Abstract
To develop an alternative genotyping tool, the genetic diversity of Encephalitozoon hellem was examined at the polar tube protein (PTP) locus. Nucleotide sequence analysis of the PTP gene divided 24 E. hellem isolates into four genotypes, compared to two genotypes identified by analysis of the internal transcribed spacer of the rRNA gene. The four PTP genotypes differed from each other by the copy number of the 60-bp central repeat as well as by point mutations. A simple PCR test was developed to differentiate E. hellem genotypes based on the difference in the size of PTP PCR products, which should facilitate the genotyping of E. hellem in clinical samples.
Encephalitozoon hellem is one of the four most common human microsporidian parasites. Thus far, humans are the only identified mammalian hosts, although microsporidiosis caused by E. hellem is probably common in birds (2, 23, 32, 34, 36). Because all human cases, except the single case mentioned here, have been reported in AIDS patients, it has been suggested that E. hellem infection in humans is opportunistic, and the parasite may be of zoonotic origin. Concurrent E. hellem infection of both humans and their companion birds, however, has not yet been documented (4).
Molecular tools have been developed and employed to delineate the transmission of human microsporidiosis. Characterization of the internal transcribed spacer (ITS) of the rRNA gene has identified three genotypes of Encephalitozoon cuniculi based on the number of GTTT repeats present: genotype I (originally isolated from a rabbit) containing three repeats, genotype II (originally isolated from a mouse) containing two repeats, and genotype III (originally isolated from a dog) containing four repeats (11). Both genotypes I and III of E. cuniculi have been found in humans, indicating that E. cuniculi of animal origin may be a source of human infection (6, 10, 25, 27, 33). ITS sequence differences of Enterocytozoon bieneusi have also been reported among different humans infected with this parasite (16, 26). Additionally, ITS sequence differences have also been shown in E. bieneusi infecting different species of domestic animals (3, 5, 6, 16, 19, 24–26). However, the zoonotic potential of E. bieneusi from animals is not yet clear.
Genetic diversity probably also exists in E. hellem. A recent ITS sequence characterization of five human isolates from Europe and Africa has identified three genotypes of E. hellem (21). The extent and significance of genetic diversity in E. hellem, however, are not yet clear. There is also a need for the development of simpler genotyping tools targeting other genes to define the epidemiology of human E. hellem infection. Recently, a gene coding for the polar tube protein (PTP) of E. hellem has been reported (15). Because the gene has long central repeats of 60 bp and the number of repeats in repetitive proteins tends to vary in other parasites, such as Plasmodium spp., we examined the sequence diversity of the PTP gene among various isolates of E. hellem.
MATERIALS AND METHODS
Parasite isolates and DNA extraction.
The E. hellem isolates used in this study included 24 human isolates from 20 patients in the United States, Puerto Rico, Italy, Switzerland, and Spain (Table 1). E. hellem diagnosis was made by a combination of electron microscopy and species-specific PCR analysis. All isolates were maintained in E6 and HLF cell cultures (37) after inoculation with patient samples, including biopsy, bronchoalveolar, sputum, and urine samples. DNA was extracted from cultured parasites using a phenol-chloroform method previously described (12). Nucleic acid from each sample was resuspended in 50 μl of distilled water and stored at −20°C before being used in PCR.
TABLE 1.
