Abstract
Alzheimer’s disease (AD) is the most common neurodegenerative disorder in elderly subjects. Recent studies verified the effects of cognitive training combined with repetitive transcranial magnetic stimulation (rTMS-COG) in AD patients. Here, we analyzed neuropsychological and neurophysiological data, derived from electroencephalography (EEG), to evaluate the effects of a 6-week protocol of rTMS-COG in 72 AD. We designed a randomized, double-blind, sham-controlled trial to evaluate efficacy of rTMS on 6 brain regions obtained by an individual MRI combined with COG related to brain areas to stimulate (i.e., syntax and grammar tasks, comprehension of lexical meaning and categorization tasks, action naming, object naming, spatial memory, spatial attention). Patients underwent neuropsychological and EEG examination before (T0), after treatment (T1), and after 40 weeks (T2), to evaluate the effects of rehabilitation therapy. “Small World” (SW) graph approach was introduced allowing us to model the architecture of brain connectivity in order to correlate it with cognitive improvements. We found that following 6 weeks of intensive daily treatment the immediate results showed an improvement in cognitive scales among AD patients. SW present no differences before and after the treatment, whereas a crucial SW modulation emerges at 40-week follow-up, emphasizing the importance of rTMS-COG rehabilitation treatment for AD. Additional results demonstrated that the delta and alpha1 SW seem to be diagnostic biomarkers of AD, whereas alpha2 SW might represent a prognostic biomarker of cognitive recovery. Derived EEG parameters can be awarded the role of diagnostic and predictive biomarkers of AD progression, and rTMS-COG can be regarded as a potentially useful treatment for AD.
Keywords: EEG, Small World, LORETA, Cognitive training, rTMS, Alzheimer
Introduction
Alzheimer’s disease (AD) is the most common neurodegenerative disorder in elderly population and the main cause of cognitive impairment. To date, the most used treatments for AD are pharmacological therapies that can transiently slow cognitive decline but do not modify the progression of the disease [1]. Limited effectiveness of pharmacological treatments has prompted the scientific community to test complementary therapeutic strategies for AD. Recent studies have verified the potential effects of cognitive training (COG) on brain plasticity [2]. In general, COG includes sessions composed of paper-and-pencil or electronic form tasks designed to involve cognitive abilities [3, 4].
Together with cognitive training one innovative strategy includes non-invasive brain stimulation techniques (NIBS) such as repetitive transcranial magnetic stimulation (rTMS). rTMS has been shown to facilitate cortical activity: It can modulate cortical excitability depending on the parameters of stimulation, and there is evidence that it is effective for many conditions, including AD [5–8]. Particularly, Cotelli and colleagues explored the effect of rTMS on the dorsolateral prefrontal cortex (DLPFC) to evaluate language abilities in control and AD groups. They have found that rTMS improves performance both in controls and AD patients [9, 10].
Based on the assumptions above, some researchers hypothesized that a treatment combining rTMS and COG may result in synergic effects more effective compared to applying the two therapies separately.
A few studies have investigated the impact of dual-stimulation rTMS-COG to evaluate improvements and long-term effects. Lee et al. [11] reported that rTMS-COG provided by the NeuroAD system (Neuronix) can improve scores of neuropsychological scales such as the Alzheimer’s Disease Assessment scale in cognitive subdomain (ADAS-Cog) which include memory and language ability. More recently, Brem and collaborators [12], in a clinical trial funded by Neuronix, has reported that rTMS-COG by NeuroAD can improve the cognitive status of AD patients with a predictive role of TMS in cognition’s change after the intervention period.
Our study builds on and overcomes some limitations of this study in three important ways. First, 72 patients were enrolled against the smaller sample size of Brem’s research. Furthermore, while in the previous study the patients were monitored only after treatment, in the present they carried out a follow-up of about 40 weeks. Again, the most important novelty was the introduction of electroencephalography (EEG) at the beginning (T0), at the end of treatment (T1), and again at 40-week follow-up (T2) together with clinical assessment of ADAS-Cog. Indeed, because of its high temporal resolution and wide use in neurodegenerative disease, EEG is crucial for the study of brain activation [13–15]; modern methods based on graph analysis on EEG data were developed to estimate brain connectivity changes. The “Small World” (SW) graph approach is hypothesized to reflect the balance between local processing and global integration, and it was introduced allowing to model the architecture of brain connectivity in order to correlate it with cognitive improvements [16–18].
Within this frame, we analyzed neuropsychological and neurophysiological data to evaluate the effects of a 6-week high-frequency rTMS-COG in mild-to-moderate AD patients [12] immediately after the end of treatment and in the long-term follow-up. Furthermore, we investigated the role of SW indexes as diagnostic and prognostic biomarkers for cognitive recovery in AD, exploring possible correlations between network abnormalities and clinical assessment.
Subjects and Methods
Inclusion and Exclusion Criteria
A dataset of 72 mild-to-moderate AD patients was recruited (T0) (54% females, age 72.43 ± 1.05 standard error, education 12.7 ± 0.59) (DSM 5, NIA-AA [19]) A total of 63 patients (Table 1), net of all patients who dropped out, completed the entire protocol with neurophysiological and EEG evaluation after the treatment (T1), and 36 of these underwent an average 40-week long-term follow-up (T2). The entire protocol is shown in Fig. 1. All the experiments were undertaken with the informed and signed consent of each participant and caregiver/family representative, according to Code of Ethics of the World Medical Association (1997), and the experimental procedures were conforming to the Declaration of Helsinki. The rTMS and EEG protocols were performed in accordance with safety guidelines procedures.
Table 1.
Demographic data of AD patients in A, B, C groups. n is the number of patients in each group; age, education, and MMSE are expressed in terms of Mean Standard Error (SE), whereas gender by means of male/female ratio (M/F)
| Group A | Group B | Group C | |
|---|---|---|---|
| n | 30 | 17 | 16 |
| Age (years) | 71.07 | 72.24 | 75.2 |
| Education | 13.87 | 11.47 | 11.81 |
| MMSE | 22.93 | 20.64 | 21 |
| Gender (M/F) | 14/16 | 10/7 | 5/11 |
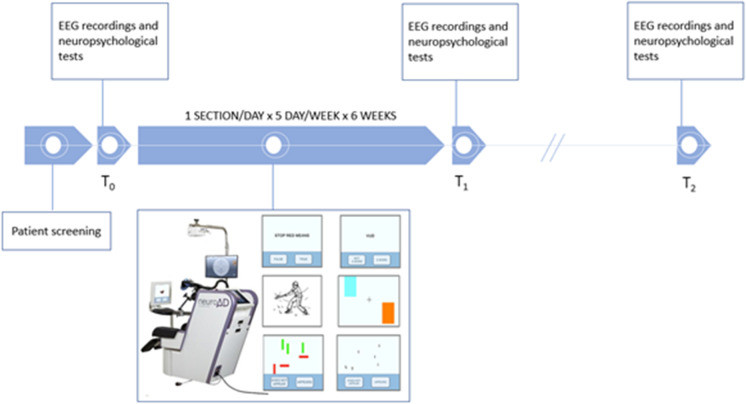
Fig. 1.
