Abstract
In 1996, a monitoring program was initiated at the teaching hospital of Amiens, France, and carried out for 3 years. All extended-spectrum β-lactamase (ESBL)-producing Enterobacter aerogenes isolates recovered from clinical specimens were collected for investigation of their epidemiological relatedness by pulsed-field gel electrophoresis and enterobacterial repetitive intergenic consensus PCR (ERIC-PCR) and determination of the type of ESBL harbored by isoelectric focusing and DNA sequencing. Molecular typing revealed the endemic coexistence, during the first 2 years, of two clones expressing, respectively, SHV-4 and TEM-24 ESBLs, while an outbreak of the TEM-24-producing strain raged in the hospital during the third year, causing the infection or colonization of 165 patients. Furthermore, this strain was identified as the prevalent clone responsible for outbreaks in many French hospitals since 1996. This study shows that TEM-24-producing E. aerogenes is an epidemic clone that is well established in the hospital's ecology and able to spread throughout wards. The management of the outbreak at the teaching hospital of Amiens, which included the reinforcement of infection control measures, failed to obtain complete eradication of the clone, which has become an endemic pathogen.
Enterobacter aerogenes is an opportunistic pathogen. It has been associated with significant nosocomial infections, including urinary tract infections, especially in catheterized patients, respiratory tract infections, and bacteremia, particularly in elderly or debilitated patients. This species is naturally resistant to aminopenicillins and older cephalosporins due to a chromosomal cephalosporinase but remains susceptible to oxyimino cephalosporins. However, overproduction of the AmpC β-lactamase (5) or plasmid-mediated extended-spectrum β-lactamases (ESBLs) can confer resistance to extended-spectrum cephalosporins (35).
Outbreaks of multiresistant E. aerogenes infections have emerged during the past decade in many countries. They were investigated by using molecular typing methods such as pulsed-field gel electrophoresis (PFGE) (2, 15, 24, 32), random amplified polymorphic DNA analysis (4, 8, 12, 13, 23), enterobacterial repetitive intergenic consensus sequence PCR (ERIC-PCR) (8, 13, 15, 21), and ribotyping (4, 21, 23). In some studies, the β-lactam resistance was characterized, giving ESBL identification (4, 8, 11, 14, 32, 35). The outbreaks occurred in the United States (21, 31, 35), Belgium (15, 24), and Austria (2). In France, ESBL-producing E. aerogenes (ESBL-EA) has become a threat since an epidemic clone producing TEM-24 ESBL has spread to nearly all French teaching hospitals, including the hospital of Amiens (8). This prevalent clone was highly resistant to all antibiotics except gentamicin, isepamicin, imipenem, and the latest cephalosporins, such as cefepime and cefpirome. Furthermore, the emergence in France of strains resistant to all β-lactams after the use of imipenem led to a therapeutic dilemma, as no antibiotic alternatives were available (7, 8).
The emergence of ESBL-EA in the hospital of Amiens, France, was detected in 1995. The increasing number of isolates found during the following months caused us to survey the situation. Our monitoring program was initiated in October 1996 and carried out for 3 years. All strains of ESBL-EA isolated from clinical specimens were collected for determination of their epidemiological relatedness by by two molecular typing methods, ERIC-PCR and PFGE analysis. In addition, the ESBLs were characterized by the determination of their isoelectric points and by determination of the nucleotide sequences of the genes that encode them.
MATERIALS AND METHODS
Hospital presentation.
The university-affiliated hospital center of Amiens, France, is a 1,750-bed teaching hospital with mainly medical (695 beds) and surgical (450 beds) care units and several intensive care units (ICU; 80 beds). The hospital is divided into two main geographical sites (a north site and a south site). The distance between these sites is about 3.5 miles.
Data collection.
The surveillance program was initiated in October 1996 after the detection and isolation of several ESBL-EA iolates in the hospital and carried on until August 1999. We included in the analysis all clinical samples from any body site that was positive for an ESBL-EA isolate. The genus and species were determined biochemically with the API 20E (bioMérieux, Marcy l'étoile, France). On the basis of an agar disk diffusion assay (1), the strains were found to be resistant to expanded-spectrum cephalosporins, and ESBL production was detected by the double-disk synergy test (25). Duplicates isolated from the same patient were excluded. We calculated the incidence rate of hospital-acquired ESBL-EA infection or colonization as the number of newly infected or colonized patients per 1,000 patient days (PD).
Six strains of TEM-24-producing E. aerogenes belonging to the prevalent clone described by Bosi et al. (8) were kindly provided by C. Bollet (Marseille, France) to be included in PFGE and ERIC-PCR studies.
Escherichia coli XL-1 blue (Stratagene, St-Quentin-en-Yvelines, France) was used as the host for plasmid transfer experiments.
PFGE analysis.
PFGE was performed with all of the ESBL-EA strains isolated during the outbreak period. Macrorestriction analysis of chromosomal DNA was done with PFGE by published procedures with XbaI (New England Biolabs, Boston, Mass.) (32). Restriction fragments of DNA were separated by PFGE with a GenPath apparatus (Bio-Rad S.A., Ivry-sur-Seine, France). Electrophoresis was performed at 6 V/cm and 14°C. The run time was 19.7 h, with pulse times ranging from 5 to 25 s. A lambda ladder (Bio-Rad) was used for molecular size markers. The gels were stained with ethidium bromide and photographed.
