Abstract
MicroRNAs (miRNAs) are potent regulators of multiple biological processes. Previous studies have demonstrated that miR-146a-5p increases in normal mice during aging, while long-living Ames dwarf (df/df) mice maintain youthful levels of this miRNA. The aim of this study was to elucidate the involvement of miR-146a-5p in modulating cellular senescence and apoptosis in visceral adipose tissue of df/df mice and cultured pre-adipocytes. To test the effects of miR-146a-5p overexpression on visceral adipose tissue, wild-type, and df/df mice, were treated with miRNA-negative control-base and df/df were transfected with 4 or 8 µg/g of a miR-146a-5p mimetic, respectively. Effects of miR-146a-5p overexpression were also evaluated in 3T3-L1 cells cultured under high and normal glucose conditions. Treatment with miR-146a-5p mimetic increased cellular senescence and inflammation and decreased pro-apoptotic factors in visceral adipose tissue of df/df mice. The miR-146a-5p mimetic induced similar effects in 3T3-L1 cells cultivated at normal but not high glucose levels. Importantly, 3T3-L1 HG cells in high glucose conditions showed significantly higher expression of miR-146a-5p than 3T3-L1 grown in normal glucose conditions. These results indicate that miR-146a-5p can be a marker for cellular senescence. This miRNA represents one of the significant SASP factors that if not precisely regulated, can accentuate inflammatory responses and stimulate senescence in surrounding non-senescent cells. The role of miR-146a-5p is different in healthy versus stressed cells, suggesting potential effects of this miRNA depend on overall organismal health, aging, and metabolic state.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11357-021-00490-3.
Keywords: miR-146a-5p, Cellular senescence, Visceral adipose tissue, Apoptosis, Pre-Adipocytes
Introduction
The Ames dwarf (df/df) mouse is a well-established model for studies of aging processes. Df/df mice are deficient in growth hormone (GH), thyroid-stimulating hormone (TSH), and prolactin due to a homozygous recessive mutation in the prophet of pituitary factor 1 (prop 1), gene, which encodes a transcription factor responsible for pituitary development [5, 52]. As a result of GH deficiency, df/df mice have secondary deficiencies in IGF-1 [14, 32, 37]. Importantly, df/df mice live between 40 and 60% longer than wild-type littermates [4, 6]. In addition to an extended lifespan, df/df mice also have an extended healthspan, with preservation of insulin sensitivity, glucose tolerance, and cognitive and neuromuscular function and protection from cancer throughout their increased lifespan [20, 22, 35, 37]. In humans, a similar condition recognized as Laron dwarfism is also characterized by deficiencies in IGF-1 and resistance to GH caused by mutations in GH receptors. Moreover, these patients are characterized by dwarfism, obesity, and severe hypoglycemia [9].
We previously showed short-term treatment with GH during early postnatal development shortens the lifespan of df/df mice, suggesting the GH signaling pathway is a major factor in regulating longevity in these mice [41]. Additionally, we showed GH-deficiency alters the pattern of circulating microRNAs (miRNAs), suggesting interactions between genotype and aging [57] and facilitating identification of miRNAs relevant to aging.
miRNAs are a class of small non-coding RNAs ranging from 18 to 25 nucleotides in length. These miRNAs mediate a number of cellular functions through post-transcriptional regulation, leading to modulation of ~ 30% of the human genome [10, 36]. Produced in the nucleus, miRNAs regulate cytoplasmic gene expression [3]. miRNAs are involved in regulating expression by either suppressing translation of protein-coding genes or cleaving target mRNAs, thereby inducing their degradation [26, 36, 46]. miRNAs appear to be important etiological or facilitating factors in the pathogenesis of age-related diseases, including type 2 diabetes mellitus, obesity, cancers, and Alzheimer’s disease [19, 31, 44]. Changes in several miRNA expression profiles are strongly associated with downstream effects on aging and longevity [13, 16, 51]. Surprisingly, miRNAs can also be found in the extracellular environment, including in serum, plasma, saliva, urine, as well as in circulating extracellular vesicles [12, 24, 38, 59, 67]. Numerous circulating miRNAs have been identified, and their impact ranges from regulating adipogenesis and glucose metabolism to modulating inflammation in human white adipose tissue [1, 23]. They also regulate hepatic lipid metabolism and suppress insulin resistance by enhancing adipose tissue endothelial function [28, 55, 64].
miR-146a-5p exhibits an interaction between genotype and aging. A previous publication from our group showed that miR-146a-5p increases in serum of wild-type mice with aging, while long-lived df/df mice maintain it at youthful levels as they age [57]. Indeed, higher levels of miR-146a-5p in old mice appear to be connected to the age-associated occurrence of osteoporosis, as miR-146-5p knock-out mice have preserved and even increased bone mineral density at older ages [47]. miR-146a-5p plays an important role in regulating inflammation [8, 30, 53, 65]. There are apparently conflicting findings indicating that miR-146a-5p can be either a negative or a positive regulator of inflammation. miR-146a-5p appears to suppress interleukin 8 (IL-8) and MCP1 in human adipocytes [46]. However, miR-146a-5p is upregulated by inflammatory factors, including interleukin 1β (IL-1β) and tumor necrosis factor-alpha (TNFα) [7]. Conversely, in df/df mice, known for maintaining a reduced inflammatory state, low levels of miR-146a-5p might contribute to their well-regulated inflammatory responses and blunted age-related increases in inflammation [57].
Adipose tissue is not simply an energy storage organ, it also influences the whole body through the secretion of adipokines, cytokines, and chemokines. These secreted factors are involved in a variety of functions including metabolism, energy balance, inflammatory, and lipid homeostasis [21, 29]. In cultured human adipocytes, mimetic-mediated overexpression of miR-146a suppresses adipocyte inflammation by targeting IRAK1 and TRAF6, reducing NF-κB activation, and inducing synthesis and secretion of inflammatory cytokines [46]. However, inhibiting IRAK1 and TRAF6 by miR-146a-5p also silences the PI3K/AKT pathway, which can lead to activation of NF-κB signaling [15, 17]. This activation subsequently induces synthesis and secretion of inflammatory cytokines, which in turn are associated with senescent cell accumulation [15, 17]. The above findings suggest miR-146a-5p is part of a negative feedback loop that regulates crosstalk between inflammatory processes and insulin signaling.
Based on the aforementioned conflicting findings concerning the role of miR-146a-5p in aging and metabolism, the central aim of this study was to elucidate the involvement of miR-146a-5p in modulating senescence and apoptosis. We examined the effects of miR-146a-5p on biomarkers of senescence, pro-apoptotic factors, and pro-inflammatory cytokines in visceral adipose tissue of long-living df/df mice and in cultured pre-adipocytes.
Materials and methods
Animals
Normal control littermates (N) and df/df female mice were housed under pathogen-free and controlled temperature (22 ± 2 °C) and light conditions (12-h light/12-h dark cycle). The University of Central Florida Animal Care and Use Committee approved all animal care and experimental procedures in this study (Protocol 19–28). This study followed recommendations from the Guide for the Care and Use of Laboratory Animals (National Institutes of Health).
Treatment of mice with a miR-146a mimetic
To compare the abilities of wild-type and df/df mice to regulate adipose tissue miR-146a-5p, we designed a custom miRIDIAN Mimetic stabilized mouse mmu-miR-146a-5p mimetic (Dharmacon, INC., USA) and a custom miRIDIAN mimetic-stabilized negative control. The miRNA-NC-based sequence derived from C. elegans miRNA has minimal sequence identities with mice or humans (Dharmacon, Inc., USA). The miR-146a-5p mimetic and negative controls were prepared using an in vivo jetPEI transfection reagent (Polyplus Transfection, Illkirch, France) according to the manufacturer’s instructions.
