Abstract
Background
Platelets support tumour progression. However, their prognostic significance and relation to circulating tumour cells (CTCs) in operable breast cancer (BrCa) are still scarcely known and, thus, merit further investigation.
Methods
Preoperative platelet counts (PCs) were compared with clinical data, CTCs, 65 serum cytokines and 770 immune-related transcripts obtained using the NanoString technology.
Results
High normal PC (hPC; defined by the 75th centile cut-off) correlated with an increased number of lymph node metastases and mesenchymal CTCs in the 70 operable BrCa patients. Patients with hPC and CTC presence revealed the shortest overall survival compared to those with no CTC/any PC or even CTC/normal PC. Adverse prognostic impact of hPC was observed only in the luminal subtype, when 247 BrCa patients were analysed. hPC correlated with high content of intratumoural stroma, specifically its phenotype related to CD8+ T and resting mast cells, and an increased concentration of cytokines related to platelet activation or even production in bone marrow (i.e. APRIL, ENA78/CXCL5, HGF, IL16, IL17a, MDC/CCL22, MCP3, MMP1 and SCF).
Conclusions
Preoperative platelets evaluated alone and in combination with CTCs have prognostic potential in non-metastatic BrCa and define patients at the highest risk of disease progression, putatively benefiting from anti-platelet therapy.
Subject terms: Breast cancer, Prognostic markers
Background
Platelets play a key role in regulating tumour cells’ survival in the blood stream and metastasis efficiency [1, 2]. They can adhere to circulating tumour cells (CTCs), support their extravasation [3, 4], induce their proliferation [5] and even recruit and activate macrophages or neutrophils to induce tumour escape from natural killer cell lysis [6]. Putatively platelets can also initiate or at least maintain epithelial-mesenchymal transition (EMT) in CTCs, which might result in the generation of a (semi-) mesenchymal phenotype [7, 8] that is assumed to indicate higher metastatic potential of CTCs [9]. This phenomenon can influence CTC detection using standard methods, such as epithelial cell marker-based isolation and characterisation; thus, it can result in false-negative enumeration of CTCs [9]. Preoperative platelet counts (PCs) and platelet-to-lymphocyte ratios have been demonstrated to predict patient outcomes in certain tumour entities [10–12], including breast cancer and even its most aggressive triple-negative subtype [13–15]. Finally, the prevention or inhibition of platelet activation by antithrombotic drugs, such as aspirin, heparin, warfarin or novel nanoparticle cocktails, can result in improved patient outcomes and even attenuate the formation of the metastatic niche [16–19].
Platelets release small molecules, such as cytokines/chemokines, which, in turn, can directly or indirectly regulate CTCs and their fate. Cytokines/chemokines mediate the communication between cells and can be secreted by primary tumours, intratumoural stromal cells, immune cells or even different cellular components of homing organs or already existing distant (micro)metastasis. However, only a few studies have investigated cytokines/chemokines in relation to CTCs, including those on breast carcinoma [20–23]. The concentrations of cytokines/chemokines differ depending on the status and characteristics of CTCs [22, 23] and correlate with patient outcomes in different solid tumours, including breast cancer [24]. Some of them are considered therapeutic targets [25], whereas others (e.g. tumour growth factor beta (TGFβ) or platelet-derived growth factor) can mediate platelet regulation of the tumour cell phenotype [1, 8].
This study aims to investigate the clinical relevance of platelets and their potential interplay with CTCs in non-metastatic breast cancer. The PCs and selected serum cytokines/chemokines are correlated with the clinical outcome, presence and phenotype of CTCs and molecular features of primary tumours to reveal a potential network of interactions.
Methods
Breast cancer patients
Cohort I: patients with CTC and primary tumour characteristics
Female breast cancer patients (n = 108) were treated in the Medical University Hospital in Gdańsk, Poland, during 2010-2013. All patients were staged I–III according to the American Joint Committee on Cancer staging manual version 7 [26]. Patients with co-morbidities or secondary cancer, undergoing neoadjuvant therapy, with lobular and mixed (i.e. lobular-ductal) breast cancer (characterised by innate loss of E-cadherin putatively falsifying the acquired EMT status) and those for whom preoperative blood count data were not available were excluded from this study. Due to fewer patients with PC beyond the normal range, the patients were limited to those with PC ranged between 150,000–400,000 platelets/µl (n = 70). Their blood counts and clinical parameters were documented (Supplementary Tables 1 and 2). Overall survival (OS) was defined as the time between the mastectomy and the patient’s death. The last follow-up was completed in February 2016, and the median follow-up was conducted after 4.1 years (range 1.6–5.3 years).
Informed consent was collected from all study participants, and the permission to conduct this experiment was taken from the Bioethical Committee of the Medical University of Gdansk (NKEBN/30/2010, approved on the 17 March 2010).
Cohort II: extended cohort of patients with different age and molecular subtypes of breast cancer
Female non-metastatic breast cancer patients without previous and concurrent malignancies, staged I–III treated in the Medical University of Gdańsk (n = 70, Gdańsk, Poland) and Medical University of Warsaw (n = 184, Warsaw, Poland), were included in this cohort based on their preoperative PC within normal range (n = 254). Their clinical parameters, including age and molecular subtype were documented (Supplementary Table 3).
The study was conducted in accordance with the Helsinki Declaration of 1975, STROBE [27] and REMARK study recommendations [28].
Blood counts
Blood counts were performed in the routinely collected preoperative blood samples in the certified institution of each involved centre (i.e. Central Clinical Laboratory of the Medical University Hospital, Gdańsk, Poland and Maria Skłodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland) by using standard automated methods. In addition, lymphocyte-to-neutrophil, lymphocyte-to-monocyte, platelet-to-lymphocyte, eosinophil-to-lymphocyte, basophil-to-lymphocyte and eosinophil-to-basophil ratios were calculated. Different cut-offs were tested in order to divide patients in regard to their PC. As a final stratification system, in the presented study patients were dichotomised into groups with normal PC (nPC) and high normal PC (hPC) according to the 75th centile cut-off equal 289,000 platelets/ µl.