Experimental protocol timeline: After the initial screening, the enrolled patients underwent EEG recording and neuropsychological evaluation before the treatment (T0). The rehabilitation treatment (rTMS-COG for group A, sham rTMS-COG for group B, and COG for group C) lasted 6 weeks and immediately after the treatment (T1) and in a long-term 40-week follow-up the EEG and neuropsychological evaluation were repeated
AD was diagnosed according to the National Institute on Aging-Alzheimer’s Association workgroups [20]. In addition, all patients included in the study needed to have fluent Italian-speaking skills as their first language and to have a brain MRI excluding any focal brain lesion that might have affected cognitive function. Patients receiving cholinesterase inhibitors and/or memantine therapy were allowed to participate, provided the medication was taken for at least 3 months prior to the beginning of the study and the dosage of which was stable in the 60 days before the start of the study.
The exclusion criteria for AD included: Clinical Dementia Rating (CDR) score of 0 or 3, a history of epilepsy, severe agitation, lack of cooperation, unstable medical conditions, alcoholism and/or drug abuse, other neurological or psychiatric disorders different from AD, or regular use of benzodiazepines or other hypnotics (up until 2 weeks before the beginning of the study). Finally, patients who had contraindications for MRI execution or TMS treatment—according to TMS questionnaire [21] (e.g., patients with cardiac pacemaker or electrodes, intracranial implants, cochlear implants, metal clips, infusion medication pumps)—were not included in the study.
Experimental study design
We designed a randomized, double-blind, sham-controlled trial of efficacy of rTMS stimulation combined with cognitive training, consisting in 3 groups. Patients were randomly assigned in similar number in treated and sham/control groups:
Group A: real rTMS stimulation and cognitive training (rTMS-COG)
Group B: sham TMS stimulation and cognitive training (equal to group A)
Group C: sham TMS stimulation and pseudo-cognitive training.
The patients in the group A received daily treatment sessions for 6 weeks, 1 session/day for 5 days/week, for a total of 30 sessions, in which rTMS was applied to six different brain regions activated during the performance of cognitive tasks with computerized cognitive training. The patients in the group B were treated with the same procedure, receiving sham rTMS and computerized cognitive training. The patients in the group C were also treated with the same protocol, applying sham rTMS interleaved with pseudo-cognitive training.
The application of combined rTMS-COG was achieved using a paid NeuroAD device (Neuronix Ltd., Israel). A rTMS figure-of-eight magnetic coil, included in the system, was positioned following stereotactic navigation on a personalized template extracted by individual volumetric MRI to stimulate 6 brain regions (Broca’s area, R-dlPFC and L-dlPFC, Wernicke’s area R-pSAC and L-pSAC). The TMS treatment intensity was set to 90% of the MT intensity at the frontal cortex because of inconvenient eye twitches and 110% of the MT intensity at the other regions. Several paradigms were developed for the tasks including syntax and grammar tasks for the Broca’s area (e.g., is the sentence “Stop red means” correct or incorrect), comprehension of lexical meaning and categorization tasks for the Wernicke’s area (e.g., is the word “vud” a word or not a word?), action naming, object naming, and spatial memory (of shapes, colors, and letters) tasks for both the R-dlPFC and the L-dlPFC brain areas (e.g., showing a picture of a wardrobe where patients have to choose between the category “furniture” or “fruit”), spatial attention (for shapes and letters) tasks for both the R-pSAC and L-pSAC brain areas (e.g., the red vertical rectangle does or does not appear). The cognitive tasks were displayed on a 22″ computer touch screen (Elo-Touch, USA) in front of the patient, who selected the answers by touching graphical buttons on the screen. Each daily treatment session lasted about 45 min/1 h, including preparation, and three brain areas were targeted and stimulated separately as described in the following. For each brain area, the treatment consisted of 20–30 trains of rTMS (2 s of 10 Hz/train and 20 pulses/train), followed by 1–4 COG tasks, during a period of 20–40 s. In addition, in order to meet safety limitations of up to 1,500 pulses a day [6] the protocol was designed so that 3 brain areas were mainly impacted by cognitive tasks each day, for which 20–30 trains composed of 2 s pulses of 10 Hz each were administrated per brain area for a total of 1200–1400 pulses per day. The sham/control groups went through the same procedure using a sham coil that imitated auditory and somatosensory effects without effective magnetic stimulation. Each group was subjected to 30 sessions of rehabilitation for 6 weeks (1 session/day for 5 days/week for 6 weeks). rTMS was applied every day to three different brain regions, and subsequently, cognitive rehabilitation was performed involving the same stimulated areas with selective tasks. The level of difficulty for the COG tasks was developed on a patient-to-patient basis, by controlling for task variables such as the time available to complete each task and the number of objects. NeuroAD cognitive training consisted of 10 difficulty levels (Very Easy, Easy, Moderate, Moderate-1, Moderate-2, Moderate-Pro, Advanced, ADV-1, ADV-2, ADV-Pro): Patients began cognitive training at the easiest, the level of difficulty of the cognitive tasks was adapted to the individual performance of the patient for the entire treatment period, if the patient was able to successfully solve 70% of cognitive exercises pertaining to a single stimulated area, he was offered exercises of the next difficulty level. Each “next level” is characterized by an increase in stimulus response time, i.e., the time range from the presentation of the stimulus to the moment in which the subject must respond, or by increasing the number of displayed target and distracter stimuli. The cortical area stimulated by the rTMS corresponded to the cognitive domain that is trained shortly after. For the patients in the sham group, pseudo-cognitive training consists in simple objects or nature movies (e.g., a flower or animals or a landscape), presented on the screen, unrelated to cognitive function, and they simply chose whether or not they liked them.
Data recordings and preprocessing
Patients underwent neuropsychological and neurophysiological examination before (T0), after the treatment (T1) and during a last follow-up after about 40 weeks (T2); in particular, they were evaluated through ADAS-Cog scale assessment and EEG clinical recording to evaluate the effects of rehabilitation therapy. EEGs were measured at rest before and after the rTMS-COG treatment, without any task to perform, in at least 6 min of resting condition. EEG signals were measured from 32 electrodes positioned according to the augmented International 10–20 system. Two separate channels, vertical and horizontal EOGs, were used to monitor eyes blinking. Impedance was kept below 5 KΩ, and the sampling rate frequency was set up at 512 Hz. The data were processed in MATLAB (MathWorks, Natick, MA) using scripts based on EEGLAB toolbox (Swartz Center for Computational Neurosciences, La Jolla, CA) [22–24]. The EEG recordings were band-pass filtered from 0.2 to 47 Hz using a finite impulse response (FIR) filter. Imported data were divided in 2-s duration epochs, and principal artifacts in the EEG recordings (i.e., eye movements, scalp muscle contraction, and cardiac activity) were removed first by means of an EEG expert visual inspection, and later with ICA algorithm [25, 26], that enables the separation of statistically independent sources from multichannel EEG recordings [25, 27, 28] as implemented in the EEGLAB. At the end of the artifact removal procedure at least 5 min remained for subjects.
