Abstract
Tendons are cable-like connective tissues that transfer both active and passive forces generated by skeletal muscle to bone. In the mature skeleton, the tendon-bone enthesis is an interfacial zone of transitional tissue located between two mechanically dissimilar tissues: compliant, fibrous tendon to rigid, dense mineralized bone. In this review, we focus on emerging areas in enthesis development related to its structure, function, and mechanobiology, as well as highlight established and emerging signaling pathways and physiological processes that influence the formation and adaptation of this important transitional tissue.
Keywords: tendon, enthesis, muscle loading, fibrocartilage
1. Introduction
During development, the absence or constraint of movement can lead to musculoskeletal disorders such as fracture-prone brittle bones, malformed and dysplastic joints, respiratory and neurological impairments, and life-long mobility problems (1). Our ability to move relies on the transmission of muscle forces to the skeleton in order to articulate joints and maintain stability during standing, walking, and sitting. Tendons are cable-like connective tissues that attach muscle to bone and are essential for the transmission of both active and passive muscle loads (2,3). In the mature skeleton, the tendon-bone enthesis is an interfacial zone of transitional tissue located between compliant, fibrous tendon to rigid, dense mineralized bone (4–7). This transitional tissue provides a mechanism of stress and strain reduction at the interface between two mechanically dissimilar tissues (5,8,9). The transmission of muscle loads from tendon to bone is essential for both enthesis development as well as healing. Complete removal of loading during enthesis healing, such as following rotator cuff injury, leads to impaired mechanical integrity following surgical repair (10) and further exacerbates poor healing outcomes following chronic injury (10–14).
Paleoanthropological studies have often inferred the mechanically-dependent adaptation of fibrocartilaginous entheses to describe occupational histories of skeletons (15). This is often evaluated from skeletal remains by examining bony structural features such as ridges, tubercles, and tuberosities (i.e., protuberances on the periosteal surface of bone). However, a direct link between bone shape and loading histories especially in paleoanthropological archives is complicated and difficult to clearly define (16,17), and the ability of the mature enthesis to adapt to mechanical loads remains contested (18–20). Nonetheless, the enthesis is at risk of overuse injuries and pathology, clinically referred to as enthesopathies, and such injuries can affect adolescent and adult patients alike. In adolescents, sports-related injuries such as Sever and Osgood-Schlatter diseases are common clinical pathologies affecting the apophysis of bone at sites of tendon-bone entheses in children between the ages of 8–15 years (21). In this review, we focus on emerging areas in enthesis development related to its structure, function, and mechanobiology, as well as highlight known and emerging signaling pathways that contribute to the formation of this important transitional tissue.
2. Structure, function, and development of the enthesis
Tendon-bone entheses are positioned on the periosteal surface of bone and are typically found at sites of “superstructure” ridges, known as tuberosities and tubercles. These superstructures give bones their three-dimensional shape (22–24). The microscopic structure of the enthesis varies depending on its anatomical location and mechanical demands and is characterized as either fibrous (i.e., periosteal, bony) or fibrocartilaginous (6). Fibrous entheses are generally found at insertion sites of stabilizing tendons, whereas fibrocartilaginous entheses are typically found at insertions of tendons that contribute to joint movement. Fibrous enthesis attach directly to bone and typically form Sharpey’s fibers, which are perforating fibers that embed into bone’s periosteal surface (25). Fibrocartilaginous entheses consist of four distinct histological zones, including aligned tendon, unmineralized fibrocartilage, mineralized fibrocartilage, and subchondral bone. A smooth and uniform basophilic tidemark distinguishes the transition between the two fibrocartilaginous zones, and this tidemark is disrupted and irregular in enthesopathy. The fibrocartilage enthesis matures during postnatal growth in response to mechanical loads from skeletal muscle and consists of cells that express both tenogenic and chondrogenic factors (24,26–29)(Figure 1).
Figure 1.
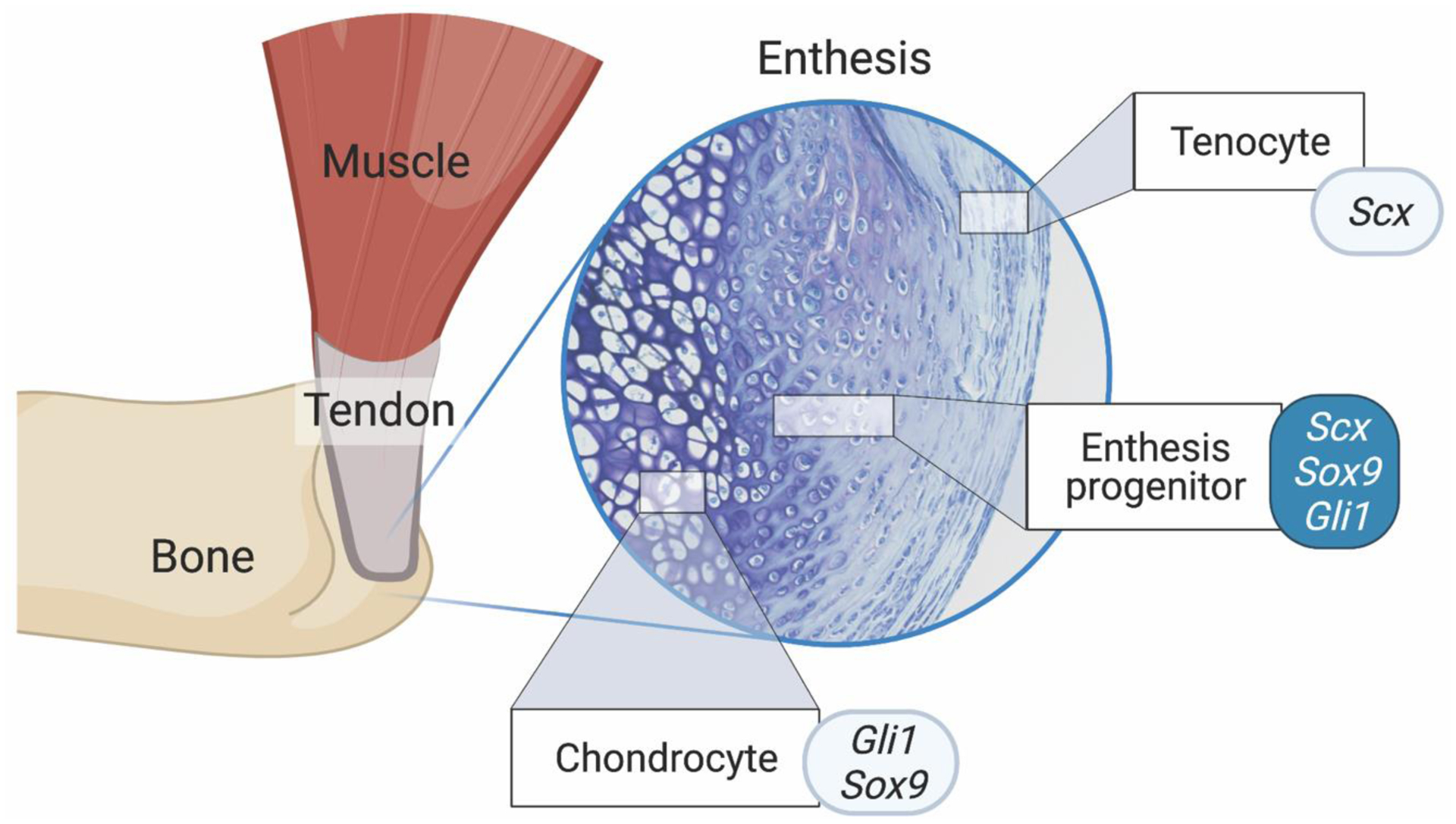
The enthesis is a transitionally graded tissue positioned between bone and tendon. The primordial enthesis develops from bi-fated progenitor cells expressing chondrogenic and tenogenic factors (i.e., Scx, Sox9, and Gli1). Created with BioRender.com.
Recent work has defined a general mechanism of isometric scaling (i.e., proportional growth of superstructure size relative to bone size) that minimizes cumulative superstructure drift along the length of bones (22). Superstructures form modularly from a distinct pool of cells that express both Scleraxis (Scx) and Sox-9, and these superstructures are reliable phenotypic readouts of enthesis development and muscle loading during embryonic and postnatal growth in the vertebrate limb (Figure 2) (23,27). The global patterning of superstructures is regulated by numerous factors, including the GLI-Krüppel family member 3 (Gli3), transforming growth factor Tgfb, bone morphogenetic protein (BMP4), and pre-B cell leukemia transcription factor (e.g., Pbx1) (23,24). Superstructure progenitors differentiate into either chondrocytes (on the cartilage side) or fibroblasts (on the tendon side) (24,27,30), and this bi-fated cell mixture is regulated by Krüppel-like (KLF) transcription factors (26). Depending on the type of enthesis (i.e., migratory or stationary), these progenitor populations are either replaced by or differentiate into Gli1+ cells that eventually become the enthesis (30,31). In the limb, the unique molecular signature of the enthesis depends on its anatomical positioning (i.e., at the epiphysis, periosteal surface, or attached to superstructures (6,24,32,33)). The Sox9+/Scx+ cells of the enthesis, sandwiched between chondrocytes and tendon fibroblasts, have recently been identified as “bi-fated,” as these cells express a mixed transcriptome of both chondrogenic and tenogenic genes (Figure 1) (26). This shared transcriptome between two otherwise distinct cell types may suggest that enthesis progenitors share regulatory elements with both chondrocytes and tendon fibroblasts, and these shared regulatory elements (e.g., KLF) act as enhancers to drive expression in enthesis cells as well as in adjacent cartilage (e.g., Col11a1 associated elements) and tendon resident cells (e.g., Col1a1 associated elements) (26).
Figure 2.