ERIC-PCR analysis.
After an overnight culture at 37°C on blood sheep agar medium, the total cellular DNA of one colony was extracted by the Chelex technique (16) and the DNA concentration was determined by UV spectrophotometry. Random amplified polymorphic DNA analysis with primer ERIC-2 was performed as previously described (13). Amplified products were monitored in 1.5% agarose gels in Tris-acetate-EDTA buffer, stained with ethidium bromide, and photographed on a UV light transilluminator. Strains were considered to be different if their profiles differed by two or more bands according to previous studies (39, 44).
Plasmid DNA purification and transformation experiment.
Plasmid DNA was purified from bacterial cells by the alkaline lysis method (6) with the QIAGEN Plasmid Midi Kit (Qiagen, Courtaboeuf, France). Transformation experiments were performed as described by Sambrook et al. (40). Transformants were selected on Mueller-Hinton agar plates containing amoxicillin (50 μg/ml). This antibiotic was obtained from Sigma (Sigma-Aldrich, St-Quentin-Fallavier, France).
PCR detection of the blaTEM and blaSHV genes.
Plasmid DNAs extracted from transformant cells were used as templates in specific PCRs for the detection of the blaTEM and blaSHV genes. Primers A and B (10) were used for amplification of the blaTEM gene; primers 1 and 3 were used for amplification of the gene coding for the SHV β-lactamase (37).
A Perkin-Elmer 9600 apparatus was used, and the reactions were run under the following conditions: 30 cycles of 1 min at 95°C, 1 min at 42°C, and 1 min at 72°C and, finally, 3 min at 72°C for the blaTEM amplification and 5 min at 95°C, followed by 30 cycles of 1 min at 95°C, 1 min at 55°C, and 1 min at 72°C and, finally, 3 min at 72°C for the blaSHV amplification. The resulting PCR products were run in 1.5% agarose gels.
The PCR product was sequenced by automated fluorescent sequencing by the dye terminator method (Perkin-Elmer, Courtaboeuf, France) with oligonucleotides A, B, C, and D (10) for the blaTEM gene or with oligonucleotides 1, 3, 8, and 13 for the blaSHV gene (37).
β-Lactamase study.
Following Trypticase soy broth culture (bioMérieux), β-lactamases were extracted from bacteria by sonication. Unbroken cells and cell envelopes were removed by centrifugation. Detection of β-lactamases and determination of pIs by analytical isoelectric focusing in polyacrylamide gels (pH range, 3.5 to 9.5) were performed as reported elsewhere (29), and the β-lactamase activity was localized by the use of an iodine starch method in agar gel (27). β-Lactamases whose pIs are known (TEM-1, pI 5.4; TEM-2, pI 5.6; TEM-3, pI 6.3; TEM-24, pI 6.5) were focused in parallel with the extracts.
RESULTS
Strain collection.
During the study period, a total of 743 strains of E. aerogenes were isolated (7 to 50 strains per month). Two hundred thirty-seven clinical isolates of ESBL-EA (0 to 33 per month), each one from a different patient, were detected and collected. Among the E. aerogenes isolates, the percentage of ESBL-EA strains varied from 0 (November 1996 and January 1997) to 66 (January 1999). These strains were recovered from 103 urine cultures, 45 stool cultures, 18 surgical wounds, 15 tracheal aspirations, 14 sputum samples, 8 central venous catheters, 8 bronchial aspirations, 8 skin swabs, 7 fluid collection cultures, 6 blood cultures, 2 vaginal swabs, 1 bronchoalveolar fluid sample, 1 nasal swab, and 1 peritoneal exudate sample. All strains were fully susceptible to imipenem on the basis of the agar disk diffusion method.
PFGE pattern and ERIC-PCR analysis.
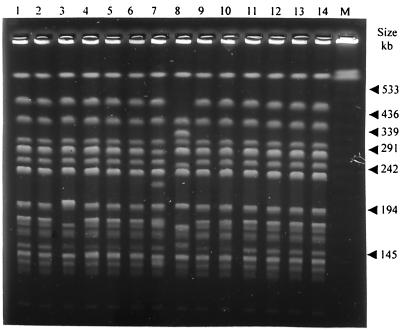
A major PFGE pattern was found in 209 isolates (0 to 33 per month). Although slight differences in the restriction patterns of some of them were found, they were considered subtypes of the epidemic clone (42). A minor PFGE pattern was also found in the analysis of 28 ESBL-EA isolates (0 to 3 per month). It differs from that of the major clone by more than seven bands. Fourteen strains belonging to the minor clone and 14 strains belonging to the major clone were selected to represent the PFGE patterns shown, respectively, in Fig. 1 and 2A. ERIC-PCR was applied to all of the ESBL-EA isolates. The results obtained with this technique were concordant with those of the PFGE analysis. Only two clones were identified among all of the ESBL-EA isolates. Representative ERIC-PCR profiles of the minor and major clones are shown, respectively, in Fig. 3 and 4A.
FIG. 1.
Representative PFGE fingerprints obtained after digestion with XbaI of 14 clinical isolates of E. aerogenes belonging to the minor clone (lanes 1 to 14). Lane M contains molecular size markers (lambda ladder).
