Abstract
We report a case of acute ischemic nephropathy in a patient with severe renal artery stenosis and bradycardia due to sick sinus syndrome. An 83-year-old Japanese woman with a history of hypertension was diagnosed with sick sinus syndrome and scheduled for pacemaker implantation. Four days prior to admission for the procedure, she experienced sudden-onset severe right flank pain that persisted for 1 day. On the day of admission, her serum creatinine level increased from 1.35 mg/dL, measured 2 weeks earlier, to 7.04 mg/dL. Laboratory examinations showed elevated C-reactive protein and lactate dehydrogenase levels. A computed tomography scan showed a severely atrophied left kidney, suggesting that it was non-functioning. Doppler ultrasonography of the right renal artery showed an extended acceleration time, suggesting proximal stenosis. Magnetic resonance imaging showed no enhancement in the proximal portions of the right renal artery, consistent with severe stenosis or occlusion. The patient developed severe bradycardia with lightheadedness; as a result, pacemaker implantation was performed on post-admission day 7. On day 10, digital subtraction angiography revealed diffuse severe stenosis of the right renal artery; intravascular ultrasonography suggested plaque rupture. Percutaneous transluminal renal angioplasty (PTRA) was performed and a drug-eluting stent was placed. On day 11, hemodialysis was performed owing to deteriorating renal function. The patient’s renal function dramatically improved shortly thereafter. This case highlights the importance of PTRA for select patients, as it can potentially save some patients from chronic dialysis, and outlines the possible implications of bradycardia in the pathogenesis of ischemic nephropathy.
Keywords: Renal artery stenosis, Sick sinus syndrome, Acute renal failure, Percutaneous transluminal renal angioplasty, Dialysis
Introduction
Between 5 and 22% of elderly patients with chronic kidney disease (CKD) have renal artery stenosis (RAS), which can cause refractory hypertension and renal impairment [1–3]. Approximately 90% of RAS cases are attributed to atherosclerosis; RAS is also caused by fibromuscular dysplasia, which is often observed in young women [4]. Treatment for RAS includes pharmacological therapy and percutaneous transluminal renal artery angioplasty (PTRA) [5]. Pharmacological treatment consists of antihypertensives for blood pressure control, statins for dyslipidemia, and aspirin or clopidogrel as antiplatelet therapy. PTRA involves balloon angioplasty with or without stent placement. Complications associated with PTRA include arterial perforation, hematoma at the puncture site, re-stenosis, and atheroembolic disease [6, 7].
The efficacy of PTRA has been controversial as previous large-scale randomized controlled trials found that patients who underwent PTRA and medical treatment had a comparable prognosis to that of patients treated with medication alone [7–9]. However, many patients enrolled in these trials had a relatively mild degree of stenosis and stable renal function [7–9]. The potential benefit of PTRA in patients with more severe stenosis and progressive renal dysfunction is yet to be evaluated in controlled trials, in part because of the difficulty of recruiting a large number of patients with such severe conditions. Nonetheless, clinical guidelines and prior studies suggest that PTRA may be indicated in patients with hemodynamically significant bilateral RAS, RAS in a solitary kidney, or rapidly progressive renal dysfunction [10–12].
Here, we report a case of acute ischemic nephropathy in a patient with severe renal artery stenosis and bradycardia due to sick sinus syndrome. The patient required temporary dialysis; however, dramatic renal functional recovery was observed after PTRA.