Human E. hellem isolates used in this study and genotyping results from analyses of ITS, SSU rRNA, and PTP genes
| Isolate | Origina | Sex/age (yr)b | Isolation (mo/yr) | Country/state | ITS genotypec | SSU rRNA genotyped | PTP genotype | Reference(s) |
|---|---|---|---|---|---|---|---|---|
| CDC:V278 | Urine | M | 11/92 | Puerto Rico | 1A | 1A | 1A | 37 |
| CDC:V242 | Urine | M/26* | 01/92 | USA/Ga. | 1A | 1A | 1A | 31, 37 |
| CDC:V274 | BAL | M/26* | 10/92 | USA/Ga. | 1A | 1A | 1A | 31, 37 |
| Le | Conjunctiva | M/46 | 12/89 | USA/N.Y. | 1A | 1A | 1A | 7, 13 |
| CDC:V257 | Urine | F/35† | 06/92 | USA/N.Y. | 1A | 1A | 1A | 31, 37 |
| CDC:V258 | Sputum | F/35† | 06/92 | USA/N.Y. | 1A | 1A | 1A | 31, 37 |
| CDC:V281 | Nasal | F/35† | 12/92 | USA/N.Y. | 1A | 1A | 1A | 31, 37 |
| Wo | Conjunctiva | M/37 | 1994 | USA/Mass. | 1A | 1A | 1A | 7, 9 |
| Ye | Cornea | M/29 | 12/89 | USA/Tex. | 1A | 1A | 1A | 7, 40 |
| Ly | Cornea | M/30 | 1990 | USA/Ohio | 1A | 1A | 1A | Unpublished data |
| He | Conjunctiva | M/33 | 12/89 | USA/N.Y. | 1A | 1A | 1B | 7, 13 |
| CDC:V213 | Urine | M/30 | 02/91 | USA/Ga. | 1A | 1A | 1B | 31, 37 |
| CDC:SP-1 | BAL | M/41 | 1996 | Spain | 1A | 1A | 1B | 22 |
| LEPV-2-93 | BAL | M/27 | 10/93 | Italy | 1A | 1A | 1B | 30, 37 |
| PV-3-93 | BAL | M/30 | 12/93 | Italy | 1A | 1A | 1B | 29, 37 |
| PV-8-95 | Sputum | F/34 | 03/95 | Italy | 1A | 1C | 1C | 14, 37 |
| VRPV-1-93 | Urine | M/33 | 09/93 | Italy | 1A | 1C | 1C | 14, 37 |
| PV-5-95 | BAL | M/38 | 01/95 | Italy | 1A | 1C | 1C | 29, 37 |
| PV-11-98 | Nasal | M/34 | 01/98 | Italy | 1A | 1C | 1C | 14, 37 |
| PV-10-97 | Ocular | M/12● | 01/97 | Italy | 1A | 1C | 1C | 14, 37 |
| PV-10-97EI | Sputum | M/12● | 01/97 | Italy | 1A | 1C | 1C | 14, 37 |
| MIPV-6-95 | BAL | F/33 | 10/95 | Italy | 1A | 1C | 1C | 14, 37 |
| PV-9-96 | BAL | M/38e | 07/96 | Italy | 1A | 1C | 1C | 28 |
| CDC:V261 | Urine | M/49 | 06/92 | Switzerland | 2B | 2B | 2B | 37, 38 |
BAL, bronchoalveolar lavage.
Superscript symbols indicate multiple samples from the same patient with the same symbol.
Genotypes 1A, 1B, and 1C had an identical ITS sequence.
Genotypes 1A and 1B had an identical SSU rRNA sequence.
HIV negative.
PCR and sequence analysis.
The complete genes coding for the SSU rRNA and ITS and a 1,253-bp fragment of the PTP gene were amplified from DNA of each sample by PCR. The primer sets used were MICRO-F (5′-CACCAGGTTGATTCTGCCTGA-3′) and 1492N4 (5′-CCAACTGAAACCTTGTTACGACTT-3′) for SSU rRNA and ss1061f (5′-GGTGGTGCATGGCCG-3′) and ls212r1 [5′-GTT(G/A)GTTTCTTTTCCTC-3′] for the ITS (39). A fragment of the PTP of 1,253 bp was amplified from DNA of all E. hellem isolates by PCR using primers 5′-ATGAAAGGTATTTCGAAGAT-3′ (nucleotides 124 to 143) and 5′-GCCTCCATGGCATACTGC-3′ (nucleotides 1359 to 1376), based on a PTP sequence (AF044915) previously published by Keohane et al. (15). The PCR products were sequenced in both directions on an ABI377 autosequencer (Applied Biosystems, Foster City, Calif.). The sequences obtained were aligned with each other and the published sequence using the Wisconsin package (version 9.0; Genetics Computer Group, Madison, Wis.).
Genotyping by direct PCR analysis of PTP.
Based on the results of PTP gene sequencing, a simple length polymorphism-based PCR genotyping technique was developed. A fragment of the PTP of 461 to 611 bp was amplified from E. hellem DNA by PCR using primers 5′-CATGCTTGCCAACACAGG-3′ (nucleotides 764 to 781 of AF044915), and 5′-TGGAGGCATTGCAATAGG-3′ (nucleotides 1207 to 1224 of AF044915). The PCR products were differentiated by electrophoresis in agarose gel, using 100-bp ladders (Life Technologies, Grand Island, N.Y.) as molecular size markers.
Nucleotide sequence accession numbers.
The SSU rRNA, ITS, and PTP nucleotide sequences of E. hellem were deposited in the GenBank database under accession no. AF33836 to AF338368 and AY024342.
RESULTS
Sequence analysis of SSU rRNA.