CTC isolation and characterisation
CTCs were isolated and enriched from preoperatively collected blood samples (at a volume of 5 ml) of Cohort I patients and phenotyped blindly to the clinico-pathological data for the gene expression of HER2/mammaglobin 1/cytokeratin 19/vimentin as previously described [26]. Briefly, the CTC-enriched fraction was isolated, and RNA was extracted, pre-amplified and quantified by the real-time polymerase chain reaction. MGB1+ and/or HER2+ samples identified as (i) CK19+/VIM− were classified as epithelial CTC-positive, whereas those identified as (ii) CK19−/VIM+ were classified as mesenchymal CTC-positive.
Cytokine/chemokine analysis
The blood samples (at a volume of 3.5 ml) for cytokine/chemokine analysis were obtained preoperatively from 36 Cohort I patients and characterised as CTC-negative (CTC−, n = 24), epithelial CTC-positive (epiCTC+, n = 7) and mesenchymal CTC-positive (mesCTC+, n = 5). The blood sera were fractionated through centrifugation at 1300–2000 × g for 10 min within 30 min after blood collection and stored at −80 °C until further analysis.
The concentrations of the serum cytokines/chemokines (i.e. APRIL, BAFF, BLC, bNGF, CD30, CD40-ligand, ENA78, Eotaxin, Eotaxin-2, Eotaxin-3, FGF-2, Fractalkine, G-CSF/CSF-3, GM-CSF, GRO-alpha/KC, HGF, IFN-alpha, IFN-gamma, IL-1alpha, IL-1beta, IL-10, IL-12p70, IL-13, IL-15, IL-16, IL-17A, I-18, IL-2, IL-2R, IL-20, IL-21, IL-22, IL-23, IL-27, IL-3, IL-31, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IP-10, I-TAC, LIF, MCP-1, MCP-2, MCP-3, M-CSF, MDC/CCL22, MIF, MIG, MIP-1alpha, MIP-1beta, MIP-2alpha, MMP1, SCF, SDF-1alpha, TNF-alpha, TNF-beta, TNF-RII, TRAIL, TSLP, Tweak and VEGF-A) were measured using a commercially available magnetic microbead-based 65-Plex Human ProcartaPlexTM Panel and a Bio-Plex® 200 system based on Luminex xMAP technology (Bio-Rad) according to the manufacturer’s instructions. The analysed blood sera were blinded to the experimenter, diluted in 1:4 ratio and measured in duplicate. The cytokine/chemokine concentrations were calculated within the range of the obtained standard curves at seven descending concentration points (experimental range 0.82–387,992 pg/ml, depending on the cytokine/chemokine).
Primary tumour characterisation
The epithelial or mesenchymal phenotype of the primary tumour of the Cohort I patients was determined immunohistochemically by pathologist on the basis of E-cadherin, N-cadherin and vimentin expressions assessed in tumour cells, as previously described [26]. Briefly, an epithelial phenotype was determined in tumours characterised by the presence of E-cadherin and absence of both N-cadherin and vimentin, whereas a mesenchymal phenotype was determined in tumours characterised by E-cadherin loss or the presence of N-cadherin or vimentin [26].
The intratumoural stromal content within the primary tumour was evaluated using tissue microarrays stained with haematoxylin and eosin. Each tumour was investigated in five fragments (single tissue cores), each having a diameter of 1 mm. The stromal content was defined by the pathologist as the percent area of non-tumour components in each individually examined fragment of tumour. The highest assessed stromal content among the five examined tumor fragments was assigned to a patient and grouped according to the 75th centile (equal to 80%) in the groups with low or high stromal content.
Profiling of immune cells infiltrating primary tumours
The abundance of 22 leukocyte subsets in primary tumours was estimated for 32 Cohort I patients (n = 32, luminal BrCa) by using the CIBERSORTx digital cytometry algorithm [29]. In brief, the previously obtained immune-related transcriptome generated using the nCounter PanCancer Immune Profiling Panel (NanoString technology; data available at NCBI GEO under accession number GSE180186) [30] was subjected to an absolute mode analysis with a leukocyte gene signature matrix (LM22) and B-mode (bulk mode) batch correction following the CIBERSORTx manual (correlation coefficients of the analysed samples ranged 0.567–0.854, all having p < 0.001). The resulting absolute proportions of the leukocyte subsets were compared according to the patient’s platelet count status (nPC vs. hPC). In addition, median-based fold changes (FCs) between those groups were evaluated. Differences were estimated using the Mann–Whitney test, with p < 0.05 considered statistically significant.
Statistics
All outcomes were documented and analysed statistically using SPSS ver. 25 licensed for the University of Gdańsk. The differences in the distribution of clinical parameters between the patients characterised by nPC and hPC were analysed using the chi-squared test or Fisher’s exact test. The relationships between blood counts or cytokine/chemokine concentrations (presented as continuous values) and (i) CTC presence/phenotype, (ii) clinical parameters and (iii) molecular signatures of tumours or their stroma were analysed by conducting the Mann–Whitney or Kruskal–Wallis test. In addition, the outcomes for blood counts and immunohistochemically assessed molecular signatures of the tumour and stroma were classified into negative and positive groups according to the cut-offs equal to their 75th centile values. The associations among CTCs, platelet status and time to OS were evaluated using the log-rank test and Kaplan–Meier plot in R statistical environment [31]. To estimate the hazard risk, a Cox-hazard-potential regression analysis (CI 95%) was conducted. All results with p < 0.05 were considered statistically significant. Cases with missing data were excluded from the analysis.
Results
High normal platelet counts correlate with worse clinical outcome and mesenchymal phenotype of CTCs
The blood count was available for 75 of the 108 previously described [26] non-metastatic breast cancer patients, including a few patients with individual blood cell-type counts beyond the normal ranges. The cut-off at the 75th centile, equal to 289,000 platelets/µl, was used to define patients with nPC and hPC. Only three patients had platelet counts above the upper cut-off of the normal range (150,000–400,000 platelets/µl), which was not sufficient to perform reliable statistical analysis of patients with clinically defined thrombocytosis. Therefore, we narrowed the current analysis to patients within the normal range of preoperative platelet counts. Consequently, 70 breast cancer patients (Supplementary Table 1) with platelets within the normal range (Supplementary Table 2) were selected to study platelets’ association with the OS and presence and phenotype of the CTC. The mean platelet count in this cohort was 257,728 platelets/µl (±SD51,257; range 160,000–390,000 platelets/µl). The other blood counts were defined beyond the normal range for some individual patients (Supplementary Table 2); however, as none of the patients were disqualified from surgery, we did not exclude any further patient from the study cohort. Note that all results presented in this study did not differ if the statistical analysis included three patients with platelet counts above 400,000 platelets/µl in the patient group with high PC (data not shown).