Mice were divided randomly into four groups (n = 6–10/group): normal controls treated with miRNA-NC-base, df/df controls treated with miRNA-NC-base, df/df treated with 4 µg/g miR-146a-5p mimetic, and df/df treated with 8 µg/g miR-146a-5p mimetic. All mice were subjected to five intraperitoneal (i.p.) injections, with 2-day intervals between each miR-146a-5p mimetic or negative control injection. Mice were euthanized, and visceral adipose tissue was collected 48 h after the last injection.
Metabolic analyses
Bodyweight was measured before each injection. Glucose tolerance testing (GTT) was performed after the 3rd injection. Mice have fasted for 16 h before intraperitoneal injections of glucose (2 g/kg body weight). Blood glucose levels were measured at 0, 15, 30, 60, and 120 min after the glucose injections using a glucometer (OneTouch Ultra 2, LifeScan).
Cell culture and high glucose-induced senescence
3T3-L1 cells were purchased from ATCC (Manassas, Virginia) and cultured in DMEM/F-12 medium (Corning) supplemented with 10% fetal bovine serum (Fisherbrand), 100 units/ml penicillin, and 100 µg/ml streptomycin (Gibco) at a relative humidity of 95%, 5%CO2, and 37 °C. To induce senescence, 3T3-L1 cells were cultured in DMEM/F-12 medium supplemented with 26 mM glucose, 10% fetal bovine serum, and 1% penicillin/streptomycin for 10 days.
For transfection, cells were cultured in 6-well plates at 2.3 × 105 cells/well in DMEM/F-12 medium with 1% penicillin/streptomycin, serum-free at either a normal glucose level (NG), 4 mM, or high glucose level (HG), 26 mM mimicking obesity model. 3T3-L1 cells were transfected with 10 nM miR-146a-5p mimetic (Dharmacon). HiPerFect Transfection Reagent (Qiagen) was used for transfection, and the protocol followed was according to the manufacturer’s recommendations. Transfection was validated after 72 h by assessing levels of miR-146a-5p by qRT-PCR.
β-galactosidase assay
β-galactosidase was assayed using a Mammalian Beta-Galactosidase Assay Kit (Thermo Scientific, Waltham, MA, USA) following the manufacturer’s protocol. Protein was extracted from 3T3-L1 NG and 3T3-L1 HG cells and from approximately 150 mg of frozen visceral adipose tissue from df/df mice. Cells and tissue samples were homogenized in M-PER Mammalian Protein Extraction Reagent. Following homogenization, 50 µL homogenates and 50 µL β-galactosidase assay reagent were added to 96-well plates and incubated for 30 min at 37 °C. Absorbance was measured at a wavelength of 405 nm.
ELISA
In visceral adipose tissue from df/df mice and 3T3-L1 NG and 3T3-L1 HG conditioned media, IL-6 was measured using a commercially available ELISA kit (Thermo Scientific, Waltham, MA, USA) following the manufacturer’s protocol.
RNA isolation and quantitative real-time PCR (qPCR)
miRNA was isolated from samples utilizing the miRNeasy Mini Kit (Qiagen) according to the manufacturer’s instructions. RNA concentration and purity were determined using a plate reader (EpochTM Microplate Spectrophotometer, BioTek, Winooski, VT, USA).
For miRNA quantification, 5 ng RNA was converted to complementary DNA (cDNA) using a TaqMan Advanced miRNA Assay kit (Applied Biosystems) according to the manufacturer’s protocol. For downstream qRT-PCR analysis, cDNA samples were diluted at 1:10 and stored at − 20 °C. To verify the relative expression of miR-146a-5p, reactions were performed in duplicates by combining 5 µL TaqMan Fast Advanced Master Mix (2 × ; Applied Biosystems), 0.5 µL TaqMan Advanced miRNA Assay reagent (20 ×), 2 µL RNase-free water, and 2.5 µL diluted cDNA/well in MicroAmp Fast Optical 96-well reaction plates (Applied Biosystems). miR-16-5p was used as the housekeeping miRNA to normalize qPCR data. Relative miRNA expression was determined by the 2-ΔΔCt method.
To evaluate mRNA expression, total RNA (1 μg) was converted to cDNA using the iScript cDNA synthesis kit (Bio-Rad) according to the manufacturer’s protocol. Reactions were set up in a MicroAmp Fast Optical 96-well reaction plate with 2 µL of 1:5 diluted cDNA, 0.2 µL of forward and reverse primers each (primers are listed below), 12.6 µL of nuclease free water, and 5 µL of Fast SYBR Green Master Mix (Applied Biosystems)/well. To normalize qRT-PCR data, β-2-microglobulin (B2M) was used as a housekeeping gene. Relative mRNA expression was determined by the 2-ΔΔCt method and student’s t-tests were used for statistical analysis.
Primers sequences were (5’ > 3’) B2M-FW: AAGTATACTCACGCCACCCA, B2M-REV: CAGGCGTATGTATCAGTCTC, P16-FW: CCCAACGCCCCGAACT, P16-REV: GCAGAAGAGCTGCTACGTGAA, BAX-FW: CCACCAGCTCTGAACAGATC, BAX-REV: CAGCTTCTTGGTGGACGCAT, Caspase 9-FW: AGCAGAGAGTAGTGAAGCTG, and Caspase 9-REV: ACACAGACATCATGAGCTCC.
RNA sequencing
Visceral adipose tissue RNA samples from each experimental group (n = 5) were randomly selected for RNA sequencing. Library preparation and sequencing were performed at Beijing Novogene Co., Ltd. All RNA-Seq data are available at the Sequence Read Archive (SRA) at NCBI, accession number PRJNA743364 ([dataset] Nunes 2021). Mapping of sequencing reads to the mouse transcriptome (Illumina iGenomes annotation for UCSC mm10, http://support.illumina.com/sequencing/sequencing_software/igenome.html) was performed using HiSat2. The number of reads aligned to its corresponding gene was calculated by HTSeq 0.12.4. Genes with an average FPKM lower than 1/100,000 of the total aligned reads in more than 50% of the samples were eliminated from further analyses. Statistical analyses for differentially expressed mRNAs were performed using R software (4.0.3) and the Bioconductor package EdgeR using the HTSeq output count. Read counts were normalized for library depth, and pairwise comparisons, measuring fold change, uncorrected p-values from the negative binomial distribution, and adjusted p-values (false discovery rate; FDR) were obtained. Genes with an FDR < 0.05 and fold change (FC) > 2.0 were considered to be up-regulated, and with FDR < 0.05 and FC < 0.5 as down-regulated. mRNAs were further processed for pathway analysis using the Generally Applicable Gene-set Enrichment (GAGE), which uses log-based fold changes as per gene statistics, and Pathview packages in R (21, 22). Enrichment of Gene Ontology (GO) terms (biological processes, molecular function, and cellular component) was also performed using the GAGE package. P-values < 0.05 were considered significant for pathways and GO Terms analysis.