The complete gene coding for SSU rRNA was sequenced for each E. hellem isolate. Three types of sequences were obtained from the 20 isolates studied. Fifteen isolates had SSU rRNA sequences identical to the genotype 1 sequence (genotype 1A or 1B in Table 2) reported before (20). Eight isolates had a similar sequence except for an insertion of G at position 162 (genotype 1C in Table 2). One isolate (CDC:V261) had an SSU rRNA sequence identical to the genotype 2 (genotype 2A or 2B in Table 2) sequence previously described (20), which had seven nucleotide base differences from genotype 1 (Tables 1 and 2).
TABLE 2.
Sequence differences in the SSU rRNA among E. hellem genotypesa
| Genotype | Nucleotide at position:b
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 119 | 160 | — | 171 | 505 | 517 | 538 | 539 | 952 | 1013 | |
| (119) | (160) | (162) | (172) | (506) | (518) | (539) | (540) | (953) | (1014) | |
| 1A | A | G | — | G | A | G | T | A | A | G |
| 1B | A | G | — | G | A | G | T | A | A | G |
| 1C | A | G | G | G | A | G | T | A | A | G |
| 2A | A | T | — | T | T | A | G | G | A | A |
| 2B | A | T | — | T | T | A | G | G | A | A |
| 2C | T | T | — | T | T | A | G | G | G | A |
Genotypes 1A and 1B = genotype 1 of Mathis et al.; genotype 2A and genotype 2B = genotype 2 of Mathis et al.; genotype 2C = genotype 3 of Mathis et al. (21).
Position in 1C shown in parentheses.
Sequence analysis of ITS.
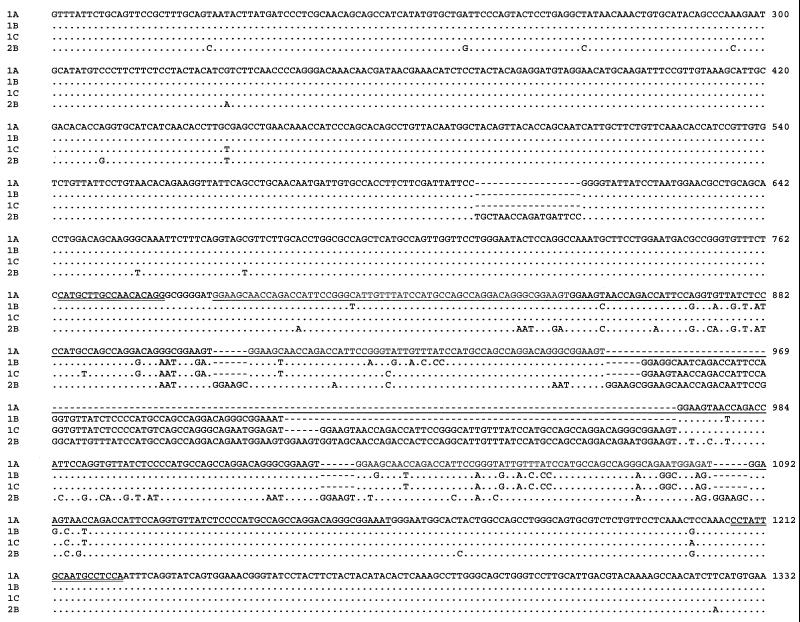
All E. hellem isolates used in this study were also sequenced for the ITS gene. Nucleotide sequences obtained for 23 of the 24 isolates were identical to the genotype 1 sequence previously reported (genotype 1A, 1B, or 1C in Fig. 1) (21). One isolate (CDC:V261), however, had an ITS sequence similar to those of genotypes 2 (genotype 2A in Fig. 1) and 3 (genotype 2C in Fig. 1) reported before (21). Differences between these and other E. hellem genotypes are shown in Fig. 1.
FIG. 1.
Sequence differences in the rRNA ITS among E. hellem genotypes. Dots denote sequence identity to genotype 1A, and dashes depict nucleotide deletions. Genotypes 1A, 1B, and 1C = genotype 1 of Mathis et al.; genotype 2A = genotype 2 of Mathis et al.; genotype 2C = genotype 3 of Mathis et al. (21).
Sequence analysis of PTP.
All E. hellem isolates were also analyzed at the PTP locus. Although a PCR product of 1,253 bp was expected from each isolate, this was only the case for 10 isolates. Other isolates had PTP PCR products larger than 1,253 bp. Four different sizes of PTP PCR products were detected, as reflected in the different migration rates in agarose gel electrophoresis (data not shown).