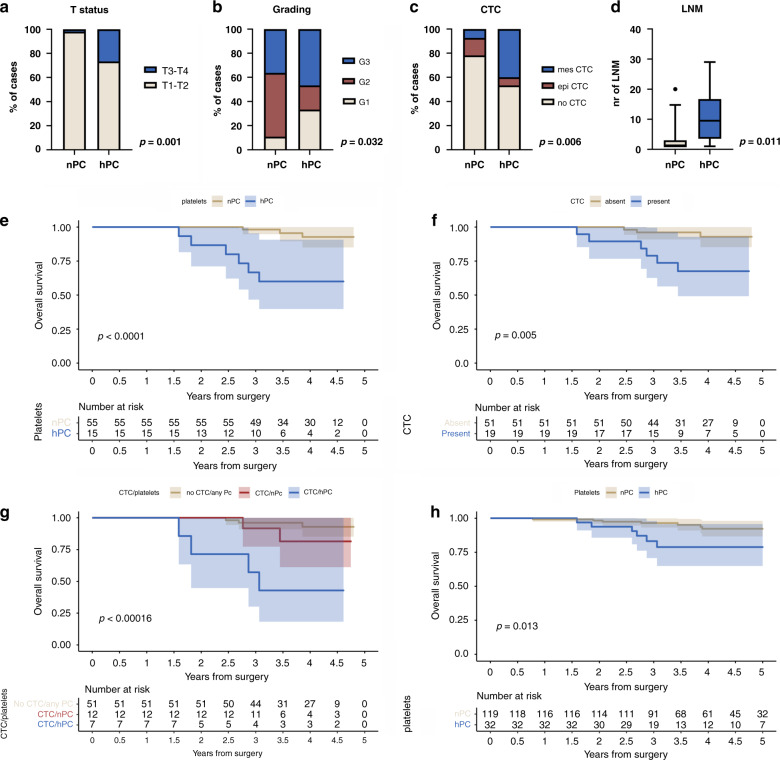
hPC found in 15 (21%) of 70 patients correlated with a higher tumour (T) status (p = 0.001, Fig. 1a, Supplementary Table 3), higher grading (p = 0.032, Fig. 1b, Supplementary Table 4) and the mesenchymal phenotype of CTCs (p = 0.006, Fig. 1c, Supplementary Table 4). PC was higher in CTC-positive patients but did not reach statistical significance (Mann–Whitney test, p = 0.125, Supplementary Fig. 1). hPC was also associated with a higher number of lymph node metastases in lymph node positive (N1) patients (n = 38, Mann–Whitney test, p = 0.011, Fig. 1d).
Fig. 1. Relationship between platelets and clinical data in breast cancer.
Relationship between hPC and higher T status (a), grading (b), CTC phenotype (n = 70) (c) and higher number of LNMs (n = 38, lymph node positive (N1) patients) (d). Survival analysis of breast cancer patients (n = 70) in the context of platelet counts (e), CTC presence (f) and combined status of platelet count and CTC (g). Survival analysis of platelet count in patients with luminal breast cancer (n = 151; panel h). CTC – circulating tumour cell, epi CTC – CTC of epithelial phenotype, mes CTC – CTC of mesenchymal phenotype, nPC – normal platelet count, hPC – high normal platelet count, LNM – lymph node metastasis, OS – overall survival.
The presence of CTC(s) (p = 0.005, Fig. 1e), particularly their mesenchymal phenotype (p = 0.011, data not shown), correlated with the worse clinical outcome in the selected cohort of patients (n = 70), as described previously [26]. The hPC correlated with a shorter OS (Kaplan–Meier plot, log-rank test, p < 0.0001, Fig. 1f; Cox-hazard-potential regression analysis, p = 0.001; HR = 9.635, CI 95% 2.396–38.742, Table 1). The patients with both CTC-positivity and hPC were characterised by a shorter OS (p < 0.001) compared to those with (i) no CTC and any PC (Cox-hazard-potential regression analysis, p = 0.001; HR = 9.635, CI 95% 2.396–38.742) or even (ii) CTCs and nPC (Cox-hazard-potential regression analysis, p = 0.078; HR = 4.634, CI 95% 0.843–25.468, Fig. 1g). Of note, if patients were stratified according to their PC and CTC phenotype (Supplementary Fig. 2A), hPC/mes CTC status was the strong predictor of a shorter time to death when compared to nPC/no CTC (Supplementary Fig. 2B, Kaplan–Meier plot, log-rank analysis, p < 0.001) or even all other patients (Supplementary Fig. 2C, Kaplan–Meier plot, log-rank analysis, p = 0.002).
Table 1.
Impact of platelets on OS of breast cancer patients in multivariate analysis.
| Parameter | Group | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|---|
| p-value | HR | CI 95% | p-value | HR | CI 95% | ||
| Age | ≥50 vs. <50 | 0.984 | 0.984 | 0.204–4.741 | – | – | – |
| T status | T3-4 vs. T1-2 | <0.001 | 1.123 | 1.057–1.193 | 0.197 | 1.052 | 0.974–1.137 |
| N status | N1 vs. N0 | 0.099 | 3.751 | 0.778–18.085 | – | – | – |
| Tumour grade | G3 vs. G1-2 | 0.723 | 0.974 | 0.842–1.127 | – | – | – |
| Hormone receptor status | neg vs. pos | 0.501 | 2.042 | 0.255–16.335 | – | – | – |
| Her2 status | pos vs. neg | 0.892 | 0.897 | 0.186–4.320 | – | – | – |
| Platelet status | hPC vs. nPC | 0.001 | 9.635 | 2.396–38.742 | 0.059 | 4.722 | 0.940–23.723 |
| CTC status | pos vs. neg | 0.013 | 5.783 | 1.445–23.138 | 0.298 | 2.397 | 0.463–12.425 |
Statistically significant results are presented in bold. Platelet range: 150,000–400,000, pts n = 69.