Statistical analysis
All data are expressed as means ± SEM. Statistical analysis was performed using GraphPad Prism 8. Significance between two groups was analyzed by student’s t-tests, comparisons of three or more groups were tested by one-way ANOVA with Tukey’s test. P < 0.05 was considered significant. For unsupervised exploratory data analysis using Principal Component Analysis (PCA) and Heatmap clustering, the top 500 genes according to the coefficient of variation (CV %) were selected. PCA and heatmap analyses were performed in R using singular value decomposition, for hierarchical clustering Pearson correlation coefficient was used as the distance metric and average distance for clustering.
Results
In vivo transfection of df/df mice with miR-146a-5p mimetic induces cellular senescence and inflammation in visceral adipose tissue
To evaluate the effect of miR-146a-5p on visceral adipose tissue, df/df mice were transfected with 4 μg/g or 8 μg/g of miR-146a-5p mimetic by five intraperitoneal (i.p.) injections within 2 days intervals. Forty-eight hours following the last injection, visceral adipose tissue was collected from all animals, and successful transfection was validated by assessing the levels of miR-146a-5p using qRT-PCR. As shown in Fig. 1A, in vivo transfection with both 4 and 8 μg/g miR-146a mimetic both significantly increased the level of miR-146a-5p in visceral adipose tissue of df/df female mice (p = 0.015 and p = 0.0006, respectively).
Fig. 1.
The miR-146a-5p mimetic increased markers of senescence and inflammation in visceral adipose tissue of df/df mice. A Expression of miR-146a-5p in visceral adipose tissue from df/df mice after miR-146a mimetic transfection. B β-galactosidase activity, C p16Ink4a expression in visceral adipose tissue from df/df mice after 4 or 8 μg/g of miR-146a mimetic injections. D IL-6 expression in visceral adipose tissue from df/df mice after 4 and 8 μg/g miR-146a mimetic injections. Values are means ± S.E.M. *p < 0.05 versus df/df control and + p < 0.05 versus all groups
To investigate if transfection of visceral adipose tissue with miR-146a-5p increases senescence, β-galactosidase activity, and p16Ink4 gene expression were evaluated. As indicated in Fig. 1B, the treatment with 8 µg/g miR-146a-5p mimetic resulted in a significant increase of β-galactosidase activity (p = 0.033). Additionally, p16Ink4a expression was increased in the 8 µg/g of miR-146a treatment group (p = 0.0018; Fig. 1C).
In addition to senescence markers, the ELISA analysis of IL-6 indicated a significant increase of this pro-inflammatory cytokine in visceral adipose tissue in response to 8 μg/g miR-146a-5p mimetic treatment (p = 0.020; Fig. 1D).
Body\weight and glucose tolerance are not influenced by miR-146a-5p overexpression
Treatment of df/df mice with the miR-146a mimetic had no influence on body weight or glucose tolerance tests (Table 1). However, three injections of 4 μg/g, but not 8 μg/g of miR-146a-5p mimetic caused a significant increase of basal glucose levels in df/df mice (p = 0.047), while 8 μg/g caused a non-significant rise in glucose levels, bringing them closer to fasting glucose levels in wild-type normal (N) animals (Table 1). There was also a significantly elevated glucose level in df/df mice treated with 8 μg/g miR-146a mimetic when measured 2 h after i.p. glucose injection (p = 0.026).
Table 1.
Body weights, glucose tolerance tests (2 h), and basal glucose in normal and df/df mice
| Normal control | df/df control | df/df miR-146a 4 μg/g | df/df miR-146a 8 μg/g | |
|---|---|---|---|---|
| Number of mice | 10 | 9 | 10 | 6 |
| Body weight (g) | 28.3 ± 1.50 | 10.7 ± 0.92 | 9.9 ± 0.79 | 10.6 ± 0.71 |
| Basal glucose (mg/dL) | 131.1 ± 6.15ab | 117.4 ± 3.29a | 140 ± 6.40b | 131.5 ± 4.77ab |
| Glucose tolerance test (2 h) | 151.4 ± 7.54b | 112 ± 9.68a | 132.9 ± 9.00ab | 152.5 ± 10.93b |
Values are means ± S.E.M. Groups that do not share a superscript differ significantly (p < 0.05)
The miR-146a-5p mimetic downregulated apoptosis signaling pathways in visceral adipose tissue of df/df mice
To determine genes regulated by miR-146a-5p, we performed RNAseq analysis of visceral adipose tissue following in vivo treatment. In order to investigate the ability of miR-146a mimetic treatment to result in distinct gene expression patterns, the 500 most variant genes based on the coefficient of variation were used for PCA and heatmap analysis (Fig. 2A–B). The analysis showed a clear distinction between the gene expression profiles of the df/df controls and df/df mice treated with 8 μg/g miR-146a-5p. Conversely, df/df control and df/df mice treated with 4 μg/g miR-146a-5p clustered closely together, indicating similar expression profiles. Next, for the identification of relevant individual genes differential expression analysis was performed. A total of 198 genes regulated by 8 μg/g miR-146a-5p mimetic transfection in visceral adipose tissue were identified, of which, 169 genes were downregulated and 29 genes up-regulated (Fig. 3A; Table S1 and S2). Additionally, 6 genes were downregulated and only 2 genes were upregulated with 4 μg/g miR-146a-5p mimetic transfection (Table S3). Venn analysis identified Cyp3a25 as a gene to be regulated by both treatments, 4 μg/g and 8 μg/g (Fig. 3B). In addition, VENN analysis was performed on downregulated genes using a less stringent cut-off of p < 0.05 and experimentally verified miR-146a-5p targets listed in Tarbase v.8 (https://doi.org/10.1093/nar/gkx1141) to identify genes potentially regulated due to direct interaction with miR-146a-5p (Fig. 3C).
Fig. 2.
miR-146a at 8 μg/g elicits greater transcriptional responses in df/df mice than miR-146a at 4 μg/g. A Principal Component Analysis (PCA) and B Heatmap clustering analysis on visceral adipose tissue transcriptional profiles identified by RNA sequencing analyses in df/df control (CTL), df/df 4 μg/g (TRT4), and df/df 8 μg/g (TRT8) using the 500 most variant genes according to the coefficient of variation (CV %)
Fig. 3.
Differential expression analysis of RNA-sequencing data in visceral adipose tissue and VENN analysis. A Volcano Plot depicts log2 transformed fold change and adjusted p-value (FDR) for df/df mice treated with 8 μg/g miR-146a-5p mimetic versus controls. B VENN analysis of overlap among genes between df/df mice treated with 4 and 8 μg/g miR-146a-5p, respectively. C Venn analysis of downregulated genes (p < 0.05) in both contrasts (4 μg/g vs. control and 8 μg/g vs. control) and previously reported miR-146a-5p target genes listed in Tarbase v.8
KEGG pathways upregulated and downregulated by target genes of the 8 μg/g miR-146a-5p transfections are shown in Tables 2 and 3, respectively. Overall, 20 pathways were upregulated and 14 pathways were downregulated. Interestingly, the apoptosis signaling pathway represented one of the downregulated pathways in visceral adipose tissue of df/df transfected with 8 µg/g miR-146a-5p mimetic (Fig. 4).
Table 2.
