Abstract
BACKGROUND:
Women with gestational glucose intolerance, defined as an abnormal initial gestational diabetes screening test, are at risk for adverse pregnancy outcomes even if they do not have gestational diabetes. We previously defined gestational diabetes physiologic subtypes based on the primary underlying physiology leading to hyperglycemia and found that women with different subtypes had differential risk of adverse outcomes. Physiologic subclassification has not yet been applied to women with gestational glucose intolerance.
OBJECTIVE:
We defined gestational glucose intolerance physiologic subtypes based on the presence of insulin resistance, insulin deficiency, or mixed pathophysiology, and aimed to determine if these subtypes are at differential risk for adverse outcomes. We hypothesized that women with the insulin resistant subtype of gestational glucose intolerance would have the greatest risk of adverse pregnancy outcomes.
STUDY DESIGN:
In a hospital-based cohort study, we studied women with gestational glucose intolerance (glucose loading test 1-hour glucose ≥140 mg/dL, N=236) and normal glucose tolerance (glucose loading test 1-hour glucose <140 mg/dL, N=1472). We applied homeostasis model assessment to fasting glucose and insulin levels at 16–20 weeks’ gestation to assess insulin resistance and deficiency and used these measures to classify women with gestational glucose intolerance into subtypes. We compared odds of adverse outcomes (large for gestational age birthweight, neonatal intensive care unit admission, pregnancy-related hypertension, cesarean delivery) in each subtype to odds in women with normal glucose tolerance using logistic regression with adjustment for age, race/ethnicity, marital status, and body mass index.
RESULTS:
Of women with gestational glucose intolerance (12% with gestational diabetes), 49% had the insulin resistant subtype (N=115), 27% had the insulin deficient subtype (N=70), 17% had the mixed pathophysiology subtype (N=40), and 5% were uncategorized (N=11). We found increased odds of large for gestational age birthweight (primary outcome), in women with the insulin resistant subtype compared to women with normal glucose tolerance (OR 2.35 [1.43–3.88], p=0.001; adjusted OR 1.74 [1.02–3.48], p=0.04). The odds of large for gestational age birthweight in women with the insulin deficient subtype were increased only after adjustment for covariates (OR 1.69 [0.84–3.38], p=0.14; adjusted OR 2.05 [1.01–4.19], p=0.048). Among secondary outcomes, there was a trend toward increased odds of neonatal intensive care unit admission in the insulin resistant subtype in an unadjusted model (OR 2.09 [0.99–4.40], p=0.05); this finding was driven by an increased risk of neonatal intensive care unit admission in women with the insulin resistant subtype and body mass index <25 kg/m2. Infants of women with other subtypes did not have increased odds of neonatal intensive care unit admission. The odds of pregnancy-related hypertension in the insulin resistant subtype were increased (OR 2.09 [1.31–3.33], p=0.002; adjusted OR 1.77 [1.07–2.92], p=0.03) compared to women with normal glucose tolerance; other subtypes did not have increased odds of pregnancy-related hypertension. There was no difference in cesarean delivery rates in nulliparous women across subtypes.
CONCLUSION:
Insulin resistant gestational glucose intolerance is a high-risk subtype for adverse pregnancy outcomes. Delineating physiologic subtypes may provide opportunities for a more personalized approach to gestational glucose intolerance.
Keywords: Adverse pregnancy outcomes, diabetes mellitus, gestational diabetes, gestational glucose intolerance, glucose intolerance, insulin deficiency, insulin resistance, large for gestational age birthweight, physiologic subtypes, pregnancy, pregnancy-related hypertension
Introduction:
Women with gestational diabetes mellitus (GDM) and their infants have an increased risk of adverse pregnancy outcomes including large for gestational age birthweight (LGA), neonatal intensive care unit (NICU) admission, pregnancy-related hypertension, and cesarean delivery.1–3 Women with gestational glucose intolerance (GGI), who have an abnormal initial screening glucose loading test (GLT), also have an increased risk of the same adverse pregnancy outcomes, whether or not they meet GDM diagnostic criteria.1, 4–14 While the American College of Gynecology (ACOG) has suggested that some women with GGI who do not meet GDM criteria may nevertheless be treated as if they have GDM,15 it is not yet known if every woman with GGI has an elevated risk of adverse pregnancy outcomes. We previously found that the risk of adverse pregnancy outcomes differs according to the underlying physiology leading to hyperglycemia in GDM;16 similar heterogeneity may be present among women with GGI.
Women with GDM, on average, have diminished insulin sensitivity (insulin resistance) and diminished insulin secretion (insulin deficiency) compared to pregnant women with normal glucose tolerance (NGT). However, there is substantial heterogeneity in the underlying physiology leading to hyperglycemia in GDM.16–19 Our previous work defined GDM subtypes based on the presence or absence of insulin resistance and/or insulin deficiency, assessed using multiple timed glucose and insulin measurements from an oral glucose tolerance test (OGTT).16 In our prior study, we found that women with an insulin resistant subtype of GDM had an increased risk of fetal overgrowth and GDM-associated pregnancy outcomes.16 In comparison, women with the insulin deficient or mixed pathophysiology subtypes of GDM did not have increased risk of adverse pregnancy outcomes compared to women with NGT.16
Here, we aimed to define physiologic subtypes of GGI (including all women who had an abnormal initial GDM screening test). Subtypes were delineated by insulin resistance, insulin deficiency, or mixed pathophysiology using homeostasis model assessment, which uses fasting glucose and insulin measures.20 We aimed to determine if GGI subtypes are at differential risk for adverse pregnancy outcomes. We hypothesized that women with the insulin resistant subtype of GGI would be at highest risk.
Materials and Methods:
Participants were from the Massachusetts General Hospital (MGH) Obstetrical Maternal Study (MOMS, N=9913), enrolled from 1998–2006.21 Institutional review board (IRB) approval and informed consent were obtained before study procedures began. A subset of women in the cohort (N=1853) provided a fasting blood sample at 16–20 weeks gestation (serum frozen at −80C). For the present study, we included women who had fasting glucose levels measured at the time of study who also had a 1-hour GLT (initial screening test for GDM) at >22 weeks gestation (median 27.7 [IQR 26.9–28.4] weeks). Of these women, those with gestational glucose intolerance (GGI: 1-hour GLT result ≥140 mg/dl) were included if they had a stored fasting serum sample available for insulin measurement or had insulin previously measured. We included all women with NGT, regardless of sample availability. For pregnancy-related hypertension analyses, we excluded women with chronic hypertension, defined as an elevated blood pressure at the initial prenatal visit occurring at <20 weeks’ gestation. For cesarean delivery analyses, only nulliparous women were included.