Case report
An 83-year-old woman with a history of hypertension was referred to the cardiology department of our hospital for the evaluation of sick sinus syndrome. Five years prior to referral, her serum creatinine level was 0.67 mg/dL. Five months prior to referral, the patient experienced lightheadedness and saw her primary care doctor who diagnosed sick sinus syndrome based on the results of a 12-lead electrocardiogram. On the day of referral, her serum creatinine level was 1.35 mg/dL. She had been taking an angiotensin II receptor blocker (ARB) for at least 5 months. Permanent pacemaker implantation was scheduled for 2 weeks later. Four days prior to hospital admission for the procedure, the patient experienced sudden-onset right flank pain, which resolved the next day. On the day of admission, her creatinine level was markedly elevated to 7.04 mg/dL; thus, the patient was referred to our nephrology department for the evaluation of acute kidney injury. She was normotensive at 133/84 mmHg, and her pulse rate was 93 beats per minute. Her weight was 59.9 kg, which was greater than her usual weight of 56 kg. Mild bilateral lower extremity edema was noted on physical examination. Her medications included 40 mg telmisartan, 12.5 mg hydrochlorothiazide, 10 mg nifedipine for hypertension, difenidol, laroxifen for osteoporosis, vonoprazan fumarate, and herbal medicines for dizziness. Blood tests showed elevated C-reactive protein (CRP; 7.96 mg/dL), lactate dehydrogenase (LDH; 603 U/L), and brain natriuretic peptide (BNP; 341.3 pg/dL) levels (Table 1), and her sodium levels were abnormal (132 mEq/L). She also had mild anemia and metabolic acidosis. Urinalysis revealed mild proteinuria and elevated renal tubular markers. Chest radiography showed cardiomegaly and mild pleural effusion. A 12-lead electrocardiogram showed sinus rhythm with a first-degree atrioventricular block and complete right bundle branch block.
Table 1.
Laboratory findings on admission
| Blood counts | |
| White blood cell | 7,000/µL |
| Red blood cell | 371X 104/µL |
| Hemoglobin | 12.2 g/dL |
| Platelet | 14.1 X 104/µL |
| Coagulation | |
| PT-INR | 1.05 |
| APTT | 32.7 s |
| Fibrinogen | 507 mg/dL |
| D-dimer | 1.4 μg/mL |
| Blood chemistry | |
| Sodium | 132 mEq/L |
| Potassium | 4.8 mEq/L |
| Chloride | 97 mEq/L |
| Calcium | 8.6 mg/dL |
| Phosphorus | 4.8 mg/dL |
| Magnesium | 2.2 mg/dL |
| Urea nitrogen | 74 mg/dL |
| Creatinine | 7.04 mg/dL |
| Total protein | 6.4 g/dL |
| Albumin | 3.1 g/dL |
| LDH | 603 U/L |
| AST | 21 U/L |
| ALT | 30 U/L |
| ALP | 128 U/L |
| Total bilirubin | 1.2 mg/dL |
| Glucose | 105 mgdL |
| C-reactive protein | 7.96 mg/dL |
| Brain natriuretic peptide | 341.3 pg/mL |
| IgG | 1297.9 mg/dL |
| IgA | 251.9 mg/dL |
| IgM | 56 mg/dL |
| Rheumatoid factor | 5.4 U/mL |
| Anti-nuclear antibody | 20 fold |
| Anti-ds-DNA IgG antibody | < 0.5 U/mL |
| CH50 | ≥ 60.0/mL |
| ASO | 12 U/mL |
| PR3-ANCA | < 1.0 U/mL |
| MPO-ANCA | < 1.0 U/mL |
| Anti-GBM antibody | < 2.0 U/mL |
| Serum electrophoresis | All negative |
| Urine electrophoresis | All negative |
| Venous blood gas | |
| pH | 7.324 |
| pCO2 | 41.7 torr |
| HCO3− | 21.1 mmol/L |
| Urine test | |
| Urine protein | 1+ |
| Urine blood | 1+ |
| Urine white blood cell | 1+ |
| Specific gravity | 1.007 |
| Urine urobilinogen | ± |
| Urine bilirubin | Negative |
| Urine sediment | |
| Red blood cell | 1-4/HPF |
| White blood cell | 1-4/HPF |
| Urine chemistry | |
| Urine UN | 325 mg/dL |
| Urine Cr | 53.3 mg/dL |
| Urine Na | 34.2 mEq/L |
| Urine K | 9.1 mEq/L |
| Urine Cl | 19.1 mEq/L |
| Urine β2-microglobulin | 10,902 µg/L |
| Urine NAG | 8.9 U/L |
PT-INR prothrombin international normalized ratio, APTT activated partial thromboplastin time, LDH lactate dehydrogenase, AST aspartate aminotransferase, ALT alanine aminotransferase, ALP alkaline phosphatase, Anti-ds-DNA anti-double-stranded DNA, ASO antistreptolysin O, GBM glomerular basement membrane, HPF high power field, NAG N-acetyl-beta-D-glucosaminidase
We first stopped telmisartan and hydrochlorothiazide to rule out the possibility of medication-induced acute kidney injury. Ultrasonography did not show a distended or collapsed inferior vena cava, suggesting that renal congestion or dehydration was unlikely. The fractional excretion of sodium and the fractional excretion of urea nitrogen were not reduced (3.4% and 58.0%, respectively). Furthermore, echocardiography did not show reduced left ventricular ejection fraction, suggesting normal cardiac output.
