Abstract
Citrate has been proposed as anticoagulation of choice in continuous renal replacement therapy (CRRT). However, little is known about the pharmacokinetics (PK) and metabolism of citrate in liver failure patients who require CRRT with regional citrate anticoagulation (RCA). This prospective clinical PK study was conducted at King Chulalongkorn Memorial Hospital between July 2019 to April 2021, evaluating seven acute liver failure (ALF) and seven acute-on-chronic liver failure (ACLF) patients who received CRRT support utilizing RCA as an anticoagulant at a citrate dose of 3 mmol/L. For evaluation of the citrate PK, we delivered citrate for 120 min and then stopped for a further 120 min. Total body clearance of citrate was 152.5 ± 50.9 and 195.6 ± 174.3 mL/min in ALF and ACLF, respectively. The ionized calcium, ionized magnesium, and pH slightly decreased after starting citrate infusion and gradually increased to baseline after stopping citrate infusion. Two of the ACLF patients displayed citrate toxicity during citrate infusion, while, no ALF patient had citrate toxicity. In summary, citrate clearance was significantly decreased in critically ill ALF and ACLF patients receiving CRRT. Citrate use as an anticoagulation in these patients is of concern for the risk of citrate toxicity.
Subject terms: Continuous renal replacement therapy, Pharmacokinetics, Liver cirrhosis
Introduction
Regional citrate anticoagulation (RCA) is the first-line anticoagulation in critically ill patients receiving continuous renal replacement therapy (CRRT)1. Many studies have shown an impressive benefit of RCA to extend circuit lifespans, reduce the incidence of hemorrhagic complications, and lower transfusion needs2–6. Citrate, the anionic salt of citric acid, anticoagulated the extracorporeal circuit by chelating ionized calcium (Ca2+), which is a key cofactor in the clotting cascade7,8. Under physiological conditions, citrate is mainly metabolized by mitochondria in the liver, skeletal muscle, and the kidney into bicarbonate within 5 min9,10. Therefore, in the setting of acute liver failure (ALF) or acute-on-chronic liver failure (ACLF), citrate metabolism may be incomplete and lead to citrate toxicity. The toxicity of citrate during CRRT is defined as citrate accumulation and presents as low Ca2+ concentrations, due to the complex binding of citrate and Ca2+ and a need of calcium substitution; metabolic acidosis, due to the decreased citric acid cycle production of bicarbonate; and a total calcium (Catot)/Ca2+ ratio of > 2.511,12.
With respect to citrate pharmacokinetics (PK) in critically ill patients with acute kidney injury (AKI), it was reported that citrate clearance is not impaired in AKI patients13. In contrast, the citrate PK in critically ill patients with cirrhosis was impaired by 50% compared to non-cirrhotic critically ill patients14. To the best of our knowledge, no study has been conducted to demonstrate the citrate PK in critically ill ALF or ACLF patients receiving CRRT.
Therefore, to address this knowledge gap of RCA in CRRT, we first evaluated the citrate PK and metabolism among critically ill acute decompensated liver failure patients receiving CRRT.
Material and methods
Trial design and oversight
This study was a prospective clinical PK study at King Chulalongkorn Memorial Hospital (KCMH) in Thailand from July 2019 to April 2021. The trial was retrospective registered at Thai Clinical Trials Registry on 07/07/2021 (TCTR20210707010) and was approved by the institutional review board of the Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand (IRB No. 694/63). The study was conducted according to the Declaration of Helsinki and Good Clinical Practice guidelines, and all study methods were carried out in accordance with relevant guidelines and regulations. Written informed consent was obtained from all patients by their close relatives or themselves (if eligible) before enrollment. All potential patients who declined to participate were received the standard treatment included CRRT, vasopressor, appropriate antibiotics, etc. for their conditions and were not disadvantaged by not participating for the study.
Patients
The inclusion criteria were critically ill ALF or ACLF adult patients with AKI, aged ≥ 18 years old, and receiving CRRT. The ALF was defined as an acute onset of severe liver injury with (i) a worsening liver function test with abnormal coagulation, usually with an INR ≥ 1.5, and (ii) any degree of hepatic encephalopathy in a patient without pre-existing liver disease, according to The Asian Pacific Association for the Study of the Liver (APASL) consensus 2014. Whereas, ACLF was defined as ALF in a patient with a pre-existing liver disease confirmed by ultrasonography or liver biopsy15,16. The exclusion criteria were severe acidosis (pH < 7.1) or severe alkalosis (pH > 7.55), blood transfusion within 24 h prior to the study, use of citrate-containing medications, severe hypocalcemia (serum ionized calcium < 0.8 mmol/L), and use of heparin as anticoagulation.
Study protocol
For the citrate PK study, CRRT was performed as continuous venovenous hemofiltration (CVVH) mode with 100% pre-dilution, using the PRISMAFLEX system version 8.1 (Baxter Healthcare /Gambro Lundia AB, Sweden) with a standard PRISMA M-100 AN69 (polyacrilonitrile) hemofilter. The pre-dilution replacement solution was infused via pre-blood pump line (white pump) of PRISMAFLEX system. Citra-HF-Pre (Nordic Medcom, Finland) was used as pre-dilution replacement solution for isotonic citrate solution (consisted of citrate 13.3 mmol/L, Na+ 139.9 mmol/L, K+ 3 mmol/L, Mg2+ 0.5 mmol/L, and Cl− 104 mmol/L) for 120 min. The pre-dilution replacement flow rate and blood flow rate was calculated to achieve the prescribed pre-filter citrate dose of 3 mmol/L and the effluent dose of 25–30 mL/kg/h. A pre-dilution replacement flow rates in weight ranges (< 60, 60–70, and > 70 kg) to match the prespecified citrate dose were provided in the Supplementary Table 1. The ultrafiltration (UF) rates were adjusted based on the patient’s fluid status. However, the UF rates were not permitted to exceed a net negative balance of 400 mL per 3 h.