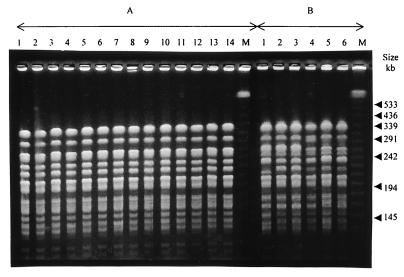
FIG. 2.
Representative PFGE fingerprints obtained after digestion with XbaI of 14 clinical isolates of E. aerogenes belonging to the major clone (A) and six strains of E. aerogenes belonging to the clone prevalent in France (B). Lanes M contain molecular size markers (lambda ladder).
FIG. 3.
Representative ERIC-PCR patterns of 14 clinical isolates of E. aerogenes belonging to the minor clone (lanes 1 to 14). Lane M contains molecular size markers (marker VI).
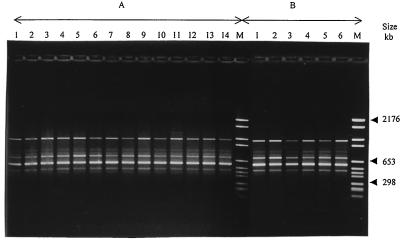
FIG. 4.
Representative ERIC-PCR patterns of 14 clinical isolates of E. aerogenes belonging to the major clone (A) and six strains of E. aerogenes belonging to the clone prevalent in France (B). Lanes M contain molecular size markers (marker VI).
Identification of ESBLs.
The plasmid contents of EAA56 and EAA89, which belong, respectively, to the major and the minor clones isolated in Amiens, were used for the transformation experiment. The transformant E. coli XLA56 expressed a single β-lactamase with an estimated pI of 6.5, in agreement with the pI of TEM-24. The PCR product of the blaTEM gene was detected. Nucleotide sequence analysis showed that it differed from the TEM-2 sequence by four substitutions leading to the amino acid replacements Glu→Lys-104, Arg→Ser-164, Ala→Thr-237, and Glu→Lys-239 (positions are numbered in accordance with the system of Ambler et al. [3]) and by one silent mutation at position 925 (A→G) (according to Sutcliffe's numbering system [41]). These substitutions are identical to those previously described for TEM-24, except for the cytidine at position 682, which is identical to the blaTEM-2 gene, instead of the silent mutation (C→T) as described previously (10).
The transformant E. coli XLA89 expressed a single β-lactamase with a pI of 7.9. The PCR product of the blaSHV gene was detected. Nucleotide sequence analysis showed that it differed from the SHV-1 sequence by three substitutions leading to the amino acid replacements Arg→Leu-205, Gly→Ser-238, and Glu→Lys-240 and by two silent mutations at positions 722 (T→C) and 796 (C→G) (positions are numbered in accordance with the coding sequence of SHV-1 [30]). These mutations are identical to those previously described for SHV-4 (22, 26), except for the silent mutations.
Evolution of ESBL-EA incidence and geographical clusters.
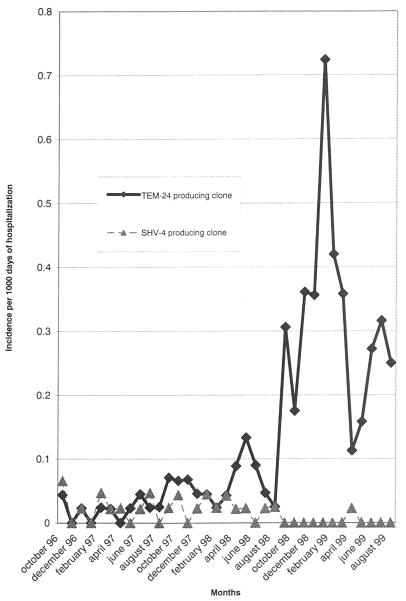
During the study period, 6,922 to 9,330 admittances per month were observed and represented 39,234 to 47,485 days of hospitalization per month. The incidence rates of the minor and major clones are presented in Table 1. The ESBL-EA incidence remained constant until August 1998 (Fig. 5). From September 1998 to January 1999, the incidence increased dramatically. This increase was exclusively due to the major clone, whereas the minor clone disappeared. The incidence reached its highest level in January 1999 (0.72/1,000 PD). From February to May 1999, the incidence decreased to the rate which had been observed in May 1998 (0.16/1,000 PD) and then increased again during the summer months.
TABLE 1.