We suspected renal artery occlusion as the cause of her acute kidney injury, based on the patient’s elevated LDH and CRP levels and the sudden onset of right flank pain. Although echocardiography did not show apparent cardiac emboli, D-dimer level was mildly elevated at 1.40 µg/mL on day 3, making it difficult to completely exclude the possibility of cardiac emboli. An abdominal computed tomography (CT) scan revealed that the left kidney was significantly smaller than the right kidney, suggesting that the left kidney was non-functional. There was no evidence of hydronephrosis. Doppler ultrasonography of the renal arteries showed an extended acceleration time for the right renal artery, suggesting severe stenosis in the proximal portion of the right renal artery. Abdominal magnetic resonance (MR) imaging and MR angiography did not detect flow signals in either renal artery, suggesting severe RAS or occlusion (Fig. 1).
Fig. 1.
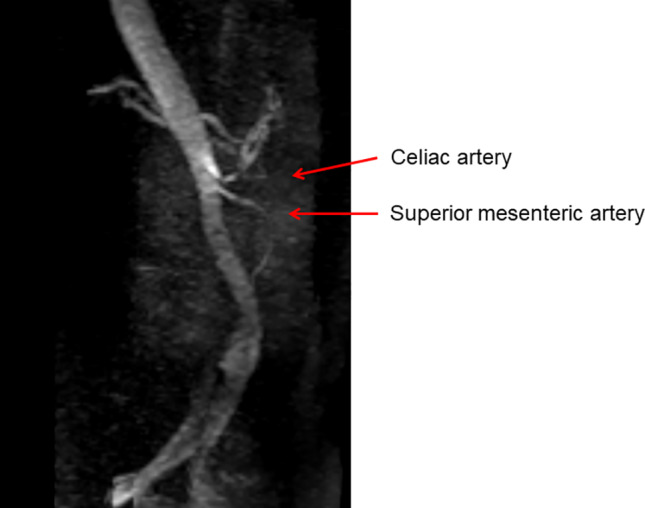
Abdominal MRI and MRA. Flow signals were not detected in both renal arteries, suggesting severe renal artery stenosis or occlusion
While investigations were being performed, the patient developed severe bradycardia with a heart rate of 20–30 beats per minute, accompanied by lightheadedness. Given that the patient was symptomatic, a temporary pacemaker was inserted on day 6 post-admission, and a permanent pacemaker was implanted on day 7 to reduce the risk of catheter-associated infection.