Functional connectivity of cortical sources analysis
Brain connectivity was computed using exact Low Resolution Electromagnetic Tomography (eLORETA) [29, 30] software on Regions Of Interest (ROIs) defined according to the Brodmann areas (Bas): 42 ROIs (BAs: 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 13, 17, 18, 19, 20, 21, 22, 23, 24, 25, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47). ROIs are needed for the estimation of electric neuronal activity that is used to analyze brain functional connectivity. Among the eLORETA current density time series of the ROIs, intracortical Lagged Linear Coherence, extracted by “all nearest voxels” for the 84 ROIs and centered on each BA of interest by a sphere of 19 mm for the resting sub-networks, was computed [29, 31] between all possible pairs of the ROIs for each of the seven independent EEG frequency bands [32] of delta (2–4 Hz), theta (4–8 Hz), alpha 1 (8–10.5 Hz), alpha 2 (10.5–13 Hz), beta 1 (13–20 Hz), beta 2 (20–30 Hz), and gamma (30 – 45 Hz) for each subject. Moving from the definition for the complex-valued coherence [33, 34] between time series x and y in the frequency band ω, which is based on the cross-spectrum given by the covariance and variances of the signals, the lagged linear coherence in the frequency band ω is reported on the following equation. [29, 35]:
where Var and C ov are variances and covariance of the signals x e y and ImCov and ReCov denote the imaginary and real part of covariance, respectively. This equation was developed to provide a measure of true physiological connectivity not affected by volume conduction and with low spatial resolution. The values of connectivity computing between all pairs of ROIs for each frequency band and for each subject were used as measure of weight of the graph in the following graph analyses.
Graph analysis
A network is a mathematical representation of a real-world complex system. It is defined by a set of nodes (vertices) and links (edges) between couples of nodes. Nodes usually represent brain regions, while links represent anatomical, functional, or effective connections, depending on the dataset. Functional connections correspond to magnitudes of temporal correlations in activity and may occur between pairs of anatomically unconnected regions. A weighted graph is a mathematical representation of vertices that may be linked through connections of variable weights. In the present study two measures of graph theory were computed using the toolkit available at http://www.brain-connectivity-toolbox.net and adapted by MATLAB scripts [22, 36, 37] to define the Small World (SW) index: the normalized Clustering Coefficient Cw and the normalized Path Length Lw [38]. The normalization was obtained dividing the values of characteristic path length and of clustering coefficient by the mean values obtained by the average measure of each parameter in all frequency bands. SW index is the ratio of these two measures [39], and it was used to describe the balance between the local connectedness and the global integration of a network.
Statistical Evaluation
A statistical ANOVA design between two factors, Groups (A, B, C) and Time (T0 and T1), was used to evaluate the statistical differences between pre- and post-treatment in terms of ADAS-Cog score through the 3 groups (A, B, C), with a statistical cutoff level of p < 0.05. The same analysis between groups and time was also used but considering A and B together, thus having a treated group and a sham one (A + B, C).
ANOVA design was likewise addressed for the data derived from EEG; in particular, the statistical analysis of the SW values was performed among three factors: Groups (A, B, C), Bands of interest (delta, theta, alpha 1, alpha 2, beta 1, beta 2, gamma), and Time (T0 and T1) and separately for groups A and group B in the factors Bands (delta, theta, alpha 1, alpha 2, beta 1, beta 2, gamma) and Time (T0, T1, T2) with a statistical cutoff level of p < 0.05. ANOVA was implemented with the software Statistica (StatSoft Inc.). The normality of the data was tested using the Kolmogorov–Smirnov test, and the hypothesis of Gaussianity could not be rejected. ANOVA was chosen since it is known to be robust with respect to the departure of normality and homoscedasticity of data being treated [40]. Greenhouse and Geisser correction was used for the protection against the violation of the sphericity assumption in the repeated-measure ANOVA.
Furthermore, in order to evaluate the possible diagnostic and predictive role of SW, in the first case Pearson’s linear correlation analysis was also performed between SW parameter and ADAS-Cog score in every considered time (T0, T1, T2) grouping all patients together. Similarly, correlation analysis was applied to establish the predictive role of SW, correlating its values at T0 and the ADAS-Cog defined at T2 considering all patients together (Bonferroni corrected to obtain p < 0.05).
Results
Behavioral results
The patients in the treatment group (A) exhibited an improvement of -1.40 points on the ADAS-Cog score at T1 immediately after the end of rTMS-COG treatment from T0; this value, albeit not statistically significant, satisfied one endpoint of this study: an improvement of ADAS-Cog score at the end of rTMS-COG treatment. However, the patients in the group B (only COG) showed an improvement of -1.76 points on the ADAS-Cog score, also in this case statistically not significant. An important result to highlight is a worsening of + 0.44 in the ADAS-Cog score in the control group (Table 2).
Table 2.
Alzheimer’s Disease Assessment scale in cognitive subdomain (ADAS-Cog) scores in A, B, C groups at T0, T1, T2 in terms of Mean ± Standard Error (SE). ADAS-Cog differences between baseline T0 and, respectively, T1 and T2 are also shown
| Measurement (ADAS—Cog) |
Group A (Mean ± SE) | Group B (Mean ± SE) | Group C (Mean ± SE) |
|---|---|---|---|
| Pre-treatment (T0) | 23.5 1.44 | 26.7 ± 2.06 | 25.8 ± 1.7 |
| Post-treatment (T1) | 22.2 1.54 | 25.3 ± 2.05 | 26.1 ± 1.7 |
| Difference (T1 – T0) | -1.40 | -1.76 | + 0.44 |
| Last follow-up (T2) |
23.5 3.1 (22 subjs) |
30.1 3.2 (13 subjs) |
52 2.8 (2 subjs) |
| Difference (T2 – T0) | + 1.59 | + 3.29 | + 15 |
Considering the group A and B together as the treated group, the statistical ANOVA for the treated and Sham patients showed a significant interaction (F (1, 61) = 3.7979, p = 0.05) between all factors (groups and Time). The Duncan post hoc analysis showed that only treated patients presented a significant decrement (p = 0.039).
At T2 follow-up, all groups presented a worsening in the ADAS-Cog score although group A presented the less one: A = + 1.59, B = + 3.29, C = + 15. Of note, 22 patients of the group A were available to come back after 40 weeks, 13 of the group B, and only two patients of group C.