Numbers of isolates per month and incidence rates of TEM-24- and SHV-4-producing E. aerogenes strains over the course of the study period
| Mo | No. of E. aerogenes isolates
|
Incidence/1,000 PD
|
|||
|---|---|---|---|---|---|
| Total non-ESBL and ESBL producers | TEM-24 producers | SHV-4 producers | TEM-24 | SHV-4 | |
| 10-96 | 13 | 2 | 3 | 0.044 | 0.066 |
| 11-96 | 12 | 0 | 0 | 0 | 0 |
| 12-96 | 16 | 1 | 1 | 0.023 | 0.023 |
| 01-97 | 7 | 0 | 0 | 0 | 0 |
| 02-97 | 20 | 1 | 2 | 0.024 | 0.047 |
| 03-97 | 18 | 1 | 1 | 0.022 | 0.022 |
| 04-97 | 19 | 0 | 1 | 0 | 0.023 |
| 05-97 | 9 | 1 | 0 | 0.023 | 0 |
| 06-97 | 9 | 2 | 1 | 0.045 | 0.022 |
| 07-97 | 15 | 1 | 2 | 0.024 | 0.047 |
| 08-97 | 17 | 1 | 0 | 0.025 | 0 |
| 09-97 | 15 | 3 | 1 | 0.071 | 0.024 |
| 10-97 | 14 | 3 | 2 | 0.066 | 0.044 |
| 11-97 | 21 | 3 | 0 | 0.068 | 0 |
| 12-97 | 22 | 2 | 1 | 0.046 | 0.023 |
| 01-98 | 22 | 2 | 2 | 0.045 | 0.045 |
| 02-98 | 15 | 1 | 1 | 0.024 | 0.024 |
| 03-98 | 22 | 2 | 2 | 0.043 | 0.043 |
| 04-98 | 17 | 4 | 1 | 0.089 | 0.022 |
| 05-98 | 21 | 6 | 1 | 0.133 | 0.023 |
| 06-98 | 26 | 4 | 0 | 0.090 | 0 |
| 07-98 | 16 | 2 | 1 | 0.047 | 0.023 |
| 08-98 | 15 | 1 | 1 | 0.025 | 0.025 |
| 09-98 | 26 | 13 | 0 | 0.306 | 0 |
| 10-98 | 16 | 8 | 0 | 0.175 | 0 |
| 11-98 | 29 | 16 | 0 | 0.361 | 0 |
| 12-98 | 35 | 15 | 0 | 0.356 | 0 |
| 01-99 | 50 | 33 | 0 | 0.724 | 0 |
| 02-99 | 41 | 18 | 0 | 0.420 | 0 |
| 03-99 | 24 | 17 | 0 | 0.358 | 0 |
| 04-99 | 26 | 5 | 1 | 0.113 | 0.023 |
| 05-99 | 25 | 7 | 0 | 0.158 | 0 |
| 06-99 | 33 | 12 | 0 | 0.272 | 0 |
| 07-99 | 23 | 13 | 2 | 0.316 | 0.049 |
| 08-99 | 34 | 10 | 0 | 0.250 | 0 |
FIG. 5.
Monthly evolution of the incidence of ESBL-EA producing strains isolated during the study period per 1,000 PD.
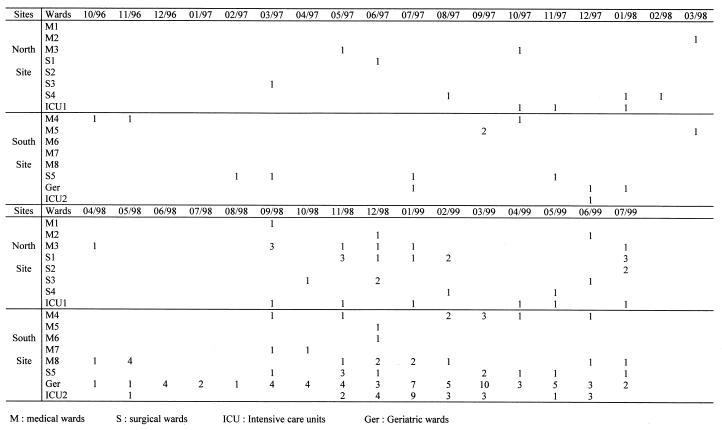
During all the entire study period, SHV-4-producing strains were recovered individually in different wards with no geographical connection. The geographical distribution of the major clone is described in Fig. 6. From October 1996 to April 1998, there was no evidence of geographical clustering. From May 1998 to August 1998, several strains of the TEM-24-producing clone were recovered in an ICU and in a geriatric ward. Since September 1998 and through the outbreak of the TEM-24-producing clone, geographical clustering between the north site (19 strains) and the south site (53 strains) was evident. Indeed, during this 5-month period, the incidence was significantly higher at the south site (0.78/1,000 versus 0.28/1,000 PD; P < 0.0001). Moreover, most of these strains were isolated in two geriatric wards (22 strains; incidence, 5.6/1,000 PD) and one medical ICU (15 strains; incidence, 16.1/1,000 PD).
FIG. 6.
Geographical distribution of the TEM-24-producing clone in the hospital wards during the study period.
Genotypic comparison of the major epidemic clone from Amiens hospital and the clone prevalent in France.
The PFGE and ERIC-PCR patterns of the six E. aerogenes strains provided by C. Bollet are presented in Fig. 2B and 4B, respectively. The TEM-24-producing E. aerogenes strains isolated at the Amiens hospital had PFGE and ERIC-PCR profiles identical to those of the clone prevalent in France that was previously described by Bosi et al. in 1999 (8).
DISCUSSION
The epidemiological situation concerning ESBL-producing enterobacteria is very dynamic and constitutes a growing worldwide problem (9, 22). The first nosocomial outbreaks caused by ESBL-producing strains occurred in 1985 in France (26, 34). Klebsiella pneumoniae was the ESBL-producing enterobacterium most frequently isolated from clinical specimens, but E. aerogenes has recently emerged as an important hospital opportunist.
