Abstract
Accurate assessment of fluid status is vital to appropriate management of hyponatremia. However, conventional parameters such as physical examination, vital signs, and laboratory markers such as natriuretic peptides suffer from limitations in this regard. Point of care ultrasonography (POCUS) is a noninvasive bedside diagnostic tool that is emerging as an adjunct to physical examination in internal medicine and subspecialties including nephrology. In this manuscript, we describe a complex case of hyponatremia, where stroke volume assessment using Doppler echocardiography has helped to objectively assess fluid status and guided therapy. A 73-year-old woman was seen for worsening serum sodium. Her urine sodium was high and there was no hypotension suggestive of euvolemia. However, POCUS demonstrated low stroke volume in the presence of normal left ventricular ejection fraction, indicative of hypovolemia. She was treated with intravenous fluids based on these findings and response was evident by normalization of the stroke volume. Clinicians should adopt a multi-parametric approach integrating all the pieces of hemodynamic puzzle when evaluating complex fluid and electrolyte disorders.
Keywords: POCUS, Point of care ultrasound, Nephrology, Physical examination, Hyponatremia
Introduction
Hyponatremia (generally defined as serum sodium < 135 mmol/L) is the most common electrolyte abnormality in hospitalized patients. It is either due to water excess relative to extracellular sodium (more common) or absolute loss of sodium (less common) [1]. Arginine vasopressin or the anti-diuretic hormone (ADH) is the prime regulator of renal water excretion and hence it is conceivable that a high ADH state is necessary for water retention leading to hyponatremia. There are several stimuli for ADH release including increased plasma osmolality, decrease in blood pressure, low effective circulating blood volume, activation of renin–angiotensin–aldosterone system, nausea, pain, stress, fear, etc [2]. Evaluation of hyponatremia involves a multi-parametric approach including careful history taking, review of vital signs, laboratory data and physical examination to assess the fluid volume status. However, it is not always easy to discern a patient’s fluid status. For example, in a study including non-edematous patients with hyponatremia, clinical assessment was able to correctly identify only 47% of hypovolemic patients and 48% of those who were euvolemic [3]. Even in patients with heart failure, the sensitivity of commonly used physical findings such as lung crackles, pedal edema and elevated jugular venous pressure has shown to be low to identify congestion [4]. In this context, point of care ultrasonography (POCUS) is emerging as a valuable adjunct to conventional assessment in evaluating patients with complex fluid and electrolyte disorders. Physicians with expertise in Doppler ultrasound can assess multiple sonographic parameters at the bedside to elucidate a patient’s hemodynamic status [5–7]. Herein, we report a case of hyponatremia, where point of care Doppler echocardiography facilitated appropriate management.
Case description
A 73-year-old woman with a history of hypothyroidism, esophagitis, hiatal hernia, and osteoarthritis initially presented after sustaining a fall at home. She was found to have undisplaced left clavicle and pubic body fractures that did not require operative intervention. However, she was found to be hyponatremic with a serum sodium of 122 mmol/L (136–145) 2 days after presentation. Sodium level was normal at presentation ~ 138 mmol/L and no labs were available during that 2-day period. After repeating the sodium level to ensure accuracy, other pertinent studies were obtained, which were notable for a low serum osmolality of 264 mOsm/kg (consistent with hypo-osmolar hyponatremia), and a low urine sodium level (< 20 mmol/L). Urine osmolality was 283 mOsm/kg. Based on the low urine sodium level and patient’s history of poor oral intake, hypovolemic hyponatremia was diagnosed, and normal saline was administered. Adequate pain control was ensured as it can be another potential stimulus for ADH, thereby contributing to hyponatremia. Serum sodium improved to 126 mmol/L. However, patient complained of abdominal distension and shortness of breath on mild exertion. Chest X-ray demonstrated mild right pleural effusion in addition to a large hiatal hernia (Fig. 1). Approximately 400 cc of pleural fluid was drained, which was transudative in nature. Left ventricular systolic function was normal on echocardiogram. Cardiothoracic surgery was consulted for the hernia, who recommended re-evaluation in the outpatient setting. Serum sodium remained stable around 126–128 mmol/L over the next 4 days. However, it again decreased to 123 mmol/L and this time, urine sodium was 46 mmol/L, fractional excretion of sodium 0.6% and urine osmolality 257 mOsm/kg. As the patient ‘appeared euvolemic’ on examination and the urine sodium increased from before, the treating physician presumed euvolemic hyponatremia. This in conjunction with lung crackles prompted diuretic therapy. Unfortunately, the serum sodium level did not improve and further trended down to 122 mmol/L, at which time nephrology consultation was requested.