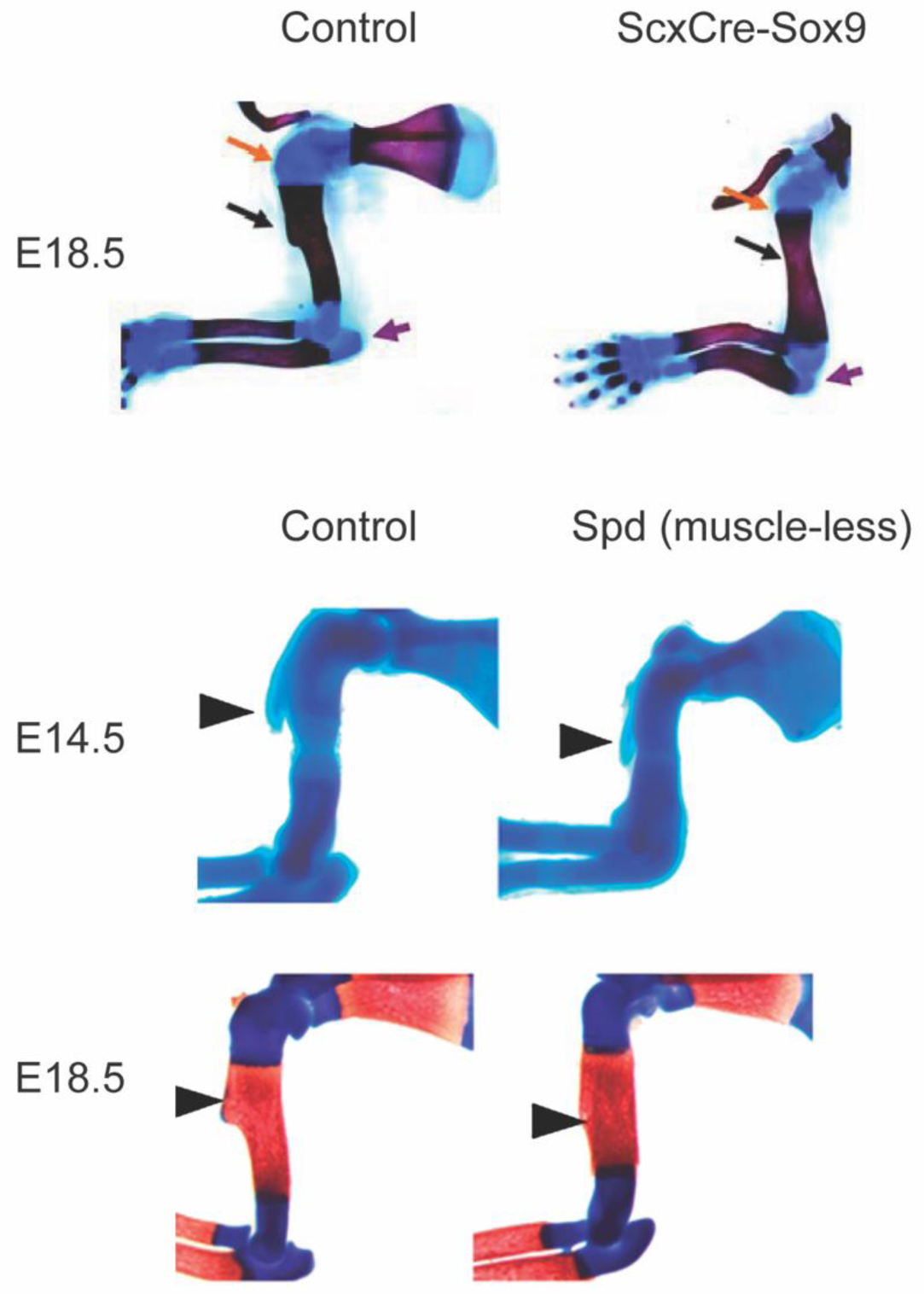
The deltoid tuberosity is a superstructure on the humerus and is a reliable readout of tendon and enthesis development as well as muscle loading in the embryonic limb. In ScxCre-Sox9 mutant mouse embryos (top row), the deltoid tuberosity (black arrow) and triceps insertion (purple arrow) fail to initiate formation. In muscleless mouse embryos, the formation of the deltoid tuberosity is initiated at embryonic day (E) 14.5; however, without muscle contraction, the tuberosity is not maintained by E18.5. Images modified with permission from (23,27).
The mechanoadaptive nature of the developing limb has been studied for decades using muscular dysgenesis models in mice. During limb development, tendon forms as an extension of the cartilage template and is later loaded by striated muscle. The formation of tendon is predominantly muscle-independent, with few exceptions (34). However, the segregation, elongation, and maintenance of tendon typically depends on applied loads upon the migration of muscle into the limb bud from the dermomyotome (23,34–37). Loss of muscle loading during embryonic growth impacts the growth but not the initiation of bone ridge formation, suggesting that the primordial superstructure emerges prior to the attachment and contraction of muscle (Figure 2) (27). These superstructures are important for skeletal function because they are three-dimensional bony structures that provide mechanical leverage to muscle for efficient movement of articular joints and also provide tendon with a stable anchorage site to bone (23). The emergence of superstructures, where entheses attach, occurs prior to muscle migration into the limb bud and is dependent on expression of the basic helix-loop-helix transcription factor, Scleraxis and Sox9 (Figure 2) (38). The maintenance of superstructures during embryonic growth relies on skeletal muscle contraction (Figure 2) (23). This has also been demonstrated during postnatal growth, and the structure and mineralization of the tendon-bone enthesis depends on muscle loading for interface maturity (28,29,39). The mineralization patterns and multi-scale structure of the enthesis have been well described in recent experimental and computational research (4,5,8,40,41). In the absence of postnatal muscle loading, fewer Gli1+ cells populate the tendon-bone interface (30) and the enthesis is less mineralized and mechanically weaker (28,39). Conversely, in some models of muscle hypertrophy (e.g., myostatin−/− mice), tuberosities are enlarged in the postnatal skeleton (42). Although technically challenging, new approaches to spatially and temporally control muscle contraction in vivo, such as use of light-activated muscle contraction using optogenetic stimulation, offer promise for use in mechanistic studies to improve our understanding of the mechano-adaptive response of the enthesis during postnatal growth (43–45). Because each enthesis is uniquely loaded depending on its anatomical location and mechanical demands, there exists a broad diversity of enthesis structure and size. For example, Felsenthal et al. recently identified divergent cell programming in migratory and stationary entheses that depends on the dynamic maintenance and replacement of Sox9+ and Gli1+ cells during postnatal growth (Figure 3) (31). Thus, developing a better understanding of enthesis diversity and mechanoadaptation is needed.
Figure 3.
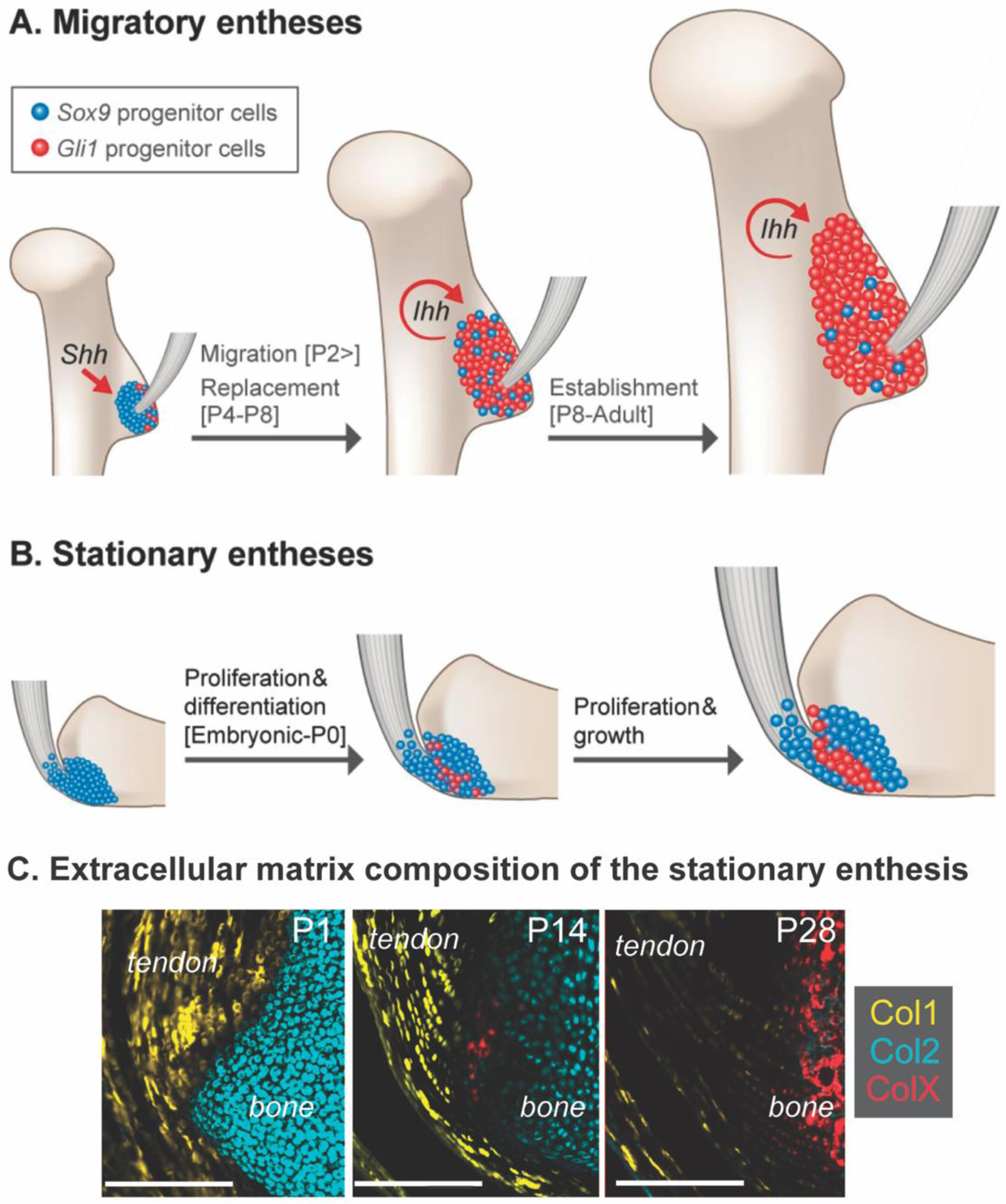
The establishment of the postnatal enthesis depends on either (A) migratory (e.g., replacement) or (B) stationary (e.g., maintenance and differentiation) of the progenitor cells at the interface between tendon and bone. (C) Enthesis maturation results in dynamic remodeling of collagen-rich extracellular matrix, with predominantly collagen type I (Col1) in tendon, collagen type II (Col2) in the cartilage template, and deposition of collagen type X at the enthesis and in the secondary ossification center. Timelines shown are representative of mouse enthesis development; P = postnatal day. Modified from (31,46)
3. Signaling pathways regulating enthesis formation and remodeling
The development of tendon and maturation of the enthesis plays a crucial role in joint shape and alignment (7,23,29,39). Tendon-bone entheses and long bone growth plates develop with overlapping and divergent cell behaviors. Most long bones develop via endochondral ossification, a process of mesenchymal differentiation and sequential replacement of cartilage with bone through expansion of growth plates. This process is mirrored in the tendon-bone enthesis, with establishment of chondrogenic cells (e.g., Sox9+) and sequential replacement or differentiation into fibrocartilage cells (e.g., Gli1+) (31). Morphologically, the development of the enthesis has been likened to a “miniature” or arrested growth plate (6,8,30,47). However, unlike growth plates in long bones which eventually fuse at skeletal maturity, the fibrocartilage of the enthesis retains the morphological features of fibrocartilage and maintain Gli1+ cells at the interface throughout postnatal growth (30).