The first ESBL-EA strains were isolated and characterized in 1988 at the teaching hospital of Clermont-Ferrand, France (14). It was found that the ESBL harbored by these strains was a TEM-24 enzyme. Since that time, several outbreaks have been reported. The overview of epidemiological studies suggests two opposite situations in the world: the epidemic situation that occurred in the United States (21, 31, 35) and Belgium (15, 24), characterized by sporadic outbreaks without any linkage and the French situation, characterized by the clonal dissemination of an ESBL-EA strain in nearly all of the hospitals in the country (8). A chronological review of the investigations conducted in France will give better insight into the purpose of this study. At the St-Marguerite hospital in Marseille, France, Davin-Regli et al. (13) conducted a 1-year prospective epidemiological study in 1994 and found a prevalent clone producing an ESBL among 185 clinical isolates. In 1996, Arpin et al. (4) reported an outbreak at the Pellegrin hospital in Bordeaux, France, caused by several clones of ceftazidime-resistant E. aerogenes producing a TEM-type or an SHV-type ESBL. In 1996, Neuwirth et al. (32) reported the characterization of 10 clinical isolates of E. aerogenes with the same PFGE pattern, collected during 1993 and 1994 at the Bocage hospital in Dijon, France, and producing a TEM-24 ESBL. All of these reports induced Bosi et al. to establish the prevalence of the TEM-24-producing clone in France (8); a representative selection of E. aerogenes isolates sent from 23 French hospital laboratories was analyzed. The prevalent E. aerogenes clone was isolated in all but two hospitals, confirming the hypothesis that this strain, bearing the large conjugative plasmid with ESBL and aminoglycoside resistance genes, had been transferred from one hospital to the others. The long-term clonal dissemination of TEM-24 ESBL in French hospitals was recently confirmed (19).
In our study, we used two molecular typing methods, PFGE and ERIC-PCR, already successfully used in previous studies (2, 8, 13, 15, 21, 24), to analyze all of the ESBL-EA strains isolated from clinical specimens at the Amiens teaching hospital during a 3-year study. The results provided by the two techniques were concordant for all of the strains, which indicates that ERIC-PCR is not only an easy and rapid method with which to test E. aerogenes strains but also a reproducible and discriminatory method.
From October 1996 to August 1998, two clones, producing SHV-4 and TEM-24 ESBL, respectively, were isolated with a low and constant rate of incidence. However, during the last year of the study, from September 1998 to August 1999, the incidence of the TEM-24 clone increased dramatically with the concomitant disappearance of the SHV-4 clone. The study revealed two successive periods: the endemic presence of two clones during the first 2 years and the outbreak of the TEM-24-producing clone during the last year.
This study demonstrates that the strain can be maintained over prolonged periods of time in the hospital environment and can cause clonal outbreaks which lead to the disappearance of other ESBL-EA clones. Moreover, a molecular epidemiological relationship was found between strains isolated in Amiens and strains isolated in other regions of France. These results suggest the clonal spread of the clone prevalent in France, described by Bosi et al. in 1999 (8), within our hospital. Unfortunately, it is impossible to determine when this strain appeared in the hospital because the monitoring of ESBLs started only with the collection of these isolates.
The TEM-24-producing E. aerogenes strain had probably been maintained in the environment, causing infections in predisposed patients. The evolution of the incidence and the geographical clustering of TEM-24-producing E. aerogenes isolates reveals the appearance of few strains in geriatric wards prior to the outbreak. Geriatric wards, where critically ill patients with low levels of resistance to exogenous colonization are cared for, are thought to be a reservoir for epidemic multidrug-resistant enterobacteria. Indeed, the investigation of Wiener et al. demonstrated the high prevalence of ESBL-producing enterobacteria in nursing homes (43). Patients admitted to geriatric wards require frequent care, which involves numerous interactions with staff members. The investigation of Denman et al. conducted in long-term care facilities in Maryland, where most of the patients were elderly, revealed breaches of hand-washing and glove use protocols potentially resulting in microbial transmission (17). This important prevalence of fecal microorganism carriage among elderly patients might be responsible for outbreaks within geriatric wards, as described by Jalaluddin et al. (24) and Rice et al. (38). The spread of epidemic strains from nursing homes to other units, especially surgical units and ICUs, can be suspected. ICUs, where patients have predisposing factors such as foreign devices, compromised immunity, and broad-spectrum antibiotic treatment, are considered to serve as breeding grounds for epidemic multidrug-resistant bacteria leading to outbreaks (2, 4, 12, 13, 15, 21, 23).
The predominance of TEM-24-producing E. aerogenes among all of the ESBL-EA isolates found is probably due to virulence determinants such as antibiotic resistance or surface factors involved in epithelial cell surface adherence. A 150-kb plasmid, extracted from K. pneumoniae, and encoding an SHV-4 ESBL, has been found to produce a surface protein which facilitates adhesion to intestinal cells (18), but the prevalent TEM-24 clone has not been shown to harbor such an adhesive factor. Moreover, there is no antibiotic resistance difference between the two epidemic ESBL-EA clones isolated at the Amiens hospital, unless the prevalent clone possesses a chromosomally encoded derepressed cephalosporinase (8), unlike the SHV-4 clone.
