Abstract
Background:
There are currently no approved therapies to treat congenital athymia, a condition of immune deficiency resulting in high early mortality due to infection and immune dysregulation. Multiple syndromic conditions, such as complete DiGeorge syndrome, 22q11.2 deletion syndrome, CHARGE (coloboma, heart defects, choanal atresia, growth or mental retardation, genital hypoplasia, and ear anomalies and/or deafness) syndrome, diabetic embryopathy, other genetic variants, and FOXN1 deficiency are associated with congenital athymia.
Objective:
This report describes 105 patients treated with cultured thymus tissue (CTT) and focuses on the outcomes of 95 patients with treatment-naïve congenital athymia.
Methods:
Ten prospective, single-arm open-label studies with patient enrollment from 1993 to 2020 form the basis of this data set. Patients were tested after administration of CTT for T cell development; all adverse events and infections were recorded.
Results:
One hundred five patients were enrolled and received CTT (full analysis set). Ten patients had diagnoses other than congenital athymia and/or received prior treatments. Ninety-five treatment-naïve congenital athymia patients were included in the efficacy analysis set (EAS). The Kaplan-Meier estimated survival rates at year 1 and year 2 after administration of CTT in the EAS were 77% (95% CI, 0.670–0.844) and 76% (95% CI, 0.657–0.834), respectively. Twenty-one patients died in the first year before developing naïve T cells, and one died in the second year after receiving CTT. Three subsequent deaths were not related to immunodeficiency. A few patients developed alopecia, autoimmune hepatitis, psoriasis, and psoriatic arthritis after year 1. Infections, autologous graft-vs-host-disease manifestations, and autoimmune cytopenias all decreased approximately 1 year after administration of CTT.
Conclusion:
Treatment with CTT led to development of naïve T cells with a 1-year survival rate of 77% and a median follow up of 7.6 years. Immune reconstitution sufficient to prevent infections and support survival typically develops 6 to12 months after administration of CTT.
Clinical Trial Registration:
The ClinicalTrials.gov registration numbers (with protocol numbers in parentheses) include NCT00576407 (668), NCT00579709 (884), NCT00566488 (931), NCT00576836 (932), NCT00579527 (950), NCT01220531 (25966), and NCT02274662 (51692).
Clinical Implications:
Cultured thymus tissue (CTT) is an investigational treatment for congenital athymia. In 105 patients, survival was 77% at 1 year; no deaths considered related to CTT occurred after year 2.
Capsule Summary:
Investigational cultured thymus tissue resulted in 1-year survival of 77% among 95 patients with congenital athymia (median follow-up, 7.6 years). Infections and autoimmunity decreased, and no immunodeficiency-related deaths occurred after year 2.
Keywords: Congenital athymia, complete DiGeorge, CHARGE syndrome, 22q11.2, thymus transplantation, RVT-802, CTT
Introduction
Congenital athymia is a rare condition[1] in which children are born without a thymus resulting in profound immunodeficiency and immune dysregulation. Naïve T cells develop in the thymus and, after leaving the thymus, fight infection and help regulate the immune system. Without a thymus, children are susceptible to life-threatening infections and immune dysregulation. Children with congenital athymia generally do not survive beyond early childhood. While hematopoietic stem cell transplants (HSCTs) have been performed in patients with congenital athymia[2, 3] only 7 of 17 patients (41%) were reported as surviving by Janda et al.[2] Currently, the only available treatment for these patients, outside of a clinical trial, is supportive care, which may include strict infection-prevention measures including immunoglobulin replacement, prophylactic antimicrobials, and protective isolation.
Congenital athymia is associated with several underlying syndromic conditions that can be severe and, in some cases, can lead to death. Complete DiGeorge anomaly (cDGA) was used to refer to patients with congenital athymia in prior publications and encompassed 22q11.2 deletion syndrome (22q11.2ds), CHARGE (coloboma, heart defects, choanal atresia, growth or mental retardation, genital hypoplasia, and ear anomalies and/or deafness) syndrome, diabetic embryopathy, and other genetic defects associated with congenital athymia. FOXN1 deficiency was a separate category of athymic patients. Comorbidities of these associated syndromic conditions that are frequently experienced by patients with congenital athymia include hypoparathyroidism, congenital heart and kidney defects, esophageal and tracheal atresia, choanal atresia, hypothyroidism, hearing loss, and developmental delay.
Congenital athymia can be detected when newborns are screened for severe combined immunodeficiency (SCID). In the United States, SCID screening has become the standard method by which congenital athymia is detected. The diagnosis is confirmed using flow cytometry in conjunction with testing for genes causing SCID. Infants with congenital athymia have very low naïve T cell counts. There is no uniformly agreed upon level of naïve T cells defining congenital athymia. Before the widespread use of newborn screening in the United States, congenital athymia was diagnosed when patients developed life-threatening infections that healthy infants are usually able to control without therapy. The diagnosis of 22q11.2ds was made in many children prior to heart surgery, but the children were rarely tested for T cell numbers, as congenital athymia is rare in 22q11.2ds.[4] Likewise, CHARGE syndrome was not associated with congenital athymia when our investigational program was initiated. More recently, newborn screening for SCID has led to early diagnosis in most patients in the United States.
Congenital athymia can present with a typical or an atypical phenotype. The typical phenotype has low T cell numbers, no rash or lymphadenopathy, and usually lack of T cell reactivity to mitogens. The atypical phenotype has oligoclonal T cell expansion, rash, lymphadenopathy, and T cell reactivity to mitogens such as phytohemagglutinin (PHA). This phenotype resembles Omenn syndrome or autologous graft versus host disease (GVHD) and typically presents at some point after birth. Patients with the atypical phenotype require treatment with immunosuppression to prevent the deleterious effects of their autoreactive T cells. Patients with the typical phenotype may transition to the atypical phenotype; although, it is unknown what triggers this transition.
Cultured thymus tissue (CTT), also known as allogeneic processed thymus tissue–agdc, is a tissue-based investigational product that is derived from donor thymus tissue and substantially manipulated to optimize viability of the tissue upon surgical implantation into the recipient.[5] The CTT is composed of many processed tissue slices; each slice is implanted in the recipient’s quadriceps muscle. The administration of CTT, previously called thymus transplantation, has been investigated at Duke University since 1993.[5–10]
The goal of this research has been to design a treatment that enables the development of naïve T cells and T cell function in order to enhance survival. This report includes specific data from all patients treated with CTT to date since the inception of this program at Duke University in 1993.
METHODS
Patients were enrolled and received CTT in 1 of 10 Institutional Review Board (IRB)–approved protocols between 1993 and 2020 after the parent(s)/guardian(s) signed an IRB–approved informed consent document (Table E1 in this article’s Online Repository at www.jacionline.org). Statistical analyses were performed by Covance Inc. (Princeton, NJ).