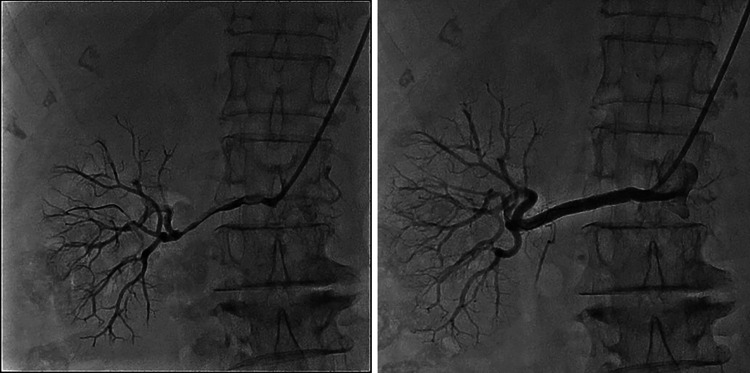
On day 7 post-admission, we performed a contrast-enhanced CT scan (Fig. 2), which showed no enhancement in the proximal portion of the right renal artery; however, the upper portion of the right kidney and a narrow branch of the right renal artery showed enhancement. The lower portion of the right kidney also showed a slight contrast enhancement in the delayed phase, suggesting that the right renal artery was not completely occluded. Digital subtraction angiography was performed on day 10, revealing diffuse severe stenosis of the right renal artery. The intravascular ultrasonography (IVUS) findings were suggestive of plaque rupture. PTRA was performed and a drug-eluting stent was placed, resulting in significantly improved renal perfusion (Fig. 3). Abdominal aortography showed very slight enhancement at the origin of the left renal artery and no enhancement in the peripheral parts. Considering the atrophy of the left kidney on CT images, severe stenosis of the left renal artery was likely.
Fig. 2.
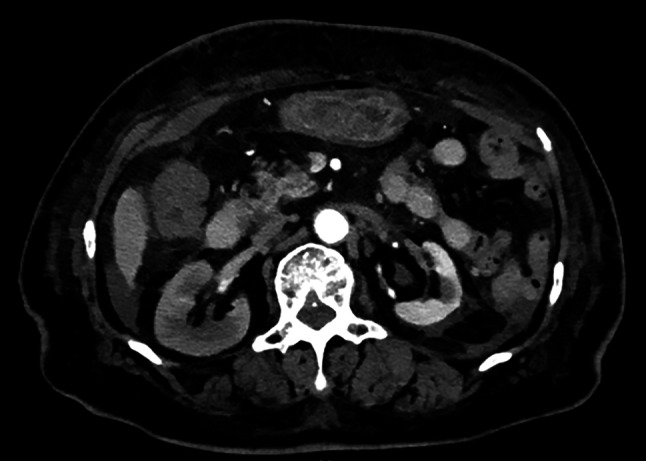
An enhanced abdominal CT scan. The left kidney was much smaller than the right kidney
Fig. 3.
The right renal artery before PTRA and drug-eluting stent placement (the left picture) and after the procedures (the right picture). Right renal artery flow was significantly improved after the procedures
Despite the improvement in renal perfusion, the patient’s renal function deteriorated with declining urine volume. Due to increasing weight and edema, we started furosemide and increased the dosage to 80 mg on day 8. We also started tolvaptan from day 5. On day 11, her serum creatinine level increased to 10.53 mg/dL and she underwent a session of hemodialysis because the risk of congestive heart failure from volume overload was imminent. Her body weight decreased from 63.9 to 62.4 kg during the hemodialysis session. Shortly thereafter, on the same day, her urine volume began to increase significantly, and her serum creatinine level began to decrease. We stopped tolvaptan on day 12 and decreased the dosage of furosemide from 80 to 40 mg on day 13. We stopped furosemide on day 14 because her body weight sharply decreased to 56.5 kg on that day without additional hemodialysis sessions. Because the patient’s blood pressure decreased from 130 to 160 mmHg before PTRA to 100 to 120 mmHg after PTRA, we stopped nifedipine. Her serum creatinine level continued to decline to 2.34 mg/dL on day 21 and she was discharged on day 22. At 2 months after discharge, her serum creatinine level had further decreased to 1.72 mg/dL. The patient’s systolic blood pressure was elevated to 140–170 mmHg; thus, we restarted nifedipine (10 mg). At 4 months after discharge, her systolic blood pressure at home was still elevated at 160–170 mmHg. Plasma renin activity and aldosterone levels were elevated at 5.1 ng/mL/h and 220.0 pg/mL, respectively, while serum potassium level was low (2.9 mEq/L). Kidney function was preserved (serum creatinine; 1.38 mg/dL). These laboratory findings raised the possibility of restenosis of the right renal artery as well as renovascular hypertension due to left RAS. We decided to continue supportive therapy by adjusting the dosage of nifedipine and monitor the patient’s clinical course.
