Abstract
To determine the source of infection for the Japanese index case of human babesiosis, we analyzed blood samples from an asymptomatic individual whose blood had been transfused into the patient. In addition, we surveyed rodents collected from near the donor's residence. Examination by microscopy and PCR failed to detect the parasite in the donor's blood obtained 8 months after the donation of the blood that was transfused. However, we were able to isolate Babesia parasites by inoculating the blood sample into SCID mice whose circulating red blood cells (RBCs) had been replaced with human RBCs. A Babesia parasite capable of propagating in human RBCs was also isolated from a field mouse (Apodemus speciosus) captured near the donor's residential area. Follow-up surveys over a 1-year period revealed that the donor continued to be asymptomatic but had consistently high immunoglobulin G (IgG) titers in serum and low levels of parasitemia which were microscopically undetectable yet which were repeatedly demonstrable by inoculation into animals. The index case patient's sera contained high titers of IgM and, subsequently, rising titers of IgG antibodies, both of which gradually diminished with the disappearance of the parasitemia. Analysis of the parasite's rRNA gene (rDNA) sequence and immunodominant antigens revealed the similarity between donor and patient isolates. The rodent isolate also had an rDNA sequence that was identical to that of the human isolates but that differed slightly from that of the human isolates by Western blot analysis. We conclude that the index case patient acquired infection by transfusion from a donor who became infected in Japan, that parasitemia in an asymptomatic carrier can persist for more than a year, and that A. speciosus serves as a reservoir of an agent of human babesiosis in Japan.
Human babesiosis due to Babesia microti (25, 26), a hemosporidian of small rodents, has increasingly been reported wherever Lyme borreliosis (11) and human granulocytic ehrlichiosis (16) are endemic. The three zoonotic agents share a life cycle that depends on rodents and a tick of the Ixodes persulcatus species complex, and this association of microbes seems to be widely distributed through the temperate zone (26). Although B. microti has long been known to parasitize rodents in northern North America (4, 6, 24, 30), Europe (7, 29), and Eurasia (22, 28), cases of parasitization of humans have been reported almost exclusively from the United States (5, 25). Cases have recently been documented in Taiwan (21) and Japan (13). Increased awareness will facilitate the detection of B. microti babesiosis in areas where the protozoon is enzootic.
We have previously reported on the Japanese index case of human babesiosis, which occurred in 1999 at Kobe City in Hyogo Prefecture, Japan (13). The patient had received a blood transfusion before the onset of babesiosis. Of the eight blood donors involved in the transfusion, only a single individual was found to be positive for specific antibody and parasite DNA, suggesting that the patient was infected via a transfusion of blood from this asymptomatic donor (20). Since neither the patient nor the donor had a history of travel abroad, the infection had presumably originated within Japan. Although no human case of babesiosis was reported in the country until 1999, human infections might simply have been undetected for many years. Indeed, more than 15 years ago, Shiota et al. (22) documented the presence of B. microti-like parasites in Japanese field mice (Apodemus speciosus), demonstrating that babesiosis has long been enzootic among Japanese wild rodents. However, because the original parasite described in their report is no longer available, the identity between that strain and the Kobe strain, which was isolated from the index case patient in our previous study (20), is not known.
The objective of the present study was to determine the source of infection for the Japanese index case of human babesiosis. Using SCID mice whose circulating red blood cells (RBCs) had been replaced with those of humans (designated hu-RBC-SCID mice) (27), we were able to isolate Babesia parasites not only from the asymptomatic blood donor but also from a field mouse captured near the donor's residential area. The antigenic and genotypic characteristics of these isolates were compared to those from the patient, confirming that B. microti is enzootic and zoonotic in Japan.
MATERIALS AND METHODS
Human blood specimens.
The detailed clinical course of the patient has been reported elsewhere (13). Heparinized blood samples were obtained from the patient, who provided informed consent, at the hospital of the Kobe University School of Medicine. Some samples were obtained on clinically important days: 28 December 1998 (admission and blood transfusion), 24 May 1999 (diagnosis and start of treatment with quinine and clindamycin), 27 July 1999 (5-day follow-up after discharge from hospital), 31 August 1999 (5 days after readmission due to recrudescence), and 11 November 1999 (4-week follow-up after the final discharge from the hospital). Blood samples from the asymptomatic donor and his or her family members (father, brother, and sister) were obtained with informed consent at the Hyogo Red Cross Blood Center of the Japanese Red Cross Society.
Experimental animals.
