Abstract
Glucocorticoids are widely used for treating underlying renal diseases and following renal transplantation and are often tapered or discontinued upon reaching end-stage renal failure. Although glucocorticoid withdrawal is the predominant cause of secondary adrenal insufficiency, no consensus has been established regarding its prevalence, clinical manifestations, or therapeutic regimen, for prevention of this pathological condition. We describe a 29-year-old woman admitted to our hospital because of 1-week history of fever, diarrhea, and general fatigue. She was affected with nephrotic syndrome and diagnosed with focal segmental glomerulonephritis at 15 years old, and had since been treated with glucocorticoids. She suffered from frequent relapse of nephrotic syndrome, which became refractory to other immunosuppressants and low-density lipoprotein apheresis, making discontinuation of glucocorticoids difficult. Renal function deteriorated gradually and hemodialysis was initiated 8 months before admission. She was infected with type A influenza roughly 2 weeks prior and treated with oseltamivir. She exhibited hypercalcemia (albumin corrected, 14.4 mg/dl) and hypoglycemia (31.0 mg/dl) for the first time. She was suspected of, and diagnosed with, adrenal insufficiency, because long-term glucocorticoid use was incidentally discontinued only 2 days before she contracted influenza. Clinical symptoms and hypercalcemia improved dramatically following initiation of treatment with hydrocortisone. Adrenal insufficiency is an unusual cause of hypercalcemia. However, hemodialysis patients tend to develop more severe hypercalcemia because of lack of urinary calcium excretion, which should not be overlooked because it may result in critical situations. In conclusion, clinicians should be aware of adrenal insufficiency with glucocorticoid withdrawal and hypercalcemia in hemodialysis patients.
Keywords: Adrenal insufficiency, Glucocorticoid withdrawal, Influenza, Hemodialysis, Hypercalcemia
Introduction
Glucocorticoids (GCs) are widely used for treating underlying renal diseases and are frequently tapered or discontinued upon development of end-stage renal disease (ESRD). Although GC withdrawal is the primary cause of secondary adrenal insufficiency (AI), assessment of adrenal function, prevalence and clinical manifestations of AI, and therapeutic regimens to predict and/or prevent AI remain inconclusive in such cases [1–3].
Hypercalcemia in hemodialysis patients is often caused by severe secondary hyperparathyroidism, and/or drugs, such as vitamin D analogs and calcium-containing phosphorous binders.
In contrast, AI is a relatively rare cause of hypercalcemia [4].
Herein, we report the case of a patient undergoing hemodialysis who experienced AI because of GC withdrawal and was subsequently infected with type A influenza that presented with hypercalcemia.
Case report
A 29-year-old woman was admitted to our hospital because of 1-week history of abdominal pain, appetite loss, general fatigue, and fever. She was diagnosed with type A influenza roughly 2 weeks prior and was treated with oseltamivir. She was affected with nephrotic syndrome (NS) and diagnosed with primary focal segmental glomerulonephritis (FSGS) at 15 years old, and had since been administered GCs. She suffered from frequent relapse of steroid-dependent NS. Alternative immunosuppressive agents were administered (e.g., cyclosporine, cyclophosphamide), although they were considered unacceptable because of adverse effects (onset of menopause, and acute kidney injury, respectively). She failed to effectively respond to mizoribine and low-density lipoprotein apheresis. She therefore required repeatedly increasing doses of GCs, which contributed to the difficulty of discontinuation.
She underwent surgery for bilateral cataracts 3 years earlier.
Her renal function deteriorated gradually, and hemodialysis was initiated 8 months before admission. The dose of prednisolone was slowly tapered (1.0–2.0 mg/day every 1–2 months until reaching 2.0 mg/day) and was completely withdrawn 2 days before she was diagnosed with influenza.
Her oral medications included omega-3-acid ethyl esters (4 g), rebamipide (100 mg × 3), fluvastatin sodium (20 mg), esomeprazole magnesium hydrate (20 mg) and furosemide (40 mg). Warfarin (1 mg) was administered for radial artery occlusion.