Discussion
We report a case of rapidly progressive renal dysfunction in a patient with severe RAS. Uniquely, the patient had concurrent severe bradycardia due to sick sinus syndrome. Although the patient temporarily required hemodialysis, her renal function dramatically improved after PTRA, which restored blood flow to the right kidney and allowed the cessation of renal replacement therapy.
Regarding the etiology of the patient’s renal impairment, ischemic nephropathy due to severe stenosis of the right renal artery was probably the primary cause, based on the angiography findings and the recovery of renal function after PTRA. Prior to the development of severe right RAS, the patient probably had non-functioning left kidney because the left kidney was severely atrophied. As for the urinary findings on admission, although the fractional excretion of urea nitrogen (FEUN) was expected to decrease owing to reduced renal perfusion caused by severe RAS, the FEUN was not low, probably because acute tubular necrosis occurred due to ischemic damage.
As for the elevation of blood pressure after discharge, we considered the possibility of restenosis of the right renal artery as well as renovascular hypertension due to left RAS. A previous report showed that the kidney could still cause renovascular hypertension in the presence of a small amount of blood flow, even if the kidney was severely atrophic and non-functional [13]. As enhanced CT images suggested the presence of some blood flow in the left renal artery, renovascular hypertension owing to left RAS could be present. However, due to lack of data on the onset of hypertension, previous renin and aldosterone levels, and blood pressure changes before and after ARB was started, the presence of renovascular hypertension due to left RAS remained speculative.
Regarding the pathogenesis of right RAS in our patient, based on the IVUS findings, we believe that the plaque in the atherosclerotic renal artery ruptured. However, the possibility of renal infarction due to thrombosis could not be fully ruled out given the presence of severe bradycardia, an episode of acute flank pain, and elevated LDH and CRP levels. Previous reports on acute renal failure following episodes of lower back or flank pain were cases of renal artery occlusion [14, 15]. Thus, the episode of acute right flank pain in our case raises the possibility that partial occlusion of the right renal artery may have occurred.
Bradycardia alone was unlikely to have induced decreased renal perfusion because stroke volume increases to compensate for bradycardia-induced hypoperfusion [16]. However, bradycardia in the presence of left ventricular dysfunction can lead to hemodynamic instability and compromised renal perfusion, leading to renal injury [17]. In this case, the preserved ejection fraction of the left ventricle shown on cardiac ultrasound and normal-to-elevated blood pressure suggest that bradycardia alone did not reduce renal perfusion. Additionally, active vasculitis was determined to be unlikely based on the absence of systemic symptoms, including fever and malaise.
To investigate the cause of renal dysfunction, we performed a contrast CT test despite severe renal impairment for the following three reasons. First, magnetic resonance angiography (MRA), even when using contrast media, was shown to have high sensitivity but low specificity [18]; thus, some patients who do not have RAS may be falsely diagnosed by MRA. Therefore, we needed to confirm the presence of RAS by contrast CT to ensure that the patient was not misdiagnosed. Second, if enhanced CT findings had not shown the presence of RAS, we could have avoided unnecessary renal angiography and procedure-associated complications, including cholesterol crystal embolization and puncture hematoma. Third, we wished to identify the characteristics of the occluded renal artery and predict the difficulty and success rate of PTRA by confirming the number of renal arteries, branching point of aorta, and lengths of occluded parts of the renal artery on enhanced CT images. Thus, the enhanced CT findings were necessary to determine whether the patient was indicated for PTRA.