NOD/shi-scid mice (8), which were originally obtained from the Central Institute of Experimental Animals, Kawasaki, Japan, were maintained in the laboratory animal facility in Rakuno-Gakuen University. Golden Syrian hamsters and C.B-17 scid mice were purchased from SLC, Inc. (Hamamatsu, Japan) and Japan CLEA (Tokyo, Japan), respectively. All animals were housed in isolators at temperatures between 22 and 25°C and were provided with a γ-ray-irradiated pellet diet and autoclaved tap water. All mice and hamsters were splenectomized and used for experiments after the surgical wounds had healed completely. Animal experimentation was carried out according to the Laboratory Animal Control Guidelines of Rakuno-Gakuen University.
Parasite isolation.
The blood samples from the patient, the asymptomatic donor, and the field mice were washed with phosphate-buffered saline (PBS; pH 7.2) by repeated centrifugation, and aliquots of RBCs were inoculated into hu-RBC-SCID mice or splenectomized hamsters for parasite isolation. To prepare hu-RBC-SCID mice, the peripheral RBCs in splenectomized NOD/shi-scid mice were replaced with human type O RBCs by the method described for our previous studies (17, 20). The rate of replacement with human RBCs in hu-RBC-SCID mice was monitored by flow cytometry (Cyto ACE150; JASCO Co., Tokyo, Japan) with biotin-labeled anti-human RBC mouse immunoglobulin G (IgG) Fab fragment (27) and phycoerythrin-labeled streptavidin (Life Technologies, Rockville, Md.) and was maintained at over 90% by repeatedly transfusing 0.5 ml of a packed cell volume of human RBCs (approximately 6 × 109) into the mice at 2- to 4-day intervals, together with administration of anti-mouse RBC monoclonal rat antibody clone 2E11 (17) and anti-erythropoietin rabbit serum (17). Blood samples were collected from the tail veins of the inoculated animals, and Giemsa-stained thin-smeared blood films were prepared to microscopically determine the percent parasitemia. The isolates were further propagated by subpassage into new animals. For the preparation of antigens or DNA, blood (parasitemia levels, 20 to 80%) was harvested by cardiocentesis from anesthetized animals, washed in PBS, and stored without cryopreservatives at −80°C.
Amplification and sequencing of parasite rDNA.
Parasite DNAs were prepared from the frozen blood stocks described above with a whole-blood DNA extraction kit (GenTLE; TaKaRa Biochemical, Otsu, Japan). Sequences encoding the eukaryotic nuclear small-subunit rRNA gene (rDNA) were amplified from the DNA samples by PCR with the primer set described by Medlin et al. (15). The specific PCR products, approximately 1.8 kb, were cloned and sequenced as described in our previous report (20). Nested PCR was used to detect parasites in human or rodent blood samples by a previously published protocol (19) except for minor modifications of the primers used. DNA samples were prepared from 500 μl of the heparinized whole-blood specimens with a DNA Extractor WB kit (Wako Pure Chemical Industries, Osaka, Japan); 1/10 of each specimen was used for the first round of PCR with the primer set of Bab1A (5′-GTCTTAGTATAAGCTTTTATACAGCG-3′) and Bab4A (5′-GATAGGTCAGAAACTTGAATGATACATCG-3′), followed by the second round of PCR with 1 μl of the first-round PCR product and the primer set of Bab2A (5′-CAGTTATAGTTTATTTGATGTTCGTTTTAC-3′) and Bab3A (5′-CGGCAAAGCCATGCGATTCGCTAAT-3′). The results of this nested PCR were confirmed by another nested PCR done independently in a different laboratory with newly designed primer sets: Bab5 (5′-AATTACCCAATCCTGACACAGG-3′) and Bab8 (5′-TTTCGCAGTAGTTCGTCTTTAACA-3′) for the first-round amplification, followed by Bab6 (5′-GACACAGGGAGGTAGTGACAAGA-3′) and Bab7 (5′-CCCAACTGCTCCTATTAACCATTAC-3′) for the second-round amplification.
Antigenic analyses.