Congenital athymia was defined in the protocols as any of the following: cDGA in which patients had athymia plus either a congenital heart defect or hypocalcemia/hypoparathyroidism, or FOXN1 deficiency. cDGA included: 22q11.2ds, CHARGE syndrome, other genetic defects associated with congenital athymia, and diabetic embryopathy. Key eligibility criteria included cDGA or FOXN1 deficiency, T cells <50/mm3 or naïve T cells (CD3+CD4+CD45RA+CD62L+ or CD3+CD8+CD45RA+CD62L+) <50/mm3, and the absence of genetic defects associated with SCID. Patients enrolled under the expanded access protocol could have naïve T cell counts >50/mm3. Exclusion criteria included heart surgery within 4 weeks before administration of CTT or anticipated within 3 months after administration of CTT, poor surgical candidate as determined by the surgeon or anesthesiologist, HIV infection, prior attempts at immune reconstitution, ventilator dependence, and cytomegalovirus (CMV) infection for patients requiring immunosuppression.
For inclusion, patients had athymia with circulating CD3+CD45RA+CD62L+ T cells <50/mm3 or <5% of the total T cell count on 2 separate flow cytometry analyses, 1 performed within 3 months and 1 performed within 1 month before administration of CTT, unless they were enrolled in the expanded access protocol, where naïve T cells could be above 50mm3. Patients with typical and atypical phenotypes were included. Patients with the typical phenotype were defined as having absence of rash and lymphadenopathy, and patients with the atypical phenotype were defined by presence of rash and lymphadenopathy.
Immunosuppression was given to patients based on the proliferative response to PHA, regardless of typical or atypical phenotype. Depending on the PHA response, patients received either rabbit anti-thymocyte globulin (RATGAM) alone or RATGAM plus a calcineurin inhibitor and/or steroids (see this article’s Online Repository at www.jacionline.org for supplementary material on immunosuppression). Patients were assessed for HLA antigens. Only patients who had previously received a HSCT with a mismatched allele required HLA matching of the thymus to that allele.
Administration
CTT is a fresh tissue-based investigational product currently manufactured at the Marcus Center for Cellular Cures (Duke University School of Medicine, Durham, NC) and administered at Duke University Medical Center in an operating suite. After manufacturing, CTT slices are inserted into pockets made in the quadriceps muscle of one or both legs.[11] Dosing was based on mm2 of CTT per recipient body surface area in m2 (See this article’s Online Repository at www.jacionline.org for supplementary material). After administration of CTT, patients continued to receive immunoglobulin G (IgG) replacement, prophylactic antimicrobials, and immunosuppressant therapy, as indicated until immune reconstitution (see this article’s Online Repository at www.jacionline.org for supplementary material on immune monitoring and safety monitoring).
Adverse events
Adverse events (AEs) were defined as unrelated, unlikely, possibly, probably, or definitely related to either protocol procedures or the CTT. Examples of AEs related to procedures were fever or seizure with the use of RATGAM; complications of surgery such as wound dehiscence, wound infection, and inflammation around stitches; and side effects from immunosuppression therapy.
After administration of CTT, the primary investigator oversaw the weaning of patients off calcineurin inhibitors, intravenous IgG, and prophylactic antimicrobials. Patients were weaned off the calcineurin inhibitor over 8 to 10 weeks once the naïve T cell percentage was over 10% of CD3 T cells.[9] Prophylaxis to treat Pneumocystis jiroveci pneumonia was discontinued when the patient was at least 9 months out from administration of CTT, no longer taking immunosuppressive therapy, had a CD4 count >200/mm3, and had a PHA response of >100,000 cpm. IgG replacement therapy could be stopped when the patient was at least 9 months out from administration of CTT, was weaned off immunosuppressive therapy, and had trough IgG levels in the normal range while receiving IgG replacement therapy. Two months after discontinuation of IgG replacement therapy, the patient’s IgG level had to be in the normal range or IgG replacement therapy was resumed.
Statistical analysis plan
The primary end point of the study was the assessment of patient survival 1 year after administration of CTT. Data on patient survival at 2 years and survival of patients who lived at least 1 year after receiving CTT were analyzed as supporting data. Secondary end points included CD3, CD4, CD8, naïve CD3, naïve CD4, and naïve CD8 T cell counts at 1 year. Proliferative responses to PHA, concanavalin A (Con A), and tetanus toxoid were also assessed. Frequency of AEs and characterization by system organ class were analyzed.
RESULTS
Patient characteristics
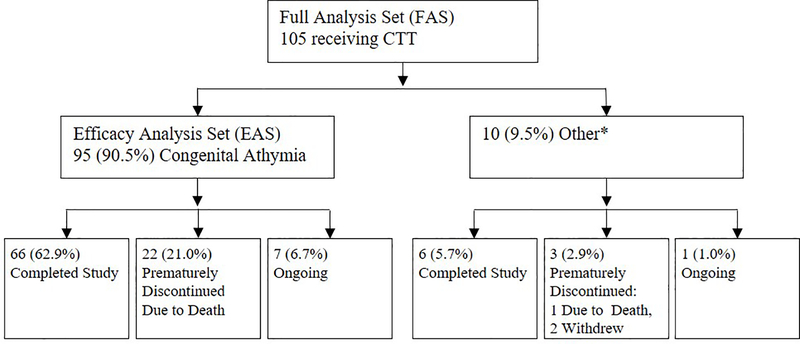
One hundred five patients were enrolled in the study and are included in the full analysis set (FAS), and 95 are included in the efficacy analysis set (EAS). Ten patients were excluded from the EAS: 2 because their ultimate diagnosis was SCID, 6 because they received previous hematopoietic stem cell transplant or fetal transplant, and 2 because their diagnoses were not definitive. The flow chart of patient disposition at end of year 2 is shown in Fig 1.
FIG 1.
Flow chart of patient disposition at the end of year 2. *“Other” includes 2 patients with SCID, 6 patients with previous hematopoietic or fetal thymic transplants, and 2 patients in which diagnosis was not definitive. CTT, cultured thymus tissue; SCID, severe combined immunodeficiency.
The baseline demographics, disease characteristics, and syndromic comorbidities of the EAS and FAS populations are shown in Table I. Heart defects associated with patients’ syndromic comorbidities are listed in Table E2 in this article’s Online Repository at www.jacionline.org. The mean patient age at the time of CTT administration was 298 days and 493 days for the EAS and FAS, respectively. Of the 95 patients with congenital athymia in the EAS, 93 had the protocol-defined diagnosis of cDGA and 2 had a diagnosis of FOXN1 deficiency; the FOXN1 cases have previously been described in detail.[12]
Table I.