Pathways upregulated by 8 μg/g miR-146a-5p mimetic in visceral adipose tissue from df/df mice
| KEGG pathway | P-value |
|---|---|
| Upregulated | |
| Oxidative phosphorylation | 0.0001 |
| Ribosome | 0.0002 |
| Fatty acid degradation | 0.0005 |
| Peroxisome | 0.0012 |
| PPAR signaling pathway | 0.0039 |
| Valine, leucine, and isoleucine degradation | 0.0048 |
| Cardiac muscle contraction | 0.0081 |
| Citrate cycle (TCA cycle) | 0.0082 |
| Metabolism of xenobiotics by cytochrome P450 | 0.0083 |
| Alanine, aspartate, and glutamate metabolism | 0.0114 |
| Drug metabolism–cytochrome P450 | 0.0158 |
| Propanoate metabolism | 0.0185 |
| Butanoate metabolism | 0.0198 |
| Retinol metabolism | 0.0251 |
| Drug metabolism–other enzymes | 0.0258 |
| Primary bile acid biosynthesis | 0.0297 |
| Tryptophan metabolism | 0.0300 |
| Glycolysis/gluconeogenesis | 0.0353 |
| Tyrosine metabolism | 0.0403 |
| Glycerophopholipid metabolism | 0.0408 |
Pathways with p < 0.05 were considered significant
Table 3.
Pathways downregulated by 8 μg/g of miR-146a-5p mimetic in visceral adipose tissue from df/df mice
| KEGG pathway | P-value |
|---|---|
| Downregulated | |
| Osteoclast differentiation | 0.0014 |
| Chemokine signaling pathway | 0.0017 |
| FcγR-mediated phagocytosis | 0.0027 |
| B cell receptor signaling pathway | 0.0045 |
| MAPK signaling pathway | 0.0088 |
| Toll-like receptor signaling pathway | 0.0200 |
| Phosphatidylinositol signaling pathway | 0.0219 |
| Apoptosis | 0.0270 |
| NOD-like receptor signaling pathway | 0.0273 |
| Neurotrophin signaling pathway | 0.0368 |
| Notch signaling pathway | 0.0401 |
| Lysosome | 0.0469 |
| Phototransduction | 0.0469 |
| Inositol phosphate metabolism | 0.0486 |
Pathways with p < 0.05 were considered significant
Fig. 4.
Representation of apoptosis signaling pathways and target genes of the miR-146a 8 μg/g mimetic in visceral adipose tissue from df/df mice. Red box: downregulated genes; Green box: upregulated genes. Grey/white boxes: not regulated
The miR-146a-5p mimetic increased markers of senescence in 3T3-L1 cells but did not affect apoptosis markers
After investigating the impact of miR-146a mimetic in vivo treatment on visceral adipose tissue of df/df mice, we also evaluated the effects of miR-146a-5p overexpression in 3T3-L1 cells cultured at a normal glucose level (3T3-L1 NG) and a high glucose level (3T3-L1 HG). Interestingly, expression of miR-146a-5p was significantly increased in 3T3-L1 cells exposed to HG compared with 3T3-L1 NG cells prior to any interventions with miR-146a-5p mimetic (p = 0.050; Fig. 5A).
Fig. 5.
The miR-146a-5p mimetic increases markers of senescence in 3T3-L1 cells. A miR-146a expression in 3T3-L1 cells maintained at normal glucose levels (4 mM; 3T3-L1 NG) or high glucose levels (26 mM; 3T3-L1 HG), B miR-146a-5p expression in 3T3-L1 NG and 3T3-L1 HG cells after miR-146a mimetic transfection, C β-galactosidase activity, D p16Ink4a gene expression. Values are means ± S.E.M. * p < 0.05 versus control 3T3-L1 NG and + p < 0.05 versus all groups
To further evaluate the effects of miR-146a-5p overexpression on cellular senescence markers, 3T3-L1 NG and HG cells were collected following 72 h of miR-146a-5p mimetic transfection. qRT-PCR was used to validate transfection by assessing miR-146a-5p levels. Transfection significantly increased miR-146a-5p in both 3T3-L1 NG (p = 0.050) and HG cells (p = 0.009; Fig. 5B).
High glucose levels in 3T3-L1 cells led to a significant elevation of β-galactosidase activity and the overexpression of miR-146a-5p significantly decreased it in 3T3-L1 HG cells (Fig. 5C). Interestingly, at normal glucose levels, miR-146a-5p significantly increased β-galactosidase activity in 3T3-L1 cells (p = 0.039; Fig. 5C). As expected, non-treated 3T3-L1 HG cells had higher p16Ink4 compared to nontreated 3T3-L1 NG cells (p = 0.031; Fig. 5D). Similarly to β-galactosidase activity, only 3T3-L1 NG miR-146a-5p transfection significantly increased p16Ink4 expression (p = 0.021; Fig. 5D). However, 3T3-L1 HG cells transfected with the miR-146a mimetic exhibited no significant difference in p16Ink4 compared to control, non-transfected cells (p = 0.996; Fig. 5D).
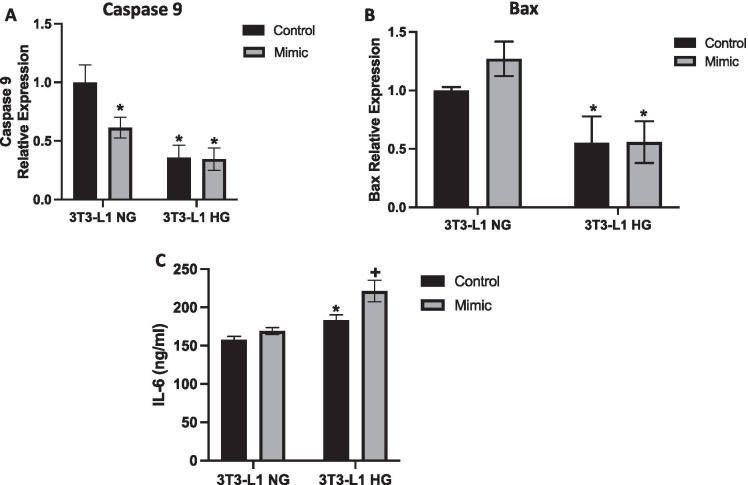
To determine effects of miR-146a overexpression on apoptotic factors we evaluated the expression of two pro-apoptotic-related genes, BAX and Caspase 9, in 3T3-L1 NG and 3T3-L1 HG cells. As expected, low expression of Caspase 9 and Bax was observed in 3T3-L1 HG cells compared to nontreated cells (p = 0.022 and p = 0.005, respectively; Fig. 6A–B). However, miR-146a-5p mimetic transfection significantly reduced caspase 9 expression in 3T3-L1 NG cells (p = 0.0076; Fig. 6A). No difference was observed in BAX expression in cells treated with the miR-146a-5p mimetic (Fig. 6B). High levels of secreted IL-6 were observed in conditioned media of 3T3-L1 NG cells and 3T3-L1 HG cells (p < 0.0001; Fig. 6C). miR-146a-5p transfection did not impact the secretion of IL-6 in 3T3-L1 NG conditioned media (Fig. 6C). However, transfection increased secreted IL-6 into conditioned media of 3T3-L1 HG cells compared to control medium (p = 0.030; Fig. 6C).
Fig. 6.