To prevent hypocalcemia, we used a constant rate of 10% calcium gluconate (0.225 mmol/mL) infused into the venous return line during citrate infusion. After 120 min, the isotonic citrate solution was stopped and switched to non-citrated isotonic solution using PRISMASOL B0 (Baxter, Italy), which consisted of Na+ 140 mmol/L, Ca2+ 1.75 mmol/L, Mg2+ 0.5 mmol/L, and Cl− 109.5 mmol/L. The concentration of KCl in the replacement fluid was 3 mmol/L, the same as the concentration in Citra-HF-Pre. The calcium gluconate infusion was also stopped after citrate infusion ended. The detail of the CRRT protocol and calcium infusion rate are shown in Supplementary Table 1.
Arterial blood samples were collected at pre-filter at baseline and after 10, 20, 40, 60, 120 (citrate infusion stopped), 125, 130, 140, 160, 180, 210, and 240 min. In addition, 10 min after the end of citrate infusion, blood samples were taken simultaneously from both the pre-filter and post-filter to calculate the citrate clearance via the hemofilter (Supplementary Fig. 1). The blood samples were sent for determination of the citrate, blood gas, electrolyte, calcium, magnesium, Ca2+, and Mg2+ concentrations. The blood gas samples were collected in a lithium heparin syringe and then analyzed by a Nova Stat Profile Prime Plus® (Nova Biomedical, Waltham, MA, USA). The citrate concentrations were measured using a commercially available kit (BOEHRINGER MANNHEIM / R-BIOPHARM Enzymatic BioAnalysis Cat. No. 10 139 076 035).
For citrate toxicity monitoring, we observed citrate accumulation, which was defined as (i) a Catot/Ca2+ ratio of > 2.5, (ii) metabolic acidosis, and (iii) low Ca2+ level. The Catot/Ca2+ ratio was monitored every 120 min.
Calculation of citrate PK
The PK parameters were performed via non-compartmental analysis using the Phoenix® WinNonlin® version 8.2 software program (Certara USA, Inc., Princeton, NJ). The area under the plasma concentration–time curve (AUC) was calculated by employing the linear trapezoidal rule. The time to reach maximum concentration (Tmax) and the maximum citrate concentration (Cmax) were measured directly from the concentration–time curve.
The volume distribution (Vd) was calculated by the standard formula; Vd = Cl/ke, whereas ke is the elimination rate constant and Cl (total drug clearance) was calculated by dose/AUC0–inf. The body citrate clearance was calculated by subtraction of the filter citrate clearance (Clfilter) from the total citrate clearance, where Clfilter was calculated based on the equations from Zheng and colleagues study13, as shown in Eq. (1);
| 1 |
where Cin and Cout are the plasma water concentrations of citrate at pre-filter (in) and post-filter (out), respectively. The blood flow rate (Qpw) was calculated from Eq. (2),
| 2 |
Data analysis
Continuous variables are presented as the mean ± standard deviation (SD) in case of a normal distribution, and as a median and interquartile range (IQR) in case of non-normally distributed variables. Categorical variables are characterized by numbers with percentages. All statistical analyses were performed using the SPSS Version 22 software (SPSS, Chicago, IL), and figures were drawn using GraphPad Prism 8 (GraphPad Software Inc., California, USA).
Ethics approval and consent to participate
This study was conducted according to the Declaration of Helsinki and Good Clinical Practice guidelines. The study protocol was approved by the institutional review board of the Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand (IRB No. 694/63). Written informed consent was obtained from all patients by their close relatives or themselves (if eligible) before enrollment.
Results
Patient characteristics
The patients’ baseline characteristics are summarized in Table 1. The causes of ALF in the ALF group were ischemic hepatitis (n = 4) and drug induced hepatitis (n = 3), whereas in the ACLF group they were ischemic hepatitis (n = 6) and autoimmune hepatitis (n = 1). The causes of cirrhosis in the ACLF group were viral hepatitis (n = 2), non-alcoholic steatohepatitis (NASH) (n = 2), alcohol, autoimmune hepatitis, and cardiac cirrhosis (n = 1 each). The mean APACHE II and SOFA scores were lower in the ALF group (19.6 ± 4.1 and 13.7 ± 3.7, respectively), than in the ACLF group (24.0 ± 5.6 and 15.6 ± 3.5, respectively).
Table 1.