On admission, her blood pressure was 121/86 mmHg, pulse was 113 beats/min, and temperature was 37.9 °C. She weighed 47.9 kg (0.3 kg below her dry weight). She was pale but not icteric. Physical examinations of the chest and abdomen were unremarkable. She exhibited moon face. No lymphadenopathy or skin lesions were observed. She had edema of the eyelids and lower legs.
Laboratory data are shown in Table 1. Hemoglobin concentration was 10.2 g/dl, platelet count was 367,000/mm3, and leukocyte count was 9,850/mm3. Percentage of eosinophils was 5.0%. Laboratory analysis also revealed the following: serum urea nitrogen: 7.3 mg/dl, creatinine: 6.38 mg/dl, uric acid: 4.5 mg/dl, sodium: 135 mEq/l, potassium: 3.1 mEq/l, chloride: 102 mEq/l, calcium (Ca): 12.0 mg/dl (adjusted Ca: 14.4 mg/dl), inorganic phosphate: 5.6 mg/dl, total protein: 3.8 g/dl, and albumin: 1.6 g/dl. No monoclonal band was detected in plasma. C-reactive protein level was 2.08 mg/dl.
Table 1.
Laboratory data
| Complete blood count | UA | 4.5 | mg/dl | int-PTH | 13 | (10–65) | pg/ml | |||
| WBC | 9850 | /mm3 | Na | 135 | mEq/l | PTH-rp | 1.1 | (< 1.1) | pmol/l | |
| Neutro | 68.0 | % | K | 3.1 | mEq/l | cortisol | 1.8 | (4.49–21.2) | µg/dl | |
| Lym | 18.1 | % | Cl | 102 | mEq/l | ACTH | 10.6 | (7.2–63.3) | pg/dl | |
| Mono | 8.6 | % | adjusted Ca | 14.4 | mg/dl | GH | 5.02 | (0.13–9.88) | ng/ml | |
| Eosino | 5.0 | % | IP | 5.6 | mg/dl | PRL* | 36.2 | (6.1–30.5) | ng/ml | |
| Baso | 0.3 | % | TP | 3.8 | g/dl | LH* | 17.0 | mIU/ml | ||
| Platelet | 36.7 × 104 | /mm3 | alb | 1.6 | g/dl | FSH* | 3.7 | mIU/ml | ||
| Hb | 10.2 | g/dl | alb | 48.7 | % | ACE | 16.9 | (7–25) | U/l | |
| Biochemistry | α1 | 5.6 | % | osteocalcin | 213.1 | (7.8–30.8) | ng/ml | |||
| AST | 23 | IU/l | α2 | 15.3 | % | calcitonin | 2.62 | (0–3.91) | pg/ml | |
| ALT | 12 | IU/l | β | 10.6 | % | TRACP-5b | 1214 | (120–420) | mU/dl | |
| ALP | 220 | IU/l | γ | 19.8 | % | PT-INR | 2.28 | |||
| LDH | 188 | IU/l | CRP | 2.08 | mg/dl | Blood gas analysis | ||||
| γ-GTP | 20 | IU/l | BS | 87 | mg/dl | pH | 7.464 | |||
| CK | 29 | IU/l | TSH | 4.47 | (0.35–4.94) | µIU/ml | HCO3 | 25.5 | mmol/l | |
| BUN | 7.3 | mg/dl | FT3 | 2.40 | (1.71–3.71) | pg/ml | BE | 2.3 | mmol/l | |
| Cr | 6.38 | mg/dl | FT4 | 0.99 | (0.7–1.48) | ng/dl | ||||
WBC white blood cells, Neutro neutrophils, Lym lymphocytes, Mono monocytes, Eosino eosinophils, Baso basophils, Hb hemoglobin, AST aspartate aminotransferase, ALT alanine aminotransferase, ALP alkaline phosphatase, LDH lactate dehydrogenase, γ-GTP γ-glutamyl transpeptidase, CK creatine kinase, BUN blood urea nitrogen, Cr creatinine, UA uric acid, Na sodium, K potassium, Cl chloride, Ca calcium, IP inorganic phosphate, TP total protein, alb albumin, CRP C-reactive protein, BS blood sugar, TSH thyroid-stimulating hormone, FT3 free triiodothyronine, FT4 free thyroxine, int-PTH intact parathyroid hormone, PTH-rp parathyroid hormone-related peptide, ACTH adrenocorticotropic hormone, GH growth hormone, PRL prolactin, LH luteinizing hormone, FSH follicle-stimulating hormone, TRACP-5b tartrate-resistant acid phosphatase-5b, PT-INR prothrombin time-international normalized ratio, ACE angiotensin converting enzyme, BE base excess
*Value at the time of ACTH stimulation test
Further laboratory measurements were as follows: thyroid-stimulating hormone (TSH): 4.47 µIU/ml, free triiodothyronine: 2.40 pg/ml, and free thyroxine: 0.99 ng/dl. Intact parathyroid hormone was 13.0 pg/ml (normal range: 10.0–65.0 pg/ml), and parathyroid hormone-related peptide was 1.1 pmol/l (normal range: < 1.1 pmol/l). Blood gas analysis showed pH was 7.464, HCO3 was 25.5 mmol/l, and base excess was 2.3.