The miR-146a-5p mimetic did not affect apoptosis but led to increased inflammation in 3T3-L1 cells. A Caspase 9 gene expression, B Bax gene expression, C IL-6 expression in conditioned medium of 3T3-L1 cells maintained at normal and high glucose levels. Values are means ± S.E.M. *p < 0.05 versus control 3T3-L1 NG and + p < 0.05 versus all groups
Discussion
There is growing interest in the regulatory role of miRNAs, with their involvement becoming apparent across a number of diseases. Studies of aging and longevity have indicated that different groups and families of miRNAs appear to be involved in regulating aging processes [49, 57, 58]. Our previous studies highlighted three such miRNAs showing high association with the aging process: miR-5107-5p, miR-342-5p, and miR-146a-5p. Interestingly, these miRNAs increased with age in serum of normal mice, while remaining unchanged in the serum of long-lived df/df mice [57]. Importantly, circulating free miRNAs can target different cell types in organs distant from their source [58]. Since miR-146a-5p has a high expression in senescent cells it was a good candidate for this study.
miR-146a-5p expression is associated with a range of normal and pathological biological processes. This miRNA can act as an anti-inflammatory factor [46, 56]. However, significant and uncontrolled overexpression of miR-146a-5p can actually promote chronic low grade inflammation [34]. Additionally, senescent cells are characterized by increased expression of miR-146a-5p and high secretory activity of this miRNA, indicating this age-associated miRNA may represent a valuable marker of senescent cell burden and aging [7, 18, 58].
Df/df mice are a well-established animal model used in studies centered on aging and longevity. As demonstrated here and in previous studies, visceral adipose tissue from df/df mice is characterized by decreased levels of inflammatory cytokines including IL-6 [33, 37]. Adipocytes secrete a number of factors that influence biological processes, including inflammation and immune responses. Accumulation of senescent cells in metabolic tissues such as adipose tissue can be detrimental to metabolic homeostasis [39, 48]. Adipose tissue senescence has been postulated to be one of the factors underlying metabolic disorders [69]. It is important to note that recent studies of cellular senescence suggest senescent cells can also have protective value, especially during early life, including protecting against the development and progression of cancer [25, 27, 29], while increased senescent cell burden with aging can be detrimental. Thus, it appears that cellular senescence should be tightly regulated, while at the same time clearing senescent cells aging may be necessary to prevent continued chronic inflammation and tissue damage due to the persistent presence of senescent cells [43, 45, 60].
In this study, we demonstrate that increased levels of senescence-associated miR-146a-5p due to in vivo treatment with a mimetic caused increased β-galactosidase activity and p16Ink4a expression in visceral adipose tissue. Increased activity of senescence-associated β-galactosidase is considered to be an outcome of cellular senescence [62]. Additionally, the expression of p16Ink4a, a regulator of senescence, has been shown to increase with aging in adipose tissue [29]. Previous studies have revealed that reduction in the percent of p16Ink4a-positive cells alleviates adipose tissue dysfunction in INK-ATTAC transgenic mice [2, 40, 61]. This observation indicates that miR-146a-5p is not only a marker of senescence, but can also accelerate senescence in healthy cells. This implies that gradual accumulation of senescent cells in adipose tissue due to aging or high-fat diet consumption could result in overexpression of cellular and, consequently, circulating miR-146a-5p, potentially targeting and spreading senescence in origin organ and other distant cells and tissues.
Different effects of miR-146a-5p have been observed across different tissues and physiological/pathological conditions. In human dendritic cells, miR-146a-5p promotes apoptosis and inhibits the production of IL-6 and TNFα [42]. In oral squamous cell carcinoma, miR-146a-5p can inhibit proliferation and accelerate apoptosis by targeting NF-κB (F. Y. [66]. In our study, RNAseq results indicate that at high-dose (8 µg/g) of the miR-146a-5p mimetic attenuated apoptosis signaling in visceral adipose tissue of df/df mice. There was no such effect at lower doses, possibly suggesting the impact of this miRNA on senescence or apoptotic pathways may be associated with the extent of dysregulation of this miRNA, which might be dependent on senescent cells’ burden and pathways targeted by this miRNA. Reduced apoptosis signaling after administering the higher doses of the miR-146a-5p mimetic could possibly be related to (1) increased senescent cell abundance, as senescent cells persist, in part, because of their resistance to apoptosis (Y. [68] and/or (2) persistence of damaged non-senescent cells despite increased inflammation, potentially further exacerbating tissue dysfunction. These possibilities and others merit further investigation. Taken together, our in vivo study indicates that overexpressing of miR-146a-5p increases IL-6 while also blocking apoptotic pathways in df/df mouse adipose tissue, which suggests that maintenance of low miR-146a-5p in old Ames dwarf mice may be an important mechanism that protects these long-living dwarfs from metabolic complications and age-associated inflammation in adipose tissue.
Several studies have demonstrated high levels of pro-inflammatory cytokines in senescent cells and tissues. Senescent cells isolated from inguinal adipose tissue exhibit increased IL-6 [2] and genetic clearance of senescent cells decreases IL-6 in adipose tissue of aged mice [61]. Consistent with increased expression of senescence markers and downregulation of pro-apoptotic pathways, we also found 8 µg/g of the miR-146a-5p mimetic caused increased IL-6, an inflammatory cytokine, in visceral adipose tissue of df/df mice. Shimada and colleagues reported similar pro-inflammatory effects induced by extracellular miR-146a-5p. They showed miR-146a-5p drives innate immune responses in the heart, coronary artery endothelial cells, and cardiac fibroblasts, leading to myocardial inflammation and cellular dysfunction [50].
To validate the direct effects of the miR-146a-5p mimetic on the senescence process in fat cells, we incubated 3T3-L1 (mouse preadipocytes) cells in a basal medium supplemented with high glucose and transfected these cells with the miR-146a-5p mimetic. Cells cultured in medium containing high glucose concentrations have been reported to exhibit premature senescence, genomic instability, and telomeric changes [11, 54]. We found that with accelerated cellular senescence in 3T3-L1 cells due to HG exposure, there was also increased expression of miRNA-146a-5p compared to 3T3-L1 in NG cells at the same passage levels. Furthermore, similarly to visceral adipose tissue from df/df mice treated with high doses of miR-146a-5p, we demonstrated that transfecting 3T3-L1 cells with the miR-146a-5p mimetic under normal glucose conditions (4 mM; 3T3-L1 NG) caused increased β-galactosidase activity and p16Ink4a expression and decreased expression of the pro-apoptotic Caspase 9 gene. Interestingly, treating 3T3-L1 HG cells with the miR-146a mimetic decreased β-galactosidase, but did not affect p16Ink4a or apoptotic genes, and slightly increased IL-6 in conditioned media. These findings suggest that miR-146a-5p is a mediator of cellular senescence, but under pre-established senescent conditions with already elevated miR-146a-5p, the miR-146a-5p mimetic has no further effects due to a negative regulatory loop signal. This also appears contrary to previous reports showing miR-146a suppresses anti-apoptotic genes and overall inflammation, consequently promoting apoptosis and reduced senescence [46, 63]. Ross and colleagues have shown transfection of miR-146a mimetics in human adipocytes prevented inflammatory responses by reducing IL-8 and MCP-1 at both the mRNA and protein levels [46]. Others have also found expression of miR-146a-5p increases in senescent cells. Bhaumik and colleagues showed that in human fibroblasts, expression of miR-146a-5p increases in an interleukin IL1-dependent manner, with high levels of IL-6 and IL-8 [7]. In our study, we observed an increase in IL-6 in visceral adipose tissue and conditioned media of 3T3-L1 HG cells transfected with the miR-146a-5p mimetic. Hence, our data support the hypothesis that overexpressing the senescence-associated miR-146a-5p in vivo or in vitro suppresses apoptotic pathways and increases secretion of inflammatory mediators in mouse pre-adipocytes thus stimulating rather than preventing senescence. However, in 3T3-L1 HG cells, this miRNA had little effect on apoptosis or inflammation, indicating the cellular stress caused by high glucose conditions may mask any additional impact of miR-146a-5p. Additionally, acute upregulation of this miRNA might be important in cells that are exposed to different stressors including high glucose, while over-expression in healthy cells or constant and unregulated increase of miR-146a-5p might be detrimental.