The indirect immunofluorescent-antibody (IFA) test was carried out by the method described previously (20), except that secondary antibody was replaced with fluorescein isothiocyanate-conjugated affinity purified goat IgG F(ab′)2 antibodies (anti-human IgM [heavy and light chains; IBL, Fujioka, Japan], anti-human IgG (Fc) [IBL], anti-human IgG, IgA, and IgM [Protos Immunoresearch, San Francisco, Calif.]). A mixture of fluorescein isothiocyanate-conjugated anti-mouse IgG plus IgM and anti-rat IgG plus IgM (Jackson ImmunoResearch Laboratories, Inc., West Grove, Pa.) was used for detection of antibodies in A. speciosus mouse serum. For the titration of specific IgM in human sera, samples were treated with protein G-conjugated Sepharose (Amersham Pharmacia Biotech, Uppsala, Sweden) to remove IgG antibodies. Western blot analysis was performed as described previously (2). Frozen stocks of Babesia-infected RBCs were thawed and washed five times at 4°C in 10 mM Tris-HCl–10 mM EDTA (pH 7.5) by centrifugation at 10,000 × g for 10 min. The resulting pellets were resuspended in 125 mM Tris-HCl (pH 6.5) containing 5% β-mercaptoethanol, 2% sodium dodecyl sulfate, 10% glycerol, and 0.1% bromophenol blue, heated at 98°C for 5 min, and vigorously vortexed. The samples were diluted such that each contained material from equivalent numbers of infected RBCs and were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis with 7.5% acrylamide gels. Proteins were electrophoretically transferred to Fluorotrans membranes (Pall BioSupport, Port Washington, N.Y.) for 1 h at 100 V. After being blocked with PBS containing 0.5% casein, the membranes were reacted with appropriately diluted immune sera, followed by reaction with secondary antibodies (alkaline phosphatase-conjugated AffiniPure goat anti-mouse IgG [heavy and light chains] or anti-Syrian hamster IgG [heavy and light chains]; Jackson ImmunoResearch Laboratories, Inc.). Immunoreactive antigens were detected with a BCIP/NBT Alkaline Phosphatase Substrates Kit IV (Vector Laboratories, Inc., Burlingame, Calif.).
RESULTS
Isolation of parasites from an asymptomatic carrier.
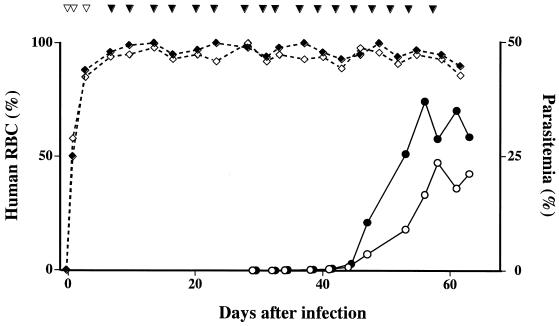
After establishing the diagnosis of B. microti babesiosis in the index case patient, we initiated a search for the blood donors who contributed to the units that had presumably infected the patient. As reported previously (20), we identified one individual whose serum contained antibody specific for B. microti. On 27 July 1999, we were able to obtain a blood sample from the implicated donor. The sample proved to be negative both by microscopy of a Giemsa-stained thin blood smear and by nested PCR specific for B. microti rDNA. However, we were able to isolate Babesia parasites by inoculation of the blood into hu-RBC-SCID mice (Fig. 1) and into splenectomized hamsters as well. It required approximately a month before parasitemia became detectable in the mice and the hamsters, indicating that the sensitivities of these experimental animals for parasite isolation were comparable and that the donor had a very low level of parasitemia. We also examined the blood samples from the donor's family members (father, brother, and sister) for detection of parasite-specific antibody and parasite DNA and for isolation of parasites by inoculation into hamsters, but all examinations gave rise to negative results.
FIG. 1.
Isolation of a Babesia parasite from an asymptomatic blood donor with hu-RBC-SCID mice. A dose of 6 × 109 RBCs from the donor was inoculated into splenectomized NOD/shi-scid mice on days 0, 1, and 3 (open arrowheads), and thereafter, the mice were repeatedly transfused with an equal dose of Babesia-free RBCs from a healthy volunteer on the days indicated by closed arrowheads. Two mice (open and closed symbols, respectively) were used, and peripheral blood samples from these mice were periodically examined for the rate of replacement with human RBCs (⋄ and ♦ on dotted lines) and for levels of parasitemia (○ and ● on solid lines).
Parasite isolation from a field mouse.
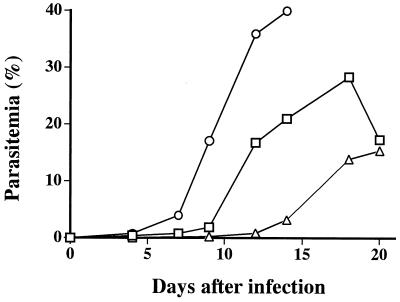
We set a total of 200 Sherman live traps at various sites within approximately 7 km of the donor's residence and captured two field mice, both of which were identified as A. speciosus. Microscopy revealed that one of the mice was parasitemic, and its serum contained antibody specific for the Kobe strain. The other mouse was negative by examination of a blood smear and by an IFA test. The blood sample from the positive mouse was divided into three portions and was inoculated into a hamster, an SCID mouse, and an hu-RBC-SCID mouse. The level of parasitemia increased the fastest in the hu-RBC-SCID mouse, followed by those in the hamster and the SCID mouse, in that order (Fig. 2). Flow cytometric analysis with the parasitized RBCs obtained from the hu-RBC-SCID mouse revealed the presence of parasites within human RBCs (data not shown).