Neurophysiological data
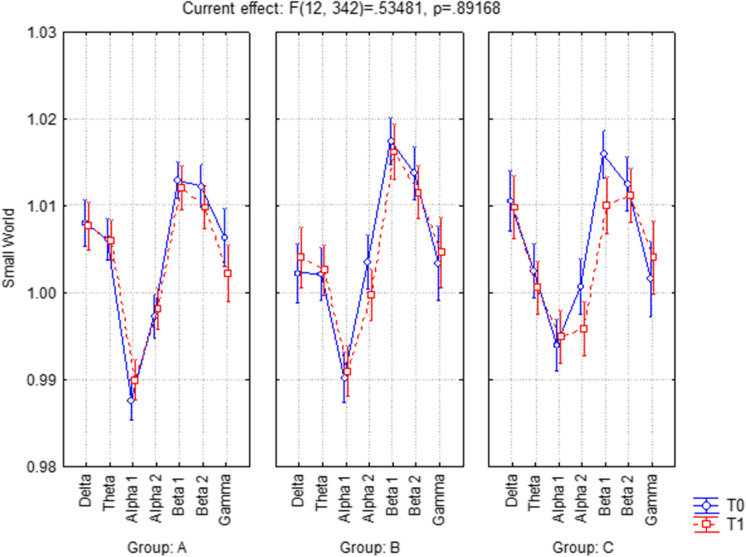
The ANOVA reported in Fig. 2 for the evaluation of the SW index showed not a significant interaction (F(12, 342) = 0.53481, p = 0.89168) between factors time (T0, T1), frequency band (delta, theta, alpha 1, alpha 2, beta 1, beta 2, and gamma), and groups (A, B, C). The conditions of the disease are stable in each group at T1 immediately after the end of the treatment.
Fig. 2.
ANOVA interaction of Small World index among groups (A, B, C), Time (T0, T1), and frequency band (delta, theta, alpha 1, alpha 2, beta 1, beta 2, and gamma). The analysis reported a not significant interaction (F(12, 342) = 0.53481, p = 0.89168) between all factors
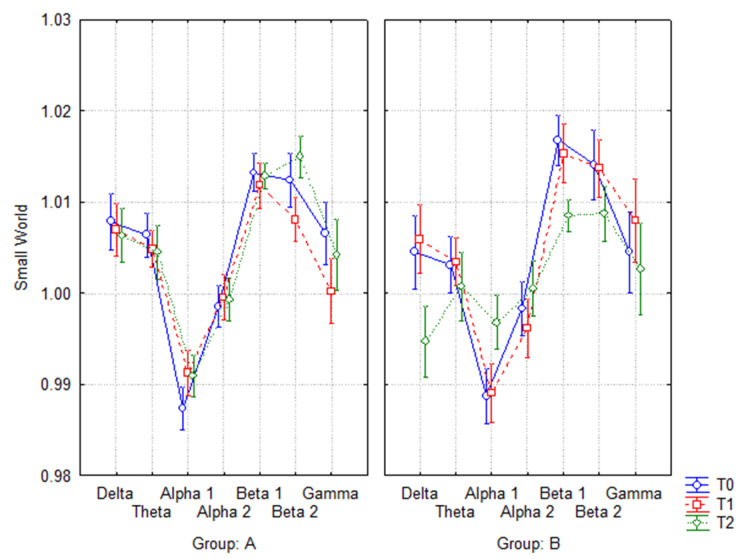
If additionally the last follow-up (T2) was considered, the ANOVA evaluation of the SW index between time (T0, T1, T2) and frequency band (delta, theta, alpha 1, alpha 2, beta 1, beta 2, and gamma) showed a not significant interaction in group A (F(12, 252) = 0.68035, p = 0.76987) (Fig. 3a), while it showed a significant one in group B (F(12, 144) = 1.9675, p = 0.03126) (Fig. 3b). The Duncan post hoc analysis showed that in group B, at the last follow-up (T2) compared to baseline, SW values presented a significant decrease in delta (p = 0.028) and beta 1 (p = 0.05), and a significant increase in alpha 1 (p = 0.05).
Fig. 3.
ANOVA interaction of Small World index in A (3a) (F(12, 252) = 0.68035, p = 0.76987) and B (3b) (F(12, 144) = 1.9675, p = 0.03126) groups at T0, T1, and T2. The Duncan post hoc analysis showed that in group B, at the last follow-up (T2) compared to baseline (T0), SW values presented a significant decrease in delta (p = 0.028), beta 1 (p = 0.05), and a significant increase in alpha 1 (p = 0.05)
Diagnostic and predictive role of SW
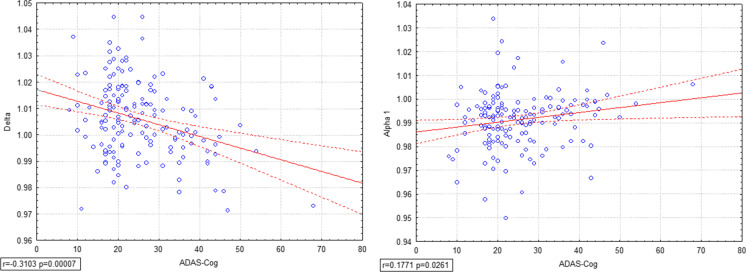
To establish the possible role of diagnostic biomarker, correlation analysis was performed considering all subjects in every time (T0, T1, T2) between the SW index in all frequency bands and the ADAS-Cog score relative to the considered time. The investigation showed a negative correlation between ADAS-Cog and delta SW index (r = -0.3103, p = 0.00007) (Fig. 4a). Furthermore, a positive correlation is present between ADAS-Cog and alpha 1 SW (r = 0.1771, p = 0.0261), namely the higher the alpha 1 SW index, the lower the ADAS-Cog score (which corresponds to a better health condition of the patient) (Fig. 4b).
Fig. 4.
Pearson’s correlation analysis was performed considering all subjects in T0, T1, T2 between Small World in every frequency and ADAS-Cog scores, showing a strong negative correlation between ADAS-Cog and delta SW (r = -0.3103, p = 0.00007) (4a) and a positive one between ADAS-Cog and alpha 1 (r = 0.1771, p = 0.0261) (4b). The lower delta SW or the higher alpha 1 SW index, the lower ADAS-Cog score, corresponding to a better cognitive condition of the patient
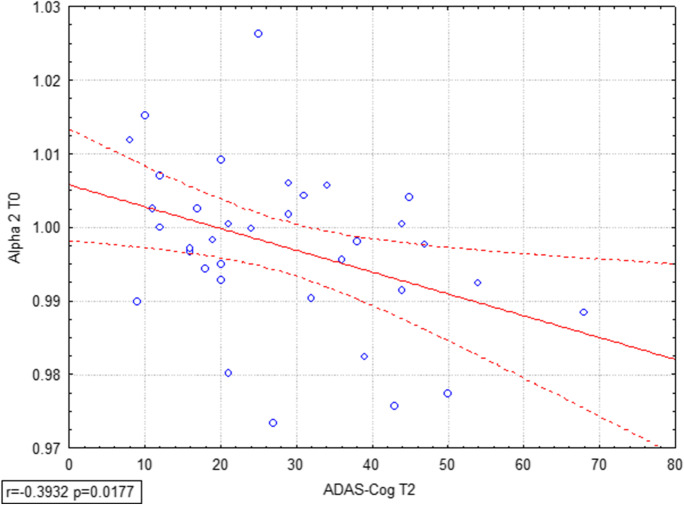
Correlation analyses were also performed considering all subjects as a group and SW indexes at the baseline in all bands; results in Fig. 5 showed that ADAS-Cog in long-term follow-up (T2) correlates negatively with SW in alpha 2 (r = -0.3932, p = 0.0177), underlining the possibility of predicting the improvement of cognition starting by the analysis of EEG data at the beginning of the training session.