Baseline characteristics and disease history
| Analysis Population | ||
|---|---|---|
| Characteristic | EAS (N = 95) | FAS (N =105) |
| Age on day of transplant (days), mean (SD) | 298 (214) | 493 (924) |
| Male, n (%) | 56 (58.9) | 60 (57.1) |
| Race, n (%) | ||
| White | 66 (69.5) | 76 (72.4) |
| Black/African American | 21 (22.1) | 21 (20.0) |
| Asian | 3 (3.2) | 3 (2.9) |
| American Indian/Alaska Native | 2 (2.1) | 2 (1.9) |
| Native Hawaiian/Other Pacific Islander | 1 (1.1) | 1 (1.0) |
| More than 1 Race | 2 (2.1) | 2 (1.9) |
| Ethnicity, n (%) | ||
| Hispanic or Latino | 18 (18.9) | 20 (19.0) |
| Not Hispanic or Latino | 77 (81.1) | 85 (81.0) |
| Congenital athymia—gene mutations, n (%) | ||
| CHD7 mutation | 12 (12.6) | 12 (11.4) |
| 22q11.2 deletion syndrome | 36 (37.9) | 38 (36.2) |
| FOXN1 mutation | 2 (2.1) | 3 (2.9) |
| TBX1 point mutation | 0 | 1 (1.0) |
| TBX2 point mutation | 1 (1.1) | 1 (1.0) |
| No genetic mutation identified* | 37 (38.9) | 41 (39.0) |
| Missing† | 7 (7.4) | 9 (8.6) |
| Age at diagnosis (days), mean (SD) | 48 (79) | 82 (173) |
| Diagnosis, n (%) | ||
| SCID | 0 | 2 (1.9) |
| FoxN1 | 2 (2.1) | 3 (2.9) |
| Partial DiGeorge | 0 | 1 (1.0) |
| Complete DiGeorge‡ | 93 (97.9) | 97 (92.4) |
| Unknown | 0 | 2 (1.9) |
| DiGeorge anomaly phenotype, n (%) | ||
| Typical DiGeorge anomaly | 50 (52.6) | 52 (49.5) |
| Atypical DiGeorge anomaly | 42 (44.2) | 44 (41.9) |
| Unknown§ | 1 (1.1) | 1 (1.0) |
| Fetal toxin exposure, n (%) | ||
| Maternal diabetes | 26 (27.4) | 29 (27.6) |
| Syndromic comorbidities, n (%) | ||
| Congenital cardiac anomaly or cardiothoracic vascular anomaly | 85 (89.5) | 91 (86.7) |
| Hypocalcemia | 81 (85.3) | 89 (84.8) |
| Diminished naïve T cell counts for age | 95 (100.0) | 105 (100.0) |
| Dysmorphic facies | 44 (46.3) | 44 (41.9) |
| Deafness or ear pinnae anomalies | 49 (51.6) | 53 (50.5) |
| Coloboma | 18 (18.9) | 18 (17.1) |
| Cleft lip | 12 (12.6) | 12 (11.4) |
| Cleft palate (frank clefting or submucous cleft) | 17 (17.9) | 17 (16.2) |
| Velopharyngeal insufficiency/hypernasal speech | 3 (3.2) | 4 (3.8) |
| Choanal atresia | 10 (10.5) | 10 (9.5) |
| Tracheal anomalies | 25 (26.3) | 25 (23.8) |
| Esophageal anomalies | 10 (10.5) | 10 (9.5) |
| Anal and/or rectal anomalies | 6 (6.3) | 6 (5.7) |
| Renal anomalies | 26 (27.4) | 27 (25.7) |
| Genital hypoplasia | 11 (11.6) | 13 (12.4) |
| Rib or vertebral anomalies | 34 (35.8) | 35 (33.3) |
| Limb anomalies | 16 (16.8) | 17 (16.2) |
| Growth or mental retardation | 49 (51.6) | 54 (51.4) |
| Other|| | 7 (7.4) | 8 (7.6) |
Includes 11 patients with CHARGE (coloboma, heart defects, atresia choanae, growth retardation, genital hypoplasia and ear abnormalities and/or deafness) who had no identified gene mutation.
No data available for the patient.
Protocol-defined complete DiGeorge diagnosis included: complete DiGeorge anomaly in which patients had athymia plus either a congenital heart defect or hypocalcemia/hypoparathroidism, 22q11.2 deletion syndrome, CHARGE syndrome, and infants of diabetic mothers.
Patient presented with GVHD after 3 unirradiated blood transfusions, at least one of which was likely cytomegalovirus seropositive.
Other conditions are swallowing difficulty; tube dependence; reflux and developmental delay; feeding intolerance; significant reflux; pyriform aperture stenosis central incisor; and CHARGE syndrome suspected based on phenotypic features of low-set ears, thin upper lip, micrognathia, significant hearing loss, micrognathia, and external ear abnormalities.
Fifty of 93 patients with cDGA had the typical phenotype. Of these patients, 15 received RATGAM and 35 did not. Of the 42 patients with the atypical cDGA phenotype, 39 received RATGAM and 2 patients treated early in the program did not. One patient received deoxycoformycin as immunosuppression therapy. One patient was not categorized as either typical or atypical because of preexisting GVHD from unirradiated blood transfusions prior to transfer to our center; this patient received cyclophosphamide and RATGAM.[8, 9] The median dose of CTT administered was 12,996 mm2 of tissue surface area/m2 recipient body surface area (range, 4902–23,755 mm2/m2) and 12,675 mm2 of tissue surface area/m2 recipient body surface area (range, 4523–23,755 mm2/m2) for the EAS and FAS, respectively (data not shown).
Outcomes
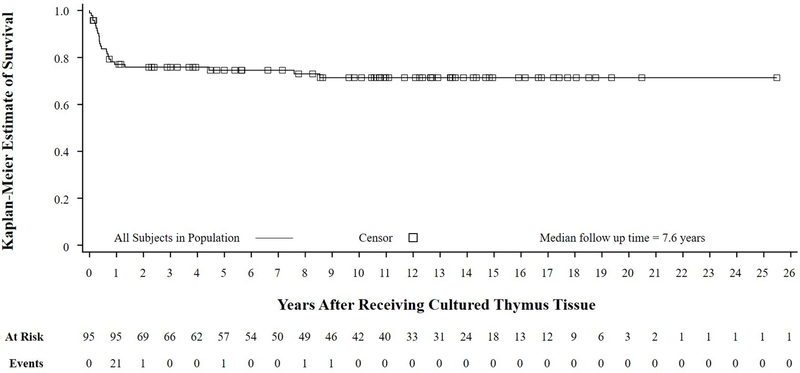
The Kaplan-Meier estimated survival rates of the EAS at year 1 and year 2 after receiving CTT are 77% and 76%, respectively (Fig 2, Table E3 in this article’s Online Repository at www.jacionline.org). The median follow-up time for the EAS was 7.6 years and ranged from 0 to 25.5 years after receiving CTT. The Kaplan-Meier estimated survival rates for the FAS is in Fig E1 (in this article’s Online Repository at www.jacionline.org) and mirrors the EAS results. For patients who were alive 1 year after receiving CTT, the estimated survival rate at a median follow-up time of 10.9 years is 93% (Fig E2 and Table E4 in this article’s Online Repository at www.jacionline.org).
FIG 2.
Kaplan Meier survival curve of EAS. This analysis shows that of the 95 patients, almost all deaths occurred in the first year. EAS, efficacy analysis set.