Baseline characteristics.
| Patient | Age/sex | Admission diagnosis | Cause of AKI | Cause of cirrhosis | Cause of ALF | APACHE II score | SOFA score | Survival status |
|---|---|---|---|---|---|---|---|---|
| 1 | 47/M | Post heart transplantation | Cardiorenal syndrome | None | Ischemic hepatitis | 16 | 16 | Alive |
| 2 | 67/F | Scleroderma renal crisis | Sepsis AKI | None | Ischemic hepatitis | 19 | 18 | Death |
| 3 | 53/M | Chikungunya viral infection | Sepsis AKI | None | Ischemic hepatitis | 16 | 13 | Alive |
| 4 | 68/M | Upper GI bleeding | Ischemic ATN | None | Ischemic hepatitis | 18 | 12 | Death |
| 5 | 57/M | Pneumonia with septic shock | Sepsis AKI | None | Drug induced hepatitis | 21 | 7 | Death |
| 6 | 46/M | Disseminated TB | Sepsis AKI | None | Drug induced hepatitis | 28 | 17 | Death |
| 7 | 81/M | Drug induced hepatitis | Sepsis AKI | None | Drug induced hepatitis | 19 | 13 | Death |
| 8 | 52/M | Massive upper GI bleeding | Hepatorenal syndrome | Alcoholic | Ischemic | 20 | 16 | Death |
| 9 | 68/F | Cryoglobulinemia | Septic AKI | HBV | Ischemic | 30 | 19 | Death |
| 10 | 53/F | PCP pneumonia | Septic AKI | Autoimmune hepatitis | Autoimmune | 15 | 15 | Death |
| 11 | 97/M | Pneumonia with septic shock | Septic AKI | NASH | Ischemic | 23 | 19 | Death |
| 12 | 85/M | Cardiogenic shock | Cardiorenal syndrome | Cardiac cirrhosis | Ischemic | 22 | 11 | Death |
| 13 | 43/M | Pneumonia with septic shock | Septic AKI | HCV | Ischemic | 30 | 11 | Death |
| 14 | 79/M | Primary bacteremia with septic shock | Septic AKI | NASH | Ischemic | 28 | 18 | Death |
AKI acute kidney injury, APACHE II score Acute Physiology and Chronic Health Evaluation II score, GI gastrointestinal, HBV hepatitis B virus, HCV hepatitis C virus, NASH non-alcoholic steatohepatitis; PCP, pneumocystis pneumonia, SOFA score Sequential Organ Failure Assessment score, TB tuberculosis.
The baseline laboratory data of the two groups are summarized in Table 2. Most laboratory values were comparable between the two groups, except for the direct bilirubin values, which were higher in the ALF group than in the ACLF group (11.3 [9.8,14.5] vs. 6.2 [3.6,11.2] mg/dL).
Table 2.
Baseline laboratory data.
| Critically ill ALF patients (N = 7) | Critically ill ACLF patients (N = 7) | |
|---|---|---|
| Hemoglobin (g/dL) | 9.1 ± 1.4 | 9.0 ± 2.2 |
| White blood cells (× 103/µL) | 12.6 ± 8.2 | 23.5 ± 11.0 |
| Platelets (× 103/µL) | 55.4 ± 33.1 | 100.4 ± 55.3 |
| BUN (mg/dL) | 30.6 ± 24.8 | 27.9 ± 11.8 |
| Cr (mg/L) | 1.5 ± 1.2 | 1.8 ± 1.0 |
| Sodium (mmol/L) | 135 ± 6 | 136 ± 5 |
| Potassium (mmol/L) | 3.7 ± 0.5 | 4.2 ± 0.8 |
| Bicarbonate (mmol/L) | 22.3 ± 2.9 | 21.7 ± 5.2 |
| Calcium (mg/dL) | 8.8 ± 0.9 | 8.3 ± 1.6 |
| Magnesium (mg/dL) | 0.9 ± 0.1 | 0.9 ± 0.1 |
| Phosphate (mg/dL) | 2.3 ± 0.9 | 4.1 ± 2.5 |
| Total bilirubin (mg/dL) | 19.5 ± 8.9 | 11.1 ± 5.1 |
| Direct bilirubin (mg/dL) | 11.3 (9.8,14.5) | 6.2 (3.6, 11.2) |
| AST (U/L) | 128 (50,355) | 165 (58,614) |
| ALT (U/L) | 120 (23,352) | 112 (21,224) |
| ALP (U/L) | 130 (78,170) | 165 (99,183) |
| Albumin (g/L) | 3.2 ± 0.7 | 3.3 ± 0.5 |
| PT (s) | 21.2 ± 8.8 | 27.4 ± 15.2 |
| INR | 1.9 (1.4, 2.6) | 1.8 (1.6, 3.9) |
| aPTT (s) | 36.3 (27.7, 52.0) | 51.5 (34.5, 84.5) |
| pH | 7.42 ± 0.06 | 7.39 ± 0.08 |
| Lactate (mmol/L) | 3.26 ± 2.83 | 5.47 ± 2.00 |
| Ionized calcium (mmol/L) | 1.18 ± 0.10 | 1.08 ± 0.22 |
| Ionized magnesium (mmol/L) | 0.59 ± 0.11 | 0.51 ± 0.07 |
ALT alanine aminotransferase, ALP alkaline phosphatase, aPTT activated partial thromboplastin time, AST aspartate aminotransferase, BUN blood urea concentration, Cr creatinine, INR international normalized ratio, PT prothrombin time.
Data are represented as mean ± SD or median (IQR).