FIG. 2.
Propagation of Babesia parasites derived from a field mouse (A. speciosus) in a hamster, an SCID mouse, and an hu-RBC-SCID mouse. The blood sample from the Apodemus mouse, which contained approximately 2 × 107 parasitized RBCs, was divided into three portions and inoculated on day 0 into a hamster (□), an NOD/shi-scid mouse (▵), and an hu-RBC-SCID mouse (○). All animals had been splenectomized prior to the experiment.
Analysis of rDNA sequence.
Approximately 1.8 kb of the rDNA target sequence was obtained from the parasites isolated from the asymptomatic donor and from the Apodemus mouse. Both sequences were exactly identical to that previously reported for the Kobe strain (DDBJ accession no. AB032434).
Antigenic analysis.
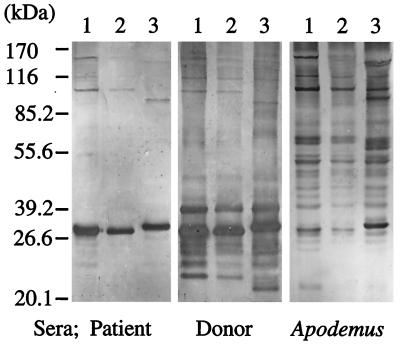
To determine whether the patient, donor, or mouse isolates might differ antigenically, we performed pairwise comparisons of their IFA titers. Whereas the sera from both the patient and the donor demonstrated comparable titers of antibodies against all the three parasite isolates (IFA titers, 1:12,800 or 1:25,600 with both serum samples), the Apodemus mouse serum showed a higher titer against homologous parasite antigens than heterologous parasite antigens (IFA titers, 1:3,200 and 1:800, respectively). When the profiles of the parasite antigens were analyzed by Western blotting, the isolates from the patient and the donor were indistinguishable but differed slightly from the Apodemus mouse-derived parasite (Fig. 3). The patient's convalescent-phase sera mainly recognized three immunodominant proteins with estimated molecular masses of approximately 28, 97, and 140 kDa, respectively, whereas the donor's sera recognized a much wider variety of additional antigens. Parasite antigens recognized by the Apodemus mouse serum sample greatly differed from those recognized by the human serum sample.
FIG. 3.
Western blot analysis of Babesia parasites isolated from the index case patient, the donor, and an Apodemus mouse (lanes 1, 2, and 3, respectively). Insoluble membrane fractions of the lysates of infected hamster RBCs were analyzed with sera obtained from the patient, the donor, and the Apodemus mouse. The relative mobilities of the molecular mass marker proteins are indicated.
Follow-up survey.
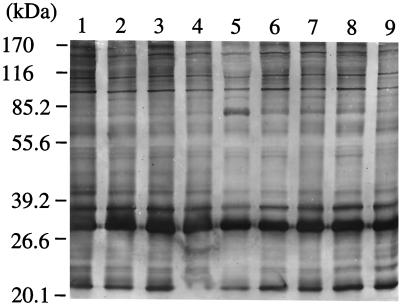
Follow-up of the patient and the asymptomatic donor demonstrated that both remained chronically parasitemic and seroreactive (Table 1). Because we found that the presence of IgG antibody significantly interfered with determination of the IgM titer by the IFA test, the serum samples were absorbed with protein G-Sepharose prior to the IgM titer determination. Throughout the survey period, the donor had apparently been healthy and did not show any clinical symptoms of babesiosis. This individual's serum consistently contained high specific IgG titers. Babesia parasites were repeatedly isolated from the donor's blood by inoculation into splenectomized hamsters, whereas detection of parasite DNA by rDNA-based PCR gave variable results. The patient had high IgM titers at both the acute and the recrudescent phases. A rapid rise in IgG titer was observed after the recrudescent phase, which was followed by gradual decreases in both IgG and IgM titers, in accordance with the disappearance of parasitemia. All the isolates obtained at various time points from the patient and the asymptomatic carrier exhibited virtually identical banding profiles when the parasite antigens were analyzed by Western blotting (Fig. 4).
TABLE 1.