DNA sequencing analysis of PCR products confirmed the presence of four PTP genotypes. Genotype 1A was generated from the smallest PCR products from the 10 E. hellem isolates, 1,253 bp in length, and each was identical to the published sequence (AF044915). In contrast, genotypes 1B, 1C, and 2B were 1,313-, 1,373- and 1,421-bp long and found in five, eight, and one isolate, respectively (Table 1 and Fig. 2). Differences in PTP sequence length among genotypes 1A, 1B, and 1C were due to variations in the number of a 60-bp tandem repeat: each of them had six, seven, and eight copies of the 60-bp repeat, respectively. Genotype 2B had three copies of the 60-bp repeat and five copies of a 66-bp repeat. In the 66-bp repeat, a 6-bp sequence (GGAAGC or GGAAGT) was repeated once at the beginning of the 60-bp repeat (Fig. 2). In addition, genotype 2B also had an 18-bp insert prior to the repeat region. Sequence variations among genotypes were also seen in the repeat and nonrepeat regions.
FIG. 2.
Sequence diversity among E. hellem genotypes in the PTP gene. Dots denote sequence identity to genotype 1A (AF044915), and dashes depict nucleotide deletions. The repeat region is underlined, and the primer sequences used in direct genotyping PCR are double underlined. The numbers at the ends of lines are nucleotide positions in AF044915.
Differentiation of E. hellem genotypes by direct PCR.
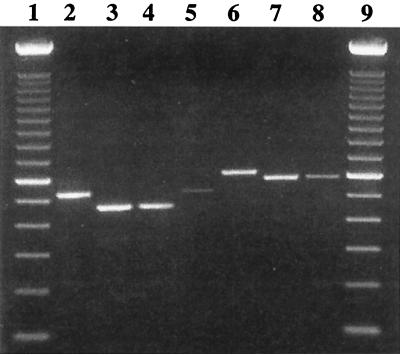
Because of the length polymorphism among E. hellem genotypes in the PTP gene, a set of primers (5′-CATGCTTGCCAACACAGG-3′ and 5′-TGGAGGCATTGCAATAGG-3′) was developed for the detection and differentiation of human E. hellem by direct PCR analysis. This primer set was designed to generate PCR products of predicted sizes of 461, 521, 581, and 611 bp for genotypes 1A, 1B, 1C, and 2B, respectively. Testing of this PCR primer set with E. hellem DNA of known genotypes produced PCR products concordant with the expected sizes, which were easily differentiated from each other in agarose gel electrophoresis (Fig. 3).
FIG. 3.
Genotyping E. hellem isolates by PCR analysis of the PTP gene. Lanes 1 and 9, 100-bp ladders; lanes 2 and 5, genotype 1B; lanes 3 and 4, genotype 1A; lane 6, genotype 2B; and lanes 7 and 8, genotype 1C.
DISCUSSION
Genotyping microsporidian parasites in clinical samples is helpful to the understanding of the transmission of human microsporidiosis. Since the discovery of three genotypes of E. cuniculi in 1995 (10), there has been increasing interest in genotyping of human-pathogenic microsporidian parasites, including E. cuniculi, E. hellem, Encephalitozoon intestinalis, and E. bieneusi (1–3, 6, 8, 10, 16–19, 21, 24–26). With the exception of the use of pulsed-field gel electrophoresis and karyotyping (1, 35), most of the genotyping studies targeted the ITS. Different genotypes have been found in E. bieneusi and E. hellem in addition to E. cuniculi (16, 19, 21, 24–26). Because most of the genotyping techniques involve DNA sequencing, these techniques are time-consuming, expensive, and not widely used in diagnostic laboratories. Therefore, alternative techniques and genetic loci are needed for better characterization of the molecular epidemiology of microsporidiosis and population and genetic structure of microsporidia.
Results of this study suggest that the PTP gene may be a good target for genotype analysis. Four genotypes of E. hellem were found in the 24 isolates analyzed at this genetic locus. This typing resolution is much higher than that produced by sequence analysis of the ITS, which yielded two genotypes. In fact, the typing resolution at the ITS locus was even lower than the sequence analysis of SSU rRNA, which divided the 24 isolates into three genotypes. The PTP gene had an additional advantage of having length polymorphism. Thus, genotypes 1A, 1B, and 1C had six, seven, and eight copies of the 60-bp central repeat. Genotype 2B also had eight copies of the central repeat, but five copies of the repeat were 66 bp in length, with a smaller 6-bp repeat at the beginning of the 60-bp repeat. This length polymorphism in PTP enabled the differentiation of the E. hellem genotypes by electrophoresis of PCR products without restriction digestion or sequence analysis.