Osteocalcin was 213.1 ng/ml (normal range: 7.8–30.8 ng/ml), tartrate-resistant acid phosphatase-5b (TRACP-5b) was 1,214 mU/dl (normal range: 120–420 mU/dl). Prothrombin time-international normalized ratio (PT-INR) was 2.28.
Blood culture was negative. A chest X-ray revealed slight right pleural effusion.
She was not prescribed vitamin D analogs or calcium-containing phosphorus binders, which can cause hypercalcemia. Dialysate Ca concentration was 3.0 mEq/L. Her serum Ca level was never previously elevated.
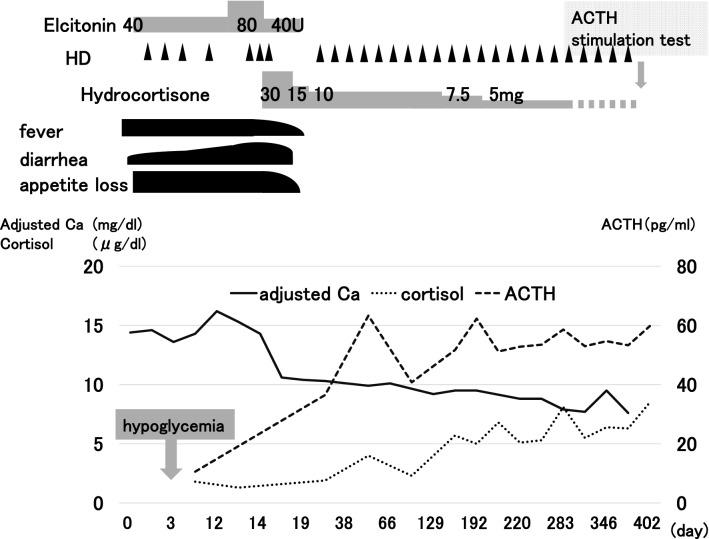
Her clinical course during hospitalization is shown in Fig. 1. Elcatonin administration and frequent hemodialysis had no effect on hypercalcemia. She became more emaciated and experienced a single episode of hypoglycemia (blood glucose: 31.0 mg/dl) early during the morning of day 3 of hospitalization (she felt lethargic). The clinical picture was interpreted as AI because of recent GC withdrawal following prolonged use just before contracting influenza. Indeed, plasma cortisol level had decreased (1.3 µg/dl) with normal adrenocorticotropic hormone (ACTH) level (10.6 pg/dl). Growth hormone (GH) level was normal (5.02 ng/ml) (Table 1).
Fig. 1.
Elcatonin administration and frequent hemodialysis had no effect on hypercalcemia. After administration of hydrocortisone (30 mg), clinical symptoms and hypercalcemia were rapidly and dramatically improved. Hydrocortisone was gradually tapered to alternate-day dose of 5.0 mg, and discontinued after continuous ACTH stimulation test, which was performed 13 months after onset of AI and revealed partial recovery thereof. HD hemodialysis, ACTH adrenocorticotropic hormone, Ca, calcium
Abdominal computed tomography (CT) revealed bilateral adrenal gland atrophy, without bleeding or calcification. CT did not reveal a pancreatic tumor, and brain magnetic resonance imaging did not show a pituitary tumor.