Citrate PK
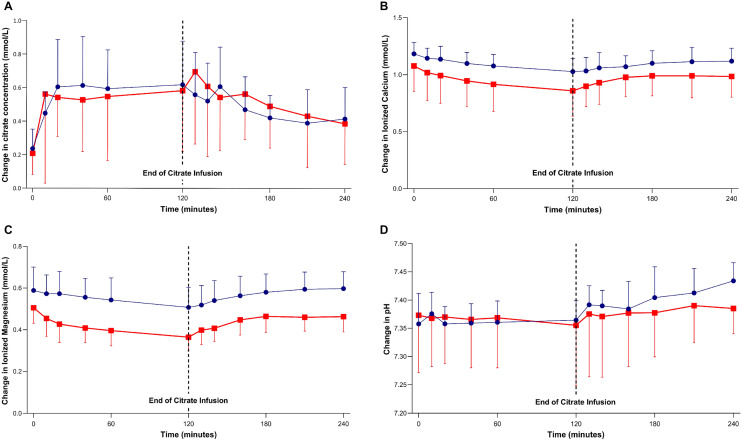
The citrate PK results are shown in Table 3, while the average citrate concentrations during citrate infusion until 120 min after the infusion was stopped are illustrated in Fig. 1A. In ALF patients, the citrate concentration gradually increased from baseline during infusion to a maximum concentration of 0.76 ± 0.27 mmol/L after 100.0 ± 60.0 min. The average body citrate clearance was 152.5 ± 50.9 mL/min. Whereas, in the ACLF patients, the time to reached a broadly similar maximum citrate concentration (0.72 ± 0.44 mmol/L) was numerically longer at 113.8 ± 32.8 min, giving a numerically higher body citrate clearance rate (195.6 ± 174.3 mL/min). After stopping citrate infusion, the citrate concentration was consistently decreased in both groups.
Table 3.
Citrate PK.
| Parameter | Critically ill ALF patients (N = 7) | Critically ill ACLF patients (N = 7) |
|---|---|---|
| AUC0−t (mmol min/L) | 124.4 ± 43.9 | 113.9 ± 73.3 |
| AUC0−inf (mmol min/L) | 267.2 ± 111.7 | 372.6 ± 399.3 |
| Tmax (min) | 100.0 ± 60.0 | 113.8 ± 32.8 |
| Vd (L) | 45.6 ± 8.0 | 58.2 ± 49.7 |
| Clbody (mL/min) | 152.5 ± 50.9 | 195.6 ± 174.3 |
| Cbaseline (mmol/L) | 0.24 ± 0.12 | 0.21 ± 0.12 |
| Cmax (mmol/L) | 0.76 ± 0.27 | 0.72 ± 0.44 |
| Total dose (mmol) | 39.9 | 39.9 |
AUC area under the plasma concentration–time curve, Tmax time to maximum concentration, Vd volume of distribution, Clbody citrate clearance by body, Cbaseline baseline citrate concentration, Cmax maximum citrate concentration.
Data are shown as mean ± SD.
Figure 1.
Comparison of ALF (blue circles) and ACLF (red squares). The results for the plasma citrate concentration (A), Ca2+ (B), Mg2+ (C), and blood pH (D) during 120 min of citrate infusion and 120 min after citrate infusion was stopped. Data are presented as the mean ± 1SD.
Change in the Ca2+ and Mg2+ concentrations
The Catot and Ca2+ levels at the baseline were not different in both groups (Table 2). After the citrate infusion was finished, the Ca2+ concentrations were slightly decreased to 1.03 ± 0.12 and 0.86 ± 0.22 mmol/L in the ALF and ACLF groups, respectively. However, the Ca2+ level tended to be lower in the ACLF than the ALF patients during citrate infusion. After the citrate infusion was stopped (after 120 min), the Ca2+ concentrations gradually increased to a baseline of 1.12 ± 0.11 and 0.98 ± 0.18 mmol/L in the ALF and ACLF groups, respectively, (Fig. 1B).
The ACLF patients tended to have lower Mg2+ levels than the ALF patients, but both reached their minimum concentration at the end of the citrate infusion (120 min) of 0.51 ± 0.1 and 0.36 ± 0.07 mmol/L in the ALF and ACLF patients, respectively. After the citrate infusion was stopped, the Mg2+ concentration was gradually increased to the baseline in both groups (0.60 ± 0.08 mmol/L vs. 0.46 ± 0.07 mmol/L in the ALF and ACLF groups, respectively) (Fig. 1C).
Metabolic effect
Metabolic profiles were comparable at baseline between the two groups (Table 2). During citrate infusion, the pH slightly decreased in both groups and gradually increased after citrate infusion was stopped (Fig. 1D). However, one patient in the ACLF group developed severe metabolic acidosis (pH < 7.2) during citrate infusion. The pH did not improve after infusion and so it could likely be explained from the underlying disease of this patient and citrate accumulation due to liver impairment. No incidence of metabolic alkalosis occurred during citrate infusion in both groups.