The primary exposure was physiologic subtype of GGI, defined with homeostasis model assessment (HOMA-2)20 using fasting glucose and insulin levels measured at 16–20 weeks gestation. Glucose levels were measured at the time of blood draw in the MGH Core Chemistry Laboratory (Boston, MA). We measured insulin levels on frozen serum using a chemiluminescent immunoassay (inter-assay variation <5.6%) at the Brigham Research Assay Core (Boston, MA). For 25 participants with GGI in whom additional samples were not available, we used insulin levels previously measured using a radioimmunoassay from Linco Research (St. Louis, MO).22 Insulin resistance and/or deficiency were defined in pregnancies with GGI as HOMA-2S (measure of insulin sensitivity) or HOMA-2B (measure of beta-cell function)20, 23 <50th percentile. The 50th percentile was determined in women with GLT 1-hour glucose <130 mg/dl who had insulin measured by the same assay as those with GGI. Women with GGI (including those with GDM) were classified into physiologic subtypes based on the presence or absence of insulin resistance/deficiency: 1) insulin resistant GGI: isolated insulin resistance, 2) insulin deficient GGI: isolated insulin deficiency, 3) mixed pathophysiology GGI: both insulin resistance and deficiency, and 4) uncategorized: neither insulin resistance nor deficiency. The referent group for examining outcomes was women with NGT (N=1472, GLT 1-hour glucose <140 mg/dl).
The primary outcome was large for gestational age birthweight (LGA), defined as birth weight >90th percentile for gestational age.24 Secondary outcomes included infant outcomes birthweight percentile (BW%)24 and NICU admission, and maternal outcomes pregnancy-related hypertension and cesarean delivery in nulliparous women. Hyperglycemia, including GDM, is known to contribute to pregnancy-related hypertension, including preeclampsia.2, 3, 12–14, 25, 26 We defined pregnancy-related hypertension as gestational hypertension or pre-eclampsia, ascertained using either prenatal outpatient blood pressures (systolic blood pressure [SBP] ≥140 mmHg or diastolic blood pressure [DBP] ≥90 mmHg after 20 weeks gestation) and/or by the notation of either of these diagnoses (as an indication for induction or cesarean delivery or complication of labor) in the inpatient delivery record. Women with chronic hypertension (defined as SBP ≥140 mmHg or DBP ≥90 mmHg at initial prenatal visit) were excluded from the pregnancy-related hypertension outcome analysis. We examined GDM diagnosis using National Diabetes Data Group (NDDG) criteria,27 which were in clinical use at MGH at the time of data collection.
We compared participant characteristics according to physiologic GGI subtype and NGT, using the Kruskal-Wallis tests for continuous variables or Chi-square tests for categorical variables. If the global p-value indicated a difference across subgroups (p <0.05), we performed post-hoc pairwise testing using the Dunn test or Chi-square tests comparing each physiologic subtype group to the NGT group (with the alpha level adjusted for three comparisons using Bonferroni’s correction). We compared odds of adverse outcomes in each subtype to odds in women with NGT using logistic (odds ratios) or linear regression (beta coefficients) with adjustment for maternal age, race/ethnicity, marital status, and BMI measured at <20 weeks gestation, plus infant sex in LGA and birthweight models. We ran secondary analyses excluding women with GDM to determine whether findings were being driven by women in this treated sub-population. To determine whether physiologic subtypes provided information on the risk of outcomes beyond that provided by conventional BMI categories, we performed stratified analyses using linear and logistic regression models, grouping women by BMI into overweight/obesity (BMI ≥25 kg/m2) and normal/underweight strata (BMI <25 kg/m2). We ran sensitivity analyses using the 25th percentile threshold (Supplemental Table 2) for HOMA-2B and HOMA-2S to delineate GGI subtypes and using a cutoff of GLT <130 mg/dl to define the NGT group (Supplemental Table 3). Analyses were run using Stata Release 16 (StataCorp LLC, College Station, TX).
Results:
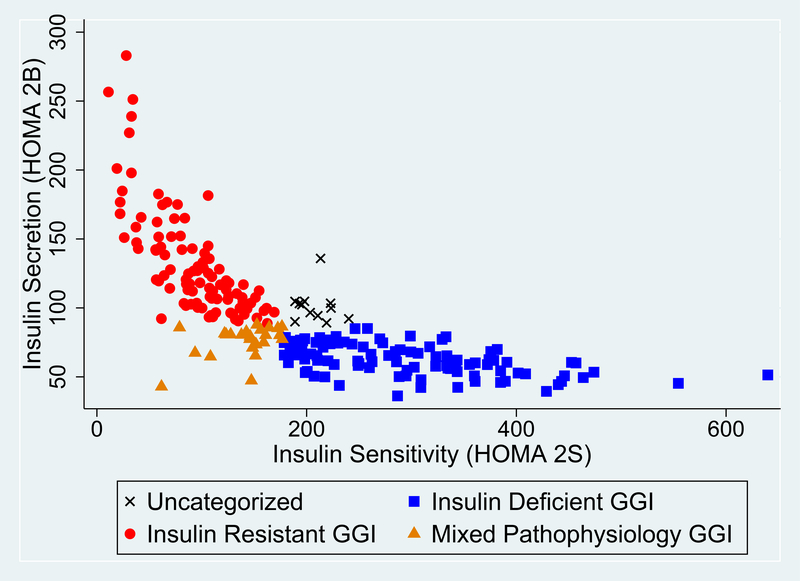
Participant characteristics by GGI subtype are given in Table 1. Of the women with GGI (GLT ≥ 140 mg/dL, N=236, Figure 1), 49% had insulin resistant GGI (N=115), 27% had insulin deficient GGI (N=70), 17% had mixed pathophysiology GGI (N=40), and 5% were uncategorized (N=11). GDM was diagnosed in N=28 (12%) women with GGI. Compared to the NGT referent group, women with the insulin deficient and mixed pathophysiology subtypes were older (Table 1). Women with insulin resistant GGI were more likely to have BMI ≥ 30kg/m2 compared to NGT; women with insulin deficient GGI were more likely to have BMI <25 kg/m2. There were no statistically significant differences in insurance status between the subtypes. Compared to NGT, women with insulin resistant GGI were less likely to be married. Women with insulin resistant GGI were also more likely to identify as Latina. Women included in our analysis were similar to those who participated in the MOMS fasting parent study (data not shown).
Table 1:
Participant Characteristics by Physiologic Subtype of Gestational Glucose Intolerance.