Fig. 5.
Pearson’s correlation analysis was performed considering all subjects as a groups Small World values at T0 and ADAS-Cog scores at T2, showing that ADAS-Cog at long-term follow-up T2 correlates negatively with alpha 2 SW (r = -0.3932, p = 0.0177), highlighting the prediction power of alpha 2 SW index
Discussion
Alzheimer’s disease (AD) is the most common neurodegenerative disorder in elderly patients. To date, the current pharmacological treatments for AD are little and transiently efficacious. The main aim of the present work was to evaluate the synergic effects of a 6-week protocol of rTMS combined with cognitive computerized training in mild-to-moderate AD patients compared to placebo groups. The effectiveness of the treatment was matched with the improvement of the patient's cognitive functions and in respect of the brain network organization after the experimental protocol and in a long-term period. Starting from the hypothesis that complex brain dynamics can be characterized by several network measures and that AD is increasingly considered a network disconnection syndrome, functional brain abnormalities can be reflected in early changes of connectivity and networks architecture taking place well in advance to symptoms onset and continuing throughout the disease progression [41–43]. AD brain network topography can be exemplified and explained by means of graph analysis parameters, such as Small World (SW), because of the progressive derangement in hub regions and long-distance connection, causing SW architecture alteration [16, 17, 37, 44, 45].
Regarding the primary objectives in our study, we found that following 6 weeks of intensive daily treatment (at T1) the immediate results showed an improvement in cognition among the AD patients after rTMS-COG treatment; particularly, the mean of ADAS-Cog scores among the group A improved by -1.40 points after rTMS-COG intervention, among group B improved by -1.76 after only cognitive training, compared to a worsening of + 0.44 points in the Control group C. After 40 weeks from treatment, we evaluated only patients in terms of variation in the ADAS-Cog scale to assess the long-term effects of the rTMS on the disease. The outcomes showed a worsening of ADAS-Cog scores compared to the baseline in all groups, with group A showing a significantly lower one (A = + 1.59, B = + 3.29, C = + 15). However, patients in group C evaluated at the long-term follow-up (T2) are only 2. We remember that group C underwent a treatment basically based on the administration of cognitive training, with sham rTMS. The reason lies in a large percentage of patients in this group whose conditions suddenly worsened over this period (about a year) and consequently were not motivated, preferring to leave the study.
A second relevant observation of the current study was to investigate whether the rTMS-Cog protocol was able to modulate cortico-cortical connectivity measured on resting-state and high-density EEG recordings via network-based graph analysis. Brain connectome is, in fact, a useful approach for understanding brain cognitive functions and its alterations [23]. Regarding the evaluation of condition after the end of the treatment, statistical analysis for the evaluation of the SW index showed not a significant variation between T0 and T1 in each group and frequency bands. The conditions of the AD patients are stable in each group at T1 immediately after the end of the treatment. A relevant result to underline stems from the SW at 40 weeks (T2) in comparison with the baseline. Namely, in line with the cognitive results (as evaluated by ADAS-Cog), patients undergoing the complete treatment protocol (group A) showed no differences in terms of SW over time, whereas the ones who were treated through only cognitive training (group B) showed a significant variation in terms of SW in delta, alpha 1, and beta 1 frequency bands.
Finally, the correlation analysis has shown a strong negative correlation between the ADAS-Cog score and delta SW and a positive correlation between the ADAS-Cog score and alpha 1 SW; namely, lower delta SW, higher the ADAS-Cog score that represents a worse cognitive condition of the patient and lower alpha 1 SW, lower the ADAS-Cog score, that emphasizes an improving cognitive state. Furthermore, a significant negative correlation between the improvement of ADAS-Cog score and baseline alpha 2 Small World index was evident, giving alpha 2 SW index the weight of recovery prediction.
In this study, we found considerable effects on dual-outcome assessment scales evaluating cognitive (ADAS-Cog) and functional change at brain networks level. ADAS-Cog is the most widely used scale to assess the level of cognitive dysfunction in AD, and studies demonstrated that ADAS-Cog is able to detect important changes at mild and moderate stages of the disease [46]. Regarding the neuropsychological results, in terms of ADAS-Cog scale, the present findings suggest a potential role of this combined rehabilitation approach in slowing down the progressive decline of cognitive functions and the advancement of disease in AD patients. This result appears remarkable in view of the fact that the disease progression in AD patients across all available and relevant literature sources is estimated as 5.5 points on ADAS-Cog per year, depending on the severity of the disease [47]. An important novelty compared to the previous studies is represented—besides the EEG connectivity analysis—by the enlargement of the time range of observation post-treatment, that we extended until 40 weeks (about 1 year after the intervention) compared with a maximum of 6 weeks as in Brem’s [12] paper. At this time point, we observed a worsening on the mean ADAS-Cog score from baseline in both treatment and sham group, that was lower in the first one compared to the second one, although not statistically significant. However, the contribution of rTMS to treatment success would seem limited from the point of view of the neuropsychological scale and similar results seem to have been achieved with cognitive training alone, at least the end of the treatment.
A further purpose of the current study was to investigate whether the rTMS-Cog protocol was able to modulate cortico-cortical connectivity measured on resting-state and high-density EEG recordings via network-based graph analysis. The parameter evaluated in the present study was the SW, a concept introduced as a model of network organization allowing an optimal balance between local specialization and global integration [38, 48, 49] SW characteristics reflect complex inhibitory and excitatory brain circuits consisting of functionally specialized regions that continuously and mutually cooperate to acquire, share, and integrate information and that are also governed by a number of variables including attention, emotion, motivation and which finally could influence network performance [50, 51]. Unfortunately, it was evident that SW between groups, bands, and time present no significant differences observed after the treatment (T1), whereas various crucial outcomes emerge at T2, considering the 40-week follow-up. In particular, by comparing SW among frequency bands in T0, T1, T2, patients in group A have shown no differences in time; meanwhile, SW values of patients in group B worsen in the different frequency bands (delta, alpha-1, and beta-1). These results are in line with a previous study in which the SW pattern, evaluated in AD and in elderly healthy subjects, presented a SW values in AD lower than normal elderly in the low frequencies band and the opposite one in the alpha band [52]. The observed SW reduction in the low-frequency bands (delta) in group B at T2 represents a more ordered structure being interpreted as a loss of efficiency of the network communication flow and functional disconnection. Miraglia and colleagues [52] showed that AD patients presented lower values of SW in delta band compared to mild cognitive impairment (MCI) patients and to normal elderly people. Additionally, several studies have revealed that an increase of alpha SW parameter correlates with a reduced brain region synchronization and integration, namely a more randomized network, reflecting a cognitive dysfunction. In line with previous evidences a reduction of SW in beta 1 was revealed. Vecchio et al. [53] have shown that beta 1 SW is lower compared to normal elderly people, also demonstrating a positive correlation of this parameter with the hippocampal volume on volumetric MRI. Assessing the subjects in the subsequent follow-ups was therefore crucial to understand whether this improvement was maintained over time and to understand whether rTMS might yield long-term effects not immediately visible. Indeed, rTMS is well-documented method that has been shown to facilitate cortical excitability for long-lasting effects: It can modulate cortical excitability, decreasing or increasing it, depending on the parameters of stimulation with an effect that seems to persist over time [5–7, 54]. Moreover, cognitive functions are regulated by synaptic neuronal activities, and synaptic strength can be altered by co-activation of input neurons. rTMS may promote the neural coactivation to change synaptic strength to further facilitate memory and language [55]. This effect has been demonstrated by other studies, e.g., Cotelli and colleagues identified that rTMS has an important role in improving language and auditory sentence comprehension [9, 56, 57] and then another report showed that rTMS can facilitate verbal responsiveness in AD patients [58].