A major challenge of studying enthesis progenitors at the transcriptional or epigenetic level is the limited number of cells that reside within a matrix dense tissue, which makes isolation for high-quality sequencing approaches difficult. Additionally, there does not yet exist an exclusive marker for enthesis progenitors that can be exclusively used for enthesis lineage tracing and targeting with Cre-lox strains in mice (Table 1). Kult et al. recently generated a compound mouse line with transgenes for Sox9-CreER, Scx-GFP, and tdTomato in an effort to sort enthesis progenitors using fluorescence-activated cell sorting (26). This approach, coupled with advancements in single-cell RNA sequencing, established a useful method to identify enthesis-specific promoters that do not overlap with adjacent limb tissues. Additionally, the use of high-precision microdissection approaches to isolate region-specific cells, such as laser-capture microdissection microscopy (29,30,48), have also shown promise, and improvements in RNA preservation prior to laser-capture methods could further improve RNA quality and rigorous downstream molecular analyses. Ideally, the identification of promoter(s) that exclusively target enthesis progenitors would allow for mechanistic studies using Cre-lox; however, there does not yet exist an “enthesis-specific” Cre strain that does not significantly overlap with other tissues (Table 1). For example, although used extensively for enthesis-related investigations, both Scx-Cre and Sox9-Cre lineages are also localized to tendon, cartilage, and/or perichondrium, as well as organs such as the kidney, brain, and lungs. Additionally, depending on the timing of induction, Gli1-CreERT2 targets a broad range of tissues with hedgehog-responsive cells including the growth plate of long bones, mesothelium, kidney, spinal cord, forebrain, and vasculature. This known and significant overlap in lineage specificity with other tissues is a challenge for enthesis-related research. Therefore, new discoveries in the identification of novel promoters that could be used for Cre-lox or CRISPR-based recombination have the potential to advance the field. For example, the development of paired recombination and inversion strategies using Cre-lox and FLP-FRT to exclusive target Sox9/Scx co-expressing cells and not Sox9- or Scx-only cells could strengthen enthesis-only specification and could improve robustness of targeting only entheseal progenitors instead of flanking tissues.
Table 1.
Previously reported Cre strains used for targeting the tendon-bone enthesis in mice.
| Cre | Enthesis prevalence | Inducible | Overlapping expression with other tissues | References |
|---|---|---|---|---|
| Constitutive Cre strains | ||||
| Scx-Cre | High | No | Yes; tendon, ligament, periosteum, trabecular bone, kidney, lung, brain, endothelial cells | (23,27,30,47,49–54) |
| Prrx1-Cre | High, for appendicular skeleton | No | Yes; all lateral plate mesoderm-derived tissues | (7,24,29,55,56) |
| Wnt1-Cre | High, for neural crest cells only | No | Yes; midbrain, dorsal spinal cord | (57) |
| Gdf5-Cre | High, for intra-articular attachments | No | Yes; articular joint tissues including ligaments, cartilage, meniscus, tendons | (46) |
| Inducible Cre strains | ||||
| Gli1-CreERT | High | Yes, tamoxifen (postnatal) | Yes; broad expression in other tissues during embryogenesis, including growth plate, mesothelium, kidney, neural stem cells, alveoli, hair follicles, heart, and vascular smooth muscle cells. | (30,31) |
| Sox9-CreERT | High, for stationary attachments | Yes, tamoxifen (embryonic) | Yes; cartilage/tendon as well as brain, lung, heart, pancreas and kidney. Sox9+ cells labeled during embryonic development are replaced by Gli1+ cells. | (24,31,58) |
| Gdf5-CreER | Moderate to low | Yes, tamoxifen (embryonic; E11.5–12.5) | Yes; proximal chondrocytes, ligaments | (59) |
| Col2-CreERT | Low or none | Yes, tamoxifen (early postnatal; <P14) | Yes; secondary ossification center, articular cartilage | (30) |
| Scx-CreERT2 | Low or none | Yes, tamoxifen | Does not label embryonic tendons or the postnatal enthesis | (60–62) |
| Cre strains (potential for enthesis targeting) | ||||
| Prrx1-CreERT2 | Not reported | Yes, tamoxifen | Yes; periosteum | (63) |
| Fgf18-CreERT2 | Not reported | Yes, tamoxifen | Yes; lung, limb bud, palate, skeleton, central nervous system, and hair follicle | (64) |
| Col1a1-CreER | Not reported | Yes, tamoxifen | Yes; osteoblasts, odontoblasts, some tendons | (65) |
| Postn-Cre | Not reported but likely | No | Yes; myofibroblasts, tendon | (66,67) |
In spite of these technical challenges related to enthesis specificity, much of what we know and understand of enthesis development has been discovered using transgenic mouse lines for the controlled specification, expression, and deletion of specific genes. Phenotypic readouts, such as the size and shape of tuberosities, provide a first-pass assessment of enthesis-related changes. The gradient morphology, mineralization, and mechanical strength of the enthesis are also important readouts for assessing the contributions of specific genes to enthesis development in transgenic strains. Some emerging pathways in enthesis development, described below, could provide insight into mechanisms of enthesis degeneration. The use of mouse and other vertebrate models (e.g., zebrafish) provide tools to mechanistically test biological pathways of enthesis development which is also critical for identifying potential regenerative strategies following injury.
3.1. TGF-β and BMP signaling
The transforming growth factor-β (TGF-β) superfamily includes a family of proteins, such as TGF-βs (TGF-β1 and TGF-β3) and bone morphogenetic proteins (BMPs, e.g., BMP2 and BMP4). TGF-β signaling is a critical pathway for joint and tendon development (60,61,68–71). The recruitment and maintenance of differentiated tendon cells is regulated by TGF-β and its receptors, including TGF-β2, TGF-β3, and TGFβR2 (60,69). Additionally, deletion of either TGFβR2 in the limb bud or BMP4 in tendon in mice leads to a complete loss of the deltoid tuberosity, suggesting it also regulates enthesis and superstructure specification (23,27,69,72). The requirement of TGFβR2 for tuberosity growth likely depends on tendon (e.g., ScxCre) but not cartilage (e.g., Col2aCre). Canonical TGFβ signaling involves ligand binding via receptors on the cell surface followed by translocation to the nucleus via the cytoplasm. These ligands may be derived from or diffuse into the tendon and enthesis from surrounding muscle, and the secretion of TGFβ is thought to be, in part, mechanically mediated (73). TGFβRs plays a role in musculoskeletal tissue crosstalk and has the potential to promote tendon regeneration (74,75). Downstream, the TGFβ and BMP subfamilies canonically signal via Smads; specifically, TGFβ signals via Smad 2/3, and BMP signals via Smads 1/5/8 (76). Recent work by Schlesinger et al. showed that loss of Smad4 leads to thinner tendons and induction of joint contracture, suggesting an essential role of BMP signaling during tendon growth (52).
The TGFβ superfamily also signals via non-Smad pathways such as MAPK and NF-κB (77). While the link has yet to be fully established in the tendon-bone enthesis, recent work by Abraham et al. showed that targeting of the NF-κB pathway via IKKβ can dramatically influence enthesis maturation as well as its ability to repair following injury (50). This work and that of others supports the need for further investigations related to crosstalk between immunomodulation and tendon-bone enthesis development.
3.2. Fibroblast growth factor (FGF) signaling
FGF ligands are secreted signaling proteins that bind to and activate a family of high affinity protein tyrosine kinase receptors (FGF receptors, FGFRs) (78). Most bones grow via endochondral ossification, a process of mesenchymal differentiation and sequential replacement of cartilage with bone through growth plates. Several key steps of endochondral ossification are dependent on activation and repression of FGFR (79) and FGF ligands such as FGF2, FGF9, and FGF18 (80–82). FGFR activation elicits a wide breadth of cellular processes and is especially important during bone development. In the growth plate of long bones, resting zone chondrocytes express low levels of Fgfr2, proliferating and pre-hypertrophic cells express high levels of Fgfr3, and hypertrophic chondrocytes express high levels of Fgfr1 (78). FGFR3 signaling in growth plate chondrocytes is especially critical for regulating bone growth and activating mutations of FGFR3 result in decreased chondrocyte hypertrophy and proliferation (79,83,84). Developing bone has distinct patterns of the FGF9 and FGF receptor expression in both intramembranous and endochondral bone formation (78). Endochondral bone development relies on the expansion of the embryonic and postnatal growth plate, which is regulated by both FGFR1 and FGFR3, whereas intramembranous formation occurs in the absence of FGFR3 and depends on FGFR1. The patterns of FGF ligands and receptors in developing bone are well established; however, the patterns of FGF signaling in the developing enthesis have only recently been elucidated (57,85,86). In the developing mouse patellar tendon, Fgfr1 is highly expressed in the tendon mid-substance and tibial insertion (85). Liang et al. showed, in mice, the mature enthesis fibrocartilage expresses Fgfr3 and Klotho, but not Fgfr1 (86), which may underly its mineralized expansion and susceptibility to enthesopathy.