| Insulin Resistant GGI | Insulin Deficient GGI | Mixed PathophysiologyGGI | Normal glucose tolerance (NGT) | p-value^ | |
|---|---|---|---|---|---|
| N (% of GGI) # | 115 (48.7%) | 70 (26.6%) | 40 (17.0%) | 1472 | |
| Participant Characteristics: | |||||
| Age (years) | 32.4 (27.8, 35.9) | 34.2 (30.1, 37.3) * | 33.4 (31.2, 36.8) * | 32.0 (28.4, 34.9) | <0.001 |
| Nulliparous | 60 (52.2%) | 41 (58.6%) | 17 (42.5%) | 812 (55.2%) | 0.36 |
| Private insurance | 77 (67.0%) | 56 (80.0%) | 30 (75.0%) | 1146 (77.9%) | 0.11 |
| Married/Partnered | 76 (66.1%) ** | 63 (90.0%) | 35 (87.5%) | 1174 (79.8%) | <0.001 |
| Race/Ethnicity: | ** | <0.001 | |||
| White | 64 (55.7%) | 54 (77.1%) | 25 (62.5%) | 1043 (70.9%) | |
| Latina | 34 (29.6%) | 5 (7.1%) | 3 (7.5%) | 227 (15.4%) | |
| Asian | 7 (6.1%) | 6 (8.6%) | 4 (10.0%) | 70 (4.8%) | |
| Black | 7 (6.1%) | 1 (1.4%) | 3 (7.5%) | 65 (4.4%) | |
| Other | 3 (2.6%) | 4 (5.7%) | 5 (12.5%) | 67 (4.6%) | |
| Maternal Measurements at 1st Prenatal Visit: | |||||
| Systolic BP (mmHg) | 112 (110, 120) | 110 (100, 120) | 112 (104, 120) | 110 (104, 120) | 0.08 |
| Diastolic BP (mmHg) | 70 (65, 80) | 70 (62, 72) | 70 (66, 74) | 70 (62, 74) | 0.07 |
| BMI (kg/m2) | 28.7 (24.9, 32.6) * | 22.8 (20.8, 24.9) * | 24.8 (23.3, 28.6) * | 24.1 (21.9, 27.4) | <0.001 |
| BMI Categories | ** | ** | <0.001 | ||
| BMI<25 | 29 (25.2%) | 53 (75.7%) | 21 (52.5%) | 868 (59.0%) | |
| BMI 25–<30 | 37 (32.2%) | 15 (21.4%) | 13 (32.5%) | 388 (26.4%) | |
| BMI>=30 | 48 (41.7%) | 2 (2.9%) | 6 (15.0%) | 210 (14.3%) | |
| Laboratory data at 16–20 Weeks Gestation | |||||
| GA at HOMA assessment (wks) | 17.3 (16.3, 18.4) | 16.7 (15.8, 17.9) | 17.6 (16.2, 19.0) | 17.1 (16.1, 18.4) | 0.12 |
| Fasting glucose (mg/dL) | 82 (78, 88) * | 77.5 (75, 82) | 87.5 (83, 94) * | 78 (74, 82) | <0.001 |
| Fasting insulin (uIU/mL) | 52.4 (39.8, 77.2) * | 16.9 (14.8, 20.5) * | 28.8 (27.1, 35.0) | 31.0 (20.3, 46.2) | <0.001 |
| Additional Pregnancy Data | |||||
| Glucose loading test (mg/dL) | 152 (145, 164) * | 154.5 (146, 165) * | 155 (147, 170) * | 108 (94, 120) | <0.001 |
| GDM diagnosis | 16 (13.9%) | 6 (8.6%) | 3 (7.5%) | -- | 0.33 |
| Gestational weight gain (lbs) | 28 (19, 36) | 29 (22, 32) | 28.5 (21, 35) | 30 (23, 36) | 0.07 |
| GA at delivery | 39.3 (38.3, 40.3) | 39.9 (38.9, 40.7) | 39.5 (38.35, 40.15) | 39.7 (38.9, 40.6) | 0.05 |
Data shown in N (%) or median (IQR).
P values were determined by Fisher’s exact test or Pearson chi-square tests for categorical variables or Kruskall-Wallis tests for continuous variables.
Bold and * indicates Dunn test results p < 0.05 when compared to Normal Glucose Tolerance (NGT). For race/ethnicity, marital status, and body mass index (BMI) categories: bold and ** indicates post-hoc Chi-square results p < 0.017 (adjusting for three comparisons) when group compared to NGT.
Total percentages do not add up to 100% as not including uncategorized (n=11, 4.7%, see Supplemental Table 1).
Abbreviations: GGI, gestational glucose intolerance defined as glucose loading test (GLT) ≥ 140mg/dl. GA, gestational age. Glucose and GLT result measured in mg/dl. BP, blood pressure. GDM, gestational diabetes diagnosis by National Diabetes Data Group (NDDG) criteria. Gestational weight gain measured by subtracting weight at first prenatal visit from weight at last prenatal visit.
Figure 1: Insulin Sensitivity and Secretion in Physiologic Subtypes of Gestational Glucose Intolerance.
Distribution of insulin sensitivity (HOMA-2S) and insulin secretion (HOMA-2B) in 236 subtyped women with gestational glucose intolerance (GGI). Women with the insulin resistant subtype of GGI (red, ●) have preserved insulin secretion and decreased insulin sensitivity. Women with the insulin deficient subtype of GGI (blue, ■) have preserved insulin sensitivity and decreased insulin secretion. Women with mixed pathophysiology GGI (orange, ▲) have a combination of insulin resistance and deficiency.
The GLT results in each GGI subtype were similar and higher than the NGT group (Table 1). As expected, given that HOMA-2 indices are calculated from these levels, there were differences in fasting glucose and insulin between subtypes (Table 1). Fasting insulin was higher in women with insulin resistant and lower in women with insulin deficient subtypes compared to women with NGT. Fasting glucose was higher in women with insulin resistant and mixed pathophysiology subtypes, and similar in women with the insulin deficient subtype compared to NGT.
Primary Outcome: LGA
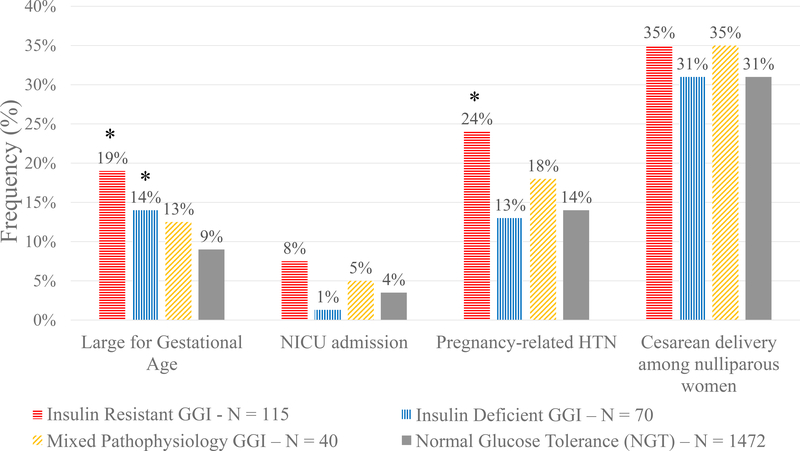
For the primary outcome of LGA, women with insulin resistant GGI had the greatest incidence of LGA (19%), while women with insulin deficient and mixed pathophysiology subtypes had a similar risk of LGA to each other (14% and 13%, respectively); the lowest risk of LGA was in the women with NGT (9%, Figure 2). In unadjusted models, women with insulin resistant GGI had significantly higher odds of an LGA birth compared to the NGT group (Table 2). These odds remained higher after adjusting for age, race/ethnicity, marital status, BMI, and infant sex (Table 2). Women with insulin deficient GGI did not have significantly higher odds of an LGA infant compared to women with NGT prior to adjustment, but after adjustment (including for BMI) the increased odds of LGA in this subtype became statistically significant (Table 2).
Figure 2: Frequency of Adverse Pregnancy Outcomes According to Physiologic Subtype of Gestational Glucose Intolerance.
Frequency was calculated as percentage of gestational glucose intolerance (GGI) for the insulin resistant, insulin deficient, and mixed pathophysiology subtypes, or percentage of normal glucose tolerance (NGT) for the referent group. Outcomes include large for gestational age infant (LGA), neonatal intensive care unit (NICU) admission, pregnancy-related hypertension (HTN), and rate of cesarean delivery among nulliparous women. Total number of women (N) and percentages of each physiologic subtype or the NGT referent group are listed. * p < 0.05 in adjusted regression models.