T cell development and function after CTT Administration
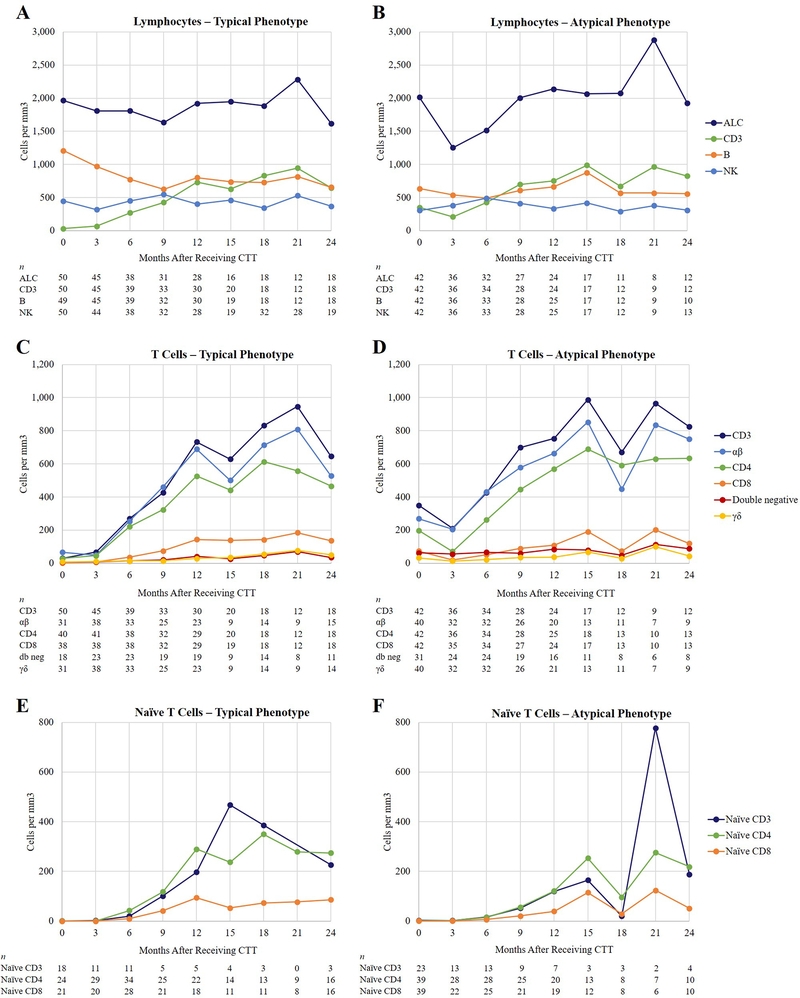
Median lymphocyte cell count results are shown in Fig 3. As seen in Fig 3, C and Fig 3, D, the median T cell counts reached their peak at approximately 1 to 2 years after CTT administration. Fig 3, C and Fig 3, D show the median CD3, CD4, CD8, αβ, and γδ T cell numbers through time. The CD3, CD4, and CD8 T cells increased but remained below the 10th percentile for age. The γδ cell counts remained low. The CD8 T cell counts remained low through year 2. Normal ranges and detailed ranges for the EAS and FAS populations of the various cell populations are found in Table E5 in this article’s Online Repository at www.jacionline.org.
FIG 3.
Flow cytometry. A, Median numbers of lymphocytes in patients with typical phenotype. B, Median numbers of lymphocytes in patients with atypical phenotype. C, T cell subsets in patients with typical phenotype. D, T cell subsets in patients with atypical phenotype. E, Naïve T cells in patients with typical phenotype. Note, there were no patient samples for month 21. F, naïve T cells in patients with atypical phenotype. Normal values for age and interquartile data are in Table E5,A and Table E5,B in this article’s Online Repository at www.jacionline.org. ALC, absolute lymphocyte count; CTT, cultured thymus tissue; db neg, double negative; NK, natural killer.
Fig 3, E and Fig 3, F show the naïve T cell numbers. Naïve T cells increased from day 0 to year 2. The patients with atypical cDGA phenotypes tended to have lower numbers of T cells immediately after receiving CTT and increased into year 2. The naïve T cell numbers start at 0 in all patients and increase, reaching the highest numbers in year 2. More detailed graphs showing the minimum, maximum, first quartile, third quartile, median and mean for naïve T cells are shown in Fig E3 in this article’s Online Repository at www.jacionline.org.
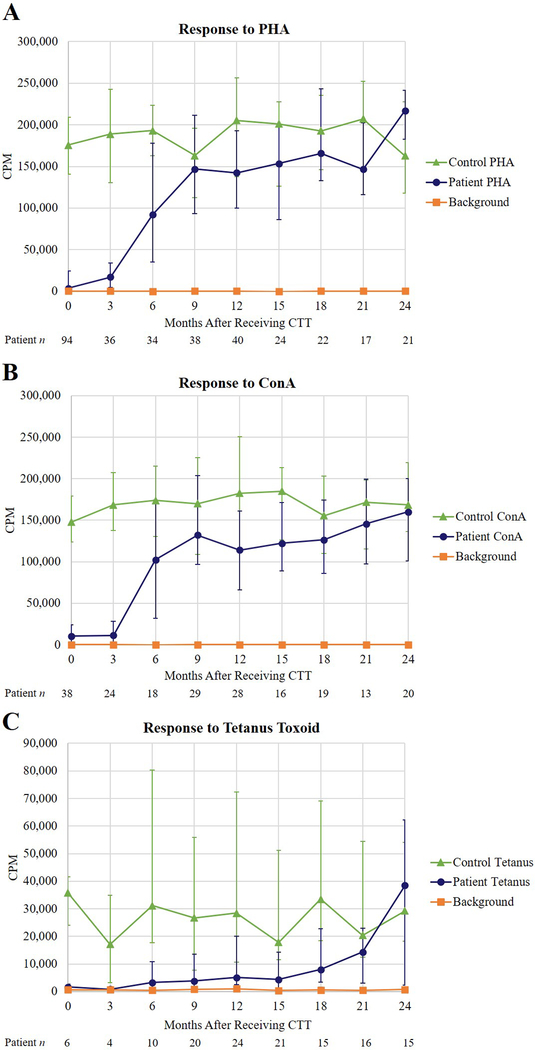
T cell function was studied by proliferative responses to mitogens and antigens. Fig 4, A and Fig 4, B show that proliferative responses to the mitogens PHA and Con A reach the normal range by 6 months after administration of CTT. The proliferative response to the antigen tetanus toxoid develops in year 2, after immunizations begin (Fig 4, C).
FIG 4.
T cell proliferative responses to mitogens and antigens. Responses to A, PHA; B, Con A; and C, tetanus toxoid. The normal response to PHA is 85,000 to 286,636 cpm, to Con A is 75,000 to 224,079 cpm, and to tetanus toxoid is 11,273 to 106,085 cpm. Con A, concanavalin A; cpm, counts per minute; PHA, phytohemagglutinin.
B and NK cells
B- and NK-cell numbers remained in the normal range in most patients (Fig 3, A and Fig 3, B). Almost all patients were able to stop IgG replacement therapy.