The indications for PTRA have been widely debated. Although large-scale randomized controlled trials failed to demonstrate the benefit of PTRA compared to pharmacological treatment [7–9], these trials have been criticized for biased patient selection [19]. One editorial argued that these trials were conducted in patients with relatively mild RAS or stable renal function; these patients are the least likely to benefit from revascularization procedures [19]. In the ASTRAL trial, approximately 40% of study subjects had moderate stenosis (50–70%), and 25% of subjects had an eGFR above 50 mL/min [8]. In the CORAL trial, 55% of subjects had moderate stenosis (< 70%) and approximately 50% had an eGFR greater than 60 mL/min/1.73 m2 [9, 20]. Moreover, patients with resistant hypertension, who are more likely to have RAS and may benefit from PTRA, were excluded from one of the randomized trials [7, 21]. Thus, the results of these trials cannot be extrapolated to patients with severe stenosis or progressive renal dysfunction. A retrospective cohort study of 263 patients with ≥ 70% stenosis in the renal artery showed that patients with rapidly declining renal function, defined as > 3 mL/min/1.73 m2 decline in eGFR per year, who received PTRA had a lower risk of cardiovascular events and incident end-stage renal disease compared to the group that did not receive PTRA [22]. Furthermore, previous case reports on patients with rapidly declining renal function, flash pulmonary edema, or resistant hypertension also demonstrated the benefit of PTRA in restoring renal function as well as ameliorating pulmonary edema and hypertension [12]. Our patient exhibited rapidly declining renal function in the presence of severe right RAS with the left kidney being most likely non-functional. The episode of acute flank pain and elevated LDH and CRP levels suggested a recent ischemic event and supported the use of PTRA. We believe that a rapid diagnostic workup should be performed when severe RAS or renal infarction is suspected so that PTRA can be performed in a timely manner to increase the chance of functional renal recovery.
Our patient’s renal function dramatically improved after PTRA, which was performed 12 days after the episode of sudden flank pain. The window for kidney viability during renal transplantation is considered to be approximately 24 h [23], while that for severe RAS is likely longer if the artery is not completely occluded [15, 24]. A previous report on a patient who required dialysis showed renal function recovery after 6 weeks of iatrogenic RAS [24]. Another report also demonstrated renal function recovery more than 24 h after oligo-anuria [15]. An MR imaging study on stenotic and contralateral kidneys demonstrated that cortical and medullary oxygenation in stenotic kidneys was maintained despite significantly reduced blood flow and kidney volume [25]. An increase in the glomerular filtration rate is associated with increased tubular sodium reabsorption, which is responsible for most renal oxygen consumption, thereby contributing to tissue hypoxia [26]. Thus, in the context of limited perfusion, the kidney may attempt to ensure oxygenation at the expense of glomerular filtration to remain viable. There is also evidence that indicates arterial-to-venous oxygen shunting also decreases with decreased renal blood flow, resulting in the preservation of renal tissue oxygenation [26]. Further mechanistic studies on renal tissue oxygenation and renal blood flow are needed to elucidate the window for kidney tissue damage in the context of severe RAS.
In summary, we encountered a case of severe ischemic nephropathy in a patient with RAS and bradycardia due to sick sinus syndrome. The patient temporarily required hemodialysis; however, her renal function improved dramatically after PTRA. This case highlights the importance of appropriate patient selection for PTRA, which has the potential to prevent some patients from undergoing chronic dialysis. Further investigation into the regenerative capacity of kidneys following severe reduction in renal blood flow would help elucidate the window for kidney damage in severe RAS and help clinicians identify which patients would benefit most from revascularization treatment.
Acknowledgements
We extend our sincere appreciation toward the Cardiology department of Yokosuka Kyosai Hospital for pacemaker placement and percutaneous transluminal renal angioplasty. We would also like to thank Editage (www.editage.com) for English language editing.
Declarations
Conflict of interest
The authors have declared that no conflict of interest exists.