Table 2:
Risk of Adverse Pregnancy Outcomes by Gestational Glucose Intolerance Physiologic Subtype.
| Outcomes | Model | Insulin Resistant GGI OR (95% CI) or β (95% CI) N = 115 |
p-value | Insulin Deficient GGI OR (95% CI) or β (95% CI) N = 70 |
p-value | Mixed Pathophysiology GGI OR (95% CI) or β (95% CI) N = 40 |
p-value | Normal Glucose Tolerance (NGT) N = 1472 |
|---|---|---|---|---|---|---|---|---|
| Primary Outcome | ||||||||
| Large for Gestational Age (LGA) | Unadjusted | 2.35 (1.43, 3.88) | 0.001 | 1.69 (0.84, 3.38) | 0.14 | 1.42 (0.55, 3.69) | 0.47 | Ref |
| Adjusted* | 1.74 (1.02, 3.48) | 0.04 | 2.05 (1.01, 4.19) | 0.048 | 1.31 (0.49, 3.48) | 0.60 | Ref | |
| Secondary Infant Outcomes | ||||||||
| Birthweight percentile (BW%) | Unadjusted | β = 6.44 (1.05, 11.83) | 0.02 | β = 0.68 (−6.13, 7.50) | 0.84 | β = 8.94 (0.03, 17.85) | 0.049 | Ref |
| Adjusted* | β = 3.75 (−1.69, 9.18) | 0.18 | β = 2.04 (−4.65, 8.73) | 0.55 | β = 7.75 (−0.99, 16.48) | 0.08 | Ref | |
| Neonatal Intensive Care Unit (NICU) admission | Unadjusted | 2.09 (0.99, 4.40) | 0.05 | 0.39 (0.05, 2.93) | 0.37 | 1.50 (0.35, 6.49) | 0.59 | Ref |
| Adjusted | 1.74 (0.78, 3.88) | 0.18 | 0.35 (0.05, 2.60) | 0.30 | 1.08 (0.25, 4.75) | 0.92 | Ref | |
| Secondary Maternal Outcomes | ||||||||
| Pregnancy-related Hypertension | Unadjusted | 2.09 (1.31, 3.33) | 0.002 | 0.89 (0.43, 1.83) | 0.74 | 1.19 (0.51, 2.75) | 0.69 | Ref |
| Adjusted | 1.77 (1.07, 2.92) | 0.03 | 0.99 (0.48, 2.08) | 0.99 | 1.07 (0.45, 2.51) | 0.88 | Ref | |
| Cesarean delivery rate among nulliparous women | Unadjusted | 1.13 (0.65, 1.97) | 0.65 | 1.01 (0.51, 1.99) | 0.97 | 1.15 (0.42, 3.14) | 0.79 | Ref |
| Adjusted | 0.81 (0.45, 1.46) | 0.48 | 1.03 (0.52, 2.07) | 0.93 | 1.13 (0.41, 3.15) | 0.81 | Ref | |
Bold odds ratios (OR) and p values indicate statistical significance with p < 0.05. GGI: gestational glucose intolerance. LGA: large for gestational age birthweight. BW%: birthweight percentile - beta coefficient from linear regression model shown. NICU: neonatal intensive care unit. Ref: referent group. OR’s shown from logistic regression models or beta coefficient from linear regression models (BW%). Regression models were adjusted for the following covariates including age, race/ethnicity, marital status, and body mass index at <20 weeks gestation. For LGA and BW% models (*), infant sex was added as a covariate.
Secondary Infant Outcomes
For our secondary infant outcome of BW%, infants of women with the insulin resistant and mixed pathophysiology subtypes had significantly higher BW% compared to the NGT group; however, these effects were attenuated and no longer statistically significant in the adjusted models (Table 2).
The incidence of NICU admission was highest in women with insulin resistant GGI, intermediate in mixed pathophysiology GGI, and lower in insulin deficient GGI compared to NGT (Figure 2). There was a trend toward increased odds of NICU admission in infants of women with insulin resistant GGI compared to infants of women with NGT; this relationship was attenuated in the fully adjusted model (Table 2).
Secondary Maternal Outcomes
There was an increased incidence of pregnancy-related hypertension in women with insulin resistant GGI (24%) compared to NGT (14%); 13% of women with insulin deficient GGI and 18% of women with mixed pathophysiology GGI had pregnancy-related hypertension as compared to 14% in the NGT group (Figure 2). In logistic regression models, unadjusted odds of women with insulin resistant GGI having pregnancy-related hypertension were greater than in NGT; these odds remained significantly higher compared to NGT in adjusted models (Table 2). In contrast, other subtypes did not have significantly greater odds of pregnancy-related hypertension compared to NGT (Table 2).
Among nulliparas, there was no difference in cesarean delivery between the subtypes and NGT (Figure and Table 2).
Analyses Excluding Women with GDM Diagnoses
Among women diagnosed with GDM (N=28, 12% of GGI), 64% (N=16) had insulin resistance, 24% (N=6) had insulin deficiency, 12% (N=3) had mixed pathophysiology, and 12% (N=3) were uncategorized (Table 1). There was no significant difference in GDM diagnosis between GGI subtypes, though the insulin resistant subtype appeared to have a greater incidence (Table 1). When women with GDM were excluded from analyses, the effect sizes observed for outcome analyses in the insulin resistant subtype were consistent with the analyses previously described in all women with GGI for LGA, pregnancy-related hypertension, NICU admission, and cesarean delivery rate (Table 3).
Table 3:
Odds of Adverse Pregnancy Outcomes By Gestational Glucose Intolerance Subtypes, Excluding Gestational Diabetes.