Donor chimerism
Three of the patients who were alive 1 year after administration of CTT had evidence of thymus donor T cell engraftment. The donor chimerism observed in one patient who had >98% donor cells at approximately 80 days after receiving CTT gradually decreased over the first year, and by the end of the second year, all T cells were from the recipient. The second patient with chimerism initially had 89% donor T cells at approximately 50 days after CTT administration, which decreased to 12% donor T cells 6 months after receiving CTT, and 23% donor T cells approximately 32 months following administration of CTT. The third patient had 75% donor T cells at 2 months after receiving CTT, but none at 10 months. No AEs related to the donor chimerism were identified.
Adverse events and infections
A summary of the severity of all AEs is presented in Table E6 in this article’s Online Repository at www.jacionline.org. AEs were reported as general AEs or infection-related AEs. Of 105 patients, there were 32 with at least 1 severe AE, 35 with at least 1 life-threatening event, and 26 AE-related deaths (discussed below). In addition, there were 2 deaths that occurred after study withdrawal in patients with SCID and therefore were not considered AE-related. Fifty-three patients had infections categorized as severe, 13 as life threatening, and 11 as fatal. AEs considered possibly related to CTT are reported in Table II and all AEs with ≥10% frequency are reported in Table E7 in this article’s Online Repository at www.jacionline.org).
Table II.
Adverse events possibly related to CTT administration by system organ class and preferred term
| FAS (N = 105) | ||
|---|---|---|
| n (%) | E | |
|
| ||
| Number of Related Adverse Events | 44 (41.9) | 87 |
| Blood and lymphatic system disorders | 21 (20.0) | 32 |
| Thrombocytopenia | 11 (10.5) | 13 |
| Neutropenia | 8 (7.6) | 8 |
| Coombs positive haemolytic anaemia | 3 (2.9) | 3 |
| Autoimmune haemolytic anaemia | 2 (1.9) | 3 |
| Haemolysis | 2 (1.9) | 2 |
| Haemolytic anaemia | 2 (1.9) | 2 |
| Immune thrombocytopenic purpura | 1 (1.0) | 1 |
| Skin and subcutaneous tissue disorders | 9 (8.6) | 10 |
| Alopecia | 4 (3.8) | 4 |
| Rash | 2 (1.9) | 2 |
| Granuloma skin | 1 (1.0) | 1 |
| Psoriasis | 1 (1.0) | 1 |
| Skin mass | 1 (1.0) | 1 |
| Stephens-Johnson syndrome | 1 (1.0) | 1 |
| General disorders and administration site conditions | 6 (5.7) | 6 |
| Pyrexia | 5 (4.8) | 5 |
| Oedema peripheral | 1 (1.0) | 1 |
| Renal and urinary disorders | 5 (4.8) | 6 |
| Proteinuria | 5 (4.8) | 5 |
| Glomerulonephritis minimal lesion | 1 (1.0) | 1 |
| Gastrointestinal disorders | 5 (4.8) | 5 |
| Diarrhoea | 3 (2.9) | 3 |
| Enteritis | 1 (1.0) | 1 |
| Ileus | 1 (1.0) | 1 |
| Injury, poisoning and procedural complications | 5 (4.8) | 5 |
| Wound dehiscence | 4 (3.8) | 4 |
| Graft haemorrhage | 1 (1.0) | 1 |
| Investigations | 4 (3.8) | 6 |
| Blood bicarbonate decreased | 2 (1.9) | 2 |
| Lymphocyte morphology abnormal | 1 (1.0) | 2 |
| Blood immunoglobulin E increased | 1 (1.0) | 1 |
| Lymphocyte count abnormal | 1 (1.0) | 1 |
| Infections and infestations | 4 (3.8) | 4 |
| Cytomegalovirus infection | 1 (1.0) | 1 |
| Staphylococcal skin infection | 1 (1.0) | 1 |
| Stitch abscess | 1 (1.0) | 1 |
| Wound infection staphylococcal | 1 (1.0) | 1 |
| Immune system disorders | 2 (1.9) | 3 |
| Graft versus Host Disease | 1 (1.0) | 1 |
| Graft versus Host Disease in gastrointestinal tract | 1 (1.0) | 1 |
| Graft versus Host Disease in skin | 1 (1.0) | 1 |
| Hepatobiliary disorders | 2 (1.9) | 2 |
| Autoimmune hepatitis | 2 (1.9) | 2 |
| Musculoskeletal and connective tissue disorders | 2 (1.9) | 2 |
| Juvenile idiopathic arthritis | 1 (1.0) | 1 |
| Psoriatic arthropathy | 1 (1.0) | 1 |
| Endocrine disorders | 1 (1.0) | 2 |
| Basedow's disease | 1 (1.0) | 1 |
| Hyperthyroidism | 1 (1.0) | 1 |
| Congenital, familial and genetic disorders | 1 (1.0) | 1 |
| Albinism | 1 (1.0) | 1 |
| Nervous system disorders | 1 (1.0) | 1 |
| Myelitis transverse | 1 (1.0) | 1 |
| Reproductive system and breast disorders | 1 (1.0) | 1 |
| Ovarian failure | 1 (1.0) | 1 |
| Vascular disorders | 1 (1.0) | 1 |
| Haematoma | 1 (1.0) | 1 |
Note: If a patient had multiple occurrences of an AE, the patient was included only once in the n for a given SOC and PT. Adverse events were coded using MedDRA version 19.1.
Related events were defined as events that were definitely, probably or possibly related to CTT or study procedures or with an unknown relationship based on investigator review.
AE, adverse event; E, number of events; FAS, full analysis set; MedDRA, Medical Dictionary for Regulatory Activities; PT, preferred term; SOC, system organ class
All AEs possibly related to CTT, or its administration, including autoimmune manifestations and signs of autologous GVHD, are reported in Table II. Cytopenias were reported for 15 patients (14.3%); a total of 22 events were reported (see Table E8 in this article’s Online Repository at www.jacionline.org). Notably, 17 of 22 events occurred in year 1, and all of these cases resolved. Of the 5 events reported in year 2, only 2 events in 1 patient are ongoing. Except for alopecia [4 patients (3.8%)], other AEs possibly reflecting symptoms of autoimmunity were reported for only 1 or 2 patients.
The rate of infection decreased over time based on a comparison of the infection rates in different time frames after CTT was administered. There was a significant decrease in the number of infections when comparing the infections occurring within the first 6 months after receiving CTT to the number of infections occurring 6 to 12 months after receiving CTT (P < .001; Table III). Infections also decreased when comparing the infections occurring within the first 12 months after receiving CTT to infections in months 12 to 24 (P < .001). For inclusion in these analyses, the patients had to be alive at the end of year 1 or year 2, respectively.
Table III.
Decreased infections 6 to 12 months compared with 0 to 6 months; 12 to 24 months compared with less than 12 months for FAS
| 1-Year Analysis | 2-Year Analysis | |||
|---|---|---|---|---|
|
| ||||
| Onset Within 6 Months | Onset 6 to ≤12 Months | Onset Within 12 Months | Onset 12 to ≤24 Months | |
|
| ||||
| No. of patients analyzed* | N = 72 | N = 69 | ||
| No. of patients with event during the interval | 67 | 38 | 68 | 35 |
| Mean (SD) No. of events per patient | 3.5 (2.6) | 2.3 (2.4) | 4.4 (3.6) | 2.7 (3.0) |
| Median (IQR†) No. of events per patient | 3.0 (1.0, 5.0) | 1.5 (1.0, 3.0) | 3.0 (2.0, 6.0) | 2.0 (1.0, 3.0) |
| P value‡ | < .001 | < .001 | ||
To be included in the 1-year analysis, patients must have been alive and on follow-up for at least 365 days after administration of cultured thymus tissue, and experienced at least one infection-related AE during one or both onset periods.