Citrate accumulation
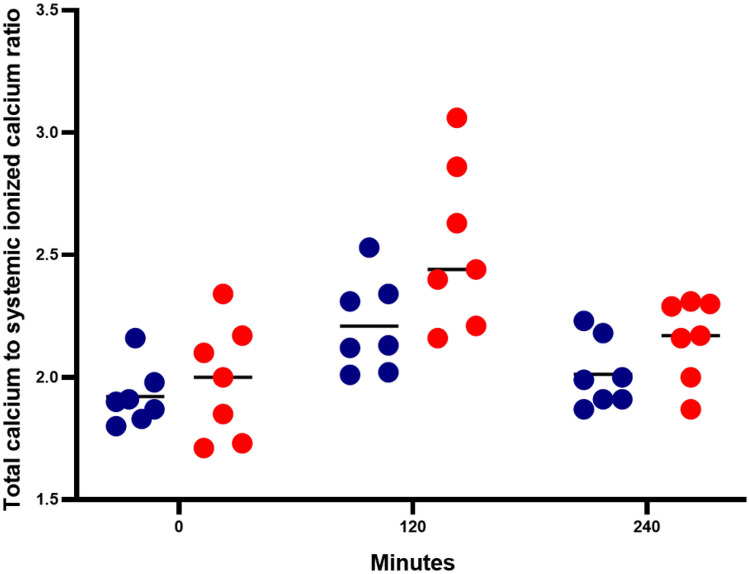
Given the risk of citrate toxicity, we monitored the Catot/Ca2+ ratio every 120 min. The average baseline Catot/Ca2+ ratio was 1.92 ± 0.12 vs. 1.99 ± 0.24 in the ALF and ACLF groups, respectively. However, after citrate infusion for 120 min, both the ALF and ACLF groups showed increased Catot/Ca2+ ratios, and this was higher in the ACLF than the ALF group (2.54 ± 0.33 vs. 2.21 ± 0.19, respectively). In the ALF group, one patient showed a Catot/Ca2+ ratio of more than 2.5 (level of 2.53) without metabolic acidosis. However, in the ACLF group, three patients had a Catot/Ca2+ ratio of > 2.5 (2.63, 2.86, and 3.06) and two of them had metabolic acidosis, confirming the diagnosis of citrate accumulation. After stopping citrate infusion all patients had a normalized Catot/Ca2+ ratio (2.01 ± 0.14 and 2.16 ± 0.17 in the ALF and ACLF group, respectively) and no evidence of citrate accumulation was found (Fig. 2).
Figure 2.
Comparison of the total calcium: systemic ionized calcium ratio between ALF (blue circles) and ACLF (red circles) at baseline, 120 min (end of 120-min citrate infusion), and 240 min (120-min after citrate infusion stopped).
Discussion
This study is the first to demonstrate citrate PK and clinical outcomes in critically ill ALF and ACLF patients receiving CRRT. Interestingly, the citrate PK were different to the results of a previous study13. Firstly, compared with the previous reported citrate clearance of 648.0 ± 347.0 mL/min in critically ill patients with CRRT13, the citrate clearance was impaired in both the ALF and ACLF patients at 152.5 ± 50.9 and 195.6 ± 174.3 mL/min, respectively. This reduced total citrate clearance was clinically confirmed by the decrease in pH and Ca2+ concentration during citrate infusion. Secondly, we found an increased Catot/Ca2+ ratio in both the ALF and ACLF groups (2.21 ± 0.19 and 2.54 ± 0.33, respectively), and two ACLF patients (28.5%) were diagnosed with citrate accumulation.
Both ALF and ACLF were common in critically ill patients receiving CRRT, and is associated with an increased risk of coagulopathy, thrombocytopenia, and bleeding17. To this concern, the Kidney Disease Proving Global Outcomes (KDIGO) Clinical Practice Guideline for AKI1 noted that severe liver failure is a major contraindication of RCA due to the risk of citrate toxicity. Our results were aligned with a previous study that citrate metabolism was decrease by approximately 50% in ALF without AKI9. However, compared to the previous studies of citrate PK and metabolism in CRRT13,14, we found the citrate clearance in ALF and ACLF patients was decreased by approximately 50% compared to that for cirrhotic patients (340 ± 185 mL/min) and approximately 75% compared to other critically ill patients (686.6 ± 353.6 mL/min). Other PK components, compared to two other studies13,14, are summarized in Supplementary Table 3.
Typically, a filter clearance (Clfilter) can be calculated by 2 methods. The first method uses sieving coefficient (SC) that is calculated by plasma and hemofiltrate concentrations as SC = C UF/C Plasma. So, the filter clearance can be determined as Clfilter = SC x ultrafiltration rate. The second one as we applied in our study utilizes plasma concentrations before entering (Cin) and after passing filter (Cout) as Clfilter = blood flow rate × ((Cin – Cout)/Cin)18,19. Welte and colleagues performed the trimethoprim/sulfametrole in critically ill patients on CRRT. The Clfilter in this study was calculated by two previously described methods. The results showed that the Clfilter of trimethoprim (1.8 vs 1.4 L/h) and sulfametrole (1.1 vs 1.1 L/h) from both calculation methods were similar20. Moreover, the second method has been used in other previously published PK studies in patients receiving RRT with other drugs21,22.
To our knowledge, citrate PK studies in patients receiving CRRT were very limited. Zheng and colleagues demonstrated citrate PK in critically ill AKI patients receiving CRRT13. They also calculated the filter clearance using Cin and Cout (second method). From aforementioned reasons and to compare the body citrate clearance (the total citrate clearance subtracting by filter citrate clearance) from Zheng’s and our results as shown in Supplementary Table 3, we decided to use the same Clfilter equation as shown in Zheng’s.
Citrate is removed by CRRT and its SC was reported as approximately 1 in previous literature23,24. However, PK changes such as volume overload, hypoalbuminemia and wide variation in protein binding are commonly seen in critically ill patients. These would affect citrate removal parameters such as SC and Clfilter from CRRT in individual patients25,26. To accurately demonstrate the Clfilter in those vulnerable patients, we decided to collect blood samples from Cin and Cout in for clearance calculation in our study.
During citrate infusion, both systemic Ca2+ and Mg2+ are chelated by the citrate, resulting in ionized hypocalcemia and ionized hypomagnesemia27. This study revealed a decreased level of both ionized hypocalcemia and ionized hypomagnesemia, which reached a minimum after 120 min (end of the citrate infusion) in both groups. However, the ACLF group had a lower ionized hypocalcemia and a lower ionized hypomagnesemia than the ALF group, which resulted from the decreased citrate clearance. Given the metabolic disturbance effect of citrate in this study, the pH tended to be shifted to an acidotic range during citrate infusion and the ACLF group had a lower average pH than the ALF group. This effect could also be explained by the impairment of the citric acid cycle production of bicarbonate. These effects were improved after stopping citrate infusion with a shift from acidification towards alkalization.