Results of follow-up surveys of the patient and the donor
| Sample source and date (yr-mo-day) | Detection of parasites
|
Reciprocal IFA titers
|
|||
|---|---|---|---|---|---|
| Microscopya | PCRb | Inoculationc | IgM | IgG | |
| Patient | |||||
| 1998-12-28d | NDe | − | ND | <100 | <100 |
| 1999-05-24 | + | + | + | 12,800 | 1,600 |
| 1999-06-25 | − | + | ND | 12,800 | 800 |
| 1999-07-27 | − | + | + | 3,200 | 1,600 |
| 1999-08-31 | + | + | + | 12,800 | 102,400 |
| 1999-10-02 | − | + | ND | 12,800 | 51,200 |
| 1999-11-01 | ND | − | ND | 1,600 | 25,600 |
| 1999-11-29 | ND | − | ND | 800 | 25,600 |
| 1999-12-27 | ND | − | ND | 400 | 6,400 |
| 2000-01-29 | ND | ND | ND | 200 | 3,200 |
| 2000-03-08 | ND | − | ND | 200 | 3,200 |
| 2000-05-22 | − | − | − | 200 | 1,600 |
| 2000-07-19 | − | − | − | 100 | 800 |
| Donor | |||||
| 1998-12-22 | ND | + | ND | 200 | 25,600 |
| 1999-06-26 | ND | − | ND | 100 | 25,600 |
| 1999-07-27 | − | − | + | 50 | 25,600 |
| 1999-08-31 | − | + | + | 25 | 12,800 |
| 1999-10-02 | − | + | + | 50 | 25,600 |
| 1999-11-11 | − | − | + | 200 | 51,200 |
| 1999-12-20 | − | − | − | 100 | 51,200 |
| 2000-01-31 | − | − | + | 50 | 25,600 |
| 2000-03-06 | − | − | + | 100 | 25,600 |
| 2000-04-24 | ND | + | ND | ND | ND |
Giemsa-stained thin-smeared blood films were microscopically observed; +, parasitemia detectable; −, not detectable.
Amplification by nested PCR. Results were confirmed in different laboratories by independent tests with different primers sets; +, amplification positive; −, negative.
Inoculation into splenectomized hamster. Blood samples from the animals were observed for 2 months; +, parasitemia detected; −, not detected.
Because a blood sample from the patient was not available on this day, the results were estimated with extracts from the cross-match test papers made prior to blood transfusion.
ND, not done.
FIG. 4.
Western blot analysis of Babesia parasites isolated from the patient and the donor at various sampling times. Isolates were obtained from the patient's blood samples obtained on 24 May 1999, 27 July 1999, and 31 August 1999 (lanes 1, 2, and 3, respectively) and from the donor's blood samples obtained on 27 July 1999, 31 August 1999, 2 October 1999, 11 November 1999, 31 January 2000, and 6 March 2000 (lanes 4 through 9, respectively). Parasite antigens were immunostained with pooled sera obtained from the donor.
DISCUSSION
In the present study we have conclusively demonstrated that the Japanese index case of human babesiosis was acquired by a blood transfusion from an asymptomatic donor who acquired the infection in Japan. The parasite was isolated from the asymptomatic blood donor and was indistinguishable from those isolated from the index case patient by rDNA sequencing and immunodominant antigen profiles. A mouse trapped near the residence of the donor was also infected with a genetically indistinguishable Babesia isolate. As we reported previously, neither the index case patient nor the donor had traveled from Japan. Thus, there is no doubt that the index case was autochthonous, although the degree of endemicity appears to be low, as the donor's family members were uninfected.
Because the donor denied prior exposure to ticks and to engaging in any relevant outdoor activities, exactly how he or she became infected is unknown. However, the donor was probably infected around his or her residential area, inasmuch as a field mouse infected with B. microti-like parasites was found there and the parasites from the mouse and the donor had identical rRNA sequences and similar antigenic properties. This field mouse was identified as A. speciosus, which Shiota et al. (22) previously identified as being commonly infected with B. microti-like parasites in Shiga Prefecture. This mouse has previously been identified as a main Japanese reservoir for Borrelia burgdorferi sensu lato (18), and it seems probable that it concurrently serves as a main reservoir of B. microti in Japan. The Babesia sp. in A. speciosus mice described by Shiota et al. (22), however, may be different from that obtained in the present study, because we recently found that a B. microti-like parasite isolated from a field mouse (A. speciosus) captured in the town of Yamanaka in Shiga Prefecture, Japan, the same place where the prototype parasite was found two decades ago, had a rDNA sequence (DDBJ accession no. AB050732) which differed from those of the Kobe strain (DDBJ accession no. AB032434) and B. microti parasites in the United States (accession no. U09833) (M. Tsuji and T. Shiota, unpublished data). There therefore appears to be at least two types of indigenous B. microti-like parasites in Japan, and epidemiological studies of these parasites are ongoing. We are actively attempting to identify the tick vectors, regions of endemicity, and prevalence of infection in wildlife and humans.