Lastly, correlation analysis was able to assess the role of diagnostic and prognostic biomarkers to SW indexes. The diagnostic role is played by delta and alpha 1 SW: In particular, a higher score of ADAS-Cog scale, that represents a worse condition in cognitive terms, may be described by a lower SW in the delta band and a higher SW in the alpha 1 band. In addition, the prognostic role is acted by alpha 2 SW analyzed at the baseline: It might be hypothesized that resting SW characteristic in EEG alpha 2 band correlates with recovery and might therefore represent a biomarker of possible functional recovery. This index could be used as a predictive factor for cognitive recovery to address patients with improving function following a rehabilitation therapy, regardless of the therapy itself.
In conclusion, rTMS combined with cognitive training can be regarded as a potentially useful treatment for AD, not modifying the neuropathological changes, but slowing down their effects on brain networks and providing important groundwork for future studies to build upon. Derived EEG parameters can be awarded the role of diagnostic and predictive biomarkers of AD progression.
Limitations and Future Perspectives
Some limitations should be taken into account as listed in the following. The number of patients enrolled is limited based on the availability and feasibility of our clinical center. The results of the present paper highlight that in the long term there are lasting effects potentially given by the rTMS application. In future studies, it would be interesting to increase the number of patients to design a fourth group of rTMS and sham cognitive training with the purpose to emphasize the results obtained in the present paper. Moreover, increase the total number of patients enrolled could be fundamental to define the correlation results which actually have a wide distribution that require a careful interpretation. Furthermore, as the present treatment of rTMS-Cog involved multiple brain networks, future trials could be designed to be more focal to involve specific regions widely demonstrated to be crucial in the alteration during AD, such as the default mode network (DMN). In addition, further researches are necessary to understand which parameters could improve the effectiveness of the treatment: The personalization of the stimulation is certainly crucial and decisive, for example defining as modified parameters, such as the frequency of stimulation, may affect the outcomes of the rehabilitation therapy. Following studies should have a protocol design, testing different rTMS parameters to establish the optimal stimulation based above all on the individuality of the patient.
Acknowledgements
This work was partially supported by the Italian Ministry of Health for Institutional Research (Ricerca corrente) and for the project “NEUROMASTER: NEUROnavigated MAgnetic STimulation in patients with mild-moderate Alzheimer disease combined with Effective cognitive Rehabilitation” (GR-2013- 510 02358430) and by Toto Holding. The authors are also grateful to the Merck Sharp & Dohme (MSD) for the sponsorship.
Author’s contribution
FV contributed to conceptualization, methodology, writing—original draft preparation; DQ and FM helped in supervision, writing—reviewing and editing; CP, RD, FL, MC, and CM were involved in methodology data curation, writing—reviewing and editing; PMR wrote and edited the review.
Data availability
The data that support the findings of this study are available on request from the corresponding author.
Declarations
Conflicts of interest
None of the authors have potential conflicts of interest to be disclosed.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Fargo KN, Aisen P, Albert M, Au R, Corrada MM, DeKosky S, et al. 2014 Report on the Milestones for the US National Plan to Address Alzheimer's Disease. Alzheimers Dement. 2014;10(5 Suppl):S430–S452. doi: 10.1016/j.jalz.2014.08.103. [DOI] [PubMed] [Google Scholar]
- 2.Trebbastoni A, Imbriano L, Podda L, Rendace L, Sacchetti ML, Campanelli A, et al. Cognitive Training in Patients with Alzheimer's Disease: Findings of a 12-month Randomized Controlled Trial. Curr Alzheimer Res. 2018;15(5):452–461. doi: 10.2174/1567205014666171113105044. [DOI] [PubMed] [Google Scholar]
- 3.Bahar-Fuchs A, Clare L, Woods B. Cognitive training and cognitive rehabilitation for mild to moderate Alzheimer's disease and vascular dementia. Cochrane Database Syst Rev. 2013(6):CD003260. [DOI] [PMC free article] [PubMed]
- 4.Kallio EL, Öhman H, Kautiainen H, Hietanen M, Pitkälä K. Cognitive Training Interventions for Patients with Alzheimer's Disease: A Systematic Review. J Alzheimers Dis. 2017;56(4):1349–1372. doi: 10.3233/JAD-160810. [DOI] [PubMed] [Google Scholar]
- 5.Kobayashi M, Pascual-Leone A. Transcranial magnetic stimulation in neurology. Lancet Neurol. 2003;2(3):145–156. doi: 10.1016/s1474-4422(03)00321-1. [DOI] [PubMed] [Google Scholar]
- 6.Rossi S, Hallett M, Rossini PM, Pascual-Leone A, Group SoTC Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 2009;120(12):2008–39. doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rossini PM, Burke D, Chen R, Cohen LG, Daskalakis Z, Di Iorio R, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: Basic principles and procedures for routine clinical and research application. An updated report from an I.F.C.N. Committee. Clin Neurophysiol. 2015;126(6):1071–107. doi: 10.1016/j.clinph.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weiler M, Stieger KC, Long JM, Rapp PR. Transcranial Magnetic Stimulation in Alzheimer's Disease: Are We Ready? eNeuro. 2020;7(1) [DOI] [PMC free article] [PubMed]
- 9.Cotelli M, Manenti R, Cappa SF, Geroldi C, Zanetti O, Rossini PM, et al. Effect of transcranial magnetic stimulation on action naming in patients with Alzheimer disease. Arch Neurol. 2006;63(11):1602–1604. doi: 10.1001/archneur.63.11.1602. [DOI] [PubMed] [Google Scholar]