Since K. pneumoniae was the first ESBL-producing enterobacterium identified; many epidemiological studies were dedicated to producing outbreaks caused by ESBL-K. pneumoniae. Complete eradication of the smallest outbreaks was achieved, but management of large nosocomial outbreaks, by reinforcement of hygiene measures or restricted use of oxyimino β-lactams, failed to eliminate the epidemic clone (33). In our hospital, we observed the same situation regarding TEM-24-producing E. aerogenes. In February 1999, 6 months after the beginning of the outbreak, a program intended to control the diffusion of multiresistant bacteria was implemented. It was based on the barrier precautions defined by the Centers for Disease Control and Prevention (20), particularly hand disinfection (with antiseptic soaps or alcohol solutions), wearing of disposable gloves and gowns when caring for carriers, and carrier identification with a “wash your hands” sign during hospitalization and at the time of patient transfer. Other reports have described the efficacy of such a program in decreasing the incidence of multiresistant bacteria (28) such as that which we observed from February to May 1999 in our hospital. The new increase observed during the summer months can be explained by understaffing of the hospital wards, which decreases compliance with isolation precautions and increases the risk of cross-transmission (36). At the end of the study, in August 1999, the incidence had just been stabilized at 0.25/1,000 days of hospitalization, which was above the incidence recorded 3 years before in October 1996.
The increasing number of TEM 24-producing E. aerogenes outbreaks is a threat to hospital ecology in France. Our study sheds new light on the epidemic behavior of this strain described by Bosi et al. in 1999 (8). Reinforcement of infection control measures, including the use of disposable gloves and the spatial segregation of patients infected or colonized with ESBL-EA, can prevent outbreaks but fails to eliminate the presence of the endemic clone.
ACKNOWLEDGMENTS
We thank Jean-Luc Saquet for critical review of the manuscript and C. Bollet for providing six strains belonging to the prevalent clone.
REFERENCES
- 1.Acar J, Chardon H, Choutet P, Courvalin P, Dabernat H, Drugeon H, Dubreuil L, Flandrois J P, Goldstein F, Morel C, Philippon A, Rouveix B, Sirot J, Thabaut A. Statement of Antibiogram Committee of the French Society for Microbiology. Pathol Biol. 1995;43:1–8. [Google Scholar]
- 2.Allerberger F, Koeuth T, Lass-Flörl C, Dierich M P, Putensen C, Schmutzhard E, Mohsenipour I, Grundmann H, Hartung D, Bauernfeind A, Eberlein E, Lupski J R. Epidemiology of infections due to multiresistant Enterobacter aerogenes in a university hospital. Eur J Clin Microbiol Infect Dis. 1996;15:517–520. doi: 10.1007/BF01691323. [DOI] [PubMed] [Google Scholar]
- 3.Ambler R P, Coulson F W, Frere J M, Ghuysen J-M, Joris B, Forsman M, Levesque R C, Tiraby G, Waley S G. A standard numbering scheme for the class A β-lactamases. J Biochem. 1991;276:269–272. doi: 10.1042/bj2760269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arpin C, Coze C, Rogues A M, Gachie J P, Bebear C, Quentin C. Epidemiological study of an outbreak due to multidrug-resistant Enterobacter aerogenes in a medical intensive care unit. J Clin Microbiol. 1996;34:2163–2169. doi: 10.1128/jcm.34.9.2163-2169.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bennet P M, Chopra I. Molecular basis of β-lactamase induction in bacteria. Antimicrob Agents Chemother. 1993;37:153–158. doi: 10.1128/aac.37.2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Birnboim H, Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979;7:1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bornet C, Davin-Regli A, Bosi C, Pages J-M, Bollet C. Imipenem resistance of Enterobacter aerogenes mediated by outer membrane permeability. J Clin Microbiol. 2000;38:1048–1052. doi: 10.1128/jcm.38.3.1048-1052.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bosi C, Davin-Regli A, Bornet C, Mallea M, Pages J-M, Bollet C. Most Enterobacter aerogenes strains in France belong to a prevalent clone. J Clin Microbiol. 1999;37:2165–2169. doi: 10.1128/jcm.37.7.2165-2169.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burwen D R, Banerjee S N, Gaynes R P. Ceftazidime resistance among selected nosocomial gram-negative bacilli in the United States. National nosocomial infections surveillance system. J Infect Dis. 1994;170:1622–1625. doi: 10.1093/infdis/170.6.1622. [DOI] [PubMed] [Google Scholar]
- 10.Chanal C, Poupart M-C, Sirot D, Labia R, Sirot J, Cluzel R. Nucleotide sequences of CAZ-2, CAZ-6, and CAZ-7 β-lactamase genes. Antimicrob Agents Chemother. 1992;36:1817–1820. doi: 10.1128/aac.36.9.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chanal C, Sirot D, Romaszko J P, Bret L, Sirot J. Survey of prevalence of extended spectrum β-lactamases among Enterobacteriaceae. J Antimicrob Chemother. 1996;38:127–132. doi: 10.1093/jac/38.1.127. [DOI] [PubMed] [Google Scholar]