We were able to demonstrate that the hu-RBC-SCID mouse model, which was developed in our previous study (20, 27), serves as an excellent experimental tool for isolation of Babesia parasites from a human carrier with a very low level of parasitemia. The sensitivity of this animal model was comparable to that of splenectomized hamsters, which have previously been reported to have the greatest susceptibility to B. microti (3). Moreover, the use of hu-RBC-SCID mice enabled us, for the first time, to demonstrate clearly that rodent-derived parasites are readily infective for human RBCs. Because an in vitro cultivation system with human RBCs has not yet been made available for B. microti, inoculation into the hu-RBC-SCID mice is the sole method for assessment of the infectivity of animal-derived parasites for humans.
The dynamics of the specific antibody responses in the patient and the asymptomatic donor provide evidence for premunition (concomitant immunity), first described during the seminal investigations of the mode of perpetuation of Texas cattle fever due to Babesia bigemina (23). The index case patient was discharged from the hospital in late July 1999, after treatment with quinine, clindamycin, and atovaquone. A month later, the patient experienced a recrudescent parasitemia (13), which resulted in boosting of not only IgG titers but also IgM titers. If recrudescence and reappearance of a specific IgM response are common during human babesiosis, detection of a high serum IgM antibody titer may not necessarily be taken as evidence for a recently acquired primary infection. Whereas the index case patient's IgG titer diminished with parasite clearance, the donor consistently had very high IgG titers. This observation is consistent with premunition, in which parasites persist despite a significant antibody response; parasitemias are controlled to a very low level by the immune response but generate enough antigenic stimuli to maintain immunity. However, antigenic variation, which has been suggested for cattle babesiosis (1), was not observed: virtually identical immunoblot profiles were seen with parasites isolated at various times from both the patient and the asymptomatic donor.
Consistent with previous reports (10), our monitoring of the index case patient and donor also demonstrated that parasitemia may persist for an extended period, implying an asymptomatic carrier state. The donor described in the present study did not show any clinical signs or symptoms of babesiosis, and the level of parasitemia was so low that a highly sensitive test, such as nested PCR, often failed to detect it. Blood donated from such individuals may therefore produce additional transfusion-associated infections. On the other hand, our ongoing epidemiological survey indicates that the prevalence of asymptomatic carriers in Japan is quite low (M. Tsuji, unpublished data). While effective chemotherapeutic protocols against human babesiosis (9, 31, 32) have already been established, an effective blood screening method for prevention of the transfusion-acquired infections has not yet been available even in the regions in the United States where babesiosis is endemic (14), where over 20 transfusion-associated cases have been reported (12). Practical measures that might be taken in Japan and at other locations where B. microti is endemic would be to inform physicians and patients about the risk of transfusion-acquired babesiosis and to develop a posttransfusion test for the rapid, sensitive, and specific detection of Babesia parasites.
ACKNOWLEDGMENTS
We thank M. Ohtake, Y. Saito, and H. Murayama, Rakuno-Gakuen University, for excellent technical assistance.
This work was supported in part by grants-in-aid from the Ministry of Education, Science and Culture of Japan (grants 11660316 and 12450139) and by Gakujutsu-Frontier Cooperative Research at Rakuno-Gakuen University.