DNA strand slippage during parasite replication probably plays a role in the evolution of length polymorphism in the PTP gene. First, this length polymophism in PTP occurred in the repeat region, and each genotype differed from the others by the deletion or insertion of one or more copies of the repeat, indicating that strand slippage by DNA polymerase during genome duplication some time in the long evolution of E. hellem was probably responsible for the length polymorphism. Second, the strand slippage theory was also supported by the insertion of a 6-bp sequence (GGAAGC or GGAAGT) in some copies of the central repeat of genotype 2B. This 6-bp sequence itself was a repetitive element, which was present at the beginning and end of each 60-bp repeat in tandem. Thus, genotypes 1A, 1B, and 1C had two copies of GGAAGC or GGAAGT at the junction of the 60-bp repeat, whereas genotype 2B had two or three copies of GGAAGC or GGAAGT tandem repeat at the junction of the 60-bp or 66-bp repeat. Third, the strand slippage theory was further supported by the insertion of an 18-bp sequence (TGCTAACCAGATGATTCC) in the nonrepeat region in genotype 2B. This 18-bp insert occurred after the sequence GATTATTCC , a variant of which (underlined) was present at the 3′ end of the insert. Again, duplication error was likely the cause of an additional insert in genotype 2B.
Among the four E. hellem genotypes found in the 24 samples, genotypes 1A, 1B, and 1C are apparently more related to each other genetically than to genotype 2B. This was reflected by the genetic distances among the four genotypes at the ITS, SSU rRNA, and PTP genes. Genotypes 1A, 1B, and 1C had identical ITS sequence, a 1-nucleotide difference in the SSU rRNA gene, and very limited sequence differences in the nonrepetitive region of the PTP gene. In contrast, genotype 2B had much different ITS and SSU rRNA sequences and more extensive changes in both the repeat and nonrepeat regions of the PTP gene (Table 2 and Fig. 1 and 2).
More E. hellem genotypes are apparently present. A previous characterization of E. hellem at the ITS and SSU rRNA loci by Mathis et al. revealed the presence of three genotypes in five isolates from humans: genotype 1 in one isolate, genotype 2 in three isolates, and genotype 3 in one isolate (21). Genotype 1 of Mathis et al. (21) had the identical ITS as genotypes 1A, 1B, and 1C and an SSU rRNA sequence identical to 1A and 1B in this study. Genotypes 2 and 3 of Mathis et al. (21). however, had ITS and SSU rRNA sequences similar but not identical to genotype 2B in this study. Thus, there are at least six E. hellem genotypes. Multiple alignment of all ITS and SSU rRNA sequences indicates that there are two groups of E. hellem parasites: genotypes 1A, 1B, and 1C are related to each other and can be grouped together, whereas genotypes 2 and 3 of Mathis et al. (21) and genotype 2B in this study are related to each other and form a second group (Table 2 and Fig. 1). Although PTP sequences are not available from genotypes 2 and 3 by Mathis et al., judged by the sequence divergence from genotypes 1A, 1B, and 1C by 2B, they are also likely to be more divergent from these genotypes. We therefore suggest renaming genotypes 2 and 3 of Mathis et al. as genotypes 2A and 2C, respectively, to reflect their relatedness to genotype 2B as described here.
The significance of the genotypic diversity in E. hellem is unclear. The only nonhuman hosts for E. hellem known are birds. Two of the avian E. hellem isolates reported have been sequenced for ITS (34, 36) and produced sequences identical to genotype 1 in humans, indicating that human E. hellem infection could be of zoonotic origin under certain circumstances. Currently, the number of E. hellem isolates genotyped is very limited and does not allow a meaningful comparison of genotype distribution between humans and birds. The data accumulated so far do suggest the presence of possible geographic segregation of certain genotypes. For example, the eight genotype 1C E. hellem isolates found in this study were all from Italy, and 10 genotype 1A isolates were all from the United States and its protectorate Puerto Rico. Similarly, the rarer E. hellem genotypes 2A (two of the three isolates in reference 21), 2B (one isolate in this study), and 2C (one isolate in reference 21) identified so far were from patients in Switzerland, with the exception of one genotype 2A isolate from Tanzania.
In summary, results of this study indicate the existence of extensive genetic diversity in E. hellem isolates from humans. This genetic diversity was previously underestimated by the analysis of ITS sequence, but now can be assessed easily by analysis of the repetitive region of the PTP gene. More extensive epidemiologic studies and characterizations of large number of isolates from humans and birds are needed to evaluate the significance of the genetic diversity and the role of birds in human E. hellem infection. These studies are now more feasible with the development of a simple E. hellem genotyping technique in this study using direct PCR.
ACKNOWLEDGMENTS
We thank Fernando Bornay-Llinares, Rainer Weber, and Ralph Bryan for providing either cultures of E. hellem or patient samples containing E. hellem, and Mary E. Bartlett and Daniel G. Colley for suggestions on improving the manuscript.
