 NS102574.
SS receives support from RO1 EY024019.
SZ receives support from K23NS114201.
Copyright Form Disclosure: Dr. Smirnakis’ institution received funding from Emmetropia; he received funding from Biogen Inc and Amgen Inc. Dr. Zafar’s institution received funding from Sage Therapeutics. Dr. Benjamin received support for article research from the National Institutes of Health (NIH). Dr. Ong’s institution received funding from NIH 2KL2TR001411. The remaining authors have disclosed that they do not have any potential conflicts of interest.
Footnotes
Conflict of Interest Statement
The authors whose names and signatures are listed above certify that they have NO affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
Reprint Statement
We do not intend to reprint this manuscript
References
- 1.Hutchinson J Four lectures on compression of the brain. In: Clark A, Down Hutchinson, et al. , editor. Clinical Lectures and Reports by the Medical and Surgical Staff of the London Hospital. London: Churchill and Sons; 1867. p. 10–55. [Google Scholar]
- 2.Reid WL, Cone WV. The Mechanism of Fixed Dilation of the Pupil: Resulting from Ipsilateral Cerebral Compresssion. Journal of the American Medical Association. 1939;112(20):2030–2034. [Google Scholar]
- 3.Ropper AH, Shafran B. Brain edema after stroke. Clinical syndrome and intracranial pressure. Arch Neurol. 1984;41(1):26–29. [DOI] [PubMed] [Google Scholar]
- 4.Fisher CM. Brain Herniation: A Revision of Classical Concepts. Canadian Journal of Neurological Sciences / ournal Canadien des Sciences Neurologiques. 1995;22(2):83–91. [DOI] [PubMed] [Google Scholar]
- 5.Posner JB, Saper CB, Schiff N, et al. Plum and Posner’s Diagnosis of Stupor and Coma. Cary, United States: Oxford University Press USA - OSO; 2007. [Google Scholar]
- 6.Ropper AH. Herniation. In: Young Wijdicks, editors. Handbook of Clinical Neurology: Elsevier; 2008. p. 79–98. [DOI] [PubMed] [Google Scholar]
- 7.Loewenfeld IE. “Simple central” anisocoria: a common condition, seldom recognized. Trans Sect Ophthalmol Am Acad Ophthalmol Otolaryngol. 1977;83(5):832–839. [PubMed] [Google Scholar]
- 8.Meyer BC. Incidence of anisocoria and difference in size of palpebral fissures in five hundred normal subjects. Arch Neurol Psychiatry. 1947;57(4):464–468. [DOI] [PubMed] [Google Scholar]
- 9.Lam BL, Thompson HS, Corbett JJ. The prevalence of simple anisocoria. Am J Ophthalmol. 1987;104(1):69–73. [DOI] [PubMed] [Google Scholar]
- 10.Olson DM, Stutzman S, Saju C, et al. Interrater Reliability of Pupillary Assessments. Neurocrit Care. 2016;24(2):251–257. [DOI] [PubMed] [Google Scholar]
- 11.Couret D, Boumaza D, Grisotto C, et al. Reliability of standard pupillometry practice in neurocritical care: an observational, double-blinded study. Crit Care. 2016;20–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61(4):344–349. [DOI] [PubMed] [Google Scholar]
- 13.Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2(7872):81–84. [DOI] [PubMed] [Google Scholar]
- 14.Kimberly WT, Bevers MB, von Kummer R, et al. Effect of IV glyburide on adjudicated edema endpoints in the GAMES-RP Trial. Neurology. 2018;91(23):2163–2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ong C, Hutch M, Barra M, et al. Effects of Osmotic Therapy on Pupil Reactivity: Quantification Using Pupillometry in Critically Ill Neurologic Patients. Neurocrit Care. 2018. [DOI] [PubMed] [Google Scholar]
- 16.Loewenfeld IE, Lowenstein O. The pupil : anatomy, physiology, and clinical applications. Boston: Butterworth-Heinemann; 1999. [Google Scholar]
- 17.Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: Building an international community of software platform partners. J Biomed Inform. 2019;95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olson DM, Stutzman SE, Atem F, et al. Establishing Normative Data for Pupillometer Assessment in Neuroscience Intensive Care: The “END-PANIC” Registry. J Neurosci Nurs. 2017;49(4):251–254. [DOI] [PubMed] [Google Scholar]
- 20.Shoyombo I, Aiyagari V, Stutzman SE, et al. Understanding the Relationship Between the Neurologic Pupil Index and Constriction Velocity Values. Sci Rep. 2018;8(1):6992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim HM, Yang HK, Hwang JM. Quantitative analysis of pupillometry in isolated third nerve palsy. PLoS One. 2018;13(11):e0208259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Larson MD, Behrends M. Portable infrared pupillometry: a review. Anesth Analg. 2015;120(6):1242–1253. [DOI] [PubMed] [Google Scholar]