HR hazard ratio, CI confidence interval, hPC high normal platelet count, nPC normal platelet count, CTC circulating tumour cells, neg negative, pos positive.
hPC showed the highest trend for association with the risk for death, however it failed to reach statistical significance in the multivariate analysis, including CTC, platelets, and T status (p = 0.059; HR = 4.722, CI 95% 0.940–23.6723, Table 1). When combined PC/CTC status was analyzed, in the univariate analysis, hPC/CTC status was associated with high risk of death (Cox-hazard-potential regression analysis, p = 0.001; HR 9.452, CI 95% 2.532–35.282). However, in the multivariate analysis, hPC/CTC did not reach statistical significance to be an independent prognostic marker (Cox-hazard-potential regression analysis, p = 0.262; HR 3.031, CI 95% 0.437–21.009, Supplementary Table 5).
To investigate the association between platelet count and OS in the context of other clinical parameters, including age and different molecular subtypes of breast cancer, we extended Cohort I by the dataset containing preoperative PCs within the normal range from non-metastatic breast cancer patients treated in the Medical University of Warsaw (n = 184, Warsaw, Poland) resulting in the Cohort II (n = 254, Supplementary Table 3). In this Cohort, PC did not correlate with any clinico-pathological parameter (Supplementary Table 6), whereas in the older patients and patients with luminal breast cancer it indicated worse outcome. Within five years after the surgery, the hPC correlated with worse OS in the sub-cohorts of all luminal cancers (i.e. luminal A + luminal B; n = 151, p = 0.013, Fig. 1h; Cox regression analysis p = 0.021, HR 3.626, CI 95% 1.216–10.817). However, it did not occur to be independent prognostic marker in multivariate analysis (Supplementary Table 7). Interestingly, in Her2-positive and triple-negative subtype of breast cancer, nPC was observed to correlate with worse prognosis but did not reach the statistical significance (Supplementary Fig. 3). Adverse effect of hPC on 5-years OS was also observed in the patients who were ≥50 (n = 92, Kaplan–Meier plot, log-rank test, p = 0.010, Supplementary Fig. 4), but was not confirmed in the multivariate analysis (Supplementary Table 8).
High normal platelet counts correlate with cytokine/chemokine profile in terms of their aggregation, activation and/or production
To identify the potential communication routes between platelets and CTCs or platelets and primary tumour or other blood cells, a broad panel of different signalling molecules (i.e. cytokines/chemokines) was screened in the sera of the selected patients.
Thirty (46%) of the 65 analysed cytokines/chemokines were detected in the blood sera of at least 4 (10%) out of the 36 examined patients (concentration range 1.17–34919 pg/µl, depending on the cytokine/chemokine concentration).
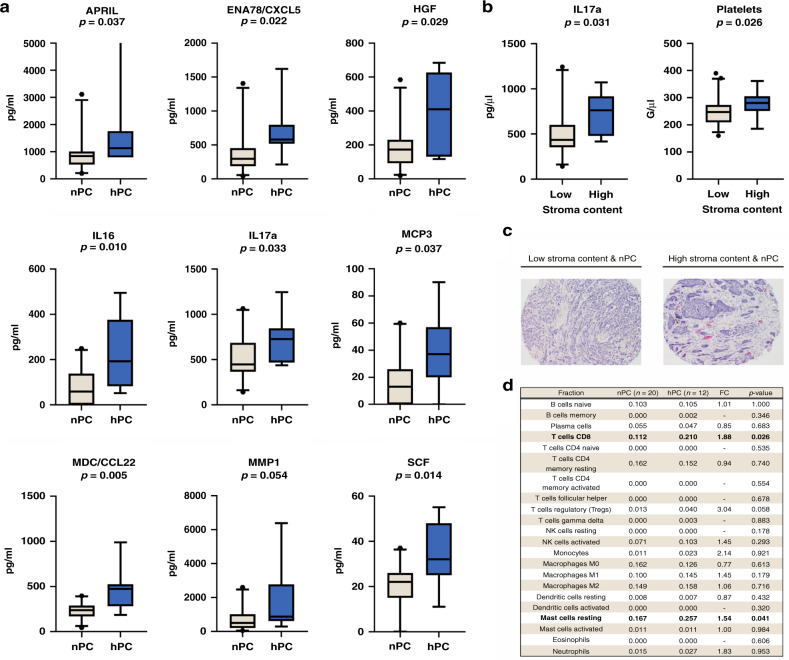
The hPC correlated with increased levels of APRIL (a proliferation-inducing ligand, also known as tumour necrosis factor superfamily member 13, TNFSF13, p = 0.037), epithelial-neutrophil activating peptide (ENA78/CXCL5, p = 0.022), hepatocyte growth factor (HGF, p = 0.029), interleukin 16 (IL16, p = 0.010), interleukin 17a (IL17a, p = 0.033), monocyte-chemotactic protein 3 (MCP3, p = 0.037), macrophage-derived chemokine (MDC/CCL22, p = 0.005), matrix metalloproteinase 1 (MMP1, p = 0.054) and stem cell factor (SCF, p = 0.014) (Fig. 2a). When the cytokine/chemokine concentrations were compared with the platelet status combined with CTC status, ENA78/CXCL5 and IL17a were significantly increased in the patients characterised by the presence of both CTC and hPC in contrast to those with no CTC/any PC or CTC/nPC (p = 0.029 and p = 0.015, respectively; data not shown).
Fig. 2. Relationship between platelets and molecular data in breast cancer patients.
Relationship between cytokine/chemokine levels and platelet status in non-metastatic breast cancer patients with nPC (n = 23) and hPC (n = 7) (a). Relationship of intratumoural stroma with higher IL17a concentration (20 patients with low stromal content vs. 10 patients with high stromal content) and higher platelet counts (53 patients with low stromal content vs. 17 patients with high stromal content) (b). Representative microscope images of low and high stromal contents in tumours of patients with nPC and hPC, haematoxylin and eosin staining, magnification ×100 (c). Leukocyte subset abundance in primary tumours according to platelet count status (CIBERSORTx analysis; for each fraction, median absolute scores in compared groups are presented; panel d). Significant correlations are presented in bold. CTC indicates circulating tumour cells, FC fold change, nPC normal platelet count, hPC high normal platelet count.