Fig. 1.
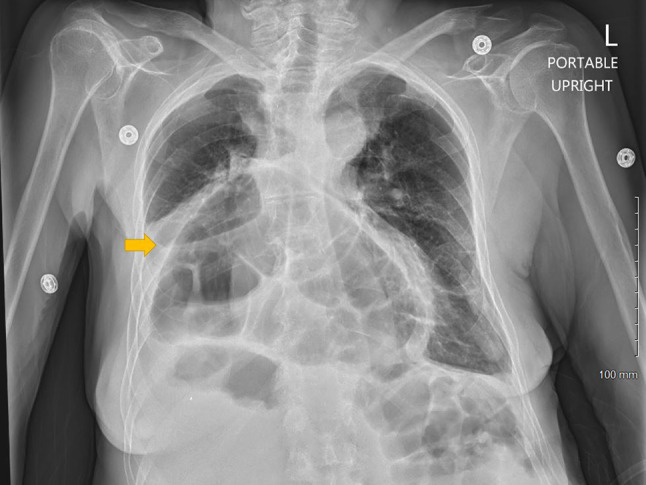
Chest radiograph demonstrating a large hiatal hernia containing transverse colon and abdominal contents (arrow) and a small right pleural effusion
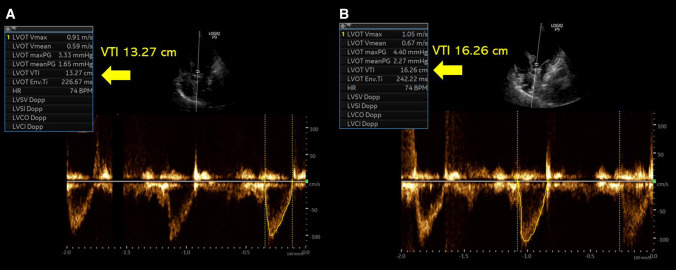
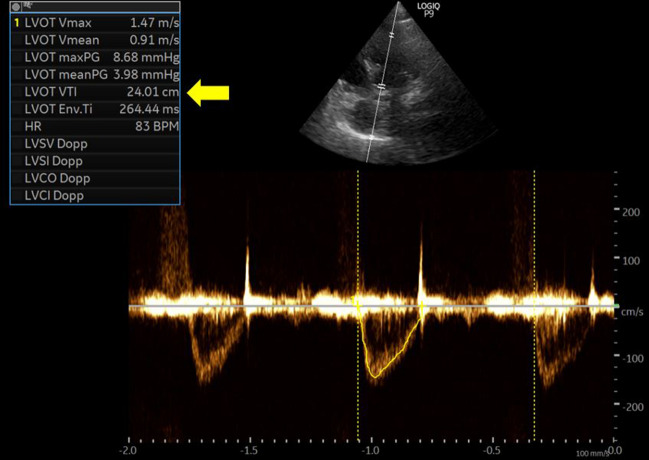
At the time of our evaluation, there was no gross sign of hypovolemia, blood pressure was 160/90 mmHg with a pulse rate of 75–80 bpm. Repeat urine sodium was 69 mmol/L and fractional excretion of sodium 0.7%. Serum creatinine was normal at 0.5 mg/dL and blood urea nitrogen 9 mg/dL. Serum thyroid stimulating hormone (TSH) level was 13 uIU/mL (0.3–0.42) but free T4 was within normal limits. Moreover, TSH level was consistent with her prior values. There was no active pain or nausea. She was not on a thiazide diuretic. We performed a POCUS evaluation to further evaluate her volume status as diuretic therapy is not typically expected to worsen ‘euvolemic’ hyponatremia, that too after two small doses. Inferior vena cava was small and collapsible, consistent with a normal right atrial pressure. Cardiac examination showed normal left ventricular systolic function. However, on pulsed wave Doppler examination of the left ventricular outflow tract, the velocity time integral (VTI), which is a surrogate for stroke volume was lower than expected. It was ~ 13 cm (normal: 18–22 cm), verified on multiple measurements, suggestive of hypovolemia (Fig. 2A). To ensure she is fluid responsive (i.e., able to increase stroke volume in response to fluids), we performed a passive leg raise test, which increased the VTI to ~ 16 cm consistent with appropriate response (> 15%) (Fig. 2B). We recommended intravenous fluid therapy with normal saline and the patient’s serum sodium improved to 130 mmol/L in 2 days. A repeat POCUS examination was undertaken to confirm the improvement in stroke volume. This time, VTI has normalized to ~ 22 cm (average of 6 readings) suggesting resolution of hypovolemia (Fig. 3). Urine sodium and osmolality at that point were 28 mmol/L and 235 mOsm/kg, respectively, with a fractional excretion of sodium 0.4%. Patient’s oral intake also picked up and protein supplementation was optimized. Serum sodium continued to improve and was 134 mml/L at the time of discharge (4 days later). Table 1 demonstrates serum sodium levels and other pertinent data during the course of patient’s hospitalization.