| Outcomes | Model | Insulin Resistant GGI OR (95% CI) or β (95% CI) N = 90 |
p-value | Insulin Deficient GGI OR (95% CI) or β (95% CI) N = 64 |
p-value | Mixed Pathophysiology GGI OR (95% CI) or β (95% CI) N = 32 |
p-value | Normal Glucose Tolerance (NGT) N = 1472 |
|---|---|---|---|---|---|---|---|---|
| Primary Outcome | ||||||||
| Large for Gestational Age (LGA) | Unadjusted | 2.49 (1.43, 4.30) | 0.001 | 1.88 (0.93, 3.78) | 0.08 | 0.66 (0.16, 2.81) | 0.58 | Ref |
| Adjusted* | 1.84 (1.02, 3.31) | 0.04 | 2.27 (1.10, 4.66) | 0.03 | 0.58 (0.13, 2.53) | 0.47 | Ref | |
| Secondary Infant Outcomes | ||||||||
| Birthweight percentile (BW%) | Unadjusted | β = 7.57 (1.50, 13.6) | 0.02 | β = 1.62 (−5.52, 8.75) | 0.66 | β = 5.07 (−4.91, 15.05) | 0.32 | Ref |
| Adjusted* | β = 4.76 (−1.31, 10.8) | 0.12 | β = 3.07 (−3.90, 10.0) | 0.39 | β = 3.95 (−5.79, 13.7) | 0.43 | Ref | |
| Neonatal Intensive Care Unit (NICU) admission | Unadjusted | 1.87 (0.78, 4.52) | 0.16 | 0.44 (0.06, 3.26) | 0.42 | 2.03 (0.46, 8.90) | 0.35 | Ref |
| Adjusted | 1.75 (0.69, 4.43) | 0.24 | 0.38 (0.05, 2.81) | 0.34 | 1.70 (0.38, 7.64) | 0.49 | Ref | |
| Secondary Maternal Outcomes | ||||||||
| Pregnancy-related Hypertension | Unadjusted | 2.11 (1.27, 3.51) | 0.004 | 0.84 (0.39, 1.81) | 0.66 | 1.29 (0.52, 3.21) | 0.58 | Ref |
| Adjusted | 1.78 (1.03, 3.08) | 0.04 | 0.95 (0.44, 2.05) | 0.89 | 1.20 (0.47, 3.04) | 0.70 | Ref | |
| Cesarean delivery rate among nulliparous women | Unadjusted | 1.29 (0.72, 2.33) | 0.40 | 1.01 (0.50, 2.04) | 0.98 | 1.26 (0.45, 3.52) | 0.65 | Ref |
| Adjusted | 0.91 (0.49, 1.70) | 0.76 | 1.02 (0.50, 2.11) | 0.94 | 1.32 (0.47, 3.74) | 0.60 | Ref | |
Women diagnosed with gestational diabetes (GDM) by National Diabetes Data Group (NDDG) criteria were excluded from these analyses. Bold odds ratios (OR) and p values indicate statistical significance with p < 0.05. GGI: gestational glucose intolerance. LGA: large for gestational age. BW%: birthweight percentile - beta coefficient from linear regression model shown. NICU: neonatal intensive care unit. Ref: referent group. OR’s shown from logistic regression models or beta coefficient from linear regression models (BW%). Regression models were adjusted for the following covariates including age, race/ethnicity, marital status, and BMI at <20 weeks gestation. For LGA and BW% models (*), infant sex was added as a covariate.
Sensitivity Analyses
When we used the 25th percentile cutoff for HOMA-2B and HOMA-2S to define insulin resistance/deficiency and categorize subtypes (instead of the 50th percentile cutoff used in the main analyses) more women were uncategorized, resulting in less informative GGI subtyping (Supplemental Table 2). We observed similar findings in primary and secondary outcomes in a sensitivity analysis where NGT definition was changed to GLT <130 mg/dl from GLT <140 mg/dl, (Supplemental Table 3).
Analyses Stratified by BMI
Primary Outcome: LGA – BMI ≥25 kg/m2:
In stratified analyses for the primary LGA outcome by BMI category, women with BMI ≥25 kg/m2 and insulin resistant GGI had an increased adjusted odds of LGA (Table 4) compared to women with NGT from the same BMI strata. Women with insulin deficient GGI and BMI ≥25 kg/m2 had similar odds of LGA to NGT women (Table 4).
Table 4:
Adverse Pregnancy Outcomes in Women with Gestational Glucose Intolerance Subtypes Stratified by BMI.
| Outcomes | Insulin Resistant GGI OR (95% CI) or β (95% CI) |
p-value | Insulin Deficient GGI OR (95% CI) or β (95% CI) |
p-value | Mixed Pathophysiology GGI OR (95% CI) or β (95% CI) |
p-value | Normal Glucose Tolerance (NGT) | |
|---|---|---|---|---|---|---|---|---|
| BMI≥25 | ||||||||
| N | 85 | -- | 17 | -- | 19 | -- | 598 | |
| Primary Outcome | LGA | 2.26 (1.29, 3.97) | 0.004 | 0.85 (0.19, 3.84) | 0.83 | 2.06 (0.63, 6.66) | 0.23 | Ref |
| Secondary Infant Outcomes | BW% | β = 7.87 (1.30, 14.4) | 0.02 | β = −8.19 (−22.3, 5.91) | 0.25 | β = 4.02 (−9.09, 17.1) | 0.55 | Ref |
| NICU admission | 1.39 (0.48, 4.02) | 0.55 | -- | -- | 1.36 (0.28, 6.61) | 0.71 | Ref | |
| Secondary Maternal Outcomes | Pregnancy-related HTN | 1.77 (1.02, 3.07) | 0.04 | 1.06 (0.33, 3.41) | 0.92 | 1.35 (0.45, 4.08) | 0.60 | Ref |
| Cesarean delivery rate among nulliparous women | 0.54 (0.26, 1.14) | 0.10 | 0.55 (0.14, 2.23) | 0.41 | 0.98 (0.16, 6.19) | 0.99 | Ref | |
| BMI<25 | ||||||||
| N | 29 | -- | 53 | -- | 21 | -- | 868 | |
| Primary Outcome | LGA | -- | -- | 2.47 (1.07, 5.71) | 0.03 | 0.57 (0.07, 4.46) | 0.60 | Ref |
| Secondary Infant Outcomes | BW% | β = −5.50 (−15.8, 4.8) | 0.30 | β = 3.78 (−3.76, 11.3) | 0.32 | β = 12.1 (0.27, 23.9) | 0.045 | Ref |
| NICU admission | 3.35 (1.04, 10.8) | 0.04 | 0.52 (0.07, 3.97) | 0.53 | -- | -- | Ref | |
| Secondary Maternal Outcomes | Pregnancy-related HTN | 1.86 (0.60, 5.70) | 0.28 | 0.91 (0.35, 2.40) | 0.85 | 0.76 (0.17, 3.33) | 0.71 | Ref |
| Cesarean delivery rate among nulliparous women | 1.74 (0.68, 4.48) | 0.25 | 1.21 (0.55, 2.71) | 0.64 | 1.35 (0.40, 4.62) | 0.63 | Ref | |
Bold odds ratios (OR) and p values indicate statistical significance with p < 0.05. GGI: gestational glucose intolerance. LGA: large for gestational age birthweight, BW%: birthweight percentile - beta coefficient from linear regression models shown (change in BW% points by GGI subtype compared to NGT). GDM: gestational diabetes by National Diabetes Data Group (NDDG) definition. HTN: hypertension. NICU: neonatal intensive care unit. Adjusted OR’s shown from logistic regression models or beta coefficient from linear regression models (BW%) stratified by women with BMI ≥ 25 kg/m2 and BMI < 25 kg/m2. Regression models were adjusted for the following covariates including age, race/ethnicity, marital status for all models, in addition to infant sex for LGA and BW% models. Women were excluded from these analyses if BMI was missing from first or second prenatal visit (missing BMI: N=7).
Primary Outcome: LGA – BMI <25 kg/m2:
Out of the 29 women in the BMI <25 kg/m2 strata with insulin resistant GGI, there were no women who had LGA births. In contrast, we found a significantly increased odds of LGA in women with insulin deficient GGI compared to NGT women in the BMI <25 kg/m2 strata (Table 4).