As previously mentioned in this review, the tendon-bone enthesis forms as an arrested growth plate with an endochondral-like zone. However, the developing enthesis differs from the growth plate during endochondral ossification in several ways. For one, the resident progenitor pool of the enthesis remains static even into postnatal maturity, whereas the expansion of the growth plate dynamically remodels and is replaced with trabecular bone throughout longitudinal bone growth. Additionally, the developing enthesis lacks a resting zone of proliferating chondrocytes, which is a critical regulator of growth plate expansion; instead, the enthesis is constrained by tendon fibroblasts. Tendon extends via cell proliferation and differentiation in the tendon anlage and then elongates following recruitment of mesenchymal progenitors (35). The enthesis relies on matrix synthesis from both fibroblasts (e.g., Scx+ cells), chondrocytes (e.g., Sox9+ cells), and fibrochondrocytes (e.g., Gli1+ cells) (Figure 1) (23,24,27,30,31). This cell fate is unique to the enthesis and strikingly different than the growth plate. Recent work studying enthesis development in the mouse mandible showed the cell fate of enthesis progenitors, specifically Scx+ cells, is regulated by FGF signaling via Fgfr2-Fgf2 signaling (57). Although the formation of the craniofacial bones and limb bones have divergent cell origins (cranial neural crest (87) vs. lateral plate mesoderm (88), respectively) and undergo different patterns of bone formation (intramembranous vs. endochondral, respectively), these findings suggest a potent role of FGF signaling during enthesis development.
3.3. Hedgehog signaling and cilia
Mineralization processes during endochondral bone formation are regulated by hedgehog (Hh) signaling (89,90). Chondrocyte maturation in the growth plate is regulated by Indian Hh, expressed by pre-hypertrophic and hypertrophic chondrocytes, via a negative-feedback loop with parathyroid hormone-related protein (PTHrP). Indian Hh induces PTHrP expression in chondrocytes further away from the growth plate in the periarticular region, which suppresses chondrocyte maturation. This process is recapitulated in the postnatal enthesis (30,31,46). Phenotypic characteristics of ScxCre-Pthrp mutant mice have enlarged superstructures at sites of fibrous entheses (47), and unloading of entheses results in reduced expression of PTHrP (91). Conversely, enthesis unloading leads to increased Gli1 expression (30,49) and ablation of Gli1+ cells as well as Hh signaling in enthesis progenitors results in a nearly complete loss of the mineralized fibrocartilage zone (30).
In vertebrates, Hh signaling relies on bidirectional intraflagellar transport (IFT) of proteins in cilia (92,93). Localization of IFT88 in the postnatal enthesis has been correlated with Gli1+ cells and tendon unloading leads to increased expression of primary cilia genes (49). Additionally, loss of cilia motor proteins such as Kif3a leads to formation of synchondroses (94), which resemble entheseal fibrocartilage. In the postnatal growth plate, primary cilia are important in Hh signaling and are required for Hh activation as well as the proteolytic processing of Gli3 to either an activator or repressor form (95). Specifically, in the absence of Hh, Gli3 is proteolytically cleaved into a short form with repressor activity (96). However, in the presence of Hh, Gli3 cleavage is inhibited and it then acts as a transcriptional activator (97). The overlap and divergent behavior of cilia and ciliary Hh signaling is an unexplored area in enthesis development that warrants further investigation.
4. Physiology and pathophysiology of the enthesis
4.1. Extracellular matrix (ECM)
The structure and function of the enthesis relies on the establishment and remodeling of its ECM. The primordial matrix of the enthesis is predominantly collagen and includes types I, III, VI, IX, and XI collagen (98). As it matures, the ECM of the enthesis undergoes a dynamic remodeling for spatially segregation of regions that richly express and deposit type I, II, and X collagens (Figure 3) (31,46,99). ECM markers of the developing enthesis include sustained expression of Col12 and Tnc, as well as Bgn during its postnatal development (31). Indeed, the enthesis is rich in hyalectans (chondroitin sulfate proteoglycans, specifically aggrecan and versican) (4,6) and small leucine-rich proteoglycans (e.g., chondroadherin and biglycan, which control fibril size and interaction with collagen) (4,26,31,85,100). These recent studies using proteomic analyses have helped better describe the ECM patterns of the enthesis, yet the dynamics and remodeling throughout growth are still unknown.
Tendon and enthesis regeneration is challenging in adult mice, however zebrafish are capable of fully regenerating tendons and this process is regulated by BMP signaling (74). In zebrafish, muscle and cartilage connective tissues may also contain signaling cues such as BMPs that, after cell ablation in these tissues, can promote directional cell recruitment (74). That ECM can be exploited for tissue regeneration is not new (101–103), yet what the composition of the matrix should be still remains unclear. Some processes of enthesis development have been mimicked in engineered strategies of enthesis regeneration, including the use of gradients in mineralization, alignment, and stiffness (104–106). Parallels in matrix remodeling during enthesis development may also be elucidated using models of scarless wound healing and tissue regeneration. During wound healing in other tissues, such as muscle and bone, the provisional matrix functions to promote proliferation and matrix deposition by migrating fibroblastic cells (102,107). This matrix-guided development has been primarily studied in organisms capable of regenerating limbs following amputation, such as in amphibians (newts, axolotl, and Xenopus), as well as in tissues that can undergo regeneration following injury, such as mouse skin (108–114). Hyaluronic acid and sulfated proteoglycans (e.g., heparan sulfate) are two key ECM components that contribute to the “pattern following” and “pattern forming” processes, respectively, of positional biochemical properties in developing and regenerating tissues. The “pattern following” cells (in newts) rely on positional information (e.g., retinoic acid (110) and hyaluronic acid (108)) in order to migrate towards or within a transitional ECM, whereas the “pattern forming” cells are responsible for synthesizing this transitional matrix (115). In axolotl, “pattern forming” cells deposit heparan sulfate proteoglycans in order to control growth factor signaling, including FGF and BMP signaling (109). The discovery of enthesis-specific ECM turnover and dynamics is likely with use of innovative techniques to visualize ECM composition and proteolysis, such as non-canonical amino acid labeling (116–118) and damaged collagen hybridization (119,120), respectively.
4.2. Enthesopathies
The presentation of inflammation, damage, and outgrowth of the mature enthesis is a clinical problem resulting in pain and dysfunction that is challenging to treat (32,121). In children, apophyseal injuries are associated with increased loading at the enthesis and can lead to painful disorders such as Sever disease, Osgood-Schlatter syndrome, and Little Leaguer’s elbow (122). Many of these conditions, especially for pediatric patients, are treated non-surgically, primarily with rest and stretching (123). Arthritic conditions, such as diffuse idiopathic spinal hyperostosis (DISH) and spondylosis, are linked to mechanical loading as well as metabolic dysfunction (124,125). Enthesopathy can be initiated by loading-induced microdamage, such as in tennis elbow and Achilles insertional tendinopathy (2,126). Degeneration and inflammation likely contribute to unresolved enthesopathy and related joint diseases, such as osteoarthritis, tendinopathy, and rotator cuff disease (50,127–129). Hallmark characteristics of unresolved tendinopathy and enthesopathy include neovascularization and increased innervation of the tendon and enthesis (130), yet the healthy enthesis is not well vascularized or innervated (131,132). Rheumatological conditions, such as fibromyalgia and psoriasis, can also manifest entheseal changes and damage which are likely not purely mechanically derived. X-linked hypophospathemia (XLH) is also a risk factor for enthesopathy, which manifests in pervasive osteophyte formation in fibrocartilaginous entheses (86). This disease, which effects mineralization of the enthesis, has been studied using Hyp mice, a model of the XLH mutation that mimics the human syndrome (including via hypophosphatemia and elevated circulating FGF-23) (86).
4.3. Future directions in enthesis research
In adult tendon, hypervascularity induces a “state-switch” that likely leads to advancement in pathology and matrix degradation (133). Yet we do not fully understand if and how the tendon-bone enthesis, and tendon more generally, is vascularized during its development or how tendon and enthesis vascularity influences its ability to heal following injury. Recent work using in vitro models of mechanical-stress deprivation models of tendon fascicles has shown that mimicking a pathophysiological environment under normal oxygen tension culture conditions can lead to pathophysiological processes associated with increased oxidative stress (e.g., activation of hypoxia-inducible factor (HIF-1) and NADPH oxidase, which produce reactive oxygen species in response to hyperoxia) (133). In vitro, low oxygen conditions (i.e., hypoxia) can promote tendon cell differentiation and maturation and limit tendon fascicle contraction (133–135). It is likely that the standard culture conditions of normoxia are in fact mimicking a pathological condition for tendon. Hypoxia can also induce a phenotypic switch from tenocyte to fibrochondrocyte in vitro which may depend on Rho/Rac GTPase signaling (136), a well-established pathway controlling cellular mechanosensing (137). The ability of a cell to respond to hypoxic stress can lead to depletion of ATP in cells (138) and Rac1 activation (139), further influencing its ability to adhere to substrates, migrate, and undergo gene transcription. However, if and how oxygenation and vascularization contribute to the development of the tendon-bone enthesis remains unexplored.