She was diagnosed with AI, and began therapy with hydrocortisone at dose of 30.0 mg. Her adjusted Ca level normalized and clinical symptoms improved rapidly and drastically within a few days (Fig. 1). She was discharged on day 21 of hospitalization.
Hydrocortisone was gradually tapered to alternate-day dose of 5.0 mg and discontinued 13 months after onset of AI, following assessment of adrenal function with a continuous ACTH stimulation test (intramuscular administration of 0.5 mg of tetracosactide acetate for 3 consecutive days). ACTH stimulation test was performed shortly after withdrawal of hydrocortisone, with close monitoring and supervision by experienced personnel. The test revealed partial recovery of AI (Table 2).
Table 2.
Continuous ACTH stimulation test
| 1 day | 3 days | |
|---|---|---|
| ACTH (pg/ml) | ||
| 06:00 | 101.3* | 3.3 |
| 16:00 | 7.7 | 2.6 |
| 23:00 | 6.1 | 2.5 |
| Cortisol (µg/dl) | ||
| 06:00 | 11.8 | 36.6 |
| 16:00 | 24.6 | 46.0 |
| 23:00 | 29.6 | 48.5 |
ACTH adrenocorticotropic hormone
*Value before ACTH loading
The levels of other anterior pituitary hormones, such as prolactin (PRL), luteinizing hormone (LH), and follicle-stimulating hormone (FSH), were all within normal ranges at the time of ACTH stimulation test, without any replacement therapy (Table 1).
After recovery of AI, TRACP-5b level decreased to 429 mU/dl, while that of osteocalcin remained high (255.4 ng/ml).
Discussion
In the present case, latent AI because of GC withdrawal became obvious through subsequent influenza infection.
Severe and refractory hypercalcemia was caused by AI.
The clinical pictures of discontinuation of long-term used GCs and hypoglycemia were suggestive of AI.
She suffered from refractory NS, which can potentially cause decreased levels of cortisol-binding protein in plasma resulting from increased urinary excretion [5].
However, unlike vitamin D-binding protein, it is obscure that reduced cortisol-binding protein exerts clinical effects in patients with NS [5]. Moreover, urinary loss of protein may be negligible in the present case because urinary output was low with 8 months of hemodialysis vintage. Therefore, it was unlikely that NS directly affected AI.
Influenza infection can induce hypoglycemia, especially in critically ill patients complicated with encephalitis including Reye syndrome [6], bacterial coinfections with sepsis, or multiple organ failure [7]. Because the patient’s condition was less severe and devoid of such complications, hypoglycemia was unlikely because of influenza infection in the present case.
Only a single episode of hypoglycemia was observed during early morning, and therefore we could not measure plasma insulin or GH levels when hypoglycemia occurred. Nonetheless, given the lack of both recurrence and a pancreatic tumor, it appeared unlikely that hypoglycemia was because of insulinoma.
GH deficiency was also unlikely because GH level was normal in a prior assessment.
On the basis of the above factors, the patient was diagnosed with AI because of GC withdrawal with hypoglycemia as an associated clinical manifestation.
AI is a critical and sometimes life-threating disease with clinical manifestation of relatively low production of GCs, with or without deficiency of mineralocorticoids and adrenal androgens. According to the underlying mechanism, AI is classified as primary or secondary. Primary AI results from destruction of adrenal glands because of autoimmune adrenalitis (Addison’s disease), tuberculosis, hemorrhage, or primary or metastatic cancer. Secondary AI results from interference of synthesis of adrenal cortisol when regions along the hypothalamic–pituitary–adrenal (HPA) axis are affected. GC withdrawal is the leading cause of secondary AI.
FSGS is the glomerular disease that most commonly leads to ESRD. It is strongly associated with refractory NS, and requires prolonged and aggressive treatment, including GCs or other immunosuppressants [8].
Chronic use of GCs impairs the HPA axis [9]. Although suppression of the HPA axis may cause atrophy of the adrenal glands, they may maintain the capacity to secrete adequate amounts of steroids for physiological requirements [1]. AI may be precipitated by stressors, such as severe infection or surgery, which increase the demand for GCs.