REFERENCES
- 1.Allred D A, Cinque R M, Lane T J, Ahrens K P. Antigenic variation of parasite-derived antigens on the surface of Babesia bovis-infected erythrocytes. Infect Immun. 1994;62:91–98. doi: 10.1128/iai.62.1.91-98.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arai S, Tsuji M, Kim S-J, Nakada K, Kirisawa R, Ohta M, Ishihara C. Antigenic and genetic diversities of Babesia ovata in persistently infected cattle. J Vet Med Sci. 1998;60:1321–1327. doi: 10.1292/jvms.60.1321. [DOI] [PubMed] [Google Scholar]
- 3.Brandt F, Healy G R, Welch M. Human babesiosis: the isolation of Babesia microti in golden hamsters. J Parasitol. 1977;63:934–937. [PubMed] [Google Scholar]
- 4.Fay F H, Rausch R L. Parasitic organisms in the blood of arvicoline rodents in Alaska. J Parasitol. 1969;55:1258–1265. [Google Scholar]
- 5.Gorenflot A, Moubri K, Precigout E, Carcy B, Schetters T P M. Human babesiosis. Ann Trop Med Parasitol. 1998;92:489–501. doi: 10.1080/00034989859465. [DOI] [PubMed] [Google Scholar]
- 6.Healy G R, Spielman A, Gleason N. Human babesiosis: reservoir of infection on Nantucket Island. Science. 1976;192:479–480. doi: 10.1126/science.769166. [DOI] [PubMed] [Google Scholar]
- 7.Hussein H S. Ixodes trianguliceps: seasonal abundance and role in the epidemiology of Babesia microti infection in north-western England. Ann Trop Med Parasitol. 1980;74:531–539. doi: 10.1080/00034983.1980.11687381. [DOI] [PubMed] [Google Scholar]
- 8.Koyanagi Y, Tanaka Y, Kira J, Ito M, Hioki K, Misawa N, Kawano Y, Yamasaki K, Tanaka R, Suzuki Y, Ueyama Y, Terada E, Tanaka T, Miyasaka M, Kobayashi T, Kumazawa Y, Yamanoto N. Primary human immunodeficiency virus type 1 viremia and central nervous system invasion in a novel hu-PBL-immunodeficient mouse strain. J Virol. 1997;71:2417–2424. doi: 10.1128/jvi.71.3.2417-2424.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krause P J, Lepore T, Sikand V K, Gadbaw J J, Jr, Burke G, Telford III S R, Brassard P, Pearl D, Azlanzadeh J, Christianson D, McGrath D, Spielman A. Atovaquone and azithromycin for the treatment of babesiosis. N Engl J Med. 2000;343:1454–1458. doi: 10.1056/NEJM200011163432004. [DOI] [PubMed] [Google Scholar]
- 10.Krause P J, Spielman A, Telford III S R, Sikand V K, McKay K, Christianson D, Pollack R J, Brassard P, Magera J, Ryan R, Persing D H. Persistent parasitemia after acute babesiosis. N Engl J Med. 1998;339:160–165. doi: 10.1056/NEJM199807163390304. [DOI] [PubMed] [Google Scholar]
- 11.Krause P J, Telford III S R, Spielman A, Sikand V, Ryan R, Christianson D, Burke G, Brassard P, Pollack R, Peck J, Persing D H. Concurrent Lyme disease and babesiosis: evidence for increased severity and duration of illness. JAMA. 1996;275:1657–1660. [PubMed] [Google Scholar]
- 12.Linden J V, Wong S J, Chu F K, Schmidt G B, Bianco C. Transfusion-associated transmission of babesiosis in New York State. Transfusion. 2000;40:285–289. doi: 10.1046/j.1537-2995.2000.40030285.x. [DOI] [PubMed] [Google Scholar]
- 13.Matsui T, Inoue R, Kajimoto K, Tamekane A, Katayama Y, Shimoyama M, Chihara K, Saito-Ito A, Tsuji M. First documentation of human babesiosis in Japan. Jpn J Clin Hematol. 2000;41:628–634. (In Japanese with English summary.) [PubMed] [Google Scholar]
- 14.McQuiston J H, Childs J E, Chamberland M E, Tabor E. Transmission of tick-borne agents of disease by blood transfusion: a review of known and potential risks in the United States. Transfusion. 2000;40:274–284. doi: 10.1046/j.1537-2995.2000.40030274.x. [DOI] [PubMed] [Google Scholar]
- 15.Medlin L, Elwood H J, Stickel S, Sogin M L. The characterization of enzymatically amplified eukaryotic 16S-like rRNA-coding regions. Gene. 1988;71:491–499. doi: 10.1016/0378-1119(88)90066-2. [DOI] [PubMed] [Google Scholar]
- 16.Mitchell P D, Reed K D, Hofkes J M. Immunoserologic evidence of coinfection with Borrelia burgdorferi, Babesia microti, and human granulocytic Ehrlichia species in residents of Wisconsin and Minnesota. J Clin Microbiol. 1996;34:724–727. doi: 10.1128/jcm.34.3.724-727.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakamura Y, Tsuji M, Arai S, Ishihara C. A method for rapid and complete substitution of the circulating erythrocytes in SCID mice with bovine erythrocytes and use of the substituted mice for bovine hemoprotozoa infections. J Immunol Methods. 1995;188:247–254. doi: 10.1016/0022-1759(95)00222-7. [DOI] [PubMed] [Google Scholar]