- 10.Cotelli M, Manenti R, Rosini S, Calabria M, Brambilla M, Bisiacchi PS, et al. Action and Object Naming in Physiological Aging: An rTMS Study. Front Aging Neurosci. 2010;2:151. doi: 10.3389/fnagi.2010.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee J, Choi BH, Oh E, Sohn EH, Lee AY. Treatment of Alzheimer's Disease with Repetitive Transcranial Magnetic Stimulation Combined with Cognitive Training: A Prospective, Randomized, Double-Blind Placebo-Controlled Study. J Clin Neurol. 2016;12(1):57–64. doi: 10.3988/jcn.2016.12.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brem AK, Di Iorio R, Fried PJ, Oliveira-Maia AJ, Marra C, Profice P, et al. Corticomotor Plasticity Predicts Clinical Efficacy of Combined Neuromodulation and Cognitive Training in Alzheimer's Disease. Front Aging Neurosci. 2020;12:200. doi: 10.3389/fnagi.2020.00200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vecchio F, Babiloni C, Lizio R, FallaniFde V, Blinowska K, Verrienti G, et al. Resting state cortical EEG rhythms in Alzheimer's disease: toward EEG markers for clinical applications: a review. Suppl Clin Neurophysiol. 2013;62:223–236. doi: 10.1016/b978-0-7020-5307-8.00015-6. [DOI] [PubMed] [Google Scholar]
- 14.Jelic V, Shigeta M, Julin P, Almkvist O, Winblad B, Wahlund LO. Quantitative electroencephalography power and coherence in Alzheimer's disease and mild cognitive impairment. Dementia. 1996;7(6):314–323. doi: 10.1159/000106897. [DOI] [PubMed] [Google Scholar]
- 15.Rossini PM, Di Iorio R, Vecchio F, Anfossi M, Babiloni C, Bozzali M, et al. Early diagnosis of Alzheimer's disease: the role of biomarkers including advanced EEG signal analysis. Report from the IFCN-sponsored panel of experts. Clin Neurophysiol. 2020;131(6):1287–310. doi: 10.1016/j.clinph.2020.03.003. [DOI] [PubMed] [Google Scholar]
- 16.Vecchio F, Miraglia F, Marra C, Quaranta D, Vita MG, Bramanti P, et al. Human brain networks in cognitive decline: a graph theoretical analysis of cortical connectivity from EEG data. J Alzheimers Dis. 2014;41(1):113–127. doi: 10.3233/JAD-132087. [DOI] [PubMed] [Google Scholar]
- 17.Vecchio F, Miraglia F, Quaranta D, Granata G, Romanello R, Marra C, et al. Cortical connectivity and memory performance in cognitive decline: A study via graph theory from EEG data. Neuroscience. 2016;316:143–150. doi: 10.1016/j.neuroscience.2015.12.036. [DOI] [PubMed] [Google Scholar]
- 18.Stam CJ, Reijneveld JC. Graph theoretical analysis of complex networks in the brain. Nonlinear Biomed Phys. 2007;1(1):3. doi: 10.1186/1753-4631-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34(7):939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 20.McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr, Kawas CH, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rossi S, Hallett M, Rossini PM, Pascual-Leone A. Screening questionnaire before TMS: an update. Clin Neurophysiol. 2011;122(8):1686. doi: 10.1016/j.clinph.2010.12.037. [DOI] [PubMed] [Google Scholar]
- 22.Miraglia F, Vecchio F, Bramanti P, Rossini PM. Small-worldness characteristics and its gender relation in specific hemispheric networks. Neuroscience. 2015;310:1–11. doi: 10.1016/j.neuroscience.2015.09.028. [DOI] [PubMed] [Google Scholar]
- 23.Miraglia F, Vecchio F, Rossini PM. Searching for signs of aging and dementia in EEG through network analysis. Behav Brain Res. 2017;317:292–300. doi: 10.1016/j.bbr.2016.09.057. [DOI] [PubMed] [Google Scholar]
- 24.Vecchio F, Miraglia F, Judica E, Cotelli M, Alù F, Rossini PM. Human brain networks: a graph theoretical analysis of cortical connectivity normative database from EEG data in healthy elderly subjects. Geroscience. 2020. [DOI] [PMC free article] [PubMed]
- 25.Miraglia F, Tomino C, Vecchio F, Alù F, Orticoni A, Judica E, et al. Assessing the dependence of the number of EEG channels in the brain networks' modulations. Brain Res Bull. 2020. [DOI] [PubMed]
- 26.Vecchio F, Tomino C, Miraglia F, Iodice F, Erra C, Di Iorio R, et al. Cortical connectivity from EEG data in acute stroke: A study via graph theory as a potential biomarker for functional recovery. Int J Psychophysiol. 2019;146:133–138. doi: 10.1016/j.ijpsycho.2019.09.012. [DOI] [PubMed] [Google Scholar]
- 27.Hoffmann S, Falkenstein M. The correction of eye blink artefacts in the EEG: a comparison of two prominent methods. PLoS One. 2008;3(8):e3004. [DOI] [PMC free article] [PubMed]
- 28.Iriarte J, Urrestarazu E, Valencia M, Alegre M, Malanda A, Viteri C, et al. Independent component analysis as a tool to eliminate artifacts in EEG: a quantitative study. J Clin Neurophysiol. 2003;20(4):249–257. doi: 10.1097/00004691-200307000-00004. [DOI] [PubMed] [Google Scholar]
- 29.Pascual-Marqui RD, Lehmann D, Koukkou M, Kochi K, Anderer P, Saletu B, et al. Assessing interactions in the brain with exact low-resolution electromagnetic tomography. Philos Trans A Math Phys Eng Sci. 1952;2011(369):3768–3784. doi: 10.1098/rsta.2011.0081. [DOI] [PubMed] [Google Scholar]
- 30.Babiloni C, Cassetta E, Binetti G, Tombini M, Del Percio C, Ferreri F, et al. Resting EEG sources correlate with attentional span in mild cognitive impairment and Alzheimer's disease. Eur J Neurosci. 2007;25(12):3742–3757. doi: 10.1111/j.1460-9568.2007.05601.x. [DOI] [PubMed] [Google Scholar]
- 31.Pascual-Marqui RD, Esslen M, Kochi K, Lehmann D. Functional imaging with low-resolution brain electromagnetic tomography (LORETA): a review. Methods Find Exp Clin Pharmacol. 2002;24(Suppl C):91–5. [PubMed] [Google Scholar]
- 32.Kubicki S, Herrmann WM, Fichte K, Freund G. Reflections on the topics: EEG frequency bands and regulation of vigilance. Pharmakopsychiatr Neuropsychopharmakol. 1979;12(2):237–245. doi: 10.1055/s-0028-1094615. [DOI] [PubMed] [Google Scholar]
- 33.Lehmann D, Faber PL, Tei S, Pascual-Marqui RD, Milz P, Kochi K. Reduced functional connectivity between cortical sources in five meditation traditions detected with lagged coherence using EEG tomography. Neuroimage. 2012;60(2):1574–1586. doi: 10.1016/j.neuroimage.2012.01.042. [DOI] [PubMed] [Google Scholar]
- 34.Nolte G, Bai O, Wheaton L, Mari Z, Vorbach S, Hallett M. Identifying true brain interaction from EEG data using the imaginary part of coherency. Clin Neurophysiol. 2004;115(10):2292–2307. doi: 10.1016/j.clinph.2004.04.029. [DOI] [PubMed] [Google Scholar]
- 35.Pascual-Marqui RD. Instantaneous and lagged measurements of linear and nonlinear dependence between groups of multivariate time series: frequency decomposition. eprint arXiv:07111455.