In the present case, long-term administration of GCs was incidentally discontinued only 2 days before influenza was contracted. It is conceivable that latent HPA suppression caused by prolonged use of GCs became obvious through influenza infection.
Despite the suppressed cortisol level, ACTH was unelevated and remained relatively low.
These observations may be compatible with a previous report that indicated that exogeneous GCs suppress secretion of ACTH as well as cortisol [10].
ACTH was elevated gradually, and eventually exceeded normal level at the time of ACTH stimulation test (Table 2). The levels of anterior pituitary hormones other than ACTH (TSH, GH, PRL, LH, FSH) were normal.
Taken together, absolute and relative suppressions of cortisol and ACTH, respectively, were believed to be because of GC withdrawal rather than hypopituitarism.
Malignancy and primary hyperparathyroidism account for most cases (90%) of hypercalcemia. Other causes include sarcoidosis, immobilization, and drugs (vitamin D analogs, calcium preparations) [11]. Notably, hypercalcemia in hemodialysis patients is often caused by severe secondary hyperparathyroidism and/or drugs, such as vitamin D analogs and calcium-containing phosphorous binders. AI is an unusual cause of hypercalcemia [12, 13].
In the present case, on the basis of the high effectiveness of hydrocortisone and absence of other factors, AI was considered the cause of hypercalcemia.
The mechanisms underlying hypercalcemia in AI are incompletely understood. However, causative mechanisms include increased intestinal and bone absorption, and decreased renal excretion of Ca [14, 15].
There is little evidence that increased intestinal Ca absorption is the mechanism underlying AI, while dietary Ca content did not affect serum Ca levels in either clinical [14] or animal [16] studies. This was also unlikely in the present case because the patient experienced loss of appetite.
Increased Ca efflux into circulation from bone with accelerated reabsorption may have been a causative factor of hypercalcemia because serum TRACP-5b was elevated. Elevation of osteocalcin was sustained even after recovery of AI, and therefore could have been related to accumulation in serum because of decreased renal clearance rather than reflecting a change in bone metabolic status because of AI [17].
It was reported that, compared with patients with preserved diuresis, anuric hemodialysis patients tend to develop more severe hypercalcemia because of AI [18]. In the present case, the primary underlying mechanism of hypercalcemia may have been decreased renal excretion concomitant with renal insufficiency.
It was also reported that concurrent hyperthyroidism may exacerbate hypercalcemia because of AI, because GCs inhibit thyroxine-induced Ca mobilization from bone. In addition, hypercalcemia was prominent, especially in the presence of decreased serum pH [19]. Except for hypothyroidism, both obvious thyrotoxicosis and euthyroid status can be sufficient to induce hypercalcemia because of AI [20, 21]. It is uncertain whether preserved thyroid function contributed to emphasize hypercalcemia in this patient. Even if this occurred, it is unlikely that low pH is involved in its mechanism, because frequent hemodialysis had no effect on hypercalcemia.
GCs are widely used for treating diseases underlying renal failure, including primary or secondary glomerulonephritis, collagen diseases (e.g., vasculitis, systemic lupus erythematosus, rheumatoid arthritis), or following renal transplantation. GCs are frequently tapered and/or discontinued upon development of end-stage renal failure or in cases considered to be non-responders (no longer expected to demonstrate improvement in clinical outcomes) to minimize and avoid adverse effects from their continued use [22]. Even if asymptomatic or unrecognized, patients treated with GCs can be affected with biochemical AI and may be at risk of manifested AI or adrenal crisis requiring prompt diagnosis and treatment, particularly when stressors including infection are present. Meanwhile, no consensus has been established regarding prevalence, clinical manifestations, or therapeutic regimens, for prevention of AI.
It was recently reported that basal cortisol levels of > 8.1 µg/dl and < 4.3 µg/dl are cutoff values for preserved adrenal function and biochemical AI, respectively. Moreover, alternate-day dosing of GCs or change to hydrocortisone prior to complete discontinuation of GCs may be effective for preventing adrenal crisis in cases with biochemical AI [23]. Further studies and case analyses are required to fully elucidate how AI can be prevented in the context of discontinuation of GCs.