In summary, our results indicate that miR-146a-5p represents a marker for cellular senescence. They also suggest that this miRNA, when not precisely regulated, can lead to deregulated inflammatory responses and enhanced senescence process in healthy cells. This is especially true for cells in close proximity to senescent cells over-expressing miR-146a-5p where the miRNA can be transferred through extracellular vesicle-mediated delivery. Similarly, the miRNA could be delivered systemically by the circulating senescent cell-derived EVs. The role of this miRNA merits further study with respect to regulation of senescence, apoptosis, and inflammation, as our study indicates that the role of miR-146a-5p might be different in healthy versus stressed cells. This suggests effects of miR-146a-5p may depend on overall organismal health, aging, and metabolic state.
Author contributuion
ADCN: performed laboratory and animal experiments, data analyses, and wrote the manuscript; MW and AS: helped in data analyses and revised drafts of the manuscript; SN: performed laboratory experiments and revised drafts of the manuscript; LY, CL, and TDS: performed laboratory experiments; JG and PDR: involved in the conceptualization of the idea, data interpretation, and revised drafts of the manuscript; KM, EB, JLK, and TT: involved in the conceptualization of the idea and data interpretation. All authors reviewed and approved the final version of the manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Funding
This work was supported by NIH grants R56 AG061414 (M.M.), R15 AG059190 (M.M), R03 AG059846 (M.M.), R21 AG062985 (M.M), R37AG13925 (JLK, TT) PO1 AG043376 (PDR), U19 AG056278 (PDR), and PO1AG062413 (JLK, TT, PDR). JLK and TT are also supported by the Connor Fund, Robert J. and Theresa W. Ryan, and the Noaber Foundation. A portion of this work was supported by NASA-Florida Space Grant Consortium, 66016A30 grant “Biomanufacturing of Vascularized Tissues”, and by the NASA (SSERVI16) Cooperative Agreement (NNH16ZDA001N) program titled “Radiation Effects on Volatiles and Exploration of Asteroids and Lunar Surfaces (REVEALS)”, 80ARC017M0007.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Arner E, Mejhert N, Kulyte A, Balwierz PJ, Pachkov M, Cormont M, … Arner P. Adipose tissue microRNAs as regulators of CCL2 production in human obesity. Diabetes. 2012; 61(8):1986-199310.2337/db11-1508 [DOI] [PMC free article] [PubMed]
- 2.Baker DJ, Wijshake T, Tchkonia T, LeBrasseur NK, Childs BG, van de Sluis B… van Deursen JM. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011; 479(7372):232-23610.1038/nature10600 [DOI] [PMC free article] [PubMed]
- 3.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 4.Bartke A, Brown-Borg H. Life extension in the dwarf mouse. Curr Top Dev Biol. 2004;63:189–225. doi: 10.1016/S0070-2153(04)63006-7. [DOI] [PubMed] [Google Scholar]
- 5.Bartke A, Brown-Borg H, Mattison J, Kinney B, Hauck S, Wright C. Prolonged longevity of hypopituitary dwarf mice. Exp Gerontol. 2001;36(1):21–28. doi: 10.1016/s0531-5565(00)00205-9. [DOI] [PubMed] [Google Scholar]
- 6.Bartke A, Wright JC, Mattison JA, Ingram DK, Miller RA, Roth GS. Extending the lifespan of long-lived mice. Nature. 2001;414(6862):412. doi: 10.1038/35106646. [DOI] [PubMed] [Google Scholar]
- 7.Bhaumik D, Scott GK, Schokrpur S, Patil CK, Orjalo AV, Rodier F. … Campisi J . MicroRNAs miR-146a/b negatively modulate the senescence-associated inflammatory mediators IL-6 and IL-8 Aging (Albany NY). 2009; 1(4):402-41110.18632/aging.100042 [DOI] [PMC free article] [PubMed]
- 8.Boldin MP, Taganov KD, Rao DS, Yang L, Zhao JL, Kalwani M, … Baltimore D. miR-146a is a significant brake on autoimmunity, myeloproliferation, and cancer in mice. J Exp Med. 2011; 208(6):1189-120110.1084/jem.20101823 [DOI] [PMC free article] [PubMed]
- 9.Cannarella R, Crafa A, La Vignera S, Condorelli RA, Calogero AE. Role of the GH-IGF1 axis on the hypothalamus-pituitary-testicular axis function: lessons from Laron syndrome. Endocr Connect. 2021;10(9):1006–1017. doi: 10.1530/EC-21-0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Catalanotto, C., Cogoni, C., & Zardo, G. (2016). MicroRNA in Control of Gene Expression: an Overview of Nuclear Functions. Int J Mol Sci, 17(10). doi:10.3390/ijms17101712 [DOI] [PMC free article] [PubMed]
- 11.Chang TC, Hsu MF, Wu KK. High glucose induces bone marrow-derived mesenchymal stem cell senescence by upregulating autophagy. PLoS ONE. 2015;10(5):e0126537. doi: 10.1371/journal.pone.0126537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cortez MA, Bueso-Ramos C, Ferdin J, Lopez-Berestein G, Sood AK, Calin GA. MicroRNAs in body fluids–the mix of hormones and biomarkers. Nat Rev Clin Oncol. 2011;8(8):467–477. doi: 10.1038/nrclinonc.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Lencastre, A., Pincus, Z., Zhou, K., Kato, M., Lee, S. S., & Slack, F. J. (2010). MicroRNAs both promote and antagonize longevity in C. elegans. Curr Biol, 20(24), 2159–2168. doi:10.1016/j.cub.2010.11.015 [DOI] [PMC free article] [PubMed]
- 14.Do A, Menon V, Zhi X, Gesing A, Wiesenborn DS, SpongA, … Masternak MM. Thyroxine modifies the effects of growth hormone in Ames dwarf mice. Aging (Albany NY). 2015; 7(4):241-25510.18632/aging.100739 [DOI] [PMC free article] [PubMed]
- 15.Fukao T, Koyasu S. PI3K and negative regulation of TLR signaling. Trends Immunol. 2003;24(7):358–363. doi: 10.1016/s1471-4906(03)00139-x. [DOI] [PubMed] [Google Scholar]
- 16.Grillari J, Hackl M, Grillari-Voglauer R. miR-17-92 cluster: ups and downs in cancer and aging. Biogerontology. 2010;11(4):501–506. doi: 10.1007/s10522-010-9272-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guha M, Mackman N. The phosphatidylinositol 3-kinase-Akt pathway limits lipopolysaccharide activation of signaling pathways and expression of inflammatory mediators in human monocytic cells. J Biol Chem. 2002;277(35):32124–32132. doi: 10.1074/jbc.M203298200. [DOI] [PubMed] [Google Scholar]
- 18.Hackl M, Brunner S, Fortschegger K, Schreiner C, Micutkova L, Muck C, … Grillari J. miR-17, miR-19b, miR-20a, and miR-106a are down-regulated in human aging. Aging Cell. 2010; 9(2):291-29610.1111/j.1474-9726.2010.00549.x [DOI] [PMC free article] [PubMed]