Another concern about using RCA in liver failure patients is the potential for citrate accumulation. This study reported a higher Catot/Ca2+ ratio (> 2.5) and two ACLF patients (28.5%) showed evidence of citrate accumulation during citrate infusion. Although, many studies have performed CRRT using RCA and reported a low incidence of citrate accumulation via intensive monitoring of the calcium and acid–base status17,28–30, our study showed the potential for citrate accumulation in patients with liver impairment.
Interestingly, our study found that the body citrate clearance in ALF was lower than in ACLF group. There might be explained by higher severity of liver injury and lower metabolic capacities to detoxify albumin bound toxin in ALF than in ACLF31,32. We showed that high incidence of low systemic Ca2+, high Catot/Ca2+ ratio, and high rate of citrate accumulation in ACLF group. This result might cause by the difference in baseline characteristics and laboratory between both groups. In our study, there was lower baseline systemic Ca2+ in ACLF group compared to ALF group (1.08 ± 0.22 mmol/L vs 1.18 ± 0.10 mmol/L) (Table 2, Fig. 1B). The systemic Ca2+ was used as a marker to of citrate related complication. Many studies of RCA among liver disease used the high calcium infusion rate or calcium-containing dialysate to avoid systemic hypocalcemia and high Catot/Ca2+ ratio17,33. However, systemic ionized hypocalcemia among critically ill patients is multifactorial cause included abnormal parathyroid hormone secretion, vitamin D deficiency, transfusion of citrated, the effect of circulating catecholamines, and disease severity34,35. Finally, the difference of clinical citrate related metabolic effect between both groups could not absolutely concluded due to the short citrate infusion times and the limited number of patients.
Of note, there was a different citrate dosing regimen between13 and our study. We applied continuous citrate infusion at 3 mmol/L as a total dose of 39.9 mmol in each case, with an average AUC0−t of 124.4 ± 43.9 and 113.9 ± 73.3 in the ALF and ACLF groups, respectively. Whereas, the previous study13 utilized a total dose of 63.7 ± 9.1 mmol, with an average AUC0−t of 79.7 ± 95.5 mmol that was comparable to healthy patients without AKI. To compare the AUC from these studies, we normalized the AUC by the total dose of 63.7 mmol. The normalized AUCs from our two liver impairment groups, based on the citrate dose from the previous study13, were 198.60 and 181.76 mmol*min/L in the ALF and ACLF groups, respectively. Clearly, the normalized AUC from the ALF and ACLF groups in this study were considerably (2.28- to 2.49-fold) higher than in the previous study13. As aforementioned, the clinical effects of citrate toxicity were revealed in some patients from our study, and so we would recommend that the citrate dosing regimens should be reduced at least two-fold to a citrate infusion dose of 1.5 mmol/L, to avoid citrate accumulation and toxicity.
This study had several strengths. This is the first reported clinical PK and metabolism of citrate in decompensated liver failure receiving CRRT, which is the knowledge gap of this area. Based on reported citrate PK changes from our study, we suggest that RCA use in these liver injury patients should be closely monitored to avoid citrate-related complications, such as citrate accumulation or severe hypocalcemia. The results of our study can be generalized to the real-life practice as the critical conditions, AKI undergoing CRRT and liver injury, are commonly seen in ICU settings.
Our study had several limitations. At first, we used only CVVH treatment for CRRT, while there is other CRRT modalities, such as CVVHD and CVVHDF, used in different clinical scenarios that might give different results. Secondly, the citrate infusion time was only 120 min and so we could not follow the clinical outcomes of long-term metabolic and citrate toxicity. A clinical study with a long-term follow up timeframe would be needed. Thirdly, our baseline citrate concentration did not start at zero mmol, which might affect the exogenous citrate level from the blood product and the impairment of citrate clearance in these patients. Fourthly, due to limited number of patients enrolled in this study, the population PK modelling and the correlation of each covariation and clinical parameters to citrate PK could not be performed. Further study with higher numbers of patients is absolutely needed. Finally, the definitions of ALF and ACLF in our study derived from The Asian Pacific Association for the Study of the Liver (APASL) consensus, which based on Asia–Pacific region. Therefore, our results may not be generalizable among other ALF and ACLF definitions such as Europe and Western region, which the definitions derived from the European Association for the Study of the Liver (EASL)36.
Conclusions
Our study demonstrated that citrate clearance in critically ill patients with decompensated liver failure was significantly impaired by 50% and 75% compared to compensated cirrhotic and other critically ill patients, respectively. Despite the recent data on the feasibility of citrate in these patients, we still emphasize using a lower dose of citrate with intensive monitoring of calcium, Ca2+, and electrolyte concentrations to prevent citrate-related complications. Further studies needed to be provided for RCA use in liver failure patients.
Supplementary Information
Acknowledgements
We thank the staff, nurses, and research coordinators from the Excellence Center for Critical Care Nephrology (EC-CCN), Faculty of Medicine, Chulalongkorn University. We also thank Miss Sasipha Tachaboon, MSc medical technologist, for laboratory procedures.