ADDENDUM IN PROOF
A recent report by Peuvel et al. (I. Peuvel, F. Delbac, G. Metenier, P. Peyret, and C. P. Vivares, Parasitology 121:581–587, 2000) showed that two human Encephalitozoon hellem isolates differed from each other in the polar tube protein gene sequences.
REFERENCES
- 1.Biderre C, Mathis A, Deplazes P, Weber R, Metenier G, Vivares C P. Molecular karyotype diversity in the microsporidian Encephalitozoon cuniculi. Parasitology. 1999;118:439–445. doi: 10.1017/s0031182099004023. [DOI] [PubMed] [Google Scholar]
- 2.Black S S, Steinohrt L A, Bertucci D C, Rogers L B, Didier E S. Encephalitozoon hellem in budgerigars (Melopsittacus undulatus) Vet Pathol. 1997;34:189–198. doi: 10.1177/030098589703400303. [DOI] [PubMed] [Google Scholar]
- 3.Breitenmoser A C, Mathis A, Burgi E, Weber R, Deplazes P. High prevalence of Enterocytozoon bieneusi in swine with four genotypes that differ from those identified in humans. Parasitology. 1999;118:447–453. doi: 10.1017/s0031182099004229. [DOI] [PubMed] [Google Scholar]
- 4.Bryan R T, Schwartz D A. Epidemiology of microsporidiosis. In: Wittner M, editor. Microsporidia and microsporidiosis. Washington, D.C.: ASM Press; 1999. pp. 502–530. [Google Scholar]
- 5.del Aguila C, Izquierdo F, Navajas R, Pieniazek N J, Miro G, Alonso A I, Da Silva A J, Fenoy S. Enterocytozoon bieneusi in animals: rabbits and dogs as new hosts. J Eukaryot Microbiol. 1999;46:8S–9S. [PubMed] [Google Scholar]
- 6.Deplazes P, Mathis A, Muller C, Weber R. Molecular epidemiology of Encephalitozoon cuniculi and first detection of Enterocytozoon bieneusi in faecal samples of pigs. J Eukaryot Microbiol. 1996;43:93S. doi: 10.1111/j.1550-7408.1996.tb05018.x. [DOI] [PubMed] [Google Scholar]
- 7.Didier E S, Didier P J, Friedberg D N, Stenson S M, Orenstein J M, Yee R W, Tio F O, Davis R M, Vossbrinck C, Millichamp N, Shadduck J A. Isolation and characterization of a new human microsporidian, Encephalitozoon hellem (n. sp), from three AIDS patients with keratoconjunctivitis. J Infect Dis. 1991;163:617–621. doi: 10.1093/infdis/163.3.617. [DOI] [PubMed] [Google Scholar]
- 8.Didier E S, Didier P J, Snowden K F, Shadduck J A. Microsporidiosis in mammals. Microbes Infect. 2000;2:709–720. doi: 10.1016/s1286-4579(00)00354-3. [DOI] [PubMed] [Google Scholar]
- 9.Didier E S, Rogers L B, Brush A D, Wong S, Traina-Dorge V, Bertucci D. Diagnosis of disseminated microsporidian Encephalitozoon hellem infection by PCR-Southern analysis and successful treatment with albendazole and fumagillin. J Clin Microbiol. 1996;34:947–952. doi: 10.1128/jcm.34.4.947-952.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Didier E S, Visvesvara G S, Baker M D, Rogers L B, Bertucci D C, De Groote M A, Vossbrinck C R. A microsporidian isolated from an AIDS patient corresponds to Encephalitozoon cuniculi III, originally isolated from domestic dogs. J Clin Microbiol. 1996;34:2835–2837. doi: 10.1128/jcm.34.11.2835-2837.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Didier E S, Vossbrinck C R, Baker M D, Rogers L B, Bertucci D C, Shadduck J A. Identification and characterization of three Encephalitozoon cuniculi strains. Parasitology. 1995;111:411–421. doi: 10.1017/s0031182000065914. [DOI] [PubMed] [Google Scholar]
- 12.Fedorko D P, Nelson N A, Cartwright C P. Identification of microsporidia in stool specimens by using PCR and restriction endonucleases. J Clin Microbiol. 1995;33:1739–1741. doi: 10.1128/jcm.33.7.1739-1741.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Friedberg D N, Stenson S M, Orenstein J M, Tierno P M, Charles N C. Microsporidial keratoconjunctivitis in acquired immunodeficiency syndrome. Arch Ophthalmol. 1990;108:504–508. doi: 10.1001/archopht.1990.01070060052047. [DOI] [PubMed] [Google Scholar]