Secondary Outcomes – Stratified by BMI:
Secondary Infant Outcome – NICU Admission:
In women with BMI ≥25 kg/m2, there was no increased risk of NICU admission in women with insulin resistant GGI compared to NGT (Table 4). There were no women with insulin deficient GGI and BMI ≥25 kg/m2 who had an infant with a NICU admission, and no higher odds of NICU admission in women with mixed pathophysiology GGI compared with NGT (Table 4). In women with BMI <25 kg/m2, there were higher odds of NICU admission in women with insulin resistant GGI compared to NGT (Table 4). The odds of women with BMI <25 kg/m2 and insulin deficient GGI having an infant requiring a NICU admission were not statistically different from NGT, and there were no NICU admissions in infants of women with mixed pathophysiology GGI with BMI <25 kg/m2 (Table 4).
Secondary Maternal Outcome – Pregnancy-Related Hypertension:
For pregnancy-related hypertension, the insulin resistant subtype appeared to convey a greater risk regardless of BMI strata, though the effect was only statistically significant in the BMI ≥25 kg/m2 strata (Table 4). There was no difference in risk of pregnancy-related hypertension in women with insulin deficient or mixed pathophysiology subtypes compared to NGT women in either BMI strata (Table 4).
Comment:
Principal Findings:
In this study of 236 women with gestational glucose intolerance (GGI), we found differential risk of adverse pregnancy outcomes depending on the physiology underlying the glucose intolerance, with insulin resistance appearing to convey the highest risk. Specifically, in unadjusted models and after adjustment for all covariates including maternal BMI, we found increased risk of LGA and pregnancy-related hypertension in women with the insulin resistant subtype of GGI. There also appeared to be a uniquely high risk of NICU admission in women with normal weight and the insulin resistant subtype.
Results in the Context of What is Known:
While we previously subtyped GDM based on insulin resistance and/or deficiency status, to our knowledge, the present analysis is the first to broaden this subclassification to GGI. Prior studies by us and others evaluating pregnancy outcomes based on GDM physiologic subtypes have found that women with insulin resistance are at an increased risk of adverse pregnancy outcomes including fetal overgrowth,16 preterm delivery and neonatal hypoglycemia,28 pregnancy-related hypertension, primary cesarean delivery, and neonatal hyperinsulinemia and adiposity.29 Our data expand these findings to women with lesser degrees of glucose intolerance in pregnancy.
Our primary outcome of LGA was significantly more common in women with insulin resistant GGI in unadjusted and adjusted analyses. This association was not entirely mediated by fetal exposure to maternal obesity,30 as adjustment for BMI did not completely attenuate the relationship between insulin resistant GGI and LGA. Women with the insulin deficient and mixed pathophysiology subtypes also had some evidence for an increased risk of fetal overgrowth. Maternal obesity,30–32 fasting16 and nocturnal hyperglycemia,33 hyperinsulinemia,34, 35 and hypertriglyceridemia36, 37 may contribute to increased risk of LGA infants in women with insulin resistance.
Among secondary maternal outcomes, women with insulin resistant GGI had an increased risk of pregnancy-related hypertension compared to women with NGT; this increased risk was independent of BMI and other covariates, and was not seen in women with the insulin deficient or mixed pathophysiology subtypes of GGI. Outside of pregnancy, insulin resistance is known to be associated with hypertension, independent of diabetes and obesity.38 Previous studies by our group and others using HOMA-IR,39 sex hormone binding globulin (SHBG),40 and urine insulin41 as markers of insulin resistance have linked insulin resistance to pregnancy-related hypertension. However, these studies did not assess the contribution of insulin resistance specifically in women with glucose intolerance.39–43
Of note, we also found evidence for an association between insulin resistant GGI and our secondary infant outcome NICU admission, which was driven by women with BMI <25 kg/m2. The mechanisms underlying this finding deserve further study.
Clinical Implications:
Studying women with GGI (rather than only those with GDM) is clinically relevant, as women with GGI who do not have GDM have been shown to be at risk for GDM-associated adverse outcomes but are currently untreated.4, 10, 11 The 2018 ACOG guidelines suggest that women with one abnormal OGTT value (a subset of women GGI) may be treated as if they have GDM, but this is up to provider discretion.15 Our current findings provide the premise for future studies of increased monitoring or treatment for women with GGI (without GDM) with insulin resistance. For example, it would be of clinical interest to determine whether women with insulin resistant GGI who are at increased risk of pregnancy-related hypertension would benefit from aspirin prophylaxis44 or glucose-lowering therapy.
Research Implications:
Typically, women with GDM are treated during pregnancy, without regard to the underlying physiologic mechanism leading to hyperglycemia.15, 45 Future prospective studies are necessary to determine if treatment during pregnancy based on GGI or GDM physiologic subtypes could reduce the risk of adverse pregnancy outcomes. While there are at least two clinical trials currently testing subtype-specific treatment of GDM using diet (NCT04187521) or medical therapy (NCT03029702), future research may benefit from including women with GGI and the same physiologic subtypes.
Strengths and Limitations:
Strengths of this study include the detailed phenotypic, laboratory, clinical, and outcome data utilized. Our use of a fasting blood sample to subtype women with GGI is another strength, as this boosts the potential for clinical translation and is unlike prior approaches, which used multiple timed glucose and insulin levels during an OGTT. The blood sample for subtyping was done in the early second trimester prior to diagnosis of GDM, which may ultimately allow for earlier intervention. Limitations include the retrospective and observational design, which is subject to bias by confounding, but we have adjusted for many potential confounders in statistical models. In addition, generalizability may be limited by inclusion of women from a single academic center. Our sample may differ from the general population of pregnant women due to older age, <50% racial/ethnic minority groups, and >60% private insurance status.
Conclusions:
Currently, pregnant women with GDM are all treated similarly, and women with GGI without GDM are not treated for hyperglycemia. Our results support using physiologic subtyping to identify insulin resistance in women with GGI, regardless of GDM diagnosis; this approach could enable targeted interventions leading to more favorable outcomes. While previous studies of GDM physiologic subtypes used full OGTT data for subtype classification, we determined subtypes of GGI based on a single fasting blood draw, making such subtyping feasible in clinical practice. Our findings imply that additional monitoring and possible treatment of women with the insulin resistant subtype of GGI (even without a GDM diagnosis) could be beneficial, given the increased risk of fetal overgrowth, pregnancy-related hypertension, and NICU admission observed in this group.
Supplementary Material
AJOG Condensation Page:
1). Condensation:
Fasting blood tests identify insulin resistant women with gestational glucose intolerance who are at risk for hyperglycemia-associated adverse pregnancy outcomes.
2). Short Title:
Gestational Glucose Intolerance Subtypes and Adverse Pregnancy Outcomes
3). AJOG at a Glance:
A. Why was this study conducted?
In a prior study, we defined physiologic subtypes of gestational diabetes (GDM, based on the underlying physiology leading to hyperglycemia) and found that these subtypes had differential risks of adverse outcomes. In this study, we expanded this subclassification framework to gestational glucose intolerance (GGI, abnormal initial GDM screen).
B. What are the key findings?
Women with GGI had a differential risk of adverse pregnancy outcomes depending on the physiology underlying the glucose intolerance, with insulin resistance conveying the greatest risk for large for gestational age birthweight, pregnancy-related hypertension, and neonatal intensive care unit admission.