- 23.Shirozu K, Setoguchi H, Tokuda K, et al. The effects of anesthetic agents on pupillary function during general anesthesia using the automated infrared quantitative pupillometer. J Clin Monit Comput. 2017;31(2):291–296. [DOI] [PubMed] [Google Scholar]
- 24.Steinhauer SR, Hakerem G. The pupillary response in cognitive psychophysiology and schizophrenia. Ann N Y Acad Sci. 1992;658:182–204. [DOI] [PubMed] [Google Scholar]
- 25.Oddo M, Sandroni C, Citerio G, et al. Quantitative versus standard pupillary light reflex for early prognostication in comatose cardiac arrest patients: an international prospective multicenter double-blinded study. Intensive Care Med. 2018;44(12):2102–2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Solari D, Rossetti AO, Carteron L, et al. Early prediction of coma recovery after cardiac arrest with blinded pupillometry. Ann Neurol. 2017;81(6):804–810. [DOI] [PubMed] [Google Scholar]
- 27.Peter JK, Eelco FMW. Fixed and dilated: the history of a classic pupil abnormality. Journal of Neurosurgery JNS. 2015;122(2):453–463. [DOI] [PubMed] [Google Scholar]
- 28.Macewen W The Pupil and Its Semiological Aspects. Am J M Sc. 1887;94:123–146. [Google Scholar]
- 29.Ropper AH, Cole D, Louis DN. Clinicopathologic Correlation in a Case of Pupillary Dilation From Cerebral Hemorrhage. Archives of Neurology. 1991;48(11):1166–1169. [DOI] [PubMed] [Google Scholar]
- 30.Weerakoon SM, Stutzman SE, Atem FD, et al. Investigation of Pupillary Changes After Carotid Endarterectomy and Carotid Stent Placement Using Automated Pupillometry. J Stroke Cerebrovasc Dis. 2020;29(5):104693. [DOI] [PubMed] [Google Scholar]
- 31.Yoo YJ, Yang HK, Hwang JM. Efficacy of digital pupillometry for diagnosis of Horner syndrome. PLoS One. 2017;12(6):e0178361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McNett M, Moran C, Grimm D, et al. Pupillometry Trends in the Setting of Increased Intracranial Pressure. J Neurosci Nurs. 2018;50(6):357–361. [DOI] [PubMed] [Google Scholar]
- 33.El Ahmadieh TY, Bedros N, Stutzman SE, et al. Automated Pupillometry as a Triage and Assessment Tool in Patients with Traumatic Brain Injury. World Neurosurg. 2021;145:163–169. [DOI] [PubMed] [Google Scholar]
- 34.Fisher CM. The neurological examination of the comatose patient. Acta neurologica scandinavica. 1969;45(S36):5. [DOI] [PubMed] [Google Scholar]
- 35.McNealy DE, Plum F. Brainstem Dysfunction with Supratentorial Mass Lesions. Archives of Neurology. 1962;7(1):10–32. [Google Scholar]
- 36.Osman M, Stutzman SE, Atem F, et al. Correlation of Objective Pupillometry to Midline Shift in Acute Stroke Patients. J Stroke Cerebrovasc Dis. 2019. [DOI] [PubMed] [Google Scholar]
- 37.Ong C, Hutch M, Smirnakis S. The Effect of Ambient Light Conditions on Quantitative Pupillometry. Neurocrit Care. 2018. [DOI] [PubMed] [Google Scholar]
- 38.IE L The Pupil: Anatomy, Physiology and Clinical Applications. Woburn MA: Butterworth-Heinemann; 1999. [Google Scholar]
- 39.Steck RP, Kong M, McCray KL, et al. Physiologic anisocoria under various lighting conditions. Clin Ophthalmol. 2018;12:85–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gross JR, McClelland CM, Lee MS. An approach to anisocoria. Curr Opin Ophthalmol. 2016;27(6):486–492. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1: Pupil Influencing Medication Categories
Supplementary Table 2: Observational Level NOA and Glasgow Coma Scale
Supplementary Table 3: Observational Level NOA and Medication Categories
Supplementary Table 4: Multivariable Models of Max Midline Shift and Death using First-Time NOA
Supplementary Table 5: Missing Data Variables of Interest
Supplementary Table 6: Radiographic Findings and New Onset Anisocoria
Supplementary Figure 1: Inclusion/Exclusion Criteria
Supplementary Figure 2: Diagnostic Breakdown of New Onset Anisocoria (NOA)
Supplementary Fig 2: Breakdown of NOA-status (patients who experienced at least one episode of NOA-Abnormal, NOA-Normal, or non-anisocoric episodes during ICU stay) among the six most frequent diagnoses.
Supplementary Figure 3: NPi Prior to First-Time New Onset Anisocoria (FT-NOA)
Supplemental Fig 3: Minimum NPi between eyes is significantly lower in up to four observations prior to FT NOA-Abnormal observations than in FT NOA-Normal observations.*Abbreviations: NOA-N = First-Time NOA-Normal, NOA-Ab = First-Time NOA-Abnormal