Informed consent
The patient’s consent was obtained for the writing of this case report.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References:
- 1.Appel RG, Bleyer AJ, Reavis S, Hansen KJ. Renovascular disease in older patients beginning renal replacement therapy. Kidney Int. 1995;48:171–176. doi: 10.1038/ki.1995.281. [DOI] [PubMed] [Google Scholar]
- 2.Tanemoto M, Saitoh H, Satoh F, Satoh H, Abe T, Ito S. Predictors of undiagnosed renal artery stenosis among Japanese patients with risk factors of atherosclerosis. Hypertens Res. 2005;28:237–242. doi: 10.1291/hypres.28.237. [DOI] [PubMed] [Google Scholar]
- 3.Kalra PA, Guo H, Kausz AT, Gilbertson DT, Liu J, Chen SC, et al. Atherosclerotic renovascular disease in United States patients aged 67 years or older: risk factors, revascularization, and prognosis. Kidney Int. 2005;68:293–301. doi: 10.1111/j.1523-1755.2005.00406.x. [DOI] [PubMed] [Google Scholar]
- 4.Safian RD, Textor SC. Renal-artery stenosis. N Engl J Med. 2001;344:431–442. doi: 10.1056/NEJM200102083440607. [DOI] [PubMed] [Google Scholar]
- 5.Balk E, Raman G, Chung M, Ip S, Tatsioni A, Alonso A, et al. Effectiveness of management strategies for renal artery stenosis: a systematic review. Ann Intern Med. 2006;145:901–912. doi: 10.7326/0003-4819-145-12-200612190-00143. [DOI] [PubMed] [Google Scholar]
- 6.Mochida Y, Ohtake T, Miyashita Y, Ishioka K, Oka M, Maesato K, et al. Long-term clinical outcome of patients with severe atherosclerotic renal artery stenosis after percutaneous transluminal renal angioplasty. Renal Replacement Therapy. 2018;4:5. doi: 10.1186/s41100-018-0147-x. [DOI] [Google Scholar]
- 7.Bax L, Woittiez AJ, Kouwenberg HJ, Mali WP, Buskens E, Beek FJ, et al. Stent placement in patients with atherosclerotic renal artery stenosis and impaired renal function: a randomized trial. Ann Intern Med. 2009;150(840–8):W150–1. doi: 10.7326/0003-4819-150-12-200906160-00119. [DOI] [PubMed] [Google Scholar]
- 8.Wheatley K, Ives N, Gray R, Kalra PA, Moss JG, ASTRAL Investigators et al. Revascularization versus medical therapy for renal-artery stenosis. N Engl J Med. 2009;361:1953–1962. doi: 10.1056/NEJMoa0905368. [DOI] [PubMed] [Google Scholar]
- 9.Cooper CJ, Murphy TP, Cutlip DE, Jamerson K, Henrich W, Reid DM, et al. Stenting and medical therapy for atherosclerotic renal-artery stenosis. N Engl J Med. 2014;370:13–22. doi: 10.1056/NEJMoa1310753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, et al. Management of patients with peripheral artery disease (compilation of 2005 and 2011 ACCF/AHA guideline recommendations): a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines. Circulation. 2013;127:1425–1443. doi: 10.1161/CIR.0b013e31828b82aa. [DOI] [PubMed] [Google Scholar]
- 11.Chonchol M, Linas S. Diagnosis and management of ischemic nephropathy. Clin J Am Soc Nephrol. 2006;1:172–181. doi: 10.2215/CJN.00940905. [DOI] [PubMed] [Google Scholar]
- 12.Yan X, Ding M, Li J, Zhan H, Zuo Y, Wang H, et al. Refractory hypertension due to renal artery stenosis in a solitary kidney: case report and literature review. Int J Clin Exp Med. 2018;11:2778–2784. [Google Scholar]
- 13.Morikawa T, Imanishi M, Suzuki H, Okada N, Okumura M, Konishi Y, et al. Mast cell chymase in the ischemic kidney of severe unilateral renovascular hypertension. Am J Kidney Dis. 2005;45:e45–e50. doi: 10.1053/j.ajkd.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 14.Campbell JE, John M, Bates MC. Surgical rescue and restoration of renal function in an anuric renal failure patient: a case report. W V Med J. 2008;104:22–24. [PubMed] [Google Scholar]