- 19.He X, He L, Hannon GJ. The guardian's little helper: microRNAs in the p53 tumor suppressor network. Cancer Res. 2007;67(23):11099–11101. doi: 10.1158/0008-5472.CAN-07-2672. [DOI] [PubMed] [Google Scholar]
- 20.Ikeno Y, Bronson RT, Hubbard GB, Lee S, Bartke A. Delayed occurrence of fatal neoplastic diseases in ames dwarf mice: correlation to extended longevity. J Gerontol A Biol Sci Med Sci. 2003;58(4):291–296. doi: 10.1093/gerona/58.4.b291. [DOI] [PubMed] [Google Scholar]
- 21.Jung UJ, Choi MS. Obesity and its metabolic complications: the role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int J Mol Sci. 2014;15(4):6184–6223. doi: 10.3390/ijms15046184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kinney-Forshee BA, Kinney NE, Steger RW, Bartke A. Could a deficiency in growth hormone signaling be beneficial to the aging brain? Physiol Behav. 2004;80(5):589–594. doi: 10.1016/j.physbeh.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 23.Kloting N, Berthold S, Kovacs P, Schon MR, Fasshauer M, Ruschke K…Bluher M. MicroRNA expression in human omental and subcutaneous adipose tissue. PLoS ONE. 2009; 4 (3): e469910.1371/journal.pone.0004699 [DOI] [PMC free article] [PubMed]
- 24.Kolenda T, Guglas K, Rys M, Bogaczynska M, Teresiak R, Blizniak … Lamperska KM. Biological role of long non-coding RNA in head and neck cancers. Rep Pract Oncol Radiother. 2017; 22(5):378-38810.1016/j.rpor.2017.07.001 [DOI] [PMC free article] [PubMed]
- 25.Krtolica A, Parrinello S, Lockett S, Desprez PY, Campisi J. Senescent fibroblasts promote epithelial cell growth and tumorigenesis: a link between cancer and aging. Proc Natl Acad Sci U S A. 2001;98(21):12072–12077. doi: 10.1073/pnas.211053698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krutzfeldt J, Stoffel M. MicroRNAs: a new class of regulatory genes affecting metabolism. Cell Metab. 2006;4(1):9–12. doi: 10.1016/j.cmet.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 27.Kuilman T, Michaloglou C, Mooi WJ, Peeper DS. The essence of senescence. Genes Dev. 2010;24(22):2463–2479. doi: 10.1101/gad.1971610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin X, Qin Y, Jia J, Lin T, Lin X, Chen L … Xiao D . MiR-155 enhances insulin sensitivity by coordinated regulation of multiple genes in mice. PLoS Genet. 2016; 12 (10):e100630810.1371/journal.pgen.1006308 [DOI] [PMC free article] [PubMed]
- 29.Liu Z, Wu KKL, Jiang X, Xu A, Cheng KKY. The role of adipose tissue senescence in obesity- and ageing-related metabolic disorders. Clin Sci (Lond) 2020;134(2):315–330. doi: 10.1042/CS20190966. [DOI] [PubMed] [Google Scholar]
- 30.Lu LF, Boldin MP, Chaudhry A, Lin LL, Taganov KD, Hanada T, … Rudensky AY. Function of miR-146a in controlling Treg cell-mediated regulation of Th1 responses. Cell. 2010; 142(6):914-92910.1016/j.cell.2010.08.012 [DOI] [PMC free article] [PubMed]
- 31.Lukiw WJ, Zhao Y, Cui JG. An NF-kappaB-sensitive micro RNA-146a-mediated inflammatory circuit in Alzheimer disease and in stressed human brain cells. J Biol Chem. 2008;283(46):31315–31322. doi: 10.1074/jbc.M805371200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Masternak MM, Al-Regaiey K, Bonkowski MS, Panici J, Sun L, Wang J … Bartke A . Divergent effects of caloric restriction on gene expression in normal and long-lived mice. J Gerontol A Biol Sci Med Sci. 2004; 59(8):784-78810.1093/gerona/59.8.b784 [DOI] [PubMed]
- 33.Masternak MM, Bartke A, Wang F, Spong A, Gesing A, Fang Y … Westbrook R. Metabolic effects of intra-abdominal fat in GHRKO mice. Aging Cell. 2012; 11(1):73-8110.1111/j.1474-9726.2011.00763.x [DOI] [PMC free article] [PubMed]
- 34.Masternak MM, Darcy J, Victoria B, Bartke A. Dwarf Mice and Aging. Prog Mol Biol Transl Sci. 2018;155:69–83. doi: 10.1016/bs.pmbts.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 35.Masternak MM, Panici JA, Bonkowski MS, Hughes LF, Bartke A. Insulin sensitivity as a key mediator of growth hormone actions on longevity. J Gerontol A Biol Sci Med Sci. 2009;64(5):516–521. doi: 10.1093/gerona/glp024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mendell JT, Olson EN. MicroRNAs in stress signaling and human disease. Cell. 2012;148(6):1172–1187. doi: 10.1016/j.cell.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Menon V, Zhi X, Hossain T, Bartke A, Spong A, Gesing A, Masternak MM. The contribution of visceral fat to improved insulin signaling in Ames dwarf mice. Aging Cell. 2014;13(3):497–506. doi: 10.1111/acel.12201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nunez Lopez YO, Victoria B, Golusinski P, Golusinski W, Masternak MM. Characteristic miRNA expression signature and random forest survival analysis identify potential cancer-driving miRNAs in a broad range of head and neck squamous cell carcinoma subtypes. Rep Pract Oncol Radiother. 2018;23(1):6–20. doi: 10.1016/j.rpor.2017.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Palmer AK, Tchkonia T, LeBrasseur NK, Chini EN, Xu M, Kirkland JL. Cellular senescence in type 2 diabetes: a therapeutic opportunity. Diabetes. 2015;64(7):2289–2298. doi: 10.2337/db14-1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Palmer AK, Xu M, Zhu Y, Pirtskhalava T, Weivoda MM, Hachfeld CM … Kirkland JL. Targeting senescent cells alleviates obesity-induced metabolic dysfunction. Aging Cell. 2019; 18(3):e1295010.1111/acel.12950 [DOI] [PMC free article] [PubMed]
- 41.Panici JA, Harper JM, Miller RA, Bartke A, Spong A, Masternak MM. Early life growth hormone treatment shortens longevity and decreases cellular stress resistance in long-lived mutant mice. FASEB J. 2010;24(12):5073–5079. doi: 10.1096/fj.10-163253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Park H, Huang X, Lu C, Cairo MS, Zhou X. MicroRNA-146a and microRNA-146b regulate human dendritic cell apoptosis and cytokine production by targeting TRAF6 and IRAK1 proteins. J Biol Chem. 2015;290(5):2831–2841. doi: 10.1074/jbc.M114.591420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pignolo RJ, Passos JF, Khosla S, Tchkonia T, Kirkland JL. Reducing senescent cell burden in aging and disease. Trends Mol Med. 2020;26(7):630–638. doi: 10.1016/j.molmed.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Poy MN, Eliasson L, Krutzfeldt J, Kuwajima S, Ma X, Macdonald PE, … Stoffel M . A pancreatic islet-specific microRNA regulates insulin secretion Nature. 2004; 432 (7014):226-23010.1038/nature03076 [DOI] [PubMed]