- 12.Davin-Regli A, Saux P, Bollet C, Gouin F, de Micco P. Investigation of outbreaks of Enterobacter aerogenes colonization and infection in intensive care units by random amplification of polymorphic DNA. J Med Microbiol. 1996;44:89–98. doi: 10.1099/00222615-44-2-89. [DOI] [PubMed] [Google Scholar]
- 13.Davin-Regli A, Monnet D, Saux P, Bosi C, Charrel R, Barthelemy A, Bollet C. Molecular epidemiology of Enterobacter aerogenes acquisition: one-year prospective study in two intensive care units. J Clin Microbiol. 1996;34:1474–1480. doi: 10.1128/jcm.34.6.1474-1480.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Champs C, Sirot D, Chanal C, Poupart M-C, Dumas M-P, Sirot J. Concomitant dissemination of three extended-spectrum β-lactamases among Enterobacteriaceae isolated in a French hospital. J Antimicrob Chemother. 1991;27:441–457. doi: 10.1093/jac/27.4.441. [DOI] [PubMed] [Google Scholar]
- 15.de Gheldre Y, Maes N, Rost F, de Ryck R, Clevenbergh P, Vincent J-L, Struelens M J. Molecular epidemiology of an outbreak of multidrug-resistant Enterobacter aerogenes infections and in vivo emergence of imipenem resistance. J Clin Microbiol. 1997;35:152–160. doi: 10.1128/jcm.35.1.152-160.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Lamballerie X, Zandotti C, Vignoli C, Bollet C, de Micco P. A one-step microbial DNA extraction method using “chelex 100” suitable for gene amplification. Res Microbiol. 1992;143:785–790. doi: 10.1016/0923-2508(92)90107-y. [DOI] [PubMed] [Google Scholar]
- 17.Denman S, Dwyer D M, Israel E, Vacek P. Handwashing and glove use in a long-term care facility—Maryland, 1992. Morb Mortal Wkly Rep. 1993;42:672–675. [PubMed] [Google Scholar]
- 18.di Martino P, Livrelli V, Sirot D, Joly B, Darfeuille-Michaud A. A new fimbrial antigen harbored by CAZ-5/SHV-4-producing Klebsiella pneumoniae strains involved in nosocomial infections. Infect Immunity. 1996;64:2266–2273. doi: 10.1128/iai.64.6.2266-2273.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Galdbart J-O, Lemann F, Aimouz D, Féron P, Lambert-Zechovsky N, Branger C. TEM-24 extended-spectrum β-lactamase-producing Enterobacter aerogenes: long-term clonal dissemination in French hospitals. Clin Microbiol Infect. 2000;6:316–323. doi: 10.1046/j.1469-0691.2000.00092.x. [DOI] [PubMed] [Google Scholar]
- 20.Garner J S The Hospital Infection Control Pratices Advisory Committee. Guidelines for isolation precautions in hospitals. Am J Infect Control. 1996;24:24–52. doi: 10.1016/s0196-6553(96)90050-4. [DOI] [PubMed] [Google Scholar]
- 21.Georghiou P R, Hamill R J, Wright C E, Versalovic J, Koeuth T, Watson D A, Lupski J R. Molecular epidemiology of infections due to Enterobacter aerogenes: identification of hospital outbreak-associated strains by molecular techniques. Clin Infect Dis. 1995;20:84–94. doi: 10.1093/clinids/20.1.84. [DOI] [PubMed] [Google Scholar]
- 22.Gniadkowski M, Dalucha A, Grzesiowski P, Hryniewicz W. Outbreak of ceftazidime-resistant Klebsiella pneumoniae in a pediatric hospital in Warsaw, Poland: clonal spread of the TEM-47 extended spectrum β-lactamase (ESBL)-producing strain and transfer of a plasmid carrying the SHV-5 like ESBL-encoding gene. Antimicrob Agents Chemother. 1998;42:3079–3085. doi: 10.1128/aac.42.12.3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grattard F, Pozzeto B, Tabard L, Petit M, Ros A, Gaudin O. Characterization of nosocomial strains of Enterobacter aerogenes by arbitrarily primed-PCR analysis and ribotyping. Infect Control Hosp Epidemiol. 1995;16:224–230. doi: 10.1086/647094. [DOI] [PubMed] [Google Scholar]
- 24.Jalaluddin S, Devaster J-M, Scheen R, Gerard M, Butzler J-P. Molecular epidemiological study of nosocomial Enterobacter aerogenes isolates in a Belgian hospital. J Clin Microbiol. 1998;36:1846–1852. doi: 10.1128/jcm.36.7.1846-1852.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jarlier V, Nicolas M H, Fournier G, Philippon A. Extended broad-spectrum β-lactamases conferring transferable resistance to newer β-lactam agents in Enterobacteriaceae: hospital prevalence and susceptibility patterns. Rev Infect Dis. 1988;10:867–878. doi: 10.1093/clinids/10.4.867. [DOI] [PubMed] [Google Scholar]
- 26.Kitzis M D, Billot-Klein D, Goldstein F W, Williamson R, Tran Van Nhieu G, Carlet J, Acar J F, Gutmann L. Dissemination of the novel plasmid-mediated β-lactamase CTX-1, which confers resistance to broad-spectrum cephalosporins, and its inhibition by β-lactamase inhibitors. Antimicrob Agents Chemother. 1988;32:9–14. doi: 10.1128/aac.32.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Labia R, Barthélémy M. L'enzymogramme des béta-lactamases: adaptation en gel de la méthode iodométrique. Ann Inst Pasteur Microbiol. 1979;130B:295–304. [PubMed] [Google Scholar]