- 14.Gatti, Sacchi S L, Novati S, Corona S, Bernuzzi A M, Moura H, Pieniazek N J, Visvesvara G S, Scaglia M. Extraintestinal microsporidiosis in AIDS patients: clinical features and advanced protocols for diagnosis and characterization of the isolates. J Eukaryot Microbiol. 1997;44:79S. doi: 10.1111/j.1550-7408.1997.tb05793.x. [DOI] [PubMed] [Google Scholar]
- 15.Keohane E M, Orr G A, Zhang H S, Takvorian P M, Cali A, Tanowitz H B, Wittner M, Weiss L M. The molecular characterization of the major polar tube protein gene from Encephalitozoon hellem, a microsporidian parasite of humans. Mol Biochem Parasitol. 1998;94:227–236. doi: 10.1016/s0166-6851(98)00071-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liguory O, David F, Sarfati C, Derouin F, Molina J M. Determination of types of Enterocytozoon bieneusi strains isolated from patients with intestinal microsporidiosis. J Clin Microbiol. 1998;36:1882–1885. doi: 10.1128/jcm.36.7.1882-1885.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liguory O, Fournier S, Sarfati C, Derouin F, Molina J M. Genetic homology among thirteen Encephalitozoon intestinalis isolates obtained from human immunodeficiency virus-infected patients with intestinal microsporidiosis. J Clin Microbiol. 2000;38:2389–2391. doi: 10.1128/jcm.38.6.2389-2391.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mathis A. Microsporidia: emerging advances in understanding the basic biology of these unique organisms. Int J Parasitol. 2000;30:795–804. doi: 10.1016/s0020-7519(00)00064-3. [DOI] [PubMed] [Google Scholar]
- 19.Mathis A, Breitenmoser A C, Deplazes P. Detection of new Enterocytozoon genotypes in faecal samples of farm dogs and a cat. Parasite. 1999;6:189–193. doi: 10.1051/parasite/1999062189. [DOI] [PubMed] [Google Scholar]
- 20.Mathis A, Michel M, Kuster H, Muller C, Weber R, Deplazes P. Two Encephalitozoon cuniculi strains of human origin are infectious to rabbits. Parasitology. 1997;114:29–35. doi: 10.1017/s0031182096008177. [DOI] [PubMed] [Google Scholar]
- 21.Mathis A, Tanner I, Weber R, Deplazes P. Genetic and phenotypic intraspecific variation in the microsporidian Encephalitozoon hellem. Int J Parasitol. 1999;29:767–770. doi: 10.1016/s0020-7519(99)00025-9. [DOI] [PubMed] [Google Scholar]
- 22.Peman J, Bornay-Llinares F J, Acosta B, Lopez-Aldeguer J, Meseguer I, Figueras M J, Hernandez A, Peset V, Gobernado M, Visvesvara G S. First report of a case of Encephalitozoon sp. microsporidiosis in a Spanish patient. Res Rev Parasitol. 1997;57:131–134. [Google Scholar]
- 23.Pulparampil N, Graham D, Phalen D, Snowden K. Encephalitozoon hellem in two eclectus parrots (Eclectus roratus): identification from archival tissues. J Eukaryot Microbiol. 1998;45:651–655. doi: 10.1111/j.1550-7408.1998.tb04562.x. [DOI] [PubMed] [Google Scholar]
- 24.Rinder H, Katzwinkel-Wladarsch S, Loscher T. Evidence for the existence of genetically distinct strains of Enterocytozoon bieneusi. Parasitol Res. 1997;83:670–672. doi: 10.1007/s004360050317. [DOI] [PubMed] [Google Scholar]
- 25.Rinder H, Katzwinkel-Wladarsch S, Thomschke A, Loscher T. Strain differentiation in microsporidia. Tokai J Exp Clin Med. 1998;23:433–437. [PubMed] [Google Scholar]
- 26.Rinder H, Thomschke A, Dengjel B, Gothe R, Loscher T, Zahler M. Close genotypic relationship between Enterocytozoon bieneusi from humans and pigs and first detection in cattle. J Parasitol. 2000;86:185–188. doi: 10.1645/0022-3395(2000)086[0185:CGRBEB]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 27.Rossi P, La Rosa G, Ludovisi A, Tamburrini A, Gomez Morales M A, Pozio E. Identification of a human isolate of Encephalitozoon cuniculi type I from Italy. Int J Parasitol. 1998;28:1361–1366. doi: 10.1016/s0020-7519(98)00122-2. [DOI] [PubMed] [Google Scholar]