To be included in the 2-year analysis, patients must have been alive and on follow-up for at least 790 days after administration of cultured thymus tissue, and experienced at least one infection-related AE during one or both onset periods.
Number of patients who had events during the analysis period.
IQR, interquartile range.
2-sided P value based on a Wilcoxon signed-rank test of the mean difference in the number of infection-related AEs per patient between the first and second intervals.
A total of 78 new viral infections were reported after CTT administration. Of these, 47 infections were reported in the first year after receiving CTT, 15 were reported in the second year after receiving CTT, and a total of 16 were reported in subsequent years. There were 4 reports each of adenoviral upper respiratory infection, adenoviral gastroenteritis, and cytomegalovirus infection; 3 reports of Epstein-Barr virus; and 1 report of adenoviral urinary tract infection. No cases of BK virus were reported. An important caveat is that we cannot be certain all patients received viral testing after leaving Duke University Medical Center.
Deaths
Among 105 patients in the FAS group, there were 28 deaths (Table IV); 26 were considered related to AEs and 2 others were reported after patients were withdrawn from the study due to SCID diagnosis following administration of CTT. Twenty-two of the 28 deaths, including 12 of the 13 infection-related deaths, occurred in the first year after administration of CTT, while the patients were still immunodeficient.
Table IV.
Causes of death.
| Day of life | Days after CTT | Causes of Death (N = 28)* |
|---|---|---|
|
| ||
| Related to Infection (n = 13) | ||
|
| ||
| 471 | 24 | Progression of underlying PIV3 pneumonia |
| 398 | 44 | Sepsis |
| 586 | 89 | Death secondary to disseminated Candida infection |
| 247 | 103 | Progressive cerebral atrophy due to CMV |
| 181 | 130 | Sepsis |
| 199 | 130 | Sepsis |
| 350 | 149 | Respiratory failure from disseminated mycobacterial infection |
| 264 | 160 | Respiratory failure due to respiratory syncytial virus |
| 416 | 234 | Respiratory failure secondary to sepsis from Candida tropicalis and C parapsilosis |
| 382 | 252 | Respiratory complications from CMV infection |
| 804 | 263 | Multiorgan system failure as seen in severe septic shock |
| 856 | 339 | Methicillin resistant Staphylococcus aureus bacteremia |
| 824 | 375 | Presumed sepsis |
|
| ||
| All Except for Infection (n = 15) | ||
|
| ||
| 107 | 0 | Hemorrhage with Nissen fundoplication surgery |
| 172 | 45 | Respiratory failure |
| 162 | 66 | Severe bilateral intraventricular hemorrhage |
| 268 | 82 | Hypoxia |
| 537 | 108 | Cardiorespiratory arrest related to anasarca |
| 5879 | 116 | Progression of EBV lymphoma resulting in intracranial bleeding and death |
| 510 | 128 | Large right parietal hemorrhage |
| 256 | 137 | Respiratory failure |
| 667 | 229 | Respiratory failure |
| 392 | 289 | Respiratory failure |
| 982 | 480 | Sudden catastrophic intracranial hemorrhage |
| 1622 | 950 | Not reported |
| 1713 | 1617 | Cardiopulmonary arrest |
| 2918 | 2769 | Respiratory failure |
| 3311 | 3116 | Respiratory failure |
CMV, cytomegalovirus; EBV, Epstein-Barr virus; PIV3, parainfluenza virus type 3
Includes 2 deaths in patients who withdrew from the study prior to having a reportable adverse event.
Multiple organisms were involved in the infection-related deaths. Parainfluenza virus 3, Candida lucitanea and Candida albicans, disseminated Candida infection, methicillin-resistant Staphylococcus aureus, mycobacterial infection, and respiratory syncytial virus were each responsible for 1 death each; 2 patients died of CMV; and 5 died of septic shock or sepsis.
Respiratory failure was responsible for 10 deaths. Conditions leading to respiratory failure and death included anasarca, superior vena cava syndrome, bronchopulmonary dysplasia, and blocked tracheostomy tube. Four of the deaths related to respiratory failure were associated with concomitant infections such as mycobacteria, CMV, and parainfluenza virus 3.
There were 5 patients for whom bleeding was a major concern and contributed to their deaths. Four of the patients had multiple recurrent bleeding events; of these,1 patient had a cerebral bleeding event presumed to be related to an infection. The fifth patient in this category died on the day that CTT administration was planned, but prior to receiving CTT, secondary to hemorrhage at the site of a calcium accretion in the inferior venae cava extending into the left hepatic vein.
DISCUSSION
CTT has been used to treat 105 pediatric patients over the past 27 years. Ninety-five of these patients were athymic, had no prior therapy, and are the focus of our reported efficacy. This group of patients had excellent survival results. Most patients in this study of CTT survived. For those who died, most deaths occurred in the first year after receiving CTT, before establishment of T cell immunity. In addition, most deaths were secondary to preexisting syndromic comorbidities, such as heart defects, or secondary to infections acquired before or shortly after receiving CTT. Immune reconstitution sufficient to support survival and prevent infections typically develops 6 to 12 months after administration of CTT.
The Kaplan-Meier survival curve for the FAS population is very similar to that of the EAS population and shows most deaths occurred in the first year. Four patients (not diagnosed with SCID and without lymphoma at the time of CTT administration) had previous HSCTs, including cord blood and peripheral blood infusions that did not result in T cell development sufficient to protect the child. All 4 patients subsequently developed naïve T cells after administration of CTT. Three of the 4 did not develop GVHD. The fourth, who was greater than 10 years old at the time of CTT administration, developed GVHD from the prior poorly matched female cord blood cells that had been given when the patient was an infant. The GVHD resolved in the second year after receiving CTT. These positive outcomes in 4 patients treated with prior HSCTs suggest that administration of CTT may be efficacious after an unsuccessful HSCTs in athymic patients. However, undergoing a prior HSCT makes it more difficult to receive CTT because it requires matching of the donated thymus tissue to any alleles in the recipient that were not fully matched by the donor stem cells in the previous HSCT.
The Kaplan-Meier survival curve of the EAS analysis set for those patients who survived past 1 year of age shows only 1 death related to lack of naïve T cell development after the first year following CTT administration. The 3 deaths occurring after the second year following CTT administration were not related to immunodeficiency. These results imply that patients without life-threatening syndromic comorbidities who survive past 2 years will likely survive well into adulthood. Five patients in our cohort are greater than 18 years old and the oldest is over 25 years old. We look forward to following up with these patients over the next decades.