The environment of the mammalian embryo is predominantly hypoxic prior to the establishment of the cardiovascular system and availability of oxygen (140). Cells are able to survive hypoxic stress depending on the stability of HIF1α (141). Additionally, when Hif1α is positively regulated, oxygen consumption and cell proliferation are tamped down while collagen synthesis is elevated even in spite of low oxygen availability (141–143). Several elegant studies have demonstrated that the fetal growth plate maintains an oxygen gradient and growth plate development relies on expression of the transcription factor HIF-1α (140–142,144). In the hypoxic growth plate, increased levels of Hif1α also lead to decreased mitochondrial respiration and oxygen consumption, ultimately promoting cell survival (145). Like the fetal growth plate, the enthesis is an avascular tissue (131). Thus, the existence of an oxygen gradient during enthesis development may be essential for collagen synthesis and metabolic demands and reprogramming.
New directions in the field related to the metabolic bioenergetics during tendon and enthesis development and adaptation are ripe for exploration, especially focused on glycolysis, lactate production, and oxidative phosphorylation. For example, the role of hypoxia and stability of Hif1α during tendon and enthesis development is unclear, as is the function of mitochondria during formation of these ECM-dense tissues. Additionally, the ability of enthesis progenitors to balance energy demands and regulate autophagy during its rapid and expansive growth remains unexplored.
5. Conclusion
The enthesis is an interfacial collagen-rich tissue essential for the joint motion and stability and functions as a stress-reducer between tendon and bone. Its development in the vertebrate skeleton is complex and mechano-adaptive, and recent discoveries have identified a unique pool of mixed tenogenic and chondrogenic cells that form and maintain this unique tissue. These bi-fated cells between tendon and bone have overlapping and divergent characteristics to an arrested growth plate, including TGF-β/BMP, FGF, and Hedgehog signaling. Future research will be required to more clearly understand the physiology of the enthesis, including identifying cellular patterns and ECM composition during the dynamic postnatal remodeling process and also elucidating the role of hypoxia and cellular metabolism during enthesis development and pathogenesis.
Highlights:
The enthesis is an interfacial zone of transitional tissue located between tendon to bone.
Entheses are found on the periosteal surface of bone on ridges, tuberosities, and tubercles.
Progenitor enthesis cells are “bi-fated” and express factors of both chondrocytes and tenocytes.
Factors that promote enthesis development include mechanical loading, TGF-beta, FGF signaling, and hedgehog signaling.
Extracellular matrix remodeling, hypoxia, and metabolism are emerging mediators of enthesis development.
Funding and Acknowledgements:
Work in the Killian Laboratory focused on enthesis development and mechanical adaptation is supported by the National Institutes of Health (R03 HD094594) and the National Science Foundation (CAREER 1944448). We also benefit from core services provided by the Musculoskeletal Health Core Center (MiMHC) at the University of Michigan at Ann Arbor (NIH P30 AR069620). Thanks to members of the Killian Laboratory past and present, especially Elahe Ganji, Connor Leek, Syeda Lamia, and LeeAnn Flowers, for thoughtful scientific discussions on the musculoskeleton’s most interesting tissue.
Abbreviations:
- Col
Collagen
- BMP
Bone morphogenetic protein
- FGF
Fibroblast growth factor
- FGFR
Fibroblast growth factor receptor
- Gli
GLI-Kruppel family member
- Hh
Hedgehog
- IFT
Intraflagellar transport protein
- PTHrP
Parathyroid hormone related protein
- Scx
Scleraxis
- Sox9
SRY-Box Transcription factor 9
- Spd
Splotched-delayed; muscle-less
- TGFβ
Transforming growth factor-beta
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Felsenthal N, Zelzer E. Mechanical regulation of musculoskeletal system development. Development. 2017. Dec 1;144(23):4271–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benjamin M, Toumi H, Ralphs JR, Bydder G, Best TM, Milz S. Where tendons and ligaments meet bone: attachment sites (‘entheses’) in relation to exercise and/or mechanical load. Journal of Anatomy. 2006. Apr;208(4):471–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zitnay JL, Weiss JA. Load transfer, damage, and failure in ligaments and tendons: DAMAGE IN LIGAMENTS AND TENDONS. J Orthop Res. 2018. Dec;36(12):3093–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rossetti L, Kuntz LA, Kunold E, Schock J, Müller KW, Grabmayr H, et al. The microstructure and micromechanics of the tendon–bone insertion. Nature Materials. 2017. Jun;16(6):664–70. [DOI] [PubMed] [Google Scholar]
- 5.Genin GM, Kent A, Birman V, Wopenka B, Pasteris JD, Marquez PJ, et al. Functional Grading of Mineral and Collagen in the Attachment of Tendon to Bone. Biophysical Journal. 2009. Aug;97(4):976–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benjamin M, Kumai T, Milz S, Boszczyk BM, Boszczyk AA, Ralphs JR. The skeletal attachment of tendons—tendon ‘entheses.’ Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology. 2002. Dec;133(4):931–45. [DOI] [PubMed] [Google Scholar]
- 7.Zelzer E, Blitz E, Killian ML, Thomopoulos S. Tendon-to-bone attachment: From development to maturity. Birth Defects Research Part C: Embryo Today: Reviews. 2014. Mar;102(1):101–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomopoulos S, Genin GM, Galatz LM. The development and morphogenesis of the tendon-to-bone insertion What development can teach us about healing. Journal of musculoskeletal & neuronal interactions. 2010;10(1):35. [PMC free article] [PubMed] [Google Scholar]
- 9.Locke RC, Peloquin JM, Lemmon EA, Szostek A, Elliott DM, Killian ML. Strain Distribution of Intact Rat Rotator Cuff Tendon-to-Bone Attachments and Attachments With Defects. Journal of Biomechanical Engineering. 2017. Nov 1;139(11):111007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hettrich CM, Rodeo SA, Hannafin JA, Ehteshami J, Shubin Stein BE. The effect of muscle paralysis using Botox on the healing of tendon to bone in a rat model. Journal of Shoulder and Elbow Surgery. 2011;20(5):688–97. [DOI] [PubMed] [Google Scholar]
- 11.Killian ML, Cavinatto LM, Ward SR, Havlioglu N, Thomopoulos S, Galatz LM. Chronic Degeneration Leads to Poor Healing of Repaired Massive Rotator Cuff Tears in Rats. The American Journal of Sports Medicine. 2015. Oct;43(10):2401–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Killian ML, Cavinatto L, Shah SA, Sato EJ, Ward SR, Havlioglu N, et al. The effects of chronic unloading and gap formation on tendon-to-bone healing in a rat model of massive rotator cuff tears. Journal of Orthopaedic Research. 2014. Mar;32(3):439–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gilotra M, Nguyen T, Christian M, Davis D, Henn RF, Hasan SA. Botulinum toxin is detrimental to repair of a chronic rotator cuff tear in a rabbit model. J Orthop Res. 2015. Aug;33(8):1152–7. [DOI] [PubMed] [Google Scholar]
- 14.Galatz LM, Charlton N, Das R, Kim HM, Havlioglu N, Thomopoulos S. Complete removal of load is detrimental to rotator cuff healing. Journal of Shoulder and Elbow Surgery. 2009;18(5):669–75. [DOI] [PubMed] [Google Scholar]
- 15.Jurmain R, Cardoso FA, Henderson C, Villotte S. Bioarchaeology’s Holy Grail: The Reconstruction of Activity. In: A Companion to Paleopathology [Internet]. John Wiley & Sons, Ltd; 2012. [cited 2021 Apr 12]. p. 531–52. Available from: https://onlinelibrary.wiley.com/doi/abs/10.1002/9781444345940.ch29 [Google Scholar]
- 16.D’Agostino MA, Olivieri I. Enthesitis. Best Practice & Research Clinical Rheumatology. 2006. Jun 1;20(3):473–86. [DOI] [PubMed] [Google Scholar]
- 17.McGonagle D, Benjamin M, Marzo-Ortega H, Emery P. Advances in the understanding of entheseal inflammation. Curr Rheumatol Rep. 2002. Dec;4(6):500–6. [DOI] [PubMed] [Google Scholar]
- 18.Wallace IJ, Winchester JM, Su A, Boyer DM, Konow N. Physical activity alters limb bone structure but not entheseal morphology. Journal of Human Evolution. 2017. Jun 1;107:14–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zumwalt A The effect of endurance exercise on the morphology of muscle attachment sites. Journal of Experimental Biology. 2006. Feb 1;209(3):444–54. [DOI] [PubMed] [Google Scholar]
- 20.Rabey KN, Green DJ, Taylor AB, Begun DR, Richmond BG, McFarlin SC. Locomotor activity influences muscle architecture and bone growth but not muscle attachment site morphology. Journal of Human Evolution. 2015. Jan 1;78:91–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nguyen JC, Sheehan SE, Davis KW, Gill KG. Sports and the Growing Musculoskeletal System: Sports Imaging Series. Radiology. 2017. Jun 19;284(1):25–42. [DOI] [PubMed] [Google Scholar]