Although AI is a relatively rare cause of hypercalcemia [4], hemodialysis patients tend to experience hypercalcemia more frequently because of decreased renal Ca excretion and use of drugs, such as vitamin D analogs and calcium-containing phosphate binders. Therefore, AI should be considered the differential diagnosis of hypercalcemia in dialysis patients with withdrawal of GCs, especially when stressors such as surgery or severe infection are present, which increase the demand for GCs.
In conclusion, clinicians should be aware of AI in patients experiencing steroid withdrawal upon development of ESRD, especially in the presence of stressors such as infection with influenza. Additionally, AI should be considered a critical disease for the differential diagnosis of hypercalcemia in hemodialysis patients.
Acknowledgements
The authors are grateful to Dr. S. Tanemura, Dr. M. Yamamoto, Dr. S. Ishiguro, and Dr. Y. Matsubayashi (Department of Hematology, Endocrinology and Metabolism, Faculty of Medicine, Niigata University, Niigata, Japan) who provided endocrinological tests.
Declarations
Conflict of interest
All the authors have declared no competing interest.
Human and animal rights
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Informed consent was obtained from the participant in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dinsen S, Baslund B, Klose M, Rasmussen AK, Friis-Hansen L, Hilsted L, Feldt-Rasmussen U. Why glucocorticoid withdrawal may sometimes be as dangerous as the treatment itself. Eur J Intern Med. 2013;24:714–720. doi: 10.1016/j.ejim.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 2.Liu D, Ahmet A, Ward L, Krishnamoorthy P, Mandelcorn ED, Leigh R, Brown JP, Cohen A, Kim H. A practical guide to the monitoring and management of the complications of systemic corticosteroid therapy. Allergy Asthma Clin Immunol. 2013;9:30. doi: 10.1186/1710-1492-9-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Broersen LH, Pereira AM, Jorgensen JO, Dekkers OM. Adrenal insufficiency in corticosteroids use: systematic review and meta-analysis. J Clin Endocrinol Metab. 2015;100:2171–2180. doi: 10.1210/jc.2015-1218. [DOI] [PubMed] [Google Scholar]
- 4.Nerup J (1974) Addison's disease–clinical studies. A report for 108 cases. Acta Endocrinol (Copenh) 76:127–41. [DOI] [PubMed]
- 5.Kaysen GA. Plasma composition in the nephrotic syndrome. Am J Nephrol. 1993;13:347–359. doi: 10.1159/000168649. [DOI] [PubMed] [Google Scholar]
- 6.Reye RD, Morgan G, Baral J. Encephalopathy and fatty degeneration of the viscera. A disease entity in childhood. Lancet. 1963;2:749–752. doi: 10.1016/S0140-6736(63)90554-3. [DOI] [PubMed] [Google Scholar]
- 7.Weis S, Carlos AR, Moita MR, Singh S, Blankenhaus B, Cardoso S, Larsen R, Rebelo S, Schauble S, Del Barrio L, Mithieux G, Rajas F, Lindig S, Bauer M, Soares MP. Metabolic Adaptation Establishes Disease Tolerance to Sepsis. Cell. 2017;169(1263–75):e14. doi: 10.1016/j.cell.2017.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haas M, Meehan SM, Karrison TG, Spargo BH. Changing etiologies of unexplained adult nephrotic syndrome: a comparison of renal biopsy findings from 1976–1979 and 1995–1997. Am J Kidney Dis. 1997;30:621–631. doi: 10.1016/S0272-6386(97)90485-6. [DOI] [PubMed] [Google Scholar]