Author contributions
P.T., W.C., P.S., N.L., S.P., and N.S. were responsible for study concept and design. S.T., P.T., and W.C. were responsible for the acquisition, analysis, or interpretation of data. A.L. was responsible for PK data analysis. P.T., W.C., and N.S. were responsible for drafting the manuscript. All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. All authors interpreted the findings, contributed to writing the manuscript, and approved the final version for publication.
Funding
The investigator-initiated study was funded by the Excellence Center for Critical Care Nephrology, King Chulalongkorn Memorial Hospital and the National Kidney Foundation of Thailand. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Data availability
On reasonable request, data from this study are available from the corresponding author.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Peerapat Thanapongsatorn and Weerachai Chaijamorn.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-05867-8.
References
- 1.Kellum JA, Lameire N, Aspelin P, Barsoum RS, Burdmann EA, Goldstein SL, et al. Kidney disease: Improving global outcomes (KDIGO) acute kidney injury work group: KDIGO clinical practice guideline for acute kidney injury. Kidney Int. Suppl. 2012;2(1):1–138. doi: 10.1038/kisup.2012.1. [DOI] [Google Scholar]
- 2.Morabito S, Pistolesi V, Tritapepe L, et al. Regional citrate anticoagulation in cardiac surgery patients at high risk of bleeding: A continuous veno-venous hemofiltration protocol with a low concentration citrate solution. Crit. Care. 2012;16(3):R111. doi: 10.1186/cc11403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zarbock A, Küllmar M, Kindgen-Milles D, et al. Effect of regional citrate anticoagulation vs systemic heparin anticoagulation during continuous kidney replacement therapy on dialysis filter life span and mortality among critically ill patients with acute kidney injury: A randomized clinical trial. JAMA. 2020;324(16):1629–1639. doi: 10.1001/jama.2020.18618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Monchi M, Berghmans D, Ledoux D, Canivet JL, Dubois B, Damas P. Citrate vs. heparin for anticoagulation in continuous venovenous hemofiltration: a prospective randomized study. Intensive Care Med. 2004;30(2):260–265. doi: 10.1007/s00134-003-2047-x. [DOI] [PubMed] [Google Scholar]
- 5.Kutsogiannis DJ, Gibney RT, Stollery D, Gao J. Regional citrate versus systemic heparin anticoagulation for continuous renal replacement in critically ill patients. Kidney Int. 2005;67(6):2361–2367. doi: 10.1111/j.1523-1755.2005.00342.x. [DOI] [PubMed] [Google Scholar]
- 6.Betjes MG, van Oosterom D, van Agteren M, van de Wetering J. Regional citrate versus heparin anticoagulation during venovenous hemofiltration in patients at low risk for bleeding: Similar hemofilter survival but significantly less bleeding. J. Nephrol. 2007;20(5):602–608. [PubMed] [Google Scholar]
- 7.Oudemans-van Straaten HM, Ostermann M. Bench-to-bedside review: Citrate for continuous renal replacement therapy, from science to practice. Crit Care. 2012;16(6):249. doi: 10.1186/cc11645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tolwani A, Wille KM. Advances in continuous renal replacement therapy: Citrate anticoagulation update. Blood Purif. 2012;34(2):88–93. doi: 10.1159/000342378. [DOI] [PubMed] [Google Scholar]
- 9.Apsner R, Schwarzenhofer M, Derfler K, Zauner C, Ratheiser K, Kranz A. Impairment of citrate metabolism in acute hepatic failure. Wien Klin Wochenschr. 1997;109(4):123–127. [PubMed] [Google Scholar]
- 10.Davenport A, Tolwani A. Citrate anticoagulation for continuous renal replacement therapy (CRRT) in patients with acute kidney injury admitted to the intensive care unit. NDT Plus. 2009;2(6):439–447. doi: 10.1093/ndtplus/sfp136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Link A, Klingele M, Speer T, et al. Total-to-ionized calcium ratio predicts mortality in continuous renal replacement therapy with citrate anticoagulation in critically ill patients. Crit. Care. 2012;16(3):R97. doi: 10.1186/cc11363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meier-Kriesche HU, Gitomer J, Finkel K, DuBose T. Increased total to ionized calcium ratio during continuous venovenous hemodialysis with regional citrate anticoagulation. Crit. Care Med. 2001;29(4):748–752. doi: 10.1097/00003246-200104000-00010. [DOI] [PubMed] [Google Scholar]
- 13.Zheng Y, Xu Z, Zhu Q, et al. Citrate pharmacokinetics in critically ill patients with acute kidney injury. PLoS ONE. 2013;8(6):e65992. doi: 10.1371/journal.pone.0065992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kramer L, Bauer E, Joukhadar C, et al. Citrate pharmacokinetics and metabolism in cirrhotic and noncirrhotic critically ill patients. Crit. Care Med. 2003;31(10):2450–2455. doi: 10.1097/01.CCM.0000084871.76568.E6. [DOI] [PubMed] [Google Scholar]
- 15.Sarin SK, Choudhury A, Sharma MK, et al. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific association for the study of the liver (APASL): an update [published correction appears in Hepatol Int. 2019 Nov;13(6):826–828] Hepatol. Int. 2019;13(4):353–390. doi: 10.1007/s12072-019-09946-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Squires JE, McKiernan P, Squires RH. Acute liver failure: An update. Clin. Liver Dis. 2018;22(4):773–805. doi: 10.1016/j.cld.2018.06.009. [DOI] [PubMed] [Google Scholar]