Fig. 2.
A Left ventricular outflow tract velocity time integral [VTI] prior to passive leg raise reading ~ 13.3 cm. B VTI after passive leg raise demonstrating increase to 16.2 cm
Fig. 3.
Follow up evaluation demonstrating a normal left ventricular outflow tract velocity time integral of ~ 24 cm (22 cm mentioned in the text was an average of multiple measurements)
Table 1.
Serum sodium levels and pertinent data during the course of hospitalization
| Day of hospitalization | Serum sodium (mmol/L) | BP (mmHg) | Weight (kg) | Urine sodium (mmol/L) | Urine osmolality (mOsm/kg) | Serum osmolality (mOsm/kg) |
|---|---|---|---|---|---|---|
| 0 (at presentation) | 138 | 159/96 | 48 | |||
| 2 | 122 | 138/84 | < 20 | 283 | 264 | |
| 4 | 126 | 131/77 | ||||
| 6 | 128 | 133/73 | ||||
| 7 | 126 | 146/77 | ||||
| 8 | 123 | 138/88 | 46 | 257 | ||
| 9 (POCUS 1) | 122 | 144/89 | 47.5 | 69 | 365 | |
| 10 | 128 | 138/84 | 49 | 351 | ||
| 11 (POCUS 2) | 130 | 125/76 | 28 | 235 | ||
| 15 | 134 | 129/74 | 50.1 | 64 | 249 |
POCUS 1 and 2 first and follow-up point of care ultrasound examinations, respectively, BP lowest blood pressure recorded in 24 h
Discussion
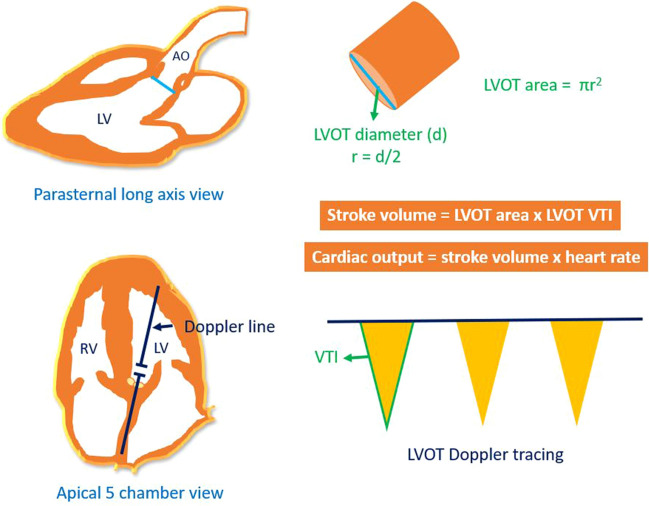
POCUS is a limited ultrasound examination performed by the clinician at the patient’s bedside to answer focused clinical questions. It is not intended to replace consultative imaging but an enhancement to physical examination. Once confined to procedural guidance, the scope of POCUS is expanding in internal medicine and nephrology, and currently includes multi-system evaluation to objectively assess hemodynamics [8–11]. In our patient, the laboratory data and physical examination seem to point toward euvolemic hyponatremia at the time of initial nephrology consult. Urine sodium was high, urine osmolality was more than 200 mOsm/kg and pain (though not significant per patient) due to fractures could explain the stimulus for ADH. Auscultation findings were mostly bowel sounds confused with crackles due to hiatal hernia. POCUS exam has uncovered hypovolemia, which would have been otherwise missed based on conventional parameters. It is likely that high urine sodium was contributed by prior diuretic administration. As cardiac flow changes precede drop in blood pressure, reduction in stroke volume can be detected before the patient decompensates. Physicians trained in Doppler ultrasonography can assess stroke volume at the bedside. Briefly, stroke volume is the product of left ventricular outflow tract (LVOT) cross sectional area and VTI. LVOT area is derived from the LVOT diameter in parasternal long-axis cardiac view using the formula πr2 (area of circle), and LVOT VTI is obtained by tracing the envelope of the systolic flow in the apical 5-chamber view (Fig. 4) [5]. As the LVOT diameter is constant for a given patient, VTI is often used as a surrogate for changes in cardiac output assuming the heart rate is relatively constant. This is what we did in our case. In addition, POCUS also allows us to assess fluid responsiveness before actually administering the fluids. This can be accomplished by the passive leg raise, which is performed by tilting the patient from a 45° semi-recumbent head-up position to a 45° leg-up position, which transfers up to 300–400 mL of blood into central circulation (auto fluid bolus). LVOT VTI is measured before and one minute after the leg raise; an increase by 10% suggests fluid responsiveness [12]. Our patient showed an increase in VTI by more than 15% and hence we could confidently administer the fluids. The fact that the repeat VTI after fluid therapy has normalized proves beyond doubt that there was a hypovolemic component to the hyponatremia. Pain and low solute intake could also be contributing to ADH release. However, POCUS allowed us to identify an additional treatable cause and potentially prevented mismanagement. It is noteworthy that while isolated assessment of the IVC is popular among novice POCUS users, a small collapsible IVC alone cannot differentiate between euvolemia and hypovolemia. Therefore, cardiac evaluation is crucial to answer this clinical question. In summary, we do not advocate over-reliance on isolated POCUS findings but would like to emphasize that adoption of this bedside diagnostic tool is a valuable addition to our armamentarium, especially when dealing with complex electrolyte disorders associated with hemodynamic derangement.