- 9.Schlaghecke R, Kornely E, Santen RT, Ridderskamp P. The effect of long-term glucocorticoid therapy on pituitary-adrenal responses to exogenous corticotropin-releasing hormone. N Engl J Med. 1992;326:226–230. doi: 10.1056/NEJM199201233260403. [DOI] [PubMed] [Google Scholar]
- 10.Salassa RM, Bennett WA, Keating FR, Jr, Sprague RG. Postoperative adrenal cortical insufficiency; occurrence in patients previously treated with cortisone. J Am Med Assoc. 1953;152:1509–1515. doi: 10.1001/jama.1953.03690160009004. [DOI] [PubMed] [Google Scholar]
- 11.Ziegler R. Hypercalcemic crisis. J Am Soc Nephrol. 2001;12(Suppl 17):S3–9. doi: 10.1681/ASN.V12suppl_1s3. [DOI] [PubMed] [Google Scholar]
- 12.Vasikaran SD, Tallis GA, Braund WJ. Secondary hypoadrenalism presenting with hypercalcaemia. Clin Endocrinol (Oxf) 1994;41:261–264. doi: 10.1111/j.1365-2265.1994.tb02540.x. [DOI] [PubMed] [Google Scholar]
- 13.Jacobs TP, Bilezikian JP. Clinical review: rare causes of hypercalcemia. J Clin Endocrinol Metab. 2005;90:6316–6322. doi: 10.1210/jc.2005-0675. [DOI] [PubMed] [Google Scholar]
- 14.Muls E, Bouillon R, Boelaert J, Lamberigts G, Van Imschoot S, Daneels R, De Moor P. Etiology of hypercalcemia in a patient with Addison's disease. Calcif Tissue Int. 1982;34:523–526. doi: 10.1007/BF02411297. [DOI] [PubMed] [Google Scholar]
- 15.Montoli A, Colussi G, Minetti L. Hypercalcaemia in Addison's disease: calciotropic hormone profile and bone histology. J Intern Med. 1992;232:535–540. doi: 10.1111/j.1365-2796.1992.tb00636.x. [DOI] [PubMed] [Google Scholar]
- 16.Walser M, Robinson BH, Duckett JW., Jr The hypercalcemia of adrenal insufficiency. J Clin Invest. 1963;42:456–465. doi: 10.1172/JCI104734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shidara K, Inaba M, Okuno S, Yamada S, Kumeda Y, Imanishi Y, Yamakawa T, Ishimura E, Nishizawa Y. Serum levels of TRAP5b, a new bone resorption marker unaffected by renal dysfunction, as a useful marker of cortical bone loss in hemodialysis patients. Calcif Tissue Int. 2008;82:278–287. doi: 10.1007/s00223-008-9127-4. [DOI] [PubMed] [Google Scholar]
- 18.Sakao Y, Sugiura T, Tsuji T, Ohashi N, Yasuda H, Fujigaki Y, Kato A. Clinical manifestation of hypercalcemia caused by adrenal insufficiency in hemodialysis patients: a case-series study. Intern Med. 2014;53:1485–1490. doi: 10.2169/internalmedicine.53.1104. [DOI] [PubMed] [Google Scholar]
- 19.Jowsey J, Simons GW. Normocalcaemia in relation to cortisone secretion. Nature. 1968;217:1277–1279. doi: 10.1038/2171277a0. [DOI] [PubMed] [Google Scholar]
- 20.Patel MC, Clayton RN. Secondary hypoadrenalism with hypercalcaemia. Clin Endocrinol (Oxf) 1994;41:839–840. doi: 10.1111/j.1365-2265.1994.tb02803.x. [DOI] [PubMed] [Google Scholar]
- 21.Kelestimur F, Bayram F. Hypercalcaemia and secondary hypoadrenalism due to Sheehan's syndrome. Clin Endocrinol (Oxf) 1995;43:769–770. doi: 10.1111/j.1365-2265.1995.tb00551.x. [DOI] [PubMed] [Google Scholar]
- 22.Ponticelli C, Graziani G. Current and emerging treatments for idiopathic focal and segmental glomerulosclerosis in adults. Expert Rev Clin Immunol. 2013;9:251–261. doi: 10.1586/eci.12.109. [DOI] [PubMed] [Google Scholar]
- 23.Karangizi AHK, Al-Shaghana M, Logan S, Criseno S, Webster R, Boelaert K, Hewins P, Harper L. Glucocorticoid induced adrenal insufficiency is common in steroid treated glomerular diseases - proposed strategy for screening and management. BMC Nephrol. 2019;20:154. doi: 10.1186/s12882-019-1354-6. [DOI] [PMC free article] [PubMed] [Google Scholar]