C. What does this study add to what is already known?
Our findings imply that a new clinical approach to women with the insulin resistant subtype of GGI could be of benefit, given the increased risk of adverse pregnancy outcomes observed in this group.
Acknowledgement:
We would like to thank Chu Yu, MS (Biostatistician at the Massachusetts General Hospital Biostatistics Center, Boston, MA) for her statistical review of the analyses in this manuscript, as well as Tanayott Thaweethai, PhD (Instructor in Investigation at the Massachusetts General Hospital Biostatistics Center and Instructor of Medicine at Harvard Medical School, Boston, MA) for his statistical consultation.
Financial support: D.J.S. was supported by a T32 training grant for Endocrinology (T32DK007028). J.B.M. was supported by UM1DK078616. C.E.P. was supported by K23DK113218, the Robert Wood Johnson Foundation’s Harold Amos Medical Faculty Development Program, and the Massachusetts General Hospital Physician Scientist Development Award.
Footnotes
Conflicts of Interest: The authors report no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Hapo Study Cooperative Research Group, Metzger BE, Lowe LP, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. May 8 2008;358(19):1991–2002. doi: 10.1056/NEJMoa0707943 [DOI] [PubMed] [Google Scholar]
- 2.Crowther CA, Hiller JE, Moss JR, McPhee AJ, Jeffries WS, Robinson JS. Effect of Treatment of Gestational Diabetes Mellitus on Pregnancy Outcomes. New England Journal of Medicine. 2005;352(24):2477–2486. doi: 10.1056/NEJMoa042973 [DOI] [PubMed] [Google Scholar]
- 3.Landon MB, Spong CY, Thom E, et al. A multicenter, randomized trial of treatment for mild gestational diabetes. N Engl J Med. Oct 1 2009;361(14):1339–48. doi: 10.1056/NEJMoa0902430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yee LM, Cheng YW, Liddell J, Block-Kurbisch I, Caughey AB. 50-Gram glucose challenge test: is it indicative of outcomes in women without gestational diabetes mellitus? The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. Sep 2011;24(9):1102–6. doi: 10.3109/14767058.2010.546450 [DOI] [PubMed] [Google Scholar]
- 5.Cheng YW, Block-Kurbisch I, Caughey AB. Carpenter-Coustan criteria compared with the national diabetes data group thresholds for gestational diabetes mellitus. Obstetrics and gynecology. Aug 2009;114(2 Pt 1):326–32. doi: 10.1097/AOG.0b013e3181ae8d85 [DOI] [PubMed] [Google Scholar]
- 6.Coustan DR, Lowe LP, Metzger BE, Dyer AR. The Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study: paving the way for new diagnostic criteria for gestational diabetes mellitus. American journal of obstetrics and gynecology. Jun 2010;202(6):654.e1–6. doi: 10.1016/j.ajog.2010.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Retnakaran R, Qi Y, Sermer M, Connelly PW, Hanley AJ, Zinman B. Glucose intolerance in pregnancy and future risk of pre-diabetes or diabetes. Diabetes care. Oct 2008;31(10):2026–31. doi: 10.2337/dc08-0972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roeckner JT, Sanchez-Ramos L, Jijon-Knupp R, Kaunitz AM. Single abnormal value on 3-hour oral glucose tolerance test during pregnancy is associated with adverse maternal and neonatal outcomes: a systematic review and metaanalysis. American journal of obstetrics and gynecology. Sep 2016;215(3):287–97. doi: 10.1016/j.ajog.2016.04.040 [DOI] [PubMed] [Google Scholar]
- 9.McLaughlin GB, Cheng YW, Caughey AB. Women with one elevated 3-hour glucose tolerance test value: are they at risk for adverse perinatal outcomes? American journal of obstetrics and gynecology. May 2006;194(5):e16–9. doi: 10.1016/j.ajog.2006.01.028 [DOI] [PubMed] [Google Scholar]
- 10.Cheng YW, McLaughlin GB, Esakoff TF, Block-Kurbisch I, Caughey AB. Glucose challenge test: screening threshold for gestational diabetes mellitus and associated outcomes. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. Dec 2007;20(12):903–8. doi: 10.1080/14767050701739384 [DOI] [PubMed] [Google Scholar]
- 11.Stamilio DM, Olsen T, Ratcliffe S, Sehdev HM, Macones GA. False-positive 1-hour glucose challenge test and adverse perinatal outcomes. Obstetrics and gynecology. Jan 2004;103(1):148–56. doi: 10.1097/01.Aog.0000109220.24211.Bd [DOI] [PubMed] [Google Scholar]
- 12.Solomon CG, Graves SW, Greene MF, Seely EW. Glucose intolerance as a predictor of hypertension in pregnancy. Hypertension. Jun 1994;23(6 Pt 1):717–21. doi: 10.1161/01.hyp.23.6.717 [DOI] [PubMed] [Google Scholar]
- 13.Suhonen L, Teramo K. Hypertension and pre-eclampsia in women with gestational glucose intolerance. Acta Obstet Gynecol Scand. May 1993;72(4):269–72. doi: 10.3109/00016349309068036 [DOI] [PubMed] [Google Scholar]
- 14.Joffe GM, Esterlitz JR, Levine RJ, et al. The relationship between abnormal glucose tolerance and hypertensive disorders of pregnancy in healthy nulliparous women. Calcium for Preeclampsia Prevention (CPEP) Study Group. American journal of obstetrics and gynecology. Oct 1998;179(4):1032–7. doi: 10.1016/s0002-9378(98)70210-8 [DOI] [PubMed] [Google Scholar]
- 15.ACOG Practice Bulletin No. 190: Gestational Diabetes Mellitus. Obstetrics and gynecology. Feb 2018;131(2):e49–e64. doi: 10.1097/aog.0000000000002501 [DOI] [PubMed] [Google Scholar]
- 16.Powe CE, Allard C, Battista MC, et al. Heterogeneous Contribution of Insulin Sensitivity and Secretion Defects to Gestational Diabetes Mellitus. Diabetes care. Jun 2016;39(6):1052–5. doi: 10.2337/dc15-2672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Catalano PM, Tyzbir ED, Roman NM, Amini SB, Sims EA. Longitudinal changes in insulin release and insulin resistance in nonobese pregnant women. American journal of obstetrics and gynecology. Dec 1991;165(6 Pt 1):1667–72. doi: 10.1016/0002-9378(91)90012-g [DOI] [PubMed] [Google Scholar]
- 18.Catalano PM, Tyzbir ED, Wolfe RR, et al. Carbohydrate metabolism during pregnancy in control subjects and women with gestational diabetes. The American journal of physiology. Jan 1993;264(1 Pt 1):E60–7. doi: 10.1152/ajpendo.1993.264.1.E60 [DOI] [PubMed] [Google Scholar]
- 19.Cheney C, Shragg P, Hollingsworth D. Demonstration of heterogeneity in gestational diabetes by a 400-kcal breakfast meal tolerance test. Obstetrics and gynecology. Jan 1985;65(1):17–23. [PubMed] [Google Scholar]
- 20.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. Jul 1985;28(7):412–9. doi: 10.1007/bf00280883 [DOI] [PubMed] [Google Scholar]