- 45.Robbins PD, Jurk D, Khosla S, Kirkland JL, LeBrasseur NK, Miller JD, … Niedernhofer LJ. Senolytic drugs: reducing senescent cell viability to extend health span. Annu Rev Pharmacol Toxicol. 2021; 61 779-80310.1146/annurev-pharmtox-050120-105018 [DOI] [PMC free article] [PubMed]
- 46.Roos J, Enlund E, Funcke JB, Tews D, Holzmann K, Debatin KM, …Fischer-Posovszky P . miR-146a-mediated suppression of the inflammatory response in human adipocytes. Sci Rep. 2016; 6:3833910.1038/srep38339 [DOI] [PMC free article] [PubMed]
- 47.Saferding V, Hofmann M, Brunner JS, Niederreiter B, Timmen M, Magilnick N… Bluml S. microRNA-146a controls age-related bone loss. Aging Cell. 2020; 19(11):e1324410.1111/acel.13244 [DOI] [PMC free article] [PubMed]
- 48.Schafer MJ, Miller JD, LeBrasseur NK. Cellular senescence: implications for metabolic disease. Mol Cell Endocrinol. 2017;455:93–102. doi: 10.1016/j.mce.2016.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schneider A, Matkovich SJ, Victoria B, Spinel L, Bartke A, Golusinski P, Masternak MM. Changes of ovarian microRNA profile in long-living Ames dwarf mice during aging. PLoS ONE. 2017;12(1):e0169213. doi: 10.1371/journal.pone.0169213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shimada BK, Yang Y, Zhu J, Wang S, Suen A, Kronstadt SM …W Chao. Extracellular miR-146a-5p induces cardiac innate immune response and cardiomyocyte dysfunction. Immunohorizons. 2020; 4 (9):561-57210.4049/immunohorizons.2000075 [DOI] [PMC free article] [PubMed]
- 51.Smith-Vikos T, Slack FJ. MicroRNAs and their roles in aging. J Cell Sci. 2012;125(Pt 1):7–17. doi: 10.1242/jcs.099200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sornson MW, Wu W, Dasen JS, Flynn SE, Norman DJ, O'Connell SM., . . . Rosenfeld MG. Pituitary lineage determination by the Prophet of Pit-1 homeodomain factor defective in Ames dwarfism. Nature, 1996; 384(6607), 327-333. doi:10.1038/384327a0 [DOI] [PubMed]
- 53.Starczynowski DT, Kuchenbauer F, Argiropoulos B, Sung S, Morin R, Muranyi A … Karsan A . Identification of miR-145 and miR-146a as mediators of the 5q- syndrome phenotype. Nat Med. 2010; 16(1):49-5810.1038/nm.2054 [DOI] [PubMed]
- 54.Stolzing A, Coleman N, Scutt A. Glucose-induced replicative senescence in mesenchymal stem cells. Rejuvenation Res. 2006;9(1):31–35. doi: 10.1089/rej.2006.9.31. [DOI] [PubMed] [Google Scholar]
- 55.Sun X, Lin J, Zhang Y, Kang S, Belkin N, Wara AK …Feinberg MW. MicroRNA-181b improves glucose homeostasis and insulin sensitivity by regulating endothelial function in white adipose tissue. Circ Res. 2016; 118 (5):810-82110.1161/CIRCRESAHA.115.308166 [DOI] [PMC free article] [PubMed]
- 56.Taganov KD, Boldin MP, Baltimore D. MicroRNAs and immunity: tiny players in a big field. Immunity. 2007;26(2):133–137. doi: 10.1016/j.immuni.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 57.Victoria B, Dhahbi JM, Nunez Lopez YO, Spinel L, Atamna H, Spindler SR, Masternak MM. Circulating microRNA signature of genotype-by-age interactions in the long-lived Ames dwarf mouse. Aging Cell. 2015;14(6):1055–1066. doi: 10.1111/acel.12373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Victoria B, Nunez Lopez YO, Masternak MM. MicroRNAs and the metabolic hallmarks of aging. Mol Cell Endocrinol. 2017;455:131–147. doi: 10.1016/j.mce.2016.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weilner S, Schraml E, Wieser M, Messner P, Schneider K, Wassermann K …Grillari J. Secreted microvesicular miR-31 inhibits osteogenic differentiation of mesenchymal stem cells. Aging Cell. 2016; 15(4):744-75410.1111/acel.12484 [DOI] [PMC free article] [PubMed]
- 60.Wyld, L., Bellantuono, I., Tchkonia, T., Morgan, J., Turner, O., Foss, F., . . . Kirkland, J. L. (2020). Senescence and cancer: a review of clinical implications of senescence and senotherapies. Cancers (Basel), 12(8). doi:10.3390/cancers12082134 [DOI] [PMC free article] [PubMed]
- 61.Xu M, Palmer AK, Ding H, Weivoda MM, Pirtskhalava T, White TA…JL Kirkland. Targeting senescent cells enhances adipogenesis and metabolic function in old age. Elife. 2015; 4:e1299710.7554/eLife.12997 [DOI] [PMC free article] [PubMed]
- 62.Yanagi, S., Tsubouchi, H., Miura, A., Matsuo, A., Matsumoto, N., & Nakazato, M. (2017). The impacts of cellular senescence in elderly pneumonia and in age-related lung diseases that increase the risk of respiratory infections. Int J Mol Sci, 18(3). doi:10.3390/ijms18030503 [DOI] [PMC free article] [PubMed]
- 63.Zhang, B., Yi, J., Zhang, C. L., Zhang, Q. H., Xu, J. F., Shen, H. Q., & Ge, D. W. (2017). miR-146a inhibits proliferation and induces apoptosis in murine osteoblastic MC3T3-E1 by regulating Bcl2. Eur Rev Med Pharmacol Sci, 21(17), 3754–3762. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/28975995 [PubMed]
- 64.Zhang M, Sun W, Zhou M, Tang Y. MicroRNA-27a regulates hepatic lipid metabolism and alleviates NAFLD via repressing FAS and SCD1. Sci Rep. 2017;7(1):14493. doi: 10.1038/s41598-017-15141-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhao JL, Rao DS, Boldin MP, Taganov KD, O'Connell RM, Baltimore D. NF-kappaB dysregulation in microRNA-146a-deficient mice drives the development of myeloid malignancies. Proc Natl Acad Sci U S A. 2011;108(22):9184–9189. doi: 10.1073/pnas.1105398108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhu FY, Gan CW, Wang MX, Sun BC, Li FJ, Qiu YH, Wang K. miR-146a-5p inhibits proliferation and promotes apoptosis of oral squamous cell carcinoma cells by regulating NF-kappaB signaling pathway. Eur Rev Med Pharmacol Sci. 2020;24(7):3717–3723. doi: 10.26355/eurrev_202004_20835. [DOI] [PubMed] [Google Scholar]
- 67.Zhu, H., & Fan, G. C. (2011). Extracellular/circulating microRNAs and their potential role in cardiovascular disease. Am J Cardiovasc Dis, 1(2), 138–149. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/22059153 [PMC free article] [PubMed]
- 68.Zhu Y, Tchkonia T, Pirtskhalava T, Gower AC, Ding H, Giorgadze N, … Kirkland JL. The Achilles' heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell. 2015; 14(4):644-65810.1111/acel.12344 [DOI] [PMC free article] [PubMed]
- 69.Zhuohao Liu KKLW, Jiang X, Aimin Xu, Cheng KKY. The role of adipose tissue senescence in obesity-and ageing-related metabolic disorders. Clin Sci. 2020;134:315–330. doi: 10.1042/CS20190966. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.