- 15.Zankl AR, Dengler TJ, Andrassy M, Volz HC, Katus HA, Zeier M. Recovery of renal function after delayed percutaneous dilation of a subtotal in-stent restenosis of the renal artery in a left solitary kidney. NDT Plus. 2009;2:236–238. doi: 10.1093/ndtplus/sfp012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iwataki M, Kim YJ, Sun BJ, Jang JY, Takeuchi M, Fukuda S, et al. Different characteristics of heart failure due to pump failure and bradyarrhythmia. J Echocardiogr. 2015;13:27–34. doi: 10.1007/s12574-014-0235-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aoun M, Tabbah R. Case report: severe bradycardia, a reversible cause of “cardio-renal-cerebral syndrome”. BMC Nephrol. 2016;17:162-016-0375-7. doi: 10.1186/s12882-016-0375-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patel ST, Mills JLS, Tynan-Cuisinier G, Goshima KR, Westerband A, Hughes JD. The limitations of magnetic resonance angiography in the diagnosis of renal artery stenosis: comparative analysis with conventional arteriography. J Vasc Surg. 2005;41:462–468. doi: 10.1016/j.jvs.2004.12.045. [DOI] [PubMed] [Google Scholar]
- 19.Mann SJ, Sos TA. Misleading results of randomized trials: the example of renal artery stenting. J Clin Hypertens. 2010;12:1–2. doi: 10.1111/j.1751-7176.2009.00216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mohan IV, Bourke V. The management of renal artery stenosis: an alternative interpretation of ASTRAL and CORAL. Eur J Vasc Endovasc Surg. 2015;49:465–473. doi: 10.1016/j.ejvs.2014.12.026. [DOI] [PubMed] [Google Scholar]
- 21.van Jaarsveld BC, Krijnen P, Derkx FH, Deinum J, Woittiez AJ, Postma CT, et al. Resistance to antihypertensive medication as predictor of renal artery stenosis: comparison of two drug regimens. J Hum Hypertens. 2001;15:669–676. doi: 10.1038/sj.jhh.1001258. [DOI] [PubMed] [Google Scholar]
- 22.Vassallo D, Ritchie J, Green D, Chrysochou C, Kalra PA. The effect of revascularization in patients with anatomically significant atherosclerotic renovascular disease presenting with high-risk clinical features. Nephrol Dial Transplant. 2018;33:497–506. doi: 10.1093/ndt/gfx025. [DOI] [PubMed] [Google Scholar]
- 23.Tennankore KK, Kim SJ, Alwayn IP, Kiberd BA. Prolonged warm ischemia time is associated with graft failure and mortality after kidney transplantation. Kidney Int. 2016;89:648–658. doi: 10.1016/j.kint.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 24.Woittiez KJ, van Buren M, Kesecioglu J. Renal artery stenosis: a classic presentation, a rare cause. BMJ Case Rep. 2012 doi: 10.1136/bcr-2012-006499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gloviczki ML, Glockner JF, Lerman LO, McKusick MA, Misra S, Grande JP, et al. Preserved oxygenation despite reduced blood flow in poststenotic kidneys in human atherosclerotic renal artery stenosis. Hypertension. 2010;55:961–966. doi: 10.1161/HYPERTENSIONAHA.109.145227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Evans RG, Gardiner BS, Smith DW, O'Connor PM. Intrarenal oxygenation: unique challenges and the biophysical basis of homeostasis. Am J Physiol Renal Physiol. 2008;295:F1259–F1270. doi: 10.1152/ajprenal.90230.2008. [DOI] [PubMed] [Google Scholar]