We have demonstrated that CTT can act similarly to normal thymus tissue to produce naïve T cells, CD3+CD4+CD45RA+CD62L+ and CD3+CD8+CD45RA+CD62L+, that can then migrate into the periphery and fight infection as CD3+CD4+ or CD3+CD8+ T cells. We previously reported the results of immunohistochemistry staining of CTT biopsies 2 – 3 months after implantation. Among 30 biopsies examined, CTT was found in 25, and all but 2 showed thymopoiesis.[13]
Thymopoiesis in the CTT results in the appearance of naïve T cells in peripheral blood, typically between 6 and 12 months after receiving CTT. Our data demonstrate that lymphocyte counts are very similar after receiving CTT in the groups with typical and atypical cDGA phenotypes. The naïve CD4 counts are also quite similar in the groups with typical and atypical cDGA phenotypes, as are the naïve CD8 counts. CD3+ T cell counts increase over time, but the median CD3+ T cell counts remain below the 10th percentile for age. The same is true for the CD4+ T cells. It is noteworthy that T cell counts under the 10th percentile for age usually result in protection from infection in patients with cDGA, similar to the protection from infection afforded to children, such as those with less severe immunodeficiencies, who have T cell counts in the lowest 10% for age. Because of the low T cell numbers, the lymphocyte count is also low for age. Of note, the CD8 count is much lower relative to the 10th percentile for age compared with the CD4 count. Anecdotally, despite the low CD8 counts, live vaccines (measles, mumps, and rubella and varicella zoster virus) have been well tolerated by our patients.
We have previously studied the appearance of T cells in the blood before and after receiving CTT.[8, 14–16] After CTT administration, T cells emigrating from the CTT develop a broad diversity of TCR-Vβ genes distributed over 20 families.[8, 15, 16] In 2 patients with the atypical cDGA phenotype that we studied in depth, T-regulatory cells were not evenly distributed across the 24 TCR-Vβ families before administration of CTT, and some TCR-Vβ families had no T-regulatory cells. Twelve months following administration of CTT, these 2 patients’ T-regulatory cells had expanded to all of the TCR-Vβ families with similar proportions as observed for CD4 T cells.[14] This distribution of T-regulatory cells after receiving CTT may explain how the rash and autoimmune cytopenias eventually resolve and allow for the discontinuation of immunosuppression in patients with the atypical cDGA phenotype.
T cell function was assessed using proliferative responses to the mitogens PHA, Con A and the antigen tetanus toxoid. Remarkably, the responses of PHA and Con A were normal by 6 months. In healthy children, 3 doses of a tetanus toxoid–containing vaccine are given at 2, 4, and 6 months of life. An additional dose is given at 15 to 18 months to ensure a good response because initial doses are often not enough to maintain an adequate response. The same number of vaccinations were given to patients with congenital athymia although the vaccines started 1 year after CTT administration. After vaccination, the proliferative responses to tetanus toxoid were similar to those of healthy children.
We have published studies of B-cell function. Immunoglobulin levels are mostly normal for age.[8, 9] Specific antibodies form to tetanus toxoid, but only in low levels to pneumococcal serotypes.[8, 9]
The data presented herein provide support for the development of immunity in a patient’s first year after receiving CTT. Infections at 6 to 12 months after CTT administration were decreased compared to the infections after 0 to 6 months. Consistent with this finding, after developing naïve T cells, patients have demonstrated the ability to overcome serious infections such as mycobacteria, parainfluenza virus 3, adenovirus, Candida albicans, varicella zoster virus, and CMV. Children with congenital athymia have immune dysregulation that leads to autoimmune phenomena such as autologous GVHD and cytopenias. Autologous GVHD with rash and lymphadenopathy usually decreases after CTT administration, likely secondary to treatment of these patients with RATGAM and calcineurin inhibitors, and does not reappear once immunosuppression is weaned, likely secondary to the reconstituted immune system. Cytopenias often develop in the first year after receiving CTT as naïve T cells begin to develop. However, as naïve T cell numbers increase toward the end of the first year after CTT administration, cytopenias resolve and the risk of new-onset cytopenias decreases.[6–10, 12, 13, 17]
AEs and infections were recorded whether or not they were related to implantation procedures or underlying syndromic comorbidities. These data show the full spectrum of challenges associated with congenital athymia.
The use of CTT administration in congenital athymia uniquely differs from the allogeneic transplants used to treat other primary immunodeficiencies in that HLA matching is not required. With mismatched hematopoietic transplants, mature donor T cells attack the mismatched allele in the recipient. In contrast, patients with congenital athymia do not have mature T cells. After receiving CTT, the recipient’s bone marrow stem cells go to the CTT to develop into recipient T cells. The recipient dendritic cells delete any developing thymocytes that bind too tightly to recipient dendritic cells (self) or donor thymic epithelium in the CTT. No T cells leave the thymus that will attack the recipient or the CTT. We have previously published the lack of effect of HLA matching on outcomes post CTT administration.[17] All patients are assessed for HLA antibodies. The only patients that require HLA matching are those who have had a prior transplant in which the donor was not fully matched to the recipient. In these exceptional cases, the allele in the bone marrow donor that is not in the recipient must be present in the donated thymus tissue.
In summary, the development of CTT as an investigational therapy for children with congenital athymia has been lifesaving. Historically, children with congenital athymia who did not receive therapy died in the first few years of life. Based on these data, families now have hope that their children, with a reconstituted immune system, will be able to live a full life.
Supplementary Material
Acknowledgments
This work could not have been performed without the grants received through the years from the NIH and the FDA in particular. We appreciate the advice we have received over the years from Nancy Bridges, MD, at the NIH, and Thomas Finn, PhD, at the FDA. The support we received from the Duke General Clinical Research Center at Duke University Medical School from November 1993 through December 2004 was instrumental in providing care for the research patients. The guidance and support we have received from the Data Safety and Monitoring Board (Chair, E. William St. Clair) from 2001 to the present was invaluable. The early support of Barton F. Haynes, MD; Rebecca Buckley, MD; Laura Hale, MD, PhD; Blythe Devlin, PhD; and Henry Rice, MD, throughout the years is very much appreciated. We acknowledge the support over the last 3 years in thymus donor screening performed by the staff under Joanne Kurtzberg, MD, at the Julian Robertson Cell and Translational Therapy Program (CT2) at Duke University and the culturing of thymus tissue at that facility since May 2016. We recognize the support given to inpatients by the Pediatric Transplantation & Cell Therapy Program at Duke University, with special thanks to Paul Martin, MD, PhD, and Vinod Prasad, MD, FRCP (London), and the input of the Division of Pediatric Allergy Immunology and Pulmonary into the care of the patients under the leadership of Division Chief John Sleasman, MD.
Funding statement:
Dr. Markert’s writing efforts were supported by the Division of Pediatric Allergy, Immunology, and Pulmonary Medicine at Duke University. Funding of the writing efforts of Gupton and McCarthy was from the Thymus Research Fund. Statistical analyses were performed by Covance, Princeton, NJ, under contract with Enzyvant, for submission to the FDA. Enzyvant has permitted these data to be used without charge.