- 28.Lucet J C, Decré D, Fichelle A, Joly-Guillou M L, Pernet M, Deblangy C, Kosmann J, Régnier B. Control of a prolonged outbreak of extended-spectrum beta-lactamase-producing Enterobacteriaceae in a university hospital. Clin Infect Dis. 1999;29:1411–1418. doi: 10.1086/313511. [DOI] [PubMed] [Google Scholar]
- 29.Mattew M, Harris A M, Marshall M J, Ross G W. The use of analytical isoelectric focusing for detection and identification of β-lactamases. J Gen Microbiol. 1975;88:169–178. doi: 10.1099/00221287-88-1-169. [DOI] [PubMed] [Google Scholar]
- 30.Mercier J, Levesque R C. Cloning of SHV-2, OHIO-1, and OXA-6 β-lactamases and cloning and sequencing of SHV-1 β-lactamase. Antimicrob Agents Chemother. 1990;34:1577–1583. doi: 10.1128/aac.34.8.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meyers H B, Fontanilla E, Mascola L. Risk factors for development of sepsis in a hospital outbreak of Enterobacter aerogenes. Am J Infect Control. 1988;16:118–122. doi: 10.1016/0196-6553(88)90050-8. [DOI] [PubMed] [Google Scholar]
- 32.Neuwirth C, Siebor E, Lopez J, Pechinot A, Kazmierczak A. Outbreak of TEM-24-producing Enterobacter aerogenes in an intensive care unit and dissemination of the extended-spectrum β-lactamase to other members of the family Enterobacteriaceae. J Clin Microbiol. 1996;34:76–79. doi: 10.1128/jcm.34.1.76-79.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pena C, Pujol M, Ardanuy C, Ricart A, Pallares R, Linares J, Ariza J, Gudiol F. Epidemiological and successful control of a large outbreak due to Klebsiella pneumoniae producing extended-spectrum β-lactamases. Antimicrob Agents Chemother. 1998;42:53–58. doi: 10.1128/aac.42.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Petit A, Sirot D, Chanal C M, Sirot J, Labia R, Gerbaud G, Cluzel R A. Novel plasmid-mediated β-lactamase in clinical isolates of Klebsiella pneumoniae more resistant to ceftazidime than to other broad-spectrum cephalosporins. Antimicrob Agents Chemother. 1988;32:626–630. doi: 10.1128/aac.32.5.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pitout J D D, Thomson K S, Hanson N D, Ehrardt A F, Coudron P, Sanders C C. Plasmid-mediated resistance to expanded-spectrum cephalosporins among Enterobacter aerogenes strains. Antimicrob Agents Chemother. 1998;42:596–600. doi: 10.1128/aac.42.3.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pittet D, Monrouga P, Perneger T The Members of the Infection Control Program. Compliance with handwashing in a teaching hospital. Ann Intern Med. 1999;130:126–130. doi: 10.7326/0003-4819-130-2-199901190-00006. [DOI] [PubMed] [Google Scholar]
- 37.Rasheed, Metchock J K B, Berkowitz F, Weigel L, Crellin J, Steward C, Hill B, Medeiros A A, Tenover F C. Evolution of extended-spectrum β-lactam resistance (SHV-8) in a strain of Escherichia coli during multiple episodes of bacteremia. Antimicrob Agents Chemother. 1997;41:647–653. doi: 10.1128/aac.41.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rice L B, Willey S H, Papanicolaou G A, Medeiros A A, Eliopoulos G M, Moellering R C, Jacoby G A. Outbreak of ceftazidime resistance caused by extended-spectrum β-lactamases at a Massachusetts chronic-care facility. Antimicrob Agents Chemother. 1990;34:2193–2199. doi: 10.1128/aac.34.11.2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rodriguez-Barradas M C, Hamill R J, Houston E D, Georghiou P R, Clarridge J E, Regnery R L, Koehler J E. Genomic fingerprinting of Bartonella species by repetitive element PCR for distinguishing species and isolates. J Clin Microbiol. 1995;33:1089–1093. doi: 10.1128/jcm.33.5.1089-1093.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 41.Sutcliffe J G. Nucleotide sequence of the ampicillin resistance gene of Escherichia coli plasmid pBR322. Proc Natl Acad Sci USA. 1978;74:3737–3741. doi: 10.1073/pnas.75.8.3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tenover F C, Arbeit R D, Goering R V, Mickelsen P A, Murray B E, Persing D H, Swaminathan B. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol. 1995;33:2233–2239. doi: 10.1128/jcm.33.9.2233-2239.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wiener J, Quinn J P, Bradford P A, Goering R V, Nathan C, Bush K, Weinstein R A. Multiple antibiotic-resistant Klebsiella and Escherichia coli in nursing homes. JAMA. 1999;281:517–523. doi: 10.1001/jama.281.6.517. [DOI] [PubMed] [Google Scholar]
- 44.Woods C R, Jr, Versalovic J, Koeuth T, Lupski J R. Analysis of relationships among isolates of Citrobacter diversus by using DNA fingerprints generated by repetitive sequence-based primers in the polymerase chain reaction. J Clin Microbiol. 1992;30:2921–2929. doi: 10.1128/jcm.30.11.2921-2929.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]