- 28.Scaglia M, Bandi C, novati S, Gatti S, Bernuzzi A M, Corona S, Sacchi L. Respiratory microsporidiosis due to Encephalitozoon hellem: the first case report in an immunocompetent subject. Parasitol Int. 1998;47:203S. [Google Scholar]
- 29.Scaglia M, Gatti S, Sacchi L, Corona S, Chichino G, Bernuzzi A M, Barbarini G, Croppo G P, Da Silva A J, Pieniazek N J, Visvesvara G S. Asymptomatic respiratory tract microsporidiosis due to Encephalitozoon hellem in three patients with AIDS. Clin Infect Dis. 1998;26:174–176. doi: 10.1086/516264. [DOI] [PubMed] [Google Scholar]
- 30.Scaglia M, Sacchi L, Gatti S, Bernuzzi A M, Polver P P, Piacentini I, Concia E, Croppo G P, Da Silva A J, Pieniazek N J, Slemenda S B, Wallace S, Leitch G J, Visvesvara G S. Isolation and identification of Encephalitozoon hellem from an Italian AIDS patient with disseminated microsporidiosis. APMIS. 1994;102:817–827. [PubMed] [Google Scholar]
- 31.Schwartz D A, Visvesvara G S, Diesenhouse M C, Weber R, Font R L, Wilson L A, Corrent G, serdarevic O N, Rosberger D F, Keenen P C, Grossniklaus H E, Hewan-Lowe K, Bryan R T. Pathologic features and immunofluorescent antibody demonstration of ocular microsporidiosis (Encephalitozoon hellem) in seven patients with acquired immunodeficiency syndrome. Am J Ophthalmol. 1993;115:285–292. doi: 10.1016/s0002-9394(14)73577-9. [DOI] [PubMed] [Google Scholar]
- 32.Snowden K, Logan K. Molecular identification of Encephalitozoon hellem in an ostrich. Avian Dis. 1999;43:779–782. [PubMed] [Google Scholar]
- 33.Snowden K, Logan K, Didier E S. Encephalitozoon cuniculi strain III is a cause of encephalitozoonosis in both humans and dogs. J Infect Dis. 1999;180:2086–2088. doi: 10.1086/315154. [DOI] [PubMed] [Google Scholar]
- 34.Snowden K F, Logan K, Phalen D N. Isolation and characterization of an avian isolate of Encephalitozoon hellem. Parasitology. 2000;121:9–14. doi: 10.1017/s0031182099005995. [DOI] [PubMed] [Google Scholar]
- 35.Sobottka I, Albrecht H, Visvesvara G S, Pieniazek N J, Deplazes P, Schwartz D A, Laufs R, Elsner H A. Inter- and intra-species karyotype variations among microsporidia of the genus Encephalitozoon as determined by pulsed-field gel electrophoresis. Scand J Infect Dis. 1999;31:555–558. doi: 10.1080/00365549950164427. [DOI] [PubMed] [Google Scholar]
- 36.Suter C, Mathis A, Hoop R, Deplazes P. Encephalitozoon hellem infection in a yellow-streaked lory (Chalcopsitta scintillata) imported from Indonesia. Vet Rec. 1998;143:694–695. [PubMed] [Google Scholar]
- 37.Visvesvara G S, Moura H, Leitch G J, Schwartz D A. Culture and propagation of microsporidia. In: Wittner M, editor. microsporidia and microsporidiosis. Washington, D.C.: ASM Press; 1999. pp. 363–392. [Google Scholar]
- 38.Weber R, Kuster H, Visvesvara G S, Bryan R T, Schwartz D A, Lüthy R. Disseminated microsporidiosis due to Encephalitozoon hellem: pulmonary colonization, microhematuria, and mild conjunctivitis in a patient with AIDS. Clin Infect Dis. 1993;17:415–419. doi: 10.1093/clinids/17.3.415. [DOI] [PubMed] [Google Scholar]
- 39.Weiss L M, Vossbrinck C R. Molecular biology, molecular phylogeny, and molecular diagnostic approaches to the microsporidia. In: Wittner M, editor. Microsporidia and microsporidiosis. Washington, D.C.: ASM Press; 1999. pp. 129–195. [Google Scholar]
- 40.Yee R W, Tio F O, Martinez A, Held K S, Shadduck J A, Didier E S. Resolution of microsporidial epithelial keratopathy in a patient with AIDS. Ophthalmology. 1991;98:196–201. doi: 10.1016/s0161-6420(91)32331-5. [DOI] [PubMed] [Google Scholar]