Funding for the research in this paper includes the following:
• M01-RR30 (The National Center for Research Resources [NCRR], Clinical Research) United States
• U19-AI38550/United States
• U01-AI38587, United States
• R01-CA28936, United States
• U01-AI38587, United States
• R01-CA28936, United States
• R01-CA61227, United States
• R21-AG16826, United States
• R21-AI44758, United States
• The American Association of Allergy, Asthma, and Immunology Women Physicians in Allergy Award, United States
• The Max Kade Foundation, United States
• Centeon Pharmaceutics
• R01-AI54843, United States
• R21-AI60967, United States
• FD-R-002606, United States
• R01-AI47040, United States
• American Academy of Allergy Asthma and Immunology, 3rd year FIT Research Award, 2006, United States
• R01-FD003528, United States
• 2R56-AI47040-11A1, Competitive Revision, United States
• 3R56-AI47040-11A1S1, Competitive Revision Supplement, United States
• The Hartwell Foundation, United States
• Orphavant Sciences GmbH (later Enzyvant Therapeutics GmbH, ETG), Switzerland
Disclosure statement:
Cultured thymus tissue (CTT) is an investigational product implanted into patients under an Investigational New Drug (IND) application with the US Food and Drug Administration. M. L. Markert is the “sponsor” of the investigations. M. L. Markert developed the technology for CTT. Duke University has licensed the technology to Enzyvant Therapeutics GmbH. M. L. Markert and Duke University have received royalties from Enzyvant. Portions of M. L. Markert’s and her research team’s salaries are being paid by funding from Enzyvant. If the technology is commercially successful in the future, M. L. Markert and Duke University may benefit financially. The salary and other items needed to create CTT are paid at cost by insurance. Enzyvant Therapeutics, Inc. reviewed the manuscript for scientific accuracy and to ensure protection of their intellectual property. Editorial assistance, funded by Enzyvant Therapeutics, Inc., was provided by Lisa M Pitchford, PhD, of JB Ashtin.
Abbreviations used
- AE
Adverse event
- 22q11.2ds
22q11.2 deletion syndrome
- cDGA
Complete DiGeorge anomaly
- CHARGE
Coloboma, heart defects, choanal atresia, growth or mental retardation, genital hypoplasia, and ear anomalies and/or deafness
- Con A
Concanavalin A
- CTT
Cultured thymus tissue
- CMV
Cytomegalovirus
- EAS
Efficacy analysis set
- FAS
Full analysis set
- GVHD
Graft versus host disease
- HSCT
Hematopoietic stem cell transplant
- IgG
Immunoglobulin G
- IRB
Institutional Review Board
- PHA
Phytohemagglutinin
- RATGAM
Rabbit antithymocyte globulin
- SCID
Severe combined immunodeficiency
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Amatuni GS, Currier RJ, Church JA, Bishop T, Grimbacher E, Nguyen AA, et al. Newborn screening for severe combined immunodeficiency and T-cell lymphopenia in California, 2010–2017. Pediatrics 2019;143(2):e20182300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Janda A, Sedlacek P, Honig M, Friedrich W, Champagne M, Matsumoto T, et al. Multicenter survey on the outcome of transplantation of hematopoietic cells in patients with the complete form of DiGeorge anomaly. Blood 2010;116(13):2229–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yamazaki Y, Urrutia R, Franco LM, Giliani S, Zhang K, Alazami AM, et al. PAX1 is essential for development and function of the human thymus. Sci Immunol 2020;5(44). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McDonald-McGinn DM, Sullivan KE. Chromosome 22q11.2 deletion syndrome (DiGeorge syndrome/velocardiofacial syndrome). Medicine 2011;90(1):1–18. [DOI] [PubMed] [Google Scholar]
- 5.Markert ML, Kostyu DD, Ward FE, McLaughlin TM, Watson TJ, Buckley RH, et al. Successful formation of a chimeric human thymus allograft following transplantation of cultured postnatal human thymus. J Immunol 1997;158(2):998–1005. [PubMed] [Google Scholar]
- 6.Markert ML, Boeck A, Hale LP, Kloster AL, McLaughlin TM, Batchvarova MN, et al. Transplantation of thymus tissue in complete DiGeorge syndrome. N Engl J Med 1999;341(16):1180–9. [DOI] [PubMed] [Google Scholar]
- 7.Markert ML, Alexieff MJ, Li J, Sarzotti M, Ozaki DA, Devlin BH, et al. Postnatal thymus transplantation with immunosuppression as treatment for DiGeorge syndrome. Blood 2004;104(8):2574–81. [DOI] [PubMed] [Google Scholar]
- 8.Markert ML, Devlin BH, Alexieff MJ, Li J, McCarthy EA, Gupton SE, et al. Review of 54 patients with complete DiGeorge anomaly enrolled in protocols for thymus transplantation: outcome of 44 consecutive transplants. Blood 2007;109(10):4539–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Markert ML, Devlin BH, McCarthy EA. Thymus transplantation. Clin Immunol 2010;135(2):236–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Markert ML, Sarzotti M, Ozaki DA, Sempowski GD, Rhein ME, Hale LP, et al. Thymus transplantation in complete DiGeorge syndrome: immunologic and safety evaluations in 12 patients. Blood 2003;102(3):1121–30. [DOI] [PubMed] [Google Scholar]
- 11.Rice HE, Skinner MA, Mahaffey SM, Oldham KT, Ing RJ, Hale LP, et al. Thymic transplantation for complete DiGeorge syndrome: medical and surgical considerations. J Pediatr Surg 2004;39(11):1607–15. [DOI] [PubMed] [Google Scholar]
- 12.Markert ML, Marques JG, Neven B, Devlin BH, McCarthy EA, Chinn IK, et al. First use of thymus transplantation therapy for FOXN1 deficiency (nude/SCID): a report of 2 cases. Blood 2011;117(2):688–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Markert ML, Li J, Devlin BH, Hoehner JC, Rice HE, Skinner MA, et al. Use of allograft biopsies to assess thymopoiesis after thymus transplantation. J Immunol 2008;180(9):6354–64. [DOI] [PubMed] [Google Scholar]
- 14.Chinn IK, Milner JD, Scheinberg P, Douek DC, Markert ML. Thymus transplantation restores the repertoires of forkhead box protein 3 (FoxP3)+ and FoxP3-T cells in complete DiGeorge anomaly. Clin Exp Immunol 2013;173(1):140–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ciupe SM, Devlin BH, Markert ML, Kepler TB. The dynamics of T-cell receptor repertoire diversity following thymus transplantation for DiGeorge anomaly. PLoS Comput Biol 2009;5(6):e1000396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kepler TB, He M, Tomfohr JK, Devlin BH, Sarzotti M, Markert ML. Statistical analysis of antigen receptor spectratype data. Bioinformatics 2005;21(16):3394–400. [DOI] [PubMed] [Google Scholar]
- 17.Markert ML, Devlin BH, Chinn IK, McCarthy EA, Li YJ. Factors affecting success of thymus transplantation for complete DiGeorge anomaly. Am J Transplant 2008;8(8):1729–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.