- 36.Miraglia F, Vecchio F, Bramanti P, Rossini PM. EEG characteristics in "eyes-open" versus "eyes-closed" conditions: Small-world network architecture in healthy aging and age-related brain degeneration. Clin Neurophysiol. 2016;127(2):1261–1268. doi: 10.1016/j.clinph.2015.07.040. [DOI] [PubMed] [Google Scholar]
- 37.Vecchio F, Miraglia F, Bramanti P, Rossini PM. Human brain networks in physiological aging: a graph theoretical analysis of cortical connectivity from EEG data. J Alzheimers Dis. 2014;41(4):1239–1249. doi: 10.3233/JAD-140090. [DOI] [PubMed] [Google Scholar]
- 38.Rubinov M, Sporns O. Complex network measures of brain connectivity: uses and interpretations. Neuroimage. 2010;52(3):1059–1069. doi: 10.1016/j.neuroimage.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 39.Vecchio F, Miraglia F, Iberite F, Lacidogna G, Guglielmi V, Marra C, et al. Sustainable method for Alzheimer dementia prediction in mild cognitive impairment: Electroencephalographic connectivity and graph theory combined with apolipoprotein E. Ann Neurol. 2018;84(2):302–314. doi: 10.1002/ana.25289. [DOI] [PubMed] [Google Scholar]
- 40.Zar JH. Biostatistical analysis. 2nd Edition ed. Englewood Cliffs: Prentice-Hall. 1984. p. 718.
- 41.D'Amelio M, Rossini PM. Brain excitability and connectivity of neuronal assemblies in Alzheimer's disease: from animal models to human findings. Prog Neurobiol. 2012;99(1):42–60. doi: 10.1016/j.pneurobio.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 42.Racz FS, Mukli P, Nagy Z, Eke A. Multifractal dynamics of resting-state functional connectivity in the prefrontal cortex. Physiol Meas. 2018;39(2):024003. doi: 10.1088/1361-6579/aaa916. [DOI] [PubMed] [Google Scholar]
- 43.Friston KaBC. Functional connectivity. 2007;12: 492–507.
- 44.Vecchio F, Miraglia F, Curcio G, Altavilla R, Scrascia F, Giambattistelli F, et al. Cortical brain connectivity evaluated by graph theory in dementia: a correlation study between functional and structural data. J Alzheimers Dis. 2015;45(3):745–756. doi: 10.3233/JAD-142484. [DOI] [PubMed] [Google Scholar]
- 45.Stam CJ. Modern network science of neurological disorders. Nat Rev Neurosci. 2014;15(10):683–695. doi: 10.1038/nrn3801. [DOI] [PubMed] [Google Scholar]
- 46.Kueper JK, Speechley M, Montero-Odasso M. The Alzheimer's Disease Assessment Scale-Cognitive Subscale (ADAS-Cog): Modifications and Responsiveness in Pre-Dementia Populations. A Narrative Review J Alzheimers Dis. 2018;63(2):423–444. doi: 10.3233/JAD-170991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ito K, Corrigan B, Zhao Q, French J, Miller R, Soares H, et al. Disease progression model for cognitive deterioration from Alzheimer's Disease Neuroimaging Initiative database. Alzheimers Dement. 2011;7(2):151–160. doi: 10.1016/j.jalz.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 48.Stam CJ. Characterization of anatomical and functional connectivity in the brain: a complex networks perspective. Int J Psychophysiol. 2010;77(3):186–194. doi: 10.1016/j.ijpsycho.2010.06.024. [DOI] [PubMed] [Google Scholar]
- 49.Liao X, Vasilakos AV, He Y. Small-world human brain networks: Perspectives and challenges. Neurosci Biobehav Rev. 2017;77:286–300. doi: 10.1016/j.neubiorev.2017.03.018. [DOI] [PubMed] [Google Scholar]
- 50.Vecchio F, Miraglia F, Quaranta D, Lacidogna G, Marra C, Rossini PM. Learning Processes and Brain Connectivity in A Cognitive-Motor Task in Neurodegeneration: Evidence from EEG Network Analysis. J Alzheimers Dis. 2018;66(2):471–481. doi: 10.3233/JAD-180342. [DOI] [PubMed] [Google Scholar]
- 51.Reijneveld JC, Ponten SC, Berendse HW, Stam CJ. The application of graph theoretical analysis to complex networks in the brain. Clin Neurophysiol. 2007;118(11):2317–2331. doi: 10.1016/j.clinph.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 52.Miraglia F, Vecchio F, Marra C, Quaranta D, Alù F, Peroni B, et al. Small World Index in Default Mode Network Predicts Progression from Mild Cognitive Impairment to Dementia. Int J Neural Syst. 2020;30(2):2050004. doi: 10.1142/S0129065720500045. [DOI] [PubMed] [Google Scholar]
- 53.Vecchio F, Miraglia F, Piludu F, Granata G, Romanello R, Caulo M, et al. "Small World" architecture in brain connectivity and hippocampal volume in Alzheimer's disease: a study via graph theory from EEG data. Brain Imaging Behav. 2017;11(2):473–485. doi: 10.1007/s11682-016-9528-3. [DOI] [PubMed] [Google Scholar]
- 54.Koch G, Martorana A, Caltagirone C. Transcranial magnetic stimulation: Emerging biomarkers and novel therapeutics in Alzheimer's disease. Neurosci Lett. 2020;719:134355. [DOI] [PubMed]
- 55.Demirtas-Tatlidede A, Vahabzadeh-Hagh AM, Pascual-Leone A. Can noninvasive brain stimulation enhance cognition in neuropsychiatric disorders? Neuropharmacology. 2013;64:566–578. doi: 10.1016/j.neuropharm.2012.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cotelli M, Calabria M, Manenti R, Rosini S, Zanetti O, Cappa SF, et al. Improved language performance in Alzheimer disease following brain stimulation. J Neurol Neurosurg Psychiatry. 2011;82(7):794–797. doi: 10.1136/jnnp.2009.197848. [DOI] [PubMed] [Google Scholar]
- 57.Cotelli M, Adenzato M, Cantoni V, Manenti R, Alberici A, Enrici I, et al. Enhancing theory of mind in behavioural variant frontotemporal dementia with transcranial direct current stimulation. Cogn Affect Behav Neurosci. 2018;18(6):1065–1075. doi: 10.3758/s13415-018-0622-4. [DOI] [PubMed] [Google Scholar]
- 58.Devi G, Voss HU, Levine D, Abrassart D, Heier L, Halper J, et al. Open-label, short-term, repetitive transcranial magnetic stimulation in patients with Alzheimer's disease with functional imaging correlates and literature review. Am J Alzheimers Dis Other Demen. 2014;29(3):248–255. doi: 10.1177/1533317513517047. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.