- 17.Zhang W, Bai M, Yu Y, et al. Safety and efficacy of regional citrate anticoagulation for continuous renal replacement therapy in liver failure patients: a systematic review and meta-analysis. Crit. Care. 2019;23(1):22. doi: 10.1186/s13054-019-2317-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pea F, Viale P, Pavan F, et al. Pharmacokinetic considerations for antimicrobial therapy in patients receiving renal replacement therapy. Clin. Pharmacokinet. 2007;46:997–1038. doi: 10.2165/00003088-200746120-00003. [DOI] [PubMed] [Google Scholar]
- 19.Weiler S, Seger C, Pfisterer H, et al. Pharmacokinetics of caspofungin in critically ill patients on continuous renal replacement therapy. Antimicrob. Agents Chemother. 2013;57:4053–4057. doi: 10.1128/AAC.00335-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Welte R, Beyer R, Hotter J, et al. Pharmacokinetics of trimethoprim/sulfametrole in critically ill patients on continuous renal replacement therapy. J. Antimicrob. Chemother. 2020;75(5):1237–1241. doi: 10.1093/jac/dkz556. [DOI] [PubMed] [Google Scholar]
- 21.Sinnollareddy MG, Roberts MS, Lipman J, et al. Pharmacokinetics of piperacillin in critically ill patients with acute kidney injury receiving sustained low-efficiency diafiltration. J. Antimicrob. Chemother. 2018;73(6):1647–1650. doi: 10.1093/jac/dky057. [DOI] [PubMed] [Google Scholar]
- 22.König C, Kluge S, Fuhrmann V, et al. Pharmacokinetics of meropenem during advanced organ support (ADVOS®) and continuous renal replacement therapy. Int. J. Artif. Organs. 2021;44(10):783–786. doi: 10.1177/03913988211021101. [DOI] [PubMed] [Google Scholar]
- 23.Chadha V, Garg U, Warady BA, Alon US. Citrate clearance in children receiving continuous venovenous renal replacement therapy. Pediatr. Nephrol. 2002;17(10):819–824. doi: 10.1007/s00467-002-0963-6. [DOI] [PubMed] [Google Scholar]
- 24.Oudemans-van Straaten HM, Ostermann M. Bench-to-bedside review: Citrate for continuous renal replacement therapy, from science to practice. Crit. Care. 2012;16(6):249. doi: 10.1186/cc11645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schetz M. Drug dosing in continuous renal replacement therapy: General rules. Curr. Opin. Crit. Care. 2007;13(6):645–651. doi: 10.1097/MCC.0b013e3282f0a3d3. [DOI] [PubMed] [Google Scholar]
- 26.Jang SM, Awdishu L. Drug dosing considerations in continuous renal replacement therapy. Semin. Dial. 2021;34(6):480–488. doi: 10.1111/sdi.12972. [DOI] [PubMed] [Google Scholar]
- 27.Zakharchenko M, Leden P, Rulíšek J, Los F, Brodska H, Balik M. Ionized magnesium and regional citrate anticoagulation for continuous renal replacement therapy. Blood Purif. 2016;41(1–3):41–47. doi: 10.1159/000440972. [DOI] [PubMed] [Google Scholar]
- 28.Wonnacott R, Josephs B, Jamieson J. CRRT regional anticoagulation using citrate in the liver failure and liver transplant population. Crit. Care Nurs. Q. 2016;39(3):241–251. doi: 10.1097/CNQ.0000000000000118. [DOI] [PubMed] [Google Scholar]
- 29.Schultheiß C, Saugel B, Phillip V, et al. Continuous venovenous hemodialysis with regional citrate anticoagulation in patients with liver failure: A prospective observational study. Crit. Care. 2012;16(4):R162. doi: 10.1186/cc11485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu Y, Bai M, Ma F, et al. Regional citrate anticoagulation versus no-anticoagulation for continuous venovenous hemofiltration in patients with liver failure and increased bleeding risk: A retrospective case-control study. PLoS ONE. 2020;15(5):e0232516. doi: 10.1371/journal.pone.0232516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin S, Smith BS. Drug dosing considerations for the critically ill patient with liver disease. Crit. Care Nurs. Clin. North Am. 2010;22(3):335–340. doi: 10.1016/j.ccell.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 32.Arroyo V, Moreau R, Jalan R. Acute-on-chronic liver failure. N. Engl. J. Med. 2020;382(22):2137–2145. doi: 10.1056/NEJMra1914900. [DOI] [PubMed] [Google Scholar]
- 33.Rhee H, Berenger B, Mehta RL, Macedo E. Regional citrate anticoagulation for continuous kidney replacement therapy with calcium-containing solutions: A cohort study. Am. J. Kidney Dis. 2021;78(4):550–559.e1. doi: 10.1053/j.ajkd.2021.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steele T, Kolamunnage-Dona R, Downey C, Toh CH, Welters I. Assessment and clinical course of hypocalcemia in critical illness. Crit Care. 2013;17(3):R106. doi: 10.1186/cc12756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burchard KW, Simms HH, Robinson A, DiAmico R, Gann DS. Hypocalcemia during sepsis: Relationship to resuscitation and hemodynamics. Arch. Surg. 1992;127(3):265–272. doi: 10.1001/archsurg.1992.01420030027005. [DOI] [PubMed] [Google Scholar]
- 36.European Association for the Study of the Liver, Clinical Practice Guidelines Panel. Wendon J, et al. EASL clinical practical guidelines on the management of acute (fulminant) liver failure. J. Hepatol. 2017;66(5):1047–1081. doi: 10.1016/j.jhep.2016.12.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
On reasonable request, data from this study are available from the corresponding author.