High normal platelet counts correlate with intratumoural resting mast cells and CD8+ T-cell phenotype
Some of the selected cytokines/chemokines are known to be produced by platelets in their alpha-granules (e.g. ENA78/CXCL5 and MMP1 [1, 32] and putatively APRIL [33]). Other cytokines/chemokines might be secreted by other components (e.g. tumour cells, intratumoural stromal cells or other blood cells). In the current study, all cytokines/chemokines that were increased in the sera of the patients with hPC were tested against clinical data, available features of primary tumour and blood count (data not shown). Apart from the correlations to hPC, IL17a correlated with a high stromal content (defined by the cut-off at the 75th centile and equal to 80% stroma within the tumour fragment; p = 0.031; Fig. 2b), whereas MDC/CCL22 correlated with a higher number of eosinophils (p = 0.005; data not shown). hPC was also observed in patients with a high intratumoural stromal content (p = 0.026, Fig. 2b, c). Therefore, additional analysis aiming to profile hPC-related stromal components and the selected cytokine/chemokine profile in these patients was performed. Interestingly, when 770 gene expression outcomes obtained using the NanoString technology in 32 primary tumours (for which both platelets and NanoString data were available) were analysed using a digital cytometry algorithm CIBERSORTx [29], hPC correlated with the increased abundance of both CD8+ T cells (FC = 1.88, p = 0.026) and resting mast cells (FC = 1.54, p = 0.041) (Fig. 2d).
When comparing patients with transcriptomic signatures indicating high and low concentrations of resting mast cells in primary tumours (using CIBERSORTx absolute mode and upper-quartile cut-off), a significant increase in the serum levels of IL17a (p = 0.033) was observed in the group with estimated high mast cell infiltration (Supplementary Fig. 5A). In addition, a comparison of signatures indicating the presence of CD8+ T cells revealed that the MMP1 (p = 0.01) and MDC/CCL22 (p = 0.01) levels were increased in tumours with high CD8+ T-cell infiltration (Supplementary Fig. 5B and C, respectively).
Discussion
Platelets are considered a primary element of the blood microenvironment modulating the efficacy of the metastatic cascade. This paper demonstrates the prognostic value of platelets in non-metastatic breast cancer. This is the first study that shows the correlation of hPC with mesenchymal CTCs and worse clinical outcome.
In this study, the platelet counts were restricted to the standard normal range (i.e. 150,000–400,000 platelets/µl), as only three patients in the CTC cohort were characterised as having higher platelet counts related to thrombocytosis, which is commonly associated with adverse outcomes. Thus, a reliable statistical analysis of the correlation of thrombocytosis or three groups of patients (i.e. patients with nPC, hPC and thrombocytosis) with CTC or clinical outcome could not be performed. However, the presented outcomes remained true even if the three patients with defined thrombocytosis were included in the subgroup of patients with high platelet counts (data not shown). In concordance with our data, Ankus et al. observed that hPC might be associated with a higher risk of cancer occurrence, including breast cancer [34], whereas in head and neck squamous cell carcinoma, hPC was correlated with worse OS [35]. In the study of a large cohort of 40,987 healthy inhabitants of seven Italian areas, the normal range of platelets was proposed to be revised in the context of age, as the range of platelet counts was significantly diminished in the proband groups above 64 years of age [36]. This observation could potentially explain why in our cohorts of patients predominantly characterised by older age, the effect of hPC classified with a relatively low cut-off (i.e. 289,000 platelets/µl) was not considered as classical thrombocytosis.
The study results corroborate the hypothesis that preoperative hPC, as well as the coincidence of CTCs, in particular mes CTC, and hPC, reflects an advanced stage of disease and poor prognosis and can have a critical impact on the metastatic process. This observation is consistent with the preclinical data obtained by Labelle et al. for a mouse model [7, 8], where the platelets supported the metastatic process. Here, the higher PC defined even within the standard normal range correlated with the worse clinical outcome and occurred more frequently in blood samples enriched by CTCs characterised by the mesenchymal phenotype, which represent a particularly aggressive type of CTCs [9]. The association of hPC with mesenchymal but not epithelial CTCs is found based on very few patients and, thus, needs further investigation. Nevertheless, hPC seems to enhance the adverse impact of CTCs on patients’ survival: patients with CTCs, in particular mes CTC, and hPC exhibit a worse prognosis more frequently than those with no CTCs and any platelet count or even CTCs and nPC. This finding is supported by the fact that hPC is the only factor that impacts OS in multivariate analysis, including the statuses of T, platelets and CTCs. Although this correlation is only borderline (which might be biased by the low number of patients included in this analysis), it indicates the postulated link between hPC and tumour progression. Finally, in our study, the platelets correlate with a higher number of established metastases in lymph nodes. Tumour dissemination to lymph nodes is considered to occur predominantly through lymphatic vessels. The correlation observed in this study suggests that vascular vessels form an alternative route and that metastatic spread is more efficient in a microenvironment abundant in platelets.
Intriguingly, in the larger cohort of patients (n = 254), hPC did not correlate to age and molecular subtype, but indicated worse clinical outcome both in older patients and luminal cancers. It might be speculated that those differences result from hormonal changes occurring both during aging and luminal cancers treatment as estrogen receptor is expressed on platelets [37, 38]. This observation, including the underlying mechanisms, should be carefully exploited for a large cohort of patients.
The relation between platelets and CTCs is being extensively studied. A series of recent data have suggested that platelets interact with CTCs, facilitating their later extravasation [1, 3, 4] and tumour cell escape from the immune response [6]. Moreover, CTCs can undergo EMT in the blood stream within a short transit time upon direct contact with platelets under platelet-derived TGFβ stimulation. In our study, hPC is associated with the mesenchymal phenotype of CTCs but not with the phenotype of tumour cells examined within primary tumours (Supplementary Table 4). However, the number of patients for whom the matched samples of primary tumour and blood are analysed is too small to observe whether the change in the phenotype of tumour cells indeed occurs in the blood stream and is platelet-dependent (10 pairs of primary tumour-blood samples with defined tumour cell phenotype; data not shown). Therefore, further studies, particularly those visualising potential interactions between platelets and CTCs, need to be conducted to prove whether platelets can initiate or only maintain EMT in tumour cells during transit through the blood stream.