- 21.Wolf M, Kettyle E, Sandler L, Ecker JL, Roberts J, Thadhani R. Obesity and preeclampsia: the potential role of inflammation. Obstetrics and gynecology. Nov 2001;98(5 Pt 1):757–62. doi: 10.1016/s0029-7844(01)01551-4 [DOI] [PubMed] [Google Scholar]
- 22.Smirnakis KV, Chasan-Taber L, Wolf M, Markenson G, Ecker JL, Thadhani R. Postpartum diabetes screening in women with a history of gestational diabetes. Obstetrics and gynecology. Dec 2005;106(6):1297–303. doi: 10.1097/01.Aog.0000189081.46925.90 [DOI] [PubMed] [Google Scholar]
- 23.Levy JC, Matthews DR, Hermans MP. Correct homeostasis model assessment (HOMA) evaluation uses the computer program. Diabetes care. Dec 1998;21(12):2191–2. doi: 10.2337/diacare.21.12.2191 [DOI] [PubMed] [Google Scholar]
- 24.Oken E, Kleinman KP, Belfort MB, Hammitt JK, Gillman MW. Associations of gestational weight gain with short- and longer-term maternal and child health outcomes. Am J Epidemiol. Jul 15 2009;170(2):173–80. doi: 10.1093/aje/kwp101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gestational Hypertension and Preeclampsia: ACOG Practice Bulletin, Number 222. Obstetrics and gynecology. Jun 2020;135(6):e237–e260. doi: 10.1097/aog.0000000000003891 [DOI] [PubMed] [Google Scholar]
- 26.Ostlund I, Haglund B, Hanson U. Gestational diabetes and preeclampsia. Eur J Obstet Gynecol Reprod Biol. Mar 15 2004;113(1):12–6. doi: 10.1016/j.ejogrb.2003.07.001 [DOI] [PubMed] [Google Scholar]
- 27.Classification and Diagnosis of Diabetes Mellitus and Other Categories of Glucose Intolerance. Diabetes. 1979;28(12):1039–1057. doi: 10.2337/diab.28.12.1039 [DOI] [PubMed] [Google Scholar]
- 28.Benhalima K, Van Crombrugge P, Moyson C, et al. Characteristics and pregnancy outcomes across gestational diabetes mellitus subtypes based on insulin resistance. Diabetologia. Nov 2019;62(11):2118–2128. doi: 10.1007/s00125-019-4961-7 [DOI] [PubMed] [Google Scholar]
- 29.Madsen LR, Gibbons KS, Ma RCW, et al. Do variations in insulin sensitivity and insulin secretion in pregnancy predict differences in obstetric and neonatal outcomes? Diabetologia. 2021/February/01 2021;64(2):304–312. doi: 10.1007/s00125-020-05323-0 [DOI] [PubMed] [Google Scholar]
- 30.Catalano PM, McIntyre HD, Cruickshank JK, et al. The Hyperglycemia and Adverse Pregnancy Outcome Study. Associations of GDM and obesity with pregnancy outcomes. 2012;35(4):780–786. doi: 10.2337/dc11-1790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lindberger E, Wikström A-K, Bergman E, et al. Association of maternal central adiposity measured by ultrasound in early mid pregnancy with infant birth size. Scientific Reports. 2020/November/12 2020;10(1):19702. doi: 10.1038/s41598-020-76741-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gao X, Yan Y, Xiang S, et al. The mutual effect of pre-pregnancy body mass index, waist circumference and gestational weight gain on obesity-related adverse pregnancy outcomes: A birth cohort study. PLoS One. 2017;12(6):e0177418. doi: 10.1371/journal.pone.0177418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Law GR, Alnaji A, Alrefaii L, et al. Suboptimal Nocturnal Glucose Control Is Associated With Large for Gestational Age in Treated Gestational Diabetes Mellitus. Diabetes care. 2019:dc182212. doi: 10.2337/dc18-2212 [DOI] [PubMed] [Google Scholar]
- 34.O’Tierney-Ginn P, Presley L, Myers S, Catalano P. Placental growth response to maternal insulin in early pregnancy. The Journal of clinical endocrinology and metabolism. Jan 2015;100(1):159–65. doi: 10.1210/jc.2014-3281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lassance L, Haghiac M, Leahy P, et al. Identification of early transcriptome signatures in placenta exposed to insulin and obesity. American journal of obstetrics and gynecology. May 2015;212(5):647.e1–11. doi: 10.1016/j.ajog.2015.02.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barbour LA, Farabi SS, Friedman JE, et al. Postprandial Triglycerides Predict Newborn Fat More Strongly than Glucose in Women with Obesity in Early Pregnancy. Obesity (Silver Spring). Aug 2018;26(8):1347–1356. doi: 10.1002/oby.22246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Layton J, Powe C, Allard C, et al. Maternal lipid profile differs by gestational diabetes physiologic subtype. Metabolism. Feb 2019;91:39–42. doi: 10.1016/j.metabol.2018.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ferrannini E, Buzzigoli G, Bonadonna R, et al. Insulin Resistance in Essential Hypertension. New England Journal of Medicine. 1987;317(6):350–357. doi: 10.1056/nejm198708063170605 [DOI] [PubMed] [Google Scholar]
- 39.Chen Z, Liu W, Sun X, Zhu L. Clinical study on the association between pregnancy-induced hypertension and insulin resistance. Exp Ther Med. 2017/May/01 2017;13(5):2065–2070. doi: 10.3892/etm.2017.4169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wolf M, Sandler L, Muñoz K, Hsu K, Ecker JL, Thadhani R. First trimester insulin resistance and subsequent preeclampsia: a prospective study. The Journal of clinical endocrinology and metabolism. Apr 2002;87(4):1563–8. doi: 10.1210/jcem.87.4.8405 [DOI] [PubMed] [Google Scholar]
- 41.Emery SP, Levine RJ, Qian C, et al. Twenty-four-hour urine insulin as a measure of hyperinsulinaemia/insulin resistance before onset of pre-eclampsia and gestational hypertension. BJOG : an international journal of obstetrics and gynaecology. Nov 2005;112(11):1479–85. doi: 10.1111/j.1471-0528.2005.00720.x [DOI] [PubMed] [Google Scholar]
- 42.Seely EW, Solomon CG. Insulin Resistance and Its Potential Role in Pregnancy-Induced Hypertension. The Journal of Clinical Endocrinology & Metabolism. 2003;88(6):2393–2398. doi: 10.1210/jc.2003-030241 [DOI] [PubMed] [Google Scholar]
- 43.Carpenter MW. Gestational Diabetes, Pregnancy Hypertension, and Late Vascular Disease. Diabetes care. 2007;30(Supplement 2):S246–S250. doi: 10.2337/dc07-s224 [DOI] [PubMed] [Google Scholar]
- 44.ACOG Committee Opinion No. 743: Low-Dose Aspirin Use During Pregnancy. Obstetrics and gynecology. Jul 2018;132(1):e44–e52. doi: 10.1097/aog.0000000000002708 [DOI] [PubMed] [Google Scholar]
- 45.2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2018. Diabetes care. Jan 2018;41(Suppl 1):S13–s27. doi: 10.2337/dc18-S002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.