- 22.Stern T, Aviram R, Rot C, Galili T, Sharir A, Kalish Achrai N, et al. Isometric Scaling in Developing Long Bones Is Achieved by an Optimal Epiphyseal Growth Balance. Lander AD, editor. PLoS Biol. 2015. Aug 4;13(8):e1002212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blitz E, Viukov S, Sharir A, Shwartz Y, Galloway JL, Pryce BA, et al. Bone Ridge Patterning during Musculoskeletal Assembly Is Mediated through SCX Regulation of Bmp4 at the Tendon-Skeleton Junction. Developmental Cell. 2009;17(6):861–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eyal S, Kult S, Rubin S, Krief S, Felsenthal N, Pineault KM, et al. Bone morphology is regulated modularly by global and regional genetic programs. Development. 2019. Jul 15;146(14):dev167882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aaron JE. Periosteal Sharpey’s fibers: a novel bone matrix regulatory system? Front Endocrin [Internet]. 2012. [cited 2021 Jun 21];3. Available from: http://journal.frontiersin.org/article/10.3389/fendo.2012.00098/abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kult S, Olender T, Osterwalder M, Markman S, Leshkowitz D, Krief S, et al. Bi-fated tendonto-bone attachment cells are regulated by shared enhancers and KLF transcription factors. eLife. 2021. Jan 15;10:e55361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blitz E, Sharir A, Akiyama H, Zelzer E. Tendon-bone attachment unit is formed modularly by a distinct pool of Scx - and Sox9 -positive progenitors. Development. 2013;140(13):2680–90. [DOI] [PubMed] [Google Scholar]
- 28.Schwartz AG, Lipner JH, Pasteris JD, Genin GM, Thomopoulos S. Muscle loading is necessary for the formation of a functional tendon enthesis. Bone. 2013. Jul;55(1):44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Killian ML, Thomopoulos S. Scleraxis is required for the development of a functional tendon enthesis. The FASEB Journal. 2016. Jan;30(1):301–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schwartz AG, Long F, Thomopoulos S, Karp S, Gaffield W, McMahon AP, et al. Enthesis fibrocartilage cells originate from a population of Hedgehog-responsive cells modulated by the loading environment. Development (Cambridge, England). 2015. Jan;142(1):196–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Felsenthal N, Rubin S, Stern T, Krief S, Pal D, Pryce BA, et al. Development of migrating tendon-bone attachments involves replacement of progenitor populations. Development [Internet]. 2018. Dec 15 [cited 2020 Mar 25];145(24). Available from: https://dev.biologists.org/content/145/24/dev165381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Benjamin M, McGonagle D. The enthesis organ concept and its relevance to the spondyloarthropathies. Adv Exp Med Biol. 2009;649:57–70. [DOI] [PubMed] [Google Scholar]
- 33.Benjamin M, Ralphs JR. Entheses--the bony attachments of tendons and ligaments. Ital J Anat Embryol. 2001;106(2 Suppl 1):151–7. [PubMed] [Google Scholar]
- 34.Huang AH, Riordan TJ, Pryce B, Weibel JL, Watson SS, Long F, et al. Musculoskeletal integration at the wrist underlies the modular development of limb tendons. Development. 2015. Jul 15;142(14):2431–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang AH, Watson SS, Wang L, Baker BM, Akiyama H, Brigande JV, et al. Requirement for scleraxis in the recruitment of mesenchymal progenitors during embryonic tendon elongation. Development. 2019. Oct 15;146(20):dev182782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bober E, Franz T, Arnold HH, Gruss P, Tremblay P. Pax-3 is required for the development of limb muscles: a possible role for the migration of dermomyotomal muscle progenitor cells. Development. 1994. Mar 1;120(3):603–12. [DOI] [PubMed] [Google Scholar]
- 37.Muscle Kardon G. and tendon morphogenesis in the avian hind limb. Development. 1998. Oct 15;125(20):4019–32. [DOI] [PubMed] [Google Scholar]
- 38.Murchison ND, Price BA, Conner DA, Keene DR, Olson EN, Tabin CJ, et al. Regulation of tendon differentiation by scleraxis distinguishes force-transmitting tendons from muscle-anchoring tendons. Development. 2007. Jul;134(14):2697–708. [DOI] [PubMed] [Google Scholar]
- 39.Thomopoulos S, Kim HM, Rothermich SY, Biederstadt C, Das R, Galatz LM. Decreased muscle loading delays maturation of the tendon enthesis during postnatal development. Journal of Orthopaedic Research. 2007;25(9):1154–63. [DOI] [PubMed] [Google Scholar]
- 40.Liu Y, Schwartz AG, Birman V, Thomopoulos S, Genin GM. Stress amplification during development of the tendon-to-bone attachment. Biomechanics and Modeling in Mechanobiology. 2014. Oct;13(5):973–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Deymier AC, Schwartz AG, Cai Z, Daulton TL, Pasteris JD, Genin GM, et al. The multiscale structural and mechanical effects of mouse supraspinatus muscle unloading on the mature enthesis. Acta Biomaterialia. 2019. Jan;83:302–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hamrick MW, McPherron AC, Lovejoy CO, Hudson J. Femoral morphology and cross-sectional geometry of adult myostatin- deficient mice. Bone. 2000;27(3):343–9. [DOI] [PubMed] [Google Scholar]
- 43.Magown P, Shettar B, Zhang Y, Rafuse VF. Direct optical activation of skeletal muscle fibres efficiently controls muscle contraction and attenuates denervation atrophy. Nat Commun. 2015. Oct 13;6:8506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bruegmann T, van Bremen T, Vogt CC, Send T, Fleischmann BK, Sasse P. Optogenetic control of contractile function in skeletal muscle. Nat Commun. 2015. Nov;6(1):7153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ganji E, Chan CS, Ward CW, Killian ML. Optogenetic activation of muscle contraction in vivo. Connective Tissue Research. 2020. Aug 10;1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dyment NA, Breidenbach AP, Schwartz AG, Russell RP, Aschbacher-Smith L, Liu H, et al. Gdf5 progenitors give rise to fibrocartilage cells that mineralize via hedgehog signaling to form the zonal enthesis. Developmental Biology. 2015. Sep;405(1):96–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang M, VanHouten JN, Nasiri AR, Johnson RL, Broadus AE. PTHrP regulates the modeling of cortical bone surfaces at fibrous insertion sites during growth. J Bone Miner Res. 2013. Mar;28(3):598–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Breidenbach AP, Aschbacher-Smith L, Lu Y, Dyment NA, Liu C-F, Liu H, et al. Ablating hedgehog signaling in tenocytes during development impairs biomechanics and matrix organization of the adult murine patellar tendon enthesis. Journal of Orthopaedic Research. 2015. Aug;33(8):1142–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fang F, Schwartz AG, Moore ER, Sup ME, Thomopoulos S. Primary cilia as the nexus of biophysical and hedgehog signaling at the tendon enthesis. Science Advances. 2020. Oct 1;6(44):eabc1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Abraham AC, Shah SA, Golman M, Song L, Li X, Kurtaliaj I, et al. Targeting the NF-κB signaling pathway in chronic tendon disease. Science Translational Medicine [Internet]. 2019. Feb 27 [cited 2021 Apr 24];11(481). Available from: https://stm.sciencemag.org/content/11/481/eaav4319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sugimoto Y, Takimoto A, Hiraki Y, Shukunami C. Generation and characterization of ScxCre transgenic mice. genesis. 2013;51(4):275–83. [DOI] [PubMed] [Google Scholar]
- 52.Schlesinger SY, Seo S, Pryce BA, Tufa SF, Keene DR, Huang AH, et al. Loss of Smad4 in the scleraxis cell lineage results in postnatal joint contracture. Developmental Biology. 2021. Feb 1;470:108–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Katz TC, Singh MK, Degenhardt K, Rivera-Feliciano J, Johnson RL, Epstein JA, et al. Distinct Compartments of the Proepicardial Organ Give Rise to Coronary Vascular Endothelial Cells. Developmental Cell. 2012. Mar 13;22(3):639–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang M, VanHouten JN, Nasiri AR, Tommasini SM, Broadus AE. Periosteal PTHrP regulates cortical bone modeling during linear growth in mice. J Anat. 2014. Jul;225(1):71–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Agarwal S, Loder S, Brownley C, Cholok D, Mangiavini L, Li J, et al. Inhibition of Hif1α prevents both trauma-induced and genetic heterotopic ossification. PNAS. 2016. Jan 19;113(3):E338–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Logan M, Martin JF, Nagy A, Lobe C, Olson EN, Tabin CJ. Expression of Cre recombinase in the developing mouse limb bud driven by aPrxl enhancer. genesis. 2002. Jun;33(2):77–80. [DOI] [PubMed] [Google Scholar]
- 57.Roberts RR, Bobzin L, Teng CS, Pal D, Tuzon CT, Schweitzer R, et al. FGF signaling patterns cell fate at the interface between tendon and bone. Development. 2019. 02;146(15). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Soeda T, Deng JM, Crombrugghe B de, Behringer RR, Nakamura T, Akiyama H. Sox9-expressing precursors are the cellular origin of the cruciate ligament of the knee joint and the limb tendons. genesis. 2010;48(11):635–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shwartz Y, Viukov S, Krief S, Zelzer E. Joint Development Involves a Continuous Influx of Gdf5-Positive Cells. Cell Rep. 2016. 21;15(12):2577–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tan G-K, Pryce BA, Stabio A, Brigande JV, Wang C, Xia Z, et al. Tgfβ signaling is critical for maintenance of the tendon cell fate. eLife. 2020. Jan 21;9:e52695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kaji DA, Howell KL, Balic Z, Hubmacher D, Huang AH. Tgfβ signaling is required for tenocyte recruitment and functional neonatal tendon regeneration. eLife. 2020. Jun 5;9:e51779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Howell K, Chien C, Bell R, Laudier D, Tufa SF, Keene DR, et al. Novel Model of Tendon Regeneration Reveals Distinct Cell Mechanisms Underlying Regenerative and Fibrotic Tendon Healing. Scientific Reports. 2017. Dec;7(1):45238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kawanami A, Matsushita T, Chan YY, Murakami S. Mice expressing GFP and CreER in osteochondro progenitor cells in the periosteum. Biochem Biophys Res Commun. 