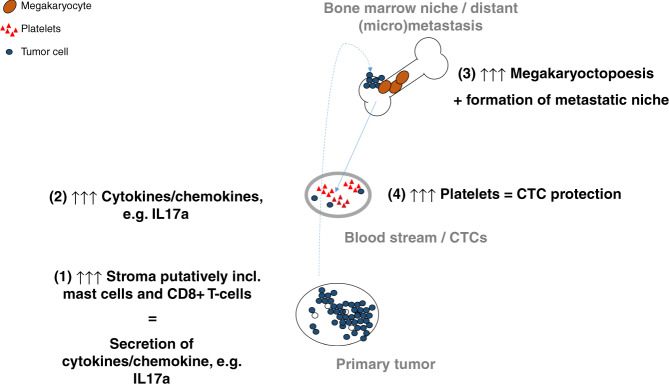
hPC is correlated with elevated levels of certain serum cytokines/chemokines, which support platelets’ pro-tumorigenic function (ENA78/CXCL5, MMP1) and play a role in their activation (MDC/CCL22) [39]. Increased levels of ENA78/CXCL5 and IL17a are observed in patients with CTCs and hPC in contrast to those without CTCs or CTCs and nPC. Interestingly, some of these selected cytokines/chemokines (APRIL, HGF, IL17a and SCF) can stimulate megakaryocytopoiesis and/or regulate platelet production [40–43]. In the current study, both higher levels of IL17a and hPC correlated with a high content of intratumoural stroma within the primary tumour. If three patients with defined thrombocytosis are added to the group of patients with hPC, SCF correlated also with a higher stromal content (data not shown). Thus, it can be speculated that the stromal component of the primary tumour secretes mediators, which in turn can modulate the bone marrow function to guide tumour dissemination and prepare the bone marrow niche for subsequent nesting of tumour cells. Putatively stroma-derived IL17a or SCF can participate in such modulation (e.g. through increased production of platelets [42, 43] that later act as pro-tumorigenic in primary tumour and blood), which consequently leads to the formation of metastasis and patients’ death (Fig. 3). In our study, as a proof-of-principle, a sub-analysis using the CIBERSORTx digital cytometry algorithm is performed to identify stromal component(s) potentially associated with hPC. The analysis results show that transcriptome signatures indicating the presence of resting mast cells and CD8+ T cells are correlated with both hPC and some identified platelet-associated cytokines. Both sub-populations of putatively identified cells can be strong activators of inflammation and produce IL17a [44–46]. Nevertheless, further in vivo studies and clinical sample analyses are required to gain sufficient knowledge on this phenomenon.
Fig. 3. Putative model of tumour progression involving regulation of platelet level by intratumoural stroma-derived factors.
The stromal components of primary tumours (e.g. CD8 + T cells and/or mast cells) secrete mediators (e.g. specific cytokines/chemokines) (1), which in turn modulate the bone marrow function (2) to guide tumour dissemination and prepare the bone marrow niche for subsequent nesting of tumour cells (3). SCF, IL17a and APRIL can participate in such modulation through the stimulation of megakaryocytes, resulting in the production of platelets that later act as premetastatic (4).
In conclusion, these outcomes indicate that the evaluation of platelets, both individually and in combination with CTCs, has prognostic potential in detecting non-metastatic breast carcinoma, particularly its luminal subtype.
The results also indicate the plausible link among the higher content of intratumoural stroma, hPC, CTCs and patients’ worse outcomes. A detailed examination of individual blood components, such as blood cells and cytokines/chemokines, is required to reveal the complex network of environmental elements hypothetically supporting tumour progression. Further studies on larger cohorts of patients with different molecular subtypes of breast cancer are required to (i) specify the interactions between primary tumour and bone marrow modulated by secreted signalling molecules, such as cytokines/chemokines, and (ii) reveal the clinical relevance of anti-platelet therapies to prevent progression in breast cancer patients who have tested positive for hPC and/or CTCs.
Supplementary information
Acknowledgements
We thank all patients who agreed to donate blood for this research. We would like to acknowledge Peter Grešner for providing statistics consultation within the services of the Centre of Biostatistics and Bioinformatics Analysis located at the Medical University of Gdańsk, Poland, working as part of “Excellence Initiative — Research University” (grant no. MNiSW 07/IDUB/2019/94).
Author contributions
Conceptualisation, NB-K and AJZ; methodology, NB-K, TK, JSz, DS and JL; formal analysis, NB-K and MP; investigation, NB-K, TK, JSz, MP, AM, DS and JL; resources, BK, KP, LK, JS and AJZ; data curation, NB-K and MP; writing — original draft preparation, NB-K and AJZ.; writing — review and editing, all authors; visualisation, NB-K and MP; supervision, NB-K and AJZ; project administration, AJZ; funding acquisition, AJZ.
Fundings
This research was funded by the National Science Centre, grant number 2016/22/E/NZ4/00664 and the National Centre for Research and Development, grant number WPC/33/HESCAP/2018.
Data availability
The data that support the findings of this work are available from the corresponding author upon request. Immune-related transcriptome data are available at NCBI GEO under accession number GSE180186.
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Primary tumour samples and blood samples were collected from the patients according to a standard protocol approved by the Medical University of Gdansk ethics committee and upon signing an informed consent. This study was performed in accordance with the tenets of the Declaration of Helsinki.
Consent for publication
All informed consent was obtained. This report does not contain any individual person’s information.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Natalia Bednarz-Knoll, Email: nbk@gumed.edu.pl.
Anna J. Żaczek, Email: azaczek@gumed.edu.pl
Supplementary information
The online version contains supplementary material available at 10.1038/s41416-021-01647-9.