Fig. 4.
Determination of stroke volume and cardiac output using echocardiography. LV, left ventricle; RV, right ventricle; Ao, aorta; LVOT, left ventricular outflow tract; r, radius; VTI, velocity time integral. Figure adapted from [5] with kind permission of the publisher, S. Karger AG, Basel
Funding
None.
Declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. IRB approval is not needed for a single case study.
Informed consent
Obtained from the patient for the publication of this case study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Liamis G, Milionis H, Elisaf M. A review of drug-induced hyponatremia. Am J Kidney Dis. 2008;52(1):144–153. doi: 10.1053/j.ajkd.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 2.Buffington MA, Abreo K. Hyponatremia: a review. J Intensive Care Med. 2016;31(4):223–236. doi: 10.1177/0885066614566794. [DOI] [PubMed] [Google Scholar]
- 3.Chung HM, Kluge R, Schrier RW, Anderson RJ. Clinical assessment of extracellular fluid volume in hyponatremia. Am J Med. 1987;83(5):905–908. doi: 10.1016/0002-9343(87)90649-8. [DOI] [PubMed] [Google Scholar]
- 4.Stevenson LW, Perloff JK. The limited reliability of physical signs for estimating hemodynamics in chronic heart failure. JAMA. 1989;261(6):884–888. doi: 10.1001/jama.1989.03420060100040. [DOI] [PubMed] [Google Scholar]
- 5.Koratala A, Kazory A. Point of care ultrasonography for objective assessment of heart failure: integration of cardiac, vascular, and extravascular determinants of volume status. Cardiorenal Med. 2021;11(1):5–17. doi: 10.1159/000510732. [DOI] [PubMed] [Google Scholar]
- 6.Koratala A, Sturgill D. Point-of-care venous Doppler ultrasound in the management of heart failure and hyponatremia. Clin Nephrol. 2021 doi: 10.5414/CN110388. [DOI] [PubMed] [Google Scholar]
- 7.Argaiz ER, Koratala A, Reisinger N. Comprehensive assessment of fluid status by point-of-care ultrasonography. Kidney. 2021 doi: 10.34067/KID.0006482020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koratala A, Teodorescu V, Niyyar VD. The nephrologist as an ultrasonographer. Adv Chronic Kidney Dis. 2020;27(3):243–252. doi: 10.1053/j.ackd.2020.03.005. [DOI] [PubMed] [Google Scholar]
- 9.Koratala A. Utilizing point-of-care ultrasound in the cardiorenal clinic to enhance patient care. J Bras Nefrol. 2021;43(1):135–136. doi: 10.1590/2175-8239-JBN-2020-0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.LoPresti CM, Schnobrich DJ, Dversdal RK, Schembri F. A road map for point-of-care ultrasound training in internal medicine residency. Ultrasound J. 2019;11(1):10. doi: 10.1186/s13089-019-0124-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smallwood N, Dachsel M. Point-of-care ultrasound (POCUS): unnecessary gadgetry or evidence-based medicine? Clin Med (Lond) 2018;18(3):219–224. doi: 10.7861/clinmedicine.18-3-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller A, Mandeville J. Predicting and measuring fluid responsiveness with echocardiography. Echo Res Pract. 2016;3(2):G1–G12. doi: 10.1530/ERP-16-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]