2009. Aug 28;386(3):477–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hagan AS, Boylan M, Smith C, Perez-Santamarina E, Kowalska K, Hung IH, et al. Generation and validation of novel conditional flox and inducible Cre alleles targeting fibroblast growth factor 18 (Fgf18). Dev Dyn. 2019. Sep;248(9):882–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ouyang Z, Chen Z, Ishikawa M, Yue X, Kawanami A, Leahy P, et al. Prx1 and 3.2kb Col1a1 promoters target distinct bone cell populations in transgenic mice. Bone. 2014. Jan;58:136–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Noack S, Seiffart V, Willbold E, Laggies S, Winkel A, Shahab-Osterloh S, et al. Periostin secreted by mesenchymal stem cells supports tendon formation in an ectopic mouse model. Stem Cells Dev. 2014. Aug 15;23(16):1844–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kanisicak O, Khalil H, Ivey MJ, Karch J, Maliken BD, Correll RN, et al. Genetic lineage tracing defines myofibroblast origin and function in the injured heart. Nat Commun. 2016. Jul 22;7:12260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Spagnoli A, O’Rear L, Chandler RL, Granero-Molto F, Mortlock DP, Gorska AE, et al. TGF-beta signaling is essential for joint morphogenesis. J Cell Biol. 2007. Jun 18;177(6):1105–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pryce BA, Watson SS, Murchison ND, Staverosky JA, Dünker N, Schweitzer R. Recruitment and maintenance of tendon progenitors by TGFbeta signaling are essential for tendon formation. Development. 2009. Apr;136(8):1351–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Havis E, Bonnin M-A, Esteves de Lima J, Charvet B, Milet C, Duprez D. TGFβ and FGF promote tendon progenitor fate and act downstream of muscle contraction to regulate tendon differentiation during chick limb development. Development. 2016. Oct 15;143(20):3839–51. [DOI] [PubMed] [Google Scholar]
- 71.Robbins JR, Evanko SP, Vogel KG. Mechanical loading and TGF-beta regulate proteoglycan synthesis in tendon. Arch Biochem Biophys. 1997. Jun 15;342(2):203–11. [DOI] [PubMed] [Google Scholar]
- 72.Seo H-S, Serra R. Deletion of Tgfbr2 in Prx1-cre expressing mesenchyme results in defects in development of the long bones and joints. Dev Biol. 2007. Oct 15;310(2):304–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Subramanian A, Kanzaki LF, Galloway JL, Schilling TF. Mechanical force regulates tendon extracellular matrix organization and tenocyte morphogenesis through TGFbeta signaling. eLife. 2018. Nov 26;7:e38069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Niu X, Subramanian A, Hwang TH, Schilling TF, Galloway JL. Tendon Cell Regeneration Is Mediated by Attachment Site-Resident Progenitors and BMP Signaling. Curr Biol. 2020. Sep 7;30(17):3277–3292.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ruschke K, Hiepen C, Becker J, Knaus P. BMPs are mediators in tissue crosstalk of the regenerating musculoskeletal system. Cell Tissue Res [Internet]. 2012. Mar [cited 2021 Apr 12];347(3). Available from: http://link.springer.com/10.1007/s00441-011-1283-6 [DOI] [PubMed] [Google Scholar]
- 76.Massagué J, Chen YG. Controlling TGF-beta signaling. Genes Dev. 2000. Mar 15;14(6):627–44. [PubMed] [Google Scholar]
- 77.Mu Y, Gudey SK, Landström M. Non-Smad signaling pathways. Cell Tissue Res. 2012. Jan;347(1):11–20. [DOI] [PubMed] [Google Scholar]
- 78.Ornitz DM, Itoh N. The Fibroblast Growth Factor signaling pathway. Wiley Interdiscip Rev Dev Biol. 2015. Jun;4(3):215–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Su N, Jin M, Chen L. Role of FGF/FGFR signaling in skeletal development and homeostasis: learning from mouse models. Bone Res. 2014;2:14003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hung IH, Yu K, Lavine KJ, Ornitz DM. FGF9 regulates early hypertrophic chondrocyte differentiation and skeletal vascularization in the developing stylopod. Developmental Biology. 2007. Jul;307(2):300–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hung IH, Schoenwolf GC, Lewandoski M, Ornitz DM. A combined series of Fgf9 and Fgf18 mutant alleles identifies unique and redundant roles in skeletal development. Developmental Biology. 2016. Mar;411(1):72–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Karuppaiah K, Yu K, Lim J, Chen J, Smith C, Long F, et al. FGF signaling in the osteoprogenitor lineage non-autonomously regulates postnatal chondrocyte proliferation and skeletal growth. Development. 2016. 15;143(10):1811–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Su N, Xu X, Li C, He Q, Zhao L, Li C, et al. Generation of Fgfr3 conditional knockout mice. International journal of biological sciences. 2010. Jun;6(4):327–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang Q, Green RP, Zhao G, Ornitz DM. Differential regulation of endochondral bone growth and joint development by FGFR1 and FGFR3 tyrosine kinase domains. Development. 2001. Oct 1;128(19):3867–76. [DOI] [PubMed] [Google Scholar]
- 85.Liu C-F, Aschbacher-Smith L, Barthelery NJ, Dyment N, Butler D, Wylie C. Spatial and Temporal Expression of Molecular Markers and Cell Signals During Normal Development of the Mouse Patellar Tendon. Tissue Engineering Part A. 2012. Mar;18(5–6):598–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liang G, Katz LD, Insogna KL, Carpenter TO, Macica CM. Survey of the Enthesopathy of X-Linked Hypophosphatemia and Its Characterization in Hyp Mice. Calcif Tissue Int. 2009. Sep;85(3):235–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chai Y, Jiang X, Ito Y, Bringas P, Han J, Rowitch DH, et al. Fate of the mammalian cranial neural crest during tooth and mandibular morphogenesis. Development. 2000. Apr;127(8):1671–9. [DOI] [PubMed] [Google Scholar]
- 88.Prummel KD, Nieuwenhuize S, Mosimann C. The lateral plate mesoderm. Development. 2020. Jun 19;147(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kronenberg HM. Developmental regulation of the growth plate. Nature. 2003. May;423(6937):332–6. [DOI] [PubMed] [Google Scholar]
- 90.Long F, Ornitz DM. Development of the Endochondral Skeleton. Cold Spring Harbor Perspectives in Biology. 2013. Jan 1;5(1):a008334–a008334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chen X, Macica C, Nasiri A, Judex S, Broadus AE. Mechanical regulation of PTHrP expression in entheses. Bone. 2007. Nov;41(5):752–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Huangfu D, Liu A, Rakeman AS, Murcia NS, Niswander L, Anderson KV. Hedgehog signalling in the mouse requires intraflagellar transport proteins. Nature. 2003. Nov;426(6962):83–7. [DOI] [PubMed] [Google Scholar]
- 93.Kopinke D, Norris AM, Mukhopadhyay S. Developmental and regenerative paradigms of cilia regulated hedgehog signaling. Seminars in Cell & Developmental Biology. 2021. Feb;110:89–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Koyama E, Young B, Nagayama M, Shibukawa Y, Enomoto-Iwamoto M, Iwamoto M, et al. Conditional Kif3a ablation causes abnormal hedgehog signaling topography, growth plate dysfunction, and excessive bone and cartilage formation during mouse skeletogenesis. Development. 2007. Jun 1;134(11):2159–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chang C-F, Serra R. Ift88 regulates Hedgehog signaling, Sfrp5 expression, and β-catenin activity in post-natal growth plate: CILIA/HEDGEHOG REGULATION OF Sfrp5. J Orthop Res. 2013. Mar;31(3):350–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang B, Fallon JF, Beachy PA. Hedgehog-Regulated Processing of Gli3 Produces an Anterior/Posterior Repressor Gradient in the Developing Vertebrate Limb. Cell. 2000. Feb;100(4):423–34. [DOI] [PubMed] [Google Scholar]
- 97.Bai CB, Stephen D, Joyner AL. All Mouse Ventral Spinal Cord Patterning by Hedgehog Is Gli Dependent and Involves an Activator Function of Gli3. Developmental Cell. 2004. Jan;6(1):103–15. [DOI] [PubMed] [Google Scholar]
- 98.Ahmed S, Nowlan NC. Initiation and emerging complexity of the collagen network during prenatal skeletal development. eCM. 2020. Feb 27;39:136–55. [DOI] [PubMed] [Google Scholar]
- 99.Galatz L, Rothermich S, VanderPloeg K, Petersen B, Sandell L, Thomopoulos S. Development of the supraspinatus tendon-to-bone insertion: Localized expression of extracellular matrix and growth factor genes. J Orthop Res. 2007. Dec;25(12):1621–8. [DOI] [PubMed] [Google Scholar]
- 100.Mouw JK, Ou G, Weaver VM. Extracellular matrix assembly: a multiscale deconstruction. Nat Rev Mol Cell Biol. 2014. Dec;15(12):771–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Badylak SF, Freytes DO, Gilbert TW. Extracellular matrix as a biological scaffold material: Structure and function. Acta Biomaterialia. 2009. Jan 1;5(1):1–13. [DOI] [PubMed] [Google Scholar]
- 102.Calve S, Simon H-G. The extracellular matrix plays an active role in muscle regeneration. The FASEB Journal. 2010. Apr 1;24(S1):181.6–181.6. [Google Scholar]
- 103.Calve S, Simon H-G. Biochemical and mechanical environment cooperatively regulate skeletal muscle regeneration. FASEB J. 2012. Jun;26(6):2538–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Font Tellado S, Bonani W, Balmayor ER, Foehr P, Motta A, Migliaresi C, et al. Fabrication and Characterization of Biphasic Silk Fibroin Scaffolds for Tendon/Ligament-to-Bone Tissue Engineering. Tissue Engineering Part A. 2017. Aug;23(15–16):859–72. [DOI] [PubMed] [Google Scholar]