References
- 1.Leblanc R, Peyruchaud O. Metastasis: new functional implications of platelets and megakaryocytes. Blood. 2016;128:24–31. doi: 10.1182/blood-2016-01-636399. [DOI] [PubMed] [Google Scholar]
- 2.Heeke S, Mograbi B, Alix-Panabières C, Hofman P. Never travel alone: the crosstalk of circulating tumor cells and the blood microenvironment. Cells. 2019;8:714. doi: 10.3390/cells8070714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bendas G, Borsig L. Cancer cell adhesion and metastasis: selectins, integrins, and the inhibitory potential of heparins. Int J Cell Biol. 2012;2012:676731. [DOI] [PMC free article] [PubMed]
- 4.Coupland LA, Chong BH, Parish CR. Platelets and P-selectin control tumor cell metastasis in an organ-specific manner and independently of NK cells. Cancer Res. 2012;72:4662–71. doi: 10.1158/0008-5472.CAN-11-4010. [DOI] [PubMed] [Google Scholar]
- 5.Mitrugno A, Pelz K, Sears R, McCarty O. Abstract B62: Role of the blood microenvironment in the upregulation of oncoproteins in circulating pancreatic cancer cells. Cancer Res. 2017;77:B62. [Google Scholar]
- 6.Lou X-L, Sun J, Gong S-Q, Yu X-F, Gong R, Deng H. Interaction between circulating cancer cells and platelets: clinical implication. Chin J Cancer Res. 2015;27:450. doi: 10.3978/j.issn.1000-9604.2015.04.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Labelle M, Begum S, Hynes RO. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell. 2011;20:576–90. doi: 10.1016/j.ccr.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Labelle M, Begum S, Hynes RO. Platelets guide the formation of early metastatic niches. Proc Natl Acad Sci USA. 2014;111:E3053–E3061. doi: 10.1073/pnas.1411082111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bednarz-Knoll N, Alix-Panabières C, Pantel K. Plasticity of disseminating cancer cells in patients with epithelial malignancies. Cancer Metastasis Rev. 2012;31:673–87. doi: 10.1007/s10555-012-9370-z. [DOI] [PubMed] [Google Scholar]
- 10.Rao XD, Zhang H, Xu ZS, Cheng H, Shen W, Wang XP. Poor prognostic role of the pretreatment platelet counts in colorectal cancer: a meta-analysis. Medicine. 2018;97:e10831. doi: 10.1097/MD.0000000000010831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grilz E, Posch F, Königsbrügge O, Schwarzinger I, Lang IM, Marosi C, et al. Association of platelet-to-lymphocyte ratio and neutrophil-to-lymphocyte ratio with the risk of thromboembolism and mortality in patients with cancer. Thrombosis Haemost. 2018;118:1875–84. doi: 10.1055/s-0038-1673401. [DOI] [PubMed] [Google Scholar]
- 12.Li L, Wang J, Meng S, Li Z, Huang Z, Sun J, et al. Peripheral blood leukocytes and platelets serve as prognostic factors in breast cancer. Cancer Biother Radiopharm. 2020;36:167–73. doi: 10.1089/cbr.2019.3032. [DOI] [PubMed] [Google Scholar]
- 13.Krenn-Pilko S, Langsenlehner U, Thurner EM, Stojakovic T, Pichler M, Gerger A, et al. The elevated preoperative platelet-to-lymphocyte ratio predicts poor prognosis in breast cancer patients. Br J Cancer. 2014;110:2524–30. doi: 10.1038/bjc.2014.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang M, Huang X, Song Y, Gao P, Sun J, Wang Z. High platelet-to-lymphocyte ratio predicts poor prognosis and clinicopathological characteristics in patients with breast cancer: a meta-analysis. BioMed Res Int. 2017;2017:9503025. [DOI] [PMC free article] [PubMed]
- 15.Vernieri C, Mennitto A, Prisciandaro M, Huber V, Milano M, Rinaldi L, et al. The neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios predict efficacy of platinum-based chemotherapy in patients with metastatic triple negative breast cancer. Sci Rep. 2018;8:1–10. doi: 10.1038/s41598-018-27075-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bruno A, Dovizio M, Tacconelli S, Contursi A, Ballerini P, Patrignani P. Antithrombotic agents and cancer. Cancers. 2018;10:253. doi: 10.3390/cancers10080253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jing L, Qu H, Wu D, Zhu C, Yang Y, Jin X, et al. Platelet-camouflaged nanococktail: Simultaneous inhibition of drug-resistant tumor growth and metastasis via a cancer cells and tumor vasculature dual-targeting strategy. Theranostics. 2018;8:2683. doi: 10.7150/thno.23654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ponert JM, Schwarz S, Haschemi R, Müller J, Pötzsch B, Bendas G, et al. The mechanisms how heparin affects the tumor cell induced VEGF and chemokine release from platelets to attenuate the early metastatic niche formation. PLoS ONE. 2018;13:e0191303. doi: 10.1371/journal.pone.0191303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Papa AL, Jiang A, Korin N, Chen MB, Langan ET, Waterhouse A, et al. Platelet decoys inhibit thrombosis and prevent metastatic tumor formation in preclinical models. Sci Transl Med. 2019;11:eaau5898. doi: 10.1126/scitranslmed.aau5898. [DOI] [PubMed] [Google Scholar]
- 20.Vilsmaier T, Rack B, König A, Friese K, Janni W, Jeschke U, et al. Influence of circulating tumour cells on production of IL-1alpha, IL-1beta and IL-12 in sera of patients with primary diagnosis of breast cancer before treatment. Anticancer Res. 2016;36:5227–36. doi: 10.21873/anticanres.11093. [DOI] [PubMed] [Google Scholar]
- 21.Divella R, Daniele A, Abbate I, Bellizzi A, Savino E, Simone G, et al. The presence of clustered circulating tumor cells (CTCs) and circulating cytokines define an aggressive phenotype in metastatic colorectal cancer. Cancer Causes Control. 2014;25:1531–41. doi: 10.1007/s10552-014-0457-4. [DOI] [PubMed] [Google Scholar]