- 18.Nakao M, Miyamoto K. Mixed infection of different Borrelia species among Apodemus speciosus mice in Hokkaido, Japan. J Clin Microbiol. 1995;33:490–492. doi: 10.1128/jcm.33.2.490-492.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Persing D H, Mathiesen D, Marshall W F, Telford S R, Spielman A, Thomford J W, Conrad P A. Detection of Babesia microti by polymerase chain reaction. J Clin Microbiol. 1992;30:2097–2103. doi: 10.1128/jcm.30.8.2097-2103.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saito-Ito A, Tsuji M, Wei Q, He S, Matsui T, Kohsaki M, Arai S, Kamiyama T, Hioki K, Ishihara C. Transfusion-acquired, autochthonous human babesiosis in Japan: isolation of Babesia microti-like parasites with hu-RBC-SCID mice. J Clin Microbiol. 2000;38:4511–4516. doi: 10.1128/jcm.38.12.4511-4516.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shih C-M, Liu L-P, Chung W-C, Ong S J, Wan C-C. Human babesiosis in Taiwan: asymptomatic infection with a Babesia microti-like organism in a Taiwanese woman. J Clin Microbiol. 1997;35:450–454. doi: 10.1128/jcm.35.2.450-454.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shiota T, Kurimoto H, Haguma N, Yoshida Y. Studies on Babesia first found in murine in Japan: epidemiology, morphology and experimental infection. Zentbl Bakteriol Mikrobiol Hyg Reihe A. 1984;256:347–355. [PubMed] [Google Scholar]
- 23.Smith T, Kilbourne F L. Investigation into the nature, causation, and prevention of Texas or southern cattle fever. Bull Bur Anim Ind US Dept Agric. 1893;1:177–304. [Google Scholar]
- 24.Steketee R W, Eckman M R, Burgess E C, Kuritsky J N, Dickerson J, Schell W L, Godsey M S, Jr, Davis J P. Babesiosis in Wisconsin: a new focus of disease transmission. JAMA. 1985;53:2675–2678. doi: 10.1001/jama.253.18.2675. [DOI] [PubMed] [Google Scholar]
- 25.Telford S R, III, Maguire J H. Babesiosis. In: Guerrant R L, Walker D H, Weller P F, editors. Tropical infectious diseases: principles, pathogens, and practice. Vol. 1. London, England: Churchill Livingstone; 1999. pp. 767–773. [Google Scholar]
- 26.Telford S R, III, Spielman A. Babesiosis of humans. In: Collier L, Balows A, Sussman M, editors. Topley and Wilson's microbiology and microbial infections. 9th ed. Vol. 5. London, England: Arnold; 1998. pp. 349–359. [Google Scholar]
- 27.Tsuji M, Ishihara C, Arai S, Hiratai R, Azuma I. Establishment of a SCID mouse model having circulating human red blood cells and a possible growth of Plasmodium falciparum in the mouse. Vaccine. 1995;13:1389–1392. doi: 10.1016/0264-410x(95)00081-b. [DOI] [PubMed] [Google Scholar]
- 28.van Peen P F D, Chang S J, Banknieder A R, Santana F J. Piroplasms from Taiwanese rodents. J Protozool. 1977;24:310–312. doi: 10.1111/j.1550-7408.1977.tb00984.x. [DOI] [PubMed] [Google Scholar]
- 29.Walter G. Transmission and course of parasitemia of Babesia microti (Hannover I strain) in the bank vole (Clethrionomys glareolus) and field vole (Microtus agrestis) Acta Trop. 1984;41:259–264. . (In German.) [PubMed] [Google Scholar]
- 30.Watkins R A, Moshier S E, O'Dell W D, Pinter A J. Splenomegaly and reticulocytosis caused by Babesia microti infections in natural populations of the montane vole, Microtus montanus. J Protozool. 1991;38:573–576. doi: 10.1111/j.1550-7408.1991.tb06082.x. [DOI] [PubMed] [Google Scholar]
- 31.Wittner M, Lederman J, Tanowitz H B, Rosenbaum G S, Weiss L M. Atovaquone in the treatment of Babesia microti infections in hamsters. Am J Trop Med Hyg. 1996;55:219–222. doi: 10.4269/ajtmh.1996.55.219. [DOI] [PubMed] [Google Scholar]
- 32.Wittner M, Rowin K S, Tanowitz H B, Hobbs J F, Saltzman S, Wenz B, Hirsch R, Chisholm E, Healy G R. Successful chemotherapy of transfusion babesiosis. Ann Intern Med. 1982;96:601–604. doi: 10.7326/0003-4819-96-5-601. [DOI] [PubMed] [Google Scholar]