- 105.Li X, Xie J, Lipner J, Yuan X, Thomopoulos S, Xia Y. Nanofiber Scaffolds with Gradations in Mineral Content for Mimicking the Tendon-to-Bone Insertion Site. Nano Letters. 2009. Jul;9(7):2763–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sun Han Chang RA, Shanley JF, Kersh ME, Harley BAC. Tough and tunable scaffold-hydrogel composite biomaterial for soft-to-hard musculoskeletal tissue interfaces. Sci Adv. 2020. Aug;6(34):eabb6763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pizzo AM, Kokini K, Vaughn LC, Waisner BZ, Voytik-Harbin SL. Extracellular matrix (ECM) microstructural composition regulates local cell-ECM biomechanics and fundamental fibroblast behavior: a multidimensional perspective. Journal of Applied Physiology. 2005. May;98(5):1909–21. [DOI] [PubMed] [Google Scholar]
- 108.Calve S, Odelberg SJ, Simon H-G. A transitional extracellular matrix instructs cell behavior during muscle regeneration. Developmental Biology. 2010. Aug 1;344(1):259–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Otsuka T, Phan AQ, Laurencin CT, Esko JD, Bryant SV, Gardiner DM. Identification of Heparan-Sulfate Rich Cells in the Loose Connective Tissues of the Axolotl (Ambystoma mexicanum) with the Potential to Mediate Growth Factor Signaling during Regeneration. Regenerative Engineering and Translational Medicine. 2020. Mar 1;6(1):7–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Vieira WA, Wells KM, Raymond MJ, De Souza L, Garcia E, McCusker CD. FGF, BMP, and RA signaling are sufficient for the induction of complete limb regeneration from non-regenerating wounds on Ambystoma mexicanum limbs. Developmental Biology. 2019. Jul 15;451(2):146–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nacu E, Glausch M, Le HQ, Damanik FFR, Schuez M, Knapp D, et al. Connective tissue cells, but not muscle cells, are involved in establishing the proximo-distal outcome of limb regeneration in the axolotl. Development. 2013. Feb 1;140(3):513–8. [DOI] [PubMed] [Google Scholar]
- 112.Gardiner DM. Regulation of Regeneration by Heparan Sulfate Proteoglycans in the Extracellular Matrix. Regenerative Engineering and Translational Medicine. 2017. Sep 1;3(3):192–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wang Y-H, Beck C. Distinct patterns of endosulfatase gene expression during Xenopus laevis limb development and regeneration. Regeneration. 2015;2(1):19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kirn-Safran C, Farach-Carson MC, Carson DD. Multifunctionality of extracellular and cell surface heparan sulfate proteoglycans. Cell Mol Life Sci. 2009. Nov 1;66(21):3421–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.McCusker C, Bryant SV, Gardiner DM. The axolotl limb blastema: cellular and molecular mechanisms driving blastema formation and limb regeneration in tetrapods: The Axolotl Limb Blastema. Regeneration. 2015. Apr;2(2):54–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dieterich DC, Lee JJ, Link AJ, Graumann J, Tirrell DA, Schuman EM. Labeling, detection and identification of newly synthesized proteomes with bioorthogonal non-canonical amino-acid tagging. Nat Protoc. 2007. Mar;2(3):532–40. [DOI] [PubMed] [Google Scholar]
- 117.Calve S, Witten AJ, Ocken AR, Kinzer-Ursem TL. Incorporation of non-canonical amino acids into the developing murine proteome. Sci Rep. 2016. 30;6:32377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Saleh AM, Wilding KM, Calve S, Bundy BC, Kinzer-Ursem TL. Non-canonical amino acid labeling in proteomics and biotechnology. J Biol Eng. 2019;13:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zitnay JL, Li Y, Qin Z, San BH, Depalle B, Reese SP, et al. Molecular level detection and localization of mechanical damage in collagen enabled by collagen hybridizing peptides. Nature Communications. 2017;8:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hwang J, Huang Y, Burwell TJ, Peterson NC, Connor J, Weiss SJ, et al. In Situ Imaging of Tissue Remodeling with Collagen Hybridizing Peptides. ACS Nano. 2017. Oct 24;11(10):9825–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Marchesoni A, De Marco G, Merashli M, McKenna F, Tinazzi I, Marzo-Ortega H, et al. The problem in differentiation between psoriatic-related polyenthesitis and fibromyalgia. Rheumatology. 2018. Jan 1;57(1):32–40. [DOI] [PubMed] [Google Scholar]
- 122.Achar S, Yamanaka J. Apophysitis and Osteochondrosis: Common Causes of Pain in Growing Bones. AFP. 2019. May 15;99(10):610–8. [PubMed] [Google Scholar]
- 123.Ladenhauf HN, Seitlinger G, Green DW. Osgood–Schlatter disease: a 2020 update of a common knee condition in children. Current Opinion in Pediatrics. 2020. Feb;32(1):107–12. [DOI] [PubMed] [Google Scholar]
- 124.Pillai S, Littlejohn G. Metabolic factors in diffuse idiopathic skeletal hyperostosis - a review of clinical data. Open Rheumatol J. 2014. Dec 19;8:116–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.McGonagle D, Tan AL, Carey J, Benjamin M. The anatomical basis for a novel classification of osteoarthritis and allied disorders. J Anat. 2010. Mar;216(3):279–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Greif DN, Emerson CP, Jose J, Toumi H, Best TM. Enthesopathy—An Underappreciated Role in Osteoarthritis? Current Sports Medicine Reports. 2020. Nov;19(11):495–7. [DOI] [PubMed] [Google Scholar]
- 127.Abraham AC, Shah SA, Thomopoulos S. Targeting Inflammation in Rotator Cuff Tendon Degeneration and Repair. Tech Shoulder Elb Surg. 2017. Sep;18(3):84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Millar NL, Murrell GAC, McInnes IB. Alarmins in tendinopathy: unravelling new mechanisms in a common disease. Rheumatology (Oxford). 2013. May;52(5):769–79. [DOI] [PubMed] [Google Scholar]
- 129.Millar NL, Silbernagel KG, Thorborg K, Kirwan PD, Galatz LM, Abrams GD, et al. Tendinopathy. Nat Rev Dis Primers. 2021. Jan 7;7(1):1. [DOI] [PubMed] [Google Scholar]
- 130.Hackett L, Millar NL, Lam P, Murrell GAC. Are the Symptoms of Calcific Tendinitis Due to Neoinnervation and/or Neovascularization? JBJS. 2016. Feb 3;98(3):186–92. [DOI] [PubMed] [Google Scholar]
- 131.Lorenz MR, Brazill JM, Beeve AT, Shen I, Scheller EL. A neuroskeletal atlas: spatial mapping and contextualization of axon subtypes innervating the long bones of C3H and B6 mice. Journal of Bone and Mineral Research [Internet]. [cited 2021 Apr 23];n/a(n/a). Available from: https://asbmr.onlinelibrary.wiley.com/doi/abs/10.1002/jbmr.4273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Shaw H, Milz S, Büettner A, Santer RM, Watson A, Benjamin M, et al. The Innervation of the Enthesis Organ of the Rat Achilles Tendon: 2426. Medicine & Science in Sports & Exercise. 2006. May;38(Supplement):S449. [Google Scholar]
- 133.Wunderli SL, Blache U, Beretta Piccoli A, Niederöst B, Holenstein CN, Passini FS, et al. Tendon response to matrix unloading is determined by the patho-physiological niche. Matrix Biology. 2020. Jul 1;89:11–26. [DOI] [PubMed] [Google Scholar]
- 134.Calejo I, Costa-Almeida R, Reis RL, Gomes ME. In vitro temporal HIF-mediated deposition of osteochondrogenic matrix governed by hypoxia and osteogenic factors synergy. Journal of Cellular Physiology. 2021;236(5):3991–4007. [DOI] [PubMed] [Google Scholar]
- 135.Lavagnino M, Oslapas AN, Gardner KL, Arnoczky SP. Hypoxia inhibits primary cilia formation and reduces cell-mediated contraction in stress-deprived rat tail tendon fascicles. Muscles Ligaments Tendons J. 2016. Sep 17;6(2):193–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.McBeath R, Edwards RW, O’Hara BJ, Maltenfort MG, Parks SM, Steplewski A, et al. Tendinosis develops from age- and oxygen tension-dependent modulation of Rac1 activity. Aging Cell. 2019;18(3):e12934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ohashi K, Fujiwara S, Mizuno K. Roles of the cytoskeleton, cell adhesion and rho signalling in mechanosensing and mechanotransduction. The Journal of Biochemistry. 2017. Mar 1;161(3):245–54. [DOI] [PubMed] [Google Scholar]
- 138.Wheaton WW, Chandel NS. Hypoxia. 2. Hypoxia regulates cellular metabolism. Am J Physiol Cell Physiol. 2011. Mar;300(3):C385–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hirota K, Semenza GL. Rac1 Activity Is Required for the Activation of Hypoxia-inducible Factor 1*. Journal of Biological Chemistry. 2001. Jun 15;276(24):21166–72. [DOI] [PubMed] [Google Scholar]
- 140.Dunwoodie SL. The role of hypoxia in development of the Mammalian embryo. Dev Cell. 2009. Dec;17(6):755–73. [DOI] [PubMed] [Google Scholar]
- 141.Schipani E, Mangiavini L, Merceron C. HIF-1α and growth plate development: what we really know. Bonekey Rep [Internet]. 2015. Aug 12 [cited 2021 Apr 23];4. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4549921/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Amarilio R, Viukov SV, Sharir A, Eshkar-Oren I, Johnson RS, Zelzer E. HIF1alpha regulation of Sox9 is necessary to maintain differentiation of hypoxic prechondrogenic cells during early skeletogenesis. Development. 2007. Nov;134(21):3917–28. [DOI] [PubMed] [Google Scholar]
- 143.Stegen S, Laperre K, Eelen G, Rinaldi G, Fraisl P, Torrekens S, et al. HIF-1α metabolically controls collagen synthesis and modification in chondrocytes. Nature. 2019. Jan;565(7740):511–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Simon MC, Keith B. The role of oxygen availability in embryonic development and stem cell function. Nat Rev Mol Cell Biol. 2008. Apr;9(4):285–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Yao Q, Khan MP, Merceron C, LaGory EL, Tata Z, Mangiavini L, et al. Suppressing Mitochondrial Respiration Is Critical for Hypoxia Tolerance in the Fetal Growth Plate. Developmental Cell. 2019. Jun 3;49(5):748–763.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]