- 22.Mego M, Cholujova D, Minarik G, Sedlackova T, Gronesova P, Karaba M, et al. CXCR4-SDF-1 interaction potentially mediates trafficking of circulating tumor cells in primary breast cancer. BMC Cancer. 2016;16:1–9. doi: 10.1186/s12885-016-2143-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smolkova B, Mego M, Horvathova Kajabova V, Cierna Z, Danihel L, Sedlackova T, et al. Expression of SOCS1 and CXCL12 proteins in primary breast cancer are associated with presence of circulating tumor cells in peripheral blood. Transl Oncol. 2016;9:184–90. doi: 10.1016/j.tranon.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lippitz BE. Cytokine patterns in patients with cancer: a systematic review. Lancet Oncol. 2013;14:e218–e228. doi: 10.1016/S1470-2045(12)70582-X. [DOI] [PubMed] [Google Scholar]
- 25.Setrerrahmane S, Xu H. Tumor-related interleukins: old validated targets for new anti-cancer drug development. Mol Cancer. 2017;16:1–17. doi: 10.1186/s12943-017-0721-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Markiewicz A, Wełnicka-Jaśkiewicz M, Seroczyńska B, Skokowski J, Majewska H, Szade J, et al. Epithelial-mesenchymal transition markers in lymph node metastases and primary breast tumors-relation to dissemination and proliferation. Am J Transl Res. 2014;6:793. [PMC free article] [PubMed] [Google Scholar]
- 27.Von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Int J Surg. 2014;12:1495–9. [Google Scholar]
- 28.McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM. REporting recommendations for tumor MARKer prognostic studies (REMARK) Breast Cancer Res Treat. 2006;100:229–35. doi: 10.1007/s10549-006-9242-8. [DOI] [PubMed] [Google Scholar]
- 29.Newman AM, Steen CB, Liu CL, Gentles AJ, Chaudhuri AA, Scherer F, et al. Determining cell type abundance and expression from bulk tissues with digital cytometry. Nat Biotechnol. 2019;37:773–82. doi: 10.1038/s41587-019-0114-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Popeda M, Stokowy T, Bednarz-Knoll N, Jurek A, Niemira M, Bielska A, et al. NF-kappa B signaling-related signatures are connected with the mesenchymal phenotype of circulating tumor cells in non-metastatic breast cancer. Cancers. 2019;11:1961. doi: 10.3390/cancers11121961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.R Core Team. R: A language and environment for statistical computing. 2013. https://www.R-project.org/.
- 32.Blair P, Flaumenhaft R. Platelet alpha-granules: basic biology and clinical correlates. Blood Rev. 2009;23:177–89. doi: 10.1016/j.blre.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sandberg WJ, Otterdal K, Gullestad L, Halvorsen B, Ragnarsson A, Frøland SS, et al. The tumour necrosis factor superfamily ligand APRIL (TNFSF13) is released upon platelet activation and expressed in atherosclerosis. Thrombosis Haemost. 2009;102:704–10. doi: 10.1160/TH08-10-0665. [DOI] [PubMed] [Google Scholar]
- 34.Ankus E, Price SJ, Ukoumunne OC, Hamilton W, Bailey SE. Cancer incidence in patients with a high normal platelet count: a cohort study using primary care data. Fam Pract. 2018;35:671–5. doi: 10.1093/fampra/cmy018. [DOI] [PubMed] [Google Scholar]
- 35.Rachidi S, Wallace K, Day TA, Alberg AJ, Li Z. Lower circulating platelet counts and antiplatelet therapy independently predict better outcomes in patients with head and neck squamous cell carcinoma. J Hematol Oncol. 2014;7:1–7. doi: 10.1186/s13045-014-0065-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Balduini CL, Noris P. Platelet count and aging. Haematologica. 2014;99:953. doi: 10.3324/haematol.2014.106260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khetawat G, Faraday N, Nealen ML, Vijayan KV, Bolton E, Noga SJ, et al. Human megakaryocytes and platelets contain the estrogen receptor beta and androgen receptor (AR): testosterone regulates AR expression. Blood. 2000;95:2289–96. [PubMed] [Google Scholar]
- 38.Jayachandran M, Miller VM. Human platelets contain estrogen receptor alpha, caveolin-1 and estrogen receptor associated proteins. Platelets. 2003;14:75–81. doi: 10.1080/0953710031000080562. [DOI] [PubMed] [Google Scholar]
- 39.Gear AR, Camerini D. Platelet chemokines and chemokine receptors: linking hemostasis, inflammation, and host defense. Microcirculation. 2003;10:335–50. doi: 10.1038/sj.mn.7800198. [DOI] [PubMed] [Google Scholar]
- 40.Uzan G, Prandini M-H, Rosa J-P, Berthier R. Hematopoietic differentiation of embryonic stem cells: an in vitro model to study gene regulation during megakaryocytopoiesis. Stem Cells. 1996;14:194–9. doi: 10.1002/stem.5530140725. [DOI] [PubMed] [Google Scholar]
- 41.Bonci D, Hahne M, Felli N, Peschle C, De Maria R. Potential role of APRIL as autocrine growth factor for megakaryocytopoiesis. Blood. 2004;104:3169–72. doi: 10.1182/blood-2003-11-3861. [DOI] [PubMed] [Google Scholar]
- 42.Avraham H, Vannier E, Cowley S, Jiang SX, Chi S, Dinarello CA, et al. Effects of the stem cell factor, c-kit ligand, on human megakaryocytic cells. Blood. 1992;79:365–71. [PubMed] [Google Scholar]
- 43.Tan W, Liu B, Barsoum A, Huang W, Kolls JK, Schwarzenberger P. Requirement of TPO/c-mpl for IL-17A-induced granulopoiesis and megakaryopoiesis. J Leukoc Biol. 2013;94:1303–8. doi: 10.1189/jlb.1212639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Varricchi G, Galdiero MR, Loffredo S, Marone G, Iannone R, Marone G, et al. Are mast cells MASTers in cancer? Front Immunol. 2017;8:424. doi: 10.3389/fimmu.2017.00424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tu JF, Pan HY, Ying XH, Lou J, Ji JS, Zou H. Mast cells comprise the major of interleukin 17-producing cells and predict a poor prognosis in hepatocellular carcinoma. Medicine. 2016;95:e3220. doi: 10.1097/MD.0000000000003220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Srenathan U, Steel K, Taams LS. IL-17+ CD8+ T cells: differentiation, phenotype and role in inflammatory disease. Immunol Lett. 2016;178:20–26. doi: 10.1016/j.imlet.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this work are available from the corresponding author upon request. Immune-related transcriptome data are available at NCBI GEO under accession number GSE180186.